Abstract
Mutations in the gene for lamin A/C (LMNA) cause a diverse range of diseases known as laminopathies. LMNA-related cardiomyopathy is a common inherited heart disease and is highly penetrant with a poor prognosis. In the past years, numerous investigations using mouse models, stem cell technologies, and patient samples have characterized the phenotypic diversity caused by specific LMNA variants and contributed to understanding the molecular mechanisms underlying the pathogenesis of heart disease. As a component of the nuclear envelope, LMNA regulates nuclear mechanostability and function, chromatin organization, and gene transcription. This review will focus on the different cardiomyopathies caused by LMNA mutations, address the role of LMNA in chromatin organization and gene regulation, and discuss how these processes go awry in heart disease.
Keywords: nuclear lamina, lamin A/C, LMNA, cardiomyopathy, epigenetics, chromatin architecture, stem cells
1. Introduction
Mutations in genes encoding proteins of the nuclear lamina result in wide-ranging clinical phenotypes collectively referred to as laminopathies [1]. Many of these diseases are caused by mutations in the gene for lamin A/C (LMNA) and affect primarily the muscles, the peripheral nerves, and the adipose tissue or cause systemic diseases such as premature aging syndromes [2]. The LMNA gene encodes A-type lamins, generated by alternative splicing, of which lamins A and C are the main splicing products [3,4]. In addition to the A-type lamins, the nuclear lamina is composed of B-type lamins, i.e., lamins B1 and B2, encoded by LMNB1 and LMNB2 genes, respectively [5,6,7,8]. LMNB2 also encodes the germ-line-specific lamin B3, produced by alternative splicing [9].
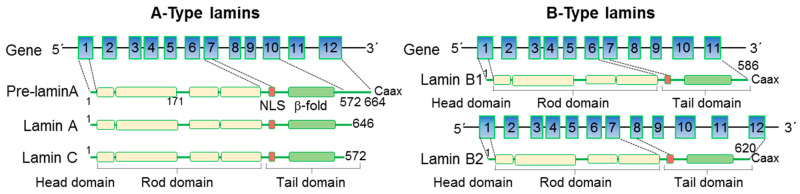
A- and B-type lamins have a common structural organization: a short “head” domain at the N-terminus followed by a central helical rod domain and a C-terminal “tail” domain. The central rod domain is composed of four coiled-coil regions that allow lamins to form parallel coiled-coil dimers and higher-order meshworks [10,11,12]. The “tail” consists of a globular region, which adopts an immunoglobulin (Ig)-like β-fold involved in protein–protein interactions. Pre-lamin A- and B-type lamins also have a CaaX motif at the C-terminus which guides protein farnesylation and carboxyl methylation, important for targeting to the nuclear envelope [10,11,12] (Figure 1).
Figure 1.
Structure of nuclear lamins. A- and B-type lamins have a conserved domain structure, consisting of a short N-terminal “head” domain, central helical coiled-coil rod domain, and a C-terminal immunoglobulin (Ig)-like β-fold domain. The nuclear localization signal (NLS) is located at the beginning of the tail domain. Pre-lamin A- and B-type lamins also have a CaaX motif at the C-terminus guiding their targeting to the inner nuclear membrane.
Both A- and B-type lamins form separate but interconnected filamentous meshworks located between the inner nuclear membrane and the peripheral heterochromatin, which on the one hand provide structural support to the nucleus and on the other hand anchor chromatin at the nuclear periphery, thereby shaping the higher-order chromatin structure [13,14,15]. In contrast to lamins B1 and B2, which are localized at the periphery and associate mainly with transcriptionally inactive chromatin [16,17], lamins A and C are also found in the nuclear interior and associate with both heterochromatin and euchromatin [18]. In addition, lamins interact with the LINC complex, which couples the nucleoskeleton with the cytoskeleton [19,20], and thereby can directly translate mechanical cues and changes in extracellular matrix mechanics into alterations in chromatin structure and transcriptional activity [21].
In the last years, a large number of studies identified distinct molecular pathways dysregulated in patients with pathogenic LMNA mutations, mouse models, or stem cells carrying LMNA mutations. Here, we summarize the current knowledge on the role of lamin A/C in diseases of the heart muscle and specifically focus on how changes in lamin-A/C-dependent chromatin architecture could be involved in the pathogenesis of cardiomyopathies.
2. LMNA-Related Dilated Cardiomyopathy
Dilated cardiomyopathy (DCM) is characterized by enlargement and dilatation of one or both ventricles of the heart, which occurs together with impaired contractility and heart function [22]. The LMNA gene is the second most commonly mutated gene in familial dilated cardiomyopathy (DCM), accounting for 5% to 8% of cases [23]. Patients carrying pathogenic LMNA mutations have a poor prognosis due to the high rate of sudden cardiac death resulting from malignant arrhythmias. Atrial fibrillation (AF), atrioventricular (AV) conduction block, ventricular tachycardia, and sudden cardiac death often precede the development of systolic dysfunction [24,25,26]. Although LMNA-related DCM is an adult-onset disease, it cannot be excluded that structural changes and arrhythmias may be present in early asymptomatic individuals [27].
To date, around 500 mutations and 300 protein variants have been reported for LMNA; detailed information on the different mutations is available through the UMD-LMNA mutation database (www.umd.be/LMNA, accessed on 3 January 2023) (Table 1). Most of the mutations associated with cardiomyopathies are located in the head and rod domains and are mostly truncation or missense mutations [28]. Heterozygous truncation mutations often result in lamin A/C haploinsufficiency due to a premature termination codon generated by insertions or deletions resulting in a frameshift, aberrant splice site, or nonsense mutations. A homozygous LMNA nonsense mutation (Y259X) has also been reported, resulting in a lethal phenotype [29]. LMNA missense mutations, on the other hand, are thought to mostly act through a dominant negative mechanism [28]. Patients carrying heterozygous mutations in LMNA in combination with mutations within other genes such as TTN, DES, SUN1/2, etc., display a particularly severe clinical cardiac phenotype [30,31,32,33,34].
Table 1.
Cardiomyopathies caused by the most well studied pathogenic LMNA mutations in patients. Dilated cardiomyopathy (DCM); left ventricular noncompaction cardiomyopathy (LVNC); arrhythmogenic right ventricular cardiomyopathy (ARVC); restrictive cardiomyopathy (RCM); Emery–Dreifuss muscular dystrophy (EDMD); limb–girdle muscular dystrophy (LGMD).
| LMNA Mutation | Disease | Clinical Features | References |
|---|---|---|---|
| p. N195K | DCM | Heart dilatation, fibrosis, arrhythmia, sinus bradycardia, atrioventricular conduction block, and atrial arrhythmias | [26,35] |
| p. R225X | DCM | Atrial fibrillation, complete atrioventricular block, ventricular tachyarrhythmia, and heart failure | [36,37] |
| p. K117fs | DCM | Atrioventricular block, ventricular tachycardia, atrial fibrillation, arrhythmias at the single-cell level | [38] |
| p.c.908_909 delCT | DCM | Atrial fibrillation, sick sinus syndrome, dilated cardiomyopathy | [39] |
| p.28insA | DCM | Dilated cardiomyopathy with conduction defects | [40] |
| p. T101 | DCM, lipodystrophy, atypical progeroid syndrome | Hypertriglyceridemia, diabetes mellitus, insulin resistance, left ventricular myocyte hypertrophy, interstitial fibrosis | [41,42,43,44] |
| p. R377H | EDMD, LGMD, DCM | Muscular dystrophy, atrial fibrillation or flutter; conduction defects | [45,46,47,48] |
| p. S143p | DCM | Atrioventricular conduction defects, left ventricular systolic dysfunction and dilatation | [49,50] |
| p.H222P | EDMD | Muscle weakness, cardiac arrhythmias | [51] |
| p. K219T | DCM | Heart dilatation, atrial fibrillation, atrioventricular block | [52,53] |
| p. E161K | DCM | Dilatation, atrial fibrillation, conduction system diseases | [40,54] |
| p. R541C | DCM, LVNC | Reduced heart contractility, left ventricle dilatation, polymorphic premature ventricular contraction, diffuse ST-T change | [41,55] |
| p. R190W | DCM, LVNC, ARVC | Left ventricular noncompaction, conduction system defect, abnormal activation of ERK1/2 signaling and sarcomeric disorganization | [56,57,58] |
| p. R644C | DCM, LVNC, ARVC, | Also leads to lipodystrophy, atypical progeria, phenotypic diversity, and low penetrance associated with the R644C mutation | [59,60] |
| p. V445E | LVNC | Ventricular tachycardia/fibrillation | [58] |
| p.c.835 delG:p.Glu279ArgfsX201 | RCM | Diastolic dysfunction, biatrial enlargement, atrial fibrillation, skeletal muscle weakness | [61] |
Since a number of LMNA mutations result in a loss of function, lamin A/C haploinsufficient (Lmna+/−) and Lmna knockout mice (Lmna-/-) have been extensively used to study the molecular mechanisms underlying LMNA loss-of-function (LOF) cardiomyopathy (Table 2). Lmna−/− mice develop DCM two weeks after birth and die within one month [62,63]. Lmna+/− mice are viable and fertile but already at ten weeks of age show AV conduction defects and atrial and ventricular arrhythmias, characteristic for patients with LMNA LOF mutations [64]. Cellular characterization revealed that Lmna haploinsufficiency results in AV node cardiomyocyte death and altered electrophysiological properties [64]. Furthermore, Lmna−/− and Lmna+/− cardiomyocytes (CMs) show premature binucleation, cell cycle withdrawal, and abnormal contractility [63,65].
Table 2.
Mouse models of laminopathies.
| Mouse Model | Gene Targeting Strategy | Disease | Homozygous Phenotype | Heterozygous Phonotype | References |
|---|---|---|---|---|---|
| Lmna−/− * | Deletion of exons 8-11; a truncated lamin A protein of 54 kDa is still expressed | DCM, EDMD | Retarded postnatal growth, conduction disorders, DCM, EDMD, death by 8 weeks of age | AV conduction defects, both atrial and ventricular arrhythmias; develop DCM by 50 weeks | [64,65] |
| Lmna−/− | Deletion of exon 2 | DCM LVNC |
Retarded postnatal growth, conduction disorders, DCM, noncompaction, death within 1 month, developmental defects | RV dilatation, RV noncompaction, developmental defects | [62,63] |
| Lmna GT−/− | A gene trap cassette inserted upstream of exon 2 of Lmna | N/A | Growth retardation at 2 weeks, impaired postnatal cardiac hypertrophy, skeletal muscle hypotrophy, defects in lipid metabolism | No apparent abnormalities | [66] |
| Myh6 cre -Lmna f/f | Conditional deletion of Lmna in cardiomyocytes | DCM | DCM, cardiac dysfunction, conduction defects, ventricular arrhythmias, fibrosis, apoptosis, and premature death within 4 weeks | Develop cardiac dilatation and dysfunction, cardiac arrhythmias, fibrosis in older mice |
[67] |
| Lamin C only |
Deletion of the last 150 nucleotides of exon 11 and the complete intron 11 | N/A | No obvious phenotype | No obvious phenotype | [68] |
| Lamin A only |
Deletion of introns 10 and 11, the last 30 bp of exon 11, and the first 24 bp of exon 12 | N/A | No apparent abnormalities | N/A | [69] |
| Pre-lamin A only | Deletion of intron 10 | N/A | No apparent abnormalities | N/A | [69] |
| Lmna N195K | Missense mutation in exon 3 | DCM | DCM, conduction defects, fibrosis, minor growth retardation, increased heart weight, death at 12–14 weeks | No obvious phenotype | [35] |
| Lmna R225X | Nonsense mutation at exon 4 causing premature truncation of both lamin A and lamin C | DCM | Retarded postnatal growth, conduction disorders, dilated cardiomyopathy, AV node fibrosis, death within postnatal 2 weeks | No apparent abnormalities | [36] |
| Lmna E82K | Transgenic mice expressing Lmna E82K under the control of α-MHC promoter | DCM | N/A | DCM, conduction defects, fibrosis, increased heart weight | [70] |
| Lmna ∆K32 | Deletion of lysine 32 of lamin A/C in exon 1 | DCM | Retarded postnatal growth, striated muscle maturation delay, metabolic defects including reduced adipose tissue and hypoglycemia, death within 3 weeks | Develop a progressive cardiac dysfunction and DCM | [71,72,73] |
| Lmna R541C | Missense mutation in exon 10 | DCM | Ventricular dilatation and reduced systolic function | N/A | [55] |
| Lmna H222P | Missense mutation in exon 4 | EDMD DCM |
Heart dilatation, conduction defects, increased fibrosis, hypertrophy defects, death by 9 months of age, developmental defects | No apparent abnormalities | [74,75,76] |
| Lmna M317K | Transgenic mice expressing Lmna M317K missense mutation under the control of α-MHC promoter | EDMD | N/A | Increased eosinophilia and fragmentation of cardiomyofibrils, nuclear pyknosis and edema without fibrosis or significant inflammation, death at 2–7 weeks of age | [77] |
| Lmna D300N | Transgenic mice expressing Lmna D300N; Myh6-tTA mice | DCM | N/A | Heart dilatation, increased heart-to-body-weight ratio, fibrosis, death within two months | [78] |
| Lmna L530P | Missense mutation in exon 9 | HGPS | Loss of subcutaneous fat, reduction in growth rate, and death by 4 weeks of age | No apparent abnormalities | [79] |
| Lmna G609G | Point mutation in exon 11 | HGPS | Shortened life span, reduced body weight, bone and cardiovascular abnormalities, death at an average of 100 days |
Develop a similar phenotype to homozygotes but at an older age, average death at 242 days | [80] |
| Lmna HG | Deletion of introns 10–11 and last 150 nucleotides of exon 11 | HGPS | Growth retardation osteoporosis, micrognathia, loss of adipose tissue, death by 3–4 weeks of age | Similar phenotype to homozygotes but less severe, death by 21 weeks | [81] |
| Lmna nHG | Deletion of introns 10 and 11 and the last 150 bp of exon 11 together with an exchange of cysteine to serine in the CaaX motif | HGPS | Weight loss, reduced subcutaneous and abdominal fat by 4–8 weeks of age, death at 17 weeks of age | Similar spectrum of disease phenotypes as Lmna HG/+ mice but less severe, death by 36 weeks of age | [82] |
| csmHG | Deletion of introns 10 and 11 and the last 150 bp of exon 11, three-nucleotide deletion (the isoleucine in progerin’s CaaX motif) | HGPS | No bone phenotype, normal body weight and survival | No bone disease, normal body weight and survival | [83] |
| G608G BAC | 164-kb BAC carrying mutated G608G human LMNA | HGPS | N/A | Progressive loss of vascular smooth muscle cells | [84] |
| tetop_LAG608G | Targeted expression of the lamin A G608G minigene using the keratin 5 transactivator | HGPS | N/A | Growth retardation, skin and teeth abnormalities, fibrosis, loss of hypodermal adipocytes | [85] |
| Keratin14-progerin | Vector expressing progerin in epidermis under the control of the keratin 14 promoter | HGPS | N/A | Severe abnormalities in skin keratinocyte nuclei, including nuclear envelope lobulation and decreased nuclear circularity | [86] |
*: Although initially this mouse model has been used as a Lmna knockout mouse model, later studies revealed that a truncated lamin A protein of 54 kDa is still expressed.
Another mutation often used for modeling the LMNA LOF mutation is the p.R225X mutation, a nonsense mutation causing premature truncation of both lamin A and lamin C splice isoforms. Patients carrying this pathogenic mutation show early onset of AF, secondary AV block, and DCM [87]. Like Lmna−/− mice, homozygote Lmna R225X mice also exhibit retarded postnatal growth, conduction disorders, and DCM [36]. Other LOF mutations, e.g., K117fs and 28insA, also lead to a DCM phenotype. LMNA p. K117fs mutation is a frameshift mutation that leads to a premature translation-termination codon [38], whereas 28insA is an adenosine insertion mutation in exon 1 resulting similarly in a premature stop codon [40]. Messenger RNAs (mRNAs) that contain a premature stop codon often undergo degradation through the nonsense-mediated mRNA decay (NMD) surveillance mechanism and thus can cause haploinsufficiency. Consistent with this, a significant decrease in lamin A/C protein levels is observed in K117fs iPSC-CMs as a result of NMD-mediated degradation of LMNA mRNA [38]. In addition to truncation mutations, which can result in LMNA haploinsufficiency, mutations such as N195K, T10I, R541S, and R337H also show reduced lamin A/C protein levels [35,41]. Patients carrying these pathogenic mutations also develop DCM [26,51,88]. It is still unclear why these mutations lead to decreased lamin A/C levels. Possible reasons could be that protein translation or the stability of lamin A/C are affected in mutant CMs. For example, although Lmna mRNA does not change, both lamin A and lamin C levels are decreased in CMs and MEFs derived from Lmna N195K/N195K mutant mice [35]. Interestingly, patients carrying different LMNA missense mutations resulting in DCM also exhibit lower protein levels [89]. To what extent the decrease in lamin A/C levels or changes in protein function result in disease pathogenesis is still largely unknown and needs further investigation.
Although it may seem that DCM is predominantly caused by LMNA haploinsufficiency, missense mutations in LMNA, which do not lead to changes in lamin A/C protein levels, also result in DCM. For example, LMNA K219T missense mutation causing severe DCM and heart failure with conduction system disease [52] does not lead to obvious changes in lamin A/C levels in K219T iPSC-CMs [53]. LMNA H222P missense mutation has been shown to cause Emery–Dreifuss muscular dystrophy (EDMD) and DCM in patients. Homozygous mice with the H222P mutation display muscular dystrophy, left ventricular dilatation, and conduction defects and die by 9 months of age [76]. Similarly to the K219T mutation, Western blot analysis of cardiac and skeletal muscle samples shows no obvious difference in lamin A/C protein levels between wild-type and Lmna H222P/H222P mice [74]. Interestingly, recent studies suggested a developmental origin of LMNA-related cardiac laminopathy. Lmna H222P/H222P embryonic hearts showed noncompaction, dilatation, and decreased heart function already at E13.5[75], while Lmna+/− and Lmna−/− embryonic hearts showed noncompaction cardiomyopathy with no decrease in ejection fraction [63]. Differentiation of mouse embryonic stem cells (ESCs) harboring the Lmna p.H222P mutation revealed decreased expression of cardiac mesoderm marker genes, such as Eomes and Mesp1 as well as cardiac progenitor (CP) markers and impaired CM differentiation. This is in stark contrast to Lmna+/− and Lmna−/− mESCs, which showed premature CM differentiation [63,75], suggesting different mechanisms behind the heart phenotype caused by lamin A/C haploinsufficiency or changes in protein functionality.
Among laminopathy-associated missense mutations, the addition of proline is the most common. Proline addition can significantly alter protein structure. For example, LMNA S143P missense mutation causes DCM and disturbs the coiled-coil domain, thus affecting lamin A/C assembly into the nuclear lamina. This results in nuclear fragility and reduced cellular stress tolerance [49]. The addition of proline might also affect protein phosphorylation through proline-directed kinases, such as the mitogen-activated protein (MAP) kinases, cyclin-dependent protein kinase 5 (CDK5), glycogen synthase 3, etc. Mutations resulting in the addition of proline often result in striated muscle disease, suggesting a common underlying mechanism [90].
3. Arrhythmogenic Right Ventricular Cardiomyopathy
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited heart muscle disorder that predominantly affects the right ventricle [91]. A progressive loss of myocytes and fibro-fatty replacement associated with arrhythmias in the right ventricular myocardium is a hallmark of the disease [92]. Mutations in desmosomal genes, such as Plakophilin 2 (PKP2), Desmoplakin (DSP), Desmoglein 2 (DSG2), Desmocollin 2 (DSC2), and junction plakoglobin (JUP), are the main cause of ARVC [93,94,95,96,97,98]. In addition, mutations in the calcium-handling protein Ryanodine Receptor 2 (RYR2) [99], Phospholamban (PLN) [100], the adherens junction protein Cadherin 2 (CDH2) [101], Integrin-Linked Kinase (ILK) [102], the signaling molecule Transforming Growth Factor-β3 (TGFB3) [103], the cytoskeletal structure protein Titin (TTN) [104], Desmin (DES) [105], transmembrane protein 43 (TMEM43), and lamin A/C (LMNA) have also been reported in ARVC [24,106,107,108].
In 2011, Quarta et al. first reported ARVC caused by mutations in LMNA. Four LMNA variants were identified: R190W, R644C, R72C, and G382V [24]. The R190W and R644C variants also cause DCM and left ventricular noncompaction (LVNC). In addition, R644C can also lead to lipodystrophy and atypical progeria, thus showing an extreme phenotypic diversity. ARVC patients with these four mutations all exhibit RV dilatation and systolic dysfunction. Histological examination of the right ventricular myocardium from R190W and G382V patients showed a loss of more than 50% of myocytes and extensive interstitial fibrosis and fatty replacement [24]. Interestingly, immunohistochemical staining showed significantly reduced plakoglobin expression at the intercalated discs in the myocardium, which could contribute to the development of ARVC [24]. M1K, W514X, and M384I mutations in LMNA have also been identified in ARVC. Patients with M1K and W514X mutations show RV dilatation, non-sustained ventricular tachycardia, and complete atrioventricular block [108]. A patient with the M384I variant not only developed ARVC but also peripheral neuropathy and peroneal muscular atrophy [109].
So far, it remains unknown how LMNA mutations result in ARVC. Since LMNA is a ubiquitously expressed protein, its mechanoprotective function in cardiomyocytes, which can limit the progressive loss of myocytes, its role in the regulation of genes involved in cardiac contractility, and its important role in regulating cell fate choices, which may result in an excess of fibroblasts and adipocytes, might be involved. Tracing back the origins of fat tissue in a mouse model of ARVC, Lombardi et al. suggested that second heart field (SHF)-derived progenitor cells switch to an adipogenic fate through nuclear plakoglobin (JUP)-mediated Wnt signaling inhibition [110]. A subset of resident cardiac fibro-adipocyte progenitor cells characterized by PDGFRAposLinnegTHY1negDDR2neg expression signatures have been shown to be a major source of adipocytes in ARVC caused by Desmoplakin (DSP) haploinsufficiency [111]. Furthermore, the endocardium, epicardium, and cardiac mesenchymal stromal cells also serve as a source of adipocytes in the heart [112,113,114]. Because the endocardium and epicardium give rise to diverse cardiac cell lineages, including mesenchyme and adipocytes [115], via endothelial-to-mesenchymal transition (EndMT) and epithelial-to-mesenchymal transition (EMT), lamin A/C function in regulating EMT [75] might also be a key mechanism driving ARVC pathogenesis.
4. Left Ventricle Noncompaction Cardiomyopathy
Left ventricular noncompaction (LVNC) cardiomyopathy is a rare congenital heart disease resulting from abnormal development of the endocardium and myocardium. Patients with LVNC exhibit a thin compact myocardium and excessive trabeculation and can eventually develop progressive cardiac dysfunction followed by heart failure. LVNC can manifest together with other cardiomyopathies and congenital heart disease [116]. Studies have identified various genes associated with LVNC, such as TTN, MYH7, TNNT2, LDB3, MYBPC3, ACTC1, DSP, CASQ2, RBM20, and the intermediate filaments DES [117] and LMNA [118], with the two most affected genes being TTN and LMNA [119]. The first reported LMNA mutant variant causing LVNC is R190W, which is also associated with familial DCM and ARVC [56]. Another pathogenic LMNA variant causing LVNC is LMNA R644C. R644C mutation carriers show an extreme phenotypic diversity, ranging from DCM and LVNC to lipodystrophy and atypical progeria [59]. Parents and colleagues reported four family members with the LMNA R644C mutation, three of whom developed left ventricular noncompaction cardiomyopathy with normal LV dimensions and function and without evidence of dysrhythmias [60]. Other mutations such as LMNA V74fs, R572C, and V445E have also been associated with LVNC. Patients with the V445E missense mutation are characterized by an arrhythmogenic form of LVNC, suggested to be due to dysfunctional SCN5A [58,119].
How LMNA mutations result in LVNC and the mechanisms underlying the high phenotypic diversity are largely unknown. Two recent studies demonstrated that Lmna H222P/H222P as well as Lmna−/− and Lmna+/− embryonic hearts exhibit noncompaction, suggesting these mouse models as important tools to study the developmental origin and the mechanisms behind LMNA-mediated noncompaction cardiomyopathy [63,75]. Interestingly, our own study revealed that Lmna LOF results in abnormal cell fate choices during cardiogenesis, i.e., promotes CM and represses endothelial cell fate. Since the crosstalk between CMs and endothelial cells is instrumental for proper cardiac development and myocardial compaction [120], abnormal cardiovascular cell fate choices and dysfunctional endothelium might also contribute to LVNC. Thus, understanding the link between alternative cell fate choices, changes in cell behavior, and tissue-specific phenotypes caused by pathogenic LMNA mutations would be an important question to address in further studies.
5. Restrictive Cardiomyopathy
Restrictive cardiomyopathy (RCM) is a rare cardiac disease characterized by increased myocardial stiffness resulting in impaired ventricular filling. Patients with RCM show enlarged atria and diastolic dysfunction, while systolic function and ventricular wall thicknesses are often normal until the later stages of the disease [121,122,123]. Although most causes of RCM are acquired, several gene mutations have also been identified in patients with RCM [121,122,123,124]. The most common mutated genes found in RCM are sarcomere-related genes such as TTN [125], TNNI3 [126], MYH7 [127], ACTC1 [128], etc. Mutations in non-sarcomere genes such as DES [129], TMEM87B [130], FLNC [131], etc., have also been reported. Recently, Paller et al. reported a truncation mutation of LMNA (c.835 delG:p.Glu279ArgfsX201) in an RCM patient who had a significant biatrial enlargement, atrial fibrillation, and skeletal muscle weakness. Both right and left ventricular size and function were normal, and histological analysis revealed cardiac hypertrophy and focal interstitial fibrosis in the endomyocardial tissue [61]. How Lmna mutations cause RCM is not known; a plausible mechanism could be the activation of profibrotic signaling, as discussed below.
6. Molecular Mechanisms Resulting in LMNA-Related Cardiomyopathy Pathogenesis
Since LMNA-related cardiomyopathies caused by distinct point mutations show phenotypic diversity, the precise molecular mechanisms resulting in disease pathogenesis are also distinct and complex. Taking into account the variety of different functions of the nuclear lamina, three central mechanisms have been suggested to drive disease pathogenesis.
The “mechanical hypothesis” proposes that disruption of the nuclear lamina causes increased nuclear fragility and increased susceptibility to mechanical stress [132]. This hypothesis is supported by observations that CMs from patients or mouse models with lamin A/C mutations exhibit nuclear rupture, DNA damage, and cell cycle arrest [63,65,88,133]. Interestingly, Lmna−/− non-CMs subjected to stretch show significantly increased DNA damage, further supporting the notion that the elevated cell death could be due to the inability of Lmna−/− CMs to respond adequately to mechanical stress [63]. Importantly, a recent study revealed that disrupting the LINC complex and thereby decoupling the nucleus/nucleoskeleton from the mechanical forces transduced by the cytoskeleton increases more than fivefold the lifespan of LMNA-deficient mice [134], pointing to therapeutic opportunities for patients carrying mutations resulting in nuclear fragility.
Myriad studies have demonstrated a role of lamins in regulating MAPK, TGF-β, Wnt–β-catenin, and Notch signaling cascades [135,136] and suggested that altered signaling is a key driver of LMNA-related dilated cardiomyopathy. For instance, LMNA-related cardiomyopathy shows a significant increase in myocardial fibrosis which contributes to left ventricular dysfunction and heart failure [24,35,137,138]. Profibrotic signaling, such as TGF-β, MAPK, and ERK signaling, is activated in Lmna H222P/H222P mice, and the partial inhibition of ERK and JNK signaling before the onset of cardiomyopathy in Lmna H222P/H222P mice significantly reduces cardiac fibrosis and prevents the development of left ventricle dilatation and decreased cardiac ejection fraction [138,139,140,141]. Indeed, therapies targeting intracellular signaling alterations are being developed in a preclinical setting [142].
Since nuclear lamins anchor chromatin at the nuclear periphery, the “chromatin hypothesis” suggests that chromatin alterations as a result of LMNA haploinsufficiency or mutation result in abnormal gene expression programs responsible for the disease phenotype [132]. In the last years, a number of studies using iPSC-CMs or mESC-CMs uncovered changes in chromatin architecture coupled to transcriptional changes in different ion channels such as SCN5A, CACNA1A/C/D, HCN4, SCN3b, and SCN4b, as well as Pdgfb pathway activation, which might explain the arrhythmogenic conduction defects in LMNA patients [38,53,63,143].
7. Epigenetics in LMNA-Related CARDIOMYOPATHIES
Lamina-associated domain reorganization and changes in chromatin architecture in LMNA-related cardiomyopathy.
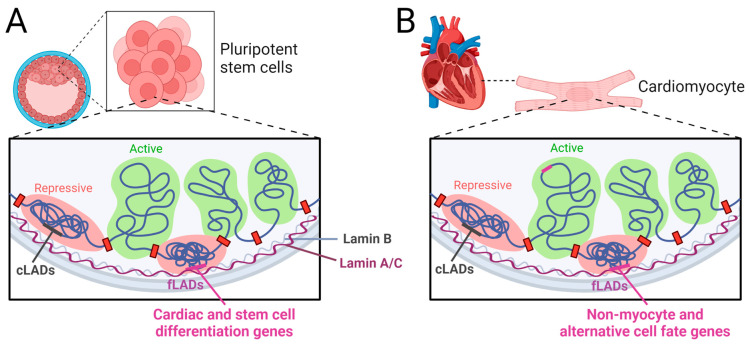
As already mentioned, the nuclear lamina shapes higher-order chromatin structure by anchoring large heterochromatic regions (~ 0.1–10 Mb stretches) at the nuclear periphery, termed lamina-associated domains (LADs). LADs are enriched in the repressive histone marks H3K9me2/3 and H3K27me3, and genes associated with LADs are mostly inactive [15]. Although most LADs are conserved between cell types (constitutive LADs (cLADs)), some chromatin nuclear lamina interactions are detected in specific cell types (facultative LADs (fLADs)) (Figure 2) [144,145]. Indeed, genome–nuclear lamina dynamics have been proposed to play a key role in cell fate decisions by “locking” or “unlocking” genes conferring cell identity at the nuclear periphery [145,146]. For example, during mESC differentiation into astrocytes (ACs), specific AC genes detach from ESC LADs resulting in gene activation. A substantial number of genes are not immediately activated upon detachment from the nuclear lamina but rather become primed for activation at a later stage [145]. Similar mechanisms also occur during CM differentiation. HDAC3 directly represses cardiac differentiation by tethering CM genes to the nuclear lamina. The loss of HDAC3 in cardiac progenitor cells releases these genomic regions from the nuclear periphery, leading to early cardiac gene expression and differentiation [147]. Our own study further showed that lamin A/C and not B-type lamins is responsible for the early activation of a transcriptional program promoting CM versus endothelial cell fate and differentiation [63]. Interestingly, lineage shifts upon LMNA loss or mutation have been reported in other tissues, suggesting that aberrant activation of genes driving an unscheduled differentiation could be a common feature of laminopathic cells [148,149,150,151]. Similar to ACs, we found two modes of regulation: (i) Lamin A/C keeps cell differentiation and cardiac morphogenesis genes silent, such as Gata4/6, Bmps, Wnts, Myl4, etc. Upon lamin A/C LOF, these genes are ectopically expressed in mESCs. (ii) Lamin A/C restricts transcriptional permissiveness at cardiac structural and contraction genes, such as Ryr2, Mybpc3, Adrb2, etc. Upon lamin A/C LOF, chromatin becomes more accessible, but this is not sufficient to elicit gene transcription in ESCs. However, during cardiac differentiation, these primed loci are readily accessible to cardiac transcription factors (TFs), resulting in aberrant cardiovascular cell fate choices, premature CM maturation, cell cycle withdrawal, and abnormal contractility. In contrast, Lmna H222P/H222P mESCs, or mESCs harboring the G609G mutation causing accelerated aging, did not show similar changes in chromatin accessibility nor in expression patterns, supporting the view that the molecular mechanisms underlying the distinct phenotypes upon lamin A/C LOF and missense mutations are different [63].
Figure 2.
Function of lamin A/C in naïve pluripotent stem cells (A) and CMs (B). In naïve pluripotent stem cells (A), lamin A/C anchors cardiac genes and genes involved in stem cell differentiation to the repressive nuclear periphery. Lamin A/C LOF leads to their detachment from the nuclear lamina accompanied with either immediate activation or epigenetic priming for activation later in cardiogenesis. These chromatin alterations result in premature cardiomyocyte differentiation, cell cycle withdrawal, and abnormal contractility. cLADs—constitutive LADs; fLADs—facultative LADs. In cardiomyocytes (B), the nuclear lamina anchors non-myocyte genes and genes involved in alternative cell fates to the nuclear periphery. Mutations in LMNA lead to disruption of the peripheral heterochromatin, resulting in activation of alternative cell fate genes. Figures were designed with BioRender.
Many recent studies have focused on the role of lamin A/C in chromatin organization in human induced pluripotent stem cell (hiPSC)-derived CMs (hiPSC-CMs) to pinpoint the molecular mechanisms associated with LMNA cardiomyopathy. For instance, in hiPSC-CMs harboring the frameshift mutation K117fs that leads to lamin A/C haploinsufficiency, chromatin accessibility is increased at lamin A/C LADs, leading to transcriptional activation. Among others, the PDGF pathway was highly activated in K117fs iPSC-CMs and its inhibition rescued the arrhythmic phenotype, suggesting that PDGF inhibitors could be beneficial in preventing fatal arrhythmias often manifested in patients with LMNA-related cardiomyopathy [38]. Notably, the authors found that many genes located in non-LAD regions are also highly upregulated in K117fs iPSC-CMs compared to controls, suggesting that mutations in lamin A/C might also result in maladaptive epigenetic remodeling at non-LAD regions. This might be mediated through changes in B-type lamin function, upregulation of pioneer transcription factors, loss of binding of repressive complexes, or other mechanisms. Indeed, although B-type lamins form distinct meshworks, the loss of A-type lamins results in alterations in B-type meshworks, suggesting that their activity might be interconnected [152]. Thus, mutation-mediated changes in lamin A/C activity might also affect lamin B1/B2 function. Interestingly, lamin B2 plays an essential function in regulating CM karyokinesis, and Lmnb2 ablation resulted in polyploid CM nuclei in neonatal mice [153]. Lmna ablation also results in increased numbers of binucleated CMs in neonatal mice [63], suggesting that lamin A/C loss might affect lamin B2 function. The activation of pioneer transcription factors, which can engage developmentally silenced genes embedded in “closed” chromatin [154,155,156,157] and induce chromatin opening, might also play a role in LMNA-related cardiomyopathies. Indeed, the pioneer cardiac TF GATA4 is activated by lamin A/C loss, and Gata4 silencing or haploinsufficiency rescues the abnormal cardiovascular cell function induced by lamin A/C deficiency [63]. Another pioneer TF, FoxO1 [158], also shows increased binding to chromatin upon Lmna LOF. FoxO TFs play key functions in stress response, cell proliferation, and apoptosis, and the longevity and suppression of FoxO activity in CMs partially rescues the cardiac phenotype and prolongs survival [159]. Additionally, the nuclear lamina may serve as a binding platform for chromatin remodelers, such as the Polycomb Group Proteins, which can initiate large-scale epigenetic alterations. This will be discussed in the following section (Figure 3).
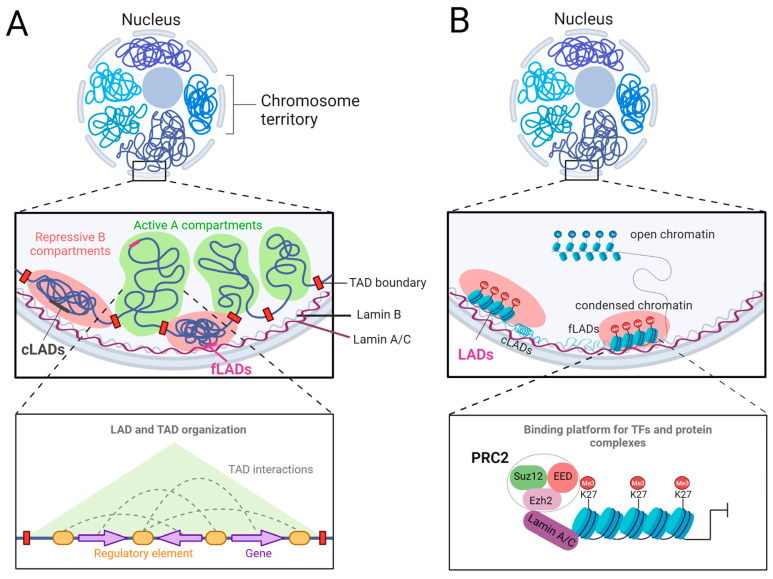
Figure 3.
Lamin-A/C-mediated chromatin organization. (A) Nuclear lamins play a key role in shaping the 3D chromatin organization by anchoring chromatin at the nuclear periphery. Mutations in LMNA might cause changes in LAD and TAD organization, leading to aberrant transcriptional programs. A- and B-type lamins form interconnected networks; thus, LMNA mutations might alter lamin B function. (B) The nuclear lamina serves as a binding platform of TFs and chromatin modifiers, for example, the PRC2 complex, which might be altered upon LMNA mutations.
Another study using an iPSC model harboring the T10I mutation in LMNA suggested a role of the nuclear lamina in safeguarding cellular identity [41]. In T10I iPSC-CMs, the peripheral heterochromatin enriched for non-myocyte lineage genes was disrupted, resulting in the activation of alternative cell fate genes. Upregulation of non-cardiac genes was also observed in iPSC-CMs carrying the R225X mutation in lamin A/C (Figure 2B). Importantly, CACNA1A, encoding a neuronal P/Q-type calcium channel, was upregulated, and pharmacological inhibition partially rescued the altered electrophysiological properties of R225X iPSC-CMs [143]. In this context, it is important to note that in contrast to mouse/human blastocysts and naïve mouse mESCs, hiPSCs cultured in standard conditions represent a primed state and do not express detectable levels of lamin A/C protein [63]. Since lamin A/C plays an important role in chromatin organization in naïve pluripotent stem cells, which is essential for normal cardiogenesis, some important aspects of lamin A/C function cannot be modeled using hiPSCs and requires studies using naïve hiPSCs carrying LMNA mutations.
In addition, chromatin and expression analysis of CMs from patients with different LMNA-related DCM mutations revealed extensive rearrangement of LMNA–chromatin interactions in DCM patients [89]. The reorganization of lamin A/C LADs is associated with altered CpG methylation and dysregulated expression of a large number of genes involved in cell metabolism, the cell cycle, and cell death. Most of the LMNA-related DCM patients’ samples used in this study showed a decrease in lamin A/C protein levels, suggesting that LMNA LOF might account for the observed DNA, chromatin, and expression changes [89].
It is still poorly understood how cell-type-specific tethering at the nuclear lamina is achieved and how mutations in lamins affect the tethering of key cell fate determinants in stem cells and in cells already committed to a certain lineage. Lamins interact with chromatin either directly [160] or indirectly through chromatin-binding proteins. Consistent with its association with both hetero- and euchromatin, lamin A/C interacts with proteins associated with both hetero- and euchromatin, e.g., LAP2α, Emerin, and BANF1 [161,162], while B-type lamins interact with the lamin B receptor (LBR), which mediates the attachment to the inner nuclear membrane, and Heterochromatin Protein 1 (HP1α) associated with heterochromatin [163]. However, all these proteins are broadly expressed and cannot account for the cell-type-specific tethering of LADs. Thus, identifying cell-type-specific interacting partners for nuclear lamins and the effect of lamin mutations on these interactions will be key in understanding the molecular mechanisms underlying the wide-ranging clinical phenotypes and may pinpoint druggable protein–protein interfaces for therapeutic applications.
Moreover, how mutations in lamin A/C affect the separation into relatively active and inactive chromatin regions is still debatable [164]. The genome is organized into higher-order structural domains referred to as topologically associated domains (TADs). TADs tend to interact based on their epigenetic status and transcriptional activity, thus dividing chromosomes into two types of large-scale compartments generally called A compartments (active) and B compartments (inactive) (Figure 3A) [165]. An analysis of A/B compartment changes revealed only ∼1.2% compartment switches in R225X iPSC-CMs with only a minimal correlation with highly dysregulated genes [143]. In contrast, during cardiac differentiation, ∼20% of the genome undergoes A/B compartment reorganization, while many others appear constitutively associated with the nuclear lamina. Interestingly, in Lmna−/− mESC, around 8% of the chromatin compartments switched from A to B and vice versa as a result of lamin A/C loss. These compartment switches highly overlap with lamin A LADs. Genes within the B/A compartment switches (inactive to active) were linked to calcium ion transmembrane transport, muscle cell differentiation, and relaxation of cardiac muscle, including genes such as Myl4, Atp2a3, Ryr2, and Camk2d, which were either activated or primed upon lamin A/C loss. Lamin A/C is expressed in naïve pluripotent stem cells, absent after the loss of pluripotency and during early differentiation, and re-expressed in CMs. This dynamic expression pattern may provide a window of opportunity for LAD and chromatin compartment reorganization, and the activation of transcriptional programs driving important developmental decisions and cell identity.
The role of Polycomb Group Proteins in LMNA-related cardiomyopathy.
As we discussed before, LADs are enriched for H3K27me3. The downregulation of lamin A/C remodels the repressive H3K27me3 and the permissive H3K4me3 histone marks, thereby enhancing transcriptional permissiveness [166]. Indeed, lamin A/C interacts with the Polycomb repressive complex 2 (PRC2) complex, which catalyzes H3K27me3 [167], and lamin A/C loss in myoblasts results in PcG protein foci disassembly, ectopic expression of Polycomb targets, and premature myogenic differentiation [167]. Polycomb Group (PcG) proteins are key epigenetic repressors during development and differentiation. The Polycomb repressive complex 2 (PRC2)-mediated deposition of H3K27me3 recruits the canonical Polycomb repressive complex 1 (PRC1) that monoubiquitinates lysine 119 of histone H2A (H2AK119ub1) and induces chromatin compaction. The core PRC2 is formed by EED, SUZ12, and the catalytic components EZH2 or EZH1 (Figure 3B) [168,169]. Both PRC1 and PRC2 play an important role in cardiac development and differentiation. EZH2 is essential for CM proliferation, survival, and postnatal cardiac homeostasis. The inactivation of Ezh2 specifically in cardiac progenitors results in ectopic transcriptional programs and lethal heart defects [170,171]. PRC2 function also ensures proper cardiac growth, and Eed ablation by TnT-Cre leads to myocardial hypoplasia and embryonic lethality [170,171]. In a mouse model of EDMD, lamin A/C loss results in PcG repositioning and de-repression of non-muscle genes in muscle satellite stem cells together with the activation of p16INK4a that induces cell cycle arrest. This aberrant transcriptional program causes impairment in self-renewal, loss of cell identity, and premature exhaustion of the quiescent satellite cell pool [172]. In a recent study using iPSC-CMs carrying the cardiac-laminopathy-associated K219T mutation, it was shown that the binding of lamin A/C together with PRC2 at the SCN5A promoter represses its expression, resulting in decreased conduction velocity [53]. Together, aberrant PRC activity upon LMNA mutation might play an important role in LMNA-related cardiomyopathies (Figure 3B).
8. Advances in Therapeutic Strategies for LMNA-Related Cardiomyopathy
The clinical management of LMNA-related DCM includes pharmacological treatment with ACE inhibitors and beta blockers and implantable cardiac defibrillators (ICDs) [173,174]. Heart transplantation or ventricular assist devices may also be required for patients in the end stages of heart failure [173,174]. The inhibition of mTOR, MAPK, and LSD1 significantly rescues the LMNA-related DCM phenotype in mice [75,138,175], and a novel and selective p38 MAPK inhibitor is now in a phase 3 clinical trial in LMNA-related DCM [176]. In addition, CRISPR/Cas9-based genome editing strategies have been used in LMNA-caused Hutchinson–Gilford Progeria Syndrome (HGPS) and show promising results [177,178,179]. By using guide RNAs (gRNAs) that target LMNA exon 11 to specifically interfere with lamin A/progerin expression, both Santiago-Fernández et al. and Beyret et al. show a reduced progerin expression and improvement in the progeria phenotype in an HGPS mouse model [177,178]. However, off-target effects, e.g., resulting from insertion and deletions during non-homologous end joining (NHEJ), are a major concern. To overcome these limitations, CRISPR/Cas9-mediated base pair editing systems have been used in HGPS mice [179]. Base pair editing systems could modify the genome without the need of double-strand DNA breaks or donor DNA templates [180]. Two classes of DNA base editors have been reported: cytosine base editors (CBEs), which convert C:G to T:A, and adenine base editors (ABEs) which convert A:T to G:C [181,182]. Systemic injection of a single dose of dual AAV9 encoding ABE and sgRNA into an HGPS mouse model significantly extends the median lifespan of the mice, improves aortic health, and fully rescues VSMC counts as well as adventitial fibrosis [179]. Despite the power of the base pair editing technology, a major limitation is the inability to edit the genome beyond four transition mutations. Prime editing represents a novel approach which is not only suitable for all transition and transversion mutations but also for small insertion and deletion mutations [183]. Similar to base pair editing, prime editing does not require double-strand DNA breaks or donor DNA templates [183] and could be used in the correction of genetic cardiomyopathies.
9. Conclusions and Perspectives
Accumulating evidence shows that epigenetic alterations play a crucial role in LMNA-related cardiomyopathies. Mutations in LMNA affect 3D genome architecture and chromatin accessibility, thereby altering gene expression programs. Several prospective target genes, such as PDGFRB, Gata4, SCN5A, and CACNA1A, have been identified using experimental models harboring different LMNA mutations, which may serve as potential therapeutic targets. As reviewed above, specific LMNA variants can cause extreme phenotypic diversity, which makes it challenging to understand the primary changes underlying disease pathogenesis and thus to design specific treatment strategies for patients. Therefore, an important question remains: how do different and specific LMNA mutations result in phenotypic diversity? Environmental factors, such as diet, exercise, and stress, as well as age, sex, and other comorbidities, might also contribute to the phenotypic variability in patients with pathogenic LMNA mutations. Identifying cell-type-specific interacting partners for nuclear lamins and the effect of lamin mutations on these interactions would also be important in understanding the wide-ranging clinical phenotypes and may pinpoint druggable protein–protein interfaces for therapeutic applications. Given the important role of lamin A/C in heart development and CM differentiation, developmental changes in asymptomatic-at-birth LMNA patients might result in late changes in heart structure and function, warranting further investigation.
Author Contributions
Conceptualization, Y.W. and G.D.; writing—original draft preparation, Y.W.; writing—review and editing, G.D.; visualization, Y.W. and G.D.; funding acquisition, G.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the SFB1366 (Project A03), SFB1550 (Project A03) funded by the DFG, and the DZHK (81Z0500202, 81X2500216), funded by BMBF.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Schreiber K.H., Kennedy B.K. When lamins go bad: Nuclear structure and disease. Cell. 2013;152:1365–1375. doi: 10.1016/j.cell.2013.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Osmanagic-Myers S., Foisner R. The structural and gene expression hypotheses in laminopathic diseases-not so different after all. Mol. Biol. Cell. 2019;30:1786–1790. doi: 10.1091/mbc.E18-10-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKeon F.D., Kirschner M.W., Caput D. Homologies in both primary and secondary structure between nuclear envelope and intermediate filament proteins. Nature. 1986;319:463–468. doi: 10.1038/319463a0. [DOI] [PubMed] [Google Scholar]
- 4.Fisher D.Z., Chaudhary N., Blobel G. cDNA sequencing of nuclear lamins A and C reveals primary and secondary structural homology to intermediate filament proteins. Proc. Natl. Acad. Sci. USA. 1986;83:6450–6454. doi: 10.1073/pnas.83.17.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruenbaum Y., Foisner R. Lamins: Nuclear intermediate filament proteins with fundamental functions in nuclear mechanics and genome regulation. Annu. Rev. Biochem. 2015;84:131–164. doi: 10.1146/annurev-biochem-060614-034115. [DOI] [PubMed] [Google Scholar]
- 6.Lin F., Worman H.J. Structural organization of the human gene (LMNB1) encoding nuclear lamin B1. Genomics. 1995;27:230–236. doi: 10.1006/geno.1995.1036. [DOI] [PubMed] [Google Scholar]
- 7.Vorburger K., Lehner C., Kitten G., Eppenberger H., Nigg E. A second higher vertebrate B-type lamin: cDNA sequence determination and in vitro processing of chicken lamin B2. J. Mol. Biol. 1989;208:405–415. doi: 10.1016/0022-2836(89)90505-6. [DOI] [PubMed] [Google Scholar]
- 8.Peter M., Kitten G., Lehner C., Vorburger K., Bailer S., Maridor G., Nigg E. Cloning and sequencing of cDNA clones encoding chicken lamins A and B1 and comparison of the primary structures of vertebrate A-and B-type lamins. J. Mol. Biol. 1989;208:393–404. doi: 10.1016/0022-2836(89)90504-4. [DOI] [PubMed] [Google Scholar]
- 9.Höger T.H., Zatloukal K., Waizenegger I., Krohne G. Characterization of a second highly conserved B-type lamin present in cells previously thought to contain only a single B-type lamin. Chromosoma. 1990;99:379–390. doi: 10.1007/BF01726689. [DOI] [PubMed] [Google Scholar]
- 10.Turgay Y., Eibauer M., Goldman A.E., Shimi T., Khayat M., Ben-Harush K., Dubrovsky-Gaupp A., Sapra K.T., Goldman R.D., Medalia O. The molecular architecture of lamins in somatic cells. Nature. 2017;543:261–264. doi: 10.1038/nature21382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burke B., Stewart C.L. The nuclear lamins: Flexibility in function. Nat. Rev. Mol. Cell Biol. 2013;14:13–24. doi: 10.1038/nrm3488. [DOI] [PubMed] [Google Scholar]
- 12.Walling B.L., Murphy P.M. Protean regulation of leukocyte function by nuclear lamins. Trends Immunol. 2021;42:323–335. doi: 10.1016/j.it.2021.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Shimi T., Kittisopikul M., Tran J., Goldman A.E., Adam S.A., Zheng Y., Jaqaman K., Goldman R.D. Structural organization of nuclear lamins A, C, B1, and B2 revealed by superresolution microscopy. Mol. Biol. Cell. 2015;26:4075–4086. doi: 10.1091/mbc.E15-07-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nmezi B., Xu J., Fu R., Armiger T.J., Rodriguez-Bey G., Powell J.S., Ma H., Sullivan M., Tu Y., Chen N.Y. Concentric organization of A-and B-type lamins predicts their distinct roles in the spatial organization and stability of the nuclear lamina. Proc. Natl. Acad. Sci. USA. 2019;116:4307–4315. doi: 10.1073/pnas.1810070116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Steensel B., Belmont A.S. Lamina-Associated Domains: Links with Chromosome Architecture, Heterochromatin, and Gene Repression. Cell. 2017;169:780–791. doi: 10.1016/j.cell.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reddy K.L., Zullo J.M., Bertolino E., Singh H. Transcriptional repression mediated by repositioning of genes to the nuclear lamina. Nature. 2008;452:243–247. doi: 10.1038/nature06727. [DOI] [PubMed] [Google Scholar]
- 17.Wen B., Wu H., Shinkai Y., Irizarry R.A., Feinberg A.P. Large histone H3 lysine 9 dimethylated chromatin blocks distinguish differentiated from embryonic stem cells. Nat. Genet. 2009;41:246–250. doi: 10.1038/ng.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gesson K., Rescheneder P., Skoruppa M.P., von Haeseler A., Dechat T., Foisner R. A-type lamins bind both hetero- and euchromatin, the latter being regulated by lamina-associated polypeptide 2 alpha. Genome Res. 2016;26:462–473. doi: 10.1101/gr.196220.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jahed Z., Mofrad M.R. The nucleus feels the force, LINCed in or not! Curr. Opin. Cell Biol. 2019;58:114–119. doi: 10.1016/j.ceb.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 20.Stroud M.J., Banerjee I., Veevers J., Chen J. Linker of nucleoskeleton and cytoskeleton complex proteins in cardiac structure, function, and disease. Circ. Res. 2014;114:538–548. doi: 10.1161/CIRCRESAHA.114.301236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lityagina O., Dobreva G. The LINC Between Mechanical Forces and Chromatin. Front. Physiol. 2021;12:710809. doi: 10.3389/fphys.2021.710809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerull B., Klaassen S., Brodehl A. Genetic Causes of Cardiac Disease. Springer Nature; Berlin/Heidelberg, Germany: 2019. The Genetic Landscape of Cardiomyopathies. [Google Scholar]
- 23.McNally E.M., Mestroni L. Dilated Cardiomyopathy: Genetic Determinants and Mechanisms. Circ. Res. 2017;121:731–748. doi: 10.1161/CIRCRESAHA.116.309396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quarta G., Syrris P., Ashworth M., Jenkins S., Zuborne Alapi K., Morgan J., Muir A., Pantazis A., McKenna W.J., Elliott P.M. Mutations in the Lamin A/C gene mimic arrhythmogenic right ventricular cardiomyopathy. Eur. Heart J. 2012;33:1128–1136. doi: 10.1093/eurheartj/ehr451. [DOI] [PubMed] [Google Scholar]
- 25.Van Rijsingen I.A., Arbustini E., Elliott P.M., Mogensen J., Hermans-van Ast J.F., Van Der Kooi A.J., Van Tintelen J.P., Van Den Berg M.P., Pilotto A., Pasotti M. Risk factors for malignant ventricular arrhythmias in lamin A/C mutation carriers: A European cohort study. J. Am. Coll. Cardiol. 2012;59:493–500. doi: 10.1016/j.jacc.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 26.Fatkin D., MacRae C., Sasaki T., Wolff M.R., Porcu M., Frenneaux M., Atherton J., Vidaillet Jr H.J., Spudich S., De Girolami U. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N. Engl. J. Med. 1999;341:1715–1724. doi: 10.1056/NEJM199912023412302. [DOI] [PubMed] [Google Scholar]
- 27.Kumar S., Baldinger S.H., Gandjbakhch E., Maury P., Sellal J.-M., Androulakis A.F., Waintraub X., Charron P., Rollin A., Richard P. Long-term arrhythmic and nonarrhythmic outcomes of lamin A/C mutation carriers. J. Am. Coll. Cardiol. 2016;68:2299–2307. doi: 10.1016/j.jacc.2016.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Nishiuchi S., Makiyama T., Aiba T., Nakajima K., Hirose S., Kohjitani H., Yamamoto Y., Harita T., Hayano M., Wuriyanghai Y. Gene-based risk stratification for cardiac disorders in LMNA mutation carriers. Circ. Cardiovasc. Genet. 2017;10:e001603. doi: 10.1161/CIRCGENETICS.116.001603. [DOI] [PubMed] [Google Scholar]
- 29.van Engelen B.G., Muchir A., Hutchison C.J., van der Kooi A.J., Bonne G., Lammens M. The lethal phenotype of a homozygous nonsense mutation in the lamin A/C gene. Neurology. 2005;64:374–376. doi: 10.1212/01.WNL.0000149763.15180.00. [DOI] [PubMed] [Google Scholar]
- 30.Roncarati R., Viviani Anselmi C., Krawitz P., Lattanzi G., von Kodolitsch Y., Perrot A., di Pasquale E., Papa L., Portararo P., Columbaro M., et al. Doubly heterozygous LMNA and TTN mutations revealed by exome sequencing in a severe form of dilated cardiomyopathy. Eur. J. Hum. Genet. 2013;21:1105–1111. doi: 10.1038/ejhg.2013.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Galata Z., Kloukina I., Kostavasili I., Varela A., Davos C.H., Makridakis M., Bonne G., Capetanaki Y. Amelioration of desmin network defects by αB-crystallin overexpression confers cardioprotection in a mouse model of dilated cardiomyopathy caused by LMNA gene mutation. J. Mol. Cell. Cardiol. 2018;125:73–86. doi: 10.1016/j.yjmcc.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 32.Maggi L., Mavroidis M., Psarras S., Capetanaki Y., Lattanzi G. Skeletal and cardiac muscle disorders caused by mutations in genes encoding intermediate filament proteins. Int. J. Mol. Sci. 2021;22:4256. doi: 10.3390/ijms22084256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vincenzo M., Michelangelo M., Costanza S., Francesca T., Lucia C., Greta A., Anna R., Fulvia B., Adelaide C.M., Giovanna L. A Single mtDNA Deletion in Association with a LMNA Gene New Frameshift Variant: A Case Report. J. Neuromuscul. Dis. 2022;9:457–462. doi: 10.3233/JND-220802. [DOI] [PubMed] [Google Scholar]
- 34.Meinke P., Mattioli E., Haque F., Antoku S., Columbaro M., Straatman K.R., Worman H.J., Gundersen G.G., Lattanzi G., Wehnert M. Muscular dystrophy-associated SUN1 and SUN2 variants disrupt nuclear-cytoskeletal connections and myonuclear organization. PLoS Genet. 2014;10:e1004605. doi: 10.1371/journal.pgen.1004605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mounkes L.C., Kozlov S.V., Rottman J.N., Stewart C.L. Expression of an LMNA-N195K variant of A-type lamins results in cardiac conduction defects and death in mice. Hum. Mol. Genet. 2005;14:2167–2180. doi: 10.1093/hmg/ddi221. [DOI] [PubMed] [Google Scholar]
- 36.Cai Z.-J., Lee Y.-K., Lau Y.-M., Ho J.C.-Y., Lai W.-H., Wong N.L.-Y., Huang D., Hai J.-j., Ng K.-M., Tse H.-F. Expression of Lmna-R225X nonsense mutation results in dilated cardiomyopathy and conduction disorders (DCM-CD) in mice: Impact of exercise training. Int. J. Cardiol. 2020;298:85–92. doi: 10.1016/j.ijcard.2019.09.058. [DOI] [PubMed] [Google Scholar]
- 37.Lee Y.K., Lau Y.M., Cai Z.J., Lai W.H., Wong L.Y., Tse H.F., Ng K.M., Siu C.W. Modeling treatment response for lamin A/C related dilated cardiomyopathy in human induced pluripotent stem cells. J. Am. Heart Assoc. 2017;6:e005677. doi: 10.1161/JAHA.117.005677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lee J., Termglinchan V., Diecke S., Itzhaki I., Lam C.K., Garg P., Lau E., Greenhaw M., Seeger T., Wu H. Activation of PDGF pathway links LMNA mutation to dilated cardiomyopathy. Nature. 2019;572:335–340. doi: 10.1038/s41586-019-1406-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.MacLeod H.M., Culley M.R., Huber J.M., McNally E.M. Lamin A/C truncation in dilated cardiomyopathy with conduction disease. BMC Med. Genet. 2003;4:4. doi: 10.1186/1471-2350-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sebillon P., Bouchier C., Bidot L., Bonne G., Ahamed K., Charron P., Drouin-Garraud V., Millaire A., Desrumeaux G., Benaiche A. Expanding the phenotype of LMNA mutations in dilated cardiomyopathy and functional consequences of these mutations. J. Med. Genet. 2003;40:560–567. doi: 10.1136/jmg.40.8.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shah P.P., Lv W., Rhoades J.H., Poleshko A., Abbey D., Caporizzo M.A., Linares-Saldana R., Heffler J.G., Sayed N., Thomas D. Pathogenic LMNA variants disrupt cardiac lamina-chromatin interactions and de-repress alternative fate genes. Cell Stem Cell. 2021;28:938–954.e939. doi: 10.1016/j.stem.2020.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hussain I., Patni N., Ueda M., Sorkina E., Valerio C.M., Cochran E., Brown R.J., Peeden J., Tikhonovich Y., Tiulpakov A. A novel generalized lipodystrophy-associated progeroid syndrome due to recurrent heterozygous LMNA p. T10I mutation. J. Clin. Endocrinol. Metab. 2018;103:1005–1014. doi: 10.1210/jc.2017-02078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sahinoz M., Khairi S., Cuttitta A., Brady G.F., Rupani A., Meral R., Tayeh M.K., Thomas P., Riebschleger M., Camelo-Piragua S. Potential association of LMNA-associated generalized lipodystrophy with juvenile dermatomyositis. Clin. Diabetes Endocrinol. 2018;4:6. doi: 10.1186/s40842-018-0058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mory P.B., Crispim F., Kasamatsu T., Gabbay M.A., Dib S.A., Moisés R.S. Atypical generalized lipoatrophy and severe insulin resistance due to a heterozygous LMNA p. T10I mutation. Arq. Bras. Endocrinol. Metabol. 2008;52:1252–1256. doi: 10.1590/S0004-27302008000800008. [DOI] [PubMed] [Google Scholar]
- 45.Yang J., Argenziano M.A., Burgos Angulo M., Bertalovitz A., Beidokhti M.N., McDonald T.V. Phenotypic Variability in iPSC-Induced Cardiomyocytes and Cardiac Fibroblasts Carrying Diverse LMNA Mutations. Front. Physiol. 2021;12:2162. doi: 10.3389/fphys.2021.778982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muchir A., Bonne G., van der Kooi A.J., van Meegen M., Baas F., Bolhuis P.A., de Visser M., Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum. Mol. Genet. 2000;9:1453–1459. doi: 10.1093/hmg/9.9.1453. [DOI] [PubMed] [Google Scholar]
- 47.Charniot J.C., Pascal C., Bouchier C., Sébillon P., Salama J., Duboscq-Bidot L., Peuchmaurd M., Desnos M., Artigou J.Y., Komajda M. Functional consequences of an LMNA mutation associated with a new cardiac and non-cardiac phenotype. Hum. Mutat. 2003;21:473–481. doi: 10.1002/humu.10170. [DOI] [PubMed] [Google Scholar]
- 48.van Tintelen J.P., Hofstra R.M., Katerberg H., Rossenbacker T., Wiesfeld A.C., du Marchie Sarvaas G.J., Wilde A.A., van Langen I.M., Nannenberg E.A., van der Kooi A.J., et al. High yield of LMNA mutations in patients with dilated cardiomyopathy and/or conduction disease referred to cardiogenetics outpatient clinics. Am. Heart J. 2007;154:1130–1139. doi: 10.1016/j.ahj.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 49.West G., Gullmets J., Virtanen L., Li S.-P., Keinänen A., Shimi T., Mauermann M., Heliö T., Kaartinen M., Ollila L. Deleterious assembly of the lamin A/C mutant p. S143P causes ER stress in familial dilated cardiomyopathy. J. Cell Sci. 2016;129:2732–2743. doi: 10.1242/jcs.184150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.West G., Turunen M., Aalto A., Virtanen L., Li S.-P., Heliö T., Meinander A., Taimen P. A heterozygous p. S143P mutation in LMNA associates with proteasome dysfunction and enhanced autophagy-mediated degradation of mutant lamins A and C. Front. Cell Dev. Biol. 2022;10:932983. doi: 10.3389/fcell.2022.932983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonne G., Mercuri E., Muchir A., Urtizberea A., Bécane H.M., Recan D., Merlini L., Wehnert M., Boor R., Reuner U., et al. Clinical and molecular genetic spectrum of autosomal dominant Emery-Dreifuss muscular dystrophy due to mutations of the lamin A/C gene. Ann. Neurol. 2000;48:170–180. doi: 10.1002/1531-8249(200008)48:2<170::AID-ANA6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 52.Perrot A., Hussein S., Ruppert V., Schmidt H.H., Wehnert M.S., Duong N.T., Posch M.G., Panek A., Dietz R., Kindermann I., et al. Identification of mutational hot spots in LMNA encoding lamin A/C in patients with familial dilated cardiomyopathy. Basic Res. Cardiol. 2009;104:90–99. doi: 10.1007/s00395-008-0748-6. [DOI] [PubMed] [Google Scholar]
- 53.Salvarani N., Crasto S., Miragoli M., Bertero A., Paulis M., Kunderfranco P., Serio S., Forni A., Lucarelli C., Dal Ferro M., et al. The K219T-Lamin mutation induces conduction defects through epigenetic inhibition of SCN5A in human cardiac laminopathy. Nat. Commun. 2019;10:2267. doi: 10.1038/s41467-019-09929-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mewborn S.K., Puckelwartz M.J., Abuisneineh F., Fahrenbach J.P., Zhang Y., MacLeod H., Dellefave L., Pytel P., Selig S., Labno C.M. Altered chromosomal positioning, compaction, and gene expression with a lamin A/C gene mutation. PLoS ONE. 2010;5:e14342. doi: 10.1371/journal.pone.0014342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang L., Sun J., Chen Z., Liu L., Sun Y., Lin J., Hu X., Zhao M., Ma Y., Lu D. The LMNA p. R541C mutation causes dilated cardiomyopathy in human and mice. Int. J. Cardiol. 2022;363:149–158. doi: 10.1016/j.ijcard.2022.06.038. [DOI] [PubMed] [Google Scholar]
- 56.Hermida-Prieto M., Monserrat L., Castro-Beiras A., Laredo R., Soler R., Peteiro J., Rodríguez E., Bouzas B., Álvarez N., Muñiz J. Familial dilated cardiomyopathy and isolated left ventricular noncompaction associated with lamin A/C gene mutations. Am. J. Cardiol. 2004;94:50–54. doi: 10.1016/j.amjcard.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 57.Chatzifrangkeskou M., Yadin D., Marais T., Chardonnet S., Cohen-Tannoudji M., Mougenot N., Schmitt A., Crasto S., Di Pasquale E., Macquart C. Cofilin-1 phosphorylation catalyzed by ERK1/2 alters cardiac actin dynamics in dilated cardiomyopathy caused by lamin A/C gene mutation. Hum. Mol. Genet. 2018;27:3060–3078. doi: 10.1093/hmg/ddy215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu Z., Shan H., Huang J., Li N., Hou C., Pu J. A novel lamin A/C gene missense mutation (445 V > E) in immunoglobulin-like fold associated with left ventricular non-compaction. Europace. 2016;18:617–622. doi: 10.1093/europace/euv044. [DOI] [PubMed] [Google Scholar]
- 59.Rankin J., Auer-Grumbach M., Bagg W., Colclough K., Duong N.T., Fenton-May J., Hattersley A., Hudson J., Jardine P., Josifova D. Extreme phenotypic diversity and nonpenetrance in families with the LMNA gene mutation R644C. Am. J. Med. Genet. Part A. 2008;146:1530–1542. doi: 10.1002/ajmg.a.32331. [DOI] [PubMed] [Google Scholar]
- 60.Parent J.J., Towbin J.A., Jefferies J.L. Left ventricular noncompaction in a family with lamin A/C gene mutation. Tex. Heart Inst. J. 2015;42:73. doi: 10.14503/THIJ-13-3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paller M.S., Martin C.M., Pierpont M.E. Restrictive cardiomyopathy: An unusual phenotype of a lamin A variant. ESC Heart Fail. 2018;5:724–726. doi: 10.1002/ehf2.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim Y., Zheng Y. Generation and characterization of a conditional deletion allele for Lmna in mice. Biochem. Biophys. Res. Commun. 2013;440:8–13. doi: 10.1016/j.bbrc.2013.08.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y., Elsherbiny A., Kessler L., Cordero J., Shi H., Serke H., Lityagina O., Trogisch F.A., Mohammadi M.M., El-Battrawy I. Lamin A/C-dependent chromatin architecture safeguards nave pluripotency to prevent aberrant cardiovascular cell fate and function. Nat. Commun. 2022;13:6663. doi: 10.1038/s41467-022-34366-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wolf C.M., Wang L., Alcalai R., Pizard A., Burgon P.G., Ahmad F., Sherwood M., Branco D.M., Wakimoto H., Fishman G.I. Lamin A/C haploinsufficiency causes dilated cardiomyopathy and apoptosis-triggered cardiac conduction system disease. J. Mol. Cell. Cardiol. 2008;44:293–303. doi: 10.1016/j.yjmcc.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nikolova V., Leimena C., Mcmahon A.C., Tan A.C., Chandar S., Jogia D., Kesteven S.H., Michalicek J., Otway R., Verheyen F. Defects in nuclear structure and function promote dilated cardiomyopathy in lamin A/C–deficient mice. J. Clin. Investig. 2004;113:357–369. doi: 10.1172/JCI200419448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kubben N., Voncken J.W., Konings G., van Weeghel M., van den Hoogenhof M.M., Gijbels M., van Erk A., Schoonderwoerd K., van den Bosch B., Dahlmans V., et al. Post-natal myogenic and adipogenic developmental: Defects and metabolic impairment upon loss of A-type lamins. Nucleus. 2011;2:195–207. doi: 10.4161/nucl.2.3.15731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Auguste G., Rouhi L., Matkovich S.J., Coarfa C., Marian A.J. BET bromodomain inhibition attenuates cardiac phenotype in myocyte-specific Lamin A/C-deficient mice. J. Clin. Investig. 2020;130:4740–4758. doi: 10.1172/JCI135922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fong L.G., Ng J.K., Lammerding J., Vickers T.A., Young S.G. Prelamin A and lamin A appear to be dispensable in the nuclear lamina. J. Clin. Investig. 2006;116:743–752. doi: 10.1172/JCI27125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Coffinier C., Jung H.J., Li Z., Nobumori C., Yun U.J., Farber E.A., Davies B.S., Weinstein M.M., Yang S.H., Lammerding J. Direct Synthesis of Lamin A, Bypassing Prelamin A Processing, Causes Misshapen Nuclei in Fibroblasts but No Detectable Pathology in Mice. J. Biol. Chem. 2010;285:20818–20826. doi: 10.1074/jbc.M110.128835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dan L., Lian H., Zhang X., Shao H., Lan H., Qin C., Zhang L., Wu G.S. LMNA E82K Mutation Activates FAS and Mitochondrial Pathways of Apoptosis in Heart Tissue Specific Transgenic Mice. PLoS ONE. 2010;5:e15167. doi: 10.1371/journal.pone.0015167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bertrand A.T., Renou L., Papadopoulos A., Beuvin M., Lacène E., Massart C., Ottolenghi C., Decostre V., Maron S., Schlossarek S., et al. DelK32-lamin A/C has abnormal location and induces incomplete tissue maturation and severe metabolic defects leading to premature death. Hum. Mol. Genet. 2012;21:1037–1048. doi: 10.1093/hmg/ddr534. [DOI] [PubMed] [Google Scholar]
- 72.Cattin M.E., Ferry A., Vignaud A., Mougenot N., Jacquet A., Wahbi K., Bertrand A.T., Bonne G. Mutation in lamin A/C sensitizes the myocardium to exercise-induced mechanical stress but has no effect on skeletal muscles in mouse. Neuromuscul. Disord. NMD. 2016;26:490–499. doi: 10.1016/j.nmd.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 73.Cattin M.E., Bertrand A.T., Schlossarek S., Le Bihan M.C., Skov Jensen S., Neuber C., Crocini C., Maron S., Lainé J., Mougenot N., et al. Heterozygous LmnadelK32 mice develop dilated cardiomyopathy through a combined pathomechanism of haploinsufficiency and peptide toxicity. Hum. Mol. Genet. 2013;22:3152–3164. doi: 10.1093/hmg/ddt172. [DOI] [PubMed] [Google Scholar]
- 74.Wada E., Kato M., Yamashita K., Kokuba H., Hayashi Y.K. Deficiency of emerin contributes differently to the pathogenesis of skeletal and cardiac muscles in LmnaH222P/H222P mutant mice. PLoS ONE. 2019;14:e0221512. doi: 10.1371/journal.pone.0221512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Guenantin A.C., Jebeniani I., Leschik J., Watrin E., Puceat M. Targeting the histone demethylase LSD1 prevents cardiomyopathy in a mouse model of laminopathy. J. Clin. Investig. 2021;131:e136488. doi: 10.1172/JCI136488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arimura T., Helbling-Leclerc A., Massart C., Varnous S., Niel F., Lacene E., Fromes Y., Toussaint M., Mura A.-M., Keller D.I. Mouse model carrying H222P-Lmna mutation develops muscular dystrophy and dilated cardiomyopathy similar to human striated muscle laminopathies. Hum. Mol. Genet. 2005;14:155–169. doi: 10.1093/hmg/ddi017. [DOI] [PubMed] [Google Scholar]
- 77.Wang Y., Herron A.J., Worman H.J. Pathology and nuclear abnormalities in hearts of transgenic mice expressing M371K lamin A encoded by an LMNA mutation causing Emery-Dreifuss muscular dystrophy. Hum. Mol. Genet. 2006;15:2479–2489. doi: 10.1093/hmg/ddl170. [DOI] [PubMed] [Google Scholar]
- 78.Chen S.N., Lombardi R., Karmouch J., Tsai J.Y., Czernuszewicz G., Taylor M.R.G., Mestroni L., Coarfa C., Gurha P., Marian A.J. DNA Damage Response/TP53 Pathway Is Activated and Contributes to the Pathogenesis of Dilated Cardiomyopathy Associated With LMNA (Lamin A/C) Mutations. Circ. Res. 2019;124:856–873. doi: 10.1161/CIRCRESAHA.118.314238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mounkes L.C., Kozlov S., Hernandez L., Sullivan T., Stewart C.L. A progeroid syndrome in mice is caused by defects in A-type lamins. Nature. 2003;423:298–301. doi: 10.1038/nature01631. [DOI] [PubMed] [Google Scholar]
- 80.Osorio F.G., Navarro C.L., Cadianos J., López-Mejía I., López-Otín C. Splicing-Directed Therapy in a New Mouse Model of Human Accelerated Aging. Sci. Transl. Med. 2011;3:106ra107. doi: 10.1126/scitranslmed.3002847. [DOI] [PubMed] [Google Scholar]
- 81.Yang S.H., Meta M., Qiao X., Frost D., Bauch J., Coffinier C., Majumdar S., Bergo M.O., Young S.G., Fong L.G. A farnesyltransferase inhibitor improves disease phenotypes in mice with a Hutchinson-Gilford progeria syndrome mutation. J. Clin. Investig. 2006;116:2115–2121. doi: 10.1172/JCI28968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yang S.H., Andres D.A., Spielmann H.P., Young S.G., Fong L.G. Progerin elicits disease phenotypes of progeria in mice whether or not it is farnesylated. J. Clin. Investig. 2008;118:3291–3300. doi: 10.1172/JCI35876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yang S.H., Chang S.Y., Ren S., Wang Y., Andres D.A., Spielmann H.P., Fong L.G., Young S.G. Absence of progeria-like disease phenotypes in knock-in mice expressing a non-farnesylated version of progerin. Hum. Mol. Genet. 2011;20:436–444. doi: 10.1093/hmg/ddq490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Varga R., Eriksson M., Erdos M.R., Olive M., Harten I., Kolodgie F., Capell B.C., Cheng J., Faddah D., Perkins S. Progressive vascular smooth muscle cell defects in a mouse model of Hutchinson–Gilford progeria syndrome. Proc. Natl. Acad. Sci. USA. 2006;103:3250–3255. doi: 10.1073/pnas.0600012103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sagelius H., Rosengardten Y., Hanif M., Erdos M.R., Rozell B., Collins F.S., Eriksson M. Targeted transgenic expression of the mutation causing Hutchinson-Gilford progeria syndrome leads to proliferative and degenerative epidermal disease. J. Cell Sci. 2008;121:969–978. doi: 10.1242/jcs.022913. [DOI] [PubMed] [Google Scholar]
- 86.Wang Y., Panteleyev A.A., Owens D.M., Djabali K., Stewart C.L., Worman H.J. Epidermal expression of the truncated prelamin A causing Hutchinson-Gilford progeria syndrome: Effects on keratinocytes, hair and skin. Hum. Mol. Genet. 2008;17:2357–2369. doi: 10.1093/hmg/ddn136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee Y.K., Jiang Y., Ran X.R., Lau Y.M., Ng K.M., Lai W.H., Siu C.W., Tse H.F. Recent advances in animal and human pluripotent stem cell modeling of cardiac laminopathy. Stem Cell Res. 2016;7:139. doi: 10.1186/s13287-016-0401-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gupta P., Bilinska Z.T., Sylvius N., Boudreau E., Veinot J.P., Labib S., Bolongo P.M., Hamza A., Jackson T., Ploski R. Genetic and ultrastructural studies in dilated cardiomyopathy patients: A large deletion in the lamin A/C gene is associated with cardiomyocyte nuclear envelope disruption. Basic Res. Cardiol. 2010;105:365–377. doi: 10.1007/s00395-010-0085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cheedipudi S.M., Matkovich S.J., Coarfa C., Hu X., Robertson M.J., Sweet M., Taylor M., Mestroni L., Cleveland J., Willerson J.T., et al. Genomic Reorganization of Lamin-Associated Domains in Cardiac Myocytes Is Associated With Differential Gene Expression and DNA Methylation in Human Dilated Cardiomyopathy. Circ. Res. 2019;124:1198–1213. doi: 10.1161/CIRCRESAHA.118.314177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lin E.W., Brady G.F., Kwan R., Nesvizhskii A.I., Omary M.B. Genotype-phenotype analysis of LMNA-related diseases predicts phenotype-selective alterations in lamin phosphorylation. FASEB J. 2020;34:9051–9073. doi: 10.1096/fj.202000500R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gerull B., Brodehl A. Insights Into Genetics and Pathophysiology of Arrhythmogenic Cardiomyopathy. Curr. Heart Fail. Rep. 2021;18:378–390. doi: 10.1007/s11897-021-00532-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marcus F.I., McKenna W.J., Sherrill D., Basso C., Bauce B., Bluemke D.A., Calkins H., Corrado D., Cox M.G., Daubert J.P. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation. 2010;121:1533–1541. doi: 10.1161/CIRCULATIONAHA.108.840827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Joshi-Mukherjee R., Coombs W., Musa H., Oxford E., Taffet S., Delmar M. Characterization of the molecular phenotype of two arrhythmogenic right ventricular cardiomyopathy (ARVC)-related plakophilin-2 (PKP2) mutations. Heart Rhythm. 2008;5:1715–1723. doi: 10.1016/j.hrthm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Awad M.M., Dalal D., Tichnell C., James C., Tucker A., Abraham T., Spevak P.J., Calkins H., Judge D.P. Recessive arrhythmogenic right ventricular dysplasia due to novel cryptic splice mutation in PKP2. Hum. Mutat. 2006;27:1157. doi: 10.1002/humu.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Z., Bowles N.E., Scherer S.E., Taylor M.D., Kearney D.L., Ge S., Nadvoretskiy V.V., DeFreitas G., Carabello B., Brandon L.I. Desmosomal dysfunction due to mutations in desmoplakin causes arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ. Res. 2006;99:646–655. doi: 10.1161/01.RES.0000241482.19382.c6. [DOI] [PubMed] [Google Scholar]
- 96.Awad M.M., Dalal D., Cho E., Amat-Alarcon N., James C., Tichnell C., Tucker A., Russell S.D., Bluemke D.A., Dietz H.C. DSG2 mutations contribute to arrhythmogenic right ventricular dysplasia/cardiomyopathy. Am. J. Hum. Genet. 2006;79:136–142. doi: 10.1086/504393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Corrado D., Thiene G. Arrhythmogenic right ventricular cardiomyopathy/dysplasia: Clinical impact of molecular genetic studies. Circulation. 2006;113:1634–1637. doi: 10.1161/CIRCULATIONAHA.105.616490. [DOI] [PubMed] [Google Scholar]
- 98.Brodehl A., Weiss J., Debus J.D., Stanasiuk C., Klauke B., Deutsch M.A., Fox H., Bax J., Ebbinghaus H., Gartner A., et al. A homozygous DSC2 deletion associated with arrhythmogenic cardiomyopathy is caused by uniparental isodisomy. J. Mol. Cell. Cardiol. 2020;141:17–29. doi: 10.1016/j.yjmcc.2020.03.006. [DOI] [PubMed] [Google Scholar]
- 99.Tiso N., Stephan D.A., Nava A., Bagattin A., Devaney J.M., Stanchi F., Larderet G., Brahmbhatt B., Brown K., Bauce B. Identification of mutations in the cardiac ryanodine receptor gene in families affected with arrhythmogenic right ventricular cardiomyopathy type 2 (ARVD2) Hum. Mol. Genet. 2001;10:189–194. doi: 10.1093/hmg/10.3.189. [DOI] [PubMed] [Google Scholar]
- 100.Zwaag P., Rijsingen I., Ruite R. Recurrent and founder mutations in the Netherlands—Phospholamban p.Arg14del mutation causes arrhythmogenic cardiomyopathy. Neth. Heart J. 2013;21:286–293. doi: 10.1007/s12471-013-0401-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mayosi B.M., Fish M., Shaboodien G., Mastantuono E., Kraus S., Wieland T., Kotta M.-C., Chin A., Laing N., Ntusi N.B.A., et al. Identification of Cadherin 2 (CDH2) Mutations in Arrhythmogenic Right Ventricular Cardiomyopathy. Circ. Cardiovasc. Genet. 2017;10:e001605. doi: 10.1161/CIRCGENETICS.116.001605. [DOI] [PubMed] [Google Scholar]
- 102.Brodehl A., Rezazadeh S., Williams T., Munsie N.M., Liedtke D., Oh T., Ferrier R., Shen Y., Jones S.J.M., Stiegler A.L., et al. Mutations in ILK, encoding integrin-linked kinase, are associated with arrhythmogenic cardiomyopathy. Transl. Res. J. Lab. Clin. Med. 2019;208:15–29. doi: 10.1016/j.trsl.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Beffagna G., Occhi G., Nava A., Vitiello L., Ditadi A., Basso C., Bauce B., Carraro G., Thiene G., Towbin J.A. Regulatory mutations in transforming growth factor-β3 gene cause arrhythmogenic right ventricular cardiomyopathy type 1. Cardiovasc. Res. 2005;65:366–373. doi: 10.1016/j.cardiores.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 104.Taylor M., Graw S., Sinagra G., Barnes C., Slavov D., Brun F., Pinamonti B., Salcedo E.E., Sauer W., Pyxaras S. Genetic variation in titin in arrhythmogenic right ventricular cardiomyopathy–overlap syndromes. Circulation. 2011;124:876–885. doi: 10.1161/CIRCULATIONAHA.110.005405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Protonotarios A., Brodehl A., Asimaki A., Jager J., Quinn E., Stanasiuk C., Ratnavadivel S., Futema M., Akhtar M.M., Gossios T.D., et al. The Novel Desmin Variant p.Leu115Ile Is Associated With a Unique Form of Biventricular Arrhythmogenic Cardiomyopathy. Can. J. Cardiol. 2021;37:857–866. doi: 10.1016/j.cjca.2020.11.017. [DOI] [PubMed] [Google Scholar]
- 106.Hodgkinson K., Connors S., Merner N., Haywood A., Young T.L., McKenna W., Gallagher B., Curtis F., Bassett A., Parfrey P. The natural history of a genetic subtype of arrhythmogenic right ventricular cardiomyopathy caused by a p. S358L mutation in TMEM43. Clin. Genet. 2013;83:321–331. doi: 10.1111/j.1399-0004.2012.01919.x. [DOI] [PubMed] [Google Scholar]
- 107.Merner N.D., Hodgkinson K.A., Haywood A.F., Connors S., French V.M., Drenckhahn J.-D., Kupprion C., Ramadanova K., Thierfelder L., McKenna W. Arrhythmogenic right ventricular cardiomyopathy type 5 is a fully penetrant, lethal arrhythmic disorder caused by a missense mutation in the TMEM43 gene. Am. J. Hum. Genet. 2008;82:809–821. doi: 10.1016/j.ajhg.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kato K., Takahashi N., Fujii Y., Umehara A., Nishiuchi S., Makiyama T., Ohno S., Horie M. LMNA cardiomyopathy detected in Japanese arrhythmogenic right ventricular cardiomyopathy cohort. J. Cardiol. 2016;68:346–351. doi: 10.1016/j.jjcc.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 109.Liang J.J., Goodsell K., Grogan M., Ackerman M.J. LMNA-mediated arrhythmogenic right ventricular cardiomyopathy and charcot-marie-tooth type 2B1: A patient-discovered unifying diagnosis. J. Cardiovasc. Electrophysiol. 2016;27:868–871. doi: 10.1111/jce.12984. [DOI] [PubMed] [Google Scholar]
- 110.Lombardi R., Dong J., Rodriguez G., Bell A., Leung T.K., Schwartz R.J., Willerson J.T., Brugada R., Marian A.J. Genetic fate mapping identifies second heart field progenitor cells as a source of adipocytes in arrhythmogenic right ventricular cardiomyopathy. Circ. Res. 2009;104:1076–1084. doi: 10.1161/CIRCRESAHA.109.196899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Lombardi R., Chen S.N., Ruggiero A., Gurha P., Czernuszewicz G.Z., Willerson J.T., Marian A.J. Cardiac fibro-adipocyte progenitors express desmosome proteins and preferentially differentiate to adipocytes upon deletion of the desmoplakin gene. Circ. Res. 2016;119:41–54. doi: 10.1161/CIRCRESAHA.115.308136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matthes S.A., Taffet S., Delmar M. Plakophilin-2 and the migration, differentiation and transformation of cells derived from the epicardium of neonatal rat hearts. Cell Commun. Adhes. 2011;18:73–84. doi: 10.3109/15419061.2011.621561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang H., Pu W., Li G., Huang X., He L., Tian X., Liu Q., Zhang L., Wu S.M., Sucov H.M. Endocardium minimally contributes to coronary endothelium in the embryonic ventricular free walls. Circ. Res. 2016;118:1880–1893. doi: 10.1161/CIRCRESAHA.116.308749. [DOI] [PubMed] [Google Scholar]
- 114.Sommariva E., Brambilla S., Carbucicchio C., Gambini E., Meraviglia V., Dello Russo A., Farina F., Casella M., Catto V., Pontone G. Cardiac mesenchymal stromal cells are a source of adipocytes in arrhythmogenic cardiomyopathy. Eur. Heart J. 2016;37:1835–1846. doi: 10.1093/eurheartj/ehv579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang H., Lui K.O., Zhou B. Endocardial Cell Plasticity in Cardiac Development, Diseases and Regeneration. Circ. Res. 2018;122:774–789. doi: 10.1161/CIRCRESAHA.117.312136. [DOI] [PubMed] [Google Scholar]
- 116.Arbustini E., Favalli V., Narula N., Serio A., Grasso M. Left Ventricular Noncompaction: A Distinct Genetic Cardiomyopathy? J. Am. Coll. Cardiol. 2016;68:949–966. doi: 10.1016/j.jacc.2016.05.096. [DOI] [PubMed] [Google Scholar]
- 117.Kulikova O., Brodehl A., Kiseleva A., Myasnikov R., Meshkov A., Stanasiuk C., Gartner A., Divashuk M., Sotnikova E., Koretskiy S., et al. The Desmin (DES) Mutation p.A337P Is Associated with Left-Ventricular Non-Compaction Cardiomyopathy. Genes. 2021;12:121. doi: 10.3390/genes12010121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Probst S., Oechslin E., Schuler P., Greutmann M., Boyé P., Knirsch W., Berger F., Thierfelder L., Jenni R., Klaassen S. Sarcomere gene mutations in isolated left ventricular noncompaction cardiomyopathy do not predict clinical phenotype. Circ. Cardiovasc. Genet. 2011;4:367–374. doi: 10.1161/CIRCGENETICS.110.959270. [DOI] [PubMed] [Google Scholar]
- 119.Sedaghat-Hamedani F., Haas J., Zhu F., Geier C., Kayvanpour E., Liss M., Lai A., Frese K., Pribe-Wolferts R., Amr A. Clinical genetics and outcome of left ventricular non-compaction cardiomyopathy. Eur. Heart J. 2017;38:3449–3460. doi: 10.1093/eurheartj/ehx545. [DOI] [PubMed] [Google Scholar]
- 120.Tian Y., Morrisey E.E. Importance of myocyte-nonmyocyte interactions in cardiac development and disease. Circ. Res. 2012;110:1023–1034. doi: 10.1161/CIRCRESAHA.111.243899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muchtar E., Blauwet L.A., Gertz M.A. Restrictive cardiomyopathy: Genetics, pathogenesis, clinical manifestations, diagnosis, and therapy. Circ. Res. 2017;121:819–837. doi: 10.1161/CIRCRESAHA.117.310982. [DOI] [PubMed] [Google Scholar]
- 122.Nihoyannopoulos P., Dawson D. Restrictive cardiomyopathies. Eur. J. Echocardiogr. 2009;10:iii23–iii33. doi: 10.1093/ejechocard/jep156. [DOI] [PubMed] [Google Scholar]
- 123.Mogensen J., Arbustini E. Restrictive cardiomyopathy. Curr. Opin. Cardiol. 2009;24:214–220. doi: 10.1097/HCO.0b013e32832a1d2e. [DOI] [PubMed] [Google Scholar]
- 124.Brodehl A., Gerull B. Genetic Insights into Primary Restrictive Cardiomyopathy. J. Clin. Med. 2022;11:2094. doi: 10.3390/jcm11082094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Peled Y., Gramlich M., Yoskovitz G., Feinberg M.S., Afek A., Polak-Charcon S., Pras E., Sela B.A., Konen E., Weissbrod O., et al. Titin mutation in familial restrictive cardiomyopathy. Int. J. Cardiol. 2014;171:24–30. doi: 10.1016/j.ijcard.2013.11.037. [DOI] [PubMed] [Google Scholar]
- 126.Kostareva A., Gudkova A., Sjöberg G., Mörner S., Semernin E., Krutikov A., Shlyakhto E., Sejersen T. Deletion in TNNI3 gene is associated with restrictive cardiomyopathy. Int. J. Cardiol. 2009;131:410–412. doi: 10.1016/j.ijcard.2007.07.108. [DOI] [PubMed] [Google Scholar]
- 127.Greenway S.C., Wilson G.J., Wilson J., George K., Kantor P.F. Sudden death in an infant with angina, restrictive cardiomyopathy, and coronary artery bridging: An unusual phenotype for a β-myosin heavy chain (MYH7) sarcomeric protein mutation. Circ. Heart Fail. 2012;5:e92–e93. doi: 10.1161/CIRCHEARTFAILURE.112.969303. [DOI] [PubMed] [Google Scholar]
- 128.Kaski J.P., Syrris P., Burch M., Tome-Esteban M.-T., Fenton M., Christiansen M., Andersen P.S., Sebire N., Ashworth M., Deanfield J.E. Idiopathic restrictive cardiomyopathy in children is caused by mutations in cardiac sarcomere protein genes. Heart. 2008;94:1478–1484. doi: 10.1136/hrt.2007.134684. [DOI] [PubMed] [Google Scholar]
- 129.Brodehl A., Pour Hakimi S.A., Stanasiuk C., Ratnavadivel S., Hendig D., Gaertner A., Gerull B., Gummert J., Paluszkiewicz L., Milting H. Restrictive Cardiomyopathy is Caused by a Novel Homozygous Desmin (DES) Mutation p.Y122H Leading to a Severe Filament Assembly Defect. Genes. 2019;10:918. doi: 10.3390/genes10110918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yu H.-C., Coughlin C.R., Geiger E.A., Salvador B.J., Elias E.R., Cavanaugh J.L., Chatfield K.C., Miyamoto S.D., Shaikh T.H. Discovery of a potentially deleterious variant in TMEM87B in a patient with a hemizygous 2q13 microdeletion suggests a recessive condition characterized by congenital heart disease and restrictive cardiomyopathy. Mol. Case Stud. 2016;2:a000844. doi: 10.1101/mcs.a000844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brodehl A., Ferrier R.A., Hamilton S.J., Greenway S.C., Brundler M.-A., Yu W., Gibson W.T., McKinnon M.L., McGillivray B.C., Alvarez N., et al. Mutations in FLNC are Associated with Familial Restrictive Cardiomyopathy. Hum. Mutat. 2016;37:269–279. doi: 10.1002/humu.22942. [DOI] [PubMed] [Google Scholar]
- 132.Brayson D., Shanahan C.M. Current insights into LMNA cardiomyopathies: Existing models and missing LINCs. Nucleus. 2017;8:17–33. doi: 10.1080/19491034.2016.1260798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cho S., Vashisth M., Abbas A., Majkut S., Vogel K., Xia Y., Ivanovska I.L., Irianto J., Tewari M., Zhu K. Mechanosensing by the lamina protects against nuclear rupture, DNA damage, and cell-cycle arrest. Dev. Cell. 2019;49:920–935.e5. doi: 10.1016/j.devcel.2019.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chai R.J., Werner H., Li P.Y., Lee Y.L., Nyein K.T., Solovei I., Luu T.D.A., Sharma B., Navasankari R., Maric M., et al. Disrupting the LINC complex by AAV mediated gene transduction prevents progression of Lamin induced cardiomyopathy. Nat. Commun. 2021;12:4722. doi: 10.1038/s41467-021-24849-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Andres V., Gonzalez J.M. Role of A-type lamins in signaling, transcription, and chromatin organization. J. Cell Biol. 2009;187:945–957. doi: 10.1083/jcb.200904124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bernasconi P., Carboni N., Ricci G., Siciliano G., Lattanzi G. Elevated TGF β2 serum levels in Emery-Dreifuss Muscular Dystrophy: Implications for myocyte and tenocyte differentiation and fibrogenic processes. Nucleus. 2018;9:292–304. doi: 10.1080/19491034.2018.1467722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chatzifrangkeskou M., Le Dour C., Wu W., Morrow J.P., Joseph L.C., Beuvin M., Sera F., Homma S., Vignier N., Mougenot N., et al. ERK1/2 directly acts on CTGF/CCN2 expression to mediate myocardial fibrosis in cardiomyopathy caused by mutations in the lamin A/C gene. Hum. Mol. Genet. 2016;25:2220–2233. doi: 10.1093/hmg/ddw090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wu W., Muchir A., Shan J., Bonne G., Worman H.J. Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene. Circulation. 2011;123:53–61. doi: 10.1161/CIRCULATIONAHA.110.970673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Muchir A., Pavlidis P., Decostre V., Herron A.J., Arimura T., Bonne G., Worman H.J. Activation of MAPK pathways links LMNA mutations to cardiomyopathy in Emery-Dreifuss muscular dystrophy. J. Clin. Investig. 2007;117:1282–1293. doi: 10.1172/JCI29042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu W., Shan J., Bonne G., Worman H.J., Muchir A. Pharmacological inhibition of c-Jun N-terminal kinase signaling prevents cardiomyopathy caused by mutation in LMNA gene. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2010;1802:632–638. doi: 10.1016/j.bbadis.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Umbarkar P., Tousif S., Singh A.P. Fibroblast GSK-3α Promotes Fibrosis via RAF-MEK-ERK Pathway in the Injured Heart. Circ. Res. 2022;131:620–636. doi: 10.1161/CIRCRESAHA.122.321431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cattin M.E., Muchir A., Bonne G. ‘State-of-the-heart’ of cardiac laminopathies. Curr. Opin. Cardiol. 2013;28:297–304. doi: 10.1097/HCO.0b013e32835f0c79. [DOI] [PubMed] [Google Scholar]
- 143.Bertero A., Fields P.A., Smith A.S., Leonard A., Beussman K., Sniadecki N.J., Kim D.-H., Tse H.-F., Pabon L., Shendure J. Chromatin compartment dynamics in a haploinsufficient model of cardiac laminopathy. J. Cell Biol. 2019;218:2919–2944. doi: 10.1083/jcb.201902117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Meuleman W., Peric-Hupkes D., Kind J., Beaudry J.B., Pagie L., Kellis M., Reinders M., Wessels L., van Steensel B. Constitutive nuclear lamina-genome interactions are highly conserved and associated with A/T-rich sequence. Genome Res. 2013;23:270–280. doi: 10.1101/gr.141028.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Peric-Hupkes D., Meuleman W., Pagie L., Bruggeman S.W., Solovei I., Brugman W., Graf S., Flicek P., Kerkhoven R.M., van Lohuizen M., et al. Molecular maps of the reorganization of genome-nuclear lamina interactions during differentiation. Mol. Cell. 2010;38:603–613. doi: 10.1016/j.molcel.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Van Bortle K., Corces V.G. Spinning the web of cell fate. Cell. 2013;152:1213–1217. doi: 10.1016/j.cell.2013.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Poleshko A., Shah P.P., Gupta M., Babu A., Morley M.P., Manderfield L.J., Ifkovits J.L., Calderon D., Aghajanian H., Sierra-Pagán J.E. Genome-nuclear lamina interactions regulate cardiac stem cell lineage restriction. Cell. 2017;171:573–587.e14. doi: 10.1016/j.cell.2017.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Oldenburg A., Briand N., Sørensen A.L., Cahyani I., Shah A., Moskaug J.Ø., Collas P. A lipodystrophy-causing lamin A mutant alters conformation and epigenetic regulation of the anti-adipogenic MIR335 locus. J. Cell Biol. 2017;216:2731–2743. doi: 10.1083/jcb.201701043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pellegrini C., Columbaro M., Schena E., Prencipe S., Andrenacci D., Iozzo P., Angela Guzzardi M., Capanni C., Mattioli E., Loi M. Altered adipocyte differentiation and unbalanced autophagy in type 2 Familial Partial Lipodystrophy: An in vitro and in vivo study of adipose tissue browning. Exp. Mol. Med. 2019;51:1–17. doi: 10.1038/s12276-019-0289-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Czapiewski R., Batrakou D.G., de Las Heras J.I., Carter R.N., Sivakumar A., Sliwinska M., Dixon C.R., Webb S., Lattanzi G., Morton N.M. Genomic loci mispositioning in Tmem120a knockout mice yields latent lipodystrophy. Nat. Commun. 2022;13:321. doi: 10.1038/s41467-021-27869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ramirez-Martinez A., Zhang Y., Chen K., Kim J., Cenik B.K., McAnally J.R., Cai C., Shelton J.M., Huang J., Brennan A., et al. The nuclear envelope protein Net39 is essential for muscle nuclear integrity and chromatin organization. Nat. Commun. 2021;12:690. doi: 10.1038/s41467-021-20987-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Shimi T., Pfleghaar K., Kojima S., Pack C.G., Solovei I., Goldman A.E., Adam S.A., Shumaker D.K., Kinjo M., Cremer T., et al. The A- and B-type nuclear lamin networks: Microdomains involved in chromatin organization and transcription. Genes Dev. 2008;22:3409–3421. doi: 10.1101/gad.1735208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Han L., Choudhury S., Mich-Basso J.D., Ammanamanchi N., Ganapathy B., Suresh S., Khaladkar M., Singh J., Maehr R., Zuppo D.A., et al. Lamin B2 Levels Regulate Polyploidization of Cardiomyocyte Nuclei and Myocardial Regeneration. Dev. Cell. 2020;53:42–59.e11. doi: 10.1016/j.devcel.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Soufi A., Garcia M.F., Jaroszewicz A., Osman N., Pellegrini M., Zaret K.S. Pioneer transcription factors target partial DNA motifs on nucleosomes to initiate reprogramming. Cell. 2015;161:555–568. doi: 10.1016/j.cell.2015.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Spitz F., Furlong E.E. Transcription factors: From enhancer binding to developmental control. Nat. Rev. Genet. 2012;13:613–626. doi: 10.1038/nrg3207. [DOI] [PubMed] [Google Scholar]
- 156.Swinstead E.E., Miranda T.B., Paakinaho V., Baek S., Goldstein I., Hawkins M., Karpova T.S., Ball D., Mazza D., Lavis L.D., et al. Steroid Receptors Reprogram FoxA1 Occupancy through Dynamic Chromatin Transitions. Cell. 2016;165:593–605. doi: 10.1016/j.cell.2016.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Zaret K.S., Carroll J.S. Pioneer transcription factors: Establishing competence for gene expression. Genes Dev. 2011;25:2227–2241. doi: 10.1101/gad.176826.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Hatta M., Cirillo L.A. Chromatin opening and stable perturbation of core histone:DNA contacts by FoxO1. J. Biol. Chem. 2007;282:35583–35593. doi: 10.1074/jbc.M704735200. [DOI] [PubMed] [Google Scholar]
- 159.Auguste G., Gurha P., Lombardi R., Coarfa C., Willerson J.T., Marian A.J. Suppression of Activated FOXO Transcription Factors in the Heart Prolongs Survival in a Mouse Model of Laminopathies. Circ. Res. 2018;122:678–692. doi: 10.1161/CIRCRESAHA.117.312052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Glass C.A., Glass J.R., Taniura H., Hasel K.W., Blevitt J.M., Gerace L. The alpha-helical rod domain of human lamins A and C contains a chromatin binding site. EMBO J. 1993;12:4413–4424. doi: 10.1002/j.1460-2075.1993.tb06126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dechat T., Korbei B., Vaughan O.A., Vlcek S., Hutchison C.J., Foisner R. Lamina-associated polypeptide 2alpha binds intranuclear A-type lamins. Pt 19J. Cell Sci. 2000;113:3473–3484. doi: 10.1242/jcs.113.19.3473. [DOI] [PubMed] [Google Scholar]
- 162.Lee K.K., Haraguchi T., Lee R.S., Koujin T., Hiraoka Y., Wilson K.L. Distinct functional domains in emerin bind lamin A and DNA-bridging protein BAF. J. Cell Sci. 2001;114:4567–4573. doi: 10.1242/jcs.114.24.4567. [DOI] [PubMed] [Google Scholar]
- 163.Ye Q., Worman H.J. Interaction between an integral protein of the nuclear envelope inner membrane and human chromodomain proteins homologous to Drosophila HP1. J. Biol. Chem. 1996;271:14653–14656. doi: 10.1074/jbc.271.25.14653. [DOI] [PubMed] [Google Scholar]
- 164.Lieberman-Aiden E., van Berkum N.L., Williams L., Imakaev M., Ragoczy T., Telling A., Amit I., Lajoie B.R., Sabo P.J., Dorschner M.O., et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326:289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rao S.S., Huntley M.H., Durand N.C., Stamenova E.K., Bochkov I.D., Robinson J.T., Sanborn A.L., Machol I., Omer A.D., Lander E.S. A 3D map of the human genome at kilobase resolution reveals principles of chromatin looping. Cell. 2014;159:1665–1680. doi: 10.1016/j.cell.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lund E., Oldenburg A.R., Delbarre E., Freberg C.T., Duband-Goulet I., Eskeland R., Buendia B., Collas P. Lamin A/C-promoter interactions specify chromatin state–dependent transcription outcomes. Genome Res. 2013;23:1580–1589. doi: 10.1101/gr.159400.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cesarini E., Mozzetta C., Marullo F., Gregoretti F., Gargiulo A., Columbaro M., Cortesi A., Antonelli L., Di Pelino S., Squarzoni S. Lamin A/C sustains PcG protein architecture, maintaining transcriptional repression at target genes. J. Cell Biol. 2015;211:533–551. doi: 10.1083/jcb.201504035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schuettengruber B., Chourrout D., Vervoort M., Leblanc B., Cavalli G. Genome regulation by polycomb and trithorax proteins. Cell. 2007;128:735–745. doi: 10.1016/j.cell.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 169.Margueron R., Reinberg D. The Polycomb complex PRC2 and its mark in life. Nature. 2011;469:343–349. doi: 10.1038/nature09784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.He A., Ma Q., Cao J., Von Gise A., Zhou P., Xie H., Zhang B., Hsing M., Christodoulou D.C., Cahan P. Polycomb repressive complex 2 regulates normal development of the mouse heart. Circ. Res. 2012;110:406–415. doi: 10.1161/CIRCRESAHA.111.252205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Delgado-Olguín P., Huang Y., Li X., Christodoulou D., Seidman C.E., Seidman J., Tarakhovsky A., Bruneau B.G. Epigenetic repression of cardiac progenitor gene expression by Ezh2 is required for postnatal cardiac homeostasis. Nat. Genet. 2012;44:343. doi: 10.1038/ng.1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Bianchi A., Mozzetta C., Pegoli G., Lucini F., Valsoni S., Rosti V., Petrini C., Cortesi A., Gregoretti F., Antonelli L. Dysfunctional polycomb transcriptional repression contributes to Lamin A/C dependent muscular dystrophy. J. Clin. Investig. 2020;130:2408–2421. doi: 10.1172/JCI128161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hershberger R.E., Morales A., Siegfried J.D. Clinical and genetic issues in dilated cardiomyopathy: A review for genetics professionals. Genet. Med. 2010;12:655–667. doi: 10.1097/GIM.0b013e3181f2481f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Hershberger R.E., Jordan E. LMNA-Related Dilated Cardiomyopathy. University of Washington; Seattle, WA, USA: 2022. [PubMed] [Google Scholar]
- 175.Ramos F.J., Chen S.C., Garelick M.G., Dai D.F., Liao C.Y., Schreiber K.H., MacKay V.L., An E.H., Strong R., Ladiges W.C., et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci. Transl. Med. 2012;4:144ra103. doi: 10.1126/scitranslmed.3003802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.MacRae C., Taylor M., Mestroni L., Moses J., Ashley E., Wheeler M., Lakdawala N., Hershberger R., Ptaszynski M., Sandor V. Proceedings of European Heart Journal. Oxford Univ Press; Oxford, UK: 2016. Phase 2 study of a797, an oral, selective p38 mitogen-activated protein kinase inhibitor, in patients with lamin a/c-related dilated cardiomyopathy; p. 1011. [Google Scholar]
- 177.Santiago-Fernández O., Osorio F.G., Quesada V., Rodríguez F., Basso S., Maeso D., Rolas L., Barkaway A., Nourshargh S., Folgueras A.R. Development of a CRISPR/Cas9-based therapy for Hutchinson–Gilford progeria syndrome. Nat. Med. 2019;25:423–426. doi: 10.1038/s41591-018-0338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Beyret E., Liao H.K., Yamamoto M., Hernandez-Benitez R., Fu Y., Erikson G. Single-dose CRISPR-Cas9 therapy extends lifespan of mice with Hutchinson-Gilford progeria syndrome. Nat. Med. 2019;25:419–422. doi: 10.1038/s41591-019-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Koblan L.W., Erdos M.R., Wilson C., Cabral W.A., Levy J.M., Xiong Z.-M., Tavarez U.L., Davison L.M., Gete Y.G., Mao X., et al. In vivo base editing rescues Hutchinson Gilford progeria syndrome in mice. Nature. 2021;589:608–614. doi: 10.1038/s41586-020-03086-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Anzalone A.V., Koblan L.W., Liu D.R. Genome editing with CRISPR–Cas nucleases, base editors, transposases and prime editors. Nat. Biotechnol. 2020;38:824–844. doi: 10.1038/s41587-020-0561-9. [DOI] [PubMed] [Google Scholar]
- 181.Gaudelli N.M., Komor A.C., Rees H.A., Packer M.S., Liu D.R. Programmable base editing of AT to GC in genomic DNA without DNA cleavage. Nature. 2017;551:464–471. doi: 10.1038/nature24644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zuris J.A., Liu D.R., Packer M.S., Komor A.C., Kim Y.B. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature. 2016;533:420–424. doi: 10.1038/nature17946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Anzalone A.V., Randolph P.B., Davis J.R., Sousa A.A., Koblan L.W., Levy J.M., Chen P.J., Christopher W., Newby G.A., Aditya R. Search-and-replace genome editing without double-strand breaks or donor DNA. Nature. 2019;576:149–157. doi: 10.1038/s41586-019-1711-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created.