Abstract
Simple Summary
Minimally invasive liver resections are nowadays performed worldwide for both benign and malignant lesions. Good short-term and safe long-term outcomes have been reported. Despite this growing implementation of the technique, challenging scenarios and debated indications still exist. There is currently a lack of high-quality evidence regarding minimally invasive liver resections in portal hypertension, advanced cirrhosis, lesions in the posterosuperior segments and large and recurrent tumors.
Abstract
Laparoscopic liver resections (LLRs) have been increasingly adopted for the treatment of hepatocellular carcinoma (HCC), with safe short- and long-term outcomes reported worldwide. Despite this, lesions in the posterosuperior segments, large and recurrent tumors, portal hypertension, and advanced cirrhosis currently represent challenging scenarios in which the safety and efficacy of the laparoscopic approach are still controversial. In this systematic review, we pooled the available evidence on the short-term outcomes of LLRs for HCC in challenging clinical scenarios. All randomized and non-randomized studies reporting LLRs for HCC in the above-mentioned settings were included. The literature search was run in the Scopus, WoS, and Pubmed databases. Case reports, reviews, meta-analyses, studies including fewer than 10 patients, non-English language studies, and studies analyzing histology other than HCC were excluded. From 566 articles, 36 studies dated between 2006 and 2022 fulfilled the selection criteria and were included in the analysis. A total of 1859 patients were included, of whom 156 had advanced cirrhosis, 194 had portal hypertension, 436 had large HCCs, 477 had lesions located in the posterosuperior segments, and 596 had recurrent HCCs. Overall, the conversion rate ranged between 4.6% and 15.5%. Mortality and morbidity ranged between 0.0% and 5.1%, and 18.6% and 34.6%, respectively. Full results according to subgroups are described in the study. Advanced cirrhosis and portal hypertension, large and recurrent tumors, and lesions located in the posterosuperior segments are challenging clinical scenarios that should be carefully approached by laparoscopy. Safe short-term outcomes can be achieved provided experienced surgeons and high-volume centers.
Keywords: laparoscopic liver resection, hepatocellular carcinoma, advanced cirrhosis, portal hypertension, large HCC, posterosuperior segments, recurrent HCC
1. Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver tumor and the third leading cause of cancer-related deaths worldwide [1,2]. Whenever feasible, liver resection (LR) is one of the treatments of choice in very early and early-stage disease, offering long-term survivals exceeding 50% at 5 years [3]. Since 1992, when the first laparoscopic liver resection (LLR) was described, minimally invasive approaches have been increasingly employed for both benign and malignant liver diseases [4]. Indeed, despite the initial skepticism from the oncological point of view, nowadays, LLRs are considered safe for the treatment of malignant tumors and are widely adopted in experienced centers for colorectal liver metastases, intrahepatic cholangiocarcinomas, and HCCs [5]. Recent meta-analyses disclosed improved short- and comparable long-term outcomes of LLRs compared to open in the setting of HCC [6,7]. However, a variety of different patients and tumor presentations were included, eventually analyzing a heterogeneous population with different risk factors from both the perioperative and long-term standpoint.
Conditions such as advanced cirrhosis (AC), portal hypertension (PH), lesions located in the posterosuperior (PS) segments, large tumors, and recurrent HCCs represent unique clinical scenarios that require careful and specific considerations in the setting of minimally invasive approaches. Indeed, these conditions are associated with increased perioperative morbidity and mortality and were initially considered as contraindications to LLRs as recommended in the Southampton Consensus Guidelines for Laparoscopic liver surgery [8]. Despite this, experienced centers have been pushing the indications in these challenging scenarios, reporting safe outcomes both in the perioperative setting and long-term survivals [9,10,11]. Nevertheless, the evidence is still limited to small studies, which have been mostly singe center and retrospective in nature. This systematic review aimed to pool all the available literature regarding LLRs in these challenging scenarios and to summarize the evidence.
2. Material and Methods
2.1. Literature Search
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement guidelines were followed for conducting and reporting this systematic review. A systematic literature search was performed independently by two of the authors (E.M.M. and G.B.) using PubMed, WoS, and Scopus databases. The search was limited to studies in humans and published in English. Case reports, reviews, and meta-analyses were excluded. No restrictions were set for the date of publication. The search strategy was based on different combinations of words for each database. For the PubMed database, the following combination was used: (repeat hepatectomy OR recurrent HCC) AND (large HCC OR large hepatocellular carcinoma) AND (laparoscopic liver resections OR minimally invasive) AND (portal hypertension) AND (advanced liver cirrhosis) AND (posterosuperior segments). The same keywords were inserted in the search manager fields of Scopus. Extensive crosschecking of the reference lists of all retrieved articles that fulfilled the inclusion criteria further broadened the search. This systematic review was registered in the PROSPERO database with the number CRD42023396942.
2.2. Study Selection
The same two authors independently screened the titles and abstracts of the studies that were identified with the electronic search. Duplicate studies were excluded. The following criteria were set: (1) studies reporting laparoscopic liver resections for the above-mentioned indications; (2) studies reporting at least one perioperative outcome. The following exclusion criteria were set: (1) studies reporting non-laparoscopic liver resections, (2) studies not reporting separate outcomes for laparoscopic liver resections and (3) studies in which it was impossible to retrieve or calculate the data of interest. In the case of more than one report from the same center, only the most recent or the highest-quality study was included in the review. Advanced cirrhosis was defined as a Child–Pugh score of B or more [12]. Portal hypertension was defined as the presence of indirect signs of clinically significant portosystemic shunts (radiological or biochemical) or by a portosystemic gradient of more than 10 mmHg [13]. Segments VII, VIII, and IVa were considered posterosuperior [14]. A size of >5 cm was considered a large HCC [15].
2.3. Data Extraction
The same two authors extracted the main data as follows: (1) first author, study type, and subgroup; (2) number and characteristics of patients including Child–Pugh and/or MELD score; (3) intraoperative characteristics including the number of major/minor hepatectomies, anatomic or non-anatomic resections, operative time, blood loss, Pringle maneuver, conversion rates, and (4) postoperative outcomes including complications, Clavien–Dindo et al. [16] grade, liver-specific complications (bile leak, ascites, and liver failure) and mortality. Liver failure was defined according to the classification of International Study Group of Liver Surgery (ISGLS) [17] Major complications were defined as Clavien–Dindo > II. Relevant texts, tables, and figures were reviewed for data extraction, and whenever further information was required, the corresponding authors of the papers were contacted by e-mail. Discrepancies between the two reviewers were resolved by consensus discussion. Quality assessment was performed according to the Newcastle–Ottawa Scale (Table 1) [18].
Table 1.
Newcastle–Ottawa scale for quality assessment of the included studies.
| Study Authors | Selection | Comparability | Outcome | Total |
|---|---|---|---|---|
| Cipriani et al. [19] | *** | ** | *** | 8 |
| Troisi et al. [20] | **** | ** | *** | 9 |
| Cai et al. [21] | *** | ** | *** | 8 |
| Beard et al. [22] | **** | ** | *** | 9 |
| Lim et al. [23] | **** | ** | *** | 9 |
| Guo et al. [24] | **** | ** | ** | 8 |
| Molina et al. [25] | *** | ** | *** | 8 |
| Zheng et al. [26] | *** | ** | *** | 8 |
| Casellas et al. [27] | **** | ** | *** | 9 |
| Ruzzenente et al. [28] | **** | ** | *** | 9 |
| Kwon et al. [29] | *** | ** | ** | 7 |
| Chiang et al. [30] | *** | ** | *** | 8 |
| Fu et al. [31] | ** | ** | *** | 7 |
| Xu et al. [32] | *** | ** | *** | 8 |
| Xiang et al. [33] | **** | ** | *** | 9 |
| Levi Sandri et al. [34] | **** | ** | *** | 9 |
| Ai et al. [35] | **** | ** | *** | 9 |
| Casaccia et al. [36] | **** | ** | ** | 8 |
| Xiang et al. [37] | **** | ** | *** | 9 |
| Lee et al. [38] | *** | ** | ** | 7 |
| Tagaytay et al. [39] | *** | ** | *** | 8 |
| Kwon et al. [40] | *** | ** | *** | 8 |
| Yoon et al. [41] | **** | ** | ** | 8 |
| Xiao et al. [42] | **** | ** | *** | 9 |
| Cherqui et al. [43] | *** | ** | ** | 7 |
| Levi Sandri et al. [44] | **** | ** | *** | 9 |
| Liu et al. [45] | *** | ** | *** | 8 |
| Belli et al. [46] | ** | ** | *** | 7 |
| Goh et al. [47] | *** | ** | ** | 7 |
| Levi Sandri et al. [48] | **** | ** | *** | 9 |
| Gon et al. [49] | *** | ** | *** | 8 |
| Zhang et al. [50] | *** | ** | ** | 7 |
| Morise et al. [51] | *** | ** | ** | 7 |
| Kanazawa et al. [52] | *** | ** | * | 6 |
| Onoe et al. [53] | *** | ** | *** | 8 |
| Miyama et al. [54] | *** | ** | *** | 8 |
Each * counts as 1 point.
3. Results
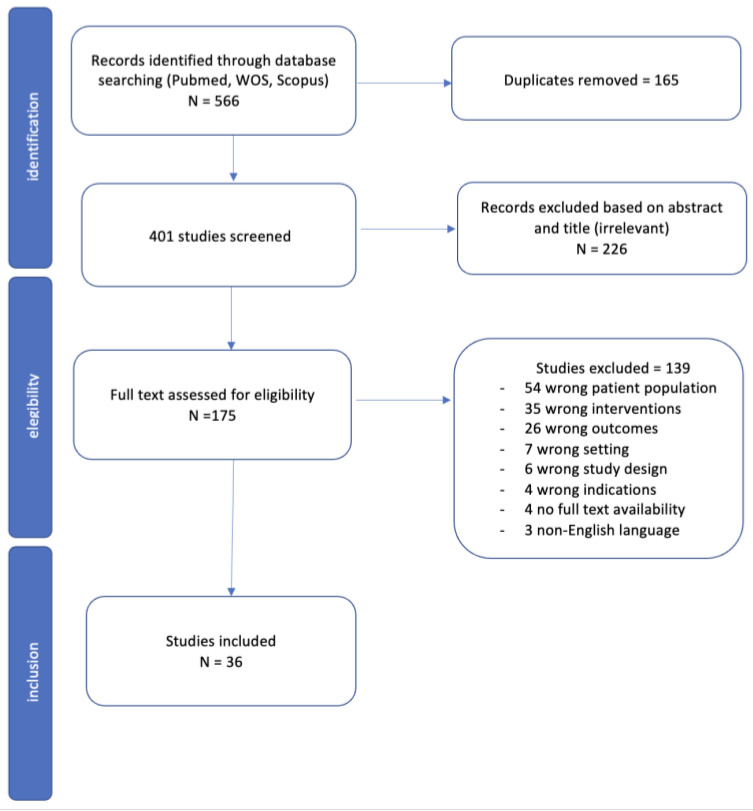
The literature search yielded 566 articles; after duplicate removal, 401 titles and abstracts were reviewed (Figure 1). Of these, 226 papers were excluded based on abstract and title; 175 articles were assessed for eligibility and full text screened. Of these, 139 articles were excluded. Finally, a total of 36 articles dated between 2006 and 2022 fulfilled the selection criteria and were included in this systematic review [19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. There was no disagreement between the authors regarding eligibility. The articles consisted of 33 retrospective and three prospective reports, gathering a total of 1859 patients. Characteristics of the included studies are summarized in Table 2.
Figure 1.
Prisma flow diagram.
Table 2.
Baseline characteristics of the included studies.
| First Author | Subgroup | Country | Type of Study | No. of Patients | Age | Gender M/F | Child–Pugh A/B/C | MELD Score |
|---|---|---|---|---|---|---|---|---|
| Cipriani et al. [19] | Advanced cirrhosis | Italy | Retro | 25 | 66 (23–88) | 14/11 | 0/25/0 | NR |
| Troisi et al. [20] | Advanced cirrhosis | Italy | Retro | 100 | 68 (27–84) | 75/25 | 0/100/0 | 9 (4–22) |
| Cai et al. [21] | Advanced cirrhosis | China | Retro | 5 | 60 (27–79) | 5/0 | 0/5/0 | NR |
| Beard et al. [22] | Advanced cirrhosis | USA | Retro | 26 | 60.5 (49–77) | 22/4 | 0/20/6 | NR |
| Lim et al. [23] | Portal hypertension | France | Prosp | 18 | 64 (52–83) | 11/7 | 18/0/0 | 8 (6–11) |
| Guo et al. [24] | Portal hypertension | China | Retro | 16 | 50 (29–70) | 9/7 | 12/4/0 | NR |
| Molina et al. [25] | Portal hypertension | Spain | Retro | 16 | 64 (50–75) | 11/5 | 16/0/0 | NR |
| Zheng et al. [26] | Portal hypertension | China | Retro | 24 | 58.5 (54–68) | 21/3 | 18/6/0 | NR |
| Casellas et al. [27] | Portal hypertension | Spain | Retro | 31 | 64 ± 8 * | 20/11 | 31/0/0 | NR |
| Ruzzenente et al. [28] | Portal hypertension | Italy | Retro | 89 | NR | 67/22 | 67/19/3 | NR |
| Kwon et al. [29] | Large HCC | Republic of Korea | Retro | 20 | 56.1 ± 12.6 * | 16/4 | NR | NR |
| Chiang et al. [30] | Large HCC | Taiwan | Retro | 37 | 58 ± 11.7 * | 30/7 | 36/1/0 | NR |
| Fu et al. [31] | Large HCC | China | Retro | 14 | 61.5 (28–77) | 10/4 | NR | 6 (6–7) |
| Xu et al. [32] | Large HCC | China | Retro | 102 | 52.5 (25–80) | 80/22 | NR | NR |
| Xiang et al. [33] | Large HCC | China | Prosp | 128 | 51 ± 11.9 * | 109/19 | 108/20/0 | NR |
| Levi Sandri et al. [34] | Large HCC | Italy | Retro | 38 | 71 (61–77) | 25/13 | 38/0/0 | 7 (6–8) |
| Ai et al. [35] | Large HCC | China | Retro | 97 | 52 (14–77) | 75/22 | 59/38/0 | NR |
| Casaccia et al. [36] | Posterosuperior segments | Italy | Retro | 22 | 66 (47–76) | 13/9 | 19/3/0 | NR |
| Xiang et al. [37] | Posterosuperior segments | China | Retro | 56 | 51.6 ± 10.2 | 47/9 | NR | NR |
| Lee et al. [38] | Posterosuperior segments | Republic of Korea | Retro | 58 | 56 (33–74) | 37/21 | 56/2/0 | NR |
| Tagaytay et al. [39] | Posterosuperior segments | Republic of Korea | Retro | 37 | 60 ± 10.58 * | 28/9 | NR | NR |
| Kwon et al. [40] | Posterosuperior segments | Republic of Korea | Retro | 149 | 57 ± 10.4 * | 115/34 | 146/1/2 | NR |
| Yoon et al. [41] | Posterosuperior segments | Republic of Korea | Retro | 25 | 53 ± 10 * | 14/11 | 23/2/0 | NR |
| Xiao et al. [42] | Posterosuperior segments | China | Retro | 41 | 52 ± 11.62 * | 34/7 | 39/2/0 | NR |
| Cherqui et al. [43] | Posterosuperior segments | France | Retro | 27 | 63 (40–76) | 22/5 | 27/0/0 | NR |
| Levi Sandri et al. [44] | Posterosuperior segments | Italy | Retro | 62 | 71 (59.5–75) | 50/12 | 62/0/0 | 7 (6–8) |
| Liu et al. [45] | Recurrent HCC | China | Retro | 30 | 56.5 (27–79) | 23/7 | 30/0/0 | NR |
| Belli et al. [46] | Recurrent HCC | Italy | Retro | 15 | 68 (58–75) | NR | 15/0/0 | NR |
| Goh et al. [47] | Recurrent HCC | Singapore | Retro | 20 | 68.5 (67–71) | 18/2 | NR | NR |
| Levi Sandri et al. [48] | Recurrent HCC | Italy | Retro | 74 | 72 (65–76) | 55/19 | 66/8/0 | 7 (7–9) |
| Gon et al. [49] | Recurrent HCC | Japan | Retro | 23 | 72 (67–79) | 18/5 | 23/0/0 | NR |
| Zhang et al. [50] | Recurrent HCC | China | Prosp | 31 | 54 (37–66) | 26/5 | NR | NR |
| Morise et al. [51] | Recurrent HCC | Japan | Retro | 238 | 67 ± 11.8 * | 181/57 | NR | NR |
| Kanazawa et al. [52] | Recurrent HCC | Japan | Retro | 20 | 70 (46–83) | 15/5 | 19/1/0 | NR |
| Onoe et al. [53] | Recurrent HCC | Japan | Retro | 30 | 71(50–85) | 23/7 | 30/0/0 | 5 (4–13) |
| Miyama et al. [54] | Recurrent HCC | Japan | Retro | 115 | 68 ± 10.8 * | 91/24 | NR | NR |
Data are expressed as median (min; max). NR, not reported. HCC, hepatocellular carcinoma. Retro, retrospective. Prosp, prospective. * Data expressed as mean ± standard deviation.
3.1. Advanced Cirrhosis
Four studies were included in the subgroup of LLRs in patients with advanced cirrhosis gathering a total of 156 patients, of whom 116 (74.4%) were male and 40 (25.6%) were female (Table 2). Median age ranged between 60 (27–79) and 68 (27–84). One-hundred and fifty patients (96.1%) were scored as Child–Pugh B and 6 (3.9%) as Child–Pugh C with a MELD score of 9 (4–22) that was reported only in one study [20]. Three studies reported the number of minor/major hepatectomies and anatomic/non-anatomic resections (Table 3). Minor hepatectomies were more frequently performed (117/131, 89.3%) as compared to major hepatectomies (14/131, 10.7%). Non-anatomic resections were performed in 74/131 (56.5%) cases, while anatomic hepatectomies were carried out in 57/131 (43.5%). Only one study described tumor localization (62% anterolateral and 38% posterosuperior segments) [20]. Operative time ranged between 99 (43–354) and 235 (84–605) minutes, while blood loss was between 50 (10–4750) and 800 (240–1000) mL (Table 3). Concerning hilar clamping, no Pringle maneuver was used in two studies, while 63/156 (40.4%) of the hepatectomies were performed under clamping among the remaining studies. Overall, 8/151 (5.3%) patients required intraoperative blood transfusions. Thirteen cases (8.3%) were converted to open. Concerning postoperative outcomes, 54 (34.6%) patients developed postoperative complications, of which 44 (81.5%) were minor and 10 (18.5%) were major (Table 4). Liver-specific morbidity was observed in 34 (21.8%) cases, with 3 (1.9%) patients experiencing liver failure, 29 (18.6%) patients experiencing ascites, and 2 (1.3%) patients experiencing bile leaks. Median hospital stay ranged between 2 (1–19) and 10 (7–15) days. Eight (5.1%) patients died within 90 days of surgery (Table 4).
Table 3.
Intraoperative characteristics of the included studies.
| First Author | Subgroup | Type of Hepatectomy Major/Minor |
Type of Resection Non-Anatomic/Anatomic |
Operative Time (min) | Pringle n (%) |
Conversion n (%) |
Blood Loss (mL) | Intraoperative Transfusions n (%) |
|---|---|---|---|---|---|---|---|---|
| Cipriani et al. [19] | Advanced cirrhosis | NR | NR | 210 (120–280) | 7 (28%) | 4 (16%) | 350 (200–1000) | 3 (12%) |
| Troisi et al. [20] | Advanced cirrhosis | 14/86 | 51/49 | 235 (84–605) | 56 (56%) | 6 (6%) | 110 (0–3270) | 1 (1-4) |
| Cai et al. [21] | Advanced cirrhosis | 0/5 | 5/0 | 135 (80–170) | 0 | 2 (40%) | 800 (240–1000) | NR |
| Beard et al. [22] | Advanced cirrhosis | 0/26 | 18/8 | 99 (43–354) | 0 | 1 (4%) | 50 (10–4750) | 4 (15%) |
| Lim et al. [23] | Portal hypertension | 2/16 | 12/6 | 240 (100–360) | NR | 2 (11%) | 300 (20–1700) | 0 (0%) |
| Guo et al. [24] | Portal hypertension | 0/16 | 16/0 | 336 ± 18 * | NR | NR | 337 ± 351 * | NR |
| Molina et al. [25] | Portal hypertension | 0/15 | 4/12 | 150 (90–215) | 6 (40) | 3 (20%) | 90 (80–1000) | 1 (7%) |
| Zheng et al. [26] | Portal hypertension | 12/12 | 15/9 | 180 (150–250) | 12 (50%) | 2 (8.3%) | 200 (100–400) | 5 (21%) |
| Casellas et al. [27] | Portal hypertension | 1/30 | 17/14 | 280 (202–338) | 25 (81%) | 1 (3%) | 415 (200–731) | 1 (3%) |
| Ruzzenente et al. [28] | Portal hypertension | 14/75 | NR | NR | NR | NR | NR | NR |
| Kwon et al. [29] | Large HCC | 11/9 | 1/19 | 358.8 ± 136 * | 0 (0%) | 2 (10%) | 600 (NR) | 5 (25%) |
| Chiang et al. [30] | Large HCC | 19/18 | 4/33 | 232 ± 91.2 * | NR | 1 (2.7%) | 623 ± 841.75 * | NR |
| Fu et al. [31] | Large HCC | 0/14 | 0/14 | 195 (90–390) | NR | 1 (7%) | 50 (10–1200) | 13 (93%) |
| Xu et al. [32] | Large HCC | 28/74 | 51/51 | 217.5 (55–470) | 50 (0–115) ** | 3 (3%) | 175 (10–1000) | 3 (3%) |
| Xiang et al. [33] | Large HCC | 28/100 | 70/58 | 234 (105–501) | NR | 12 (9.4%) | 456 (50–2000) | 23 (18%) |
| Levi Sandri et al. [34] | Large HCC | 12/26 | 9/29 | 225 (159–270) | 10 (26%) | 7 (18.4%) | 300 (75–800) | NR |
| Ai et al. [35] | Large HCC | 5/92 | 24/73 | 245 ± 105 * | NR | 9 (9%) | 460 ± 426 * | 5 (4.5%) |
| Casaccia et al. [36] | Posterosuperior segments | 0/22 | 15/7 | 300 (120–560) | 1 (4.5%) | 1 (4.5%) | 55 (20–1400) | 10 (45.4%) |
| Xiang et al. [37] | Posterosuperior segments | 14/42 | 31/25 | 217.5 ± 63.7 * | NR | 10 (17.9%) | 295 ± 187 * | 9 (16.1%) |
| Lee et al. [38] | Posterosuperior segments | 8/50 | 16/42 | 355 (165–930) | NR | 8 (13.8%) | 600 (130–14,300) | NR |
| Tagaytay et al. [39] | Posterosuperior segments | 0/37 | 25/12 | 215 ± 70 * | NR | 1 (2.7%) | 201 ± 254 * | 1 (1.8%) |
| Kwon et al. [40] | Posterosuperior segments | 28/121 | 73/76 | 362 ± 180.7 * | 60 (40%) | 28 (19%) | 1376 ± 2509 * | 22 (15%) |
| Yoon et al. [41] | Posterosuperior segments | 6/19 | 7/18 | 347 ± 117.9 * | NR | 4 (16%) | 986 ± 920.8 * | 10 (40%) |
| Xiao et al. [42] | Posterosuperior segments | 6/35 | 7/34 | 242 ± 73.6 * | NR | 3 (7.3%) | 272 ± 170 * | 3 (7.3%) |
| Cherqui et al. [43] | Posterosuperior segments | 1/26 | 10/17 | 240 (150–360) | NR | 7 (26%) | 338 ± 182 * | 3 (15%) |
| Levi Sandri et al. [44] | Posterosuperior segments | 12/50 | 32/30 | 240 (172–300) | 32 (18–45) ** | 12 (18%) | 200 (50–300) | 5 (8%) |
| Liu et al. [45] | Recurrent HCC | 1/29 | 19/11 | 200.5 (68–525) | 0 | 4 (13.3%) | 100 (10–600) | 0 (0%) |
| Belli et al. [46] | Recurrent HCC | 0/15 | 7/8 | 84 (40–130) | 9 | 1 (6.6%) | NR | NR |
| Goh et al. [47] | Recurrent HCC | 2/18 | 0/20 | 315 (181–395) | 4 (20%) | 3 (15%) | 200 (100–450) | 2 (10%) |
| Levi Sandri et al. [48] | Recurrent HCC | 5/69 | 47/27 | 210 (150–300) | NR | 9 (12.1%) | 100 (50–225) | 5 (6.7%) |
| Gon et al. [49] | Recurrent HCC | 0/23 | 21/2 | 286 (251–417) | NR | 1 (4%) | 10 (10–50) | 0 (0%) |
| Zhang et al. [50] | Recurrent HCC | 0/31 | 19/12 | 116 ± 37.5 * | NR | 0 (0%) | 117.5 ± 35.5 * | NR |
| Morise et al. [51] | Recurrent HCC | 9/229 | NR | 272 ± 187 * | NR | 0 (0%) | 268 ± 730 * | 22 (9%) |
| Kanazawa et al. [52] | Recurrent HCC | 0/20 | NR | 239 (69–658) | NR | 2 (10%) | 78 (1–1500) | 0 (0%) |
| Onoe et al. [53] | Recurrent HCC | 0/30 | 27/3 | 276 (125–589) | NR | 2 (6.75%) | 100 (0–1050) | NR |
| Miyama et al. [54] | Recurrent HCC | 1/114 | 108/7 | 260 ± 158 * | NR | NR | 283 ± 823 * | 12 (10%) |
Data are expressed as median (min–max). NR, not reported. HCC, hepatocellular carcinoma. * Data expressed as mean ± standard deviation. ** Mean time of clamping.
Table 4.
Post operative outcomes of the included studies.
| First Author | Subgroup | Morbidity n (%) |
CD 0-II n (%) |
CD III-IV n (%) |
Liver Failure n (%) |
Ascites n (%) |
Bile Leak n (%) |
Mortality n (%) |
|---|---|---|---|---|---|---|---|---|
| Cipriani et al. [19] | Advanced cirrhosis | 9 (36%) | 7 (78%) | 2 (22%) | 1 (4%) | 3 (12%) | 1 (4%) | 4 (16%) |
| Troisi et al. [20] | Advanced cirrhosis | 38 (38%) | 31 (81.5%) | 7 (18.5%) | 2 (5%) | 26 (68.4%) | 1 (3%) | 2 (2%) |
| Cai et al. [21] | Advanced cirrhosis | 1 (20%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Beard et al. [22] | Advanced cirrhosis | 6 (23%) | 5 (19%) | 1 (4%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (8%) |
| Lim et al. [23] | Portal hypertension | 7 (39%) | 7 (100%) | 0 (0%) | 2 (28.5%) | 2 (28.5%) | 0 (0%) | 0 (0%) |
| Guo et al. [24] | Portal hypertension | 6 (37.5%) | NR | NR | NR | NR | NR | 0 (0%) |
| Molina et al. [25] | Portal hypertension | 6 (40%) | 4 (67%) | 2 (33%) | 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) |
| Zheng et al. [26] | Portal hypertension | 8 (33%) | 5 (62.5%) | 3 (37.5%) | 1 (12.5%) | 2 (25%) | 0 (0%) | 0 (0%) |
| Casellas et al. [27] | Portal hypertension | 16 (52%) | 14 (93%) | 2 (7%) | 3 (19%) | 5 (31%) | 0 (0%) | 0 (0%) |
| Ruzzenente et al. [28] | Portal hypertension | 26 (29%) | 15 (57%) | 11 (42%) | NR | NR | NR | 0 (0%) |
| Kwon et al. [29] | Large HCC | 3 (15%) | 3 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Chiang et al. [30] | Large HCC | 7 (18.9%) | 6 (85%) | 1 (15%) | 1 (14%) | 3 (43%) | 1 (14%) | 0 (0%) |
| Fu et al. [31] | Large HCC | 1 (7%) | 1 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Xu et al. [32] | Large HCC | 20 (19%) | 15 (75%) | 5 (25%) | 4 (20%) | 5 (25%) | 4 (20%) | 0 (0%) |
| Xiang et al. [33] | Large HCC | 26 (20.3%) | 13 (50%) | 13 (50%) | 2 (7.7%) | 0 (0%) | 0 (0%) | 1 (0.78%) |
| Levi Sandri et al. [34] | Large HCC | 20 (52%) | 17 (85%) | 3 (15%) | 0 (0%) | NR | NR | 0 (0%) |
| Ai et al. [35] | Large HCC | 10 (10%) | 10 (100%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Casaccia et al. [36] | Posterosuperior segments | 4 (18%) | 4 (100%) | 0 (0%) | NR | NR | NR | 0 (0%) |
| Xiang et al. [37] | Posterosuperior segments | 9 (16.1%) | 9 (100%) | 0 (0%) | 0 (0%) | 1 (11%) | 1 (11%) | 0 (0%) |
| Lee et al. [38] | Posterosuperior segments | 10 (17.2%) | 4 (40%) | 6 (60%) | NR | NR | NR | 0 (0%) |
| Tagaytay et al. [39] | Posterosuperior segments | 3 (8.1%) | 2 (67%) | 1 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Kwon et al. [40] | Posterosuperior segments | 28 (19%) | 14 (50%) | 14 (50%) | NR | NR | NR | 0 (0%) |
| Yoon et al. [41] | Posterosuperior segments | 7 (28%) | 7 (100%) | 0 (0%) | NR | NR | NR | 0 (0%) |
| Xiao et al. [42] | Posterosuperior segments | 7 (17%) | 5 (71%) | 2 (29%) | 0 (0%) | 1 (14%) | 1 (14%) | 0 (0%) |
| Cherqui et al. [43] | Posterosuperior segments | 9 (33%) | NR | NR | 1 (4%) | 2 (7%) | 0 (0%) | 0 (0%) |
| Levi Sandri et al. [44] | Posterosuperior segments | NR | NR | NR | NR | NR | NR | 0 (0%) |
| Liu et al. [45] | Recurrent HCC | 2 (6.7%) | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 1 (50%) | 0 (0%) |
| Belli et al. [46] | Recurrent HCC | 4 (26.6%) | 4 (100%) | 0 (0%) | 0 (0%) | 1 (25%) | 0 (0%) | 0 (0%) |
| Goh et al. [47] | Recurrent HCC | 2 (10%) | 2 (100%) | 0 (0%) | NR | NR | NR | 0 (0%) |
| Levi Sandri et al. [48] | Recurrent HCC | 17 (22.9%) | 5 (29%) | 12 (71%) | NR | 3 (3.7%) | 1 (1.7%) | 0 (0%) |
| Gon et al. [49] | Recurrent HCC | 2 (9%) | 1 (50%) | 1 (50%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Zhang et al. [50] | Recurrent HCC | NR | NR | 0 (0%) | NR | NR | NR | 1 (3%) |
| Morise et al. [51] | Recurrent HCC | 36 (15%) | 7 (19%) | 29 (81%) | 2 (5.5%) | 5 (14%) | 15 (42%) | 1 (0.4%) |
| Kanazawa et al. [52] | Recurrent HCC | 1 (5%) | 0 (0%) | 1 (100%) | 0 (0%) | 1 (100%) | 0 (0%) | 0 (0%) |
| Onoe et al. [53] | Recurrent HCC | 30 (100%) | 28 (93.3%) | 2 (6.7%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| Miyama et al. [54] | Recurrent HCC | 15 (13%) | 10 (67%) | 5 (22%) | NR | NR | NR | 0 (0%) |
Data are expressed as median (range). NR, not reported. HCC, hepatocellular carcinoma. CD, Clavien–Dindo.
Comparative Results between Open vs. Minimally Invasive Surgery in Advanced Cirrhosis
Only Troisi et al. compared open vs. laparoscopic surgery in advanced cirrhosis. All patients were scored as Child–Pugh B. Laparoscopy was associated with lower blood loss (median 110 mL versus 400 mL in the open group; p = 0.004), lower morbidity (38% vs. 51%; p = 0.041) and fewer major complications (7% vs. 21%; p = 0.010) [20].
3.2. Portal Hypertension
Six studies were included in the subgroup of LLRs in patients with portal hypertension with a total of 194 patients, 139 (71.6%) male and 55 (28.4%) female with a median age between 50 (29–70) and 64 (52–83). One-hundred and sixty-two (83.5%) patients were scored as Child–Pugh A, 29 (14.9%) were scored as Child–Pugh B, and 3 (1.5%) were scored as Child–Pugh C, with a MELD score of 8 (6–11) that was reported only in one study [23] (Table 2). Tumor size ranged between 2.0 (1.1–5.7) and 3.3 (2.0–4.8) cm. The majority of patients underwent a minor hepatectomy (165, 85.1%), while major hepatectomies were performed in 29 (14.9%) cases. Non-anatomic resections were conducted in 64/105 (61.0%) patients, while 41/105 (39.0%) underwent an anatomical hepatectomy (Table 3). Only one study reported tumor’s location (77% anterolateral and 23% posterosuperior segments) [27]. Operative time ranged between 150 (90–215) and 336 ± 18 min. Blood loss ranged between 90 (80–1000) and 415 (200–731) mL. Pringle maneuver was performed in 43/71 cases (60.6%). Intraoperative blood transfusions were needed in 7/89 patients (7.9%). Conversion to open happened in 8/89 (9.0%) cases (Table 3). Regarding postoperative outcomes, 69 (35.5%) patients developed postoperative complications of which 45 (71.4%) were minor and 18 (28.6%) were major. Liver-specific morbidity was reported in 16/89 (17.9%) cases with 89 (6.7%) patients developing liver failure and 10/89 (11.2%) experiencing ascites. Hospital stays ranged between 3 (2–20) and 13.5 (9–24) days. In this subgroup, neither bile leak nor 90-day mortality was observed (Table 4).
Comparative Results between Open vs. Minimally Invasive Surgery in Portal Hypertension
Only Ruzzenente et al. reported comparative results between laparoscopic and open surgery in portal hypertension. They found that patients undergoing laparoscopic approach had shorter hospital stay (>7 days: open 55% vs. laparoscopic 29%, p < 0.001) as well as lower morbidity (open: 42% vs. laparoscopic: 29%, p = 0.001) [28].
3.3. Large HCC
Seven studies were included in the subgroup of patients with large HCC, with a total of 436 individuals, 345 (79.1%) males, and 91 (20.9%) females. Age ranged between 51 ± 11.9 and 71 (61–77). Two hundred and forty-one patients (80.3%) were scored as Child–Pugh A, and 59/300 (19.7%) were scored as Child–Pugh B (Table 2). No Child–Pugh C patients were reported in this subgroup. Tumor size ranged between 6 (5.5–10) and 7.8 ± 2.15 cm. Only one study described tumor locations (73 (71.56%) anterolateral and 29 (28.44%) posterosuperior segments) [32]. Three hundred and thirty-three patients (76.4%) underwent a minor hepatectomy, while 103 (23.6%) were submitted to a major resection. Anatomic hepatectomies were performed in 277 patients (63.5%) (Table 3). Operative time ranged between 195 (90–390) and 358 ± 136 min. Pringle maneuver was applied in 10/58 (17.2%) cases. Blood loss ranged between 50 (10–1200) and 623 ± 841.7 mL. Forty-nine (13.6%) patients required intraoperative blood transfusions. Thirty five (8.0%) cases were converted to laparotomy (Table 3). Only one study described the reason for conversion (four cases for uncontrollable bleeding, two cases for oncological safety and three cases for tumor encroaching on the diaphragmatic muscle) [35]. Regarding postoperative outcomes, 87 (19.9%) patients developed complications, of which 65 (74.7%) were minor and 22 (25.3%) were major (Table 4). Liver-specific morbidity was observed in 20/398 (5.0%) cases, with 7/436 patients (1.6%) developing liver failure, 8/398 (2.0%) developing ascites, and 5/398 (1.3%) developing bile leak. Median hospital stay ranged between 6 (4–8) and 11.4 ± 3.1 days. One patient (0.2%) died within 90 days of surgery.
Comparative Results between Open vs. Minimally Invasive Surgery in Large HCC
Four studies compared the postoperative results of open vs. laparoscopic surgery [30,31,33,35] in the setting of large lesions. All of them showed shorter hospital stay in the laparoscopic group. Xiang et al. and Ai et al. showed lower rates of postoperative complications in the laparoscopic group [33,35]. Chiang et al. and Fu et al. found a lower blood loss [30,31]. No differences were found in terms of postoperative mortality.
3.4. Posterosuperior Segments
Nine studies were included in the subgroup of LLRs in patients with HCC located in the posterosuperior segments with a total of 477 patients, 360 (75.5%) male and 117 (24.5%) female with an age ranging between 51.6 ± 10.2 and 71 (59.5–75) (Table 2). Three hundred and seventy-two patients (96.9%) were scored as Child–Pugh A, 10/384 (2.6%) were scored as Child–Pugh B and 2/384 (0.5%) were scored as Child–Pugh C. Tumor size ranged between 2.31 ± 0.78 and 4.22 ± 2.05 cm. Major hepatectomies were performed in 75 (15.7%) cases, while 402 (84.3%) underwent a minor resection. In 216 (45.2%) cases, a non-anatomic resection was performed as compared to 261 (54.8%) in which the resection was anatomic (Table 3). Operative time ranged between 215 ± 70 and 362 ± 180.7 min. Pringle maneuver was applied in 61/171 (35.7%) cases. Blood loss ranged between 55 (20–1400) and 1376 ± 2509 mL. Sixty-three (15.0%) patients required an intraoperative blood transfusion, and conversion to open was necessary in 15.5% of cases (Table 3). Regarding postoperative outcomes, 77/415 (18.6%) patients had complications of which 45 (66.2%) were minor and 23 (33.8%) were major. Liver-specific morbidity was observed in 7/161 (4.3%) cases, with 1/161 (0.6%) patient developing liver failure, 4/161 (2.4%) experiencing ascites, and 2 (1.2%) experiencing bile leaks (Table 4). Hospital stay ranged between 5 (3–7) and 10.5 ± 2.7 days. Mortality at 90 days was 0%.
Comparative Results between Open vs. Minimally Invasive Surgery for Lesions Located in Posterosuperior Segments
Three studies compare the results of laparoscopic and open surgery for HCC located in posterosuperior segments [39,40,42]. All of the studies showed a lower morbidity rate and shorter hospital stay in the laparoscopic group. Only Tagaytay et al. found lower blood loss (218.11 vs. 358.92 mL, p = 0.046) and shorter operative time (7.03 vs. 11.78 days, p = 0.001) in the laparoscopic group. No differences were found in terms of 90-day mortality.
3.5. Recurrent HCC
Ten studies were included in the subgroup of repeat LLRs in patients with recurrent HCC with a total of 596 patients, 450 (77.4%) male and 131 (22.6%) female with a median age between 54 (37–66) and 72 (67–79). One hundred and eighty-three patients (95.3%) were Child–Pugh A, and 9/192 (4.7%) were Child–Pugh B. No Child–Pugh C patients were reported in this subgroup (Table 2). Tumor size ranged between 1.25 (0.8–3.5) and 3.8 (3.3–4.5) cm. Only two studies reported on the location of tumors (254 (71.95%) anterior segments and 99 (28.05%) posterior segments) [51,54]. Minor hepatectomies were performed in the vast majority of cases (578, 97.0%) and 248/338 (73.4%) patients underwent a non-anatomic resection. The median time interval from the first operation ranged between 3.9 (0.2–16) and 32 (3–136) months. In 318 (77%) cases, the first operation was performed by open and in 95 (23%), it was performed by laparoscopy. The site of recurrence was described only in two studies and was shown to be ipsilateral in 40 (65.5%) cases and controlateral in 21 (34.5%) cases [49,50]. Operative time ranged between 84 (40–130) and 315 (181–395) min (Table 3). Pringle maneuver was applied in 13/65 (0.2%) cases, which was probably due to difficult surgical anatomy because of re-operation. Blood loss ranged between 10 (10–50) and 283 ± 823 mL, and 41/520 (7.9%) patients required intraoperative blood transfusions. Conversion to laparotomy happened in 22/481 (4.6%). Regarding postoperative outcomes, 109/565 (19.3%) patients developed complications of which 58 (53.3%) were minor and 51 (46.7%) were major (Table 4). Two patients (0.5%) experienced liver failure, 10 (2.3%) developed ascites, and 17 (3.9%) developed a bile leak. The hospital stay ranged between 4 (3–5) and 11.7 ± 11.5 days. Two patients died within 90 days from surgery with a mortality rate of 0.34%.
Comparative Results between Open vs. Minimally Invasive Surgery for Recurrent HCC
Eight studies compared the results of laparoscopic vs. open surgery for recurrent HCCs [45,47,49,50,51,52,53,54]. All of them showed a shorter hospital stay in the laparoscopic group. The majority found lower blood loss [45,49,50,51,53] and only three studies reported lower postoperative morbidity rate in the laparoscopic group [45,52,54]. Concerning operative time, Morise et al. and Goh et al. reported longer operative time, while Zhang et al. reported shorter operative time in the laparoscopic group [47,50,51]. Gon et al. showed shorter operative time in the laparoscopic group only if the recurrent HCC was located in the controlateral parenhcyma from the previous resection [49]. No statistically significant differences in 90-day mortality was observed.
4. Discussion
Despite the recent advances in surgical techniques and the widespread adoption of minimally invasive approaches for liver resections, patients with advanced cirrhosis, portal hypertension, large and recurrent lesions, and tumors located in the posterosuperior segments still represent a challenge even in the most experienced hands. Indeed, perioperative complications in the above-mentioned settings are potentially high, and long-term outcomes are still under investigation [15]. Careful preoperative evaluation and assessment of potential risk factors is key to guide a thorough discussion of potential risks and benefits, thereby selecting patients and minimizing unexpected events.
Patients with advanced cirrhosis and portal hypertension represent one of the most difficult clinical scenarios in the management of HCC [3]. Indeed, these patients may present with impaired performance status, sarcopenia, encephalopathy, ascites, and severe portosystemic shunts. Therapeutic alternatives such as liver transplantation and locoregional options might come into play, but many patients still undergo resection. The decision of whether to operate on patients with such advanced conditions represents a dilemma. Perioperative risks are high, with increased rates of postoperative morbidity, especially liver failure and ascites [17,55,56]. In this setting, minimally invasive approaches could be beneficial to improve postoperative outcomes [7,9,21]. Indeed, the abdominal cavity is respected as compared to a large open incision, avoiding the interruption of portosystemic shunts, manipulation of the liver is reduced, and the abdominal cavity is not exposed to the air, thus avoiding electrolyte imbalances [57]. However, the LLRs in such patients are technically more challenging. Adhesions are well vascularized, there is an increased bleeding during the transection, and the parenchyma is stiff, thus limiting exposure. According to our review, only four papers have been reported describing LLRs on AC, thus limiting the evidence in this setting. Furthermore, most patients with advanced cirrhosis were scored as Child–Pugh B, while only six patients were scored as C. The literature on liver resection in Child–Pugh C patients is limited both in open and laparoscopic surgery because of the questionable postoperative outcomes [15]. In our opinion, therapeutic alternatives should be well discussed in such patients, as no sufficient data are available so far to support resection, especially in laparoscopy. Although minor and non-anatomical resections were more frequent in these subgroups, intraoperative blood loss was high, the Pringle maneuver was frequently applied (40.4% in AC and 60.6% in PH), and conversion rates were high (8.3% in AC and 9.0% in PH), confirming the technical complexity of these procedures. Despite the potential advantages of the minimally invasive approach, according to our review, AC and PH had the highest rates of morbidity, especially postoperative liver failure (up to 6.7% in PH), ascites production (up to 18.6% in AC) and the highest chance of dying after surgery (5.1% mortality in AC). This confirms that the presence of clinically significant portal hypertension and advanced cirrhosis are important prognostic factors for worse postoperative outcomes, especially in terms of liver decompensation surrogates. For this reason, these very high-risk patients, when considered for surgery, should be managed by experienced surgeons in high volume centers and should be well selected to improve the outcomes.
Large HCCs represent another common surgical dilemma to approach by laparoscopy. These lesions frequently require major hepatectomies and/or anatomic resections. The dissection of the hilar structures, the large parenchymal transection, the major vasculobiliary structures encountered and the extensive mobilizations require specific learning curve, as each of these steps have specific technical challenges [8,58,59]. This is enhanced when dealing with large lesions, since exposure and mobilization are further limited [60]. Notwithstanding, perioperative outcomes were good with no major blood loss or high rates of conversions to open, and only 20% of patients were developing postoperative morbidity, mostly minor in severity. A cutoff of 5 cm was applied by most of the included studies to define large lesions [29,30,31,32,33,34,35]. Together with the dimensions of the tumor that should be further categorized, we also believe that localization of the lesion should be considered in future studies, as perioperative outcomes could be very different between a lesion located close to the hilum or at the periphery. Dimensions and localization would therefore allow for a more precise selection of patients, thereby improving outcomes.
Posterosuperior segments were initially considered as a contraindication to the laparoscopic approach, being defined as the non-laparoscopic segments [61]. Thanks to the widespread adoption of minimally invasive approaches and to the learning curves, nowadays, lesions in the PS segments are frequently approached by laparoscopy, with good short and long-term outcomes for both benign and malignant lesions [62,63]. However, few reports on HCCs in the PS segments exist, as this still represents a challenging indication, especially in cirrhotic patients. According to our review, intraoperative and postoperative outcomes were good, with a morbidity rate as high as 18.6%, thereby disclosing the safety and efficacy of such approach. However, conversion to open was high (15.5%) as was the need for Pringle maneuver (36%), again stressing the technical complexity and thereby confirming the need for advanced technical skills.
Despite the good long-term outcomes of liver resections for HCC, as much as 70% of patients will experience recurrence of their tumor [3,64]. Salvage liver transplantation, for those eligible, represents a valid treatment. However, repeat liver resection could also be used in selected patients, as outcomes are good both in the short and long-term. According to our review, most resections were minor, reflecting the fact that a parenchymal sparing policy is very important in these patients that have already undergone a previous resection. Unnecessary sacrifice of healthy parenchyma should be minimized. We found that repeat resections for recurrent HCCs require long operative time. This is reasonable considering adhesions from previous surgery that can often be vascularized in cirrhotic patients, thereby prolonging the dissection and exposure as well as preparation of the Pringle maneuver. Indeed, the Pringle maneuver was rarely applied (only 0.2% of cases), reflecting the fact that during repeat resections, the pedicle is difficult to sling given previous maneuvers in the area. This makes the liver transection phase potentially riskier, as bleeding cannot be controlled by hilar clamping.
This systematic review has some limitations; first, it is mainly based on retrospective studies, including mostly small and single-center studies. While the evidence is limited for advanced cirrhosis and portal hypertension, more patients have been reported in the setting of large and recurrent lesions and in posterosuperior segments. The wide inclusion period of the studies might also limit the conclusions, since technical evolutions have happened and are still happening in the field of LLRs. Therefore, we need more data to compare minimally invasive surgery and open surgery in the mentioned situations. In this setting, robotics has been increasingly used in the most recent years: from initial skepticism due to the lack of substantial literature to a worldwide adoption of this technique with similar outcomes as compared to laparoscopy [65]. This review was limited to patients operated on by laparoscopy, and conclusions should therefore not be generalized to robotics. Future studies investigating the role of robotic liver resections in challenging scenarios such as the ones depicted in this review are warranted. Long-term outcomes also have been rarely disclosed in these settings [66,67,68]. Further studies should clarify the oncological safety. To our knowledge, this is the first review that includes all the challenging indications for LLRs for HCC. Only Yin et al. explored the role of LLRs in posterosuperior segments, but no pooled evidence exists concerning AC, PH, large lesions, tumors in the PS segments and repeat LLRs [69].
5. Conclusions
Laparoscopic liver resections for HCC have good short- and long-term outcomes. Advanced cirrhosis and portal hypertension, large and recurrent tumors and lesions located in the posterosuperior segments are challenging clinical scenarios that should be carefully approached by laparoscopy. Safe short-term outcomes can be achieved provided experienced surgeons and high-volume centers. Advanced cirrhosis and portal hypertension are the riskiest scenarios. The selection of patients is key in these settings.
Acknowledgments
The authors would like to thank Matilde Berardi for her substantial support during manuscript drafting.
Author Contributions
Conceptualization, G.B. and E.M.M.; methodology, M.C.; software, G.M.; validation, R.L.M., S.F. and N.G.; formal analysis, M.A.; investigation, A.L.; resources, E.G.; data curation, D.C.; writing—original draft preparation, L.D.C.; writing—review and editing, E.M.M. and G.B.; visualization, D.V.; supervision, G.B.; project administration, G.M.E.; funding acquisition, P.M. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ferlay J., Soerjomataram I., Dikshit R., Eser S., Mathers C., Rebelo M., Parkin D.M., Forman D., Bray F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer. 2015;136:E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2020. CA Cancer J. Clin. 2020;70:7–30. doi: 10.3322/caac.21590. [DOI] [PubMed] [Google Scholar]
- 3.Reig M., Forner A., Rimola J., Ferrer-Fàbrega J., Burrel M., Garcia-Criado Á., Kelley R.K., Galle P.R., Mazzaferro V., Salem R., et al. BCLC strategy for prognosis prediction and treatment recommendation: The 2022 update. J. Hepatol. 2022;76:681–693. doi: 10.1016/j.jhep.2021.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gagner M., Rheault M., Dubuc J. Laparoscopic partial hepatectomy for liver tumor. Surg. Endosc. 1992;6:97–98. [Google Scholar]
- 5.Ciria R., Cherqui D., Geller D.A., Briceno J., Wakabayashi G. Comparative Short-term Benefits of Laparoscopic Liver Resection: 9000 Cases and Climbing. Ann. Surg. 2016;263:761–777. doi: 10.1097/SLA.0000000000001413. [DOI] [PubMed] [Google Scholar]
- 6.Ciria R., Gomez-Luque I., Ocaña S., Cipriani F., Halls M., Briceño J., Okuda Y., Troisi R., Rotellar F., Soubrane O., et al. A Systematic Review and Meta-Analysis Comparing the Short- and Long-Term Outcomes for Laparoscopic and Open Liver Resections for Hepatocellular Carcinoma: Updated Results from the European Guidelines Meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann. Surg. Oncol. 2019;26:252–263. doi: 10.1245/s10434-018-6926-3. [DOI] [PubMed] [Google Scholar]
- 7.Morise Z., Ciria R., Cherqui D., Chen K.-H., Belli G., Wakabayashi G. Can we expand the indications for laparoscopic liver resection? A systematic review and meta-analysis of laparoscopic liver resection for patients with hepatocellular carcinoma and chronic liver disease. J. Hepato-Biliary-Pancreatic Sci. 2015;22:342–352. doi: 10.1002/jhbp.215. [DOI] [PubMed] [Google Scholar]
- 8.Abu Hilal M., Aldrighetti L., Dagher I., Edwin B., Troisi R.I., Alikhanov R., Aroori S., Belli G., Besselink M., Briceno J., et al. The Southampton Consensus Guidelines for Laparoscopic Liver Surgery: From Indication to Implementation. Ann. Surg. 2018;268:11–18. doi: 10.1097/SLA.0000000000002524. [DOI] [PubMed] [Google Scholar]
- 9.Berardi G., Morise Z., Sposito C., Igarashi K., Panetta V., Simonelli I., Kim S., Goh B.K., Kubo S., Tanaka S., et al. Development of a nomogram to predict outcome after liver resection for hepatocellular carcinoma in Child-Pugh B cirrhosis. J. Hepatol. 2020;72:75–84. doi: 10.1016/j.jhep.2019.08.032. [DOI] [PubMed] [Google Scholar]
- 10.D’Hondt M., Ovaere S., Knol J., Vandeputte M., Parmentier I., De Meyere C., Vansteenkiste F., Besselink M., Pottel H., Verslype C. Laparoscopic right posterior sectionectomy: Single-center experience and technical aspects. Langenbeck’s Arch. Surg. 2019;404:21–29. doi: 10.1007/s00423-018-1731-9. [DOI] [PubMed] [Google Scholar]
- 11.Azoulay D., Ramos E., Casellas-Robert M., Salloum C., Lladó L., Nadler R., Busquets J., Caula-Freixa C., Mils K., Lopez-Ben S., et al. Liver resection for hepatocellular carcinoma in patients with clinically significant portal hypertension. JHEP Rep. 2020;3:100190. doi: 10.1016/j.jhepr.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Child C.G., Turcotte J.G. Surgery and portal hypertension. Major Probl. Clin. Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 13.Berzigotti A., Reig M., Abraldes J.G., Bosch J., Bruix J. Portal hypertension and the outcome of surgery for hepatocellular carcinoma in compensated cirrhosis: A systematic review and meta-analysis. Hepatology. 2015;61:526–536. doi: 10.1002/hep.27431. [DOI] [PubMed] [Google Scholar]
- 14.Berardi G., Aghayan D., Fretland Å.A., Elberm H., Cipriani F., Spagnoli A., Montalti R., Ceelen W.P., Aldrighetti L., Abu Hilal M., et al. Multicentre analysis of the learning curve for laparoscopic liver resection of the posterosuperior segments. Br. J. Surg. 2019;106:1512–1522. doi: 10.1002/bjs.11286. [DOI] [PubMed] [Google Scholar]
- 15.European Association for the Study of the Liver EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J. Hepatol. 2018;69:182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 16.Dindo D., Demartines N., Clavien P.A. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann. Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahbari N.N., Garden O.J., Padbury R., Brooke-Smith M., Crawford M., Adam R., Koch M., Makuuchi M., Dematteo R.P., Christophi C., et al. Posthepatectomy liver failure: A definition and grading by the International Study Group of Liver Surgery (ISGLS) Surgery. 2011;149:713–724. doi: 10.1016/j.surg.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur. J. Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Cipriani F., Fantini C., Ratti F., Lauro R., Tranchart H., Halls M., Scuderi V., Barkhatov L., Edwin B., Troisi R.I., et al. Laparoscopic liver resections for hepatocellular carcinoma. Can we extend the surgical indication in cirrhotic patients? Surg. Endosc. 2018;32:617–626. doi: 10.1007/s00464-017-5711-x. [DOI] [PubMed] [Google Scholar]
- 20.Troisi R.I., Berardi G., Morise Z., Cipriani F., Ariizumi S., Sposito C., Panetta V., Simonelli I., Kim S., Goh B.K.P., et al. Laparoscopic and open liver resection for hepatocellular carcinoma with Child–Pugh B cirrhosis: Multicentre propensity score-matched study. Br. J. Surg. 2021;108:196–204. doi: 10.1093/bjs/znaa041. [DOI] [PubMed] [Google Scholar]
- 21.Cai X., Liang X., Yu T., Liang Y., Jing R., Jiang W., Li J., Ying H. Liver cirrhosis grading Child-Pugh class B: A Goliath to challenge in laparoscopic liver resection?—Prior experience and matched comparisons. HepatoBiliary Surg. Nutr. 2015;4:391–397. doi: 10.3978/j.issn.2304-3881.2015.09.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beard R.E., Wang Y., Khan S., Marsh J.W., Tsung A., Geller D.A. Laparoscopic liver resection for hepatocellular carcinoma in early and advanced cirrhosis. HPB. 2018;20:521–529. doi: 10.1016/j.hpb.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Lim C., Osseis M., Lahat E., Doussot A., Sotirov D., Hemery F., Lantéri-Minet M., Feray C., Salloum C., Azoulay D. Safety of laparoscopic hepatectomy in patients with hepatocellular carcinoma and portal hypertension: Interim analysis of an open prospective study. Surg. Endosc. 2019;33:811–820. doi: 10.1007/s00464-018-6347-1. [DOI] [PubMed] [Google Scholar]
- 24.Guo P., Liao S., Li J., Zheng S. Clinical application of combined laparoscopic surgery in the treatment of primary hepatocellular carcinoma with portal hypertension: A report of 16 cases. Transl. Cancer Res. 2019;8:330–337. doi: 10.21037/tcr.2019.01.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Molina V., Sampson-Dávila J., Ferrer J., Fondevila C., del Gobbo R.D., Calatayud D., Bruix J., García-Valdecasas J.C., Fuster J. Benefits of laparoscopic liver resection in patients with hepatocellular carcinoma and portal hypertension: A case-matched study. Surg. Endosc. 2018;32:2345–2354. doi: 10.1007/s00464-017-5930-1. [DOI] [PubMed] [Google Scholar]
- 26.Zheng J., Feng X., Liang Y., Cai J., Shi Z., Kirih M.A., Tao L., Liang X. Safety and feasibility of laparoscopic liver resection for hepatocellular carcinoma with clinically significant portal hypertension: A propensity score-matched study. Surg. Endosc. 2021;35:3267–3278. doi: 10.1007/s00464-020-07763-6. [DOI] [PubMed] [Google Scholar]
- 27.Casellas-Robert M., Lim C., Lopez-Ben S., Lladó L., Salloum C., Codina-Font J., Comas-Cufí M., Ramos E., Figueras J., Azoulay D. Laparoscopic Liver Resection for Hepatocellular Carcinoma in Child–Pugh A Patients with and Without Portal Hypertension: A Multicentre Study. World J. Surg. 2020;44:3915–3922. doi: 10.1007/s00268-020-05687-9. [DOI] [PubMed] [Google Scholar]
- 28.Ruzzenente A., Bagante F., Ratti F., Alaimo L., Marques H.P., da Silva S.G., Soubrane O., Endo I., Sahara K., Beal E., et al. Minimally Invasive Versus Open Liver Resection for Hepatocellular Carcinoma in the Setting of Portal Vein Hypertension: Results of an International Multi-institutional Analysis. Ann. Surg. Oncol. 2020;27:3360–3371. doi: 10.1245/s10434-020-08444-3. [DOI] [PubMed] [Google Scholar]
- 29.Kwon Y., Han H.-S., Yoon Y.-S., Cho J.Y. Are Large Hepatocellular Carcinomas Still a Contraindication for Laparoscopic Liver Resection? J. Laparoendosc. Adv. Surg. Tech. A. 2015;25:98–102. doi: 10.1089/lap.2014.0226. [DOI] [PubMed] [Google Scholar]
- 30.Chiang M.-H., Tsai K.-Y., Chen H.-A., Wang W.-Y., Huang M.-T. Comparison of surgical outcomes for laparoscopic liver resection of large hepatocellular carcinomas: A retrospective observation from single-center experience. Asian J. Surg. 2021;44:1376–1382. doi: 10.1016/j.asjsur.2021.03.027. [DOI] [PubMed] [Google Scholar]
- 31.Fu X.-T., Tang Z., Shi Y.-H., Zhou J., Liu W.-R., Gao Q., Ding G.-Y., Chen J.-F., Song K., Wang X.-Y., et al. Laparoscopic Versus Open Left Lateral Segmentectomy for Large Hepatocellular Carcinoma: A Propensity Score–Matched Analysis. Surg. Laparosc. Endosc. Percutaneous Tech. 2019;29:513–519. doi: 10.1097/SLE.0000000000000723. [DOI] [PubMed] [Google Scholar]
- 32.Xu H., Liu F., Hao X., Wei Y., Li B., Wen T., Wang W., Yang J. Laparoscopically anatomical versus non-anatomical liver resection for large hepatocellular carcinoma. HPB. 2020;22:136–143. doi: 10.1016/j.hpb.2019.06.008. [DOI] [PubMed] [Google Scholar]
- 33.Xiang L., Li J., Chen J., Wang X., Guo P., Fan Y., Zheng S. Prospective cohort study of laparoscopic and open hepatectomy for hepatocellular carcinoma. Br. J. Surg. 2016;103:1895–1901. doi: 10.1002/bjs.10294. [DOI] [PubMed] [Google Scholar]
- 34.Sandri G.B.L., Spoletini G., Vennarecci G., Francone E., Abu Hilal M., Ettorre G.M. Laparoscopic liver resection for large HCC: Short- and long-term outcomes in relation to tumor size. Surg. Endosc. 2018;32:4772–4779. doi: 10.1007/s00464-018-6225-x. [DOI] [PubMed] [Google Scholar]
- 35.Ai J.-H., Li J.-W., Chen J., Bie P., Wang S.-G., Zheng S.-G. Feasibility and Safety of Laparoscopic Liver Resection for Hepatocellular Carcinoma with a Tumor Size of 5–10 cm. PLoS ONE. 2013;8:e72328. doi: 10.1371/journal.pone.0072328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Casaccia M., Andorno E., Di Domenico S., Nardi I., Bottino G., Gelli M., Valente U. Laparoscopic liver resection for hepatocellular carcinoma in cirrhotic patients. Feasibility of nonanatomic resection in difficult tumor locations. J. Minimal Access Surg. 2011;7:222–226. doi: 10.4103/0972-9941.85644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang L., Xiao L., Li J., Chen J., Fan Y., Zheng S. Safety and Feasibility of Laparoscopic Hepatectomy for Hepatocellular Carcinoma in the Posterosuperior Liver Segments. World J. Surg. 2015;39:1202–1209. doi: 10.1007/s00268-015-2946-3. [DOI] [PubMed] [Google Scholar]
- 38.Lee W., Han H.-S., Yoon Y.-S., Cho J.Y., Choi Y., Shin H.K., Jang J.Y., Choi H., Kwon S.U. Comparison of laparoscopic liver resection for hepatocellular carcinoma located in the posterosuperior segments or anterolateral segments: A case-matched analysis. Surgery. 2016;160:1219–1226. doi: 10.1016/j.surg.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Tagaytay T.G., Han D.H., Chong J.U., Hwang H.S., Choi G.H., Kim K.S. Laparoscopic Minor Liver Resections for Hepatocellular Carcinoma in the Posterosuperior Segments Using the Rubber Band Technique. World J. Surg. 2022;46:1151–1160. doi: 10.1007/s00268-022-06468-2. [DOI] [PubMed] [Google Scholar]
- 40.Kwon Y., Cho J.Y., Han H.-S., Yoon Y.-S., Lee H.W., Lee J.S., Lee B., Kim M. Improved Outcomes of Laparoscopic Liver Resection for Hepatocellular Carcinoma Located in Posterosuperior Segments of the Liver. World J. Surg. 2021;45:1178–1185. doi: 10.1007/s00268-020-05912-5. [DOI] [PubMed] [Google Scholar]
- 41.Yoon Y.-S., Han H.-S., Cho J.Y., Ahn K.S. Total laparoscopic liver resection for hepatocellular carcinoma located in all segments of the liver. Surg. Endosc. 2010;24:1630–1637. doi: 10.1007/s00464-009-0823-6. [DOI] [PubMed] [Google Scholar]
- 42.Xiao L., Xiang L.-J., Li J.-W., Chen J., Fan Y.-D., Zheng S.-G. Laparoscopic versus open liver resection for hepatocellular carcinoma in posterosuperior segments. Surg. Endosc. 2015;29:2994–3001. doi: 10.1007/s00464-015-4214-x. [DOI] [PubMed] [Google Scholar]
- 43.Cherqui D., Laurent A., Tayar C., Chang S., Van Nhieu J.T., Loriau J., Karoui M., Duvoux C., Dhumeaux D., Fagniez P.-L. Laparoscopic Liver Resection for Peripheral Hepatocellular Carcinoma in Patients with Chronic Liver Disease: Midterm results and per-spectives. Ann. Surg. 2006;243:499–506. doi: 10.1097/01.sla.0000206017.29651.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sandri G.B.L., Ettorre G.M., Aldrighetti L., Cillo U., Valle R.D., Guglielmi A., Mazzaferro V., Ferrero A., Di Benedetto F., I Go MILS Group on HCC Laparoscopic liver resection of hepatocellular carcinoma located in unfavorable segments: A propensity score-matched analysis from the I Go MILS (Italian Group of Minimally Invasive Liver Surgery) Registry. Surg. Endosc. 2019;33:1451–1458. doi: 10.1007/s00464-018-6426-3. [DOI] [PubMed] [Google Scholar]
- 45.Liu K., Chen Y., Wu X., Huang Z., Lin Z., Jiang J., Tan W., Zhang L. Laparoscopic liver re-resection is feasible for patients with posthepatectomy hepatocellular carcinoma recurrence: A propensity score matching study. Surg. Endosc. 2017;31:4790–4798. doi: 10.1007/s00464-017-5556-3. [DOI] [PubMed] [Google Scholar]
- 46.Belli G., Cioffi L., Fantini C., D’Agostino A., Russo G., Limongelli P., Belli A. Laparoscopic redo surgery for recurrent hepatocellular carcinoma in cirrhotic patients: Feasibility, safety, and results. Surg. Endosc. 2009;23:1807–1811. doi: 10.1007/s00464-009-0344-3. [DOI] [PubMed] [Google Scholar]
- 47.Goh B.K.P., Syn N., Teo J.-Y., Guo Y.-X., Lee S.-Y., Cheow P.-C., Chow P.K.H., Ooi L.L.P.J., Chung A.Y.F., Chan C.-Y. Perioperative Outcomes of Laparoscopic Repeat Liver Resection for Recurrent HCC: Comparison with Open Repeat Liver Resection for Recurrent HCC and Laparoscopic Resection for Primary HCC. World J. Surg. 2019;43:878–885. doi: 10.1007/s00268-018-4828-y. [DOI] [PubMed] [Google Scholar]
- 48.Sandri G.B.L., Colasanti M., Aldrighetti L., Guglielmi A., Cillo U., Mazzaferro V., Valle R.D., De Carlis L., Gruttadauria S., Di Benedetto F., et al. Is minimally invasive liver surgery a reasonable option in recurrent HCC? A snapshot from the I Go MILS registry. Updat. Surg. 2022;74:87–96. doi: 10.1007/s13304-021-01161-w. [DOI] [PubMed] [Google Scholar]
- 49.Gon H., Kido M., Tanaka M., Kuramitsu K., Komatsu S., Awazu M., So S., Toyama H., Fukumoto T. Laparoscopic repeat hepatectomy is a more favorable treatment than open repeat hepatectomy for contralateral recurrent hepatocellular carcinoma cases. Surg. Endosc. 2021;35:2896–2906. doi: 10.1007/s00464-020-07728-9. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J., Zhou Z.-G., Huang Z.-X., Yang K.-L., Chen J.-C., Chen J.-B., Xu L., Chen M.-S., Zhang Y.-J. Prospective, single-center cohort study analyzing the efficacy of complete laparoscopic resection on recurrent hepatocellular carcinoma. Chin. J. Cancer. 2016;35:25. doi: 10.1186/s40880-016-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Morise Z., Aldrighetti L., Belli G., Ratti F., Belli A., Cherqui D., Tanabe M., Wakabayashi G., Cheung T.T., Lo C.M., et al. Laparoscopic repeat liver resection for hepatocellular carcinoma: A multicentre propensity score-based study. Br. J. Surg. 2020;107:889–895. doi: 10.1002/bjs.11436. [DOI] [PubMed] [Google Scholar]
- 52.Kanazawa A., Tsukamoto T., Shimizu S., Kodai S., Yamamoto S., Yamazoe S., Ohira G., Nakajima T. Laparoscopic liver resection for treating recurrent hepatocellular carcinoma. J. Hepato-Biliary-Pancreatic Sci. 2013;20:512–517. doi: 10.1007/s00534-012-0592-9. [DOI] [PubMed] [Google Scholar]
- 53.Onoe T., Yamaguchi M., Irei T., Ishiyama K., Sudo T., Hadano N., Kojima M., Kubota H., Ide R., Tazawa H., et al. Feasibility and efficacy of repeat laparoscopic liver resection for recurrent hepatocellular carcinoma. Surg. Endosc. 2020;34:4574–4581. doi: 10.1007/s00464-019-07246-3. [DOI] [PubMed] [Google Scholar]
- 54.Miyama A., Morise Z., Aldrighetti L., Belli G., Ratti F., Cheung T.-T., Lo C.-M., Tanaka S., Kubo S., Okamura Y., et al. Multicenter Propensity Score-Based Study of Laparoscopic Repeat Liver Resection for Hepatocellular Carcinoma: A Subgroup Analysis of Cases with Tumors Far from Major Vessels. Cancers. 2021;13:3187. doi: 10.3390/cancers13133187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bruix J., Castells A., Bosch J., Feu F., Fuster J., Garcia-Pagan J.C., Visa J., Bru C., Rodes J. Surgical resection of hepatocellular carcinoma in cirrhotic patients: Prognostic value of preoperative portal pressure. Gastroenterology. 1996;111:1018–1022. doi: 10.1016/S0016-5085(96)70070-7. [DOI] [PubMed] [Google Scholar]
- 56.Northup P.G., Wanamaker R.C., Lee V.D., Adams R.B., Berg C.L. Model for End-Stage Liver Disease (MELD) Predicts Nontransplant Surgical Mortality in Patients with Cirrhosis. Ann. Surg. 2005;242:244–251. doi: 10.1097/01.sla.0000171327.29262.e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morise Z., Sugioka A., Kawabe N., Umemoto S., Nagata H., Ohshima H., Kawase J., Arakawa S., Yoshida R. Pure laparoscopic hepatectomy for hepatocellular carcinoma patients with severe liver cirrhosis. Asian J. Endosc. Surg. 2011;4:143–146. doi: 10.1111/j.1758-5910.2011.00081.x. [DOI] [PubMed] [Google Scholar]
- 58.Wakabayashi G., Cherqui D., A Geller D., Buell J.F., Kaneko H., Han H.S., Asbun H., Oʼrourke N., Tanabe M., Koffron A.J., et al. Recommendations for laparoscopic liver resection: A report from the second international consensus conference held in Morioka. Ann. Surg. 2015;261:619–629. doi: 10.1097/SLA.0000000000001184. [DOI] [PubMed] [Google Scholar]
- 59.Pietrasz D., Fuks D., Subar D., Donatelli G., Ferretti C., Lamer C., Portigliotti L., Ward M., Cowan J., Nomi T., et al. Laparoscopic extended liver resection: Are postoperative outcomes different? Surg. Endosc. 2018;32:4833–4840. doi: 10.1007/s00464-018-6234-9. [DOI] [PubMed] [Google Scholar]
- 60.Martin A.N., Narayanan S., Turrentine F.E., Bauer T.W., Adams R.B., Stukenborg G.J., Zaydfudim V.M. Clinical Factors and Postoperative Impact of Bile Leak After Liver Resection. J. Gastrointest. Surg. 2018;22:661–667. doi: 10.1007/s11605-017-3650-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buell J.F., Cherqui D., Geller D.A., O’Rourke N., Iannitti D., Dagher I., Koffron A.J., Thomas M., Gayet B., Han H.S., et al. The International Position on Laparoscopic Liver Surgery: The Louisville Statement, 2008. Ann. Surg. 2009;250:825–830. doi: 10.1097/SLA.0b013e3181b3b2d8. [DOI] [PubMed] [Google Scholar]
- 62.Berardi G., Wakabayashi G., Igarashi K., Ozaki T., Toyota N., Tsuchiya A., Nishikawa K. Full Laparoscopic Anatomical Segment 8 Resection for Hepatocellular Carcinoma Using the Glissonian Approach with Indocyanine Green Dye Fluorescence. Ann. Surg. Oncol. 2019;26:2577–2578. doi: 10.1245/s10434-019-07422-8. [DOI] [PubMed] [Google Scholar]
- 63.Van der Poel M.J., Huisman F., Busch O.R., Abu Hilal M., van Gulik T.M., Tanis P.J., Besselink M.G. Stepwise introduction of laparoscopic liver surgery: Validation of guideline recommendations. HPB. 2017;19:894–900. doi: 10.1016/j.hpb.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 64.Tabrizian P., Jibara G., Shrager B., Schwartz M., Roayaie S. Recurrence of Hepatocellular Cancer After Resection: Patterns, treat-ments, and prognosis. Ann. Surg. 2015;261:947–955. doi: 10.1097/SLA.0000000000000710. [DOI] [PubMed] [Google Scholar]
- 65.Ciria R., Berardi G., Alconchel F., Briceño J., Choi G.H., Wu Y., Sugioka A., Troisi R.I., Salloum C., Soubrane O., et al. The impact of robotics in liver surgery: A worldwide systematic review and short-term outcomes meta-analysis on 2,728 cases. J. Hepato-Biliary-Pancreatic Sci. 2022;29:181–197. doi: 10.1002/jhbp.869. [DOI] [PubMed] [Google Scholar]
- 66.Nomi T., Kaibori M., Tanaka S., Hirokawa F., Hokuto D., Noda T., Ueno M., Nakai T., Ikoma H., Iida H., et al. Short- and long-term outcomes of laparoscopic versus open repeat liver resection for hepatocellular carcinoma: A multicenter study. J. Hepato-Biliary-Pancreat. Sci. doi: 10.1002/jhbp.1222. [DOI] [PubMed] [Google Scholar]
- 67.Zhang K.-J., Liang L., Diao Y.-K., Xie Y.-M., Wang D.-D., Xu F.-Q., Ye T.-W., Lu W.-F., Cheng J., Shen G.-L., et al. Short- and long-term outcomes of laparoscopic versus open liver resection for large hepatocellular carcinoma: A propensity score study. Surg. Today. 2022;53:322–331. doi: 10.1007/s00595-022-02576-7. [DOI] [PubMed] [Google Scholar]
- 68.Belli G., Fantini C., Belli A., Limongelli P. Laparoscopic Liver Resection for Hepatocellular Carcinoma in Cirrhosis: Long-Term Outcomes. Dig. Surg. 2011;28:134–140. doi: 10.1159/000323824. [DOI] [PubMed] [Google Scholar]
- 69.Yin Z., Jin H., Ma T., Wang H., Huang B., Jian Z. Laparoscopic hepatectomy versus open hepatectomy in the management of posterosuperior segments of the Liver: A systematic review and meta-analysis. Int. J. Surg. 2018;60:101–110. doi: 10.1016/j.ijsu.2018.10.040. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.