Abstract
Simple Summary
Breast cancer remains the fourth-leading cause of death worldwide, and therapeutic improvement is warranted. The phosphatidylinositol 3-kinase (PI3K) pathway is one of the major pathways in oncogenesis, and PI3K alterations are common in all breast cancer subtypes. Despite modest clinical activity and a high toxicity rate, pan-PI3K inhibitors paved the way for selective PI3K inhibitor development. In this overview, we cover the past, the present, and potential paths, as well as the therapeutic challenges to come for this therapeutic class.
Abstract
The phosphatidylinositol 3-kinase (PI3K) pathway is one of the most altered pathways in human cancers, and it plays a central role in cellular growth, survival, metabolism, and cellular mobility, making it a particularly interesting therapeutic target. Recently, pan-inhibitors and then selective p110α subunit inhibitors of PI3K were developed. Breast cancer is the most frequent cancer in women and, despite therapeutic progress in recent years, advanced breast cancers remain incurable and early breast cancers are at risk of relapse. Breast cancer is divided in three molecular subtypes, each with its own molecular biology. However, PI3K mutations are found in all breast cancer subtypes in three main “hotspots”. In this review, we report the results of the most recent and main ongoing studies evaluating pan-PI3K inhibitors and selective PI3K inhibitors in each breast cancer subtype. In addition, we discuss the future of their development, the various potential mechanisms of resistance to these inhibitors and the ways to circumvent them.
Keywords: breast cancer, PI3KCA, pan-PI3K inhibitor, selective PI3K inhibitor, resistance mechanism, overview, target therapy
1. Introduction
Worldwide, in 2020, breast cancer (BC) represented the most frequent cancer, with approximately 2.3 million cases, and the fourth-leading cause of death, with approximately 685,000 deaths [1]. In early BC, which represents approximately 90% of cases, the treatment is based on surgery, radiotherapy, and, depending on the molecular subtype, on endocrine therapy (ET), chemotherapy, targeted therapy, and immune therapy. In advanced BC, the standard of care is based on systemic therapy and varies according to the molecular subtype. When the human epidermal growth factor (HER2) is overexpressed (HER2+), the treatment includes a combination of either cytotoxics (or less commonly ET) with anti-HER2 agents, including anti-HER2 monoclonal antibodies trastuzumab and pertuzumab, or tyrosine kinase inhibitors, such as lapatinib or tucatinib. In trastuzumab-resistant disease, antibody–drug conjugates (ADCs), such as trastuzumab emtansine and, most recently, trastuzumab deruxtecan, are recommended [2]. In triple-negative breast cancers (TNBCs), the standard of care is chemotherapy, which may be combined with an immune checkpoint inhibitor when PD-L1 is expressed, and, more recently, ADCs, such as sacituzumab govitecan or trastuzumab deruxtecan. In the Estrogen Receptor (ER)/Progesterone Receptor (PR)-positive/HER2-negative subtype (hormone receptor HR+/HER2−), the treatment relies upon ET in combination with a CDK4/6 inhibitor [3,4,5]. The therapeutic management is characterized by multiple lines of ET, possibly in combination with other targeted therapies, until the appearance of a clear ET resistance, followed by the use of palliative chemotherapy, which may include trastuzumab deruxtecan in the case of HER2 low expression. In both advanced stages of TN and HR+/HER2− subtypes, PARP inhibitors (olaparib, talazoparib) are also an option in the case of germline BRCA1 or BRCA2 mutations [6,7,8,9].
While very significant progress, including an improvement in overall survival, has been made during the last two decades in all the molecular subtypes, advanced BC is still considered as an incurable disease and the disease recurrence almost invariably occurs after periods of remission on specific systemic treatments. In this context, several attempts have been made to improve the overall efficacy of the already established standard of care. Amongst them, the therapeutic modulation of the phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway has been one of the most investigated strategies to improve the survival at advanced stages of the disease in each molecular subtype. After various generations of mTOR inhibitors failed to demonstrate an improvement in overall survival, and while Akt inhibitors are currently under comparative evaluation, PI3K inhibitors have been developed during the last 10 years, mitigating clinical achievements. In this review, we examine the main features of this important biological pathway and the rationale for its therapeutic targeting in BC, as well as the main clinical results that have been obtained and the next perspectives for further clinical experimentations.
2. The Phosphatidylinositol 3-Kinase (PI3K) Pathway Molecular Alterations in Breast Cancer and the Rationale for Therapeutic Targeting
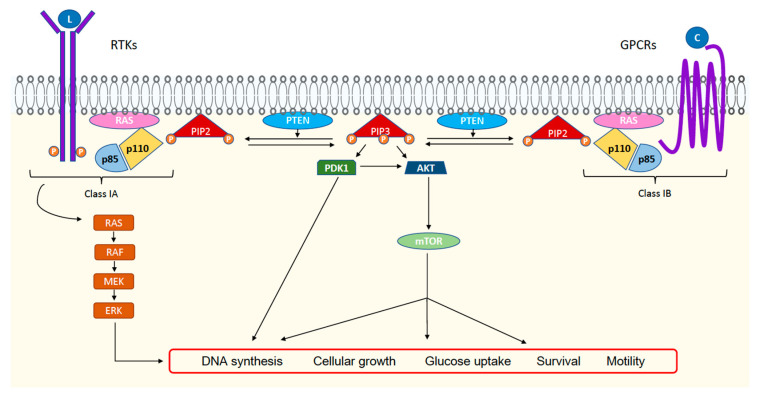
The PI3K pathway is one of the most frequently altered pathways in human tumors [10]. PI3K belongs to the family of intracellular lipid kinases and can be divided into three classes. Class I is activated by insulin or other growth factors and can be further divided into classes IA and IB. Class IA PI3K is associated with tyrosine kinase receptors (RTKs) and class IB is associated with heterotrimeric G-protein-coupled receptors (GPCRs). Both are composed of a catalytic subunit and a regulatory subunit. For class IA PI3K, which is the most directly involved in carcinogenesis and is encoded by the PIK3CA gene, the catalytic subunit isoforms are p110α, p110β, p110γ, or p110δ. The catalytic subunit associates with the regulatory subunit (encoded by PIK3R1, PIK3R2, or PIK3R3) [11,12], which inhibits the kinase activity of p110, stabilizes p110, and promotes a negative retrocontrol [11]. When an RTK is activated by growth factors, PI3K is recruited to the plasma membrane and is activated, thus phosphorylating phosphatidylinositol 4,5 biphosphate (PIP2) to generate phosphatidylinositol 3,4,5 triphosphate (PIP3). PIP3 recruits and binds AKT or other proteins with a pleckstrin-homology (PH) domain such as 3-phosphoinositide–dependent protein kinase 1 (PDK1). Then, AKT is phosphorylated and activated by PDK1 and mammalian target of rapamycin complex 2 (mTORC2) [13], triggering several phosphorylation-based signaling cascades. PI3K plays a central role in several cellular processes, including DNA synthesis, metabolism, and action on actin cytoskeleton [14,15]. The tumor suppressor phosphatase and tensin homolog (PTEN) negatively regulates the action of PIP3 via 3′-phosphatase activity, thus transforming PIP3 into PIP2 [16], which stops the phosphorylation cascade (Figure 1).
Figure 1.
Summary diagram of the PI3K pathway and cellular activation pathways.
Class IA features tyrosine kinase receptors (RTKs) and class IB has G-protein-coupled receptors (GPCRs). When a growth factor (L) or a chemokine (C) activates the receptor, PI3K is recruited to the cellular membrane and is activated, thus phosphorylating phosphatidylinositol 4,5 biphosphate (PIP2) to generate phosphatidylinositol 3,4,5 triphosphate (PIP3). PIP3 recruits and binds to protein kinase B (AKT). Then, AKT is phosphorylated and activated by 3-phosphoinositide-dependent protein kinase 1 (PDK1) and mammalian target of rapamycin complex (mTOR), triggering several phosphorylation-based signaling cascades. PI3K plays a central role in several cellular processes, including DNA synthesis, cellular growth, metabolism, survival, and cell motility.
Alteration of the PI3K pathway is one of the most common genetic alterations in human cancers [17]. Each actor in the pathway may be concerned with different alterations (mutations, deletions, or amplifications), with PIK3CA and PTEN at the top of the list and being, respectively, the second and third most highly mutated genes in human cancers [10,18,19]. The PTEN tumor suppressor gene [20,21] is commonly inactivated in sporadic cancers. PTEN loss induces an increase in PIP3, resulting in a constitutive activation of the PI3K pathway, which promotes cell proliferation and cell cycle progression. AKT1 gain-of-function mutations may also be found and similarly activate the pathway. PIK3CA mutations are found in approximately 30% of different solid tumors [19,22,23]. The catalogue of somatic mutations in cancer (COSMIC) shows that 80% of PIK3CA mutations concern only three “hotspots” and are located on the kinase and helical domains of the catalytic subunits. These “hotspots” H1047R, E542K, and E545K may be found in a large range of tumors, including breast, colorectal, endometrial, and cervical cancers, and glioblastoma [24]. In the kinase domain, the H1047R mutation (exon 20) enhances the linking of p110 to the cell membrane without the requirement of RAS. In the helical domain, the E542K and E545K mutations (exon 9) disturb the interaction with SH2 domains and, consequently, block the action of a regulatory subunit [25,26]. PIK3CA mutations promote the resistance to apoptosis, lead to an increased invasive capacity via a possible epithelial-to-mesenchymal transition, induce a chromosomal instability, and favor an immunosuppressive environment [27,28,29,30]. Across all BC subtypes, the prevalence of PIK3CA mutations varies between 25 and 40%, the highest being in HR+/HER2− BC [31]. A meta-analysis found a positive association between the PIK3CA mutation and ER expression, while for HER2 overexpression, the results are not clear and need more exploration. In TNBC, PIK3CA mutations were also associated with the presence of the androgen receptor (AR) [32]. PIK3CA mutations are associated with an improvement in recurrence-free survival (RFS) in early BC patients but have no impact on the distant disease-free survival (DFS) or overall survival (OS) [33]. In HR+/HER2− BC, PIK3CA mutations do not predict the response to ET at an early stage but the results are unclear in the metastatic setting. A meta-analysis, including 1929 patients mixing all subtypes and early and metastatic stages of BC, found that the PIK3CA mutation is an independent poor-prognosis factor [34]. Recently, Mosele et al. found distinct results according to the BC subtypes. HR+/HER2− metastatic BC with the PIK3CA mutation appeared to be less sensitive to chemotherapy and had a worse survival than wild-type PIK3CA. In TNBC, the data were opposite and the patients with the PIK3CA mutation had better survival than patients with wild-type tumors. This may be due to the fact that some TNBCs were initially ER+ tumors that lost ER expression when tumors acquired the PIK3CA mutations. It might also be due to AR+ TNBCs, which are enriched in PIK3CA mutations and are thought to have a more indolent natural history compared to the other subtypes of TNBC [32]. Several studies showed a different prognostic impact between the two “hotspot” mutations on exon 9 and 20, but the results are unclear and contradictory [35,36,37,38]. In the neo-adjuvant setting, a series of patients (n = 92) with early TNBC treated with an anthracycline-based regimen and PIK3CA exon 20 mutation had a lower rate of pathological complete response (pCR); in this trial, exon 20 mutation occurred in only seven patients [39]. Hu et al. also suggested that, in TNBC cell lines and in 50 patients with early TNBC, the PIK3CA mutation induced chemoresistance and promoted relapse [40].
An analysis of the PIK3CA-mutated cancer genome revealed a co-occurrence of multiple PI3K alterations in approximately 15% of BC cases and these alterations were more frequently mutations in cis on the same allele [41]. Multiple pathway alterations lead to hyperactivation compared to a unique alteration, suggesting enhanced oncogenic addiction of double PIK3CA mutations and, therefore, a possible increased sensitivity to PI3Kα inhibitors [41]. Activated HER2-HER3 receptors also solicit the PI3K pathway, which suggests that PIK3CA mutations may confer some resistance to anti-HER2 therapy [42]. Pre-clinical data found that the PIK3CA mutation may confer resistance to trastuzumab and lapatinib [43]. In HER2+ BC, the predictive or prognostic value of PIK3CA mutational status was also controversial. In early BC, a study failed to show an impact of PIK3CA mutation for trastuzumab sensitivity when administered in the neo-adjuvant or adjuvant setting. Another group conducted by Loibl et al. found that the PIK3CA mutation was associated with a significant reduction in pCR in a meta-analysis, including 967 patients treated in the neo-adjuvant setting with trastuzumab or lapatinib. In the metastatic setting, the results are also unclear. In the CLEOPATRA trial (a randomized phase III study in metastatic HER2+ BC receiving trastuzumab plus docetaxel with or without pertuzumab as first-line treatment), Baselga et al. showed that wild-type PIK3CA status was associated with a better prognosis, independently from the treatment effect. With lapatinib, a small-molecule HER2 inhibitor, or pertuzumab, another anti-HER2 monoclonal antibody, the PIK3CA mutations did not predict a benefit from these therapies [37,44,45,46].
In vitro, the treatment of cell lines by PI3K inhibitors leads to an arrest of proliferation [47]. A direct cytotoxic effect was also reported with a pan-PIK3 inhibitor or a dual inhibitor PI3Kγ-PI3Kα. In the ER+ BC cell line, the appearance of hormone resistance was associated with hyperactivation of the PI3K pathway. The PIK3CA and mTOR inhibitors allow one to induce apoptosis of hormone-independent cells and, consequently, delays the hormone resistance phase [48]. A recent meta-analysis, including eight studies with 2670 patients, showed that the PIK3CA mutation is a favorable predictive factor of objective response rate (ORR) (odds ratio: 1.98) and PFS (hazard ratio (HR) = 0.65) in HR+ BC treated by a PI3K inhibitor [49].
3. Clinical Development of PI3K Inhibitors
3.1. Pan-PI3K Inhibitors
These therapies inhibit the kinase activity of all four isoforms of class I PI3K: α, β, γ, and δ.
3.1.1. Buparlisib (BKM120)
Buparlisib is a 2,6-dimorpholino pyrimidine derivative, which inhibits both PI3K (p110 subunit) and also mTOR, although to a lesser extent [50]. At the preclinical level, it has antitumor activity, preferentially in PIK3CA-mutated cell lines, inducing apoptosis in the endocrine-sensitive BC cell lines when combined with estrogen deprivation. Additionally, it demonstrated synergism with trastuzumab in trastuzumab-resistant HER2+ models [51].
Oral buparlisib was first tested in a phase I study involving advanced solid tumors. Approximately 40% of patients experienced a serious adverse event (SAE), including hyperglycemia, skin rash, asthenia, and mood disorder. A dose of 100 mg/day was the recommended dose and a clinical benefit rate (CBR, i.e., objective responses and stable disease for more than 24 weeks) of 41% was observed [52]. A phase I study tested buparlisib in combination with capecitabine in 25 advanced BC patients from all molecular subtypes. The main side effects observed during the study included nausea, hyperglycemia, rash, diarrhea, mucositis, depression, and anxiety. Approximately 50% of patients had a grade ≥3 adverse event (AE). Despite encouraging results with a 68% CBR % [53], the development of buparlisib in association with capecitabine was stopped. In phase Ib studies focusing on HR+/HER2− BC, the 100 mg/day dose was confirmed as safe in association with letrozole [54] or fulvestrant [55], and the observed CBRs were promising. A phase II study examined the efficacy of buparlisib in association with tamoxifen in advanced BC pretreated by ET. The median PFS was 6.1 months but reached 8.7 months in PIK3CA-mutated patients. The study was prematurely stopped because of the significant rate of buparlisib-related toxicity [56].
In the BELLE-2 trial, buparlisib was investigated in HR+/HER2− BCs whose disease progressed on or after aromatase inhibitor (AI) use. Patients (n = 1147) received an association of fulvestrant with buparlisib or placebo. Median PFS was significantly but marginally increased in the patients treated with buparlisib (6.9 versus 5.0 months with an HR of 0.78; 95% CI 0.67–0.89, p = 0.00021), but the final OS results did not find any significant difference. Regarding the tolerance, 78% of patients receiving the combination experienced grade 3 or 4 adverse events versus 34% in the placebo arm. The main side effects were hyperglycemia, rash, anxiety and some severe mood disturbances (depression and suicide attempts), and elevated ALAT and ASAT [57]. The benefit of buparlisib was similar in patients with PI3K-pathway-activated BC as in the overall cohort. In the BELLE-3 study, buparlisib versus placebo was combined with fulvestrant for HR+/HER2− BC patients (n = 432) progressing on or after prior ET and mTOR inhibitors [58]. The toxicity profile was similar to the one described in BELLE-2. Again, a numerically modest increase in PFS was noted in the buparlisib-treated patients versus placebo-treated ones (3.9 versus 1.8 months with a HR of 0.67; 95% CI 0.53–0.84, p = 0.0003), but no effect on OS was noted. In BELLE-3, the subgroup of PIK3CA-mutated BC seemed to derive a higher benefit from buparlisib: 4.7 versus 1.4 months, with a better HR of 0.39 (95% CI 0.23–0.65) than in the overall cohort. Both phase III trials suggested focusing on the search for more selective PI3K inhibitors because the clinical benefit was modest compared to the toxicity profile [58,59]. The BELLE-4, phase II/III study tested the addition of paclitaxel to buparlisib for HER2- BC but did not improve PFS. The median PFS was 8.0 months in the experimental arm versus 9.2 months in the placebo arm, with HR of 1.18 (95% CI 0.82–1.68); the study was stopped due to lack of efficacy [60].
A phase II study enrolling 50 TNBC patients did not find any response to buparlisib in this subtype [61]. Buparlisib was also explored in association with trastuzumab in a phase Ib trial enrolling trastuzumab-resistant advanced HER2+ BC. The disease control rate was 75% in the population, including approximately 40% of patients with an activated PI3K pathway. However, SAEs were observed in 67% of patients, including hyperglycemia, rash, and diarrhea. Serious mood disorders occurred for 17% of patients [62]. With an ORR of 10%, this study failed to reach its primary endpoint [63]. Our group evaluated the association of buparlisib and lapatinib in a phase Ib study for trastuzumab-resistant HER2+ advanced BC (n = 24). Buparlisib at 80 mg once a day (QD) and lapatinib at 1000 mg QD were feasible with an expected toxicity profile. The CBR was 29%, and one patient experienced a complete response (CR) (4.2%). The SAEs most frequently observed were diarrhea, rash, liver toxicity, hyperglycemia, and mood disorders [64]. NeoPHOEBE trial, a phase II randomized and double-blind study, explored the association of buparlisib, trastuzumab, and paclitaxel in a neo-adjuvant setting. Patients (n = 50) with HER2+ early BC were included. Only 16% of patients had PIK3CA mutations. The study was prematurely stopped due to unacceptable liver toxicity [65].
3.1.2. Pictilisib (GDC-0941)
Pictilisib potently and selectively inhibits all PI3K isoforms, although being particularly effective on p110α, with a much lesser effect on mTOR [66]. The first-in-human study of pictilisib enrolled several tumor types and found preliminary signs of antitumor activity as a single agent with nausea, diarrhea, vomiting, and rash being frequent AEs [67]. A BC-specific phase Ib trial was conducted, evaluating pictilisib plus paclitaxel with bevacizumab or trastuzumab, according to the molecular subtype. The response rate ranged from 23 to 53%. In a separate cohort enrolling only HR+/HER2− metastatic BC, and receiving pictilisib in association with letrozole, the ORR was 33.3%. All patients experienced an AE and the rate of SAEs was 72.5%, including neutropenia, rash, peripheral neuropathy, pneumonia, and venous thromboembolism [68].
The randomized, double-blind, placebo-controlled, FERGI trial evaluated pictilisib in combination with fulvestrant in post-menopausal women with an advanced or metastatic HR+/HER2− BC resistant to an aromatase inhibitor (AI). The median PFS was 6.6 months versus 5.1 months in the first part enrolling an unselected population, and 6.5 months versus 5.1 months in the second part enrolling only PIK3CA-mutated patients [69]. In the PEGGY study, a multicenter, placebo-controlled, phase II randomized trial enrolling 183 patients with locally recurrent or metastatic BC, the addition of pictilisib to paclitaxel did not improve the PFS at interim analysis and the trial was prematurely stopped. The median PFS was 8.2 months in the pictilisib group versus 7.8 months in the placebo group. Approximately 30 patients had PIK3CA mutations in both groups, and in this population, the median PFS was 7.3 months for the pictilisib group and 5.8 months for the placebo group [70].
In the neo-adjuvant setting, pictilisib in association with anastrozole was compared with anastrozole alone in a phase II study, enrolling 75 patients with newly diagnosed operable HR+/HER2− BC. A significantly different decrease in the geometric Ki67 average was observed: 83.8% for pictilisib/anastrozole versus 66.0% for the anastrozole group. Geometric Ki67 was used because of high variability in the determination of Ki67 and the approximate lognormal distribution of the data. Ki67 on day 15 was expressed as geometric mean proportions of the baseline and transformed into mean suppression (defined as one minus the geometric means of the proportional changes) [71]. In a subsequent study focusing on a similar population, the ratio of geometric Ki67 average suppression was 0.48 for PIK3CA helical domain mutations, 0.63 for wild type, and 1.17 for kinase domain mutations. These results suggested that early BC with helical domain mutations had a poor response to anastrozole monotherapy (mean Ki67 suppression 53.9%) and that it could be increased by the addition of pictilisib (mean Ki-67 suppression 78.1%). Conversely, BC with PIK3CA kinase domain mutations responded well to anastrozole and did not seem to benefit from pictilisib [72]. No data are available in subtypes other than HR+/HER2−, and given the disappointing results in this subtype, the clinical development of pictilisib was halted.
3.1.3. Copanlisib (BAY 80-6946)
Although displaying activity against all isoforms of PI3K, copanlisib has a higher inhibitory effect on p110α than on other isoforms and demonstrates predominant antitumor activity in PIK3CA-mutated cell lines [73]. A first-in-human study of intravenous copanlisib was conducted in patients with advanced solid cancer. In this study, 16 patients had BC and two of them experienced a partial response (PR). Nausea and hyperglycemia were the main toxicities [74]. A phase Ib trial in patients with advanced solid tumor showed no benefit for an association of weekly copanlisib plus refametinib (MEK inhibitor). In this trial, only four patients had BC and no objective response was observed. Grade 3/4 toxicity occurred in 44 patients (68.8%) with mainly hypertension, diarrhea, and rash [75].
A phase Ib/II study explored the association of copanlisib at 45 or 60 mg intravenous (IV) with weekly trastuzumab in patients with metastatic HER2+ BC who progressed after prior anti-HER2 therapy. Most adverse events were hyperglycemia, fatigue, nausea, and hypertension. Stable disease (SD) occurred in six patients (50%) with no objective response. In the phase II part (n = 20), the CBR was 30% and the duration of response was 15.0 weeks. No other clinical result is available with copanlisib in the other BC subtypes, and the clinical development of this drug has been abandoned for solid tumors, whereas it is FDA-registered in adult patients with relapsed follicular lymphoma who have received at least two prior systemic therapies.
3.2. PI3Kα-Specific Inhibitors
PI3Kα-specific inhibitors are a group of selective oral inhibitors of the PI3K catalytic subunit p110α class I. Other subunits can be inhibited, but all members of this class have a significant decrease in the effect on PI3Kβ to limit the risk of side effects in common [76].
3.2.1. Alpelisib (BYL719)
With a half-maximum inhibitory concentration in vitro on p110α less than 5 nM, compared to more than 1150 on p110β, alpelisib has demonstrated major antitumor activity in various preclinical models of cancer, notably when associated with PIK3CA alterations [77]. A first-in-human study determined the maximal tolerated dose (MTD) of oral alpelisib at 400 mg QD or 150 mg twice a day. Objective tumor responses were observed at doses superior or equal to 270 mg QD, with at least two cycles of experimental treatment, while SD occurred at 180 mg QD. Patients with wild-type PIK3CA had no clinical benefit. The most common toxicities were hyperglycemia, diarrhea, nausea, decreased appetite, and vomiting [78]. Baselga et al. explored alpelisib plus everolimus in patients with advanced solid tumor, and alpelisib plus everolimus plus exemestane for post-menopausal patients with advanced HR+ BC. For the triple association, the MTD was 200 mg for alpelisib, 2.5 mg for everolimus, and 25 mg for exemestane, QD. Main SAEs were fatigue and transaminitis. In a BC-specific dose-expansion cohort (n = 11), CBR was 50%, including 25% of PR [79,80]. Alpelisib was also tested in association with LSZ102, a selective estrogen receptor degrader (SERD), in patients with prior ET failure. Independently of the PIK3CA- or ESR1-mutational status, the median PFS and CBR were 1.8 months and 1.3% for LSZ102 alone and 3.5 months and 20.9% for LSZ102/alpelisib, respectively. In the LSZ102/alpelisib group, patients experienced diarrhea, nausea, hyperglycemia, vomiting, anemia, rash, and transaminitis [81]. A phase Ib study tested alpelisib in combination with letrozole in post-menopausal patients with metastatic HR+/HER2− BC and endocrine-resistant disease. In combination with letrozole, the MTD of alpelisib was 300 mg QD. Side effects were similar to those observed in previous phase I studies. In this trial, CBR was 44% for the PIK3CA-mutated patients and 20% for the wild-type PIK3CA patients, and was it independent from the presence of FGFR1/2 amplification and KRAS and TP53 mutations [82]. Another phase Ib study focused on the same population, but alpelisib was associated with fulvestrant. The association was safe and found the same recommended dose of 300 mg. The median PFS was 9.1 months versus 4.7 months and the objective response rate was 29% versus 0% for patients with PIK3CA-mutated BC versus those with PIK3CA wild BC, respectively [83]. A phase Ib in premenopausal patients with advanced HR+/HER2− BC evaluated the MTD and preliminary efficacy of tamoxifen plus goserelin, with or without alpelisib. MTD was 350 mg QD and the median PFS was 25.2 months. All patients experienced adverse events, including rash, weight loss, stomatitis, nausea, hyperglycemia, alopecia, and diarrhea. Grade 3/4 adverse events were found in 50% of patients [84].
SOLAR-1 study was a randomized, phase III trial enrolling patients with HR+/HER2− advanced BC and disease progression after a prior AI. It compared alpelisib versus placebo in combination with fulvestrant. In the cohort of patients with PIK3CA-mutated BC, 169 patients were treated with alpelisib and 172 with placebo. In this population, the median PFS was significantly higher in the alpelisib group compared to the placebo group (11 months versus 5.7 months, HR: 0.65, 95% CI, 0.50 to 0.85, p < 0.001). In the cohort of patients without PIK3CA mutation, no significant difference was found. Grade 3/4 adverse events occurred in 76% of the cases in the alpelisib group versus 35.5% in the placebo group. The most common side effects were hyperglycemia (63.7%), diarrhea (57.7%), nausea (44.7%), and rash (35.6%) [85]. An anti-histaminic preventive treatment decreased the incidence of rash (26.7% versus 64.1%) [86]. Quality of life (QoL) was also assessed in the SOLAR-1 trial, and no significant difference in the functional status was found for alpelisib versus placebo, with an overall change from baseline at −3.50 versus 0.27, respectively. The main differences concerned diarrhea, anorexia, nausea, and asthenia. Median time to reach 10% of deterioration for QoL was not statistically different between the two arms [87]. The final analysis concerning OS showed that the median OS was 39.3 months in the alpelisib group and 31.4 in the placebo group (HR 0.86, 95% CI 0.64–1.15, p = 0.15). Thus, the OS results did not reach statistical significance. For patients with lung and/or liver metastases, the median OS was 37.2 months and 22.8 months, and the median time to chemotherapy was 23.3 months and 14.8 months in the alpelisib and placebo groups, respectively.
The SOLAR-1 results led the FDA to approve “alpelisib in combination with fulvestrant for post-menopausal women, and men, with HR+/HER2− PIK3CA-mutated advanced or metastatic BC following progression on or after an endocrine-based regimen”, whereas the EMA approved alpelisib in a similar population but only “after an hormone treatment used alone has failed”. In SOLAR-1, only 5 to 7% of patients had received a prior CDK 4/6 inhibitor-based ET, which has become the standard of care in this population, making per-label use according to EMA registration very unlikely. Rugo et al. conducted BYLieve, a phase II multicenter, non-comparative study, for advanced HR+/HER2− BC treated with alpelisib in association with fulvestrant after previous administration of CDK 4/6 inhibitors. In this trial, the median PFS was 7.3 months, and the toxicity rate of grade 3/4 was similar to SOLAR-1, with 67% of patients facing adverse events [88]. Results were similar in cohort A, including patients with prior CDK 4/6 inhibitors plus AI, and cohort B, including patients with prior CDK 4/6 inhibitors plus fulvestrant [89]. Similar activity was found in cohorts A and B, whether patients received less or more than 6 months of treatment with a CDK4/6 inhibitor [90,91]. Bello et al. examined the data from the French early-access program of alpelisib in combination with fulvestrant in heavily pre-treated HR+/HER2− advanced BC, including CDK4/6 inhibitors, in a real-life setting (median of four previous lines of systemic treatments for advanced disease). They found a median PFS of 5.3 months (95% CI, 4.7–6.0) and a 6-month CBR of 45.3% (95% CI, 37.8–52.8), but nearly 40% of patients discontinued alpelisib due to adverse events [92].
The SAFIR02-BREAST and SAFIR-PI3K phase II randomized studies enrolled 1462 patients with metastatic HER2− BC and evaluated the maintenance with targeted therapies (including alpelisib with fulvestrant), guided by genomics versus chemotherapy. Patients with endocrine-resistant disease had a PIK3CA-mutated advanced BC, which did not progress after 6–8 cycles of first- or second-line chemotherapy. The study found a significant longer PFS for the patients with alterations in the ESMO Scale for Clinical Actionability of molecular Targets (ESCAT), equal to I or II. The median PFS was 9.1 months in the maintenance with targeted therapy group versus 2.4 months in the maintenance chemotherapy group (HR 0.41; p < 0.001). For all patients, the median PFS of the two groups was not significantly different, with an HR of 0.77 (95% CI: 0.56–1.06, p = 0.109) [93].
Letrozole plus alpelisib was compared to letrozole plus placebo in the neoadjuvant setting in the NEO-ORB trial. This was a randomized, phase II, placebo-controlled trial. Post-menopausal patients (n = 257) were included, 127 patients had a PIK3CA mutation, and 130 were wild-type PIK3CA. The composite primary endpoint (ORR and pCR) was not met. In the patients with PIK3CA-mutated BC, the ORR was 43% and 45% in the alpelisib- and placebo-treated groups, respectively, whereas in the patients with wild-type PIK3CA BC, the ORR was 63% and 61%. The pCR rates were low and not statistically different in all groups [94].
In advanced TNBC, a phase I/II study evaluated Alpelisib in association with Nab-paclitaxel and found an ORR of 59%, including 7% of CR. The median PFS was 8.7 months. Patients with a PIK3CA mutation had a median PFS of 11.9 months versus 7.5 months for patients with Wild-type PIK3CA status. They also found a higher median PFS in patients without pre-diabetic or diabetic metabolic status, with 11.9 versus 7.5 months [95]. In the neoadjuvant setting, a phase II trial is evaluating the association of alpelisib with nab-paclitaxel in patients with early refractory TNBC or with suboptimal response to initial anthracycline-based chemotherapy and displaying PIK3CA or PTEN alterations [96]. Recently, a phase I trial evaluated the combination of alpelisib and olaparib in pretreated advanced TNBC or any subtype with germline BRCA-mutated BC. The hypothesis was that PI3K inhibition could induce a functional homologous recombination deficiency (HRD), leading to higher sensitivity to PARP inhibitors. The recommended phase II dose was a reduced posology of both agents (200 mg olaparib, 200 mg alpelisib). The main toxicities were hyperglycemia, rash, fatigue, anorexia, and nausea. The ORR and CBR were 18% and 35%, respectively. Importantly, none of the responders had BRCA mutation or any other HRD gene mutation, as well any alteration in the PI3K pathway, consistent with a functional synergism between the two drugs [97].
A phase I trial examined the combination of alpelisib with trastuzumab emtansine (TDM-1) in patients with pretreated HER2+ metastatic BC. No unexpected signal of toxicity was seen and the ORR and CBR were 43% and 71%, respectively, including 30% and 60% in patients with prior resistance toTDM-1. The median PFS was 8.1 months, consistent with the central position of the PI3K pathway in the mechanisms of resistance to anti-HER2 treatment [98]. Another phase I study, including HER2+ BC with PIK3CA mutations, investigated alpelisib in combination with trastuzumab and LJM716 (an HER3-targeted antibody). The study revealed significant gastrointestinal toxicity for the triple association and was stopped prematurely [99]. A phase III, multicenter, randomized, double-blind, placebo-controlled trial, evaluating maintenance Alpelisib in association with trastuzumab and pertuzumab for metastatic HER2+ BC with PIK3CA mutation, is ongoing [100].
3.2.2. Taselisib (GDC-0032)
Taselisib inhibits tumor cell proliferation and induces apoptosis by potently and selectively inhibiting p110α, particularly in the context of activating PIK3CA mutation. Of note, its mechanisms of action also include a proteasome-mediated degradation specific to the mutant oncoprotein [101]. A phase I trial of taselisib was conducted enrolling patients with advanced solid tumors. The side effects were similar to those of alpelisib, including diarrhea, hyperglycemia, decreased appetite, nausea, rash, stomatitis, and vomiting. The ORR was 36% in the subset of BC with PIK3CA mutations, while no response was observed in patients with wild-type PIK3CA tumors [102]. In an additional large phase I trial of 166 patients with PIK3CA-mutated cancer, the ORR was 9%. In a subset of 17 patients with advanced TNBC, the ORR was 5.9%. Nevertheless, the response rate seemed to be higher in the case of helical domain mutations. Mutations in TP53 and PTEN seemed to be associated with resistance to taselisib [103]. A phase Ib study explored taselisib in combination with tamoxifen in patients with HR+ metastatic BC whose disease progressed after at least one prior line of ET (n = 30). The frequently observed adverse events were diarrhea, mucositis, and hyperglycemia. The ORR was 24%, and 40% of patients had an SD at 6 months or more [104]. A phase Ib dose-escalation and dose-expansion trial evaluated the association of taselisib plus taxane in metastatic BC and non-small-cell lung cancer (NSCLC). Seventy patients had BC, seven had NSCLC, and one had both. SAEs occurred in 90.5% (fatigue, diarrhea, nausea, and neutropenia), with 14.5% of adverse events leading to death. The ORR was 35% in the docetaxel arm and 20.4% in the paclitaxel arm. Despite interesting activity signals, such major toxicity limited further development of these associations [105].
A phase Ib trial enrolling heavily pretreated advanced HR+/HER2− BC patients evaluated taselisib with letrozole (n = 28). The ORR was 38% in the PIK3CA-mutated group and 9% in the wild-type PIK3CA group. The patients experienced diarrhea, hyperglycemia, and mucosal inflammation grade 3 or 4 adverse events, in 14%, 7%, and 7%, respectively [106]. The LORELEI trial was a phase II, multicenter, randomized, double-blind, placebo-controlled study of letrozole in association with taselisib versus letrozole, with placebo for early HR+/HER2− BC in the neoadjuvant setting (n = 334), 46% of them displaying PIK3CA mutations. The ORR was 50% in the taselisib group versus 39% in the placebo group (p = 0.049), but the pCR rate was very low in both arms (1 to 2%). An AE ≥ grade 3 occurred in 12% of patients, mainly with digestive or infectious toxicity [107]. Taselisib was also investigated in combination with fulvestrant in post-menopausal women with advanced HR+/HER2− BC, in a non-comparative phase II study enrolling 87 patients. For patients with PIK3CA-mutated BC, the ORR and CBR were 29.5% and 38.5%, respectively. Approximately 50% of patients experienced a grade 3 or more AE, which was colitis in 13% of cases. Of note, 21% of patients harbored both ESR1 and PIK3CA mutations [108,109]. These results led to a phase III study, the SANDPIPER trial, for PIK3CA-mutant advanced HR+/HER2− BC resistant to ET, evaluating fulvestrant associated with taselisib (n = 430) versus placebo (n = 176). The toxicity was significant, with 49.5% of patients experiencing an SAE in the taselisib arm versus 16.4% in the placebo arm. The most frequent AEs were diarrhea, hyperglycemia, rash, stomatitis, and colitis. A statistically significant but relatively limited improvement in PFS (7.4 months versus 5.4 months, HR 0.70, p = 0.0037) was observed in the taselisib-treated patients. The ORR was also increased with taselisib to 28.0% versus 11.9% with the placebo [110]. A multicenter, randomized phase II trial, enrolling patients with metastatic BC whose disease progressed after at least one prior line of ET, evaluated tamoxifen in association with taselisib or placebo. The median PFS was 3.2 months in the placebo arm and 4.8 months in the taselisib arm (unstratified HR 0.62, 95% CI 0.43–0.93) and CBR was 14.5% versus 22.4%. Approximately 24% of patients harbored a PIK3CA mutation. The toxicity rate of grade ≥ 3 adverse events was 44% in the taselisib arm versus 5% in the placebo arm, with no new safety signal [111].
Lehmann et al. conducted a phase Ib/II study in patients with metastatic AR+ BC to evaluate enzalutamide in combination with taselisib or placebo. Phase I included patients with HR+ BC and TNBC, whereas phase II included only patients with TNBC. In TNBC, the CBR was 35.7% in the taselisib arm versus 0% in the placebo arm. The CBR was 42.9% in PIK3CA-mutated patients versus 28.6% in wild-type PIK3CA. The CBR was 75% in the luminal androgen receptor (LAR) subtype versus 12.5% in the other subtypes (p = 0.06) [112]. In HER2+ BC, a phase Ib trial evaluated taselisib in combination with T-DM1 in metastatic BC, including 23% displaying PIK3CA mutations. Grade 3–4 toxicities included pneumonitis and thrombocytopenia at rates of 15.3% and 19.2%, respectively. The median PFS was 7.6 months and ORR was 33%, ranging from 40% to 22%, whether PIK3CA mutations were present or not, respectively [113].
Table 1 summarizes the phase III trials with PI3K inhibitors in advanced BC.
Table 1.
Phase III clinical trials of main PI3K inhibitors in breast cancer and approvals.
| Molecule | PIK3CA Subunit Target | Trial (Reference) |
Number of Patients |
Patients | Drugs | Results | Approval (FDA/EMA) |
|---|---|---|---|---|---|---|---|
| Buparlisib | Pan PI3K Inhibitor | BELLE-2 [59] |
1147 | HR+/HER2− ABC progressed on or after AI and a maximum of one previous line of CT | Fulvestrant + Buparlisib or Placebo |
mPFS 6.9 vs. 5.0 months HR 0.78 (95% CI 0.67–0.89, p = 0·00021) |
No approval |
| BELLE-3 [58] |
432 | HR+/HER2− ABC progressed on or after prior ET and mTOR inhibitors | Fulvestrant + Buparlisib or Placebo |
mPFS 3.9 vs. 1.8 months HR 0.67 (95% CI 0.53–0.84, p = 0·00030) |
|||
| BELLE-4 [60] |
416 | HR+/HER2− ABC receiving first-line CT | Paclitaxel + Buparlisib or Placebo |
mPFS 8.0 vs. 9.2 months HR 1.18 (95% CI 0.82–1.68) |
|||
| Alpelisib | p110α selective PI3K inhibitor | SOLAR-1 [85] |
341 | HR+/HER2− ABC with PIK3CA mutation and disease progression on or after prior AI | Fulvestrant + Alpelisib or Placebo |
mPFS 11.0 vs. 5.7 months HR 0.65 (95% CI, 0.50 to 0.85; p < 0.001) |
* FDA: for HR+ HER2− ABC PI3K-mutated who had received ET previously * EMA: for HR+ HER2− ABC PI3K-mutated who had received ET alone previously |
| Taselisib | Dual p110α/δ selective PI3K inhibitor | SANDPIPER [110] |
631 | HR+/HER2− ABC PIK3CA mutated resistant to ET | Fulvestrant + Taselisib or Placebo |
mPFS 7.4 vs. 5.4 months HR 0.70 (95% CI, 0.56–0.89 p = 0.0037) |
No approval |
FDA: food and drug administration; EMA: European medicine agency; HR: hazard ratio; HR: hormone receptor; HER2: human epidermal receptor 2; ABC: advanced breast cancer; mPFS: median progression-free survival; CI: confidence interval; vs.: versus; AI: aromatase inhibitor; ET: endocrine therapy; CT: chemotherapy. * Definition of the approval.
3.2.3. Inavolisib (GDC-0077)
Inavolisib is a recently developed, orally administered, strong p110α inhibitor, which induces a specific degradation of the mutated form of PIK3CA, as already described with taselisib, associated with an exquisite activity in PIK3CA-mutated cancer preclinical models [114,115]. A first-in-human, phase I dose-escalation, multi-arm trial of inavolisib included patients with advanced or metastatic tumors harboring PIK3CA mutations. The main significant toxicities included hyperglycemia, lymphopenia, fatigue, nausea, and weight loss. The CBR was estimated at 45%, with approximately 20% of patients with PR [116]. This phase I trial also examined the association of inavolisib with letrozole, with or without palbociclib. No DLT was observed at the recommended dose of inavolisib, which was 9 mg QD in both arms. The major SAEs were hyperglycemia, fatigue, hypokalemia, hyperglycemia and neutropenia, leukopenia, and thrombocytopenia in patients receiving inavolisib, without or with palbociclib, respectively. The CBR without or with palbociclib was 35% and 76%, respectively. A pharmacokinetic exploration did not find any interaction between the three drugs, and a pharmacodynamics study found an important downregulation of the PI3K pathway via a reduction in phosphorylated AKT [116].
In a phase Ib trial, the association of inavolisib with fulvestrant was examined in post-menopausal women with PIK3CA-mutated metastatic BC. The toxicity profile was similar to those previously described, except for a higher rate of hyperamylasemia and hyperlipasemia. The CBR was 60%, including 36% of patients achieving PR [117]. The association of inavolisib with fulvestrant and palbociclib was also examined in a phase Ib study enrolling PIK3CA-mutated HR+/HER2− metastatic BC patients. The grade ≥ 3 toxicities included hyperglycemia (47%) and neutropenia (47%). The CBR was evaluated at 61%, and 28% of patients had a PR [118].
3.2.4. Serabelisib (TAK-117)
Serabelisib is a novel PI3K inhibitor, with a very high level of selectivity against p110α and a strong ability to induce an inhibition of cell proliferation and apoptosis. A first-in-human phase I trial of oral serabelisib enrolled patients with advanced solid tumors and tested continuous or discontinuous regimens. The rate of grade ≥ 3 AEs ranged from 22% to 35%, depending on the drug regimen. The most frequent adverse events were ALAT/ASAT elevation and hyperglycemia. The response rate was low, and the use of serabelisib as a single agent required an intermittent schedule. Thus, given the limited efficacy, the development in monotherapy was stopped [119]. Nevertheless, the association of TAK-228 (oral mTORC1/2 inhibitor) with serabelisib was examined in metastatic TNBC, with the hypothesis that it could increase the tumor genomic instability and, consequently, increased the TNBC sensitivity to chemotherapy and immunotherapy. In a small-sized study of 10 patients with pre-treated TNBC, one PR, three SDs and six progressive diseases (PDs) were observed. Interestingly, a durable response to pembrolizumab after progression under this regimen was noted in 3 out of 10 patients [120]. The association of serabelisib-TAK-228 was also tested with paclitaxel, in patients with advanced ovarian, endometrial, or BC. The ORR was 47% in all cohorts, and the median PFS was approximately 11 months. The proportion of drug-related grade ≥ 3 adverse events was 9% [121].
Table 2 summarizes the ongoing trials of PI3K inhibitors in BC.
Table 2.
Ongoing phase I–III trials of PI3K inhibitors in breast cancer.
| Indication: Molecular Subtype | Population | Trial | Phase | Drug | Number of Patients | Primary Endpoint |
|---|---|---|---|---|---|---|
| HR+ HER2− |
ABC Postmenopausal Post AI + CDK4/6i ≤1 line of prior CT > or =1 prior line |
NCT05038735 / EPIK-B5 |
III | Fulvestrant + Alpelisib or Placebo |
234 | PFS |
| ABC Postmenopausal Pre/peri-menopausal (if LHRH agonist) |
NCT04191499 / INAVO 120 |
II/III | Palbociclib + Fulvestrant + Inavolisib or Placebo |
400 | PFS | |
| ABC Postmenopausal Pre/peri-menopausal (if LHRH agonist) |
NCT05646862 / INAVO 121 |
III | Fulvestrant + Alpelisib or Inavolisib |
400 | PFS | |
| ABC | NCT04355520 | I/II | Fulvestrant + TQ-B3525 (Selective PI3K α/δ inhibitor) |
42 | DLT | |
| ABC | NCT05504213 | I | Fulvestrant + HS-10352 (Selective PI3K α inhibitor) |
224 | MTD MAD ORR |
|
| ABC | NCT03056755 | II | Fulvestrant + Letrozole + Goserelin + Leuprolide + Alpelisib |
384 | PFS 6months | |
| ABC |
NCT05631795 / ALPINIST |
IV | Fulvestrant + Alpelisib |
100 | SAE ADR |
|
| ABC | NCT04856371 | I | Fulvestrant + Letrozole + Palbociclib + CYH33 (Selective PI3K α inhibitor) |
228 | DLT | |
| BC Ovarian cancer Solid tumor DDR gene mut +/− PIK3CA mut, Progressed prior PARP inhibitor |
NCT04586335 | I | Olaparib + CYH33 (Selective PI3K α inhibitor) |
350 | DLT ORR |
|
| ABC/Stage IV | NCT05216432 | I | Fulvestrant + RLY-2608 (Selective PI3K α inhibitor) |
190 | MTD AE SAE |
|
| ABC/Stage IV | NCT05501886 | III | Palbociclib + Fulvestrant + Alpelisib or Gedatolisib (Dual PI3K/mTOR Inhibitors) |
701 | PFS | |
| Stage IV | NCT03939897 | I/II | Abemaciclib + Fulvestrant + Copanlisib |
204 | DLT PFS |
|
| TNBC | ABC or stage IV ≤1 line of prior CT Part A—PIK3CAmut regardless of PTEN Part B1—PIK3CAmut PTEN loss Part B2—PTEN loss without PIK3CAmut |
NCT04251533 / EPIK-B3 |
III | Nab-paclitaxel + Alpelisib or Placebo |
137 | PFS (A and B2) ORR (B1) |
| Stage IV |
NCT05660083 / MpBC |
II | Nab-paclitaxel + Alpelisib + L-NMMA (iNOS inhibitor) |
36 | R2PD ORR |
|
| BC Renal cell carcinoma |
NCT03961698 / MARIO-3 |
II | Nab-paclitaxel + Bevacizumab + Atezolizumab + IPI-549 (Selective PI3K γ Inhibitor) |
91 | CR | |
| Multi tumor | NCT02637531 | I | Nivolumab + IPI-549 (Selective PI3K γ Inhibitor) |
219 | DLT AE |
|
| Multi tumor | NCT02646748 | I | Pembrolizumab + INCB050465 (Selective PI3K δ inhibitor) |
159 | Safety | |
| ABC AR+ PTEN positive |
NCT03207529 | I | Enzalutamide + Alpelisib |
28 | MTD | |
| HER2+ and/or HR+ | Depending on each group | NCT03006172 | I | A—Inavo B—Inavo + Palb/Let C—Inavo + Let D—Inavo + Fulv E—Inavo + Palb/Fulv F—Inavo + Palb/Fulv/Met, G—Inavo + Trastu/Pertu/HT |
256 | DLT R2PD SAE |
| Early BC |
NCT05306041 / GeparPiPPa |
II | Neoadjuvant: PHESGO + HT + Inavolisib |
170 | pCR | |
| ABC/stage IV |
NCT03767335 / B-PRECISE-01 |
I | Trastuzumab + MEN1611 (Selective PI3K α inhibitor) +/− Fulvestrant |
62 | MTD | |
| ABC |
NCT05063786 / ALPHABET |
III | Exp: Trastuzumab + Alpelisib +/− Fulvestrant Control: Trastuzumab + Vinorelbine + Capecitabine + Eribulin |
300 | PFS | |
| HER2+ | ABC |
NCT04208178 / EPIK-B2 |
III | Trastuzumab + Pertuzumab + Alpelisib or Placebo |
548 | DLT PFS |
| Stage IV | NCT04108858 | I/II | Trastuzumab + Pertuzumab + Copanlisib |
12 | SAE DLT PFS |
|
| ABC/Stage IV No limit of prior lines |
NCT02390427 | I | Trastuzumab + Pertuzumab + Trastuzumab emtansine + Paclitaxel + Taselisib |
68 | MTD | |
| ABC/stage IV |
NCT02705859 / Panther |
I | Trastuzumab + Copanlisib |
26 | MTD CBR |
|
| Stage IV | NCT05230810 | I/II | Fulvestrant + Tucatinib + Alpelisib |
40 | Safety PFS |
ABC: advanced breast cancer; HR: hormone receptor; HER2: human epidermal receptor 2; TNBC: triple negative breast cancer; AR: androgen receptor; HT: hormone therapy; PFS: progression-free survival; PFS2: next treatment progression-free survival; OS: overall survival; ORR: objective response rate; CBR: clinical benefice rate; DOR: duration of response; DOCR: duration of complete response; DCR: disease control rate; BOR: best overall response rate; TTR: time to response; TTCR: time to complete response; QoL: quality of life; AUC: Area Under the Concentration Time-Curve; Cmax: Maximum Plasma Concentration; Cmin: Minimum Plasma Concentration; AE: adverse events; SAE: serious adverse events; ADR: Adverse Drug Reactions; DLT: dose limiting toxicities; TTD: time to deterioration; MTD: maximum tolerated dose; RP2D: recommended phase II dose; MAD: Maximum applicable dose; TILs: Tumor Infiltrating Lymphocytes; IHC: immunohistochemistry; PK: pharmacokinetics markers; ctDNA: circulating tumor DNA; TTFST: time to first subsequent therapy; TTF: time to treatment failure; Inavo: Inavolisib; Palb: Palbociclib; Fulv: Fulvestrant; Let: Letrozole; Met: Metformin; Trastu: Trastuzumab; Pertu: Pertuzumab.
4. PI3K Inhibitors or CDK4/6 Inhibitors or Both in HR+/HER2− Disease?
While accumulating data have shown a survival benefit for adding a CDK4/6 inhibitor to ET in endocrine-resistant disease, no clinical trial has been performed comparing this class of drugs versus therapies targeting the PI3K/Akt/mTOR pathway, including PI3K inhibitors, when associated with ET. A recent study conducted by Han et al. used a network analysis, allowing for an indirect comparison between these two approaches, and it found an improvement in median PFS in favor of CDK4/6 inhibitors, as compared with PI3K inhibitors. This study included randomized trials evaluating the benefit and toxicity of CDK4/6 inhibitors or PI3K/AKT/mTOR inhibitors with ET between 2010 and 2019. A large cohort of 9771 patients was analyzed by a generic inverse variance method, allowing them to indirectly estimate the pooled HR and using a Bayesian framework to validate models with Markov Chain Monte Carlo methods. PFS was superior in the CDK4/6 inhibitors plus ET group compared to the PI3K inhibitors group (HR, 0.73; 95% CI, 0.62–0.86), while no statistical difference was shown in the AKT and mTOR inhibitors group. The benefits were observed in all subgroups, all metastatic lines, and in both visceral and bone-only diseases. The benefit of CDK4/6 inhibitors was also observed in OS, with a pooled HR at 0.78 (95% CI, 0.65–0.94) when they were compared with the PI3K and mTOR inhibitors. A risk ratio (RR) was used to evaluate grade ≥ 3 AEs. Cytopenia had a higher rate with CDK4/6 inhibitors. For cytopenia, the RR was significantly different in favor of abemaciclib versus palbociclib (RR = 0.024) and ribociclib (RR = 0.018). Hyperglycemia had a higher rate with the PI3K and mTOR inhibitors. RR was similar in the CDK4/6 and PI3K/AKT/mTOR inhibitors groups [122]. A second meta-analysis identified 79 phase II/III studies, including post-menopausal women, with HR+/HER2− BC, whose disease progressed after first-line treatment with AI or ET, and explored the CDK4/6 inhibitors or PI3K/AKT/mTOR inhibitors in association with fulvestrant. For the analysis, only eight studies were included. The authors showed that the CDK4/6 inhibitors (abemaciclib, palbociclib, and ribociclib) were superior in PFS, ORR, and OS compared to the PI3K inhibitors (pictilisib, alpelisib, and buparlisib) [123].
Robust pre-clinical data suggested a synergistic effect to the combination of CDK4/6 inhibitors with PI3K inhibitors (buparlisib and alpelisib) in HR+/HER2− BC. In a mouse model, O’Brien et al. demonstrated that the association of a CDK4/6 inhibitor to an ET significantly inhibited tumor growth compared to ET alone. Moreover, PI3K inhibitors in addition to CDK4/6 inhibitors and ET induced a tumor regression and one CR was also observed [124]. In an in vitro and in vivo study of TNBC cell lines with PIK3CA mutations, Asghar et al. found a synergistic effect of CDK4/6 inhibitors with PIK3CA inhibitors [125].
Concerning HR+/HER2− metastatic BC, a phase Ib trial tested the safety and preliminary efficiency of the association of ribociclib (continuous versus discontinuous) and fulvestrant, with/without alpelisib or buparlisib. Both arms with triple associations were suspended due to unacceptable toxicity and were not continued in phase II investigations [126]. Alpelisib was also explored in association with ribociclib and letrozole in a phase Ib/II trial dedicated to post-menopausal women. Three arms were tested, A1: letrozole/ribociclib (n = 41), A2: letrozole/alpelisib (n = 21), and A3: letrozole/ribociclib/alpelisib (n = 36). In the A3 patients, 22% had SD and 19% had PD. The safety profile was acceptable with the triple association, with grade 3/4 adverse events occurring in 22% for neutropenia, 17% for hyperglycemia, 11% for fatigue, and 6% for nausea. Arm A3 showed a considerable reduction in the Ki76 staining on tumor tissue compared to the A1 or A2 arms [127]. These results must be validated in a large, randomized trial. The triple association of palbociclib, taselisib, and fulvestrant was also explored in a phase Ib trial, conducted by Pascual et al., in patients with PIK3CA-mutated, HR+/HER2− advanced BC. In this population, with a median of three prior systemic therapy lines for advanced disease, the ORR was 37.5%. The high baseline cyclin E1 expression and early detection of ctDNA were associated with a shorter PFS. With the triple association, the most frequent grade 3/4 AEs were neutropenia (47.4%) and leucopenia (16.7%) [128].
Concerning the association of CDK4/6 inhibitors and PIK3CA inhibitors in TNBC, no trial was published. A pre-clinical study showed a synergistic effect, including a complete and durable response to combined CDK4/6 and PIK3CA inhibitors, in a mouse model [129]. For HER2+ BC, several preclinical studies demonstrated a favorable impact of CDK4/6 inhibitors and PIK3CA inhibitors, but their association was not evaluated at the clinical level [130].
5. Resistance to PI3K Inhibitors
Despite the central role in tumorigenesis of PI3K, only a modest benefit of PIK3CA inhibitors was observed in the clinical trials for both pan-PIK3CA and PIK3CAα-specific inhibitors. Thus, the clinical benefit could be limited by several resistance mechanisms. Figure 2 summarizes the main mechanisms of resistance to PI3K inhibitors and the corresponding therapeutic strategies to overcome them.
Figure 2.
Summary diagram of main mechanisms of resistance to PI3K inhibitors and potential inhibitors to counteract.
In BC and in prostate cancers, the role of the proviral integration of Moloney virus (PIM) kinase family has been highlighted. Song et al. found that PIM1 kinase promotes resistance and prevents cell death by decreasing the cellular level of ROS, which consequently attenuates the toxicity of PI3K inhibitors [131]. Furthermore, PIM1 conferred an acquired resistance to the PI3K inhibitors, since it appeared that PIM1 overexpression and PIK3CA mutations were mutually exclusive in treatment-naïve BC. In tumor biopsies obtained on disease progression under PI3K inhibitors, PIM1 was found to be overexpressed [132]. Based on the above-mentioned data, IBL-302, a PIM/PI3K/mTOR inhibitor, was developed. Pre-clinical data in BC cell lines demonstrated an antitumor efficacy in monotherapy and in combination with trastuzumab for HER2+ BC cell lines resistant to trastuzumab [133]. In BC cell lines, Litchfield et al. reported that abemaciclib can inhibit mTOR by blocking both PIM kinases and CDK4/6, which resulted in blocking the PI3K/Akt/mTOR pathway and reduced cell growth. When abemaciclib was associated with alpelisib in PIK3CA-mutated HR+ BC, it had a synergistic effect in neutralizing mTOR activation and increasing the effects on cellular proliferation. In fact, mTOR may be activated by three different pathways that are independent of each other, Akt via PI3K, PIM kinase, and CDK4. These results suggested a potential novel approach to counteract the acquired resistance mechanisms to PI3K inhibitors [134].
A network-based mathematical model was developed in HR+ PIK3CA-mutated BC cell lines, to identify the determinants of sensitivity or resistance to alpelisib. A novel resistance mechanism was proposed, involving Forkhead Box O 3 (FOXO3) downregulation. In addition, this model predicted the efficacy of alpelisib in combination with myeloid cell leukemia-1 (MCL1) inhibitors, a BH3 mimetic, which was further validated in BC cell lines [135]. Under physiological conditions, AKT negatively regulates FOXO3. AKT downregulation by PI3K inhibitors induces an activation of FOXO3 that can, therefore, be drawn into the nucleus and, then, activates transcription factors, leading to upregulation of RTK, such as HER2 or HER3. In BC, SGK3 is significantly higher in HR-positive disease than in TNBC. PI3K/AKT/mTOR pathway inhibition induced upregulation of the serum and glucocorticoid regulated kinase 3 (SGK3), and SGK3 can phosphorylate various substrates with several substrates in common with AKT and can activate mTOR independently of AKT. This is related to a strong homology between the two catalytic subunits [136]. An in vitro study showed, in PIK3CA-mutated BC cell lines, that both AKT and ERK were suppressed with pictilisib or alpelisib. Moreover, if RAS mutation co-occurred, the PI3K inhibitor failed to suppress the ERK pathway [137]. In BC cell lines, the upregulation of RPS6KA2 (RSK3) or RPS6KA6 (RSK4) promoted resistance to a PI3K inhibitor, and an RSK inhibitor in addition to PI3K inhibitor could overcome the resistance [138]. For patients with PIK3CA-mutated head and neck and esophageal squamous cell carcinomas, Elkabets et al. showed a novel acquired resistance mechanism to alpelisib. Patients who progressed after alpelisib presented an overexpression of AXL. AXL activated the epidermal growth factor receptor (EGFR) by dimerization/phosphorylation and then activated protein kinase C that led to mTOR activatione [139].
A higher activity of alpelisib has been associated with complete inhibition of mTORC1, and an increase in mTORC1 activity was found in BC cell lines with PIK3CA mutation and disease progression under alpelisib. Accordingly, the association of an mTOR inhibitor to alpelisib seemed to reverse the drug resistance [140]. Cai et al., using a whole-genome screen on HR+ PIK3CA-mutated BC cell lines, found that the loss of several genes (TSC1, TSC2, TBC1D7, AKT1S1, STK11…), which negatively regulate mTORC1, conferred resistance to PI3K inhibitors, and confirmed that mTOR inhibition could overcome resistance. Genomic alterations in mTORC1 regulators were found in 15% of HR+ PIK3CA-mutated BC samples, all of them being mutually exclusive [141].
Juric et al. analyzed progressive metastatic sites of patients who died after alpelisib treatment. Compared to the first biopsy, all sites harbored PTEN loss. Moreover, the PTEN alterations were different depending on sites, and all genomic alterations in PTEN led to resistance [142]. Razavi et al. examined biological samples from HR+ BC patients treated with alpelisib and AI in a phase I/II trial and searched for mechanisms of resistance. PTEN loss and ESR1 mutation were associated with resistance [143]. PTEN alterations (either mutation or homozygous deletions) could be found at baseline, associated with rapid disease progression, but also in the post-treatment samples, consistent with an acquired mechanism of resistance. Of note, PTEN-deficient cells are dependent on p110β for maintaining downstream AKT signaling and, thus, could be more sensitive to PI3Kβ inhibition or a combination of PI3Kα and PI3Kβ [144,145].
Hyperglycemia was one of the most frequent AEs found with PIK3CA inhibitors, with rates equal to 64% in SOLAR-1 [85], 43% in BELLE-2 [57], 37% in BELLE-3 [58], and 40% in the SANDPIPER trial [110]. For patients in which alpelisib was stopped due to unacceptable toxicity, hyperglycemia represented approximately 6%. Importantly, such hyperglycemia might induce plasma insulin rebound, thus leading to restoration of PI3K signaling and, ultimately, to treatment resistance [146]. In addition to the impact on the metabolism, an in vivo study showed that hyperglycemia could influence the BC tumor microenvironment. In co-culture in hyperglycemia compared to normal glycemia, adipocytes produced more inflammatory cytokines (IL-6, IL-8) and growth factors (VEGF, IGF1) and induced a more aggressive and invasive phenotype for BC cells in zebrafish [147]. An ongoing phase II study, entitled “Alpelisib, Fulvestrant and Dapagliflozin (SGLT2 inhibitor) for the Treatment of ER-positive, HER2-negative, PIK3CA-Mutant Metastatic Breast Cancer”, includes the same population as the SOLAR-1 trial to establish if the addition of dapagliflozin, an SGLT-2 inhibitor, could significantly reduce hyperglycemia of any grade (NCT05025735). Another phase II study in the same population examined if the association of dapagliflozin and metformin could reduce grade 3 hyperglycemia (EPIK-B4/NCT04899349). A Phase Ib/II study of serabelisib in combination with canagliflozin (an SGLT-2 inhibitor) in advanced solid tumors with PIK3CA mutations or KRAS mutations, including BC, is testing the efficiency of this association (NCT04073680). A phase II trial, “Preventing High Blood Sugar in People Being Treated for Metastatic Breast Cancer”, with three experimental arms in a similar population as SOLAR-1,a is exploring the rate of hyperglycemia grade 3 a with ketogenic diet, low-carbohydrate diet, or canagliflozin in combination with alpelisib and fulvestrant (NCT05090358).
Some of the adaptive mechanisms of resistance to PI3K inhibitors are described here (shown in yellow in the figure), along with their consequences (shown with red dotted arrows) and potential therapeutic possibilities (shown with green arrows). Loss of the tumor suppressor phosphatase and tensin homolog (PTEN) can appear before or after PI3K inhibitor use and lead to phosphatidylinositol 3,4,5 triphosphate (PIP3) overexpression. All PI3K inhibitors induce a disruption in the carbohydrate metabolism with hyperglycemia; hyperglycemia brings feedback that leads to insulin upregulation and reactivation of tyrosine kinase receptors (RTKs). Activation of mTOR, independent of protein kinase B (AKT), represents an important part of acquired resistance to PI3K inhibitors. We can find many paths of resistance, including the serum and glucocorticoid-regulated kinase 3 (SGK3), the proviral integration of Moloney virus (PIM), or PKC via activation of AXL and RTKs; these molecules induce activation of mTOR and, therefore, the pathway. AKT inactivation induces hypophosphorylation of Forkhead Box O 3 (FOXO3) and allows for the nuclear localization of FOXO3. FOXO3 induces myeloid cell leukemia-1 (MCL1) activation and upregulation of RTKs, leading to survival and overexpression of the PI3K pathway, respectively. In the RAS/MEK/ERK pathway, the PI3K inhibitor can inhibit ERK; however, the appearance of the KRAS mutation can induce activation of the pathway, despite ERK inhibition, and it has been observed that PI3K inhibitors induce upregulation of RPS6KA2 (RSK3) and RPS6KA6 (RSK4). L: ligand; IR: insulin receptor; PKC: protein kinase C; G: glucose; I: insulin.
6. Conclusions
PI3K inhibitors, including pan-inhibitors and selective inhibitors, are at the beginning of their development in BC. Although they have led to few authorizations so far, their field of evolution is considerable with the development of new more selective inhibitors. Pan-inhibitors have not shown the expected efficacy, but they have opened the way to PI3Kα-specific inhibitors. All BC subtypes have their own metabolic pathway, and a better understanding of specific mechanisms of resistance to these drugs in each subtype is necessary to adapt our therapeutic strategies. Despite the central role of PI3K in oncogenesis, only a modest antitumor activity has been observed, and the future of these drugs rests on the right choice of treatment association to limit the appearance of resistance via another molecular pathway. However, this is a toxicity profile that should not be overlooked, and learning to manage these drugs to facilitate their use as well as discovering predictive biomarkers of responses are the new challenges we must meet.
Acknowledgments
This work was supported by Institut Paoli-Calmettes.
Author Contributions
Conceptualization, A.B. and A.G.; validation, F.B. and A.G.; writing—original draft preparation, A.B.; writing—review and editing, F.B. and A.G.; supervision, A.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO Estimated Age-Standardized Incidence Rates (World) in 2020, World, Both Sexes, All Ages (excl. NMSC) 2020. [(accessed on 15 November 2022)]. Available online: https://gco.iarc.fr/today/online-analysis-multi-bars.
- 2.Baselga J., Cortés J., Kim S.-B., Im S.-A., Hegg R., Im Y.-H., Roman L., Pedrini J.L., Pienkowski T., Knott A., et al. Pertuzumab plus Trastuzumab plus Docetaxel for Metastatic Breast Cancer. N. Engl. J. Med. 2012;366:109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Finn R.S., Martin M., Rugo H.S., Jones S.E., Im S.-A., Gelmon K.A., Harbeck N., Lipatov O.N., Walshe J.M., Moulder S.L., et al. PALOMA-2: Primary results from a phase III trial of palbociclib (P) with letrozole (L) compared with letrozole alone in postmenopausal women with ER+/HER2− advanced breast cancer (ABC) J. Clin. Oncol. 2016;34:507. doi: 10.1200/JCO.2016.34.15_suppl.507. [DOI] [Google Scholar]
- 4.O’Shaughnessy J., Petrakova K., Sonke G.S., Conte P., Arteaga C.L., Cameron D.A., Hart L.L., Villanueva C., Jakobsen E., Beck J.T., et al. Ribociclib plus letrozole versus letrozole alone in patients with de novo HR+, HER2− advanced breast cancer in the randomized MONALEESA-2 trial. Breast Cancer Res. Treat. 2018;168:127–134. doi: 10.1007/s10549-017-4518-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnston S., Martin M., Di Leo A., Im S.-A., Awada A., Forrester T., Frenzel M., Hardebeck M.C., Cox J., Barriga S., et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer. 2019;5:5. doi: 10.1038/s41523-018-0097-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner N.C., Telli M.L., Rugo H.S., Mailliez A., Ettl J., Grischke E.-M., Mina L.A., Balmaña J., Fasching P.A., Hurvitz S.A., et al. A Phase II Study of Talazoparib after Platinum or Cytotoxic Nonplatinum Regimens in Patients with Advanced Breast Cancer and Germline BRCA1/2 Mutations (ABRAZO) Clin. Cancer Res. 2019;25:2717–2724. doi: 10.1158/1078-0432.CCR-18-1891. [DOI] [PubMed] [Google Scholar]
- 7.Litton J.K., Rugo H.S., Ettl J., Hurvitz S.A., Gonçalves A., Lee K.-H., Fehrenbacher L., Yerushalmi R., Mina L.A., Martin M., et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N. Engl. J. Med. 2018;379:753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sammons S., Tan T.J.Y., Traina T.A., Kim S.-B., Im Y.-H., Bachelder C., Marcom P.K., Dent R.A. Dora: A randomized phase II multicenter maintenance study of olaparib alone or olaparib in combination with durvalumab in platinum responsive advanced triple-negative breast cancer (aTNBC) J. Clin. Oncol. 2019;37:TPS1113. doi: 10.1200/JCO.2019.37.15_suppl.TPS1113. [DOI] [Google Scholar]
- 9.Eikesdal H.P., Yndestad S., Elzawahry A., Llop-Guevara A., Gilje B., Blix E.S., Espelid H., Lundgren S., Geisler J., Vagstad G., et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann. Oncol. 2021;32:240–249. doi: 10.1016/j.annonc.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Fruman D.A., Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Backer J.M. The Regulation of Class IA PI 3-Kinases by Inter-Subunit Interactions. In: Rommel C., Vanhaesebroeck B., Vogt P.K., editors. Phosphoinositide 3-Kinase in Health and Disease. Volume 346. Springer; Berlin/Heidelberg, Germany: 2010. pp. 87–114. Current Topics in Microbiology and Immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burke J.E., Williams R.L. Synergy in activating class I PI3Ks. Trends Biochem. Sci. 2015;40:88–100. doi: 10.1016/j.tibs.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Toker A. Achieving specificity in Akt signaling in cancer. Adv. Biol. Regul. 2012;52:78–87. doi: 10.1016/j.advenzreg.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vanhaesebroeck B., Leevers S.J., Ahmadi K., Timms J., Katso R., Driscoll P.C., Woscholski R., Parker P.J., Waterfield M.D. Synthesis and Function of 3-Phosphorylated Inositol Lipids. Annu. Rev. Biochem. 2001;70:535–602. doi: 10.1146/annurev.biochem.70.1.535. [DOI] [PubMed] [Google Scholar]
- 15.Goncalves M.D., Hopkins B.D., Cantley L.C. Phosphatidylinositol 3-Kinase, Growth Disorders, and Cancer. N. Engl. J. Med. 2018;379:2052–2062. doi: 10.1056/NEJMra1704560. [DOI] [PubMed] [Google Scholar]
- 16.Maehama T., Dixon J.E. The Tumor Suppressor, PTEN/MMAC1, Dephosphorylates the Lipid Second Messenger, Phosphatidylinositol 3,4,5-Trisphosphate. J. Biol. Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y., Kwok-Shing Ng P., Kucherlapati M., Chen F., Liu Y., Tsang Y.H., de Velasco G., Jeong K.J., Akbani R., Hadjipanayis A., et al. A Pan-Cancer Proteogenomic Atlas of PI3K/AKT/mTOR Pathway Alterations. Cancer Cell. 2017;31:820–832.e3. doi: 10.1016/j.ccell.2017.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Keniry M., Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 20.Li J. PTEN, a Putative Protein Tyrosine Phosphatase Gene Mutated in Human Brain, Breast, and Prostate Cancer. Science. 1997;275:1943–1947. doi: 10.1126/science.275.5308.1943. [DOI] [PubMed] [Google Scholar]
- 21.Teng D.H., Hu R., Lin H., Davis T., Iliev D., Frye C., Swedlund B., Hansen K.L., Vinson V.L., Gumpper K.L., et al. MMAC1/PTEN mutations in primary tumor specimens and tumor cell lines. Cancer Res. 1997;57:5221–5225. [PubMed] [Google Scholar]
- 22.Samuels Y., Ericson K. Oncogenic PI3K and its role in cancer. Curr. Opin. Oncol. 2006;18:77–82. doi: 10.1097/01.cco.0000198021.99347.b9. [DOI] [PubMed] [Google Scholar]
- 23.Álvarez-Garcia V., Tawil Y., Wise H.M., Leslie N.R. Mechanisms of PTEN loss in cancer: It’s all about diversity. Semin. Cancer Biol. 2019;59:66–79. doi: 10.1016/j.semcancer.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 24.Sanger Institute The Catalogue of Somatic Mutation in Cancer (COSMIC) [(accessed on 1 December 2022)]. Available online: https://cancer.sanger.ac.uk/cosmic/gene/analysis?ln=PIK3CA#distribution.
- 25.Fruman D.A., Chiu H., Hopkins B.D., Bagrodia S., Cantley L.C., Abraham R.T. The PI3K Pathway in Human Disease. Cell. 2017;170:605–635. doi: 10.1016/j.cell.2017.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karakas B., Bachman K.E., Park B.H. Mutation of the PIK3CA oncogene in human cancers. Br. J. Cancer. 2006;94:455–459. doi: 10.1038/sj.bjc.6602970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Samuels Y., Diaz L.A., Schmidt-Kittler O., Cummins J.M., DeLong L., Cheong I., Rago C., Huso D.L., Lengauer C., Kinzler K.W., et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 28.Bonelli M.A., Cavazzoni A., Saccani F., Alfieri R.R., Quaini F., La Monica S., Galetti M., Cretella D., Caffarra C., Madeddu D., et al. Inhibition of PI3K Pathway Reduces Invasiveness and Epithelial-to-Mesenchymal Transition in Squamous Lung Cancer Cell Lines Harboring PIK3CA Gene Alterations. Mol. Cancer. 2015;14:1916–1927. doi: 10.1158/1535-7163.MCT-14-0892. [DOI] [PubMed] [Google Scholar]
- 29.Biswas S.K. Metabolic Reprogramming of Immune Cells in Cancer Progression. Immunity. 2015;43:435–449. doi: 10.1016/j.immuni.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 30.Vanhaesebroeck B., Perry M.W.D., Brown J.R., André F., Okkenhaug K. PI3K inhibitors are finally coming of age. Nat. Rev. Drug Discov. 2021;20:741–769. doi: 10.1038/s41573-021-00209-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mollon L.E., Anderson E.J., Dean J.L., Warholak T.L., Aizer A., Platt E.A., Tang D.H., Davis L.E. A Systematic Literature Review of the Prognostic and Predictive Value of PIK3CA Mutations in HR+/HER2− Metastatic Breast Cancer. Clin. Breast Cancer. 2020;20:e232–e243. doi: 10.1016/j.clbc.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Mosele F., Stefanovska B., Lusque A., Tran Dien A., Garberis I., Droin N., Le Tourneau C., Sablin M.-P., Lacroix L., Enrico D., et al. Outcome and molecular landscape of patients with PIK3CA-mutated metastatic breast cancer. Ann. Oncol. 2020;31:377–386. doi: 10.1016/j.annonc.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Pang B., Cheng S., Sun S.-P., An C., Liu Z.-Y., Feng X., Liu G.-J. Prognostic role of PIK3CA mutations and their association with hormone receptor expression in breast cancer: A meta-analysis. Sci. Rep. 2015;4:6255. doi: 10.1038/srep06255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sobhani N., Roviello G., Corona S.P., Scaltriti M., Ianza A., Bortul M., Zanconati F., Generali D. The prognostic value of PI3K mutational status in breast cancer: A meta-analysis. J. Cell. Biochem. 2018;119:4287–4292. doi: 10.1002/jcb.26687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbareschi M., Buttitta F., Felicioni L., Cotrupi S., Barassi F., Del Grammastro M., Ferro A., Dalla Palma P., Galligioni E., Marchetti A. Different Prognostic Roles of Mutations in the Helical and Kinase Domains of the PIK3CA Gene in Breast Carcinomas. Clin. Cancer Res. 2007;13:6064–6069. doi: 10.1158/1078-0432.CCR-07-0266. [DOI] [PubMed] [Google Scholar]
- 36.Zhao L., Vogt P.K. Helical domain and kinase domain mutations in p110 of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc. Natl. Acad. Sci. USA. 2008;105:2652–2657. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mukohara T. PI3K mutations in breast cancer: Prognostic and therapeutic implications. Breast Cancer Targets Ther. 2015;7:111–123. doi: 10.2147/BCTT.S60696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mangone F., Bobrovnitchaia I., Salaorni S., Manuli E., Nagai M. PIK3CA exon 20 mutations are associated with poor prognosis in breast cancer patients. Clinics. 2012;67:1285–1290. doi: 10.6061/clinics/2012(11)11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo S., Loibl S., von Minckwitz G., Darb-Esfahani S., Lederer B., Denkert C. PIK3CA H1047R Mutation Associated with a Lower Pathological Complete Response Rate in Triple-Negative Breast Cancer Patients Treated with Anthracycline-Taxane-Based Neoadjuvant Chemotherapy. Cancer Res. Treat. 2020;52:689–696. doi: 10.4143/crt.2019.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hu H., Zhu J., Zhong Y., Geng R., Ji Y., Guan Q., Hong C., Wei Y., Min N., Qi A., et al. PIK3CA mutation confers resistance to chemotherapy in triple-negative breast cancer by inhibiting apoptosis and activating the PI3K/AKT/mTOR signaling pathway. Ann. Transl. Med. 2021;9:410. doi: 10.21037/atm-21-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vasan N., Razavi P., Johnson J.L., Shao H., Shah H., Antoine A., Ladewig E., Gorelick A., Lin T.-Y., Toska E., et al. Double PIK3CA mutations in cis increase oncogenicity and sensitivity to PI3Kα inhibitors. Science. 2019;366:714–723. doi: 10.1126/science.aaw9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ruiz-Saenz A., Dreyer C., Campbell M.R., Steri V., Gulizia N., Moasser M.M. HER2 Amplification in Tumors Activates PI3K/Akt Signaling Independent of HER3. Cancer Res. 2018;78:3645–3658. doi: 10.1158/0008-5472.CAN-18-0430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang L., Zhang Q., Zhang J., Sun S., Guo H., Jia Z., Wang B., Shao Z., Wang Z., Hu X. PI3K pathway activation results in low efficacy of both trastuzumab and lapatinib. BMC Cancer. 2011;11:248. doi: 10.1186/1471-2407-11-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chakrabarty A., Rexer B.N., Wang S.E., Cook R.S., Engelman J.A., Arteaga C.L. H1047R phosphatidylinositol 3-kinase mutant enhances HER2-mediated transformation by heregulin production and activation of HER3. Oncogene. 2010;29:5193–5203. doi: 10.1038/onc.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baselga J., Cortés J., Im S.-A., Clark E., Ross G., Kiermaier A., Swain S.M. Biomarker Analyses in CLEOPATRA: A Phase III, Placebo-Controlled Study of Pertuzumab in Human Epidermal Growth Factor Receptor 2–Positive, First-Line Metastatic Breast Cancer. J. Clin. Oncol. 2014;32:3753–3761. doi: 10.1200/JCO.2013.54.5384. [DOI] [PubMed] [Google Scholar]
- 46.Loibl S., Majewski I., Guarneri V., Nekljudova V., Holmes E., Bria E., Denkert C., Schem C., Sotiriou C., Loi S., et al. PIK3CA mutations are associated with reduced pathological complete response rates in primary HER2-positive breast cancer: Pooled analysis of 967 patients from five prospective trials investigating lapatinib and trastuzumab. Ann. Oncol. 2016;27:1519–1525. doi: 10.1093/annonc/mdw197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morris J.Z., Tissenbaum H.A., Ruvkun G. A phosphatidylinositol-3-OH kinase family member regulating longevity and diapause in Caenorhabditis elegans. Nature. 1996;382:536–539. doi: 10.1038/382536a0. [DOI] [PubMed] [Google Scholar]
- 48.Miller T.W., Hennessy B.T., González-Angulo A.M., Fox E.M., Mills G.B., Chen H., Higham C., García-Echeverría C., Shyr Y., Arteaga C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor–positive human breast cancer. J. Clin. Investig. 2010;120:2406–2413. doi: 10.1172/JCI41680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang M., Li J., Huang J., Luo M. The Predictive Role of PIK3CA Mutation Status on PI3K Inhibitors in HR+ Breast Cancer Therapy: A Systematic Review and Meta-Analysis. Biomed Res. Int. 2020;2020:1598037. doi: 10.1155/2020/7451576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rodon J., Dienstmann R., Serra V., Tabernero J. Development of PI3K inhibitors: Lessons learned from early clinical trials. Nat. Rev. Clin. Oncol. 2013;10:143–153. doi: 10.1038/nrclinonc.2013.10. [DOI] [PubMed] [Google Scholar]
- 51.O’Brien N.A., McDonald K., Tong L., von Euw E., Kalous O., Conklin D., Hurvitz S.A., di Tomaso E., Schnell C., Linnartz R., et al. Targeting PI3K/mTOR Overcomes Resistance to HER2-Targeted Therapy Independent of Feedback Activation of AKT. Clin. Cancer Res. 2014;20:3507–3520. doi: 10.1158/1078-0432.CCR-13-2769. [DOI] [PubMed] [Google Scholar]
- 52.Rodon J., Braña I., Siu L.L., De Jonge M.J., Homji N., Mills D., Di Tomaso E., Sarr C., Trandafir L., Massacesi C., et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Investig. New Drugs. 2014;32:670–681. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 53.McRee A.J., Marcom P.K., Moore D.T., Zamboni W.C., Kornblum Z.A., Hu Z., Phipps R., Anders C.K., Reeder-Hayes K., Carey L.A., et al. A Phase I Trial of the PI3K Inhibitor Buparlisib Combined With Capecitabine in Patients with Metastatic Breast Cancer. Clin. Breast Cancer. 2018;18:289–297. doi: 10.1016/j.clbc.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mayer I.A., Abramson V.G., Isakoff S.J., Forero A., Balko J.M., Kuba M.G., Sanders M.E., Yap J.T., Van den Abbeele A.D., Li Y., et al. Stand up to cancer phase Ib study of pan-phosphoinositide-3-kinase inhibitor buparlisib with letrozole in estrogen receptor-positive/human epidermal growth factor receptor 2-negative metastatic breast cancer. J. Clin. Oncol. 2014;32:1202–1209. doi: 10.1200/JCO.2013.54.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma Y., Aymeric L., Locher C., Mattarollo S.R., Delahaye N.F., Pereira P., Boucontet L., Apetoh L., Ghiringhelli F., Casares N., et al. Contribution of IL-17–producing γδ T cells to the efficacy of anticancer chemotherapy. J. Exp. Med. 2011;208:491–503. doi: 10.1084/jem.20100269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Welt A., Wiesweg M., Theurer S., Abenhardt W., Groschek M., Müller L., Schröder J., Tewes M., Chiabudini M., Potthoff K., et al. Buparlisib in combination with tamoxifen in pretreated patients with hormone receptor-positive, HER2-negative advanced breast cancer molecularly stratified for PIK3CA mutations and loss of PTEN expression. Cancer Med. 2020;9:4527–4539. doi: 10.1002/cam4.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Campone M., Im S.-A., Iwata H., Clemons M., Ito Y., Awada A., Chia S., Jagiełło-Gruszfeld A., Pistilli B., Tseng L.-M., et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant for postmenopausal, hormone receptor-positive, human epidermal growth factor receptor 2-negative, advanced breast cancer: Overall survival results from BELLE-2. Eur. J. Cancer. 2018;103:147–154. doi: 10.1016/j.ejca.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 58.Di Leo A., Johnston S., Lee K.S., Ciruelos E., Lønning P.E., Janni W., O’Regan R., Mouret-Reynier M.-A., Kalev D., Egle D., et al. Buparlisib plus fulvestrant in postmenopausal women with hormone-receptor-positive, HER2-negative, advanced breast cancer progressing on or after mTOR inhibition (BELLE-3): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2018;19:87–100. doi: 10.1016/S1470-2045(17)30688-5. [DOI] [PubMed] [Google Scholar]
- 59.Baselga J., Im S.-A., Iwata H., Cortés J., De Laurentiis M., Jiang Z., Arteaga C.L., Jonat W., Clemons M., Ito Y., et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18:904–916. doi: 10.1016/S1470-2045(17)30376-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Martín M., Chan A., Dirix L., O’Shaughnessy J., Hegg R., Manikhas A., Shtivelband M., Krivorotko P., Batista López N., Campone M., et al. A randomized adaptive phase II/III study of buparlisib, a pan-class I PI3K inhibitor, combined with paclitaxel for the treatment of HER2− advanced breast cancer (BELLE-4) Ann. Oncol. 2017;28:313–320. doi: 10.1093/annonc/mdw562. [DOI] [PubMed] [Google Scholar]
- 61.Garrido-Castro A.C., Saura C., Barroso-Sousa R., Guo H., Ciruelos E., Bermejo B., Gavilá J., Serra V., Prat A., Paré L., et al. Phase 2 study of buparlisib (BKM120), a pan-class I PI3K inhibitor, in patients with metastatic triple-negative breast cancer. Breast Cancer Res. 2020;22:120. doi: 10.1186/s13058-020-01354-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Saura C., Bendell J., Jerusalem G., Su S., Ru Q., De Buck S., Mills D., Ruquet S., Bosch A., Urruticoechea A., et al. Phase Ib Study of Buparlisib plus Trastuzumab in Patients with HER2-Positive Advanced or Metastatic Breast Cancer That Has Progressed on Trastuzumab-Based Therapy. Clin. Cancer Res. 2014;20:1935–1945. doi: 10.1158/1078-0432.CCR-13-1070. [DOI] [PubMed] [Google Scholar]
- 63.Pistilli B., Pluard T., Urruticoechea A., Farci D., Kong A., Bachelot T., Chan S., Han H.S., Jerusalem G., Urban P., et al. Phase II study of buparlisib (BKM120) and trastuzumab in patients with HER2+ locally advanced or metastatic breast cancer resistant to trastuzumab-based therapy. Breast Cancer Res. Treat. 2018;168:357–364. doi: 10.1007/s10549-017-4596-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Guerin M., Rezai K., Isambert N., Campone M., Autret A., Pakradouni J., Provansal M., Camerlo J., Sabatier R., Bertucci F., et al. PIKHER2: A phase IB study evaluating buparlisib in combination with lapatinib in trastuzumab-resistant HER2-positive advanced breast cancer. Eur. J. Cancer. 2017;86:28–36. doi: 10.1016/j.ejca.2017.08.025. [DOI] [PubMed] [Google Scholar]
- 65.Loibl S., de la Pena L., Nekljudova V., Zardavas D., Michiels S., Denkert C., Rezai M., Bermejo B., Untch M., Lee S.C., et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: A randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE) Eur. J. Cancer. 2017;85:133–145. doi: 10.1016/j.ejca.2017.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Folkes A.J., Ahmadi K., Alderton W.K., Alix S., Baker S.J., Box G., Chuckowree I.S., Clarke P.A., Depledge P., Eccles S.A., et al. The Identification of 2-(1H-Indazol-4-yl)-6-(4-methanesulfonyl-piperazin-1-ylmethyl)-4-morpholin-4-yl-thieno[3,2- d]pyrimidine (GDC-0941) as a Potent, Selective, Orally Bioavailable Inhibitor of Class I PI3 Kinase for the Treatment of Cancer. J. Med. Chem. 2008;51:5522–5532. doi: 10.1021/jm800295d. [DOI] [PubMed] [Google Scholar]
- 67.Sarker D., Ang J.E., Baird R., Kristeleit R., Shah K., Moreno V., Clarke P.A., Raynaud F.I., Levy G., Ware J.A., et al. First-in-Human Phase I Study of Pictilisib (GDC-0941), a Potent Pan–Class I Phosphatidylinositol-3-Kinase (PI3K) Inhibitor, in Patients with Advanced Solid Tumors. Clin. Cancer Res. 2015;21:77–86. doi: 10.1158/1078-0432.CCR-14-0947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schöffski P., Cresta S., Mayer I.A., Wildiers H., Damian S., Gendreau S., Rooney I., Morrissey K.M., Spoerke J.M., Ng V.W., et al. A phase Ib study of pictilisib (GDC-0941) in combination with paclitaxel, with and without bevacizumab or trastuzumab, and with letrozole in advanced breast cancer. Breast Cancer Res. 2018;20:109. doi: 10.1186/s13058-018-1015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krop I.E., Mayer I.A., Ganju V., Dickler M., Johnston S., Morales S., Yardley D.A., Melichar B., Forero-Torres A., Lee S.C., et al. Pictilisib for oestrogen receptor-positive, aromatase inhibitor-resistant, advanced or metastatic breast cancer (FERGI): A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2016;17:811–821. doi: 10.1016/S1470-2045(16)00106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vuylsteke P., Huizing M., Petrakova K., Roylance R., Laing R., Chan S., Abell F., Gendreau S., Rooney I., Apt D., et al. Pictilisib PI3Kinase inhibitor (a phosphatidylinositol 3-kinase [PI3K] inhibitor) plus paclitaxel for the treatment of hormone receptor-positive, HER2-negative, locally recurrent, or metastatic breast cancer: Interim analysis of the multicentre, placebo-controlled, phase II randomised PEGGY study. Ann. Oncol. 2016;27:2059–2066. doi: 10.1093/annonc/mdw320. [DOI] [PubMed] [Google Scholar]
- 71.Schmid P., Pinder S.E., Wheatley D., Macaskill J., Zammit C., Hu J., Price R., Bundred N., Hadad S., Shia A., et al. Phase II Randomized Preoperative Window-of-Opportunity Study of the PI3K Inhibitor Pictilisib Plus Anastrozole Compared With Anastrozole Alone in Patients With Estrogen Receptor–Positive Breast Cancer. J. Clin. Oncol. 2016;34:1987–1994. doi: 10.1200/JCO.2015.63.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schmid P., Pinder S., Wheatley D., Zummit C., Macaskill E., Hu J., Price R., Bundred N., Hadad S., Shia A., et al. Abstract P2-08-02: Interaction of PIK3CA mutation subclasses with response to preoperative treatment with the PI3K inhibitor pictilisib in patients with estrogen receptor-positive breast cancer. Am. Assoc. Cancer Res. 2019;79:P2-08. doi: 10.1158/1538-7445.SABCS18-P2-08-02. [DOI] [Google Scholar]
- 73.Liu N., Rowley B.R., Bull C.O., Schneider C., Haegebarth A., Schatz C.A., Fracasso P.R., Wilkie D.P., Hentemann M., Wilhelm S.M., et al. BAY 80-6946 Is a Highly Selective Intravenous PI3K Inhibitor with Potent p110α and p110δ Activities in Tumor Cell Lines and Xenograft Models. Mol. Cancer Ther. 2013;12:2319–2330. doi: 10.1158/1535-7163.MCT-12-0993-T. [DOI] [PubMed] [Google Scholar]
- 74.Patnaik A., Appleman L.J., Tolcher A.W., Papadopoulos K.P., Beeram M., Rasco D.W., Weiss G.J., Sachdev J.C., Chadha M., Fulk M., et al. First-in-human phase I study of copanlisib (BAY 80-6946), an intravenous pan-class I phosphatidylinositol 3-kinase inhibitor, in patients with advanced solid tumors and non-Hodgkin’s lymphomas. Ann. Oncol. 2016;27:1928–1940. doi: 10.1093/annonc/mdw282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ramanathan R.K., Von Hoff D.D., Eskens F., Blumenschein G., Richards D., Genvresse I., Reschke S., Granvil C., Skubala A., Peña C., et al. Phase Ib Trial of the PI3K Inhibitor Copanlisib Combined with the Allosteric MEK Inhibitor Refametinib in Patients with Advanced Cancer. Target. Oncol. 2020;15:163–174. doi: 10.1007/s11523-020-00714-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Heffron T.P., Wei B., Olivero A., Staben S.T., Tsui V., Do S., Dotson J., Folkes A.J., Goldsmith P., Goldsmith R., et al. Rational Design of Phosphoinositide 3-Kinase α Inhibitors That Exhibit Selectivity over the Phosphoinositide 3-Kinase β Isoform. J. Med. Chem. 2011;54:7815–7833. doi: 10.1021/jm2007084. [DOI] [PubMed] [Google Scholar]
- 77.Fritsch C., Huang A., Chatenay-Rivauday C., Schnell C., Reddy A., Liu M., Kauffmann A., Guthy D., Erdmann D., De Pover A., et al. Characterization of the Novel and Specific PI3Kα Inhibitor NVP-BYL719 and Development of the Patient Stratification Strategy for Clinical Trials. Mol. Cancer. 2014;13:1117–1129. doi: 10.1158/1535-7163.MCT-13-0865. [DOI] [PubMed] [Google Scholar]
- 78.Juric D., Rodon J., Tabernero J., Janku F., Burris H.A., Schellens J.H.M., Middleton M.R., Berlin J., Schuler M., Gil-Martin M., et al. Phosphatidylinositol 3-Kinase α–Selective Inhibition With Alpelisib (BYL719) in PIK3CA-Altered Solid Tumors: Results From the First-in-Human Study. J. Clin. Oncol. 2018;36:1291–1299. doi: 10.1200/JCO.2017.72.7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Baselga J., Curigliano G., Martín M., André F., Beck J.T., Tortora G., Wilke C., Charbonnier L., Blumenstein L., Donnet V., et al. Abstract CT061: A phase Ib study of alpelisib (BYL719) + everolimus ± exemestane in patients with advanced solid tumors or HR+/HER2−breast cancer. Am. Assoc. Cancer Res. 2016;76:CT061. doi: 10.1158/1538-7445.AM2016-CT061. [DOI] [Google Scholar]
- 80.Curigliano G., Martin M., Jhaveri K., Beck J.T., Tortora G., Fazio N., Maur M., Hubner R.A., Lahner H., Donnet V., et al. Alpelisib in combination with everolimus ± exemestane in solid tumours: Phase Ib randomised, open-label, multicentre study. Eur. J. Cancer. 2021;151:49–62. doi: 10.1016/j.ejca.2021.03.042. [DOI] [PubMed] [Google Scholar]
- 81.Jhaveri K., Juric D., Yap Y.-S., Cresta S., Layman R.M., Duhoux F.P., Terret C., Takahashi S., Huober J., Kundamal N., et al. A Phase I Study of LSZ102, an Oral Selective Estrogen Receptor Degrader, with or without Ribociclib or Alpelisib, in Patients with Estrogen Receptor–Positive Breast Cancer. Clin. Cancer Res. 2021;27:5760–5770. doi: 10.1158/1078-0432.CCR-21-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mayer I.A., Abramson V.G., Formisano L., Balko J.M., Estrada M.V., Sanders M.E., Juric D., Solit D., Berger M.F., Won H.H., et al. A Phase Ib Study of Alpelisib (BYL719), a PI3Kα-Specific Inhibitor, with Letrozole in ER+/HER2− Metastatic Breast Cancer. Clin. Cancer Res. 2017;23:26–34. doi: 10.1158/1078-0432.CCR-16-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Juric D., Janku F., Rodón J., Burris H.A., Mayer I.A., Schuler M., Seggewiss-Bernhardt R., Gil-Martin M., Middleton M.R., Baselga J., et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor–Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA Oncol. 2019;5:e184475. doi: 10.1001/jamaoncol.2018.4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lu Y.-S., Lee K.S., Chao T.-Y., Tseng L.-M., Chitapanarux I., Chen S.-C., Liu C.-T., Sohn J., Kim J.H., Chang Y.-C., et al. A Phase Ib Study of Alpelisib or Buparlisib Combined with Tamoxifen Plus Goserelin in Premenopausal Women with HR-Positive HER2-Negative Advanced Breast Cancer. Clin. Cancer Res. 2021;27:408–417. doi: 10.1158/1078-0432.CCR-20-1008. [DOI] [PubMed] [Google Scholar]
- 85.André F., Ciruelos E., Rubovszky G., Campone M., Loibl S., Rugo H.S., Iwata H., Conte P., Mayer I.A., Kaufman B., et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. N. Engl. J. Med. 2019;380:1929–1940. doi: 10.1056/NEJMoa1813904. [DOI] [PubMed] [Google Scholar]
- 86.Rugo H.S., André F., Yamashita T., Cerda H., Toledano I., Stemmer S.M., Jurado J.C., Juric D., Mayer I., Ciruelos E.M., et al. Time course and management of key adverse events during the randomized phase III SOLAR-1 study of PI3K inhibitor alpelisib plus fulvestrant in patients with HR-positive advanced breast cancer. Ann. Oncol. 2020;31:1001–1010. doi: 10.1016/j.annonc.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 87.Ciruelos E.M., Rugo H.S., Mayer I.A., Levy C., Forget F., Delgado Mingorance J.I., Safra T., Masuda N., Park Y.H., Juric D., et al. Patient-Reported Outcomes in Patients with PIK3CA-Mutated Hormone Receptor–Positive, Human Epidermal Growth Factor Receptor 2–Negative Advanced Breast Cancer from SOLAR-1. J. Clin. Oncol. 2021;39:2005–2015. doi: 10.1200/JCO.20.01139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rugo H.S., Lerebours F., Ciruelos E., Drullinsky P., Ruiz-Borrego M., Neven P., Park Y.H., Prat A., Bachelot T., Juric D., et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021;22:489–498. doi: 10.1016/S1470-2045(21)00034-6. [DOI] [PubMed] [Google Scholar]
- 89.Juric D., Turner N., Prat A., Chia S., Ciruelos E. Abstract P5-13-03: Alpelisib + endocrine therapy (ET) in patients with hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−), PIK3CA-mutated advanced breast cancer (ABC) previously treated with cyclin-dependent kinase 4/6 inhibitor (CDK4/6i): Biomarker analyses from the Phase II BYLieve study. Cancer Res. 2022;82:P5-13. [Google Scholar]
- 90.Chia S., Ciruelos E., Rugo H., Lerebours F. Abstract P1-18-08: Effect of Duration of Prior Cyclin-Dependent Kinase 4/6 Inhibitor (CDK4/6i) Therapy (≤6 mo or >6 mo) on Alpelisib Benefit in Patients With Hormone Receptor-Positive (HR+), Human Epidermal Growth Factor Receptor 2-Negative (HER2−), PIK3CA-Mutated Advanced Breast Cancer (ABC) from BYLieve. Cancer Res. 2022;82:P1-18. [Google Scholar]
- 91.Cardoso F., Juric D., Lerebours F., Krop I., Ruiz Borrego M., Neven P., Park Y.H., Yardley D., Jhaveri K., Arce C., et al. 175P Alpelisib (ALP) + endocrine therapy (ET) in patients (pts) with PIK3CA-mutated, hormone receptor-positive (HR+), human epidermal growth factor receptor 2-negative (HER2−) advanced breast cancer (ABC): Subgroup analyses from the BYLieve study. Ann. Oncol. 2022;33:S206–S207. doi: 10.1016/j.annonc.2022.03.194. [DOI] [Google Scholar]
- 92.Bello D., Bertucci A., De La Motte Rouge T., Blonz C., Akla S., Grenier J., Bailleux C., Benderra M.A., Simon H., Desmoulins I., et al. Alpelisib and fulvestrant efficacy in HR-positive HER2-negative PIK3CA -mutant advanced breast cancer: Data from the French early access program. J. Clin. Oncol. 2021;39:1064. doi: 10.1200/JCO.2021.39.15_suppl.1064. [DOI] [Google Scholar]
- 93.André F., Goncalves A., Filleron T., Dalenc F. Clinical utility of molecular tumor profiling: Results from the randomized trial SAFIR01-BREAST. Cancer Res. 2022;82:GS1-10. doi: 10.1158/1538-7445.SABCS21-GS1-10. [DOI] [Google Scholar]
- 94.Mayer I.A., Prat A., Egle D., Blau S., Fidalgo J.A.P., Gnant M., Fasching P.A., Colleoni M., Wolff A.C., Winer E.P., et al. A Phase II Randomized Study of Neoadjuvant Letrozole Plus Alpelisib for Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer (NEO-ORB) Clin. Cancer Res. 2019;25:2975–2987. doi: 10.1158/1078-0432.CCR-18-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sharma P., Abramson V.G., O’Dea A., Nye L., Mayer I., Pathak H.B., Hoffmann M., Stecklein S.R., Elia M., Lewis S., et al. Clinical and Biomarker Results from Phase I/II Study of PI3K Inhibitor Alpelisib plus Nab-paclitaxel in HER2-Negative Metastatic Breast Cancer. Clin. Cancer Res. 2021;27:3896–3904. doi: 10.1158/1078-0432.CCR-20-4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Damodaran S., Litton J.K., Hess K.R., Eppig C.T., Grzegorzewski K.J., Meric-Bernstam F., Wistuba I.I., White J.B., Rauch G.M., Candelaria R.P., et al. Abstract OT2-06-01: A phase-2 trial of neoadjuvant alpelisib and nab-paclitaxel in anthracycline refractory triple negative breast cancers with PIK3CA or PTEN alterations. Am. Assoc. Cancer Res. 2020;80:OT2-06. doi: 10.1158/1538-7445.SABCS19-OT2-06-01. [DOI] [Google Scholar]
- 97.Batalini F., Xiong N., Tayob N., Polak M., Eismann J., Cantley L.C., Shapiro G.I., Adalsteinsson V., Winer E.P., Konstantinopoulos P.A., et al. Phase 1b Clinical Trial with Alpelisib plus Olaparib for Patients with Advanced Triple-Negative Breast Cancer. Clin. Cancer Res. 2022;28:1493–1499. doi: 10.1158/1078-0432.CCR-21-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jain S., Shah A.N., Santa-Maria C.A., Siziopikou K., Rademaker A., Helenowski I., Cristofanilli M., Gradishar W.J. Phase I study of alpelisib (BYL-719) and trastuzumab emtansine (T-DM1) in HER2-positive metastatic breast cancer (MBC) after trastuzumab and taxane therapy. Breast Cancer Res. Treat. 2018;171:371–381. doi: 10.1007/s10549-018-4792-0. [DOI] [PubMed] [Google Scholar]
- 99.Jhaveri K., Drago J.Z., Shah P.D., Wang R., Pareja F., Ratzon F., Iasonos A., Patil S., Rosen N., Fornier M.N., et al. A Phase I Study of Alpelisib in Combination with Trastuzumab and LJM716 in Patients with PIK3CA-Mutated HER2-Positive Metastatic Breast Cancer. Clin. Cancer Res. 2021;27:3867–3875. doi: 10.1158/1078-0432.CCR-21-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hurvitz S.A., Chia S.K.L., Ciruelos E.M., Hu X., Im S.-A., Janni W., Jerusalem G., Lacouture M., O’Regan R., Rugo H.S., et al. 352TiP EPIK-B2: A phase III study of alpelisib (ALP) as maintenance therapy with trastuzumab (T) and pertuzumab (P) in patients (pts) with PIK3CA-mutated (mut) human epidermal growth factor receptor-2–positive (HER2+) advanced breast cancer (ABC) Ann. Oncol. 2020;31:S389–S390. doi: 10.1016/j.annonc.2020.08.454. [DOI] [Google Scholar]
- 101.Ndubaku C.O., Heffron T.P., Staben S.T., Baumgardner M., Blaquiere N., Bradley E., Bull R., Do S., Dotson J., Dudley D., et al. Discovery of 2-{3-[2-(1-Isopropyl-3-methyl-1 H-1,2–4-triazol-5-yl)-5,6-dihydrobenzo[f]imidazo[1,2-d][1,4]oxazepin-9-yl]-1 H-pyrazol-1-yl}-2-methylpropanamide (GDC-0032): A β-Sparing Phosphoinositide 3-Kinase Inhibitor with High Unbound Exposure and Robust in Vivo Antitumor Activity. J. Med. Chem. 2013;56:4597–4610. doi: 10.1021/jm4003632. [DOI] [PubMed] [Google Scholar]
- 102.Juric D., Krop I., Ramanathan R.K., Wilson T.R., Ware J.A., Sanabria Bohorquez S.M., Savage H.M., Sampath D., Salphati L., Lin R.S., et al. Phase I Dose-Escalation Study of Taselisib, an Oral PI3K Inhibitor, in Patients with Advanced Solid Tumors. Cancer Discov. 2017;7:704–715. doi: 10.1158/2159-8290.CD-16-1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Jhaveri K., Chang M.T., Juric D., Saura C., Gambardella V., Melnyk A., Patel M.R., Ribrag V., Ma C.X., Aljumaily R., et al. Phase I Basket Study of Taselisib, an Isoform-Selective PI3K Inhibitor, in Patients with PIK3CA-Mutant Cancers. Clin. Cancer Res. 2021;27:447–459. doi: 10.1158/1078-0432.CCR-20-2657. [DOI] [PubMed] [Google Scholar]
- 104.Baird R.D., van Rossum A.G.J., Oliveira M., Beelen K., Gao M., Schrier M., Mandjes I.A.M., Garcia-Corbacho J., Vallier A.-L., Dougall G., et al. POSEIDON Trial Phase 1b Results: Safety, Efficacy and Circulating Tumor DNA Response of the Beta Isoform-Sparing PI3K Inhibitor Taselisib (GDC-0032) Combined with Tamoxifen in Hormone Receptor Positive Metastatic Breast Cancer Patients. Clin. Cancer Res. 2019;25:6598–6605. doi: 10.1158/1078-0432.CCR-19-0508. [DOI] [PubMed] [Google Scholar]
- 105.Abramson V.G., Oliveira M., Cervantes A., Wildiers H., Patel M.R., Bauer T.M., Bedard P.L., Becerra C., Richey S., Wei M.C., et al. A phase Ib, open-label, dose-escalation study of the safety and pharmacology of taselisib (GDC-0032) in combination with either docetaxel or paclitaxel in patients with HER2-negative, locally advanced, or metastatic breast cancer. Breast Cancer Res. Treat. 2019;178:121–133. doi: 10.1007/s10549-019-05360-3. [DOI] [PubMed] [Google Scholar]
- 106.Saura C., Sachdev J., Patel M.R., Cervantes A., Juric D., Infante J.R., Richards D., Sanabria S., Lu X., Ware J., et al. Abstract PD5-2: Ph1b study of the PI3K inhibitor taselisib (GDC-0032) in combination with letrozole in patients with hormone receptor-positive advanced breast cancer. Am. Assoc. Cancer Res. 2015;75:PD5-2. doi: 10.1158/1538-7445.SABCS14-PD5-2. [DOI] [Google Scholar]
- 107.Saura C., Hlauschek D., Oliveira M., Zardavas D., Jallitsch-Halper A., de la Peña L., Nuciforo P., Ballestrero A., Dubsky P., Lombard J.M., et al. Neoadjuvant letrozole plus taselisib versus letrozole plus placebo in postmenopausal women with oestrogen receptor-positive, HER2-negative, early-stage breast cancer (LORELEI): A multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2019;20:1226–1238. doi: 10.1016/S1470-2045(19)30334-1. [DOI] [PubMed] [Google Scholar]
- 108.Dickler M., Saura C., Oliveira M., Richards D., Krop I., Cervantes A., Stout T., Jin H., Savage H., Wilson T., et al. Abstract P6-12-01: Phase II study of taselisib (GDC-0032) plus fulvestrant in HER2-negative, hormone receptor-positive advanced breast cancer: Analysis by PIK3CA and ESR1 mutation status from circulating tumor DNA. Am. Assoc. Cancer Res. 2017;77:P6-12. doi: 10.1158/1538-7445.SABCS16-P6-12-01. [DOI] [Google Scholar]
- 109.Dickler M.N., Saura C., Richards D.A., Krop I.E., Cervantes A., Bedard P.L., Patel M.R., Pusztai L., Oliveira M., Cardenas A.K., et al. Phase II Study of Taselisib (GDC-0032) in Combination with Fulvestrant in Patients with HER2-Negative, Hormone Receptor–Positive Advanced Breast Cancer. Clin. Cancer Res. 2018;24:4380–4387. doi: 10.1158/1078-0432.CCR-18-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dent S., Cortés J., Im Y.-H., Diéras V., Harbeck N., Krop I.E., Wilson T.R., Cui N., Schimmoller F., Hsu J.Y., et al. Phase III randomized study of taselisib or placebo with fulvestrant in estrogen receptor-positive, PIK3CA-mutant, HER2-negative, advanced breast cancer: The SANDPIPER trial. Ann. Oncol. 2021;32:197–207. doi: 10.1016/j.annonc.2020.10.596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Oliveira M., Baird R.D., Voorthuis R.A.B., De Boo L., van Rossum A.G.J., Garrigos Cubells L., Muñoz S., López-García D., Saura Manich C., Schrier M., et al. LBA18 POSEIDON randomized phase II trial: Tamoxifen (TAM) + taselisib or placebo (PLA) in patients (pts) with hormone receptor positive (HR+)/HER2− metastatic breast cancer (MBC) Ann. Oncol. 2021;32:S1291–S1292. doi: 10.1016/j.annonc.2021.08.2091. [DOI] [Google Scholar]
- 112.Lehmann B.D., Abramson V.G., Sanders M.E., Mayer E.L., Haddad T.C., Nanda R., Van Poznak C., Storniolo A.M., Nangia J.R., Gonzalez-Ericsson P.I., et al. TBCRC 032 IB/II Multicenter Study: Molecular Insights to AR Antagonist and PI3K Inhibitor Efficacy in Patients with AR+ Metastatic Triple-Negative Breast Cancer. Clin. Cancer Res. 2020;26:2111–2123. doi: 10.1158/1078-0432.CCR-19-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Metzger Filho O., Goel S., Barry W.T., Hamilton E.P., Tolaney S.M., Yardley D.A., Rees R., Demeo M., Mills C., Hafner M., et al. A mouse-human phase I co-clinical trial of taselisib in combination with TDM1 in advanced HER2-positive breast cancer (MBC) J. Clin. Oncol. 2017;35:1030. doi: 10.1200/JCO.2017.35.15_suppl.1030. [DOI] [Google Scholar]
- 114.Hanan E.J., Braun M.-G., Heald R.A., MacLeod C., Chan C., Clausen S., Edgar K.A., Eigenbrot C., Elliott R., Endres N., et al. Discovery of GDC-0077 (Inavolisib), a Highly Selective Inhibitor and Degrader of Mutant PI3Kα. J. Med. Chem. 2022;65:16589–16621. doi: 10.1021/acs.jmedchem.2c01422. [DOI] [PubMed] [Google Scholar]
- 115.Song K.W., Edgar K.A., Hanan E.J., Hafner M., Oeh J., Merchant M., Sampath D., Nannini M.A., Hong R., Phu L., et al. RTK-Dependent Inducible Degradation of Mutant PI3Kα Drives GDC-0077 (Inavolisib) Efficacy. Cancer Discov. 2022;12:204–219. doi: 10.1158/2159-8290.CD-21-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Juric D., Kalinsky K., Oliveira M., Cervantes A., Bedard P., Krop I., Hamilton E., Schmid P., Varga A., Turner N., et al. Abstract OT1-08-04: A first-in-human phase Ia dose escalation study of GDC-0077, a p110a-selective and mutant-degrading PI3K inhibitor, in patients with PIK3CA -mutant solid tumors. Am. Assoc. Cancer Res. 2020;80:OT1-08. doi: 10.1158/1538-7445.SABCS19-OT1-08-04. [DOI] [Google Scholar]
- 117.Kalinsky K., Juric D., Bedard P.L., Oliveira M., Cervantes A., Hamilton E., Krop I.E., Turner N., Schmid P., Varga A., et al. Abstract CT109: A phase I/Ib study evaluating GDC-0077 plus fulvestrant in patients with PIK3CA-mutant, hormone receptor-positive/HER2-negative breast cancer. Am. Assoc. Cancer Res. 2020;80:CT109. doi: 10.1158/1538-7445.AM2020-CT109. [DOI] [Google Scholar]
- 118.Bedard P.L., Jhaveri K., Cervantes A., Gambardella V., Hamilton E., Italiano A., Kalinsky K., Krop I.E., Oliveira M., Saura C., et al. Abstract PD1-02: A phase I/Ib study evaluating GDC-0077 + palbociclib (palbo) + fulvestrant in patients (pts) with PIK3CA -mutant (mut), hormone receptor-positive/HER2-negative metastatic breast cancer (HR+/HER2− mBC) Am. Assoc. Cancer Res. 2021;81:PD1-02. doi: 10.1158/1538-7445.SABCS20-PD1-02. [DOI] [Google Scholar]
- 119.Juric D., de Bono J.S., LoRusso P.M., Nemunaitis J., Heath E.I., Kwak E.L., Macarulla Mercadé T., Geuna E., Jose de Miguel-Luken M., Patel C., et al. A First-in-Human, Phase I, Dose-Escalation Study of TAK-117, a Selective PI3Kα Isoform Inhibitor, in Patients with Advanced Solid Malignancies. Clin. Cancer Res. 2017;23:5015–5023. doi: 10.1158/1078-0432.CCR-16-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.O’Shaughnessy J., Rodriguez E.S.R., Ontiveros P., Wenstrup R., Pramparo T., Lang J., Zismann V., Briones N., Hendricks W., Espina V., et al. Abstract PS11-25: Pilot trial of priming with oral TAK-228 and TAK-117 (PIKTOR) to increase DNA damage repair deficiency (DDRD) followed by cisplatin (cis) and nab paclitaxel (nab pac) in chemotherapy-pretreated metastatic triple negative breast cancer (metTNBC) pts. Am. Assoc. Cancer Res. 2021;81:PS11-25. [Google Scholar]
- 121.Starks D., Rojas-Espaillat L.A., Dey N., De P., Leyland-Jones B., Williams C.B. Final results of phase 1 evaluation of the safety and clinical activity of sapanisertib in combination with serabelisib and paclitaxel in patients with advanced ovarian, endometrial, or breast cancer. J. Clin. Oncol. 2021;39:5569. doi: 10.1200/JCO.2021.39.15_suppl.5569. [DOI] [Google Scholar]
- 122.Han Y., Wang J., Wang Z., Xu B. Comparative efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors in women with hormone receptor-positive, HER2-negative metastatic breast cancer: A systematic review and network meta-analysis. Curr. Probl. Cancer. 2020;44:100606. doi: 10.1016/j.currproblcancer.2020.100606. [DOI] [PubMed] [Google Scholar]
- 123.Leung J.H., Leung H.W.C., Wang S.-Y., Huang S.-S., Chan A.L.F. Efficacy and safety of CDK4/6 and PI3K/AKT/mTOR inhibitors as second-line treatment in postmenopausal patients with hormone receptor-positive, HER-2-negative metastatic breast cancer: A network meta-analysis. Expert Opin. Drug Saf. 2021;20:949–957. doi: 10.1080/14740338.2021.1931116. [DOI] [PubMed] [Google Scholar]
- 124.O’Brien N.A., Tomaso E.D., Ayala R., Tong L., Issakhanian S., Linnartz R., Finn R.S., Hirawat S., Slamon D.J. Abstract 4756: In vivo efficacy of combined targeting of CDK4/6, ER and PI3K signaling in ER+ breast cancer. Am. Assoc. Cancer Res. 2014;74:4756. doi: 10.1158/1538-7445.AM2014-4756. [DOI] [Google Scholar]
- 125.Asghar U.S., Barr A.R., Cutts R., Beaney M., Babina I., Sampath D., Giltnane J., Lacap J.A., Crocker L., Young A., et al. Single-Cell Dynamics Determines Response to CDK4/6 Inhibition in Triple-Negative Breast Cancer. Clin. Cancer Res. 2017;23:5561–5572. doi: 10.1158/1078-0432.CCR-17-0369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tolaney S.M., Im Y.-H., Calvo E., Lu Y.-S., Hamilton E., Forero-Torres A., Bachelot T., Maur M., Fasolo A., Tiedt R., et al. Phase Ib Study of Ribociclib plus Fulvestrant and Ribociclib plus Fulvestrant plus PI3K Inhibitor (Alpelisib or Buparlisib) for HR+ Advanced Breast Cancer. Clin. Cancer Res. 2021;27:418–428. doi: 10.1158/1078-0432.CCR-20-0645. [DOI] [PubMed] [Google Scholar]
- 127.Juric D., Ismail-Khan R., Campone M., García-Estévez L., Becerra C., De Boer R., Hamilton E., Mayer I., Hui R., Lathrop K., et al. Abstract P3-14-01: Phase Ib/II study of ribociclib and alpelisib and letrozole in ER+, HER2− breast cancer: Safety, preliminary efficacy and molecular analysis. Am. Assoc. Cancer Res. 2016;76:P3-14. doi: 10.1158/1538-7445.SABCS15-P3-14-01. [DOI] [Google Scholar]
- 128.Pascual J., Lim J.S.J., Macpherson I.R., Armstrong A.C., Ring A., Okines A.F.C., Cutts R.J., Herrera-Abreu M.T., Garcia-Murillas I., Pearson A., et al. Triplet Therapy with Palbociclib, Taselisib, and Fulvestrant in PIK3CA-Mutant Breast Cancer and Doublet Palbociclib and Taselisib in Pathway-Mutant Solid Cancers. Cancer Discov. 2021;11:92–107. doi: 10.1158/2159-8290.CD-20-0553. [DOI] [PubMed] [Google Scholar]
- 129.Teo Z.L., Versaci S., Dushyanthen S., Caramia F., Savas P., Mintoff C.P., Zethoven M., Virassamy B., Luen S.J., McArthur G.A., et al. Combined CDK4/6 and PI3Kα Inhibition Is Synergistic and Immunogenic in Triple-Negative Breast Cancer. Cancer Res. 2017;77:6340–6352. doi: 10.1158/0008-5472.CAN-17-2210. [DOI] [PubMed] [Google Scholar]
- 130.Agostinetto E., Debien V., Marta G.N., Lambertini M., Piccart-Gebhart M., de Azambuja E. CDK4/6 and PI3K inhibitors: A new promise for patients with HER2-positive breast cancer. Eur. J. Clin. Investig. 2021;51:e13535. doi: 10.1111/eci.13535. [DOI] [PubMed] [Google Scholar]
- 131.Song J.H., Singh N., Luevano L.A., Padi S.K.R., Okumura K., Olive V., Black S.M., Warfel N.A., Goodrich D.W., Kraft A.S. Mechanisms Behind Resistance to PI3K Inhibitor Treatment Induced by the PIM Kinase. Mol. Cancer. 2018;17:2710–2721. doi: 10.1158/1535-7163.MCT-18-0374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Le X., Antony R., Razavi P., Treacy D.J., Luo F., Ghandi M., Castel P., Scaltriti M., Baselga J., Garraway L.A. Systematic Functional Characterization of Resistance to PI3K Inhibition in Breast Cancer. Cancer Discov. 2016;6:1134–1147. doi: 10.1158/2159-8290.CD-16-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kennedy S.P., O’Neill M., Cunningham D., Morris P.G., Toomey S., Blanco-Aparicio C., Martinez S., Pastor J., Eustace A.J., Hennessy B.T. Preclinical evaluation of a novel triple-acting PIM/PI3K/mTOR inhibitor, IBL-302, in breast cancer. Oncogene. 2020;39:3028–3040. doi: 10.1038/s41388-020-1202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Litchfield L.M., Boehnke K., Brahmachary M., Mur C., Bi C., Stephens J.R., Sauder J.M., Gutiérrez S.M., McNulty A.M., Ye X.S., et al. Combined inhibition of PIM and CDK4/6 suppresses both mTOR signaling and Rb phosphorylation and potentiates PI3K inhibition in cancer cells. Oncotarget. 2020;11:1478–1492. doi: 10.18632/oncotarget.27539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Gómez Tejeda Zañudo J., Mao P., Alcon C., Kowalski K., Johnson G.N., Xu G., Baselga J., Scaltriti M., Letai A., Montero J., et al. Cell Line-Specific Network Models of ER+ Breast Cancer Identify Potential PI3Kα Inhibitor Resistance Mechanisms and Drug Combinations. Cancer Res. 2021;81:4603–4617. doi: 10.1158/0008-5472.CAN-21-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wright S.C.E., Vasilevski N., Serra V., Rodon J., Eichhorn P.J.A. Mechanisms of Resistance to PI3K Inhibitors in Cancer: Adaptive Responses, Drug Tolerance and Cellular Plasticity. Cancers. 2021;13:1538. doi: 10.3390/cancers13071538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ebi H., Costa C., Faber A.C., Nishtala M., Kotani H., Juric D., Della Pelle P., Song Y., Yano S., Mino-Kenudson M., et al. PI3K regulates MEK/ERK signaling in breast cancer via the Rac-GEF, P-Rex1. Proc. Natl. Acad. Sci. USA. 2013;110:21124–21129. doi: 10.1073/pnas.1314124110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Serra V., Eichhorn P.J.A., García-García C., Ibrahim Y.H., Prudkin L., Sánchez G., Rodríguez O., Antón P., Parra J.-L., Marlow S., et al. RSK3/4 mediate resistance to PI3K pathway inhibitors in breast cancer. J. Clin. Investig. 2013;123:2551–2563. doi: 10.1172/JCI66343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Elkabets M., Pazarentzos E., Juric D., Sheng Q., Pelossof R.A., Brook S., Benzaken A.O., Rodon J., Morse N., Yan J.J., et al. AXL mediates resistance to PI3Kα inhibition by activating the EGFR/PKC/mTOR axis in head and neck and esophageal squamous cell carcinomas. Cancer Cell. 2015;27:533–546. doi: 10.1016/j.ccell.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Elkabets M., Vora S., Juric D., Morse N., Mino-Kenudson M., Muranen T., Tao J., Campos A.B., Rodon J., Ibrahim Y.H., et al. mTORC1 Inhibition Is Required for Sensitivity to PI3K p110α Inhibitors in PIK3CA-Mutant Breast Cancer. Sci. Transl. Med. 2013;5:196ra99. doi: 10.1126/scitranslmed.3005747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Cai Y., Xu G., Wu F., Michelini F., Chan C., Qu X., Selenica P., Ladewig E., Castel P., Cheng Y., et al. Genomic Alterations in PIK3CA-Mutated Breast Cancer Result in mTORC1 Activation and Limit the Sensitivity to PI3Kα Inhibitors. Cancer Res. 2021;81:2470–2480. doi: 10.1158/0008-5472.CAN-20-3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Juric D., Castel P., Griffith M., Griffith O.L., Won H.H., Ellis H., Ebbesen S.H., Ainscough B.J., Ramu A., Iyer G., et al. Convergent loss of PTEN leads to clinical resistance to a PI(3)Kα inhibitor. Nature. 2015;518:240–244. doi: 10.1038/nature13948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Razavi P., Dickler M.N., Shah P.D., Toy W., Brown D.N., Won H.H., Li B.T., Shen R., Vasan N., Modi S., et al. Alterations in PTEN and ESR1 promote clinical resistance to alpelisib plus aromatase inhibitors. Nat. Cancer. 2020;1:382–393. doi: 10.1038/s43018-020-0047-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hosford S.R., Dillon L.M., Bouley S.J., Rosati R., Yang W., Chen V.S., Demidenko E., Morra R.P., Miller T.W. Combined Inhibition of Both p110α and p110β Isoforms of Phosphatidylinositol 3-Kinase Is Required for Sustained Therapeutic Effect in PTEN-Deficient, ER+ Breast Cancer. Clin. Cancer Res. 2017;23:2795–2805. doi: 10.1158/1078-0432.CCR-15-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Costa C., Ebi H., Martini M., Beausoleil S.A., Faber A.C., Jakubik C.T., Huang A., Wang Y., Nishtala M., Hall B., et al. Measurement of PIP3 Levels Reveals an Unexpected Role for p110β in Early Adaptive Responses to p110α-Specific Inhibitors in Luminal Breast Cancer. Cancer Cell. 2015;27:97–108. doi: 10.1016/j.ccell.2014.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hopkins B.D., Pauli C., Du X., Wang D.G., Li X., Wu D., Amadiume S.C., Goncalves M.D., Hodakoski C., Lundquist M.R., et al. Suppression of insulin feedback enhances the efficacy of PI3K inhibitors. Nature. 2018;560:499–503. doi: 10.1038/s41586-018-0343-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ambrosio M.R., Mosca G., Migliaccio T., Liguoro D., Nele G., Schonauer F., D’Andrea F., Liotti F., Prevete N., Melillo R.M., et al. Glucose Enhances Pro-Tumorigenic Functions of Mammary Adipose-Derived Mesenchymal Stromal/Stem Cells on Breast Cancer Cell Lines. Cancers. 2022;14:5421. doi: 10.3390/cancers14215421. [DOI] [PMC free article] [PubMed] [Google Scholar]