Abstract
Neuroendocrine (NE) differentiation in prostatic adenocarcinomas has been reported to be an early marker for development of androgen independence. Secretion of mitogenic peptides from nondividing NE cells is thought to contribute to a more aggressive disease by promoting the proliferation of surrounding tumor cells. We undertook studies to determine whether the prostate cancer cell line LNCaP could be induced to acquire NE characteristics by treatment with agents that are found in the complex environment in which progression of prostate cancer towards androgen independence occurs. We found that cotreatment of LNCaP cells with agents that signal through cyclic AMP-dependent protein kinase (PKA), such as epinephrine and forskolin, and with the cytokine interleukin-6 (IL-6) promoted the acquisition of an NE morphological phenotype above that seen with single agents. Convergent IL-6 and PKA signaling also resulted in potentiated mitogen-activated protein kinase (MAPK) activation without affecting the level of signal transducer and activator of transcription or PKA activation observed with these agents alone. Cotreatment with epinephrine and IL-6 synergistically increased c-fos transcription as well as transcription from the β4 nicotinic acetylcholine receptor subunit promoter. Potentiated transcription from these elements was shown to be dependent on the MAPK pathway. Most importantly, cotreatment with PKA activators and IL-6 resulted in increased secretion of mitogenic neuropeptides. These results indicate that PKA and IL-6 signaling participates in gene transcriptional changes that reflect acquisition of an NE phenotype by LNCaP cells and suggest that similar signaling mechanisms, particularly at sites of metastasis, may be responsible for the increased NE content of many advanced prostate carcinomas.
The androgen-responsive prostate tumor cell line LNCaP has emerged as a useful model for characterizing the development of cells with a neuroendocrine (NE) phenotype from prostatic adenocarcinoma cells. LNCaP cells have now been shown to acquire NE characteristics in response to a number of culture conditions, including increased intracellular cyclic AMP (cAMP) levels (3, 14), long-term androgen deprivation (39), and stimulation with the cytokines interleukin-1β (IL-1β) and IL-6 (17, 34).
Stimulation with activators of adenylate cyclase, such as forskolin (Fsk), and epinephrine (Epi) or isoproternol reversibly induces acquisition of NE characteristics by LNCaP cells (14). These characteristics include rounding of the cell body; appearance of long, branched neurite-like processes; development of secretory vesicles; inhibition of mitotic activity; increased expression of NE markers such as serotonin and neuron-specific enolase (NSE); and secretion of mitogenic neuropeptides such as neurotensin and parathyroid hormone-related peptide (PTHrP). Epi-induced NE differentiation of LNCaP cells involves increases in intracellular cAMP (14) and activation of cAMP-dependent protein kinase (PKA) (13). PKA-mediated signaling was found to be required and sufficient for NE differentiation in response to Epi, Fsk, and isoproternal but not IL-6 (13). Serum deprivation is capable of inducing some of these same NE characteristics (39) and results in increased steady-state cAMP levels (9). These findings suggest that tumor cell phenotypes may be dynamic and determined in part by the balance of differentiation and mitogenic factors in the local environment, and they indicate that PKA plays a central role in the acquisition of NE characteristics by LNCaP cells.
The pleiotropic cytokine IL-6 is a key mediator of host immune defense responses due to its wide range of effects on T-cell, B-cell, and macrophage responses (21, 27). In addition, IL-6 has been implicated in the pathology of numerous malignancies and autoimmune diseases (41). In prostate cancer, IL-6 and its receptor are candidate mediators of morbidity (47). Elevated levels of IL-6 receptor have been detected in prostatic hyperplasia and carcinoma tissues (40), while increased IL-6 levels are found in the circulation of patients with metastatic disease (1, 47) and correlate with hormone-refractory disease (18).
In LNCaP cells, IL-6 has been suggested to have both growth-promoting and -inhibiting activities. Several studies have indicated that IL-6 can mediate growth arrest (15, 43) and acquisition of NE characteristics, including development of a neuritic morphology and increased synthesis of NSE (34) and the granin family member chromogranin A (17), through activation of the eph-like tyrosine kinase Etk (34) and signal transducer and activator of transcription 3 (STAT3) (44). These results are contradicted by reports that IL-6-expressing LNCaP cells exhibit increased growth rates and clonogenesis correlated with increased STAT3 activation (29) and that IL-6 enhances androgen-induced growth by either autocrine or paracrine mechanisms (31).
In vivo, cytokine signaling occurs in the context of numerous other autocrine and paracrine stimuli, including adenylate cyclase activators. The metastatic sites in which progression of prostate cancer towards androgen independence occurs, such as lymph node and bone, represent such environments. We have therefore investigated the ability of IL-6 to affect NE differentiation in the context of PKA-mediated signaling in an effort to resolve the conflicting effects on LNCaP cells reported for IL-6. We undertook studies to determine whether the acquisition of NE characteristics by LNCaP cells could be enhanced following cotreatment with either the β-adrenergic receptor agonist Epi or the adenylate cyclase agonist Fsk and the cytokine IL-6. We found that these two different classes of factors synergize with one another to enhance the development of NE characteristics by LNCaP cells.
MATERIALS AND METHODS
Cell culture and treatments.
LNCaP cells were obtained from L. W. Chung (University of Virginia, Charlottesville) and maintained in T-medium (Gibco-BRL, Gaithersburg, Md.) containing 5% fetal bovine serum (FBS) (Gibco-BRL) at 37°C in a humidified, 5% CO2 environment (45). Fsk, Epi, isobutylmethylxanthine (IBMX), and epidermal growth factor (EGF) were purchased from Sigma (St. Louis, Mo.). IL-6 was obtained from Calbiochem (La Jolla, Calif.). Cells were treated in the presence of normal growth medium containing serum by directly adding the differentiation agents to the culture medium at the indicated concentrations, except for the analysis of c-fos transcriptional activity in Fig. 7. The mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinase kinase (MEK) activation inhibitors PD098059 and U0126 were purchased from Biomol (Plymouth Meeting, Pa.) and Calbiochem, respectively.
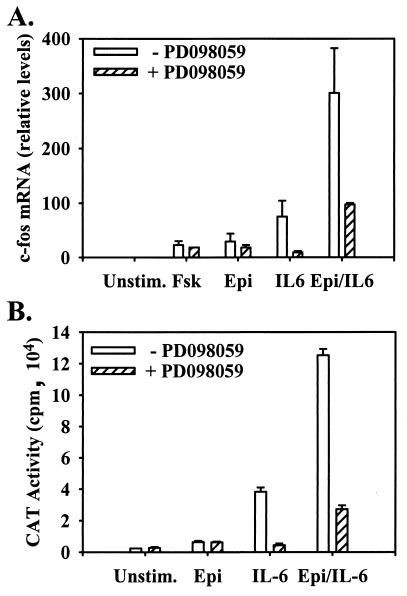
FIG. 7.
Potentiation of c-fos transcription in LNCaP cells by IL-6 and Epi cotreatment is eliminated by inhibition of the MEK-MAPK pathway. LNCaP cells were cultured overnight in serum-free, phenol red-free RPMI 1640 and stimulated for 1 h with vehicle (DMSO) (Unstim.), 10 μM Fsk, 10 μM Epi, 2 nM IL-6, or Epi and IL-6 (Epi/IL-6). (A) RNase protection analysis using a riboprobe spanning the first two exons of human c-fos was performed for the treatments in the presence and absence of the MEK inhibitor PD098059, quantitated with a phosphorimager, and expressed relative to unstimulated cells. (mean ± SEM; n = 3). (B) c-fos promoter-driven CAT expression was assessed in LNCaP cells transfected with pFosCAT and treated as for panel A (mean ± SEM; n = 3).
Mitotic activity measurements. (i) [3H]thymidine labeling.
Mitotic activity was assessed by [3H]thymidine incorporation as previously described (14). Briefly, 105 LNCaP cells were plated in 35-mm-diameter culture dishes and treated with the appropriate agents as indicated. [3H]thymidine (20 Ci/mmol) (New England Nuclear, Boston, Mass.) was added at 10 μCi per well and left for 24 h prior to harvesting of the cells for mitotic activity analysis. Cells were counted with a hemocytometer and lysed in 10% cold trichloroacetic acid. The resulting precipitate was pelleted and solubilized in 0.2 ml of 0.4 N sodium hydroxide, and the acid-insoluble radioactivity was measured by liquid scintillation counting. The mitotic activity of each treatment population was calculated as the mean acid-insoluble 3H counts per minute per cell ± standard error of the mean (SEM) for three independent experiments, each performed in triplicate, normalized to the mitotic activity of the respective untreated cultures.
(ii) BrdU labeling.
Bromodeoxyuridine (BrdU) (Sigma) labeling was performed by adding 100 μM BrdU to the culture medium of cells during the last 24 h of incubation. BrdU incorporation into DNA was assessed by immunofluorescence microscopy of paraformaldehyde-fixed cells using anti-BrdU–fluorescein isothiocyanate antibody (Boeringer-Mannheim, Indianapolis, Ind.) as previously described (13). The mitotic activity of each treatment population was calculated as the percent BrdU incorporation relative to that for untreated cells for three independent experiments ± SEM. The relatively long [3H]thymidine and BrdU labeling time represents ∼75% of the doubling time of LNCaP cells cultured in T-medium. This length of time was used in order to accommodate the very low probability that LNCaP cells would undergoing DNA synthesis upon NE differentiation as previously reported (13, 14). We have not detected any measurable cytotoxicity attributable to these labeling treatments.
Morphological analysis.
Live LNCaP cells were imaged by photomicroscopy using phase-contrast optics (Leica, Rijswijk, The Netherlands). The numbers of neuritic branch points and cells bodies were counted in photomicrographs of random fields of each respective treatment and expressed as branch points per 100 cells. The data presented are the mean derived from counting at least 200 cells per treatment from four independent experiments ± SEM.
Immunoblotting.
After treatment, cells were washed with phosphate-buffered saline (50 mM Na phosphate, 150 mM NaCl, pH 7.4) and lysed in HO buffer (50 mM HEPES, 100 mM NaCl, 1% Nonidet P-40, 2 mM EDTA, 1 μg of leupeptin per ml, 2 μg of aprotinin per ml, 0.5 mM Na-vanadate, 40 mM p-nitrophenyl phosphate, 2 μM microcystin, pH 7.2) on ice. Lysates were clarified by centrifugation at 10,000 × g for 10 min at 4°C and processed for sodium dodecyl sulfate-polyacrylamide gel electrophoresis and electrophoretic transfer to nitrocellulose (Schleicher and Schuell, Keene, N.H.), as described previously (19). STAT3 activation was determined by immunoblotting with phospho-specific STAT3 antibodies directed against phosphotyrosine 705 (Calbiochem) and phosphoserine 727 (Upstate Biotechnology, Lake Placid, N.Y.). Total STAT3 was determined using a phosphorylation-independent STAT3 antibody (Santa Cruz Biotechnology, Santa Cruz, Calif.). Antibodies specific for STAT1, STAT5a, and STAT5b were generous gifts of C. Silva (University of Virginia). Phosphorylated MAPK was detected by blotting with anti-ACTIVE MAPK (Promega, Madison, Wis.). STAT tyrosine phosphorylation was detected with horseradish peroxidase (HRP)-conjugated antiphosphotyrosine antibody RC-20 (Transduction Laboratories). The anti-βIII tubulin monoclonal antibody TuJ1 was provided by A. Frankfurter (University of Virginia), and anti-NSE monoclonal antibody was purchased from DAKO Inc. (Carpinteria, Calif.). The latter two antibodies were visualized by enhanced chemiluminescence (Amersham Pharmacia, Buckinghamshire, England) using HRP-conjugated anti-mouse secondary antibody. All other primary antibodies were detected with HRP-conjugated anti-rabbit antibody or HRP-conjugated protein A (Amersham Life Sciences). All immunoblots shown are representative of at least three independent experiments.
Kinase assays.
PKA activity was assessed by immune-complex kinase assay as previously described (13). Briefly, cells were pretreated with 50 μM PD098059 or an equal volume of dimethyl sulfoxide (DMSO) (as a vehicle control) for 10 min prior to agonist stimulation. After the indicated treatments, cell lysates were prepared as described above in HO buffer containing 100 μM IBMX to inhibit intrinsic cyclic nucleotide phosphodiesterase activity and subjected to immunoprecipitation using 500 μg of lysate protein and 1 μg of rabbit polyclonal anti-PKA CIα catalytic subunit antibody (Santa Cruz Biotechnology). Immune complexes were collected on protein A-Sepharose (Sigma) and washed three times with HO lysis buffer and once with kinase buffer (50 mM HEPES [pH 7.4], 10 mM MgCl2, 1 mM EGTA, 0.014% Tween 20). Immune complexes were incubated at 30°C for 10 min in 50 μl of kinase buffer containing 1 mM [γ-32P]ATP (1 μCi/nmol) (New England Nuclear) and a 28 μM concentration of the synthetic PKA peptide substrate Kemptide (Sigma). Reactions were terminated by the addition of 10 μl of 1 M HCl, and 35 μl of the reaction mixture was spotted onto 1-cm2 strips of phosphocellulose (P81; Whatman, Kent, United Kingdom). The P81 strips were washed four times for 10 min each in 75 mM H3PO4 and once in methanol, dried, and counted by Cherenkov radiation.
PKA-specific activity is defined as the picomoles of phosphate incorporated into Kemptide substrate per unit of PKA CIα immunoprecipitated. The amount of immunoprecipitated PKA was determined by densitometric analysis of anti-PKA catalytic subunit immunoblots prepared from the remaining immune-complex kinase assay reaction mixture. Under these conditions, less than 20% of the peptide substrate was phosphorylated. All values within a given experiment were normalized to the relative specific activity of PKA from untreated cells, which was set to an arbitrary value of 1.
RNase protection assay.
Cells (0.5 × 106 to 1 × 106) were serum starved overnight in serum-free, phenol red-free RPMI 1640 and stimulated for 1 h with either vehicle (DMSO), 10 μM Fsk, 10 μM Epi, 2 nM IL-6, or Epi and IL-6. Preparation of total cytoplasmic RNA was performed using an RNeasy purification kit (Qiagen, Valencia, Calif.). RNase protection assays were performed as previously described (20). c-fos probe was transcribed from NarI-digested pB1cfos (a generous gift of D. Engel, University of Virginia). The probe (10,000 to 15,000 cpm) and 10 μg of RNA were added to the hybridization mixtures.
CAT and luciferase assays.
LNCaP cells (3 × 105) were plated into 60-mm-diameter dishes, and 3 μg of either a chloramphenicol acetyltransferase (CAT) reporter plasmid for the c-fos promoter, −356/fos-CAT (a generous gift of D. Engel), or a luciferase reporter plasmid for the β4 nicotinic acetylcholine receptor (nAChR) promoter, pX1B4BH (6), was transfected using Lipofectin (Gibco-BRL) as described by the manufacturer. For CAT assays, cells were treated as indicated and lysates were assayed for luciferase activity as directed by PharMingen (San Diego, Calif.) and assayed in a Monolight 2010 luminometer (Analytical Luminescence Laboratory). Results were normalized to protein content and represent the mean ± SEM from three independent reporter plasmid transfection experiments.
EIAs.
Conditioned culture medium from LNCaP cells was prepared by plating 2 × 105 cells/well in 24-well culture dishes. Cells were allowed to adhere overnight and treated with the appropriate agents as indicated in 0.5 ml of culture medium. Medium from the cells was collected, cleared by centrifugation (14,000 × g, 10 min) and stored at −70°C prior to analysis. Detection of the neurosecretory peptides PTHrP and neurotensin was performed using enzyme-linked immunoassays (EIAs) for the respective peptides (Peninsula Laboratories, Inc., Belmont, Calif.) as recommended by the manufacturer. All treatments were assessed in four independent experiments, each performed in duplicate.
Data analysis.
Results are depicted as the mean ± SEM from at least three independent experiments. Photomicrographs of representative fields and immunoblots are provided to demonstrate the primary data. Statistical significance of paired treatments was assessed by Student's t test.
RESULTS
Cotreatment of LNCaP cells with Epi and IL-6 enhances NE differentiation.
The androgen-responsive prostate cancer cell line LNCaP can be induced to acquire characteristics of NE or amine precursor and decarboxylation cells associated with many prostatic adenocarcinomas. To determine if NE differentiation agonists could act cooperatively to enhance the acquisition of NE characteristics, cells were treated with maximally active concentrations of Epi and IL-6, acquired from dose-response curves of activation for PKA and STAT3 (data not shown), alone or in combination. Photomicrographs are presented in Fig. 1A to D to illustrate the morphological changes induced in response to the different treatments. After 2 days, treatment of LNCaP cells with 5 μM Epi resulted in a moderate level of morphological differentiation as evidenced by the appearance of neuritic extensions that often possessed growth cone-like structures. Fewer growth cone-like structures and longer neuritic processes were induced by 2 nM IL-6 than by Epi treatment. When treated with Epi and IL-6 together, however, cells appeared to differentiate more rapidly and to a greater degree than with either treatment alone.
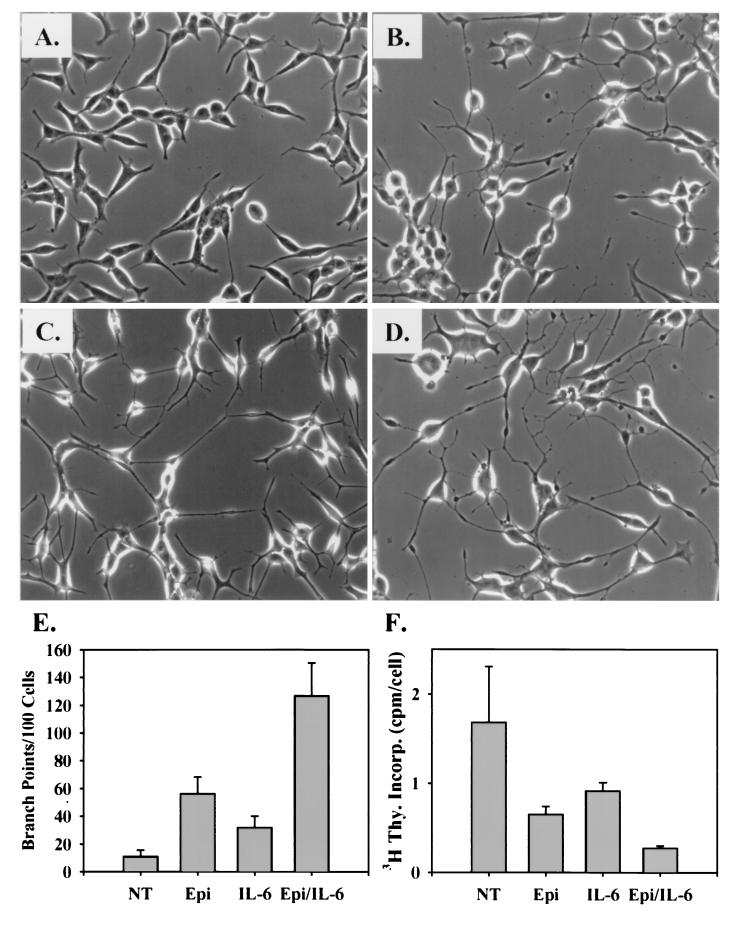
FIG. 1.
Synergistic acquisition of an NE morphology by Epi and IL-6 cotreatment. (A to D) LNCaP cells seeded at ∼30% confluence in T-medium plus 5% FBS were treated with DMSO alone (A) 5 μM Epi and 500 μM IBMX (B), 2 nM IL-6 (C), or both Epi and IL-6 (D). Cell morphology was photographed at a magnification of 20 × 2 days after treatment. (E) Neuritic branch points and cell bodies were counted as described in Materials and Methods and expressed as the mean number of branch points per 100 cells ± SEM for each treatment from four independent experiments. (F) Mitotic activity was measured during the last 24 h of the indicated treatments by incorporation of [3H]thymidine (3H Thy. Incorp.) and expressed as the mean counts per minute per cell ± SEM from three independent experiments. NT, DMSO alone.
Neuritic branching has been used as a direct measure of neuronal morphogenesis in response to neurogenic factors (11, 28, 35) and was used in this study to quantitate the influence of Epi and IL-6 treatment on the acquisition of the neuritic morphology (Fig. 1E). We found that while untreated LNCaP cells normally exhibit short, rarely branched cellular processes and cells treated with Epi or IL-6 exhibit modest branching, cells treated with both Epi and IL-6 exhibit neuritic branching two- and fourfold greater than that with either agent alone, respectively. Therefore, cotreating cells with Epi and IL-6 resulted in a greater-than-additive increased rate of morphological differentiation.
We (14) and others (9, 34) have previously demonstrated a direct correlation between morphological differentiation and inhibition of mitotic activity of LNCaP cells undergoing NE differentiation. We therefore used [3H]thymidine incorporation to measure the mitotic activity of cells treated with Epi, IL-6, and both agents (Fig. 1F). Relative to untreated cells, the mitotic activity of Epi-treated cells was reduced by ∼60%, that of IL-6-treated cells was reduced by ∼45%, and that of Epi- and IL-6-treated cells was reduced by ∼85%. These results directly correlate with the neuritic branching index and support our initial analysis that cotreating cells with Epi and IL-6 enhances the extent of NE differentiation of LNCaP cells.
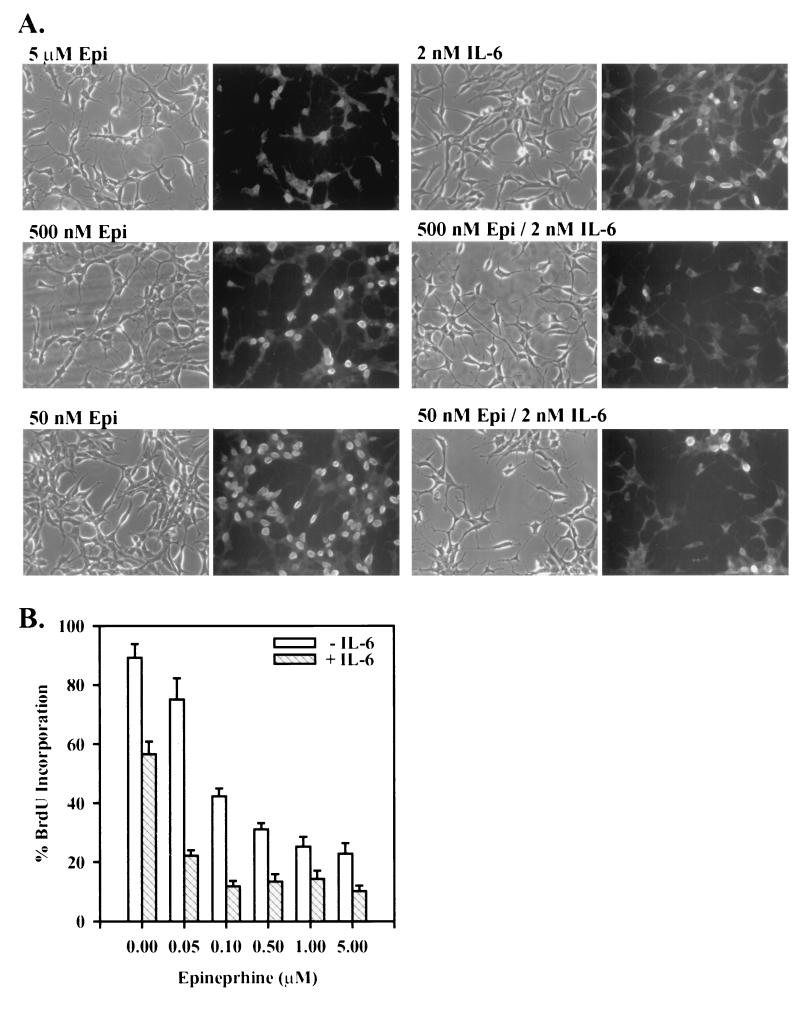
Epi appeared to have a more dramatic effect on NE differentiation than IL-6. Therefore, in order to test the ability of Epi and IL-6 to potentiate differentiation when one component is present at suboptimal concentrations, LNCaP cells were treated with Epi at doses ranging from 50 nM to 5 μM. At the lowest concentrations of Epi, little if any morphological change was observed (Fig. 2A) and cells continued to proliferate at about 80% of the rate of untreated LNCaP cells as measured by BrdU incorporation (Fig. 2B), whereas cells treated with the maximal Epi dosage underwent the expected morphological transformation and exhibited a mitotic activity ∼20% of that of untreated cells. The mitotic activity of cells treated with 2 nM IL-6 alone was ∼65% of that of untreated cells. The addition of IL-6, however, increased the degree of morphological transformation and significantly decreased the mitotic activity of cells treated with the corresponding concentrations of Epi alone (Fig 2B). This synergistic response is illustrated by the ability of 2 nM IL-6 to decrease the mitotic activity of LNCaP cells treated with 50 nM Epi fourfold, to a level observed with 5 μM Epi alone. These results indicate that IL-6 can potentiate the ability of suboptimal Epi doses to induce acquisition of an NE phenotype by LNCaP cells.
FIG. 2.
Synergistic inhibition of mitotic activity by suboptimal doses of Epi and IL-6. (A) Representative phase-contrast and BrdU-staining photomicrographs of LNCaP cells cultured in T-medium plus 5% FBS and treated with Epi at concentrations ranging from 50 nM to 5 μM in both the absence (left micrographs) and presence (right micrographs) of 2 nM IL-6 for 2 days. Cells were incubated in 100 μM BrdU for the last 24 h, fixed, and stained with a fluorescein isothiocyanate-conjugated anti-BrdU antibody. (B) The mitotic activity was determined as the percent BrdU-positive cells ± SEM by counting 100 to 200 cells per treatment in random fields from three independent experiments.
IL-6 and Epi differentially regulate expression of NE markers.
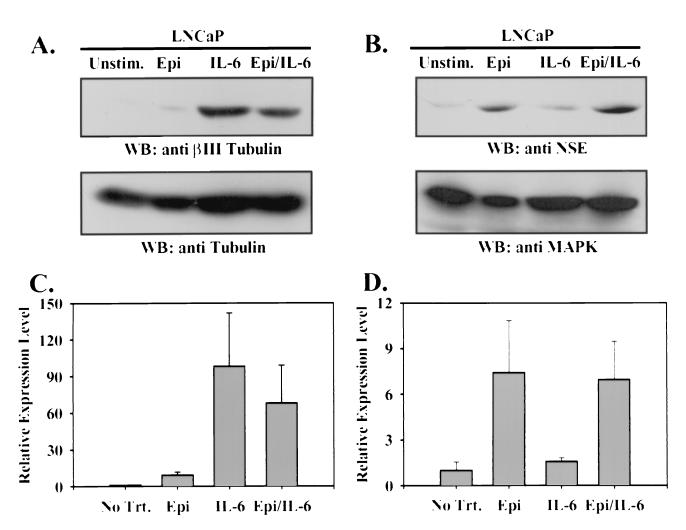
NE-like LNCaP cells were tested for expression of molecular markers found preferentially in cells of neuronal origin. By immunoblot analysis, expression of a neuronal isoform of tubulin, βIII tubulin (24–26), was essentially undetectable in LNCaP cells under normal culture conditions and was preferentially increased in response to IL-6 treatment compared to Epi treatment (Fig. 3A). On average, βIII tubulin expression was 10-fold higher in IL-6-treated cells than in Epi-treated cells. Conversely, while NSE expression was detectable at basal levels in unstimulated LNCaP cells, it was increased preferentially in response to Epi (7-fold) but only marginally in response to IL-6 (1.5-fold) (Fig. 3B). In cells treated with both Epi and IL-6, expression of neither marker was significantly affected relative to treatment with the predominant inducing agent alone. However, expression of βIII tubulin in Epi- and IL-6-treated cells was consistently lower than that in cells treated with IL-6 alone. Bovine adrenal chromaffin cells, a well characterized NE cell line, showed high expression of both neuronal markers, while murine fibroblasts did not express detectable levels of either molecule (data not shown). These results illustrate at a biochemical level the differential signaling responses activated in LNCaP cells by IL-6 and Epi.
FIG. 3.
Epi and IL-6 induce expression of distinct neuronal markers. Immunoblots (WB) were prepared from 50 μg of whole-cell lysate of LNCaP cells cultured for 3 days in T-medium plus 5% FBS (Unstim.) or stimulated with Epi and IBMX (Epi), IL-6, or Epi, IBMX, and IL-6 (Epi/IL-6) as for Fig. 1 with anti-βIII tubulin antiserum (A, upper panel), total tubulin (A, lower panel), anti-NSE antiserum (B, upper panel), or anti-MAPK antiserum (B, lower panel). Expression of each marker was quantitated by densitometric scanning of the autoradiograms and expressed as the mean fold change in βIII tubulin expression relative to total tubulin expression (C) and the fold change in NSE expression relative to MAPK expression (D), normalized to the ratio in unstimulated cells ± SEM from three independent experiments.
Cotreatment with Epi and IL-6 potentiates secretion of mitogenic neuropeptides.
The role proposed for NE cells in prostate cancer progression is the ability to secrete factors that support the growth and survival of surrounding tumor cells under androgen-deprived conditions. We previously demonstrated that LNCaP cells induced to undergo NE differentiation by activation of adenlyate cyclase secrete PTHrP and neurotensin (14). These neuropeptides have been shown to increase mitogenesis of prostate tumor cells (23, 37). To determine whether IL-6 could also induce secretion of these factors and whether Epi and IL-6 cooperatively affect secretion of PTHrP and neurotensin, EIAs were performed on conditioned medium from LNCaP cells following treatment with Epi, IL-6, or Epi and IL-6 (Table 1). Relative to unstimulated cells, IL-6 induced a twofold increase in PTHrP accumulation in conditioned medium, compared to an almost threefold increase observed in response to Epi, while cells stimulated with both agents exhibited an eightfold increase in PTHrP accumulation. Neurotensin accumulation was not significantly induced by IL-6 treatment, but it was increased over sixfold by Epi treatment alone. However, as was the case for PTHrP secretion, when LNCaP cells were treated with Epi and IL-6, neurotensin levels were elevated to 25 times those in the conditioned medium from unstimulated cells. Since the neuropeptide level detected in the conditioned medium of untreated cells was equivalent to the threshold detection level of each respective EIA, the fold increases shown here may minimize the actual increases. These results demonstrate the ability of Epi and IL-6 to potentiate secretion of mitogenic factors for prostatic NE cells over the levels seen in response to treatment with either agent alone.
TABLE 1.
Epi- and IL-6-induced mitogenic neuropeptide secretiona
| Treatment | Amt (ng/106 cells)b of:
|
|
|---|---|---|
| PTHrP | Neurotensin | |
| No stimulation | 0.189 ± 0.071 | 0.102 ± 0.007 |
| Epi | 0.539 ± 0.080c | 0.672 ± 0.318c |
| IL-6 | 0.386 ± 0.261 | 0.116 ± 0.039 |
| Epi plus IL-6 | 1.513 ± 0.398cd | 2.570 ± 1.073cd |
LNCaP cells were cultured in serum-free T-medium and either left unstimulated or treated with 10 μM Epi and 500 μM IBMX (Epi), 2 nM IL-6, or both Epi and IL-6 with IBMX (Epi plus IL-6) for 6 days. PTHrP and neurotensin levels in conditioned medium or T-medium alone (not shown) were measured using an EIA for the respective peptides.
Means ± SEM from three independent experiments are shown.
Significantly greater than value for unstimulated cells by two-sided, two-tailed Student's t test (P < 0.05).
Significantly greater than value for cells stimulated with Epi or IL-6 alone by Student's t test (P < 0.05).
STAT3 is the predominant STAT family member activated in response to IL-6 treatment.
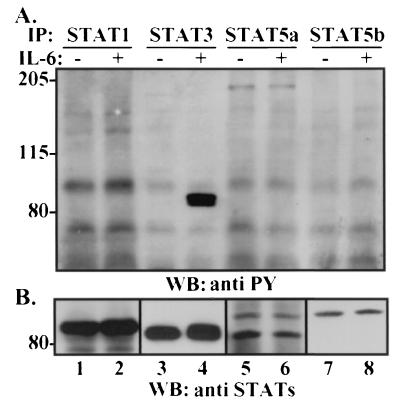
Although IL-6 has been suggested to play a role in prostate cancer progression, its function has not been resolved. Furthermore, while Etk, phosphatidylinositol 3-kinase (PI3K), and the human EGF receptor family member HER2 have been implicated in downstream signaling from the IL-6 receptor (33, 34), the best-characterized effector pathway for IL-6 is activation of members of the STAT family of transcription factors. Using dominant-negative strategies, it has been suggested that STAT3 activation is required for IL-6-induced differentiation of LNCaP cells (44). Those authors did not, however, determine whether other STAT molecules are activated upon IL-6-induced NE differentiation and whether overexpression of dominant-negative STAT3 influenced the activation of other potentially activate STATs. To examine this question, a panel of antibodies was used to determine the STAT family expression profile in LNCaP cells and their respective levels of tyrosine phosphorylation, as a measure of STAT activation. By immunoblotting, we were able to readily detect STAT1, STAT3, STAT5a, and STAT5b proteins in LNCaP lysates (Fig. 4B). Of these STATs, tyrosine phosphorylation of only STAT3 was detected upon IL-6 treatment (Fig. 4A, lane 4). Treatment with Epi alone activated none of the STATs examined (see Fig. 6; and data not shown). Thus, in agreement with Spiotto and Chung (44), we found STAT3 to be the only STAT activated in response to IL-6 stimulation of LNCaP cells.
FIG. 4.
STAT3 is the predominant STAT activated by IL-6 in LNCaP cells. LNCaP cells were cultured in T-medium plus 5% FBS and either not treated or treated with 2 nM IL-6 for 20 min. Cytosolic lysates (500 μg) were immunoprecipitated (IP) with antibodies to STAT1 (lanes 1 and 2), STAT3 (lanes 3 and 4), STAT5a (lanes 5 and 6), and STAT5b (lanes 7 and 8) and subjected to immunoblot analysis (WB) with antiphosphotyrosine (anti PY) antibody (A) and antibodies against the cognate STAT proteins (B). The antiphosphotyrosine immunoblot is shown overexposed to demonstrate that phosphotyrosine could be detected only in STAT3 under these conditions. Numbers on the left are molecular masses in kilodaltons.
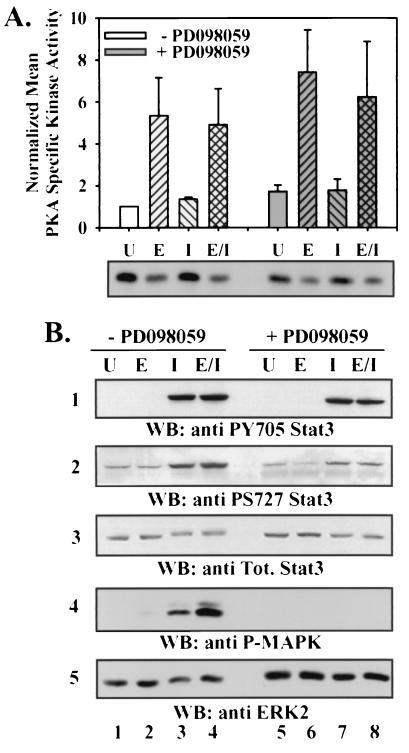
FIG. 6.
Effect of Epi and IL-6 treatment and MEK inhibition on PKA and STAT3 activation. LNCaP cells were cultured in T-medium plus 5% FBS in the absence (white bars; lanes 1 to 4) or presence (shaded bars; lanes 5 to 8) of 50 μM PD098059 for 10 min and left untreated (U) (lanes 1 and 5) or stimulated with 10 μM Epi (E) (lanes 2 and 6), 2 nM IL-6 (I) (lanes 3 and 7), or both Epi and IL-6 (E/I) (lanes 4 and 8) for 20 min. (A) PKA catalytic subunit (CIα) immunoprecipitates were split and subjected to immune-complex kinase assay and immunoblotting of the PKA catalytic subunit. PKA activity was normalized to levels of catalytic subunit protein in the immunoprecipitates and to basal activity in unstimulated, non-PD098059-treated cells. Error bars indicate SEMs. (B) Cytosolic lysates (50 μg) were subjected to immunoblot analysis (WB) for phosphorylation of STAT3 Y705 (panel 1), phosphorylation of STAT3 S727 (panel 2), and activated MAPK (panel 4), as well as for total (Tot.) STAT3 (panel 3) and total ERK2 (panel 5).
Synergistic activation of MAPK in LNCaP cells stimulated with Fsk and IL-6.
Having shown that IL-6 and adenylate cyclase activators can signal through separate pathways, we next addressed potential signaling mechanisms that might account for the observed biological synergism between Epi and IL-6. We reasoned that synergism of signaling was likely to result from convergence of common signaling pathways, as well as signaling pathways distinct for each agonist.
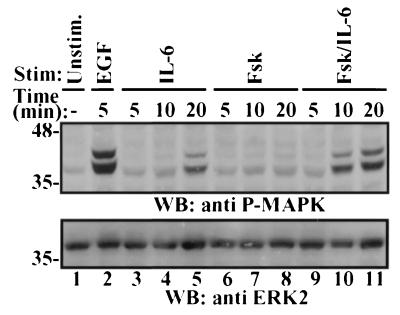
Previous reports indicated that Fsk or IL-6 stimulation of LNCaP cells results in activation of MAPK (10, 33), although the magnitude of MAPK activation by these agents is relatively modest compared to stimulation with EGF. We reasoned that activation of the MAPK signaling cascade, which has been shown to be sufficient to induce NE differentiation of the pheochromocytoma cell line PC12 (12), might be a point of convergence for adenylate cyclase- and IL-6-stimulated signaling pathways. To test this hypothesis, lysates from cells stimulated with Fsk or IL-6 over a 20-min time course were analyzed for levels of activated MAPK (Fig. 5). IL-6 treatment resulted in a twofold increase in activated MAPK levels by 10 min after stimulation, a sevenfold increase by 20 min, and decay thereafter. In contrast, Fsk induced a twofold increase in activated MAPK levels 20 min after stimulation, and this level persisted for the next 40 min. However, when cells were treated with both Fsk and IL-6, each at maximal NE differentiating doses, MAPK activation occurred more rapidly and to a greater extent than that in response to either treatment alone. This treatment increased activated MAPK levels greater than 7-fold in 10 min and 15-fold by 20 min, and the levels decreased slowly thereafter. The level observed in response to Fsk and IL-6 treatment for 20 min was ∼75% of that detected in response to EGF stimulation after 5 min. Identical results were observed when Epi was substituted for Fsk in these same experiments (data not shown). This enhanced activation suggests convergent and synergistic signaling through the MAPK pathway.
FIG. 5.
Potentiation of MAPK activation in response to Fsk and IL-6 cotreatment. LNCaP cells were cultured in T-medium plus 5% FBS and left unstimulated (Unstim.) (lane 1) or treated with 100 ng of EGF per ml (lane 2), 10 μM Fsk (lanes 3 to 5), 2 nM IL-6 (lanes 6 to 8), or both Fsk and IL-6 (lanes 9 to 11) for the indicated times. Cytosolic lysates (50 μg) were subjected to immunoblot analysis (WB) with activated MAPK antibody (upper panel) and with total ERK2 antibody (lower panel) in parallel gels. Numbers on the left are molecular masses in kilodaltons.
PKA and IL-6 signaling pathways are not hyperactivated by cotreatment with Epi and IL-6.
Since we previously demonstrated that activation of PKA is both necessary and sufficient for stimulation of certain NE characteristics by adenylate cyclase activators (13), we assessed whether PKA activation could be affected by cotreatment of LNCaP cells with IL-6 and Epi (Fig. 6A). While PKA activation was significantly enhanced in response to Epi stimulation (P < 0.05), PKA was not activated by stimulation with IL-6 alone. PKA activation in response to the combined treatment of LNCaP cells with IL-6 and Epi was not distinguishable from that of cells stimulated with Epi alone. IBMX alone had no effect on basal PKA activity, MAPK or STAT3 phosphorylation, or NE marker expression and did not significantly alter these responses in cells treated with Epi or IL-6 alone or in combination (data not shown). These results indicate that IL-6 does not enhance Epi-induced NE differentiation by enhancing activation of PKA during the acute phase of agonist treatment.
We next assessed whether cotreatment of LNCaP cells with Epi and IL-6 could enhance the extent of STAT3 activation. Phosphorylation of tyrosine 705 (Y705) of STAT3 is known to be required for dimerization and nuclear localization, while phosphorylation of serine 727 (S727) is also required for maximal transcriptional activation (49). STAT3 Y705 and S727 phosphorylation levels were compared relative to total STAT3 levels in immunoblots of lysates from appropriately treated cells as measures of activation of this transcription factor (Fig. 6B, panels 1 to 3). Phosphorylation of both Y705 and S727 of STAT3 increased in a time-dependent fashion in response to IL-6 treatment, peaking at about 20 min after stimulation and persisting for at least 24 h (data not shown). Furthermore, the kinetics and magnitude of Y705 and S727 phosphorylation in response to dual treatment with Epi and IL-6 were indistinguishable from those in response to treatment with IL-6 alone. In response to Epi treatment, no increase in STAT3 phosphorylation was detected over the times tested. Levels of STAT3 phosphorylation at Y705 and S727 in cells cotreated with Epi and IL-6 were not significantly different from those in cells treated with IL-6 alone. These results indicate that Epi does not alter the extent of IL-6-induced activation of STAT3.
MEK inhibitors do not affect PKA or STAT3 signaling events.
In order to determine whether inhibition of MAPK activation affected either of the distinct signaling pathways activated by Epi or IL-6, we assessed PKA and STAT3 activation in the presence of the MEK inhibitor PD098059. Pretreating LNCaP cells with PD098059 blocked MAPK activation under all conditions tested (Fig. 6B, panels 4 and 5). Pretreatment of LNCaP cells with PD098059 did not decrease the ability of Epi to activate PKA but rather caused a modest, reproducible, but statistically insignificant (P < 0.05) increase in both basal and stimulated PKA activities (Fig. 6A). PD098059 pretreatment also had no effect on IL-6-mediated STAT3 Y705 phosphorylation induced by IL-6 when used alone or in combination with Epi (Fig. 6B, panel 1). The level of S727 phosphorylation from lysates of PD098059-treated cells under all differentiation conditions was ∼30% lower than that from the corresponding lysates from cells treated with vehicle (Fig. 6B, panel 2). Nevertheless, the fold increase in phosphorylation of S727 upon IL-6 or Epi and IL-6 treatment remained relatively constant (∼4.3- and ∼3.7-fold in the absence and presence of PD098059, respectively) and was not significantly different (P < 0.05). This result suggests that MAPK may regulate basal STAT3 S727 phosphorylation in LNCaP cells but does not account for the inducible phosphorylation of S727 and the fact that MAPK is not the kinase primarily responsible for phosphorylation of STAT3 S727 in response to IL-6 treatment in LNCaP cells, consistent with recent findings that kinases such as mTOR (mammalian target of rapamycin) may be the major mediator of cytokine-induced S727 phosphorylation of STAT3 (51).
Potentiation of c-fos expression in Epi- and IL-6-treated LNCaP cells is MAPK dependent.
These observations indicate that MEK inhibitors can be used to assess the contribution of this pathway to downstream events that culminate in NE differentiation. Since Epi and IL-6 treatment of LNCaP cells results in activation of well-described transcription factor activation pathways, we tested whether de novo gene expression was required for NE differentiation in response to treatment with these agents. We found that the general transcription inhibitor actinomycin D was capable of inhibiting morphological changes that LNCaP cells undergo in response to Epi, IL-6, or cotreatment with these agents over a 16-h time course (data not shown), suggesting that transcriptional events play a key role in NE differentiation.
To examine the abilities of the various differentiation conditions to induce changes in specific gene transcription, we initially chose the c-fos gene as an indicator. c-fos is an immediate-early gene whose transcription is up-regulated in response to a wide variety of stimuli, and it has also been shown to play a crucial role in PC12 differentiation in response to nerve growth factor (17). Its promoter is known to contain elements responsive to PKA (4), cytokine (36), and MAPK activation (32) by way of CREB, STAT, and Elk activation, respectively. Using RNase protection analysis (Fig. 7A), we found that while at 1 h after Fsk or Epi stimulation, endogenous c-fos transcription was increased 25 to 30-fold over that in untreated cells and 80-fold in response to IL-6 stimulation, Epi and IL-6 cotreatment induced a greater-than-300-fold increase in c-fos mRNA levels. This result indicated that the combination of Epi and IL-6 resulted in a synergistic increase in c-fos transcription.
Since induction of endogenous c-fos transcription in response to Epi, Fsk, IL-6, or both Epi and IL-6 correlated with the ability of the respective treatments to induce MAPK activation, the effect of the MEK inhibitor PD098059 on c-fos gene transcription was tested. Treatment of LNCaP cells with PD098059 had little effect on the induction of c-fos transcription by Fsk or Epi but reduced IL-6-mediated c-fos transcription by almost 90% and reduced the effects of Epi plus IL-6 by 70% (Fig. 7A). These results were confirmed by using an exogenous c-fos promoter-mediated CAT transcriptional reporter assay (Fig. 7B). Together, these observations indicate that cotreatment with Epi and IL-6 affects gene transcriptional changes in LNCaP cells and that MAPK is an important mediator of such transcriptional events.
Synergistic activation of β4 nAChR promoter in LNCaP cells cotreated with Epi and IL-6 requires MAPK.
The β4 subunit of nAChR is found predominantly in neuronal cells of the central and peripheral nervous systems (5). The promoter element for this gene is not as well characterized as the c-fos promoter but has been shown to require transcriptional enhancer and activation factors expressed in neuronal cells (6). In preliminary experiments, a transcriptional reporter construct containing the β4 nAChR promoter upstream of a luciferase gene was used to test the ability of cells to support neuronal cell-specific transcriptional events. LNCaP cells transfected with the β4 nAChR promoter-driven luciferase construct showed little transcriptional activity from this promoter element when untreated or when treated with Epi or IL-6, but when they were cotreated with Epi and IL-6, LNCaP cells exhibited transcriptional activity proportional to that observed in PC12 cells stimulated with nerve growth factor (data not shown). This reporter construct was not responsive in similarly treated C3H10t½ murine fibroblasts.
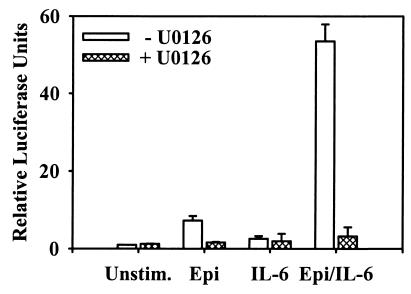
In subsequent experiments using LNCaP cells only, Epi was found to induce approximately a 7-fold increase in transcriptional activation of the β4 nAChR-Luc reporter, while IL-6 did not induce a significant change in reporter activity and the combined treatment resulted in over a 50-fold increase in reporter activity. LNCaP cells transfected with the β4 nAChR luciferase reporter construct and pretreated with the MEK activation inhibitor U0126 were unable to activate transcription in response to any of the differentiation agents (Fig. 8). In these experiments, U0126 was used because of the ability to inhibit MEK activation over a prolonged time course (42) (data not shown). These results indicate that the β4 nAChR promoter can be synergistically activated by Epi and IL-6 cotreatment in a MAPK-dependent manner. However, since IL-6 is unable to activate transcription from the β4 nAChR promoter reporter construct, MAPK would appear to be insufficient (in conjunction with STAT3 activation) to promote transcription. Therefore, a unique convergence of signaling events initiated by cotreatment of LNCaP cells with Epi and IL-6 appears to promote its activation.
FIG. 8.
β4 nAChR subunit promoter activity in LNCaP NE cells. Cells were transfected with β4 nAChR luciferase reporter construct, cultured for 2 days in T-medium plus 5% FBS, and stimulated for 12 h with vehicle (DMSO) (Unstim.), 10 μM Epi, 2 nM IL-6, or Epi and IL-6 (Epi/IL-6) in the presence or absence of 50 μM UO126. Luminometer data were expressed as light units relative to unstimulated cells (mean relative change ± SEM; n = 4).
DISCUSSION
Prostate tumors have a propensity for establishing metastatic lesions in lymph nodes and bone. The idea that factors present at these sites provide prostate tumor cells growth and/or survival advantages not normally available in the prostate gland prompted studies to identify these metastatic and growth-enhancing factors and investigate their mechanism of action. Neoplasia-derived NE cells are not detected in benign prostatic hyperplasia, prostatic intraepithelial neoplasia, or primary tumor foci within the prostate gland (8) but are found in increasing numbers in prostate tumors that exhibit features of progression to androgen independence (7, 46). Current therapies for prostate cancer are effective at treating early-stage encapsulated disease, but no successful therapies exist for recurrent, androgen-independent, excapsulated prostate tumors. It is in these later stages of progression that NE cells are speculated to play a crucial role and would therefore be a novel therapeutic target. That leads to the question of how NE cells arise and in what way they provide a growth advantage to the tumor in suboptimal survival conditions.
In this study, we assessed the ability of two factors that are found in bone marrow and lymph nodes and that have individually been shown to influence the NE status of the prostate tumor model, LNCaP, to act cooperatively to enhance the extent of NE differentiation. It is presumed that Epi reaches both the bone marrow and lymph system via the vasculature, while other β2-adrenergic receptor and adenylate cyclase agonists are known to be present in these tissues. We have demonstrated that Epi stimulation is sufficient to induce NE differentiation of LNCaP cells (14). When prostate tumor cells reach the bone and lymph, high levels of interleukins could additionally influence their phenotype, including the degree of NE differentiation of prostate tumor cells.
While IL-6 has been reported to induce NE differentiation of LNCaP cells, a direct comparison with NE characteristics induced by β-adrenergic receptor agonists or PKA has not been described. We have found that IL-6-induced NE differentiation was distinguishable from Epi-induced NE differentiation. At maximally active concentrations, Epi preferentially induced mitotic arrest, acquisition of a neuritic morphology, secretion of neurotensin and PTHrP, and expression of NSE, while IL-6 preferentially promoted extension of unbranched neurites, more modest growth arrest, and βIII tubulin expression. While we conclude that IL-6 does promote acquisition of certain NE characteristics by LNCaP cells, more importantly, we conclude that together with β2-adrenergic receptor or adenylate cyclase agonists, IL-6 induces a higher degree of NE differentiation than either agent alone as measured by the degree of inhibition of mitotic activity, development of branched neuritic processes, and potentiated accumulation of neurotensin and PTHrP in the conditioned medium of cells stimulated with both Epi and IL-6.
PTHrP was shown to be present in human prostate tumor tissue sections, with 100% of poorly differentiated, metastatic tumors staining positively (2). The prostate cancer cell lines LNCaP, DU145, and PC3 all secrete PTHrP into conditioned medium (23). Furthermore, PTHrP peptide (amino acids 1 to 34) can increase DNA synthesis in the androgen-independent cell lines PC3 and DU145 and can also increase DNA synthesis in LNCaP cells in the presence of androgen (23). Neurotensin receptors are present on PC3 cells, and these cells respond to neurotensin by increasing thymidine incorporation (37). LNCaP cells also express the neurotensin receptor and are capable of undergoing growth stimulation by neurotensin in the absence of androgen (38). Taken together, these data indicate that NE cells are secreting known mitogenic factors for prostate cancer cells.
We have observed that NE differentiation requires transcription as demonstrated by the observation that actinomycin D inhibits the rapid morphological changes seen in LNCaP cells 12 to 16 h after the addition of differentiation agents (data not shown). Because maximal expression of NE characteristics occurs in LNCaP cells over a 2- to 5-day period, it is reasonable to propose that many cellular processes are modified to achieve the gross cellular changes seen. We suggest that the transcriptional profile of these epithelial cells must be sufficiently altered to change levels not only of proteins required for secretion of mitogenic peptides but also of cytoskeletal and cell cycle proteins required for morphological transformation and mitotic arrest. Examples of such genes are those encoding subunits of nAChR, a receptor on adrenal chromaffin cells that regulates secretion of catecholamines (30). In this report, we show that inhibitors of MAPK activation reduced the immediate-early gene response and decreased transcription from the promoter of the neuronal cell-specific gene, the β4 nAChR subunit. Neither Epi nor IL-6 alone induced significant transcription from the β4 nAChR promoter, indicating that only cells responding to the synergistic action of Epi and IL-6 express a gene whose product is associated with neuronal secretory activity.
Elongation and branching are fundamental properties of neurite morphogenesis and have been used as measures of the ability of immunophilin agonists (11), bone morphogenic proteins (35), and serotonin (28) to promote neuronal differentiation. These morphological changes are well correlated with increased neurotransmitter release and expression of neuronal markers, such as NSE. The ability of Epi and IL-6 to promote similar morphological and molecular properties in LNCaP cells suggests that common intracellular signaling events may be utilized in the normal establishment of neuronal circuits and in NE differentiation of prostatic adenocarcinomas.
Our data, together with published accounts, indicate that stimulation by Epi and IL-6 activates distinct signaling mechanisms which in some instances can overlap by convergent signaling to induce more NE markers than does either treatment alone. The presence of additional environmental factors, such as androgen deprivation, which also induces NE differentiation in LNCaP cells (39), may induce a more pronounced NE phenotype that provides even greater support for aggressive tumor cell growth in the bone marrow and lymph nodes. Therapeutic strategies can be developed to inhibit NE differentiation only if the underlying molecular mechanisms required for these events are established.
Our results indicate that the high degree of NE differentiation seen in response to cotreatment with Epi and IL-6 is dependent on cross talk from intracellular signaling pathways directly downstream of the agonists. While adenylate cyclase activators induced PKA activation, such treatments did not induce STAT activation in LNCaP cells. In comparison, IL-6 readily induced STAT activation but had no effect on PKA activity in these cells. While these treatments have been shown to activate MAPK individually, in this report, we demonstrate that cotreating LNCaP cells with adenylate cyclase activators and IL-6 increased the rate and potentiated the magnitude of MAPK activation without altering the level of PKA or STAT3 activation observed in response to either agent alone. The cooperative effect at the level of MAPK activation correlates with the increase in morphological changes and inhibition of mitotic activity seen in response to cotreatment with Epi and IL-6, suggesting that synergistic activation of MAPK may be a component of the enhanced differentiation exhibited by LNCaP cells. Unfortunately, we have been unable to test this hypothesis directly due to the toxic effects of the MEK inhibitors over the time course required to measure morphological changes or neuropeptide secretion.
There are a number of ways that Epi and IL-6 might cross talk with the MAPK pathway. Stimulation of LNCaP cells with Epi results in increased cAMP levels (14), activation of PKA (13), and activation of MAPK, presumably through phosphorylation of the small G-protein Rap1 (10). Such an event has been demonstrated to facilitate the interaction of Rap1 with B-Raf (22, 48, 50). Additionally, increased intracellular cAMP levels have been reported to stimulate the cAMP-activated guanine nucleotide exchange factor for Rap1, Epac (16), which can modulate signaling to the MAPK pathway independently of PKA activation (48). While recruitment of adapter proteins such as Grb2 and Shc could activate Ras indirectly via the IL-6 receptor, in LNCaP cells IL-6 has been reported to activate MAPK through association of HER2 with the IL-6 receptor (33). Thus, the results accumulated to date suggest that the MAPK pathway may be an appropriate target for development of novel adjuvant therapies against prostate cancer progression.
ACKNOWLEDGMENTS
We thank Corinne Silva for providing antibodies for STAT family members, Tony Frankfurter for providing antibodies to βIII tubulin, Paul Gardner for providing the β4 nAChR subunit luciferase construct, Dan Engel for c-fos transcription reagents, Gina Rossi for expert technical assistance, and C. E. Meyers, M. J. Weber, J. T. Parsons, L. W. Chung, D. Engel, and R. Sikes for their conceptual insights.
This work was supported by grants from the DHHS (NCI PO1 40042, NCI R21 69848, and NCI RO1 76649) and generous support from the ARCS Foundation and the Jeffress Foundation.
REFERENCES
- 1.Adler H L, McCurdy M A, Kattan M W, Timme T L, Scardino P T, Thompson T C. Elevated levels of circulating interleukin-6 and transforming growth factor-beta1 in patients with metastatic prostatic carcinoma. J Urol. 1999;161:182–187. [PubMed] [Google Scholar]
- 2.Asadi F, Farraj M, Sharifi R, Malakouti S, Antar S, Kukreja S. Enhanced expression of parathyroid hormone-related protein in prostate cancer as compared with benign prostatic hyperplasia. Hum Pathol. 1996;27:1319–1323. doi: 10.1016/s0046-8177(96)90344-5. [DOI] [PubMed] [Google Scholar]
- 3.Bang Y J, Pirnia F, Fang W G, Kang W K, Sartor O, Whitesell L, Ha M J, Tsokos M, Sheahan M D, Nguyen P. Terminal neuroendocrine differentiation of human prostate carcinoma cells in response to increased intracellular cyclic AMP. Proc Natl Acad Sci USA. 1994;91:5330–5334. doi: 10.1073/pnas.91.12.5330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkowitz L A, Riabowol K T, Gilman M Z. Multiple sequence elements of a single functional class are required for cyclic AMP responsiveness of the mouse c-fos promoter. Mol Cell Biol. 1989;9:4272–4281. doi: 10.1128/mcb.9.10.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bigger C B, Casanova E A, Gardner P D. Transcriptional regulation of neuronal nicotinic acetylcholine receptor genes. Functional interactions between Sp1 and the rat beta4 subunit gene promoter. J Biol Chem. 1996;271:32842–32848. doi: 10.1074/jbc.271.51.32842. [DOI] [PubMed] [Google Scholar]
- 6.Bigger C B, Melnikova I N, Gardner P D. Sp1 and Sp3 regulate expression of the neuronal nicotinic acetylcholine receptor beta4 subunit gene. J Biol Chem. 1997;272:25976–25982. doi: 10.1074/jbc.272.41.25976. [DOI] [PubMed] [Google Scholar]
- 7.Bonkhoff H, Stein U, Remberger K. Endocrine-paracrine cell types in the prostate and prostatic adenocarcinoma are postmitotic cells. Hum Pathol. 1995;26:167–170. doi: 10.1016/0046-8177(95)90033-0. [DOI] [PubMed] [Google Scholar]
- 8.Bonkhoff H, Wernert N, Dhom G, Remberger K. Relation of endocrine-paracrine cells to cell proliferation in normal, hyperplastic, and neoplastic human prostate. Prostate. 1991;19:91–98. doi: 10.1002/pros.2990190202. [DOI] [PubMed] [Google Scholar]
- 9.Burchardt T, Burchardt M, Chen M W, Cao Y, de la Taille T A, Shabsigh A, Hayek O, Dorai T, Buttyan R. Transdifferentiation of prostate cancer cells to a neuroendocrine cell phenotype in vitro and in vivo. J Urol. 1999;162:1800–1805. [PubMed] [Google Scholar]
- 10.Chen T, Cho R W, Stork P J, Weber M J. Elevation of cyclic adenosine 3′,5′-monophosphate potentiates activation of mitogen-activated protein kinase by growth factors in LNCaP prostate cancer cells. Cancer Res. 1999;59:213–218. [PubMed] [Google Scholar]
- 11.Costantini L C, Isacson O. Immunophilin ligands and GDNF enhance neurite branching or elongation from developing dopamine neurons in culture. Exp Neurol. 2000;164:60–70. doi: 10.1006/exnr.2000.7417. [DOI] [PubMed] [Google Scholar]
- 12.Cowley S, Paterson H, Kemp P, Marshall C J. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 13.Cox M E, Deeble P D, Bissonette E A, Parsons S J. Activated 3′,5′-cyclic AMP-dependent protein kinase is sufficient to induce neuroendocrine-like differentiation of the LNCaP prostate tumor cell line. J Biol Chem. 2000;275:13812–13818. doi: 10.1074/jbc.275.18.13812. [DOI] [PubMed] [Google Scholar]
- 14.Cox M E, Deeble P D, Lakhani S, Parsons S J. Acquisition of neuroendocrine characteristics by prostate tumor cells is reversible: implications for prostate cancer progression. Cancer Res. 1999;59:3821–3830. [PubMed] [Google Scholar]
- 15.Degeorges A, Tatoud R, Fauvel-Lafeve F, Podgorniak M P, Millot G, de Cremoux P, Calvo F. Stromal cells from human benign prostate hyperplasia produce a growth-inhibitory factor for LNCaP prostate cancer cells, identified as interleukin-6. Int J Cancer. 1996;68:207–214. doi: 10.1002/(SICI)1097-0215(19961009)68:2<207::AID-IJC12>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 16.de Rooij J, Zwartkruis F J, Verheijen M H, Cool R H, Nijman S M, Wittinghofer A, Bos J L. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- 17.Diaz M, Abdul M, Hoosein N. Modulation of neuroendocrine differentiation in prostate cancer by interleukin-1 and -2. Prostate Suppl. 1998;8:32–36. [PubMed] [Google Scholar]
- 18.Drachenberg D E, Elgamal A A, Rowbotham R, Peterson M, Murphy G P. Circulating levels of interleukin-6 in patients with hormone refractory prostate cancer. Prostate. 1999;41:127–133. doi: 10.1002/(sici)1097-0045(19991001)41:2<127::aid-pros7>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 19.Ely C M, Oddie K M, Litz J S, Rossomando A J, Kanner S B, Sturgill T W, Parsons S J. A 42-kD tyrosine kinase substrate linked to chromaffin cell secretion exhibits an associated MAP kinase activity and is highly related to a 42-kD mitogen-stimulated protein in fibroblasts. J Cell Biol. 1990;110:731–742. doi: 10.1083/jcb.110.3.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Engel D A, Muller U, Gedrich R W, Eubanks J S, Shenk T. Induction of c-fos mRNA and AP-1 DNA-binding activity by cAMP in cooperation with either the adenovirus 243- or the adenovirus 289-amino acid E1A protein. Proc Natl Acad Sci USA. 1991;88:3957–3961. doi: 10.1073/pnas.88.9.3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hirano T. Interleukin 6 and its receptor: ten years later. Int Rev Immunol. 1998;16:249–284. doi: 10.3109/08830189809042997. [DOI] [PubMed] [Google Scholar]
- 22.Hu C D, Kariya K, Okada T, Qi X, Song C, Kataoka T. Effect of phosphorylation on activities of Rap1A to interact with Raf-1 and to suppress Ras-dependent Raf-1 activation. J Biol Chem. 1999;274:48–51. doi: 10.1074/jbc.274.1.48. [DOI] [PubMed] [Google Scholar]
- 23.Iwamura M, Abrahamsson P A, Foss K A, Wu G, Cockett A T, Deftos L J. Parathyroid hormone-related protein: a potential autocrine growth regulator in human prostate cancer cell lines. Urology. 1994;43:675–679. doi: 10.1016/0090-4295(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 24.Karkavelas G, Katsetos C D, Geddes J F, Herman M M, Vinores S A, Cooper H S, Provencio J, Frankfurter A. Class III beta-tubulin isotype (beta III) in the adrenal medulla II. Localization in primary human pheochromocytomas. Anat Rec. 1998;250:344–350. doi: 10.1002/(SICI)1097-0185(199803)250:3<344::AID-AR9>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 25.Katsetos C D, Herman M M, Balin B J, Vinores S A, Hessler R B, Arking E J, Karkavelas G, Frankfurter A. Class III beta-tubulin isotype (beta III) in the adrenal medulla III. Differential expression of neuronal and glial antigens identifies two distinct populations of neuronal and glial-like (sustentacular) cells in the PC12 rat pheochromocytoma cell line maintained in a Gelfoam matrix system. Anat Rec. 1998;250:351–365. doi: 10.1002/(SICI)1097-0185(199803)250:3<351::AID-AR10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 26.Katsetos C D, Karkavelas G, Herman M M, Vinores S A, Provencio J, Spano A J, Frankfurter A. Class III beta-tubulin isotype (beta III) in the adrenal medulla I. Localization in the developing human adrenal medulla. Anat Rec. 1998;250:335–343. doi: 10.1002/(SICI)1097-0185(199803)250:3<335::AID-AR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 27.Kishimoto T, Akira S, Taga T. Interleukin-6 and its receptor: a paradigm for cytokines. Science. 1992;258:593–597. doi: 10.1126/science.1411569. [DOI] [PubMed] [Google Scholar]
- 28.Lieske V, Bennett-Clarke C A, Rhoades R W. Effects of serotonin on neurite outgrowth from thalamic neurons in vitro. Neuroscience. 1999;90:967–974. doi: 10.1016/s0306-4522(98)00501-6. [DOI] [PubMed] [Google Scholar]
- 29.Lou W, Ni Z, Dyer K, Tweardy D J, Gao A C. Interleukin-6 induces prostate cancer cell growth accompanied by activation of stat3 signaling pathway. Prostate. 2000;42:239–242. doi: 10.1002/(sici)1097-0045(20000215)42:3<239::aid-pros10>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 30.Mizobe F, Livett B G. Nicotine stimulates secretion of both catecholamines and acetylcholinesterase from cultured adrenal chromaffin cells. J Neurosci. 1983;3:871–876. doi: 10.1523/JNEUROSCI.03-04-00871.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okamoto M, Lee C, Oyasu R. Interleukin-6 as a paracrine and autocrine growth factor in human prostatic carcinoma cells in vitro. Cancer Res. 1997;57:141–146. [PubMed] [Google Scholar]
- 32.Price M A, Hill C, Treisman R. Integration of growth factor signals at the c-fos serum response element. Philos Trans R Soc London Ser B. 1996;351:551–559. doi: 10.1098/rstb.1996.0054. [DOI] [PubMed] [Google Scholar]
- 33.Qiu Y, Ravi L, Kung H J. Requirement of ErbB2 for signalling by interleukin-6 in prostate carcinoma cells. Nature. 1998;393:83–85. doi: 10.1038/30012. [DOI] [PubMed] [Google Scholar]
- 34.Qiu Y, Robinson D, Pretlow T G, Kung H J. Etk/Bmx, a tyrosine kinase with a pleckstrin-homology domain, is an effector of phosphatidylinositol 3′-kinase and is involved in interleukin 6-induced neuroendocrine differentiation of prostate cancer cells. Proc Natl Acad Sci USA. 1998;95:3644–3649. doi: 10.1073/pnas.95.7.3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiriz J, Espejo M, Ventura F, Ambrosio S, Alberch J. Bone morphogenetic protein-2 promotes dissociated effects on the number and differentiation of cultured ventral mesencephalic dopaminergic neurons. J Neurobiol. 1999;38:161–170. [PubMed] [Google Scholar]
- 36.Sadowski H B, Shuai K, Darnell J E, Jr, Gilman M Z. A common nuclear signal transduction pathway activated by growth factor and cytokine receptors. Science. 1993;261:1739–1744. doi: 10.1126/science.8397445. [DOI] [PubMed] [Google Scholar]
- 37.Seethalakshmi L, Mitra S P, Dobner P R, Menon M, Carraway R E. Neurotensin receptor expression in prostate cancer cell line and growth effect of NT at physiological concentrations. Prostate. 1997;31:183–192. doi: 10.1002/(sici)1097-0045(19970515)31:3<183::aid-pros7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 38.Sehgal I, Powers S, Huntley B, Powis G, Pittelkow M, Maihle N J. Neurotensin is an autocrine trophic factor stimulated by androgen withdrawal in human prostate cancer. Proc Natl Acad Sci USA. 1994;91:4673–4677. doi: 10.1073/pnas.91.11.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shen R, Dorai T, Szaboles M, Katz A E, Olsson C A, Buttyan R. Transdifferentiation of cultured human prostate cells to a neuroendocrine cell phenotype in a hormone-depleted medium. Urol Res. 1997;3:67–75. doi: 10.1016/s1078-1439(97)00039-2. [DOI] [PubMed] [Google Scholar]
- 40.Siegsmund M J, Yamazaki H, Pastan I. Interleukin 6 receptor mRNA in prostate carcinomas and benign prostate hyperplasia. J Urol. 1994;151:1396–1399. doi: 10.1016/s0022-5347(17)35267-9. [DOI] [PubMed] [Google Scholar]
- 41.Simpson R J, Hammacher A, Smith D K, Matthews J M, Ward L D. Interleukin-6: structure-function relationships. Protein Sci. 1997;6:929–955. doi: 10.1002/pro.5560060501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Slack J K, Catling A D, Eblen S T, Weber M J, Parsons J T. c-Raf-mediated inhibition of epidermal growth factor-stimulated cell migration. J Biol Chem. 1999;274:27177–27184. doi: 10.1074/jbc.274.38.27177. [DOI] [PubMed] [Google Scholar]
- 43.Spiotto M T, Chung T D. STAT3 mediates IL-6-induced growth inhibition in the human prostate cancer cell line LNCaP. Prostate. 2000;42:88–98. doi: 10.1002/(sici)1097-0045(20000201)42:2<88::aid-pros2>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 44.Spiotto M T, Chung T D. STAT3 mediates IL-6-induced neuroendocrine differentiation in prostate cancer cells. Prostate. 2000;42:186–195. doi: 10.1002/(sici)1097-0045(20000215)42:3<186::aid-pros4>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 45.Thalmann G N, Anezinis P E, Chang S M, Zhau H E, Kim E E, Hopwood V L, Pathak S, von Eschenbach A C, Chung L W. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. . (Erratum, 54:3953.) [PubMed] [Google Scholar]
- 46.Theodorescu D, Broder S R, Boyd J C, Mills S E, Frierson H F. Cathepsin D and chromogranin a as predictors of long term disease specific survival after radical prostatectomy for localized carcinoma of the prostate. Cancer. 1997;80:2109–2119. [PubMed] [Google Scholar]
- 47.Twillie D A, Eisenberger M A, Carducci M A, Hseih W S, Kim W Y, Simons J W. Interleukin-6: a candidate mediator of human prostate cancer morbidity. Urology. 1995;45:542–549. doi: 10.1016/S0090-4295(99)80034-X. [DOI] [PubMed] [Google Scholar]
- 48.Vossler M R, Yao H, York R D, Pan M G, Rim C S, Stork P J. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 49.Wen Z, Zhong Z, Darnell J E., Jr Maximal activation of transcription by Stat1 and Stat3 requires both tyrosine and serine phosphorylation. Cell. 1995;82:241–250. doi: 10.1016/0092-8674(95)90311-9. [DOI] [PubMed] [Google Scholar]
- 50.Yao H, York R D, Misra-Press A, Carr D W, Stork P J. The cyclic adenosine monophosphate-dependent protein kinase (PKA) is required for the sustained activation of mitogen-activated kinases and gene expression by nerve growth factor. J Biol Chem. 1998;273:8240–8247. doi: 10.1074/jbc.273.14.8240. [DOI] [PubMed] [Google Scholar]
- 51.Yokogami K, Wakisaka S, Avruch J, Reeves S A. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]