Abstract
The aim of this study was to investigate the link between self-reported health (SRH) and mortality in older adults. In total, 505 studies were found in PubMed and Scopus, of which 26 were included in this review. In total, 6 of the 26 studies included did not find any evidence of an association between SRH and mortality. Of the 21 studies that included community dwellers, 16 found a significant relationship between SRH and mortality. In total, 17 studies involved patients with no specific medical conditions; among these, 12 found a significant link between SRH and mortality. Among the studies in adults with specific medical conditions, eight showed a significant association between SRH and mortality. Among the 20 studies that definitely included people younger than 80 years, 14 found a significant association between SRH and mortality. Of the twenty-six studies, four examined short-term mortality; seven, medium-term mortality; and eighteen, long-term mortality. Among these, a significant association between SRH and mortality was found in 3, 7, and 12 studies, respectively. This study supports the existence of a significant relation between SRH and mortality. A better understanding of the components of SRH might help guide preventive health policies aimed at delaying mortality in the long term.
Keywords: self-rated health, mortality, older adults, prediction
1. Introduction
Numerous studies have investigated the predictive value of self-reported health (SRH) on mortality or adverse health outcomes in both young and old adults [1,2,3]. Overall, the results of these studies, particularly regarding the link between SRH and mortality, widely vary according to the age and sex of the population studied, the length of follow-up, or the presence or absence of specific diseases [4]. It is, therefore, difficult to know with any certainty what weight should be given to patients’ SRH. This difficulty is particularly marked among older adults, who are often frail and multimorbid, and who may have a life expectancy that is limited by one or more chronic diseases. SRH is a valuable assessment, because it covers multiple components and is easy to collect. Several authors [5,6] have shown the multiple domains are encompassed by the term self-reported health. However, the contribution of each individual component to the overall evaluation remains to be determined and seems to vary according to the context (gender, socio-economic or educational level, age category, religion, etc.). The evaluation of SRH yields a more comprehensive view of an individual’s health and may be more accurate than a purely medical evaluation. Moreover, it allows physicians to understand complex predictive factors of health, such as chronic inflammatory status [7,8]. Finally, SRH can be evaluated by asking a single, simple question [9].
In this systematic review, we aimed to determine whether there is a significant link between SRH and mortality in older adults.
2. Methods
2.1. Search Strategy
Before launching the literature search, we ensured that no systematic review had previously been conducted on this specific topic and in this particular population, by means of verification in PubMed, Scopus, Prospero, and the Cochrane library.
This was only a systematic review. A comprehensive literature search was performed in PubMed and Scopus. The search covered all publications up to and including 23 March 2022, with no specific start date specified. Search terms were defined by two senior researchers (L.G. and M.D.) and included the following keywords in the title and/or the abstract: (“obesity paradox” OR “reverse epidemiology” OR “body mass index”) AND (mortality OR death OR survival) (“self-rated health” OR “perceived health” OR “subjective health” OR “health report” OR “quality of life”) AND (mortality OR outcome OR survival OR death) AND (Age OR old OR elder*). Filters were applied to select studies in the English or French language and studies only including human subjects and to exclude the following publication types: reviews, case reports and case series, editorials, and correspondence. Reference lists were manually checked for additional studies. Study selection was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. This study was registered with PROSPERO (an International prospective register of systematic reviews), under the number CRD42022329082.
2.2. Study Selection Criteria
Study eligibility criteria were defined prior to performing the literature search by two senior researchers (L.G. and M.D.) according to the PICOS framework. Studies were eligible for inclusion if they reported data on self-rated health. The population of the studies included people aged 65 years or older, of any sex, ethnicity, or living place. The groups to be compared were defined according to their levels of self-rated health (SRH). The outcome was death, whatever the timepoint. Basic science articles, reviews, case reports and case series, editorials, and correspondence were excluded.
2.3. Data Extraction
Data analysis was performed using Covidence systematic review software© (Veritas Health Innovation, Melbourne, Australia), available at www.covidence.org (accessed on 23 March 2022). After eliminating duplicates, two senior researchers (L.G. and M.D.) independently reviewed the titles and abstracts of all articles. In case of disagreement about whether or not to include an article, the case was discussed until consensus was reached. Overlap between studies in the results reported was checked. We independently extracted the data, using the same data extraction form. For descriptive analyses, the following data were extracted: publication year, country where the study was conducted, study design, study setting, medical condition (if any), sample size, and age (mean or median and their statistical dispersion parameters, when available). To analyse the relation between SRH and mortality, the following information was collected: outcome (death or survival), type of analysis (whether adjusted or not), SRH levels, statistical estimates (hazard ratios, odds ratios, rate ratios, and rates) and their respective 95% confidence intervals (95% CIs), and the level of significance (p-values).
2.4. Quality Assessment
The quality of the included studies was assessed independently by two researchers (L.G. and M.D.) using the Newcastle–Ottawa Scale (NOS) [10]. The NOS consists of three quality parameters: selection, comparability, and outcome assessment. The “selection” criterion is scored between 0 and 4 points; the “comparability” criterion is scored between 0 and 2 points; and the “outcome” criterion is scored between 0 and 3 points. The sum of the scores of these three criteria gives an NOS total score between 0 and 9 points. NOS scores of 7 or over were considered to be of high quality, while 5–6 indicated moderate quality, and scores under 5 indicated low quality. Disagreement was resolved by means of a joint review of the manuscript to reach consensus, and the opinion of a third researcher was requested when necessary. When appropriate and possible, certain parameters were calculated from available data (e.g., pooled mean age and/or standard deviations, odds ratios, rate ratios, etc.).
3. Results
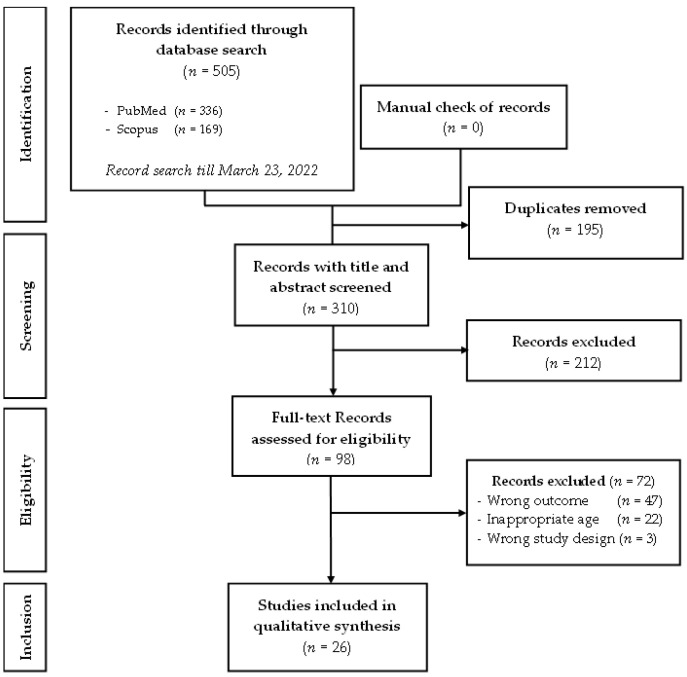
In total, 505 studies were identified during the literature search (Figure 1). Among these, 195 duplicates were excluded. After examination of the titles and abstracts of the remaining 310 studies, 98 articles were retained for full-text assessment. After reading the full text of these 98 studies, 72 were excluded for one or more of the following reasons: inappropriate age of the study population, wrong study design, or wrong outcome. Thus, 26 studies were included in the final review.
Figure 1.
PRISMA flow diagram of the records included in the systematic review.
Table 1 summarizes the characteristics of the studies included in the review. All studies were observational cohorts. The average age of the population included in the studies was >80 years in two articles [11,12] and was not specified in five articles [13,14,15,16,17]. The two articles [15,16] with a mean population age of over 80 were performed on the same cohort, with evaluation of mortality at different timepoints.
Table 1.
Description of the studies included in the present systematic review.
| Author(s), Year | Country | Study Setting | Medical Conditions | Sample Size | Age (Years) |
|---|---|---|---|---|---|
| Wuorela et al., 2020 [18] | Finland | Community | No specific conditions (men only) | 1008 | 70.0 ± 0.0 * |
| Godaert et al., 2018 [11] | France | Hospital, emergency | Hospitalised for an acute condition | 223 | 85.1 ± 5.5 * |
| Godard-Sebillotte et al., 2016 [12] | France | Hospital, emergency | Hospitalised for an acute condition | 223 | 85.1 ± 5.5 * |
| Mavaddat et al., 2016 [19] | England and Wales | Community including care homes | No specific conditions | 11,957 | 74.8 ± 6.6 * |
| Brown et al., 2015 [20] | USA | Community | No specific conditions; exclusion of end-stage renal disease at baseline | 191,001 | 75.0 ± x.x * |
| Gurland et al., 2014 [21] | USA | Community | No specific conditions | 2128 | 76.0 ± 5.8 * |
| Shen et al., 2014 [15] | Hong Kong | Health centres | No specific conditions | 66,814 | ≥65 |
| Fernández-Ruiz et al., 2013 [22] | Spain | Community | No specific conditions | 4958 | 74.1 ± 6.8 * |
| Puts et al., 2013 [2] | Canada | Hospital | Cancer | 112 | 74.2 ± 6.0 * |
| Ernstein et al., 2011 [23] | Norway | Community | No specific conditions; exclusion of cardiovascular disease at baseline | 5808 | 76.0 ± 4.9 * |
| Khang et al., 2010 [24] | Korea | Community | No specific conditions | 1448 | ≥65 |
| Ford et al., 2008 [25] | Australia | Community | No specific conditions (women only) | 12,422 | 70–75 ♦ |
| Johansson et al., 2008 [26] | Sweden | Community | Presence of signs or symptoms associated with chronic heart failure | 448 | 73.0 ± 5.6 * |
| Okamoto et al., 2008 [27] | Japan | Community | No specific conditions | 784 | ≥65 |
| Lee et al., 2007 [28] | USA | Community | No specific conditions | 6298 | ≥70 |
| Van den Brink et al., 2005 [29] | Finland, Italy, and Netherlands | Community | No specific conditions (men only) | 1141 | 76.5 ± 4.4 * |
| Baron-Epel et al., 2004 [30] | Israel | Community | No specific conditions | 1138 | 77.5 (70–101) # |
| Walker et al., 2004 [31] | Canada | Community | No specific conditions | 8697 | 75.7 ± 7.1 * |
| Bath, 2003 [32] | UK | Community | No specific conditions | 1042 | ≥65 |
| Helmer et al., 1999 [33] | France | Community | No specific conditions | 3660 | 75.2 (65–101) # |
| Yu et al., 1998 [17] | Shanghai | Community | No specific conditions | 3094 | ≥65 |
| Leung et al., 1997 [34] | Taiwan | Long-term facility | No specific conditions | 411 | 77.5 ± x.x * |
| Schoenfeld et al., 1994 [35] | USA | Community | Aging successfully | 1037 | 70–79 ♦ |
| Tsuji et al., 1994 [16] | Japan | Community | No specific conditions | 2252 | 65–113 ♦ |
| Pijls et al., 1993 [13] | Netherlands | Community | No specific conditions (men only) | 783 | 65–85 ♦ |
| Rakowski et al., 1993 [14] | USA | Community | No specific conditions | 5630 | ≥70 |
* mean ± standard deviation; # mean (range); ♦ range; x: not defined.
The main results of the included studies are summarized in Table 2. As shown in Table 2, 6 of the 26 studies did not find any evidence of an association between SRH and mortality [2,25,27,29,32,33]. Among the 21 studies that included community dwellers, 16 found a significant relationship between worse SRH and higher mortality rates [13,14,16,17,18,19,20,21,22,23,24,26,28,30,31,35]. A total of 17 studies involved patients with no specific medical conditions; among them, 12 found a significant link between worse SRH and higher mortality rates [13,14,15,16,17,18,19,20,21,22,24,30]. Among the studies including individuals with specific medical conditions, eight showed a significant association between SRH and mortality [11,12,23,26,28,31,34,35]. When only specific mortality was considered (six studies), the relationship with SRH was always significant [13,15,16,17,23,26]. Two studies involved people over the age of 80 years. They both showed a significant association between SRH and mortality [11,12]. Among the 20 studies that definitely included people younger than 80 years (but older than 65), 14 found a significant association between SRH and mortality [17,18,19,20,21,22,23,24,26,28,30,31,34,35]. Of the 26 studies, 4 examined short-term mortality (<one year), while 7 examined medium-term mortality (one to five years), and 18 studied long-term mortality (five years or over). Of these, a significant association between SRH and mortality was found in 3 [11,12,20], 7 [11,16,19,20,28,34,35], and 12 studies [13,14,15,17,18,21,22,23,24,26,30,31], respectively. When SRH was considered as a dichotomous variable (in 11 studies), it was significantly associated with mortality in 9 cases [11,12,18,19,23,24,28,30,31], and when considered as a non-dichotomous variable (in 17 studies), the association between worse SRH and higher mortality rates was significant in 12 cases [13,14,15,16,17,18,20,21,22,26,34,35].
Table 2.
Outcome and results of association between SRH and mortality in aged adults.
| Author(s), Year | Outcome | Medical Conditions |
Analysis | Results | ||
|---|---|---|---|---|---|---|
| SRH Levels | Estimates (95% CI) |
p | ||||
| Wuorela et al., 2020 [18] | 5-year mortality | No specific conditions | aHR | Good/rather good | Reference | |
| Poor | 2.17 (1.42–3.31) | <0.001 | ||||
| 10-year mortality | Good | Reference | ||||
| Rather good | 2.29 (1.24–4.23) | 0.009 | ||||
| Poor | 4.08 (2.14–7.77) | <0.001 | ||||
| 27-year mortality | Good | Reference | ||||
| Rather good | 1.19 (0.94–1.51) | 0.14 | ||||
| Poor | 1.62 (1.23–2.13 | <0.001 | ||||
| Godaert et al., 2018 [11] | 6-month mortality | Hospitalised for an acute condition | aHR | Very good/good | Reference | |
| Medium to very poor | 2.7 (1.6–4.7) | 0.0003 | ||||
| 1-year mortality | Very good/good | Reference | ||||
| Medium to very poor | 2.4 (1.5–4.0) | 0.0006 | ||||
| 2-year-mortality | Very good/good | Reference | ||||
| Medium to very poor | 1.9 (1.3–2.9) | 0.002 | ||||
| 3-year mortality | Very good/good | Reference | ||||
| Medium to very poor | 1.6 (1.1–2.4) | 0.01 | ||||
| Godard-Sebillotte et al., 2016 [12] | 6-week mortality | Hospitalised for an acute condition | aHR | Very good/good | Reference | |
| Medium to very poor | 2.61 (1.18–5.77) | 0.02 | ||||
| Mavaddat et al., 2016 [19] | 2-year mortality | No specific conditions, no prior history of stroke | aOR | Excellent/good | Reference | |
| Fair/poor | 1.7 (1.4–2.0) | S | ||||
| No specific conditions, prior history of stroke | Excellent/good | Reference | ||||
| Fair/poor | 1.1 (0.7–1.8) | NS | ||||
| 13-year mortality | No specific conditions, no prior history of stroke | aHR | Excellent | Reference | ||
| Good | 1.2 (1.0–1.4) | S | ||||
| Fair | 1.3 (1.1–1.6) | S | ||||
| Poor | 1.2 (0.9–1.7) | NS | ||||
| No specific conditions, prior history of stroke | Excellent | Reference | ||||
| Good | 0.8 (0.5–1.3) | NS | ||||
| Fair | 0.8 (0.4–1.3) | NS | ||||
| Poor | 1.1 (0.6–2.1) | NS | ||||
| Brown et al., 2015 [20] | 90-day mortality | No specific conditions, no end-stage renal disease | aHR | Excellent | Reference | |
| Very good | 1.00 (0.56–1.78) | NS | ||||
| Good | 1.65 (0.95–2.85) | NS | ||||
| Fair | 3.03 (1.73–5.30) | <0.001 | ||||
| Poor | 7.36 (4.08–13.25) | <0.001 | ||||
| Maximum follow-up mortality (>2.5 years) | aHR | Excellent | Reference | |||
| Very good | 1.13 (1.01–1.27) | <0.05 | ||||
| Good | 1.60 (1.43–1.79) | <0.001 | ||||
| Fair | 2.52 (2.25–2.83) | <0.001 | ||||
| Poor | 4.24 (3.73–4.82) | <0.001 | ||||
| Gurland et al., 2014 [21] | 16-year survival | No specific conditions | aHR | Poor | Reference | |
| Excellent | 0.69 (0.54–0.89) | S | ||||
| Good | 0.79 (0.63–0.99) | S | ||||
| Fair | 0.77 (0.62–0.96) | S | ||||
| Shen et al., 2014 [15] | 10-year all-cause mortality | No specific conditions | aHR | Better | Reference | |
| Normal | 0.86 (0.81–0.91) | S | ||||
| Worse | 0.91 (0.86–0.96) | S | ||||
| 10-year cardiovascular disease mortality | Better | Reference | ||||
| Normal | 0.85 (0.77–0.94) | S | ||||
| Worse | 0.84 (0.76–0.94) | S | ||||
| 10-year stroke mortality | Better | Reference | ||||
| Normal | 0.83 (0.70–0.99) | S | ||||
| Worse | 0.88 (0.76–1.05) | NS | ||||
| 10-year ischemic heart disease mortality | Better | Reference | ||||
| Normal | 0.88 (0.74–1.03) | NS | ||||
| Worse | 0.84 (0.71–0.99) | S | ||||
| 10-year all-cancer mortality | Better | Reference | ||||
| Normal | 0.90 (0.81–0.99) | S | ||||
| Worse | 0.97 (0.87–1.08) | NS | ||||
| 10-year all-respiratory disease mortality | Better | Reference | ||||
| Normal | 0.85 (0.75–0.96) | S | ||||
| Worse | 0.93 (0.82–1.06) | NS | ||||
| Fernández-Ruiz et al., 2013 [22] | 13-year all-cause mortality | No specific conditions | aHR | Very good | Reference | |
| Good | 0.95 (0.81–1.12) | NS | ||||
| Fair | 1.22 (1.03–1.44) | <0.05 | ||||
| Poor/very poor | 1.39 (1.15–1.69) | <0.01 | ||||
| Puts et al., 2013 [2] | 12-month mortality | Newly diagnosed cancer | aHR | Good/excellent | Reference | |
| Fair/poor/very poor | 1.33 (0.50–3.53) | NS | ||||
| Ernstein et al., 2011 [23] | 10-year IHD mortality | No specific conditions; exclusion of cardiovascular disease at baseline (men) | aHR | Very good/good | Reference | |
| Fair/poor | 1.23 (0.91–1.67) | NS | ||||
| No specific conditions; exclusion of cardiovascular disease at baseline (women) | Very good/good | Reference | ||||
| Fair/poor | 1.61 (1.14–2.29) | S | ||||
| 10-year all-cause mortality | No specific conditions; exclusion of cardiovascular disease at baseline (men) | Very good/good | Reference | |||
| Fair/poor | 1.42 (1.25–1.61) | S | ||||
| No specific conditions; exclusion of cardiovascular disease at baseline(women) | Very good/good | Reference | ||||
| Fair/poor | 1.60 (1.39–1.84) | S | ||||
| Khang et al., 2010 [24] | Long-term mortality | No specific conditions, non-institutionalized population, men | aHR | Very good/good/fair | Reference | |
| Very poor/poor | 2.21 (1.47–3.33) | S | ||||
| No specific conditions, non-institutionalized population (women) | Very good/good/fair | Reference | ||||
| Very poor/poor | 2.05 (1.33–3.15) | S | ||||
| Ford et al., 2008 [25] | Long-term mortality | No specific conditions (women only) | aHR | Excellent | Reference | |
| Very good | 1.04 (0.77–1.41) | NS | ||||
| Good | 1.27 (0.95–1.70) | NS | ||||
| Fair | 2.10 (1.56–2.83) | S | ||||
| Poor | 3.83 (2.73–5.38) | S | ||||
| Johansson et al., 2008 [26] | 10-year cardiovascular mortality | Presence of signs or symptoms associated with chronic heart failure | aHR | Very good | Reference | |
| Good | 3.4 (1.4–7.8) | 0.005 | ||||
| Poor | 4.1 (1.8–9.4) | 0.001 | ||||
| Okamoto et al., 2008 [27] | 6-year mortality | No specific conditions (men) | aHR | Fair/Poor | Reference | 0.04 # |
| Good | 0.63 (0.32–0.98) | |||||
| Excellent | 0.48 (0.14–1.07) | |||||
| No specific conditions (women) | Fair/poor | Reference | 0.40 # | |||
| Good | 0.78 (0.41–1.33) | |||||
| Excellent | 0.74 (0.21–1.32) | |||||
| Lee et al., 2007 [28] | 4-year mortality | No specific conditions (Black Americans of ≥80 years) | aOR | Good | Reference | |
| Poor | 1.9 (1.1–3.2) | S | ||||
| No specific conditions (White Americans of ≥80 years) | Good | Reference | ||||
| Poor | 2.0 (1.7–2.5) | S | ||||
| Van den Brink et al., 2005 [29] | 10-year mortality | No specific conditions (only men born between 1900 and 1920) | aHR | Healthy | Reference | |
| Not healthy | 1.19 (0.97–1.46) | NS | ||||
| Baron-Epel et al., 2004 [30] | 91-month mortality | No specific conditions (men) | aHR | Sub-optimal | Reference | |
| Optimal | 1.33 (1.10–1.61) | <0.01 | ||||
| No specific conditions (women) | aHR | Sub-optimal | Reference | |||
| Optimal | 1.40 (1.17–1.67) | <0.01 | ||||
| Walker et al., 2004 [31] | 5-year mortality | No specific conditions, cognitively intact | aHR | Good | Reference | |
| Poor | 1.57 (1.38–1.78) | S | ||||
| No specific conditions, mild to moderate cognitive impairment | Good | Reference | ||||
| Poor | 1.26 (1.01–1.59) | S | ||||
| No specific conditions, severe cognitive impairment | Good | Reference | ||||
| Poor | 1.00 (0.76–1.31) | NS | ||||
| Bath, 2003 [32] | 4-year mortality | No specific conditions (men) | aHR | Excellent | Reference | |
| Good | 0.67 (0.35–1.29) | NS | ||||
| Average | 1.17 (0.55–2.50) | NS | ||||
| Fair | 0.62 (0.25–1.53) | NS | ||||
| Poor | 0.87 (0.32–2.33) | NS | ||||
| No specific conditions (women) | aHR | Excellent | Reference | |||
| Good | 1.44 (0.63–3.29) | NS | ||||
| Average | 1.15 (0.44–2.98) | NS | ||||
| Fair | 1.13 (0.41–3.06) | NS | ||||
| Poor | 1.98 (0.63–6.25) | NS | ||||
| 12-year mortality | No specific conditions (men) | aHR | Excellent | Reference | ||
| Good | 0.94 (0.66–1.34) | NS | ||||
| Average | 1.16 (0.73–1.83) | NS | ||||
| Fair | 1.01 (0.61–1.66) | NS | ||||
| Poor | 1.54 (0.84–2.83) | NS | ||||
| No specific conditions (women) | aHR | Excellent | Reference | |||
| Good | 1.09 (0.76–1.57) | NS | ||||
| Average | 0.84 (0.54–1.31) | NS | ||||
| Fair | 1.17 (0.75–1.84) | NS | ||||
| Poor | 1.30 (0.72–2.36) | NS | ||||
| Helmer et al., 1999 [33] | 5-year mortality | No specific conditions | aHR | Very good | Reference | |
| Good | 1.93 (1.15–3.23) | <0.05 | ||||
| Fair | 2.01 (1.16–3.46) | <0.05 | ||||
| Bad/very bad | 1.87 (0.99–3.55) | NS | ||||
| Yu et al., 1998 [17] | 5-year mortality | No specific conditions (aged 65–74 years) | aHR | Excellent/good | Reference | |
| Fair | 2.16 (1.44–3.25) | <0.001 | ||||
| Poor | 1.93 (1.20–3.11) | 0.007 | ||||
| No specific conditions (aged 75 years and older) | Excellent/good | Reference | ||||
| Fair | 1.14 (0.87–1.49) | 0.338 | ||||
| Poor | 1.34 (0.95–1.88) | 0.092 | ||||
| Leung et al., 1997 [34] | 3-year mortality | No specific conditions (living in institutions) | aHR | Good | Reference | |
| Average | 4.05 (0.93–17.70) | NS | ||||
| Fair/poor | 6.00 (1.39–25.90) | S | ||||
| Schoenfeld et al., 1994 [35] | 3-year mortality | Aging successfully | aOR | Excellent | Reference | 0.0001 # |
| Good | 2.69 (2.15–3.38) | |||||
| Fair | 7.26 (4.61–11.44) | |||||
| Poor/bad | 19.56 (9.89–38.68) | |||||
| Tsuji et al., 1994 [16] | 3-year all-cause mortality | No specific conditions | aHR | Excellent/good | Reference | |
| Fair | 2.23 (1.53–3.26) | S | ||||
| Poor | 3.07 (1.50–6.26) | S | ||||
| 3-year cancer mortality | Excellent/good | Reference | ||||
| Fair | 3.41 (1.86–6.24) | S | ||||
| Poor | 13.61 (3.47–53.42) | S | ||||
| 3-year stroke mortality | Excellent/good | Reference | ||||
| Fair | 2.44 (0.97–6.15) | NS | ||||
| Poor | 2.48 (0.68–9.07) | NS | ||||
| 3-year heart disease mortality | Excellent/good | Reference | ||||
| Fair | 0.96 (0.32–2.86) | NS | ||||
| Poor | 1.34 (0.21–8.50) | NS | ||||
| Pijls et al., 1993 [13] | 5-year all-cause mortality | No specific conditions (men) | aHR | Healthy | Reference | <0.001 # |
| Rather healthy | 1.3 (0.9–1.8) | |||||
| Moderately healthy | 2.4 (1.5–3.8) | |||||
| Not healthy | 5.4 (2.7–11.0) | |||||
| 5-year cardiovascular diseases mortality | Healthy | Reference | 0.09 # | |||
| Rather healthy | 1.3 (0.8–2.2) | |||||
| Moderately healthy /not healthy |
1.9 (0.9–3.8) | |||||
| 5-year cancer mortality | Healthy | Reference | 0.003 # | |||
| Rather healthy | 1.1 (0.6–2.1) | |||||
| Moderately healthy /not healthy |
4.2 (1.9–9.4) | |||||
| Rakowski et al., 1993 [14] | Long-term mortality | No specific conditions | OR | Excellent | Reference | |
| Very good | 1.22 (0.98–1.53) | NS | ||||
| Good | 1.48 (1.21–1.82) | S | ||||
| Fair | 2.40 (1.93–3.00) | S | ||||
| Poor | 4.49 (3.50–5.77) | S | ||||
95% CI, 95% confidence interval; aHR, adjusted hazard ratio; OR, non-adjusted odds ratio; aOR, adjusted odds ratio; S, significant; NS, not significant; # p for trend.
The quality of the included studies, as assessed using the NOS, is summarized in Table 3. The quality was considered high for all 26 studies.
Table 3.
Quality assessment of the different studies included in this systematic review performed using the Newcastle–Ottawa scale (NOS).
| Author(s), Year | Study Design | Selection | Comparability | Outcome | Total Score | Quality Rating |
|---|---|---|---|---|---|---|
| Wuorela et al., 2020 [18] | Longitudinal | **** | ** | *** | 9 | High |
| Godaert et al., 2018 [11] | Longitudinal | **** | ** | *** | 9 | High |
| Godard-Sebillotte et al., 2016 [12] | Longitudinal | **** | ** | *** | 9 | High |
| Mavaddat et al., 2016 [19] | Longitudinal | *** | ** | *** | 8 | High |
| Brown et al., 2015 [20] | Longitudinal | *** | ** | *** | 8 | High |
| Gurland et al., 2014 [21] | Longitudinal | **** | ** | *** | 9 | High |
| Shen et al., 2014 [15] | Longitudinal | **** | ** | *** | 9 | High |
| Fernández-Ruiz et al., 2013 [22] | Longitudinal | **** | ** | *** | 9 | High |
| Puts et al., 2013 [2] | Longitudinal | *** | ** | *** | 8 | High |
| Ernstein et al., 2011 [23] | Longitudinal | *** | ** | *** | 8 | High |
| Khang et al., 2010 [24] | Longitudinal | *** | ** | ** | 7 | High |
| Ford et al., 2008 [25] | Longitudinal | *** | ** | *** | 8 | High |
| Johansson et al., 2008 [26] | Longitudinal | *** | ** | *** | 8 | High |
| Okamoto et al., 2008 [27] | Longitudinal | **** | ** | *** | 9 | High |
| Lee et al., 2007 [28] | Longitudinal | **** | ** | *** | 9 | High |
| Van den Brink et al., 2005 [29] | Longitudinal | *** | ** | *** | 8 | High |
| Baron-Epel et al., 2004 [30] | Longitudinal | **** | ** | *** | 9 | High |
| Walker et al., 2004 [31] | Longitudinal | **** | ** | *** | 9 | High |
| Bath, 2003 [32] | Longitudinal | **** | ** | *** | 8 | High |
| Helmer et al., 1999 [33] | Longitudinal | **** | ** | *** | 9 | High |
| Yu et al., 1998 [17] | Longitudinal | **** | ** | *** | 9 | High |
| Leung et al., 1997 [34] | Longitudinal | ** | ** | *** | 7 | High |
| Schoenfeld et al., 1994 [35] | Longitudinal | **** | ** | *** | 9 | High |
| Tsuji et al., 1994 [16] | Longitudinal | *** | ** | *** | 8 | High |
| Pijls et al., 1993 [13] | Longitudinal | *** | ** | *** | 8 | High |
| Rakowski et al., 1993 [14] | Longitudinal | **** | ** | *** | 9 | High |
NOS scores ≥7 were considered to indicate high-quality studies, and scores of 5–6 indicated moderate quality. The sum of the stars constitutes the Total score (for the first row: 4 stars for selection, two stars for Comparability, and three stars for outcome equal 9 stars (total score equals 9).
4. Discussion
In this systematic review of the predictive relationship between self-rated health (SRH) and mortality in people aged 65 years or over, we included 26 studies [2,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35], of which 4 investigated short-term mortality (≤1 year) [2,11,12,20], 8 investigated medium-term (>1 year and <5 years) mortality [11,16,19,20,28,32,34,35], and 18 investigated long-term mortality (≥5 years) [13,14,15,17,18,19,21,22,23,24,25,26,27,29,30,31,32,33]. Five articles studied the relationship between SRH and mortality at several timepoints [11,18,19,20,32]. The majority of articles concerned populations without a specific medical condition at inclusion [13,14,15,16,17,18,19,20,21,22,24,25,27,29,30,32,33].
For the two studies that included people aged 80 years or over, the authors showed a significant relationship between SRH and all-cause mortality at each timepoint (6 weeks; 6 months; and 1, 2 and 3 years). However, it seems difficult to extrapolate these results, as they all concern the same population, hospitalised via the emergency department for an acute condition.
In the community-dwelling population, with a mean age of <80 years at inclusion, not selected for the presence of any specific pathology, our systematic review supports a predictive relationship between SRH and all-cause mortality at each time point. Of 17 articles [13,14,15,16,17,18,19,20,21,22,24,25,27,29,30,32,33] studying all-cause mortality, 12 [13,14,15,16,17,18,19,20,21,22,24,30] found a predictive relationship between SRH and death (i.e., 70.5% of the articles studied). Three articles [16,19,20] were specific to medium-term and nine [13,14,15,17,18,21,22,24,30] to long-term all-cause mortality. These results are consistent with previous studies of younger adults showing that SRH is predictive of all-cause mortality in the medium (<5 years) [36,37] and long term [3]. The persistence of a long-term predictive link is remarkable in the elderly population, as these are often fragile individuals, with multiple causes of death.
SRH is a composite concept that encompasses medical, social, cultural, religious, ethnical, and individual dimensions. Several authors have attempted to characterise the different dimensions of health under the term “SRH” [1,5,6,38,39,40]. The share of each dimension in the overall subjective feeling varies from one individual to another, explaining the variable strength of the link between SRH and mortality according to gender, culture, ethnicity, socio-economic level. and even age group [33,36,41,42,43]. Zajacova et al. [44] showed that the individual criteria taken into account when assessing SRH varied from one sex to another as well as according to the period of life. Younger women tended to assess their SRH more unfavourably than men of the same age, while older women had a more favourable view of their SRH than men of the same age. This trend is even more salient if socio-economic factors (such as education, marital status, or income) are taken into account. As people age, the SRH is generally poorer, in both sexes, and this worsens as health problems and loss of autonomy increase. This illustrates the likely important role of medical criteria and functional status in the assessment of SRH with advancing age. Zajacova et al. [44] pointed out that all health indicators (physical health such as functioning or pain, mental health such as depressive symptoms, and health behaviours) are significantly associated with SRH, regardless of age or sex. Cott et al. [45] made the same observation in adult populations with one or more chronic diseases.
SRH is also associated with other factors known to predict outcome in the elderly population, such as interkeukin-6 (IL-6) [7]. Arnberg et al. [7] found that good or very good SRH was associated with low levels of systemic markers of inflammation in a population with a median age of 74 years (range of 60–93 at inclusion). Christian et al. [8] reported similar findings. Taken together, these data confirm that the collection of SRH in routine practice would be a simple and effective way of complementing the usual medical assessment to extrapolate an individual’s life trajectory.
Throughout life, including in the older population, the SRH seems to be a fairly accurate assessment of an individual’s functional capacities and even functional reserves for coping with the hazards of life, as evidenced by the predictive link with all-cause mortality demonstrated at all ages of adult life and at all timepoints. SRH is easily collected [9], even in people with mild-to-moderate cognitive impairment [31,46].
The methods used to collect SRH are variable. In our systematic review, some authors chose to assess the SRH on a value scale from excellent to very poor (excellent, very good, good, fair, poor, and very poor). Others chose to class SRH on a binary scale (SRH (excellent, very good or good) versus (fair, poor or very poor)). Of the 11 authors who evaluated SRH on a binary scale, 9 (i.e., 81.8%) found a predictive link between SRH and mortality in the short, medium, or long term [11,12,18,19,23,24,28,30,31]. Among the authors who treated the SRH according to a multiple choice scale, 12 (i.e., 70.6%) [13,14,15,16,17,18,20,21,22,26,34,35] showed a predictive link between SRH and mortality for at least one time point. The predictive capacity of the SRH with respect to mortality seems to be better when SRH is treated as a binary variable, most likely because there is greater statistical power with a dichotomous variable than with a non-binary, categorical one.
The predictive link between SRH and specific mortality in specific medical conditions seems to be more difficult to establish, because it is less well documented. In this systematic review, three articles investigated mortality linked to cancer [13,15,16], and two of them found a significant predictive relationship between SRH and cancer-related death in the medium [16] and long term [13]. Five articles investigated cardiovascular mortality [13,15,16,23,26], of which four [13,15,23,26] found that SRH significantly predicted cardiovascular death in the long term in a population with a mean age of <80 years.
5. Conclusions
SRH seems to be a good criterion for assessing the risk of mortality in the short, medium, or long term in a population of elderly subjects living at home according to the articles studied in this systematic review. SRH assessment is complementary to so-called objective medical measures. SRH is simple to collect, which makes it easy to use for health professionals and acceptable to the population. Its composite nature makes it possible to take into account an individual’s health in a global manner.
A better understanding of the components of SRH and their respective weight at each age might help to guide preventive health policies aimed at delaying mortality in the long term. However, there are currently no studies that have established that improving the criteria comprising SRH would make it possible to reduce mortality.
Moreover, as the weight of each criterion seems to vary according to the individual and the age considered, targeted interventions may not be very effective. The composite nature of the SRH concept should encourage us to implement comprehensive prevention strategies from the outset, individualised and variable over time for greater effectiveness.
Prevention strategies should be implemented early in the life of the individual and continue throughout life. The identification of poor SRH in a patient should prompt healthcare providers to promptly look for associated modifiable factors in an attempt to improve them.
Acknowledgments
To Fiona Ecarnot (University of Franche-Comté, Besançon, France), for editorial assistance.
Author Contributions
Conceptualization, L.G. and M.D.; Methodology, M.D. and L.G.; Software, M.D. and L.G.; Validation, M.D., E.C. and L.G.; Formal analysis, M.D. and L.G.; Writing—original draft preparation, M.D. and L.G.; Writing—review and editing, M.D., E.C. and L.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data can be make available at moustapha.drame@chu-martinique.fr.
Conflicts of Interest
The authors declare no conflict of interest related to this work.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Murata C., Kondo T., Tamakoshi K., Yatsuya H., Toyoshima H. Determinants of self-rated health: Could health status explain the association between self-rated health and mortality? Arch. Gerontol. Geriatr. 2006;43:369–380. doi: 10.1016/j.archger.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 2.Puts M., Monette J., Girre V., Sourial N., Wolfson C., Monette M., Batist G., Bergman H. The relationship of self-rated health with functional status, toxicity and mortality: Results of a prospective pilot study of older patients with newly-diagnosed cancer. J. Geriatr. Oncol. 2013;4:319–326. doi: 10.1016/j.jgo.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 3.Mutz J., Lewis C.M. Cross-classification between self-rated health and health status: Longitudinal analyses of all-cause mortality and leading causes of death in the UK. Sci. Rep. 2022;12:459. doi: 10.1038/s41598-021-04016-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roth D.L., Skarupski K.A., Crews D.C., Howard V.J., Locher J.L. Distinct age and self-rated health crossover mortality effects for African Americans: Evidence from a national cohort study. Soc. Sci. Med. 2016;156:12–20. doi: 10.1016/j.socscimed.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Layes A., Asada Y., Kepart G. Whiners and deniers—What does self-rated health measure? Soc. Sci. Med. 2012;75:1–9. doi: 10.1016/j.socscimed.2011.10.030. [DOI] [PubMed] [Google Scholar]
- 6.Perruccio A.V., Katz J.N., Losina E. Health burden in chronic disease: Multimorbidity is associated with self-rated health more than medical comorbidity alone. J. Clin. Epidemiol. 2012;65:100–106. doi: 10.1016/j.jclinepi.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arnberg F.K., Lekander M., Morey J.N., Segerstrom S.C. Self-rated health and interleukin-6: Longitudinal relationships in older adults. Brain Behav. Immun. 2016;54:226–232. doi: 10.1016/j.bbi.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christian L.M., Glaser R., Porter K., Malarkey W.B., Beversdorf D., Kiecolt-Glaser J.K. Poorer self-rated health is associated with elevated inflammatory markers among older adults. Psychoneuroendocrinology. 2011;36:1495–1504. doi: 10.1016/j.psyneuen.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayers P.M., Sprangers M.A. Understanding self-rated health. Lancet. 2002;359:187–188. doi: 10.1016/S0140-6736(02)07466-4. [DOI] [PubMed] [Google Scholar]
- 10.Wells G.A., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Non-Randomised Studies in Meta-Analyses. [(accessed on 25 November 2020)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 11.Godaert L., Godard-Sebillotte C., Allard Saint-Albin L., Bousquet L., Bourdel-Marchasson I., Fanon J.L., Drame M. Self-rated health as a predictor of mid-term and long-term mortality in older Afro-Caribbeans hospitalised via the emergency department. Qual. Life Res. 2017;27:91–96. doi: 10.1007/s11136-017-1693-3. [DOI] [PubMed] [Google Scholar]
- 12.Godard-Sebillotte C., Drame M., Basileu T., Fanon J.L., Godaert L. Is self-rated health an independent prognostic factor of six-week mortality in older patients hospitalized for an acute condition? Qual. Life Res. 2016;25:2335–2340. doi: 10.1007/s11136-016-1252-3. [DOI] [PubMed] [Google Scholar]
- 13.Pijls L.T., Feskens E.J., Kromhout D. Self-rated health, mortality, and chronic diseases in elderly men. The Zutphen Study, 1985–1990. Am. J. Epidemiol. 1993;138:840–848. doi: 10.1093/oxfordjournals.aje.a116787. [DOI] [PubMed] [Google Scholar]
- 14.Rakowski W., Fleishman J.A., Mor V., Bryant S.A. Self-Assessments of Health and Mortality among Older Persons: Do Questions Other than Global Self-Rated Health Predict Mortality? Res. Aging. 1993;15:92–116. doi: 10.1177/0164027593151005. [DOI] [Google Scholar]
- 15.Shen C., Schooling C.M., Chan W.M., Zhou J.X., Johnston J.M., Lee S.Y., Lam T.H. Self-rated health and mortality in a prospective Chinese elderly cohort study in Hong Kong. Prev. Med. 2014;67:112–118. doi: 10.1016/j.ypmed.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Tsuji I., Minami Y., Keyl P.M., Hisamichi S., Asano H., Sato M., Shinoda K. The predictive power of self-rated health, activities of daily living, and ambulatory activity for cause-specific mortality among the elderly: A three-year follow-up in urban Japan. J. Am. Geriatr. Soc. 1994;42:153–156. doi: 10.1111/j.1532-5415.1994.tb04944.x. [DOI] [PubMed] [Google Scholar]
- 17.Yu E.S., Kean Y.M., Slymen D.J., Liu W.T., Zhang M., Katzman R. Self-perceived health and 5-year mortality risks among the elderly in Shanghai, China. Am. J. Epidemiol. 1998;147:880–890. doi: 10.1093/oxfordjournals.aje.a009542. [DOI] [PubMed] [Google Scholar]
- 18.Wuorela M., Lavonius S., Salminen M., Vahlberg T., Viitanen M., Viikari L. Self-rated health and objective health status as predictors of all-cause mortality among older people: A prospective study with a 5-, 10-, and 27-year follow-up. BMC Geriatr. 2020;20:120. doi: 10.1186/s12877-020-01516-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mavaddat N., van der Linde R., Parker R., Savva G., Kinmonth A.L., Brayne C., Mant J. Relationship of Self-Rated Health to Stroke Incidence and Mortality in Older Individuals with and without a History of Stroke: A Longitudinal Study of the MRC Cognitive Function and Ageing (CFAS) Population. PLoS ONE. 2016;11:e0150178. doi: 10.1371/journal.pone.0150178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown D.S., Thompson W.W., Zack M.M., Arnold S.E., Barile J.P. Associations between health-related quality of life and mortality in older adults. Prev. Sci. Off. J. Soc. Prev. Res. 2015;16:21–30. doi: 10.1007/s11121-013-0437-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurland B., Teresi J.A., Eimicke J.P., Maurer M.S., Reid M.C. Quality of life impacts on 16-year survival of an older ethnically diverse cohort. Int. J. Geriatr. Psychiatry. 2014;29:533–545. doi: 10.1002/gps.4038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Ruiz M., Guerra-Vales J.M., Trincado R., Fernandez R., Medrano M.J., Villarejo A., Benito-Leon J., Bermejo-Pareja F. The ability of self-rated health to predict mortality among community-dwelling elderly individuals differs according to the specific cause of death: Data from the NEDICES cohort. Gerontology. 2013;59:368–377. doi: 10.1159/000348781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ernstsen L., Nilsen S.M., Espnes G.A., Krokstad S. The predictive ability of self-rated health on ischaemic heart disease and all-cause mortality in elderly women and men: The Nord-Trondelag Health Study (HUNT) Age Ageing. 2011;40:105–111. doi: 10.1093/ageing/afq141. [DOI] [PubMed] [Google Scholar]
- 24.Khang Y.H., Kim H.R. Self-rated health and mortality: Gender- and age-specific contributions of explanatory factors in South Korea. Int. J. Public Health. 2010;55:279–289. doi: 10.1007/s00038-010-0121-z. [DOI] [PubMed] [Google Scholar]
- 25.Ford J., Spallek M., Dobson A. Self-rated health and a healthy lifestyle are the most important predictors of survival in elderly women. Age Ageing. 2008;37:194–200. doi: 10.1093/ageing/afm171. [DOI] [PubMed] [Google Scholar]
- 26.Johansson P., Brostrom A., Dahlstrom U., Alehagen U. Global perceived health and ten-year cardiovascular mortality in elderly primary care patients with possible heart failure. Eur. J. Heart Fail. 2008;10:1040–1047. doi: 10.1016/j.ejheart.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Okamoto K., Momose Y., Fujino A., Osawa Y. Gender differences in the relationship between self-rated health (SRH) and 6-year mortality risks among the elderly in Japan. Arch. Gerontol. Geriatr. 2008;47:311–317. doi: 10.1016/j.archger.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 28.Lee S.J., Moody-Ayers S.Y., Landefeld C.S., Walter L.C., Lindquist K., Segal M.R., Covinsky K.E. The relationship between self-rated health and mortality in older black and white Americans. J. Am. Geriatr. Soc. 2007;55:1624–1629. doi: 10.1111/j.1532-5415.2007.01360.x. [DOI] [PubMed] [Google Scholar]
- 29.Van den Brink C.L., Tijhuis M., van den Bos G.A., Giampaoli S., Nissinen A., Kromhout D. The contribution of self-rated health and depressive symptoms to disability severity as a predictor of 10-year mortality in European elderly men. Am. J. Public Health. 2005;95:2029–2034. doi: 10.2105/AJPH.2004.050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baron-Epel O., Shemy G., Carmel S. Prediction of survival: A comparison between two subjective health measures in an elderly population. Soc. Sci. Med. 2004;58:2035–2043. doi: 10.1016/S0277-9536(03)00412-X. [DOI] [PubMed] [Google Scholar]
- 31.Walker J.D., Maxwell C.J., Hogan D.B., Ebly E.M. Does self-rated health predict survival in older persons with cognitive impairment? J. Am. Geriatr. Soc. 2004;52:1895–1900. doi: 10.1111/j.1532-5415.2004.52515.x. [DOI] [PubMed] [Google Scholar]
- 32.Bath P.A. Differences between older men and women in the self-rated health-mortality relationship. Gerontologist. 2003;43:387–395; discussion 372–385. doi: 10.1093/geront/43.3.387. [DOI] [PubMed] [Google Scholar]
- 33.Helmer C., Barberger-Gateau P., Letenneur L., Dartigues J.F. Subjective health and mortality in French elderly women and men. J. Gerontol. B Psychol. Sci. Soc. Sci. 1999;54:S84–S92. doi: 10.1093/geronb/54B.2.S84. [DOI] [PubMed] [Google Scholar]
- 34.Leung K.K., Tang L.Y., Lue B.H. Self-rated health and mortality in Chinese institutional elderly persons. J. Clin. Epidemiol. 1997;50:1107–1116. doi: 10.1016/S0895-4356(97)00153-4. [DOI] [PubMed] [Google Scholar]
- 35.Schoenfeld D.E., Malmrose L.C., Blazer D.G., Gold D.T., Seeman T.E. Self-rated health and mortality in the high-functioning elderly--A closer look at healthy individuals: MacArthur field study of successful aging. J. Gerontol. 1994;49:M109–M115. doi: 10.1093/geronj/49.3.M109. [DOI] [PubMed] [Google Scholar]
- 36.Singh-Manoux A., Gueguen A., Martikainen P., Ferrie J., Marmot M., Shipley M. Self-rated health and mortality: Short- and long-term associations in the Whitehall II study. Psychosom. Med. 2007;69:138–143. doi: 10.1097/PSY.0b013e318030483a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamayo-Fonseca N., Quesada J.A., Nolasco A., Melchor I., Moncho J., Pereyra-Zamora P., Lopez R., Calabuig J., Barber X. Self-rated health and mortality: A follow-up study of a Spanish population. Public Health. 2013;127:1097–1104. doi: 10.1016/j.puhe.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 38.Hays J.C., Schoenfeld D.E., Blazer D.G. Determinants of Poor Self-Rated Health in Late Life. Am. J. Geriatr. Psychiatry. 1996;4:188–196. doi: 10.1097/00019442-199622430-00002. [DOI] [PubMed] [Google Scholar]
- 39.Hu Y.-N., Hu G.-C., Hsu C.-Y., Hsieh S.-F., Li C.-C. Assessment of Individual Activities of Daily Living and its Association with Self-Rated Health in Elderly People of Taiwan. Int. J. Gerontol. 2012;6:117–121. doi: 10.1016/j.ijge.2012.01.024. [DOI] [Google Scholar]
- 40.Machon M., Vergara I., Dorronsoro M., Vrotsou K., Larranaga I. Self-perceived health in functionally independent older people: Associated factors. BMC Geriatr. 2016;16:66. doi: 10.1186/s12877-016-0239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh-Manoux A., Dugravot A., Shipley M.J., Ferrie J.E., Martikainen P., Goldberg M., Zins M., Cohort G. The association between self-rated health and mortality in different socioeconomic groups in the GAZEL cohort study. Int. J. Epidemiol. 2007;36:1222–1228. doi: 10.1093/ije/dym170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J.H., Lee K.S. Self-rated health and its determinants in Japan and South Korea. Public Health. 2013;127:834–843. doi: 10.1016/j.puhe.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 43.Jylhä M., Guralnik J., Ferrucci L., Jokela J., Heikkinen E. Is self-rated health comparable across cultures and genders? J. Gerontol. 1998;53:S144–S152. doi: 10.1093/geronb/53B.3.S144. [DOI] [PubMed] [Google Scholar]
- 44.Zajacova A., Huzurbazar S., Todd M. Gender and the structure of self-rated health across the adult life span. Soc. Sci. Med. 2017;187:58–66. doi: 10.1016/j.socscimed.2017.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cott C.A., Gignac M.A., Badley E.M. Determinants of self rated health for Canadians with chronic disease and disability. J. Epidemiol. Community Health. 1999;53:731–736. doi: 10.1136/jech.53.11.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Waldorff F.B., Nielsen A.B., Waldemar G. Self-rated health in patients with mild Alzheimer’s disease: Baseline data from the Danish Alzheimer Intervention Study. Arch. Gerontol. Geriatr. 2010;50:1–5. doi: 10.1016/j.archger.2008.12.010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data can be make available at moustapha.drame@chu-martinique.fr.