Abstract
Simple Summary
In this study, in both patients, with and without a family history of GC, young age at H. pylori eradication was significantly associated with a reduced risk of GC. The gap in the risk of GC between patients with and without a family history of GC widened as the age at the time of H. pylori eradication increased. Our findings suggest that the early treatment of H. pylori infection can further maximize the preventive effects of GC.
Abstract
Introduction: This study compares the risk of GC according to age at H. pylori eradication, stratified based on the presence of family history of GC using a population-based large cohort. Method: We analyzed individuals who underwent GC screening between 2013 and 2014 and received H. pylori eradication therapy before screening. Results: Among 1,888,815 H. pylori-treated patients, 2610/294,706 and 9332/1,594,109 patients with and without a family history of GC, respectively, developed GC. After adjusting for confounders, including age at screening, the adjusted hazard ratios (95% confidence intervals) for GC comparison, 70–74, 65–69, 60–64, 55–59, 50–54, 45–49, and <45 years with ≥75 years at H. pylori eradication were 0.98 (0.79–1.21), 0.88 (0.74–1.05), 0.76 (0.59–0.99), 0.62 (0.44–0.88), 0.57 (0.36–0.90), 0.38 (0.22–0.66), and 0.34 (0.17–0.67), respectively, among patients with a family history of GC (p < 0.001) and 1.01 (0.91–1.13), 0.95 (0.86–1.04), 0.86 (0.75–0.98), 0.67 (0.56–0.81), 0.56 (0.44–0.71), 0.51 (0.38–0.68), and 0.33 (0.23–0.47), respectively, among patients without a family history of GC (p < 0.001). Conclusion: In patients with and without a family history of GC, young age at H. pylori eradication was significantly associated with a reduced risk of GC, suggesting that the early treatment of H. pylori infection can maximize GC prevention.
Keywords: gastric cancer, Helicobacter pylori, eradication, age
1. Introduction
Gastric cancer (GC) is a serious disease worldwide. With over one million new cases each year, GC is the fifth most commonly diagnosed cancer and the fourth most common cause of cancer death globally [1]. Epidemiologic studies have consistently reported an association between Helicobacter pylori (H. pylori) infection and GC risk. Based on these results, H. pylori is categorized by the International Agency for Cancer Research as a group 1 carcinogen [2].
In the past, there was not much evidence that H. pylori eradication reduces the risk of GC, whereas in recent years, this evidence is getting stronger as an increasing number of long-term follow-up randomized controlled trials (RCTs) that prove this have been published [3,4,5]. In a meta-analysis updated in 2020 that assessed seven RCTs involving 8323 healthy individuals, H. pylori eradication significantly reduced the incidence of GC by 46% and reduced mortality from GC by 39% [5].
H. pylori infection generally occurs in infancy or early childhood and persists for a lifetime if left untreated [6]. Although the effect of H. pylori eradication on GC prevention has become clear, it remains questionable as to when the commencement of eradication therapy is more effective at preventing GC. Recent meta-analyses have demonstrated that patients with gastric dysplasia or intestinal metaplasia did not benefit from H. pylori eradication in terms of the risk of GC, implying a point of no return in the gastric carcinogenesis cascade associated with H. pylori [7,8]. In line with this, the Maastricht V consensus guideline recommends that the risk of GC can be more effectively reduced by H. pylori eradication before the development of atrophy and intestinal metaplasia [9]. Based on this theory and the existing literature, it can be expected that the prevention of GC can be further maximized if H. pylori eradication is conducted earlier. Surprisingly, however, there have been no studies to prove this seemingly obvious hypothesis. While most previous studies have compared the risk of GC between patients who received H. pylori eradication therapy and those who did not, no study has evaluated the effectiveness according to the timing of eradication among H. pylori-treated patients.
Therefore, this study aimed to evaluate the association between age at the time of H. pylori eradication and the risk of GC by using a population-based large cohort of H. pylori-treated patients in Korea. Since the effectiveness of eradication therapy can differ between patients with and without a family history of GC, we conducted analyses after stratifying the study population according to the family history of GC status.
2. Materials and Methods
2.1. Data Source and Identification of the Study Population
This was a retrospective cohort study of participants who underwent GC screening. Data were obtained from a customized dataset of the National Health Insurance Service-National Health Information Database (NHIS-NHID) in Korea. Participants with a family history of any cancer and an age- and sex-matched random sample of participants without a family history of cancer were included. The NHIS is a compulsory health insurance system that covers all forms of medical services of the entire Korean population [10]. All Koreans aged ≥40 years are provided biennial esophagogastroduodenoscopy screening under the National Cancer Screening Programme [11].
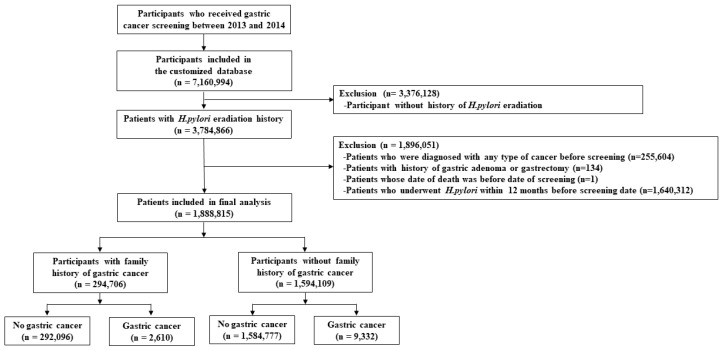
Our initial database included claims data from 2011 to 2018. Since previous prevalent GC or other types of cases may confound the association for incidence, we used a washout period of 2 years from 2011 to 2012 and excluded subjects who had history of hospital visits due to any type of cancer during the period. Accordingly, we analyzed participants who had undergone GC screening between 1 January 2013, and 31 December 2014, and had received H. pylori eradication therapy before screening. Our target study cohort included 7,160,994 adults aged ≥40 years who underwent GC screening between 2013 and 2014. Next, we selected 3,784,866 patients who had received H. pylori eradication therapy before screening. Of these, 255,604 and 134 patients were excluded because of a history of any type of cancer including GC and a history of gastric adenoma or gastrectomy during the washout period. One patient was excluded because the date of death was recorded prior to the screening date. In addition, 1,640,312 patients who received H. pylori eradication therapy within 12 months before the screening date were excluded because 12 months or less may not be sufficient to evaluate the effect of the therapy. Finally, 1,888,815 patients were included in the analysis. Since a family history of GC is a well-known high-risk factor for GC [12,13], we analyzed the association between age at H. pylori eradication and GC risk under stratification based on family history of GC. Among them, 294,706 patients had a family history of GC, while 1,594,109 patients did not (Figure 1).
Figure 1.
Flowchart for the selection of the study population. H. pylori, Helicobacter pylori.
Approval for this study was granted by the Institutional Review Board (IRB) of Ewha Womans University Mokdong Hospital (IRB No. 2020-08-030). Informed consent was waived because all screened populations agreed to transfer their screening results to NHIS-NHID, and the NHIS database was constructed after the data was anonymized.
2.2. Main Outcome and Definition of Variables
This study analyzed the association between age at the time of H. pylori eradication and the risk of GC as a top priority. In addition, we stratified these analyses based on family history of GC. We considered that a family history of GC was a powerful risk factor for GC [12,13], which can strongly impact the main research topic (the association between age at H. pylori eradication and GC risk). Thus, we analyzed the association between age at H. pylori eradication and GC risk under stratification based on family history of GC, rather than correcting it as a confounding factor in multivariate analyses.
The main outcome of this study was incident GC. GC was defined as a combination of the International Classification of Disease (ICD)-10 code for GC (C16) and the rare and intractable disease (RID) code for cancer. The RID code is related to the cost sharing of the out-of-pocket money for diseases with a high financial burden in Korea, including cancer, and the use of this code in combination with ICD-10 codes increases the accuracy of GC ascertainment [14]. A Korean study investigated the validity of cancer diagnosis based on the NHIS database vs. the National Cancer Registry database in Korea [14]. In this study, the sensitivity and positive predictive value (PPV) of the primary diagnosis-based definition of GC were 96.0% (95% confidence interval [95% CI] 96.0–96.1) and 94.1% (95% CI 94.0–94.2), respectively, and the sensitivity and PPV of the RID-based definition of GC were 95.7% (95% CI 95.7–95.8) and 93.9% (95% CI 93.8–94.0), respectively [14].
Gastric adenoma was defined as those diagnosed with ICD-10 codes D00.2 or D13.1 and a history of endoscopic resection or gastrectomy within 6 months after the above-mentioned diagnosis. To ascertain the newly diagnosed GC cases, we excluded participants diagnosed with any cancer and participants who had a history of gastrectomy or gastric adenoma during the washout period. All participants were followed until the date of GC, date of death, or 31 December 2018, whichever came first.
We defined H. pylori eradication therapy as the simultaneous prescription of a proton pump inhibitor (PPI), clarithromycin, and amoxicillin or a PPI, tetracycline, and metronidazole within 7 days from the date of first prescription of any of the above-mentioned drugs from 1 January 2011 to 12 months before the date of GC screening. This definition includes both first-line and second-line eradication therapies approved in Korea, as well as combination and sequential therapies [15]. In our analysis, age at H. pylori eradication was categorized into the following 5-year age groups: ≥75, 70–74, 65–69, 60–64, 55–59, 50–54, 45–49, and <45 years.
Information on the family history of cancer (yes/no), smoking habits (never, former, and current smokers), alcohol consumption (0 or 1/week and ≥2/week), weekly physical activity (no and yes [≥1/week]), and comorbidity status (yes/no) was obtained through a self-administered questionnaire. All participants reported whether they had any first-degree relatives (FDRs), including parents, siblings, and children, who were previously diagnosed with cancer. Based on this information, a family history of GC was defined as GC with ≥1 FDR, while no family history of GC was defined as the absence of a family history of any type of cancer. Comorbidity status was defined as having at least one of the following: hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, or stroke, based on the questionnaire. Height and weight were measured during screening and used to calculate body mass index (BMI) as weight in kilograms divided by height in meters squared. Obesity status was defined as a BMI ≥ 25 kg/m2.
To infer the indications for H. pylori eradication, the following diagnostic codes (ICD-10 codes) at the time of prescribing eradication therapy were identified: K25 for gastric ulcer, K26 for duodenal ulcer, “both K25 and K26” or “K27” for gastric and duodenal ulcer, and C88.4 for gastric mucosa-associated lymphoid tissue lymphoma.
2.3. Statistical Analysis
Baseline characteristics between participants with and without a family history of GC were compared using Student’s t-test for continuous variables and the χ2 test for categorical variables. The Grey test was conducted for statistical comparisons of cumulative incidence functions among the age groups of H. pylori eradication [16]. Crude and adjusted Cox proportional hazards regression analysis was conducted to calculate the hazard ratios (HR) and 95% CIs to quantify the risk of GC. For the adjusted analysis, age at screening, sex, obesity status, smoking habit, alcohol consumption, physical activity, and comorbidity status were adjusted. All analyses for the association between age at H. pylori eradication and GC risk were stratified based on the status of family history of GC. Kaplan–Meier curves were used to test the proportional hazards assumptions and to identify parallel lines of the log-log survival distribution function. All p-values were two-sided with a type I error (α < 0.05) that is considered statistically significant. Statistical analyses were performed using SAS statistical software (version 9.4, SAS Institute).
3. Results
3.1. Baseline Characteristics of the Study Population
Among the 1,888,815 subjects selected by inclusion and exclusion criteria, 294,706 (15.6%) had a family history of GC. The baseline characteristics of the patients with and without a family history of GC are shown in Table 1. The mean follow-up time in the study population was 4.77 years, with a maximum of 6 (median, 4.75; interquartile range [IQR], 4.28–5.22) years. The mean age of the patients was 57.1 ± 9.8 years, and 61.1% were men.
Table 1.
Baseline characteristics of all H. pylori-treated patients.
| Characteristics | Family History of GC (n = 294,706) |
No Family History of GC (n = 1,594,109) |
p-Value |
|---|---|---|---|
| Age at screening (years) | 57.1 ± 9.7 | 57.1 ± 9.8 | <0.001 |
| Age group at screening | |||
| 40–49 years | 66,376 (22.5) | 367,285 (23.0) | <0.001 |
| 50–59 years | 109,451 (37.1) | 579,176 (36.3) | |
| 60–69 years | 82,100 (27.9) | 440,026 (27.6) | |
| ≥70 years | 36,779 (12.5) | 207,622 (13.0) | |
| Sex | |||
| Female | 123,644 (42.0) | 613,556 (38.5) | <0.001 |
| Male | 171,062 (58.0) | 980,553 (61.5) | |
| Obesity (BMI ≥ 25 kg/m2) | |||
| No | 190,779 (64.7) | 1,021,777 (64.1) | <0.001 |
| Yes | 103,902 (35.3) | 572,168 (35.9) | |
| Unknown | 25 (0.0) | 164 (0.0) | |
| Smoking habit | |||
| Never | 195,926 (66.5) | 1,115,474 (70.0) | <0.001 |
| Former | 51,370 (17.4) | 232,825 (14.6) | |
| Current | 47,309 (16.1) | 245,282 (15.4) | |
| Unknown | 101 (0.0) | 528 (0.0) | |
| Alcohol consumption | |||
| 0 or 1 time per week | 227,576 (77.2) | 1,252,957 (78.6) | <0.001 |
| ≥2 times per week | 66,897 (22.7) | 340,069 (21.3) | |
| Unknown | 233 (0.1) | 1083 (0.1) | |
| Physical activity | |||
| No | 66,836 (22.7) | 394,668 (24.8) | <0.001 |
| Yes (≥1 time per week) | 227,552 (77.2) | 1,197,894 (75.2) | |
| Unknown | 318 (0.1) | 1547 (0.1) | |
| Comorbidities | |||
| Hypertension | 80,762 (27.4) | 462,299 (29.0) | <0.001 |
| Diabetes mellitus | 30,353 (10.3) | 170,240 (10.7) | |
| Dyslipidemia | 23,646 (8.0) | 120,842 (7.6) | |
| Ischemic heart disease | 11,569 (3.9) | 61,131 (3.8) | |
| Stroke | 3656 (1.2) | 19,605 (1.2) | |
| Comorbidity status (yes) | 108,743 (36.9) | 606,308 (38.0) | <0.001 |
| Indication for H. pylori eradication | |||
| Gastric ulcer | 61,147 (20.7) | 320,248 (20.1) | <0.001 |
| Duodenal ulcer | 15,229 (5.2) | 79,547 (5.0) | |
| Gastric and duodenal ulcer a | 13,469 (4.6) | 74,606 (4.7) | |
| Gastric MALT lymphoma | 4 (0.0) | 3 (0.0) | |
| H. pylori-associated gastritis | 204,857 (69.5) | 1,119,705 (70.2) |
GC, gastric cancer; BMI, body mass index; H. pylori, Helicobacter pylori; MALT, mucosa-associated lymphoid tissue. Values are presented as mean ± standard deviation or number (%). a Gastric ulcer and duodenal ulcer/ gastroduodenal ulcer unspecified.
3.2. Risk of GC According to Age at H. pylori Eradication in Patients with a Family History
The mean follow-up period was 4.78 years, with a maximum of 6 (median, 4.76; IQR, 4.29–5.25) years in patients with a family history of GC. During the follow-up period, among 294,706 patients with a family history of GC, 2610 (0.9%) developed GC.
Table 2 shows the risk of GC in relation to age at H. pylori eradication in patients with a family history of GC.
Table 2.
Risk of gastric cancer according to age at H. pylori eradication in patients with a family history of gastric cancer.
| Variables | N | Person–Years | Incidence (95% CI) a |
Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Age at H. pylori eradication | |||||
| ≥75 years | 214 | 45,998 | 4.65 (4.03–5.28) | 1 (Reference) | 1 (Reference) |
| 70–74 years | 287 | 69,429 | 4.13 (3.66–4.61) | 0.89 (0.75–1.06) | 0.98 (0.79–1.21) |
| 65–69 years | 553 | 172,833 | 3.20 (2.93–3.47) | 0.69 (0.59–0.81) | 0.88 (0.74–1.05) |
| 60–64 years | 396 | 162,193 | 2.44 (2.20–2.68) | 0.52 (0.44–0.62) | 0.76 (0.59–0.99) |
| 55–59 years | 544 | 314,374 | 1.73 (1.59–1.88) | 0.37 (0.32–0.44) | 0.62 (0.44–0.88) |
| 50–54 years | 311 | 225,437 | 1.38 (1.23–1.53) | 0.30 (0.25–0.35) | 0.57 (0.36–0.90) |
| 45–49 years | 157 | 187,873 | 0.84 (0.70–0.97) | 0.18 (0.15–0.22) | 0.38 (0.22–0.66) |
| 40–44 years | 148 | 229,125 | 0.65 (0.54–0.75) | 0.14 (0.11–0.17) | 0.34 (0.17–0.67) |
| p for trend | <0.001 | <0.001 | |||
| Age at screening, per year | 1.06 (1.06–1.06) | 1.03 (1.01–1.05) | |||
| Sex | |||||
| Female | 970 | 822,475 | 1.18 (1.11–1.25) | 1 (Reference) | 1 (Reference) |
| Male | 1640 | 584,787 | 2.80 (2.67–2.94) | 2.37 (2.19–2.57) | 2.09 (1.88–2.34) |
| Obesity (BMI ≥ 25 kg/m2) | |||||
| No | 1681 | 910,886 | 1.85 (1.76–1.93) | 1 (Reference) | 1 (Reference) |
| Yes | 928 | 496,257 | 1.87 (1.75–1.99) | 1.01 (0.94–1.10) | 0.95 (0.87–1.03) |
| Smoking habit | |||||
| Never | 1348 | 940,476 | 1.43 (1.36–1.51) | 1 (Reference) | 1 (Reference) |
| Former | 700 | 243,307 | 2.88 (2.66–3.09) | 2.00 (1.83–2.19) | 1.17 (1.04–1.31) |
| Current | 561 | 222,999 | 2.52 (2.31– 2.72) | 1.75 (1.59–1.93) | 1.38 (1.22–1.56) |
| Alcohol consumption | |||||
| 0 or 1 time/week | 1869 | 1,089,055 | 1.72 (1.64–1.79) | 1 (Reference) | 1 (Reference) |
| ≥2 times/week | 740 | 317,127 | 2.33 (2.17–2.50) | 1.36 (1.25–1.48) | 1.08 (0.98–1.19) |
| Physical activity | |||||
| No | 603 | 320,047 | 1.88 (1.73–2.03) | 1 (Reference) | 1 (Reference) |
| Yes (≥1 time/week) | 2000 | 1,085,671 | 1.84 (1.76–1.92) | 0.98 (0.89–1.07) | 0.97 (0.88–1.06) |
| Comorbidity status b | |||||
| No | 1353 | 887,775 | 1.52 (1.44–1.61) | 1 (Reference) | 1 (Reference) |
| Yes | 1257 | 519,487 | 2.42 (2.29–2.55) | 1.59 (1.47–1.72) | 1.03 (0.95–1.12) |
H. pylori, Helicobacter pylori; CI, confidence interval; HR, hazard ratio; BMI, body mass index. a Incidence rate was calculated as cases per 1000 person–years. b Comorbidity status was defined as having at least one of hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, and stroke.
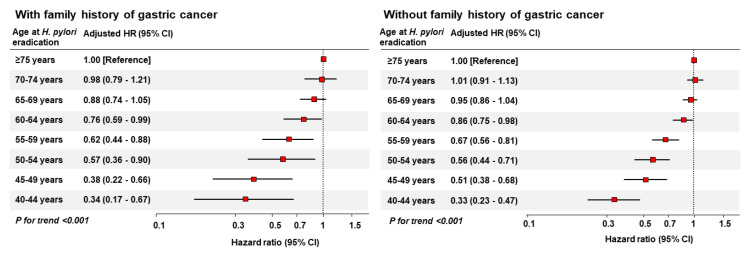
In the crude model, decreasing age at H. pylori eradication was significantly associated with a decreased risk of GC. Even after adjusting for age at screening, sex, obesity, smoking habit, alcohol consumption, physical activity, and comorbidities, younger age at H. pylori eradication was significantly associated with a decreased risk of GC (p trend < 0.001). The adjusted HRs (95% CIs) for GC comparing 70–74, 65–69, 60–64, 55–59, 50–54, 45–49, and <45 years with ≥75 years at H. pylori eradication were 0.98 (0.79–1.21), 0.88 (0.74–1.05), 0.76 (0.59–0.99), 0.62 (0.44–0.88), 0.57 (0.36–0.90), 0.38 (0.22–0.66), and 0.34 (0.17–0.67), respectively, which are illustrated in Figure 2. Age at screening, male sex, and former or current smoking status were also identified as independent risk factors for GC.
Figure 2.
Risk of gastric cancer according to age at H. pylori eradication in patients with and without a family history of gastric cancer. H. pylori, Helicobacter pylori; HR, hazard ratio; CI, confidence interval.
3.3. Risk of GC According to Age at H. pylori Eradication in Patients without a Family History
The mean follow-up period was 4.76 years, with a maximum of 6 (median, 4.75; IQR 4.27–5.22) years in patients without a family history of GC. During the follow-up period, among 1,594,109 patients without a family history of GC, 9332 (0.6%) developed GC. Table 3 shows the risk of GC in relation to age at H. pylori eradication in patients without a family history of GC.
Table 3.
Risk of gastric cancer according to age at H. pylori eradication in patients without a family history of gastric cancer.
| Variables | N | Person–Years | Incidence (95% CI) a |
Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|---|
| Age at H. pylori eradication | |||||
| ≥75 years | 827 | 262,139 | 3.15 (2.94–3.37) | 1 (Reference) | 1 (Reference) |
| 70–74 years | 1027 | 390,647 | 2.63 (2.47–2.79) | 0.84 (0.76–0.92) | 1.01 (0.91–1.13) |
| 65–69 years | 2039 | 953,595 | 2.14 (2.05–2.23) | 0.68 (0.63–0.74) | 0.95 (0.86–1.04) |
| 60–64 years | 1462 | 861,892 | 1.70 (1.61–1.78) | 0.54 (0.49–0.59) | 0.86 (0.75–0.98) |
| 55–59 years | 1884 | 1,669,636 | 1.13 (1.08–1.18) | 0.36 (0.33–0.39) | 0.67 (0.56–0.81) |
| 50–54 years | 953 | 1,181,730 | 0.81 (0.76–0.86) | 0.26 (0.23–0.28) | 0.56 (0.44–0.71) |
| 45–49 years | 663 | 988,615 | 0.67 (0.62–0.72) | 0.21 (0.19–0.24) | 0.51 (0.38–0.68) |
| 40–44 years | 477 | 1,285,235 | 0.37 (0.34–0.40) | 0.12 (0.11–0.13) | 0.33 (0.23–0.47) |
| p for trend | <0.001 | <0.001 | |||
| Age at screening, per year | 1.06 (1.06–1.06) | 1.03 (1.02–1.04) | |||
| Sex | |||||
| Female | 3539 | 4,700,031 | 0.75 (0.73–0.78) | 1 (Reference) | 1 (Reference) |
| Male | 5793 | 2,893,458 | 2.00 (1.95–2.05) | 2.65 (2.54–2.76) | 2.35 (2.23–2.49) |
| Obesity (BMI ≥ 25 kg/m2) | |||||
| No | 5800 | 4,865,695 | 1.19 (1.16–1.22) | 1 (Reference) | 1 (Reference) |
| Yes | 3531 | 2,727,062 | 1.29 (1.25–1.34) | 1.09 (1.04–1.13) | 1.02 (0.98–1.07) |
| Smoking habit | |||||
| Never | 5094 | 5,336,735 | 0.95 (0.93–0.98) | 1 (Reference) | 1 (Reference) |
| Former | 2140 | 1,099,546 | 1.95 (1.86–2.03) | 2.03 (1.93–2.14) | 1.12 (1.05–1.19) |
| Current | 2094 | 1,154,625 | 1.81 (1.74–1.89) | 1.90 (1.80–1.99) | 1.39 (1.31–1.48) |
| Alcohol consumption | |||||
| 0 or 1 time/week | 6741 | 5,979,969 | 1.13 (1.10–1.15) | 1 (Reference) | 1 (Reference) |
| ≥2 times/week | 2589 | 1,608,489 | 1.61 (1.55–1.67) | 1.43 (1.36–1.49) | 1.06 (1.01–1.12) |
| Physical activity | |||||
| No | 2496 | 1,884,265 | 1.32 (1.27–1.38) | 1 (Reference) | 1 (Reference) |
| Yes (≥1 time/week) | 6827 | 5,701,664 | 1.20 (1.17–1.23) | 0.90 (0.86–0.94) | 0.92 (0.88–0.97) |
| Comorbidity status b | |||||
| No | 4699 | 4,704,372 | 1.00 (0.97–1.03) | 1 (Reference) | 1 (Reference) |
| Yes | 4633 | 2,889,117 | 1.60 (1.56–1.65) | 1.61 (1.54–1.67) | 1.02 (0.98–1.06) |
H. pylori, Helicobacter pylori; CI, confidence interval; HR, hazard ratio; BMI, body mass index; a Incidence rate was calculated as cases per 1000 person–years. b Comorbidity status was defined as having at least one of hypertension, diabetes mellitus, dyslipidemia, ischemic heart disease, and stroke.
The results were similar to those of patients without a family history of GC. Even after adjusting for confounders, including age at screening, a decrease in the age at H. pylori eradication was significantly associated with a decrease in the risk of GC even in patients without a family history of GC (p trend < 0.001). The adjusted HRs (95% CIs) for GC comparing 70–74, 65–69, 60–64, 55–59, 50–54, 45–49, and <45 years with ≥75 years at H. pylori eradication were 1.01 (0.91–1.13), 0.95 (0.86–1.04), 0.86 (0.75–0.98), 0.67 (0.56–0.81), 0.56 (0.44–0.71), 0.51 (0.38–0.68), and 0.33 (0.23–0.47), respectively (Figure 2). Additionally, age at screening, male sex, former or current smoking, and alcohol consumption were found to be independent risk factors for GC, whereas physical activity (≥one time/week) was an independent protective factor against GC.
During the study period, most patients received standard triple therapy. The proportions of patients who received standard triple therapy and sequential/combination therapy were 93.1% (n = 1,757,599) and 6.8% (n = 127,566), respectively. We compared family history status, age at H. pylori eradication, and the development of GC between patients treated with standard triple therapy and those with sequential/concomitant therapy (Table 4). After adjusting for confounders, patients who received sequential or concomitant therapy showed a 1.62-fold increased risk of GC compared with those who received standard triple therapy.
Table 4.
Distribution of H. pylori eradication regimens according to family history, age group and cancer development status.
| Factors | H. pylori Eradication Regimen | |||||
|---|---|---|---|---|---|---|
| Standard Triple Therapy a | Sequential or Concomitant Therapy b | p-Value | ||||
| n | % | n | % | |||
| n = 1,757,599 | n = 127,566 | |||||
| Characteristics | ||||||
| Family history status | ||||||
| No | 1,483,863 | 84.4 | 107,309 | 84.1 | 0.004 | |
| Yes | 273,736 | 15.6 | 20,257 | 15.9 | ||
| Age group | ||||||
| ≥75 years | 63,261 | 3.6 | 2437 | 1.9 | <0.001 | |
| 70–74 years | 92,193 | 5.2 | 3841 | 3.0 | ||
| 65–69 years | 223,100 | 12.7 | 10,609 | 8.3 | ||
| 60–64 years | 202,709 | 11.5 | 11,110 | 8.7 | ||
| 55–59 years | 387,240 | 22.0 | 25,883 | 20.3 | ||
| 50–54 years | 271,491 | 15.4 | 22,461 | 17.6 | ||
| 45–49 years | 227,723 | 13.0 | 21,077 | 16.5 | ||
| 40–44 years | 289,882 | 16.5 | 30,148 | 23.6 | ||
| Cancer development | ||||||
| No | 1,746,513 | 99.4 | 126,745 | 99.4 | 0.312 | |
| Yes | 11,086 | 0.6 | 821 | 0.6 | ||
| Risk of gastric cancer development | ||||||
| Crude HR | Reference | 1.22 (1.13–1.31) | ||||
| Adjusted HR c | Reference | 1.62 (1.16–2.25) | ||||
a Standard triple therapy was defined as the concomitant prescription of proton pump inhibitors (PPI), clarithromycin, and amoxicillin. b Sequential therapy was defined as the concomitant prescription of PPI and amoxicillin for 5 days followed by PPI, clarithromycin, and metronidazole for 5 days. Concomitant therapy was defined as the concomitant prescription of PPI, clarithromycin, amoxicillin, and metronidazole. c Hazard ratios from the multivariate model were adjusted for age at screening, sex, obesity, smoking habit, alcohol consumption, physical activity, and comorbidities.
In this study, we also analyzed the association between age at H. pylori eradication and the risk of gastric adenoma (Table 5). After adjusting for confounders, a decrease in the age at H. pylori eradication was significantly associated with a decrease in the risk of gastric adenoma in both patients with and without a family history of GC (p trend < 0.001).
Table 5.
Risk of gastric adenoma according to the age at H. pylori eradication in total study population and the patients with and without family history of gastric cancer.
| Variables | N | Crude HR (95% CI) | Adjusted HR a (95% CI) |
|---|---|---|---|
| Total | |||
| Age at H. pylori eradication | n = 6899 | ||
| ≥60 years | 3756 | 1 (Reference) | 1 (Reference) |
| 55–59 years | 1292 | 0.64 (0.60–0.68) | 0.72 (0.66–0.78) |
| 50–54 years | 1130 | 0.47 (0.44–0.51) | 0.56 (0.50–0.63) |
| 45–49 years | 439 | 0.29 (0.26–0.32) | 0.35 (0.30–0.41) |
| 40–44 years | 282 | 0.14 (0.13–0.16) | 0.18 (0.15–0.22) |
| p for trend | <0.001 | <0.001 | |
| With family history | |||
| Age at H. pylori eradication | n = 1450 | ||
| ≥60 years | 775 | 1 (Reference) | 1 (Reference) |
| 55–59 years | 264 | 0.62 (0.54–0.71) | 0.68 (0.56–0.82) |
| 50–54 years | 251 | 0.49 (0.43–0.57) | 0.57 (0.45–0.73) |
| 45–49 years | 96 | 0.29 (0.24–0.36) | 0.35 (0.25–0.49) |
| 40–44 years | 64 | 0.16 (0.12–0.21) | 0.20 (0.13–0.31) |
| p for trend | <0.001 | <0.001 | |
| Without family history | |||
| Age at H. pylori eradication | n = 5449 | ||
| ≥60 years | 2981 | 1 (Reference) | 1 (Reference) |
| 55–59 years | 1028 | 0.65 (0.60–0.69) | 0.73 (0.66–0.80) |
| 50–54 years | 879 | 0.47 (0.43–0.50) | 0.56 (0.49–0.63) |
| 45–49 years | 343 | 0.28 (0.25–0.32) | 0.35 (0.30–0.42) |
| 40–44 years | 218 | 0.14 (0.12–0.16) | 0.18 (0.15–0.22) |
| p for trend | <0.001 | <0.001 | |
H. pylori, Helicobacter pylori; HR, hazard ratio; CI, confidence interval; a Hazard ratios from multivariate model were adjusted for age at screening, sex, obesity, smoking habit, alcohol consumption, physical activity, and comorbidities. Regression model for total population was additionally adjusted for family history of gastric cancer status.
To adjust for the influence of age itself on GC development, we analyzed the risk of GC according to age at H. pylori eradication when all participants within an age group were followed up until all reached a comparable age (Table 6). In each age group, the oldest one was used as reference and was compared with risk in other groups. As a result, we observed a significant decrease in the HRs for GC in the younger age groups, especially in the age groups 40–44, 50–54, 55–59, and 60–64. These findings can support our hypothesis that an earlier age at H. pylori eradication decreased the risk of GC.
Table 6.
Risk of gastric cancer according to age at H. pylori eradication by age group.
| Age at H. pylori Eradication a | Total Population | With Family History | Without Family History | ||||
|---|---|---|---|---|---|---|---|
| HR (95% CI) b | p c | HR (95% CI) b | p c | HR (95% CI) b | p c | ||
| 40–44 | Age 44→45 | Ref. | <0.001 | Ref. | 0.353 | Ref. | <0.001 |
| Age 43→45 | 0.98 (0.75–1.29) | 1.24 (0.71–2.16) | 0.91 (0.67–1.25) | ||||
| Age 42→45 | 0.86 (0.67–1.11) | 0.83 (0.48–1.43) | 0.87 (0.66–1.15) | ||||
| Age 41→45 | 0.78 (0.58–1.06) | 1.16 (0.64–2.09) | 0.68 (0.47–0.97) | ||||
| Age 40→45 | 0.70 (0.56–0.86) | 0.85 (0.54–1.34) | 0.66 (0.52–0.83) | ||||
| 45–49 | Age 49→50 | Ref. | 0.308 | Ref. | 0.693 | Ref. | 0.345 |
| Age 48→50 | 1.09 (0.89–1.33) | 1.10 (0.68–1.75) | 1.09 (0.87–1.36) | ||||
| Age 47→50 | 1.15 (0.92–1.44) | 1.14 (0.68–1.92) | 1.15 (0.89–1.48) | ||||
| Age 46→50 | 0.88 (0.71–1.10) | 0.96 (0.59–1.57) | 0.86 (0.68–1.10) | ||||
| Age 45→50 | 0.96 (0.76–1.23) | 0.91 (0.52–1.62) | 0.98 (0.75–1.28) | ||||
| 50–54 | Age 54→55 | Ref. | <0.001 | Ref. | 0.120 | Ref. | <0.001 |
| Age 53→55 | 0.84 (0.72–0.98) | 0.88 (0.65–1.20) | 0.83 (0.70–0.98) | ||||
| Age 52→55 | 0.80 (0.70–0.91) | 0.83 (0.64–1.09) | 0.79 (0.68–0.92) | ||||
| Age 51→55 | 0.79 (0.68–0.92) | 0.77 (0.56–1.06) | 0.80 (0.67–0.95) | ||||
| Age 50→55 | 0.75 (0.65–0.86) | 0.83 (0.63–1.09) | 0.73 (0.63–0.86) | ||||
| 55–59 | Age 59→60 | Ref. | <0.001 | Ref. | 0.022 | Ref. | <0.001 |
| Age 58→60 | 0.88 (0.76–1.01) | 0.88 (0.66–1.17) | 0.88 (0.75–1.02) | ||||
| Age 57→60 | 0.85 (0.73–0.98) | 0.76 (0.55–1.06) | 0.87 (0.73–1.03) | ||||
| Age 56→60 | 0.78 (0.68–0.90) | 0.79 (0.60–1.06) | 0.78 (0.67–0.91) | ||||
| Age 55→60 | 0.71 (0.60–0.83) | 0.67 (0.48–0.94) | 0.72 (0.60–0.86) | ||||
| 60–64 | Age 64→65 | Ref. | <0.001 | Ref. | 0.001 | Ref. | 0.005 |
| Age 63→65 | 0.85 (0.75–0.97) | 0.86 (0.66–1.12) | 0.85 (0.74–0.99) | ||||
| Age 62→65 | 0.89 (0.79–0.99) | 0.80 (0.64–1.01) | 0.91 (0.80–1.03) | ||||
| Age 61→65 | 0.78 (0.68–0.89) | 0.75 (0.57–0.99) | 0.79 (0.67–0.91) | ||||
| Age 60→65 | 0.82 (0.73–0.91) | 0.68 (0.54–0.86) | 0.86 (0.76–0.97) | ||||
| 65–69 | Age 69→70 | Ref. | 0.039 | Ref. | 0.150 | Ref. | 0.113 |
| Age 68→70 | 0.85 (0.75–0.97) | 0.84 (0.61–1.14) | 1.10 (0.94–1.30) | ||||
| Age 67→70 | 0.89 (0.79–0.99) | 0.78 (0.55–1.12) | 1.00 (0.83–1.20) | ||||
| Age 66→70 | 0.78 (0.68–0.89) | 0.81 (0.60–1.10) | 0.95 (0.80–1.12) | ||||
| Age 65→70 | 0.82 (0.73–0.91) | 0.73 (0.52–1.03) | 0.92 (0.77–1.10) | ||||
| 70–74 | Age 74→75 | Ref. | 0.079 | Ref. | 0.149 | Ref. | 0.234 |
| Age 73→75 | 1.04 (0.90–1.20) | 1.09 (0.75–1.60) | 0.93 (0.75–1.15) | ||||
| Age 72→75 | 0.95 (0.80–1.11) | 1.05 (0.76–1.45) | 0.97 (0.82–1.16) | ||||
| Age 71→75 | 0.92 (0.79–1.06) | 0.87 (0.59–1.26) | 0.85 (0.70–1.03) | ||||
| Age 70→75 | 0.88 (0.75–1.02) | 0.85 (0.62–1.16) | 0.89 (0.76–1.05) | ||||
| ≥75 | Age ≥79→age + 1 d | Ref. | 0.529 | Ref. | 0.437 | Ref. | 0.739 |
| Age 78→80 | 1.10 (0.88–1.38) | 1.38 (0.84–2.25) | 1.04 (0.81–1.34) | ||||
| Age 77→80 | 0.91 (0.71–1.17) | 1.01 (0.57–1.80) | 0.89 (0.67–1.17) | ||||
| Age 76→80 | 0.99 (0.81–1.20) | 1.33 (0.87–2.03) | 0.91 (0.73–1.14) | ||||
| Age 75→80 | 0.96 (0.77–1.18) | 0.65 (0.38–1.14) | 1.03 (0.81–1.29) | ||||
HR, hazard ratio; CI, confidence interval; a Participants within an age group were followed up until all reached comparable age. b Hazard ratios from multivariate model were adjusted for sex, obesity, smoking habit, alcohol consumption, physical activity, and comorbidity status. Regression model for total population was additionally adjusted for family history of gastric cancer status. c p for trend. d There was no age-limitation for age at H. pylori eradication; thus, for those who received H. pylori eradication at an age ≥79, the follow-up period was set to 1 year (age + 1). For example, people who received H. pylori eradication at the age of 82, the follow-up period was set to 1 year (up to the age of 83).
4. Discussion
In this population-based study, we found that in both patients with and without a family history of GC, the younger the patient’s age at the time of H. pylori eradication, the lower the risk of GC. Because age itself is an important risk factor for GC, age at screening was adjusted, but our results were, nevertheless, significantly maintained. Our findings suggest that GC prevention can be further maximized by eradicating H. pylori in the early stages of infection. To the best of our knowledge, this is the first study to identify the effect of the timing of H. pylori eradication on GC risk among H. pylori-treated patients.
Treatment of H. pylori infection has consistently been shown to reduce the risk of GC in recent meta-analyses [3,4,5]. However, since H. pylori infection is usually acquired early in life and persists for a lifetime, the question remains as to when treatment is the most optimal. Given that H. pylori infection leads to a sequence of events known as Correa’s cascade, a stepwise progression from chronic gastric inflammation, atrophy, intestinal metaplasia, dysplasia, to GC [17], it can be theoretically expected that blocking this series of processes at an early stage will increase the effectiveness of GC prevention. Several studies have supported this hypothesis. Two meta-analyses have revealed that the role of H. pylori eradication therapy after the development of pre-neoplastic changes, such as intestinal metaplasia or dysplasia is unclear [7,8]. A meta-analysis also highlighted that severe atrophy and intestinal metaplasia may increase the risk of metachronous GC after endoscopic resection in H. pylori eradicated patients [18]. In addition, some studies have reported that the effect of H. pylori eradication is affected by the degree of atrophic gastritis. Therefore, the effect on GC prevention is higher in patients with mild atrophic gastritis than in patients with extensive or severe atrophic gastritis [19,20]. These results suggest that there is a point of no return in the gastric carcinogenesis cascade associated with H. pylori; thus, H. pylori eradication should be performed before atrophy, or intestinal metaplasia occurs.
To date, no studies have compared the risk of GC according to age at the time of eradication among H. pylori-infected patients. Moreover, only two studies have evaluated the appropriate timing for H. pylori eradication. A large cohort study of 371,813 patients diagnosed with H. pylori infection in the United States showed a 13% increase in the risk of GC for each 5-year increase in age at H. pylori diagnosis (sub-HR 1.13; 95% CI 1.11–1.15) [21]. Although this study analyzed the age at which H. pylori infection was diagnosed, not the age at which H. pylori infection was treated, it suggests that diagnosing and treating H. pylori infection at a younger age may be beneficial in preventing GC. Another study of 73,237 patients in Hong Kong treated for H. pylori infection reported that H. pylori-treated patients aged <40 and 40–59 years did not differ in GC incidence compared to the general population, whereas H. pylori-treated patients aged ≥60 years showed an 18% reduction in GC incidence compared to the general population (standardized incidence ratio 0.82; 95% CI 0.69–0.97) [22]. However, a limitation of this study was that the comparison group was the general population, which included both H. pylori-infected and uninfected individuals rather than those who did not receive eradication therapy. Therefore, it is not possible to determine whether eradication therapy had a greater effect on the prevention of GC in the elderly than in the young population. This is because the most common age for GC diagnosis is over 60 [23], and most patients aged <40 and 40–59 years have not yet been followed up to the age at which GC is most prevalent. If the follow-up period was extended sufficiently, it would have been possible to confirm the effect of reducing the risk of GC even in individuals aged <60. Rather, it is reasonable to interpret these results as being able to prevent GC through H. pylori eradication, even for those aged >60.
Although our results revealed that treating H. pylori infection at a younger age can increase the effectiveness of GC prevention, there is still insufficient evidence as to when H. pylori eradication treatment should be started. If H. pylori eradication is performed in childhood, the chance of re-infection may be high, and antibiotic resistance may increase due to early exposure to antibiotics. Therefore, we would like to recommend H. pylori eradication in early adulthood after adolescence.
Another valuable finding of our study was that younger age at H. pylori eradication was associated with a lower risk of gastric adenoma. These findings support that H. pylori eradication may have a role in GC prevention by influencing the early stages of adenoma initiation and development in the gastric carcinogenesis, and further clearly suggest the benefit of early eradication.
Another finding of our study was that, even if H. pylori eradication treatment was administered, the gap in the risk of GC between patients with and without a family history of GC widened as the age at the time of H. pylori eradication increased. These findings emphasize the importance of early eradication for patients with a family history, a high-risk group for GC.
Our study had several limitations. First, the results of H. pylori eradication were not available in the NHIS-NHID. As this study was conducted retrospectively, a follow-up test to confirm the effectiveness of H. pylori eradication was not performed for all the study subjects. Additionally, information regarding whether the test was conducted is available, but information concerning the results is not available due to the nature of our cohort data. However, our results may have been more significant if we only evaluated patients who were successful in eradicating H. pylori, as shown in previous studies [21,24]. In addition, there was limited information regarding H. pylori infection, exposure time, and recurrence in our current database. Therefore, it was difficult to analyze the effect of this confounding factor on the outcome (risk of GC) and to control its influence as a confounding variable. Especially, the duration between H. pylori infection and eradication therapy may be a major factor in the development of dysplasia, which may serve as a confounder in our results. Second, the effect of H. pylori eradication in patients in their 20s or 30s could not be evaluated. Since the national GC screening program was provided to individuals aged ≥40 years and we were able to extract the prescription drug code from 2 years before the screening, the age at which H. pylori eradication therapy was performed could be analyzed from the age of 38. Third, there was limited data to analyze the efficacy of the type of each H. pylori eradication regimen in reducing GC risk. These results may be influenced by the proportion of each regimen used as the first-line therapy. However, in previously published Korean guidelines (2013) [25], sequential or concomitant therapy was not recommended as a first-line therapy because of the limited number of studies on them at that time. Since our study period coincided with the time when the 2013 guideline was implemented, almost all participants in our study were treated with standard triple therapy as a first-line regimen. Fourth, the follow-up observation period for GC occurrence was short. If we had been able to follow up younger adults under the age of 40 who received H. pylori eradication treatment for a longer period, the effect of early eradication on GC prevention might have been more clearly identified. Fifth, this study included gastric or duodenal ulcers as indications for H. pylori eradication treatment. However, it cannot be attributed to H. pylori infection itself, as a number of causes can cause gastric or duodenal ulcers. Lastly, although H. pylori infection is more related to non-cardiac GC [26], an analysis according to GC location was not performed because such information was not available in the NHIS-NHID.
5. Conclusions
In conclusion, a younger age at H. pylori eradication was significantly associated with a lower risk of GC in patients with and without a family history of GC. The gap in the incidence of GC between patients with and without a family history of GC widened as age at the time of eradication of H. pylori increased. Our results strongly support screening for H. pylori infection in early adulthood and the administration of early eradication therapy when the infection is confirmed, to maximize GC prevention. To verify our results, further prospective studies with long-term follow-up and detailed data on H. pylori infection and its eradication are needed.
Author Contributions
Y.S.J.: Conceptualization, Investigation, Methodology, Project administration, Validation, Writing—original draft; M.T.X.T.: Data curation, Formal analysis, Investigation, Methodology, Writing—original draft; H.S.: Data curation, Formal analysis; B.P.: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing—review and editing; C.M.M.: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Writing—review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study protocol was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board of Ewha Womans University Mokdong Hospital (approval No. 2020-08-030).
Informed Consent Statement
Patient consent was waived because the present study was a retrospective study using only de-identified data.
Data Availability Statement
The materials and data supporting the findings of the current study are available from the corresponding author upon reasonable request and IRB approval.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (2020R1A2C1010786 and 2020R1A5A2019210; Chang Mo Moon).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Alexander G.A., Brawley O.W. Association of Helicobacter pylori infection with gastric cancer. Mil. Med. 2000;165:21–27. doi: 10.1093/milmed/165.1.21. [DOI] [PubMed] [Google Scholar]
- 3.Fuccio L., Zagari R.M., Eusebi L.H., Laterza L., Cennamo V., Ceroni L., Grilli D., Bazzoli F. Meta-analysis: Can Helicobacter pylori eradication treatment reduce the risk for gastric cancer? Ann. Intern. Med. 2009;151:121–128. doi: 10.7326/0003-4819-151-2-200907210-00009. [DOI] [PubMed] [Google Scholar]
- 4.Ford A.C., Forman D., Hunt R.H., Yuan Y., Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer in healthy asymptomatic infected individuals: Systematic review and meta-analysis of randomised controlled trials. BMJ. 2014;348:g3174. doi: 10.1136/bmj.g3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ford A.C., Yuan Y., Moayyedi P. Helicobacter pylori eradication therapy to prevent gastric cancer: Systematic review and meta-analysis. Gut. 2020;69:2113–2121. doi: 10.1136/gutjnl-2020-320839. [DOI] [PubMed] [Google Scholar]
- 6.Park J.S., Jun J.S., Seo J.H., Youn H.S., Rhee K.H. Changing prevalence of Helicobacter pylori infection in children and adolescents. Clin. Exp. Pediatr. 2021;64:21–25. doi: 10.3345/cep.2019.01543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen H.N., Wang Z., Li X., Zhou Z.G. Helicobacter pylori eradication cannot reduce the risk of gastric cancer in patients with intestinal metaplasia and dysplasia: Evidence from a meta-analysis. Gastric Cancer. 2016;19:166–175. doi: 10.1007/s10120-015-0462-7. [DOI] [PubMed] [Google Scholar]
- 8.Rokkas T., Rokka A., Portincasa P. A systematic review and meta-analysis of the role of Helicobacter pylori eradication in preventing gastric cancer. Ann. Gastroenterol. 2017;30:414–423. doi: 10.20524/aog.2017.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Malfertheiner P., Megraud F., O’Morain C.A., Gisbert J.P., Kuipers E.J., Axon A.T., Bazzoli F., Gasbarrini A., Atherton J., Graham D.Y., et al. Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut. 2017;66:6–30. doi: 10.1136/gutjnl-2016-312288. [DOI] [PubMed] [Google Scholar]
- 10.Bahk J., Kim Y.Y., Kang H.Y., Lee J., Kim I., Lee J., Yun S.C., Park J.H., Shin S.A., Khang Y.H. Using the National Health Information Database of the National Health Insurance Service in Korea for Monitoring Mortality and Life Expectancy at National and Local Levels. J. Korean Med. Sci. 2017;32:1764–1770. doi: 10.3346/jkms.2017.32.11.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheol Seong S., Kim Y.Y., Khang Y.H., Heon Park J., Kang H.J., Lee H., Do C.H., Song J.S., Hyon Bang J., Ha S., et al. Data Resource Profile: The National Health Information Database of the National Health Insurance Service in South Korea. Int. J. Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yaghoobi M., McNabb-Baltar J., Bijarchi R., Hunt R.H. What is the quantitative risk of gastric cancer in the first-degree relatives of patients? A meta-analysis. World J. Gastroenterol. 2017;23:2435–2442. doi: 10.3748/wjg.v23.i13.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vitelli-Storelli F., Rubin-Garcia M., Pelucchi C., Benavente Y., Bonzi R., Rota M., Palli D., Ferraroni M., Lunet N., Morais S., et al. Family History and Gastric Cancer Risk: A Pooled Investigation in the Stomach Cancer Pooling (STOP) Project Consortium. Cancers. 2021;13:3844. doi: 10.3390/cancers13153844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang M.S., Park M., Back J.H., Lee G.H., Shin J.H., Kim K., Seo H.J., Kim Y.A. Validation of Cancer Diagnosis Based on the National Health Insurance Service Database versus the National Cancer Registry Database in Korea. Cancer Res. Treat. 2022;54:352–361. doi: 10.4143/crt.2021.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim H.J., Kim Y.J., Seo S.I., Shin W.G., Park C.H. Impact of the timing of Helicobacter pylori eradication on the risk of development of metachronous lesions after treatment of early gastric cancer: A population-based cohort study. Gastrointest. Endosc. 2020;92:613–622.e611. doi: 10.1016/j.gie.2020.05.029. [DOI] [PubMed] [Google Scholar]
- 16.Gray R.J. A Class of K-Sample Tests for Comparing the Cumulative Incidence of a Competing Risk. Ann. Stat. 1988;16:1141–1154. doi: 10.1214/aos/1176350951. [DOI] [Google Scholar]
- 17.Fox J.G., Wang T.C. Inflammation, atrophy, and gastric cancer. J. Clin. Investig. 2007;117:60–69. doi: 10.1172/JCI30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karbalaei M., Keikha M. Statistical proof of Helicobacter pylori eradication in preventing metachronous gastric cancer after endoscopic resection in an East Asian population. World J. Gastrointest. Surg. 2022;14:867–873. doi: 10.4240/wjgs.v14.i8.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yanaoka K., Oka M., Ohata H., Yoshimura N., Deguchi H., Mukoubayashi C., Enomoto S., Inoue I., Iguchi M., Maekita T., et al. Eradication of Helicobacter pylori prevents cancer development in subjects with mild gastric atrophy identified by serum pepsinogen levels. Int. J. Cancer. 2009;125:2697–2703. doi: 10.1002/ijc.24591. [DOI] [PubMed] [Google Scholar]
- 20.Kato M., Hayashi Y., Nishida T., Oshita M., Nakanishi F., Yamaguchi S., Kitamura S., Nishihara A., Akasaka T., Ogiyama H., et al. Helicobacter pylori eradication prevents secondary gastric cancer in patients with mild-to-moderate atrophic gastritis. J. Gastroenterol. Hepatol. 2021;36:2083–2090. doi: 10.1111/jgh.15396. [DOI] [PubMed] [Google Scholar]
- 21.Kumar S., Metz D.C., Ellenberg S., Kaplan D.E., Goldberg D.S. Risk Factors and Incidence of Gastric Cancer After Detection of Helicobacter pylori Infection: A Large Cohort Study. Gastroenterology. 2020;158:527–536.e527. doi: 10.1053/j.gastro.2019.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leung W.K., Wong I.O.L., Cheung K.S., Yeung K.F., Chan E.W., Wong A.Y.S., Chen L., Wong I.C.K., Graham D.Y. Effects of Helicobacter pylori Treatment on Incidence of Gastric Cancer in Older Individuals. Gastroenterology. 2018;155:67–75. doi: 10.1053/j.gastro.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 23.Zhang T., Chen H., Zhang Y., Yin X., Man J., Yang X., Lu M. Global changing trends in incidence and mortality of gastric cancer by age and sex, 1990–2019: Findings from Global Burden of Disease Study. J. Cancer. 2021;12:6695–6705. doi: 10.7150/jca.62734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Choi I.J., Kim C.G., Lee J.Y., Kim Y.I., Kook M.C., Park B., Joo J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020;382:427–436. doi: 10.1056/NEJMoa1909666. [DOI] [PubMed] [Google Scholar]
- 25.Kim S.G., Jung H.K., Lee H.L., Jang J.Y., Lee H., Kim C.G., Shin W.G., Shin E.S., Lee Y.C., Korean College of Helicobacter and Upper Gastrointestinal Research Guidelines for the diagnosis and treatment of Helicobacter pylori infection in Korea, 2013 revised edition. J. Gastroenterol. Hepatol. 2014;29:1371–1386. doi: 10.1111/jgh.12607. [DOI] [PubMed] [Google Scholar]
- 26.Liou J.M., Lee Y.C., El-Omar E.M., Wu M.S. Efficacy and Long-Term Safety of H. pylori Eradication for Gastric Cancer Prevention. Cancers. 2019;11:593. doi: 10.3390/cancers11050593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The materials and data supporting the findings of the current study are available from the corresponding author upon reasonable request and IRB approval.