Abstract
Systemic lupus erythematosus (SLE) potentially involves multiple parts of the ocular system, including the lacrimal glands and the cornea. The present study sought to assess the risk of aqueous-deficient dry eye disease (DED) and corneal surface damage in patients with SLE. We conducted a population-based cohort study using Taiwan’s National Health Insurance research database to compare the risks of DED and corneal surface damage between subjects with and without SLE. Proportional hazard regression analyses were used to calculate the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for the study outcomes. The propensity score matching procedure generated 5083 matched pairs with 78,817 person-years of follow-up for analyses. The incidence of DED was 31.90 and 7.66 per 1000 person-years in patients with and without SLE, respectively. After adjusting for covariates, SLE was significantly associated with DED (aHR: 3.30, 95% CI: 2.88–3.78, p < 0.0001) and secondary Sjögren’s syndrome (aHR: 9.03, 95% CI: 6.86–11.88, p < 0.0001). Subgroup analyses demonstrated that the increased risk of DED was augmented among patients with age < 65 years and female sex. In addition, patients with SLE had a higher risk of corneal surface damage (aHR: 1.81, 95% CI: 1.35–2.41, p < 0.0001) compared to control subjects, including recurrent corneal erosion (aHR: 2.98, 95% CI: 1.63–5.46, p = 0.0004) and corneal scar (aHR: 2.23, 95% CI: 1.08–4.61, p = 0.0302). In this 12-year nationwide cohort study, we found that SLE was associated with increased risks of DED and corneal surface damage. Regular ophthalmology surveillance should be considered to prevent sight-threatening sequelae among patients with SLE.
Keywords: complication, corneal erosion, keratoconjunctivitis sicca, ocular manifestation, peripheral ulcerative keratitis
1. Introduction
Dry eye disease (DED) is a multifactorial disorder that is characterized by the disruption of tear film homeostasis [1]. Tear film instability and hyperosmolarity, ocular surface inflammation and damage, and neurosensory abnormalities play etiological roles in developing ocular symptoms, including punctate epithelial keratitis, filamentary keratitis, superior limbic keratoconjunctivitis, lid parallel conjunctival folds, and lid wiper epitheliopathy [2]. The prevalence of DED varies globally, ranging from 5 to 50% across different countries and regions [3]. In Taiwan, the prevalence rate of DED was reported to be 5% to 34%, with females and the elderly in the majority [4,5,6,7]. It should be noted that patients with DED have a significantly higher risk of corneal surface damage due to progressive ocular surface inflammation and disruption [3]. Recurrent corneal erosion, corneal ulcers, and corneal scars represent common findings among patients with severe corneal surface damage. Previous studies have revealed several risk factors for DED-associated corneal surface damage, including younger age, female sex, diabetes mellitus, and autoimmune diseases (e.g., rheumatoid arthritis) [8]. Importantly, DED symptoms have an adverse impact on patients’ visual functions, daily activities, work productivity, and vision-related quality of life [9].
Systemic lupus erythematosus (SLE) is a chronic, complex, and multifaceted autoimmune disorder, while its etiology remains largely unclear [10]. SLE predominantly affects females, especially in their 20 s and 30 s [10]. The prevalence of SLE varies across different countries [11]. In Taiwan, the prevalence rate was reported to be 97.5 per 100,000 population [12]. Approximately one-third of SLE patients suffer from ocular involvement, of which keratoconjunctivitis sicca represents the most common manifestation [13,14,15,16]. In a previous report, the risks of DED, cataracts, and glaucoma were significantly higher in patients with SLE [17]. However, there is limited population-based data demonstrating the association between SLE and DED or serious corneal surface damage. The relationship between SLE and DED is not completely clarified due to some methodological drawbacks of previous studies, including small sample size (n < 1000) [14,16,17], insufficient adjustment for confounders [14,16,17], and restriction to single institutions [14,16] or specific populations (children) [14]. In addition, few studies have evaluated the potential impact of SLE on the development of corneal surface damage, and relevant risk factors remain largely unknown [14,16,17]. In this population-based cohort study, we used Taiwan’s National Health Insurance (NHI) research database to evaluate the temporal relationship between SLE and DED or corneal surface damage. Based on the current literature [13,14,15,16,17], we hypothesized that SLE was associated with both DED and corneal surface damage in this 12-year nationwide cohort.
2. Material and Methods
2.1. Data Source
This study obtained ethical approval from the Taipei Medical University-Joint Institutional Review Board (approval no. TMU-JIRB-N202210011; date of approval on 6 October 2022). Written informed consent was waived by the Institutional Review Board due to the retrospective nature of this research. All methods were performed following the Declaration of Helsinki 2013 and relevant study guidelines [18]. Taiwan’s National Health Insurance program was launched in March 1995 and offered insurance to more than 99% of 23.3 million Taiwanese residents. The NHI research database contains comprehensive claims data of the insured beneficiaries, including demographic characteristics (e.g., date of birth and sex), medical diagnoses, prescription drugs, and medical expenditures. The NHI research database has been widely used for public health statistics and risk assessment [19,20,21]. In the present study, we included subjects from the three Longitudinal Health Insurance Databases (LHID2000, LHID2005, and LHID2010), which contains original claims data of 1 million randomly sampled beneficiaries from the original NHI research database in the years 2000, 2005, and 2010, respectively [22].
2.2. Inclusion and Exclusion Criteria
Patients who had at least 2 rheumatology clinic visits with the diagnoses of SLE between 1 January 2002 and 30 June 2013 were included consecutively. We utilized the International Classification of Diseases, 9th Revision, Clinical Modification (ICD-9-CM) codes to ascertain the diagnoses of SLE, coexisting diseases, and ocular disorders (Supplementary Table S1). The index date was defined as the date of the first SLE diagnosis. Patients were excluded due to the following conditions: any previous diagnoses of DED, corneal ulcers, recurrent corneal erosion, corneal scars, interstitial and deep keratitis, corneal neovascularization, ocular burns, or open globe injury in the ophthalmology service before the index date. Subjects were also excluded if they had been prescribed eye lubricants before the index date or died in the follow-up period.
2.3. Outcome Assessment
The primary outcome was DED, which was defined as the diagnosis made twice by certified ophthalmologists with the prescriptions of cyclosporine ophthalmic emulsion in the ophthalmology care service (Supplementary Table S1). In the reimbursement regulations of Taiwan’s National Health Insurance, cyclosporine ophthalmic emulsion treatment can be used when patients’ Schirmer test scores are less than 5 mm in 5 min [5,8]. The secondary outcomes included secondary Sjögren’s syndrome (SS) and severe forms of corneal surface damage, which were defined as any diagnosis of corneal ulcers, recurrent corneal erosion, or corneal scars made twice by certified ophthalmologists.
2.4. Covariates for Model Adjustment
Insurance premium was classified into $0–$500, $501–$800, and >$800 United States dollars per month. The ICD-9-CM codes of physicians’ diagnoses within 24 months before the index date were employed to determine the following comorbidities, chosen based on data availability and existing literature: hypertension, diabetes mellitus, coronary artery disease, chronic obstructive pulmonary disease, chronic liver disease, chronic kidney disease, cerebrovascular disease, thyroid disease, major depressive disorder, anxiety disorder, sleeping disorder, and cancer (Supplementary Table S1) [23]. The Charlson comorbidity index score was calculated to evaluate the comorbidity level of included subjects [24]. We also evaluated the concurrent prescription of systemic corticosteroids within 6 months after the index date. The numbers of hospitalizations and emergency visits within 24 months before the index date were analyzed to assess the level of medical resource utilization of the studied patients.
2.5. Statistical Analysis
A non-parsimonious multivariable logistic regression model was used to calculate a propensity score for SLE and non-SLE subjects. Each SLE subject was matched to a non-SLE control using the nearest neighbor matching algorithm within a tolerance limit of 0.05 and without replacement to balance the distributions of age, sex, and monthly insurance premium between the two groups [25]. Baseline patient characteristics were compared between matched pairs using the absolute standardized mean difference [26]. We used multivariable Cox proportional hazards regression models to calculate the adjusted hazard ratio (aHR) and 95% confidence interval (CI) for the study outcomes. The multivariable models adjusted for the variables of age, sex, monthly insurance premium, coexisting diseases, Charlson comorbidity index score, use of systemic corticosteroids, number of hospitalizations, and number of emergency room visits. The Kaplan-Meier curves and log-rank tests were used to compare the cumulative incidence of ophthalmological outcomes between the two groups. Stratified analyses were also conducted by age≥ or <65 years, male or female, different Charlson comorbidity index scores, and use of systemic corticosteroids or not to examine the risk of DED within these strata. A two-sided p-value of <0.05 was considered statistically significant. All the statistical analyses were conducted using Statistics Analysis System (SAS), Version 9.4 (SAS Institute Inc., Cary, NC, USA).
3. Results
3.1. Baseline Patient Characteristics
The matching procedure generated 5083 matched pairs with 78,817 person-years of follow-up for analyses (Supplementary Figure S1). The baseline distributions of demographic and patient characteristics are shown in Table 1. Noticeably, patients with SLE were more likely to have more comorbidities, higher Charlson comorbidity index scores, prescriptions of systemic corticosteroids, and greater numbers of hospitalizations and emergency room visits.
Table 1.
Baseline characteristics of subjects with and without systemic lupus erythematosus.
| SLE n = 5083 |
Non-SLE n = 5083 |
ASMD | |||
|---|---|---|---|---|---|
| Age (years), mean (SD) | 36.1 | 16.1 | 36.1 | 16.1 | <0.0001 |
| Sex, female, n (%) | 4161 | 81.9 | 4161 | 81.9 | <0.0001 |
| Insurance premium (USD/month), n (%) | <0.0001 | ||||
| 0–500 | 1921 | 37.8 | 1921 | 37.8 | |
| 501–800 | 1561 | 30.7 | 1561 | 30.7 | |
| ≥801 | 1601 | 31.5 | 1601 | 31.5 | |
| Coexisting diseases, n (%) | |||||
| Hypertension | 527 | 10.4 | 337 | 6.6 | 0.2690 |
| Diabetes mellitus | 239 | 4.7 | 175 | 3.4 | 0.1791 |
| Coronary artery disease | 200 | 3.9 | 107 | 2.1 | 0.3553 |
| Chronic obstructive pulmonary disease | 189 | 3.7 | 100 | 2.0 | 0.3609 |
| Chronic liver disease | 383 | 7.5 | 172 | 3.4 | 0.4656 |
| Chronic kidney disease | 98 | 1.9 | 21 | 0.4 | 0.8577 |
| Cerebrovascular disease | 133 | 2.6 | 63 | 1.2 | 0.4197 |
| Thyroid disease | 172 | 3.4 | 40 | 0.8 | 0.8188 |
| Major depressive disorder | 55 | 1.1 | 23 | 0.5 | 0.4842 |
| Anxiety disorder | 501 | 9.9 | 243 | 4.8 | 0.4291 |
| Sleeping disorder | 456 | 9.0 | 232 | 4.6 | 0.3986 |
| Cancer | 109 | 2.1 | 55 | 1.1 | 0.3831 |
| Charlson comorbidity index | 0.6027 | ||||
| 0 | 3435 | 67.58 | 4741 | 93.27 | |
| 1 | 1248 | 24.55 | 246 | 4.84 | |
| 2 | 386 | 7.59 | 74 | 1.46 | |
| ≥3 | 14 | 0.28 | 22 | 0.43 | |
| Use of systemic corticosteroids, n (%) | 2680 | 52.72 | 585 | 11.51 | 1.1847 |
| Number of hospitalizations, n (%) | 0.2569 | ||||
| 0 | 4307 | 84.73 | 4713 | 92.72 | |
| 1 | 545 | 10.72 | 295 | 5.80 | |
| 2 | 132 | 2.60 | 49 | 0.96 | |
| ≥3 | 99 | 1.95 | 26 | 0.51 | |
| Number of emergency room visits, n (%) | 0.3028 | ||||
| 0 | 3709 | 72.97 | 4309 | 84.77 | |
| 1 | 809 | 15.92 | 534 | 10.51 | |
| 2 | 320 | 6.30 | 154 | 3.03 | |
| ≥3 | 245 | 4.82 | 86 | 1.69 | |
Abbreviation: ASMD = absolute standardized mean difference; SD = standard deviation; SLE = systemic lupus erythematosus; USD = United States Dollar.
3.2. Dry Eye Disease
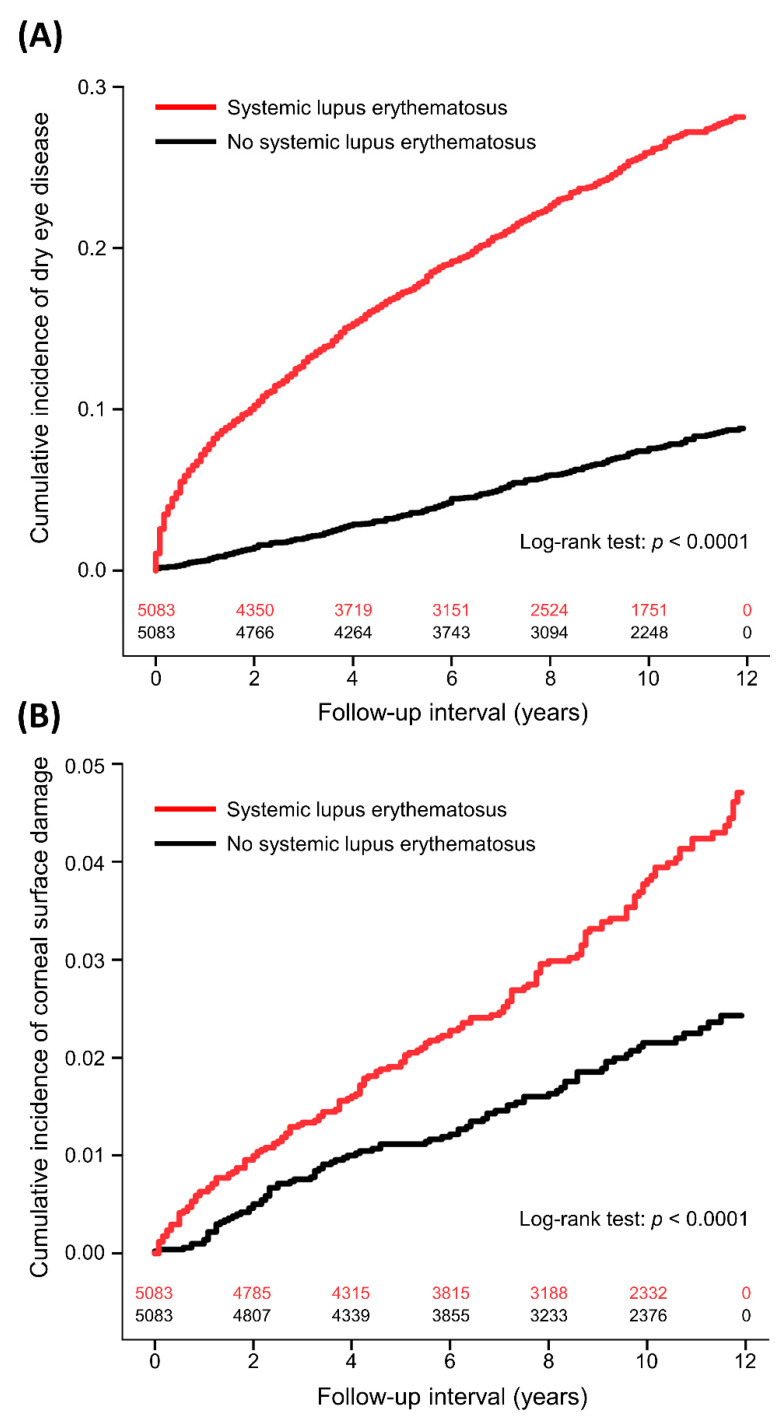
The incidence of DED was 31.90 and 7.66 per 1000 person-years in the SLE and non-SLE groups, respectively (Table 2). The interval between enrollment and DED diagnosis was median 2.6 (interquartile range: 0.6–5.6) years in the SLE patients and 5.1 (2.3–7.5) years in the non-SLE controls (p < 0.0001). The results of univariate and multivariable proportional hazards regression analyses for DED were shown in Table 3. After adjusting for covariates, SLE was significantly associated with increased DED compared to non-SLE controls (aHR: 3.30, 95% CI: 2.88–3.78, p < 0.0001). Figure 1A demonstrates the cumulative incidence of DED in the two groups. SLE was also linked to secondary SS (aHR: 9.03, 95% CI: 6.86–11.88, p < 0.0001). Other independent factors for DED were age (aHR: 1.03), female sex (aHR: 2.56), monthly insurance premium (501–800 vs. 0–500 USD, aHR: 0.92; ≥801 vs. 0–500 USD, aHR: 1.20), hypertension (aHR: 0.78), cerebrovascular disease (aHR: 0.68), sleeping disorder (aHR: 1.24), Charlson comorbidity index (1 vs. 0, aHR: 1.45; 2 vs. 0, aHR: 1.35; ≥3 vs. 0, aHR: 0.74), and use of systemic corticosteroids (aHR: 1.41). Subgroup analyses showed that the aHR for DED was higher in patients with age < 65 years (aHR: 3.48) and female sex (aHR: 3.47) compared to those with age ≥ 65 years (aHR: 1.99) and male sex (aHR: 2.20), respectively (Table 4).
Table 2.
Risks of dry eye disease, Sjögren’s syndrome, and corneal surface damage for patients with and without systemic lupus erythematosus.
| SLE n = 5083 |
Non-SLE n = 5083 |
Outcome Risk | |||||
|---|---|---|---|---|---|---|---|
| Study Outcome | Incident Case | Incidence per 1000 Person-Years | Incident Case | Incidence per 1000 Person-Years | IRR | aHR (95% CI) † | p |
| Dry eye disease | 1165 | 31.90 | 324 | 7.66 | 4.16 | 3.30 (2.88–3.78) | <0.0001 |
| Sjögren’s syndrome | 693 | 17.54 | 61 | 1.40 | 12.53 | 9.03 (6.86–11.88) | <0.0001 |
| Corneal surface damage | 169 | 3.93 | 92 | 2.12 | 1.85 | 1.81 (1.35–2.41) | <0.0001 |
| Corneal ulcer | 98 | 2.28 | 62 | 1.43 | 1.59 | 1.36 (0.94–1.98) | 0.1061 |
| Recurrent corneal erosion | 48 | 1.12 | 17 | 0.39 | 2.87 | 2.98 (1.63–5.46) | 0.0004 |
| Corneal scar | 24 | 0.56 | 14 | 0.32 | 1.75 | 2.23 (1.08–4.61) | 0.0302 |
Abbreviation: aHR = adjusted hazard ratio; CI = confidence interval; IRR = incidence rate ratio; SLE = systemic lupus erythematosus. †: Adjusted for age (continuous), sex, insurance premium (categorical), coexisting diseases, Charlson comorbidity index score, use of systemic corticosteroids, number of hospitalizations, and number of emergency room visits.
Table 3.
Univariate and multivariable analyses for dry eye disease.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| cHR | 95% CI | p | aHR | 95% CI | p | |
| Systemic lupus erythematosus | 4.06 | 3.59–4.60 | <0.0001 | 3.30 | 2.88–3.78 | <0.0001 |
| Age (years) | 1.02 | 1.02–1.02 | <0.0001 | 1.03 | 1.02–1.03 | <0.0001 |
| Sex, female vs. male | 2.28 | 1.91–2.72 | <0.0001 | 2.56 | 2.14–3.06 | <0.0001 |
| Insurance premium (USD/month) | 0.0583 | 0.0004 | ||||
| 501–800 vs. 0–500 | 0.86 | 0.76–0.98 | 0.0181 | 0.92 | 0.81–1.05 | 0.2162 |
| ≥801 vs. 0–500 | 0.95 | 0.85–1.08 | 0.4483 | 1.20 | 1.06–1.36 | 0.0053 |
| Coexisting diseases | ||||||
| Hypertension | 1.42 | 1.20–1.68 | <0.0001 | 0.78 | 0.64–0.95 | 0.0131 |
| Diabetes mellitus | 1.30 | 1.01–1.66 | 0.0398 | 0.80 | 0.61–1.04 | 0.1003 |
| Coronary artery disease | 1.92 | 1.51–2.45 | <0.0001 | 1.21 | 0.93–1.57 | 0.1673 |
| COPD | 1.32 | 0.99–1.75 | 0.0554 | 0.89 | 0.67–1.19 | 0.4422 |
| Chronic liver disease | 1.56 | 1.29–1.89 | <0.0001 | 1.07 | 0.87–1.31 | 0.5078 |
| Chronic kidney disease | 1.50 | 1.00–2.25 | 0.0485 | 0.76 | 0.50–1.16 | 0.2055 |
| Cerebrovascular disease | 1.28 | 0.90–1.83 | 0.1694 | 0.68 | 0.47–0.99 | 0.0436 |
| Thyroid disease | 2.11 | 1.60–2.78 | <0.0001 | 1.27 | 0.96–1.69 | 0.0944 |
| Major depressive disorder | 1.60 | 0.96–2.67 | 0.0688 | 0.99 | 0.59–1.67 | 0.9637 |
| Anxiety disorder | 1.73 | 1.46–2.05 | <0.0001 | 1.16 | 0.96–1.40 | 0.1154 |
| Sleeping disorder | 1.76 | 1.47–2.10 | <0.0001 | 1.24 | 1.02–1.50 | 0.0314 |
| Cancer | 1.66 | 1.16–2.37 | 0.0053 | 1.13 | 0.79–1.62 | 0.5159 |
| Charlson comorbidity index | <0.0001 | <0.0001 | ||||
| 1 vs. 0 | 2.53 | 2.26–2.84 | <0.0001 | 1.45 | 1.28–1.64 | <0.0001 |
| 2 vs. 0 | 2.20 | 1.82–2.66 | <0.0001 | 1.35 | 1.10–1.64 | 0.0038 |
| ≥3 vs. 0 | 0.97 | 0.36–2.58 | 0.9428 | 0.74 | 0.28–1.99 | 0.5506 |
| Use of systemic corticosteroids | 2.40 | 2.17–2.66 | <0.0001 | 1.41 | 1.25–1.58 | <0.0001 |
| Number of hospitalizations | 0.5954 | 0.3859 | ||||
| 1 vs. 0 | 1.10 | 0.91–1.33 | 0.3201 | 0.91 | 0.74–1.11 | 0.3432 |
| 2 vs. 0 | 1.20 | 0.81–1.76 | 0.3670 | 0.81 | 0.54–1.22 | 0.3156 |
| ≥3 vs. 0 | 1.12 | 0.68–1.83 | 0.6577 | 0.69 | 0.41–1.19 | 0.1811 |
| Number of emergency room visits | 0.0203 | 0.6522 | ||||
| 1 vs. 0 | 1.17 | 1.00–1.36 | 0.0469 | 1.03 | 0.88–1.20 | 0.7448 |
| 2 vs. 0 | 1.11 | 0.86–1.43 | 0.4246 | 0.88 | 0.67–1.14 | 0.3161 |
| ≥3 vs. 0 | 1.43 | 1.08–1.89 | 0.0119 | 1.09 | 0.80–1.48 | 0.5893 |
Abbreviation: aHR = adjusted hazard ratio; COPD = chronic obstruction pulmonary disease; cHR = crude hazard ratio; USD = United States Dollar.
Figure 1.
Cumulative incidence of dry eye disease (A) and corneal surface damage (B) between patients with and without systemic lupus erythematosus with number of subjects at risk.
Table 4.
Subgroup analyses of dry eye disease for patients with and without systemic lupus erythematosus.
| SLE n = 5083 |
Non-SLE n = 5083 |
Outcome Risk | |||||
|---|---|---|---|---|---|---|---|
| Subgroup | Incident Case | Incidence per 1000 Person-Years | Incident Case | Incidence per 1000 Person-Years | IRR | aHR (95% CI) † | p |
| All patients | 1165 | 31.90 | 324 | 7.66 | 4.16 | 3.30 (2.88–3.78) | <0.0001 |
| Age ≥ 65 years | 73 | 40.25 | 36 | 17.26 | 2.33 | 1.99 (1.30–3.06) | 0.0016 |
| Age < 65 years | 1092 | 31.47 | 288 | 7.16 | 4.40 | 3.48 (3.02–4.02) | <0.0001 |
| Male | 97 | 13.81 | 37 | 4.96 | 2.78 | 2.20 (1.46–3.31) | 0.0002 |
| Female | 1068 | 36.21 | 287 | 8.24 | 4.39 | 3.47 (3.00–4.00) | <0.0001 |
| CCI score = 0 | 662 | 26.80 | 291 | 7.41 | 3.62 | 3.20 (2.76–3.70) | <0.0001 |
| CCI score = 1 | 394 | 44.98 | 20 | 9.21 | 4.88 | 4.29 (2.67–6.91) | <0.0001 |
| CCI score = 2 | 106 | 35.82 | 12 | 17.84 | 2.01 | 2.45 (1.26–4.77) | 0.0085 |
| CCI score ≥ 3 | 3 | 29.93 | 1 | 5.68 | 5.27 | 8.12 (0.03–2021.97) | 0.4568 |
| Use of systemic corticosteroids | 707 | 38.30 | 51 | 10.09 | 3.80 | 3.45 (2.58–4.62) | <0.0001 |
| No use of systemic corticosteroids | 458 | 25.36 | 273 | 7.33 | 3.46 | 3.27 (2.80–3.83) | <0.0001 |
Abbreviation: aHR = adjusted hazard ratio; CCI = Charlson comorbidity index; CI = confidence interval; IRR = incidence rate ratio; SLE = systemic lupus erythematosus. †: Adjusted for age (continuous), sex, insurance premium (categorical), coexisting diseases, Charlson comorbidity index score, use of systemic corticosteroids, number of hospitalizations, and number of emergency room visits.
3.3. Corneal Surface Damage
The incidence of corneal surface damage was 3.93 and 2.12 per 1000 person-years in the SLE and non-SLE groups, respectively (Table 2). The time to corneal surface damage was median 4.3 years (interquartile range: 1.7–7.8) in the SLE patients and 3.8 years (interquartile range: 2.0–7.4) in the non-SLE controls (p = 0.9610). The multivariable model showed that SLE was significantly associated with increased corneal surface damage (aHR: 1.81, 95% CI: 1.35–2.41, p < 0.0001; Table 5 and Figure 1B). Further analyses showed that SLE was significantly associated with higher risks of recurrent corneal erosion (aHR: 2.98, 95% CI: 1.63–5.46, p = 0.0004) and corneal scar (aHR: 2.23, 95% CI: 1.08–4.61, p = 0.0302). Another independent factor for corneal surface damage was female sex (aHR: 1.94, 95% CI: 1.28–2.94, p = 0.0017).
Table 5.
Univariate and multivariable analyses for corneal surface damage.
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| cHR | 95% CI | p | aHR | 95% CI | p | |
| Systemic lupus erythematosus | 1.85 | 1.44–2.39 | <0.0001 | 1.81 | 1.35–2.41 | <0.0001 |
| Age (years) | 1.00 | 0.99–1.01 | 0.8792 | 1.00 | 0.99–1.01 | 0.8177 |
| Sex, female vs. male | 2.00 | 1.33–3.03 | 0.0010 | 1.94 | 1.28–2.94 | 0.0017 |
| Insurance premium (USD/month) | 0.0729 | 0.0603 | ||||
| 501–800 vs. 0–500 | 0.88 | 0.66–1.17 | 0.3719 | 0.84 | 0.63–1.13 | 0.2521 |
| ≥801 vs. 0–500 | 0.70 | 0.52–0.95 | 0.0221 | 0.69 | 0.51–0.94 | 0.0181 |
| Coexisting diseases | ||||||
| Hypertension | 0.86 | 0.52–1.42 | 0.5514 | 0.95 | 0.54–1.67 | 0.8461 |
| Diabetes mellitus | 0.45 | 0.17–1.20 | 0.1100 | 0.46 | 0.16–1.27 | 0.1334 |
| Coronary artery disease | 1.07 | 0.50–2.26 | 0.8634 | 1.18 | 0.53–2.63 | 0.6919 |
| COPD | 0.75 | 0.31–1.81 | 0.5217 | 0.76 | 0.31–1.88 | 0.5540 |
| Chronic liver disease | 0.86 | 0.47–1.58 | 0.6315 | 0.84 | 0.45–1.57 | 0.5835 |
| Chronic kidney disease | 0.34 | 0.05–2.45 | 0.2863 | 0.30 | 0.04–2.19 | 0.2355 |
| Cerebrovascular disease | 0.47 | 0.12–1.90 | 0.2893 | 0.47 | 0.11–1.94 | 0.2947 |
| Thyroid disease | 0.85 | 0.32–2.27 | 0.7389 | 0.74 | 0.27–2.02 | 0.5601 |
| Major depressive disorder | 0.59 | 0.08–4.18 | 0.5937 | 0.51 | 0.07–3.74 | 0.5073 |
| Anxiety disorder | 1.26 | 0.80–1.99 | 0.3239 | 1.16 | 0.71–1.92 | 0.5504 |
| Sleeping disorder | 1.26 | 0.77–2.07 | 0.3539 | 1.17 | 0.69–2.00 | 0.5555 |
| Cancer | 0.89 | 0.29–2.79 | 0.8466 | 0.94 | 0.30–2.99 | 0.9187 |
| Charlson Comorbidity Index | 0.0338 | 0.3330 | ||||
| 1 vs. 0 | 1.51 | 1.12–2.03 | 0.0070 | 1.22 | 0.87–1.70 | 0.2451 |
| 2 vs. 0 | 1.07 | 0.61–1.88 | 0.8100 | 0.92 | 0.51–1.66 | 0.7833 |
| ≥3 vs. 0 | 2.54 | 0.63–10.22 | 0.1903 | 2.69 | 0.66–10.95 | 0.1670 |
| Use of systemic corticosteroids | 1.39 | 1.08–1.78 | 0.0099 | 1.02 | 0.76–1.36 | 0.8950 |
| Number of hospitalizations | 0.7740 | 0.6070 | ||||
| 1 vs. 0 | 0.88 | 0.53–1.46 | 0.6153 | 0.78 | 0.46–1.33 | 0.3650 |
| 2 vs. 0 | 0.81 | 0.26–2.54 | 0.7236 | 0.66 | 0.20–2.13 | 0.4873 |
| ≥3 vs. 0 | 0.42 | 0.06–2.97 | 0.3819 | 0.40 | 0.05–3.04 | 0.3772 |
| Number of emergency room visits | 0.1484 | 0.1790 | ||||
| 1 vs. 0 | 1.49 | 1.06–2.09 | 0.0229 | 1.48 | 1.04–2.10 | 0.0301 |
| 2 vs. 0 | 0.97 | 0.50–1.90 | 0.9336 | 1.00 | 0.51–2.00 | 0.9901 |
| ≥3 vs. 0 | 1.20 | 0.56–2.54 | 0.6441 | 1.29 | 0.58–2.90 | 0.5316 |
Abbreviation: aHR = adjusted hazard ratio; COPD = chronic obstruction pulmonary disease; cHR = crude hazard ratio; USD = United States Dollar.
4. Discussion
The present study demonstrated that patients with SLE exhibited significantly greater risks of DED and corneal surface damage, especially for recurrent corneal erosion compared with age, sex and insurance premium-matched controls. Subgroup analyses further revealed that the higher SLE-associated risk of DED was observed in subjects of males and females, age ≥ 65 and <65 years old, and those with or without systemic corticosteroid treatment. Considering the devastating impact of DED and corneal surface damage on visual functions, patients with SLE should be alerted on these corneal disorders.
SLE is the third most common autoimmune disorder in Taiwan [27]. The prevalence rate of SLE remarkably increased during the 21st century [28]. Although the ocular symptoms are not included into the 11 diagnostic criteria of SLE, they are not uncommon and about one-third of patients are suffered [29]. Keratoconjunctivitis sicca is the most ocular manifestation of SLE [30], while all the parts of eye, including sclera, uvea, retina, and optic nerve, are possibly involved [31]. There are several reasons accounting for the association between SLE and DED. One is the comorbidity of Sjögren’s syndrome, which causes the reduction of tear. On the other hand, the infiltration of immune cells and immune complex into the epithelial basement membrane are also evident [32], and the increase in proinflammatory cytokine, i.e., interleukin-17, is detected in the tear film of SLE patients [33,34]. The present study also found a significant association between SLE with secondary SS in Taiwanese patients. However, the adjusted risk of DED was similar between SLE patients with and without systemic corticosteroid compared with controls, which may indicate the consequence of an ocular-specific inflammatory response in SLE patients, and topical immunosuppressants are more suitable for the management of DED [35,36].
Corneal ulcer is defined as the lesion of the corneal epithelium, which is a major threat of vision [37]. Without the proper treatment, patients may only rely on the corneal transplant for regaining their vision [38]. Most of the corneal ulcers result from the infection, including bacteria, virus, fungus, and protozoa [38]. On the other hand, non-infectious corneal ulcers, usually present as peripheral ulcerative keratitis (PUK), are highly associated with autoimmune diseases [39]. The fibrocyte and macrophage infiltration in the corneal matrix triggers the inflammatory response, and the accumulation of immune complex is found in the capillary network of cornea in PUK [40,41]. In the present study, the overall risk of corneal surface damage was significantly higher in patients with SLE. Despite that there was only a trend toward increased corneal ulcers in SLE patients, referring to recurrent corneal erosion and corneal scar, more severe types of corneal damage, there were significantly increased risks in SLE patients. The lack of significance in the corneal ulcer among SLE patients may result from other types of PUK, such as infectious or contact lens-related keratitis.
The strength of the present study was the delineation of the association between SLE and DED or corneal surface damage. Meanwhile, the population-based study provided reliable epidemiological evidence and good generalizability about the risk assessment. DED causes substantial discomfort for the SLE patients, and the management of these ocular manifestations of SLE should be emphasized. In addition, despite that SLE is not the major contributor of PUK, it did increase the risk of recurrent corneal ulcer and corneal scar, which may exert a devastating effect on eyesight. However, there were some limitations to the present study. First, since the NHI research database was diagnosis and treatment-based, the laboratory data was unavailable. Therefore, the severity and progression of SLE, DED and corneal surface damage could not be further evaluated. Second, the lack of some social habit information (e.g., alcohol and tobacco consumption) and physical examination data (e.g., body mass index, blood pressure, and visual acuity) might also introduce a bias to the analytical results. Third, we only matched the variables of age, sex and monthly insurance premium between the two groups in the propensity-score matching process in order to increase the sample size and statistical power of matched datasets. Given the fact that the incidence of corneal surface injury was relatively low (approximately 2% to 5% in the 12-year follow-up), a large patient sample is essential to detect a potential risk difference between SLE and non-SLE populations. Fourth, because the use of corticosteroids is a known risk factor for DED and corneal surface damage, the imbalance in the distribution of corticosteroid prescriptions might bias the study results. Further studies are needed to evaluate the potential impact of corticosteroids and immunosuppressants on corneal diseases among SLE patients. Finally, our cohort was only followed up until the 31 December 2013, due to the regulations of the NHI research database.
5. Conclusions
The present study demonstrated a higher risk of DED and severe forms of corneal surface damage in patients with SLE. Considering the increasing prevalence of SLE, the vision issues, which affect the quality of life substantially, should be empathized with the rheumatologists and ophthalmologists. Prophylactic and therapeutic management should be further developed for this susceptible population.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijerph20053776/s1, Table S1: ICD-9-CM codes of exposure factors, coexisting diseases, and study outcomes; Figure S1: Flow diagram for patient selection.
Author Contributions
Conceptualization, Y.-H.T. and S.-C.L.; methodology, Y.-H.T.; software, Y.-H.T.; validation, C.-T.H.; formal analysis, Y.-H.T.; investigation, C.-H.T. and S.-C.L.; resources, Y.-G.C. and S.-C.L.; data curation, Y.-X.D. and T.-J.C.; writing—original draft preparation, C.-H.T., Y.-H.T. and C.-T.H.; writing—review and editing, Y.-X.D., T.-J.C., Y.-G.C. and S.-C.L.; project administration, Y.-H.T.; funding acquisition, Y.-H.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Institutional Review Board of Taipei Medical University (TMU-JIRB-N202210011; date of approval on 6 October 2022).
Informed Consent Statement
Patient consent was waived by the Institutional Review Board.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Taipei Medical University, Taiwan, grant number TMU110-AE1-B11. The APC was funded by Shuang Ho Hospital, Taipei Medical University, New Taipei City, Taiwan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Craig J.P., Nichols K.K., Akpek E.K., Caffery B., Dua H.S., Joo C.K., Liu Z., Nelson J.D., Nichols J.J., Tsubota K., et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017;15:276–283. doi: 10.1016/j.jtos.2017.05.008. [DOI] [PubMed] [Google Scholar]
- 2.Yu L., Yu C., Dong H., Mu Y., Zhang R., Zhang Q., Liang W., Li W., Wang X., Zhang L. Recent developments about the pathogenesis of dry eye disease: Based on immune inflammatory mechanisms. Front. Pharmacol. 2021;12:732887. doi: 10.3389/fphar.2021.732887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stapleton F., Alves M., Bunya V.Y., Jalbert I., Lekhanont K., Malet F., Na K.S., Schaumberg D., Uchino M., Vehof J., et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017;15:334–365. doi: 10.1016/j.jtos.2017.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Lin P.Y., Tsai S.Y., Cheng C.Y., Liu J.H., Chou P., Hsu W.M. Prevalence of dry eye among an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology. 2003;110:1096–1101. doi: 10.1016/S0161-6420(03)00262-8. [DOI] [PubMed] [Google Scholar]
- 5.Kuo Y.K., Lin I.C., Chien L.N., Lin T.Y., How Y.T., Chen K.H., Dusting G.J., Tseng C.L. Dry eye disease: A review of epidemiology in Taiwan, and its clinical treatment and merits. J. Clin. Med. 2019;8:1227. doi: 10.3390/jcm8081227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pan L.Y., Kuo Y.K., Chen T.H., Sun C.C. Dry eye disease in patients with type II diabetes mellitus: A retrospective, population-based cohort study in Taiwan. Front. Med. 2022;9:980714. doi: 10.3389/fmed.2022.980714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin I.C., Kuo Y.K., Liu H.Y., Chien L.N. Trends in diagnosed dry eye disease incidence, 2001 to 2015: A nationwide population-based study in Taiwan. Cornea. 2022;41:1372–1377. doi: 10.1097/ICO.0000000000002987. [DOI] [PubMed] [Google Scholar]
- 8.Hung N., Kang E.Y., Lee T.W., Chen T.H., Shyu Y.C., Sun C.C. The risks of corneal surface damage in aqueous-deficient dry eye disease: A 17-year population-based study in Taiwan. Am. J. Ophthalmol. 2021;227:231–239. doi: 10.1016/j.ajo.2021.03.013. [DOI] [PubMed] [Google Scholar]
- 9.Hossain P., Siffel C., Joseph C., Meunier J., Markowitz J.T., Dana R. Patient-reported burden of dry eye disease in the UK: A cross-sectional web-based survey. BMJ Open. 2021;11:e039209. doi: 10.1136/bmjopen-2020-039209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fortuna G., Brennan M.T. Systemic lupus erythematosus: Epidemiology, pathophysiology, manifestations, and management. Dent. Clin. N. Am. 2013;57:631–655. doi: 10.1016/j.cden.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 11.Barber M.R.W., Drenkard C., Falasinnu T., Hoi A., Mak A., Kow N.Y., Svenungsson E., Peterson J., Clarke A.E., Ramsey-Goldman R. Global epidemiology of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2021;17:515–532. doi: 10.1038/s41584-021-00668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yeh K.W., Yu C.H., Chan P.C., Horng J.T., Huang J.L. Burden of systemic lupus erythematosus in Taiwan: A population-based survey. Rheumatol. Int. 2013;33:1805–1811. doi: 10.1007/s00296-012-2643-6. [DOI] [PubMed] [Google Scholar]
- 13.Palejwala N.V., Walia H.S., Yeh S. Ocular manifestations of systemic lupus erythematosus: A review of the literature. Autoimmune Dis. 2012;2012:290898. doi: 10.1155/2012/290898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ong Tone S., Elbaz U., Silverman E., Levy D., Williams S., Mireskandari K., Ali A. Evaluation of dry eye disease in children with systemic lupus erythematosus and healthy controls. Cornea. 2019;38:581–586. doi: 10.1097/ICO.0000000000001902. [DOI] [PubMed] [Google Scholar]
- 15.Kemeny-Beke A., Szodoray P. Ocular manifestations of rheumatic diseases. Int. Ophthalmol. 2020;40:503–510. doi: 10.1007/s10792-019-01183-9. [DOI] [PubMed] [Google Scholar]
- 16.Sitaula R., Shah D.N., Singh D. The spectrum of ocular involvement in systemic lupus erythematosus in a tertiary eye care center in Nepal. Ocul. Immunol. Inflamm. 2011;19:422–425. doi: 10.3109/09273948.2011.610023. [DOI] [PubMed] [Google Scholar]
- 17.Hsu C.S., Hsu C.W., Lu M.C., Koo M. Risks of ophthalmic disorders in patients with systemic lupus erythematosus: A secondary cohort analysis of population-based claims data. BMC Ophthalmol. 2020;20:96. doi: 10.1186/s12886-020-01360-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., STROBE Initiative Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. BMJ. 2007;335:806–808. doi: 10.1136/bmj.39335.541782.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ting H.C., Ma S.H., Tai Y.H., Dai Y.X., Chang Y.T., Chen T.J., Chen M.H. Association between alopecia areata and retinal diseases: A nationwide population-based cohort study. J. Am. Acad. Dermatol. 2022;87:771–778. doi: 10.1016/j.jaad.2021.10.045. [DOI] [PubMed] [Google Scholar]
- 20.Tai C.Y., Liu H.Y., Cata J.P., Dai Y.X., Chen M.H., Chen J.T., Chen T.J., Wu H.L., Cherng Y.G., Li C.C., et al. The association between general anesthesia and new postoperative uses of sedative-hypnotics: A nationwide matched cohort study. J. Clin. Med. 2022;11:3360. doi: 10.3390/jcm11123360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai S.C., Wang C.W., Wu Y.M., Dai Y.X., Chen T.J., Wu H.L., Cherng Y.G., Tai Y.H. Rheumatoid arthritis associated with dry eye disease and corneal surface damage: A nationwide matched cohort study. Int. J. Environ. Res. Public Health. 2023;20:1584. doi: 10.3390/ijerph20021584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Health Insurance Research Database Data Subsets. [(accessed on 25 January 2023)]. Available online: https://nhird.nhri.org.tw/en/Data_Subsets.html.
- 23.Shanti Y., Shehada R., Bakkar M.M., Qaddumi J. Prevalence and associated risk factors of dry eye disease in 16 northern West bank towns in Palestine: A cross-sectional study. BMC Ophthalmol. 2020;20:26. doi: 10.1186/s12886-019-1290-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li B., Evans D., Faris P., Dean S., Quan H. Risk adjustment performance of Charlson and Elixhauser comorbidities in ICD-9 and ICD-10 administrative databases. BMC Health Serv. Res. 2008;8:12. doi: 10.1186/1472-6963-8-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Austin P.C. A comparison of 12 algorithms for matching on the propensity score. Stat. Med. 2014;33:1057–1069. doi: 10.1002/sim.6004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin P.C. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat. Med. 2009;28:3083–3107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.See L.C., Kuo C.F., Chou I.J., Chiou M.J., Yu K.H. Sex- and age-specific incidence of autoimmune rheumatic diseases in the Chinese population: A Taiwan population-based study. Semin. Arthritis Rheum. 2013;43:381–386. doi: 10.1016/j.semarthrit.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Leong P.Y., Huang J.Y., Chiou J.Y., Bai Y.C., Wei J.C. The prevalence and incidence of systemic lupus erythematosus in Taiwan: A nationwide population-based study. Sci. Rep. 2021;11:5631. doi: 10.1038/s41598-021-84957-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan E.M., Cohen A.S., Fries J.F., Masi A.T., McShane D.J., Rothfield N.F., Schaller J.G., Talal N., Winchester R.J. The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1982;25:1271–1277. doi: 10.1002/art.1780251101. [DOI] [PubMed] [Google Scholar]
- 30.Jensen J.L., Bergem H.O., Gilboe I.M., Husby G., Axéll T. Oral and ocular sicca symptoms and findings are prevalent in systemic lupus erythematosus. J. Oral Pathol. Med. 1999;28:317–322. doi: 10.1111/j.1600-0714.1999.tb02047.x. [DOI] [PubMed] [Google Scholar]
- 31.Read R.W. Clinical mini-review: Systemic lupus erythematosus and the eye. Ocul. Immunol. Inflamm. 2004;12:87–99. doi: 10.1080/09273940490895308. [DOI] [PubMed] [Google Scholar]
- 32.Heiligenhaus A., Dutt J.E., Foster C.S. Histology and immunopathology of systemic lupus erythematosus affecting the conjunctiva. Eye. 1996;10:425–432. doi: 10.1038/eye.1996.94. [DOI] [PubMed] [Google Scholar]
- 33.Oh J.Y., Kim M.K., Choi H.J., Ko J.H., Kang E.J., Lee H.J., Wee W.R., Lee J.H. Investigating the relationship between serum interleukin-17 levels and systemic immune-mediated disease in patients with dry eye syndrome. Korean J. Ophthalmol. 2011;25:73–76. doi: 10.3341/kjo.2011.25.2.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frith P., Burge S.M., Millard P.R., Wojnarowska F. External ocular findings in lupus erythematosus: A clinical and immunopathological study. Br. J. Ophthalmol. 1990;74:163–167. doi: 10.1136/bjo.74.3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung H.H., Ji Y.S., Sung M.S., Kim K.K., Yoon K.C. Long-term outcome of treatment with topical corticosteroids for severe dry eye associated with Sjögren's syndrome. Chonnam Med. J. 2015;51:26–32. doi: 10.4068/cmj.2015.51.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prinz J., Maffulli N., Fuest M., Walter P., Bell A., Migliorini F. Efficacy of topical administration of corticosteroids for the management of dry eye disease: Systematic review and meta-analysis. Life. 2022;12:1932. doi: 10.3390/life12111932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed F., House R.J., Feldman B.H. Corneal abrasions and corneal foreign bodies. Prim. Care. 2015;42:363–375. doi: 10.1016/j.pop.2015.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Sharma S. Keratitis. Biosci. Rep. 2001;21:419–444. doi: 10.1023/A:1017939725776. [DOI] [PubMed] [Google Scholar]
- 39.Cao Y., Zhang W., Wu J., Zhang H., Zhou H. Peripheral ulcerative keratitis associated with autoimmune disease: Pathogenesis and treatment. J. Ophthalmol. 2017;2017:7298026. doi: 10.1155/2017/7298026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Riley G.P., Harrall R.L., Watson P.G., Cawston T.E., Hazleman B.L. Collagenase (MMP-1) and TIMP-1 in destructive corneal disease associated with rheumatoid arthritis. Eye. 1995;9:703–718. doi: 10.1038/eye.1995.182. [DOI] [PubMed] [Google Scholar]
- 41.Watanabe R., Ishii T., Yoshida M., Takada N., Yokokura S., Shirota Y., Fujii H., Harigae H. Ulcerative keratitis in patients with rheumatoid arthritis in the modern biologic era: A series of eight cases and literature review. Int. J. Rheum. Dis. 2017;20:225–230. doi: 10.1111/1756-185X.12688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.