Abstract
The Drosophila melanogaster GAGA factor (encoded by the Trithorax-like [Trl] gene) is required for correct chromatin architecture at diverse chromosomal sites. The Trl gene encodes two alternatively spliced isoforms of the GAGA factor (GAGA-519 and GAGA-581) that are identical except for the length and sequence of the C-terminal glutamine-rich (Q) domain. In vitro and tissue culture experiments failed to find any functional difference between the two isoforms. We made a set of transgenes that constitutively express cDNAs coding for either of the isoforms with the goal of elucidating their roles in vivo. Phenotypic analysis of the transgenes in Trl mutant background led us to the conclusion that GAGA-519 and GAGA-581 perform different, albeit largely overlapping, functions. We also expressed a fusion protein with LacZ disrupting the Q domain of GAGA-519. This LacZ fusion protein compensated for the loss of wild-type GAGA factor to a surprisingly large extent. This suggests that the Q domain either is not required for the essential functions performed by the GAGA protein or is exclusively used for tetramer formation. These results are inconsistent with a major role of the Q domain in chromatin remodeling or transcriptional activation. We also found that GAGA-LacZ was able to associate with sites not normally occupied by the GAGA factor, pointing to a role of the Q domain in binding site choice in vivo.
The packaging of DNA into chromatin restricts its accessibility and interferes with many DNA-dependent processes such as replication and transcription (11). To circumvent this problem of accessibility, regions of chromatin that are nucleosome free and hypersensitive to low levels of nucleases (e.g., DNase I) are established (39). One protein that seems to have an important role in generating and/or maintaining nucleosome-free regions of chromatin is the Drosophila melanogaster GAGA factor (6, 16, 17, 31, 38). GAGA binding sites are found in the promoters and enhancers of many Drosophila genes including hsp70, hsp26, fushi tarazu (ftz), engrailed (en), and the bithorax complex (BX-C) gene Ultrabithorax (Ubx) (16, 18, 30, 38). In in vitro chromatin assembly experiments, the GAGA factor cooperates with the ATP-dependent nucleosome-remodeling factor to displace nucleosomes from promoters containing GAGA binding sites. In in vivo transgene assays, mutations in the hsp70 and hsp26 GAGA binding sites interfere with the formation of nucleosome-free regions of chromatin over each promoter and reduce transcriptional activity (21, 29). The role of the GAGA factor is not, however, limited to promoters and enhancers. GAGA binding sites are also present in Polycomb response elements (the iab-7 PRE and a bxd PRE) and a domain boundary (Fab-7) from BX-C (18, 19). GAGA protein associates with these BX-C regulatory elements in vivo, and the GAGA sites in the PREs are critical for Polycomb-dependent silencing (19, 22). Finally, in diploid nuclei the GAGA factor localizes not only to euchromatin but also to heterochromatin, where it is thought to bind to the (AAGAG)n satellite DNA (26, 27). This heterochromatic localization is particularly evident during the rapid nuclear divisions in precellular blastoderm embryos.
Mutations in the gene encoding the GAGA factor, Trithorax-like (Trl), have been isolated (4, 12). The original Trl allele, Trl13C, is a weak hypomorph and has reduced viability. Like mutations in other Trithorax group genes, it dominantly enhances the haltere-to-wing transformations observed in animals heterozygous for a Ubx mutation. A presumed null allele, TrlR67, was generated by the imprecise excision of the Trl13C P-element (12). There also are two ethyl methanesulfonate (EMS) alleles that have properties quite similar to TrlR67. Trl is an essential gene, and animals homozygous for TrlR67 or either of the EMS alleles die before they reach the third larval instar. On the other hand, when TrlR67 or the EMS mutants are combined with the hypomorphic Trl13C allele, a few of the animals survive to adulthood.
Since Trl is essential for viability, adult phenotypes can be studied only by using adult-viable allelic combinations. Flies homozygous for the hypomorphic Trl13C mutation have homeotic transformations from the sixth abdominal segment to the fifth and are female sterile (12). The abdominal transformations indicate that Trl is required for the proper regulation of Abdominal B (Abd-B), a gene in the bithorax complex. The female sterility can be attributed to two different defects (4). First, Trl13C females produce many fewer eggs than do wild-type females. Second, fewer than 10% of the embryos from Trl13C mothers hatch even when fertilized by wild-type males. Only very little GAGA protein is expressed in embryos from Trl13C mothers, and more than 70% do not develop beyond the cellular blastoderm stage. This early embryonic arrest is due to disruptions in the nuclear division cycles during cycles 10 to 14 after the nuclei have migrated to the surface of the embryo. Unlike what is observed for the wild type, the nuclear division cycles in Trl13C embryos are asynchronous and a range of mitotic defects is observed. These defects include incomplete chromosome condensation, asymmetric chromosome segregation, severe chromosome fragmentation, and the loss of nuclei from the surface. It is thought that abnormalities in the chromatin organization of the heterochromatic (AAGAG)n satellite DNA are responsible for the mitotic defects. While high levels of GAGA are associated with centromeric heterochromatin in wild-type embryos, there is little or no heterochromatic protein in most Trl13C embryos (4). The few embryos that successfully cellularize and begin gastrulation display defects in expression of genes such as ftz and en that have GAGA binding sites in their regulatory regions (4). These defects are typically seen in nuclei that have little or no GAGA protein.
GAGA is synthesized in sufficient quantities from maternally deposited mRNA to sustain development through embryogenesis. However, maternal message is not the only source of GAGA in the embryo, and roughly midway through gastrulation zygotic Trl transcripts can be detected. In addition to the mRNAs encoding the GAGA-519 isoform, alternatively spliced transcripts encoding a second major GAGA isoform, GAGA-581, are expressed.
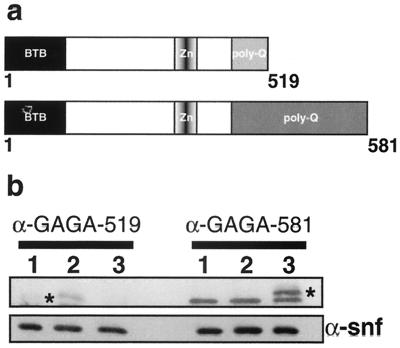
The GAGA-519 and GAGA-581 proteins have three distinct domains (Fig. 1a). The first two domains are present in both proteins, while the C-terminal domains, which are encoded by alternative 3′ exons, are unique. The N-terminal 120 amino acids (aa) comprise a so-called BTB/POZ (Bric a brac, Tramtrack, Broad-complex/Poxvirus, Zinc finger) domain. The BTB/POZ domain is found in a variety of proteins from yeast to human (2, 10, 15, 28). The central part of the protein contains a single zinc finger flanked by two small basic regions. The zinc finger and flanking basic regions are responsible for the DNA binding activity of the GAGA protein (24, 25). The C-terminal Q domains of the GAGA-519 and the GAGA-581 proteins are both highly glutamine rich, but they have distinct sequences and lengths (3).
FIG. 1.
(a) Schematic representation of the GAGA isoform constructs used in this study. Both transgenes are driven by the hsp83 promoter. (b) Western blot showing expression of the hsp83:GAGA-519 and hsp83:GAGA-581 transgenes. Lanes: 1, embryos from a cross of w1 males and females; 2, embryos from a cross of 4Xhsp83:GAGA-519; Trl81.1/Trl109.2 males and females; 3, embryos from a cross of 4Xhsp83:GAGA-581; TrlR67/Trl109.2 males and females. The first three lanes (left half of the gel) were probed with the antibody against GAGA-519 (α-GAGA-519). The weak band corresponding to the GAGA-519 protein in lane 1 is indicated with a star. The right half of the gel was probed with the GAGA-581 antibody (α-GAGA-581). This antibody recognizes a nonspecific band that appears in all three lanes. The band corresponding to the GAGA-581 protein is indicated with a star. α-Snf antibody was used as a loading control.
While there are already some indications as to likely functions of the BTB/POZ and Zn-finger domains, the role of the C-terminal Q domains is not well understood. Chromatin assembly experiments indicate that the Q domains are dispensable for chromatin remodeling at least in vitro (1). Although the Q domain of the GAGA-519 protein seems to be important for transcriptional activation in transient-transfection assays (33), it is not at all certain whether the ability to affect transcription in these assays (3, 30, 33) is relevant to GAGA function in flies. Perhaps the most compelling evidence that the Q domain may be important comes from the studies of Wilkins and Lis (37). They showed that while the 519 Q domain is not required for DNA binding, it does alter the DNA binding properties of the protein. The 519 Q domain can itself interact with single-stranded DNA and may promote DNA distortion upon GAGA factor binding. In addition, the 519 Q domain mediates the tetramerization of the intact GAGA factor and in isolation can form multimeric complexes (1, 37).
How these activities of the 519 Q domain relate to the in vivo functions of the GAGA factor is not known. Moreover, it is not at all clear why there are two GAGA isoforms with completely different Q domains. The GAGA-519 and GAGA-581 proteins behave indistinguishably in in vitro DNA binding assays and in tissue culture transient-transfection experiments, and they colocalize in polytene chromosomes (3). On the other hand, the striking conservation of the two isoforms between distantly related species of fruit flies (20) suggests that these isoforms may have distinct functions. This possibility is supported by the differences in the stage- and tissue-specific patterns of expression of the GAGA-519 and GAGA-581 proteins (3).
In the studies reported here we have used rescue constructs to examine whether the GAGA-519 and GAGA-581 are functionally equivalent in vivo. We find that GAGA-519 and GAGA-581 have overlapping but not identical functions. To further elucidate the role of the Q domain, we replaced it with the bacterial protein β-galactosidase. Surprisingly, this GAGA-LacZ fusion protein has partial function, suggesting that the Q domain may not have a major role in chromatin remodeling or gene regulation.
MATERIALS AND METHODS
Fly stocks.
Trl13C is a P-element-induced allele (the P-element is located in the first intron of the gene) (12). TrlR67 (12) was generated by excision of the P-element that generated the Trl13C allele. This mutation has sequences deleted from the 5′ half of the Trl transcription unit. Trl81.1 and Trl109.2 are independently isolated EMS-induced alleles (A. J. Greenberg, unpublished data).
All crosses were performed in w1 background using the TM6b, Tb Sb Hu e balancer chromosome to ensure consistency of genetic background. Some crosses were repeated using the TM3, Ser e balancer with indistinguishable results (data not shown).
Construction of transgenic flies.
The GAGA-581 cDNA was a generous gift of C. Benyajati. The GAGA-519 cDNA was obtained from K. Bhat. The GAGA-LacZ fusion was obtained by joining the LacZ coding sequence bearing a 3′-untranslated region with a simian virus 40 poly(A) site at the SphI site of GAGA-519. The GAGA-519 cDNA and the cDNA coding for the fusion protein were inserted into the XhoI-SalI region of the hsp83CasPeR vector (5). The 3′ end of the GAGA-519 cDNA was then substituted for the 3′ end of a GAGA-581 cDNA. All plasmids were purified with Qiagen columns and injected into embryos from the w1 stock. All the transgenes were marked by mini-white and were monitored by eye color.
Both hsp83:GAGA-519 lines (#2 and #3) described here were on the second chromosome. One of the hsp83:GAGA-581 lines (#12) was on the second, while the other (#13) was on the third. Two of the hsp83:GAGA-LacZ lines (#12 and #8) were on the third chromosome, and one (#6) was on the second. All lines exhibited comparable yellow-orange eye color with the exception of the hsp83:GAGA-LacZ line #6, which was pale yellow. All third-chromosome lines recombined readily with Trl alleles.
For simplicity, hsp83:GAGA-519-2 hsp83:GAGA-519-3; Trl81.1/Trl109.2 flies are referred to as 4Xhsp83:GAGA-519; Trl81.1/Trl109.2, and hsp83:GAGA-581-12; hsp83:GAGA-581-13 TrlR67/hsp83:GAGA-581-13 Trl109.2 flies are referred to as 4Xhsp83:GAGA-581; TrlR67/Trl109.2.
Genetic crosses.
All crosses were performed at 22°C in an incubator. Flies were grown on standard cornmeal media.
In order to minimize the influences of the environment and genetic background, the viabilities of Trl13C/TrlR67 flies that carried each of the transgenes were compared to those of their siblings that did not. These were generated by crossing w1; Tg/+; Trl13C/TM6b males or females to w1; +/+; TrlR67/TM6b males or females if the transgene was on the second chromosome and w1; Tg Trl13C/Trl13C males to w1; TrlR67/TM6b females if it was on the third.
The Trl-null flies carrying four copies of hsp83:GAGA-519 or hsp83:GAGA-581 were obtained from a cross of parents heterozygous for null Trl alleles. To avoid second-site mutation effects, the flies were made trans-heterozygous for different null alleles.
Tests for female fertility and maternal effect.
Single females were placed in laying blocks with three males each. The eggs produced by each female over a 24-h period were counted, and the numbers were averaged over 7 to 10 days. At least 4, but usually 10 females of each genotype were scored. The eggs produced were then left for an additional 48 h, and the hatch rate was calculated. All the tests were performed in an incubator at 22°C.
Statistics.
χ2 analysis was used when comparing numbers of progeny from one cross. When comparisons between crosses were made, two-tailed Fisher's exact test using 2 × 2 contingency tables was utilized. This test was used for comparing percentages of males with A6-to-A5 transformations as well.
Western blots.
About 10 0- to 6-h-old embryos' worth of extract was loaded onto a sodium dodecyl sulfate–8% polyacrylamide gel. Western blotting was performed as described in reference 9. Blots were probed with a 1:1,000 dilution of rabbit α-GAGA-581 or a 1:500 dilution of rabbit α-GAGA-519 antibody (3). Crude sera were affinity purified as described in reference 3. For a loading control the bottom part of the filter was cut off and probed with a 1:15 dilution of anti-Snf monoclonal antibody 4G3 raised in mouse (8). Horseradish peroxidase-conjugated secondary antibodies at dilutions of 1:1,000 (α-mouse) and 1:10,000 (α-rabbit) were used. Signals were detected with the Lumi-Light (Boehringer Mannheim) chemiluminescence system.
Staining of polytene chromosomes.
Larvae were grown at 22°C in plastic bottles on standard media regularly supplemented with water and yeast. When the first third-instar larvae began to appear, the bottles were switched to 29°C for 3 days to induce a high level of GAGA-LacZ expression. Salivary glands were dissected in phosphate-buffered saline (PBS) with 1% Tween 20 (Sigma) and fixed for 2 min in 1 drop of solution containing 50% acetic acid, 3.7% formaldehyde, and 1% Tween 20. Chromosomes were spread and stored in PBS with 0.2% Tween 20 and 1% bovine serum albumin (PBSTB) at 4°C. Affinity-purified rabbit α-GAGA-581 (3) was used at a dilution of 1:50. A mouse α-LacZ monoclonal antibody (obtained from the Developmental Studies Hybridoma Bank, University of Iowa) was used at a dilution of 1:50. Alexa-488- and Alexa-546-conjugated goat secondary antibodies (Molecular Probes) were used at a dilution of 1:1,000 in PBSTB with 5% normal goat serum. DNA was detected with Hoechst (0.5 μg/ml in water). Images were collected with the Zeiss LSM 510 confocal microscope.
Staining of embryos.
Embryos were collected, fixed, and stained according to a standard protocol (4). Females of all genotypes were raised at 22°C. Embryos were collected at 22°C, except for embryos from 2Xhsp83:GAGA-LacZ mothers. These were collected at 29°C. DNA was visualized using TOTO-1 at a dilution of 1:300. α-GAGA-519 and α-GAGA-581 were used at dilutions of 1:25 and 1:50, respectively. Staining was visualized by fluorescence using biotinylated goat α-rabbit or α-mouse antibodies and Alexa-488-conjugated streptavidin. Images were collected with the Zeiss LSM 510 confocal microscope.
RESULTS
GAGA transgenes express the GAGA-519 and GAGA-581 isoforms.
To examine the in vivo functions of the two GAGA isoforms, we used an hsp83 promoter to drive the expression of cDNAs encoding the GAGA-519 and GAGA-581 proteins (Fig. 1a). The hsp83 promoter is active at room temperature in most fly tissues and cell types and can be upregulated by raising the flies at elevated temperatures (5). This promoter has been used previously to drive the expression of cDNA rescue constructs for ubiquitously expressed genes like transformer, doublesex, and zw-5 (14, 34, 35). Mini-white transgenes containing the hsp83:GAGA-519 and hsp83:GAGA-581 expression constructs were introduced into flies and multiple independent insertion lines of each were obtained. Two lines for each transgene were then selected for further analysis. The appropriate GAGA isoform is expressed by each transgene. In the experiment shown in Fig. 1b, embryos were collected from Trl-null mutant females rescued by four copies of either the hsp83:GAGA-519 or the hsp83:GAGA-581 transgene and from wild type. Western blots were prepared from each sample and probed with antibodies specific to the GAGA-519 or GAGA-581 isoforms (see the legend to Fig. 1b). The maternally encoded GAGA-519 isoform (Fig. 1b, lane 1) is observed in 0- to 6-h-old wild-type embryos. This isoform is also found in embryos from 4Xhsp83:GAGA-519 mothers (lane 2), but it is not present in embryos from 4Xhsp83:GAGA-581 mothers (lane 3). Note that offspring from Trl mutant mothers that have four copies of the hsp83:GAGA-519 transgene have higher levels of the GAGA-519 isoform than are found in wild-type embryos. Finally, the GAGA-581 isoform can be detected in embryos from 4Xhsp83:GAGA-581 mothers (see lanes 4 to 6) but not in embryos from wild-type or 4Xhsp83:GAGA-519 mothers. This was expected since little if any of this isoform is contributed maternally (3).
Both GAGA isoforms rescue the lethal effects of Trl mutant combinations.
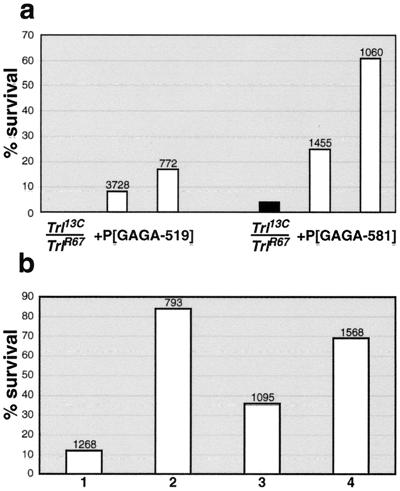
Flies homozygous for the hypomorphic Trl13C mutation have reduced viability. This lethality is enhanced when Trl13C is trans to one of the null Trl alleles such as TrlR67, and only a few percent of the mutant flies survive to the adult stage (Fig. 2a). We found that a single copy of either the hsp83:GAGA-519 or the hsp83:GAGA-581 transgene can rescue the lethality of the Trl13C/TrlR67mutant combination (Fig. 2a). In a separate set of experiments with an hsp70 promoter, Granok et al. (personal communication) also found that a cDNA encoding the GAGA-519 isoform can rescue the lethal effects of Trl13C; however, the rescuing activity in their experiments was much less than that obtained with our hsp83 transgenes, presumably because heat shock is required to activate the hsp70 promoter. A comparison of the extent of rescue with the hsp83 transgenes indicates that rescuing activity is influenced by chromosomal-position effects. Nevertheless, both of the hsp83:GAGA-581 lines are more effective in restoring viability than either of the hsp83:GAGA-519 lines.
FIG. 2.
GAGA transgenes rescue viability of hemizygous Trl13C flies. Viability was calculated as the percentage of the expected numbers based on Mendelian ratios. Numbers above bars indicate the total number of flies scored in a cross. (a) Siblings with (white columns) and without (black columns) a transgene were compared. Different bars of the same genotype represent different lines of a transgene. The viability of Trl13C/TrlR67 flies represents an average between two crosses involving different lines of the same transgene. All P values were smaller than 10−4, obtained by the χ2 test. (b) Viability of Trl-null flies rescued by four copies of hsp83:GAGA-519, four copies of hsp83:GAGA-581, or two copies of each. Genotypes of flies examined were 4Xhsp83:GAGA-519; Trl109.2/Trl81.1 (Bar 1); 4Xhsp83:GAGA-581; Trl109.2/TrlR67 (Bar 2); 2Xhsp83:GAGA-581/2Xhsp83:GAGA-519; Trl109.2/Trl81.1 (Bar 3); 2Xhsp83:GAGA-581/2Xhsp83:GAGA-519; Trl109.2/TrlR67 (Bar 4). The rescue was significant in each case, with all P values smaller than 10−4 by Fisher's exact test.
We also tested whether the hsp83:GAGA-519 or hsp83:GAGA-581 transgenes can rescue Trl-null mutations. We found that the zygotic lethality of null allelic combinations could not be rescued by either a single or two copies of the transgenes. However, it was possible to rescue Trl-null mutant combinations using four copies of the transgenes (Fig. 2b). Although GAGA-581 appears to be more active than GAGA-519 in this situation as well, for technical reasons we were able to assay the activity of only four copies of each transgene using a different combination of Trl-null alleles. Hence, the relative activity of the hsp83:GAGA-519 and hsp83:GAGA-581 transgenes is best assessed by comparing the viability of equivalent Trl mutant combinations that have either four copies of one transgene or two copies of each transgene. As can be seen in Fig. 2b (compare the first and third bars), replacing two of the four copies of hsp83:GAGA-519 with two copies of hsp83:GAGA-581 improves the viability of this Trl mutant combination. Conversely, replacing two copies of hsp83:GAGA-581 with two copies of hsp83:GAGA-519 reduces the viability of the other Trl mutant combination (Fig. 2b, compare the second and fourth bars).
The GAGA-519 and GAGA-581 transgenes have overlapping but not equivalent activities.
The results described in the previous section indicate that the hsp83:GAGA-519 transgene is less effective in rescuing the zygotic lethality associated with loss of Trl function than is hsp83:GAGA-581. An obvious question is whether there are any other differences in the activities of the two transgenes. To address this question we compared the abilities of the two transgenes to rescue several other Trl mutant phenotypes. We used two different transgene-Trl mutant combinations for these experiments. In the first, we tested the rescuing activity of a single copy of the transgene in a Trl13C homozygous mutant background. Since the Trl13C allele is hypomorphic, it should be possible to detect some amelioration of the phenotype even if the transgene has only weak activity. In the second, we tested the rescuing activity of four copies of the transgene in Trl-null background. In this background, a much higher level of activity should be required to rescue the mutant phenotypes.
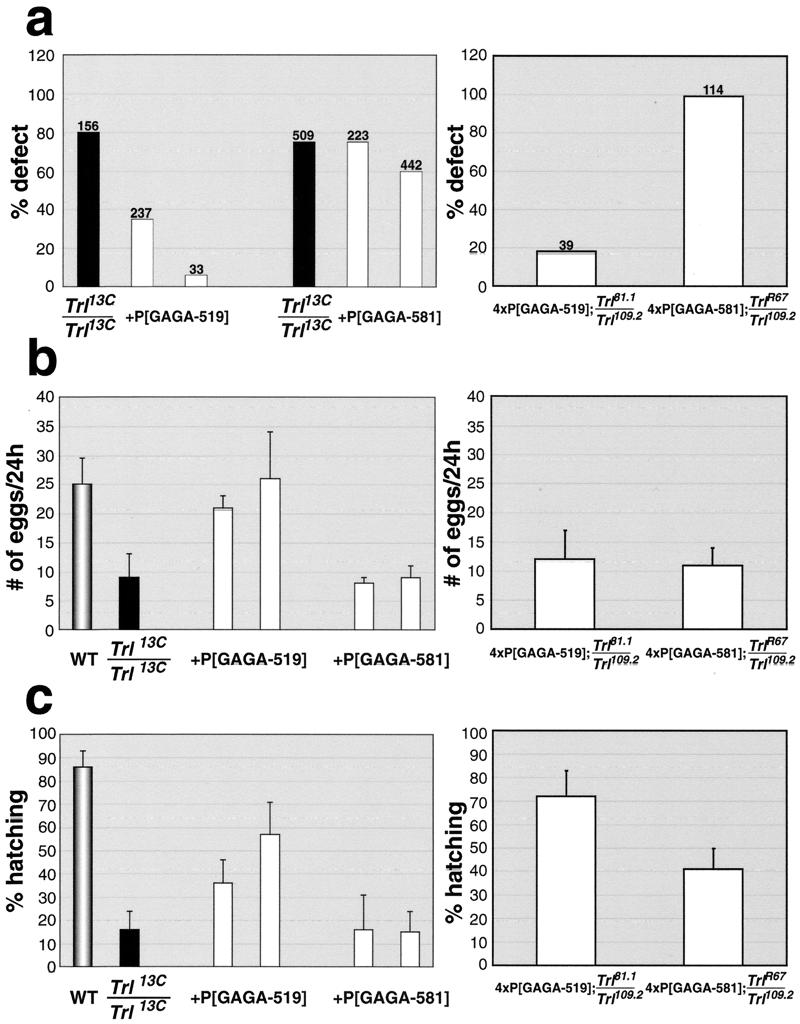
Abdominal transformation.
About 80% of Trl13C homozygous males show a transformation of the sixth abdominal segment towards the fifth, indicative of a loss of Abd-B activity (12). Since the introduction of a single copy of the hsp83:GAGA-519 transgene into the Trl13C mutant background suppresses this transformation, the GAGA-519 isoform can rescue this defect in Abd-B expression (Fig. 3a, left panel). By contrast, the hsp83:GAGA-581 transgene has little rescuing activity. Similar results were obtained with four copies of the transgene in the Trl-null mutant background. All of the surviving hsp83:GAGA-581 Trl mutant males had the abdominal transformation (Fig. 3a, right panel). In contrast, less than 20% of the hsp83:GAGA-519 Trl mutant males had this transformation.
FIG. 3.
Rescue of Trl phenotypes by GAGA-519 and GAGA-581. (a) Rescue of abdominal transformations in males. Siblings with (white columns) and without (black columns) a transgene were compared. The number above each bar represents the total number of males examined. Values for Trl13C-homozygous males shown represent averages between the crosses involving different lines of the same transgene. Rescue by GAGA-519 was highly significant, with all P values less than 10−8, calculated by Fisher's exact test. (b) Rescue of female fertility. Error bars represent 95% confidence intervals. The numbers of eggs laid by single females were measured as described in Materials and Methods. In the left panel, the gray column corresponds to the wild-type females, the black column corresponds to the Trl13C-homozygous females, and the white columns correspond to Trl13C-homozygous females carrying the transgenes. (c) Rescue of maternal effect lethality. Error bars represent 95% confidence intervals. The hatch rate was determined after 48 h at 22°C, as described in Materials and Methods. The colors of the columns correspond to the same genotypes as for panel b.
Egg-laying defects.
Trl13C females produce a reduced number of eggs compared to the wild type (Fig. 3b, left panel). This egg-laying defect can be rescued by the hsp83:GAGA-519 transgene, but not by the hsp83:GAGA-581 transgene. Interestingly, the two transgenes are equally effective in rescuing the egg-laying defects of Trl-null females (Fig. 3b, right panel).
Maternal-effect lethality.
Fewer than 20% of the embryos produced by homozygous Trl13C mothers survive to the larval stage, even when fertilized by wild-type males (Fig. 3c, left panel). The hsp83:GAGA-519 transgene partially rescues this maternal-effect lethality, while hsp83:GAGA-581 does not. In the Trl-null background, the hsp83:GAGA-581 transgene is about half as active as hsp83:GAGA-519 (Fig. 3c, right panel).
Nuclear division and transcription defects.
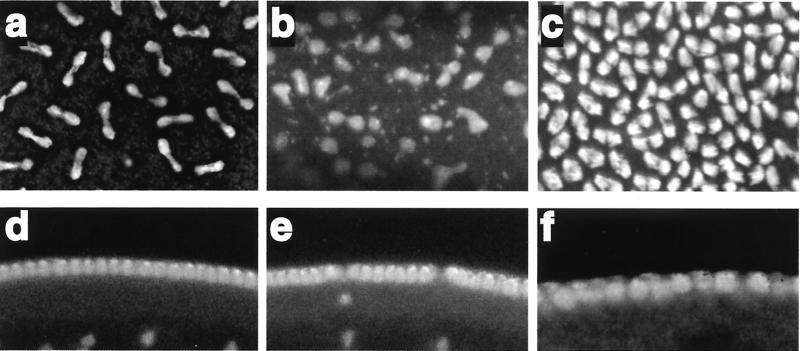
A large proportion of the embryos from Trl13C mothers have severe defects in nuclear division during the syncytial blastoderm stage. Consequently, the hsp83:GAGA-581 transgene might be less able to rescue the maternal-effect lethality of Trl mutations because the GAGA-581 isoform cannot completely support nuclear division. To determine if this is the case, we examined embryos from wild-type, homozygous Trl13C and 4X hsp83:GAGA-581; TrlR67/Trl109.2 mothers. As reported previously (4), the majority of embryos laid by homozygous Trl13C mothers exhibited a variety of nuclear division and mitotic abnormalities (Fig. 4b), leading to the loss of nuclei from the surface of the embryo later on (Fig. 4e). In contrast, none of the embryos produced by 4Xhsp83:GAGA-581; TrlR67/Trl109.2 mothers exhibited such defects (Fig. 4c), and the nuclear monolayer was intact at subsequent stages of embryogenesis (Fig. 4e). Nevertheless, fewer than half of these embryos hatched into first-instar larvae (Fig. 3c, right panel).
FIG. 4.
GAGA-581 can rescue Trl nuclear defects. (a to c) Nuclei in mitosis. (d to f) Embryos at syncytial blastoderm stage. Nuclei were visualized with TOTO-1. (a and d) Embryos from w1 mothers. (b and e) Embryos from Trl13C mothers. (c and f) Embryos from 4Xhsp83:GAGA-581; Trl109.2/TrlR67 mothers. All females were fertilized by w1 males.
These findings argue that the GAGA-581 isoform is capable of supporting nuclear division in the early embryo. Hence, the failure to more completely rescue maternal-effect lethality is most likely due to an inability to function in some other GAGA-519-dependent process such as zygotic gene expression. With this possibility in mind, we examined ftz expression in embryos produced by Trl13C mothers that either have or do not have a copy of the hsp83:GAGA-581 transgene. We found that the GAGA-581 isoform rescues the Trl13C defects in ftz expression (not shown). This would suggest that the misexpression of some other gene or some other Trl-dependent process is not fully rescued by the GAGA-581 protein.
Distribution of the two isoforms on mitotic chromosomes in early embryos.
In wild-type embryos, high levels of the GAGA-519 protein localize to centromeric heterochromatin and can be visualized as brightly labeled dots in mitotic chromosomes (Fig. 5a). (Note that even greater amounts of centromeric GAGA-519 protein are present in embryos from 4Xhsp83:GAGA-519 mothers). In contrast, in embryos from Trl13C homozygous mothers, where there are nuclear division defects, there is little or no GAGA protein in heterochromatin (4). Since the GAGA-581 protein is able to rescue the nuclear division defects, we expected that it should localize to centromeric heterochromatin like the GAGA-519 protein. Figure 5 shows that this is the case. The GAGA-581 protein was not detected in mitotic chromosomes of wild-type embryos but was localized to centromeric heterochromatin in embryos produced by mothers carrying four copies of the hsp83:GAGA-581 transgene. Moreover, this localization was observed even in embryos produced by Trl mutant mothers.
FIG. 5.
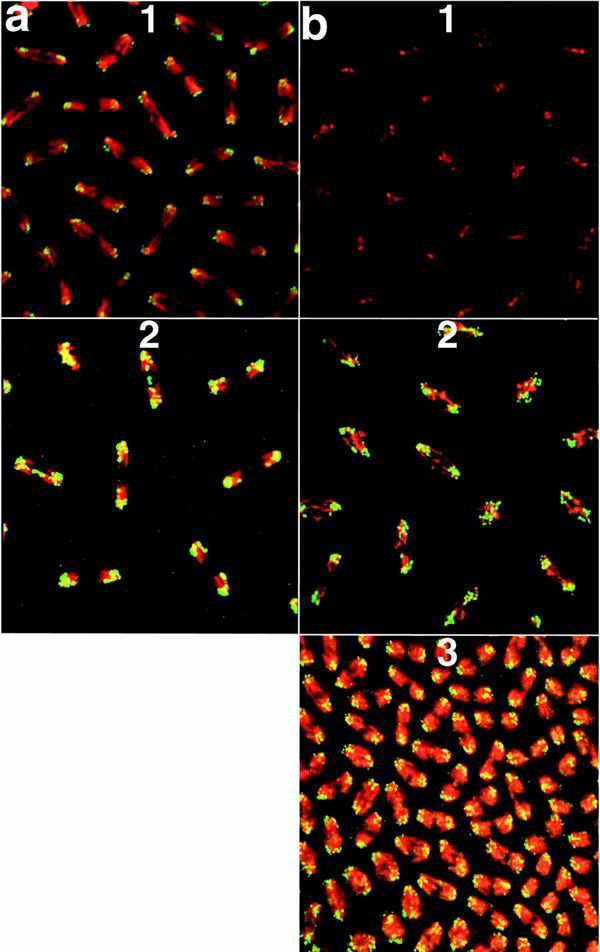
Association of GAGA-519 and GAGA-581 with centric heterochromatin. DNA is in red, and protein is in green. Chromosomes are shown in anaphase, at the time they are being separated by microtubules. Centromeres are pointing outward, and telomeres are oriented inward. All embryos are at the syncytial blastoderm stage (stage 4 [36]) of embryogenesis but have undergone different numbers of nuclear divisions. Embryos were stained with the GAGA-519-specific antibody (a) or with the GAGA-581-specific antibody (b). DNA was visualized with TOTO-1. The genotypes of embryos were as follows: a1 and b1, wild type; a2, 4Xhsp83: GAGA-519; b2, Xhsp83: GAGA-581; b3, 4Xhsp83GAGA-581; Trl109.2/TrlR67.
GAGA-LacZ fusion protein retains substantial GAGA activity.
Although the GAGA-519 and GAGA-581 proteins have distinct properties in vivo, they can, in many instances, function interchangeably. This led us to wonder whether the Q domains could be replaced by a heterologous protein. To test this possibility, we fused the coding sequences for the first 495 aa of GAGA-519 to sequences encoding the bacterial β-galactosidase protein. As illustrated in Fig. 6a, this partially eliminates the GAGA-519 Q domain and replaces it with β-galactosidase. The resulting fusion protein retains only 13 of the 30 glutamine residues found in the GAGA-519 Q domain. Three independent hsp83:GAGA-LacZ transgenic lines were selected for further study. Expression of the GAGA-LacZ fusion protein was verified by Western blotting (not shown) and by X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) staining of embryos (data not shown) and salivary glands (Fig. 7a).
FIG. 6.
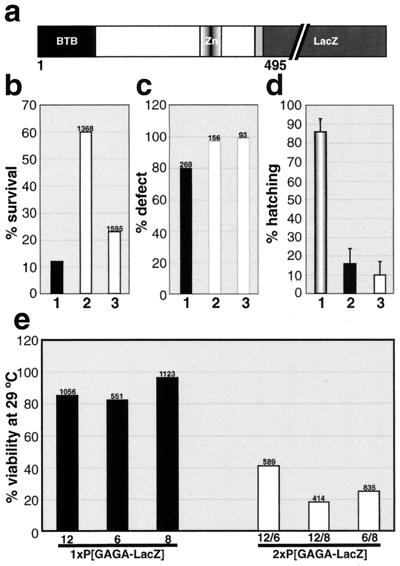
Effects of hsp83:GAGA-LacZ on viability and Trl loss of function phenotypes. (a) A schematic representation of the LacZ fusion protein. Expression was driven with the hsp83 promoter. (b) One copy of hsp83:GAGA-LacZ rescues viability of Trl13C/TrlR67 flies. Genotypes represented are as follows: 1, Trl13C/TrlR67; 2, 1Xhsp83:GAGA-LacZ #6; Trl13C/TrlR67; 3, 1Xhsp83:GAGA-LacZ #8; Trl13C/TrlR67. Siblings with and without the transgene were compared. The viability of Trl13C/TrlR67 represents an average between two crosses involving different lines of the transgene. All P values were smaller than 10−4, obtained by the χ2 test. (c) Rescue of abdominal transformations in males. The genotypes of males examined were as follows: bar 1, Trl13C/Trl13C; bar 2, 1Xhsp83: GAGA-LacZ #6; Trl13C/Trl13C; bar 3, 1Xhsp83: GAGA-LacZ #8; Trl13C/Trl13C. Siblings with and without the transgene were compared. The number above each bar represents the total number of males examined. Values for Trl13C-homozygous males shown represent averages between two crosses involving different lines of the transgene. (d) Rescue of maternal-effect lethality. The genotypes of females shown are as follows: bar 1, w1; bar 2, Trl13C/Trl13C; bar 3, 1Xhsp83:GAGA-LacZ #6; Trl13C/Trl13C. Error bars represent 95% confidence intervals. The hatch rate was determined after 48 h at 22°C, as described in Materials and Methods. (e) Lethal effects of the hsp83:GAGA-LacZ transgene in one copy (black bars) and two copies (white bars). The number above each bar indicates the total number of flies scored in a cross. The viability of flies with one copy of the transgene was compared to that of w1 siblings. The viability of flies carrying two copies of the transgene was compared to that of heterozygous siblings. Effects in two copies were assayed in trans-heterozygotes to avoid site of insertion effects. All P values for two-copy lethality were less than 10−11, calculated using the χ2 test.
FIG. 7.
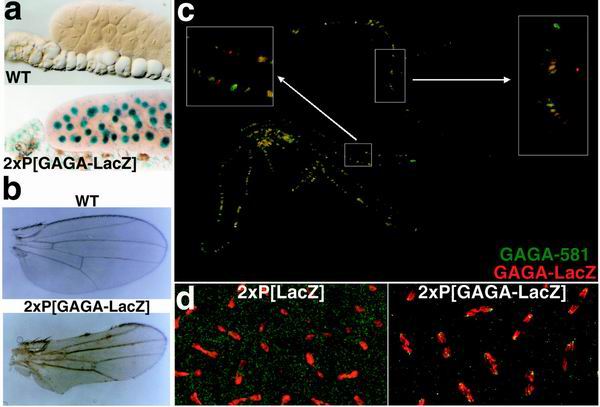
Localization of the hsp83:GAGA-LacZ transgene. (a) Salivary glands from wild-type (WT) and 2Xhsp83:GAGA-LacZ larvae stained with X-Gal. Line #8 of hsp83:GAGA-LacZ was used. Other lines showed similar staining patterns and intensities (data not shown). (b) Wing defects associated with GAGA-LacZ. Flies were raised at 29°C, and wings were dissected off and mounted in Hoyer's mountant. (c) Imperfect colocalization of GAGA-581 (green) and GAGA-LacZ (red). The overlap is yellow. Polytene chromosomes were double stained using antibodies against GAGA-581 and LacZ, as described in Materials and Methods. (d) Association of GAGA-LacZ with centric heterochromatin. DNA is in red, and protein is in green. As in Fig. 4, chromosomes are shown in anaphase, at the time they are being separated by microtubules. Centromeres are pointing outward, and telomeres are oriented inward. Embryos were stained with an antibody against LacZ; DNA was visualized with TOTO-1 (see Materials and Methods).
We then examined the biological activity of the hsp83:GAGA-LacZ transgene. As shown in Fig. 6b, the zygotic lethality of the Trl13C/TrlR67 mutant combination can be rescued by a single copy of the hsp83:GAGA-LacZ transgene. In fact, one of the transgenic lines appears to rescue zygotic lethality to almost the same extent as the hsp83:GAGA-581 transgene. On the other hand, the hsp83:GAGA-LacZ transgene does not rescue either the A6-to-A5 abdominal transformation or the maternal-effect lethality of Trl13C/TrlR67 flies (Fig. 6c and d). The fusion protein does, however, localize to centromeric heterochromatin in the mitotic chromosomes of early embryos (Fig. 7d). The activity of hsp83:GAGA-LacZ transgenes could not be assayed in Trl-null background because we were unable to obtain flies carrying more than two copies of the transgene even in a wild-type background (see below).
GAGA-LacZ binds sites not associated with wild-type GAGA proteins.
Although the hsp83:GAGA-LacZ transgene is able to rescue some of the phenotypic effects of Trl mutations, the fusion also has some unusual activities. While a single copy of the hsp83:GAGA-LacZ transgene has little effect on viability, two copies of the transgene significantly reduced viability at higher temperatures. As shown in Fig. 6e, the viability of all three pairwise combinations of hsp83:GAGA-LacZ inserts was less than 50% at 29°C. Similar lethal effects are observed when there are two copies of the same insert. The 2Xhsp83:GAGA-LacZ flies that survive at 29°C exhibit phenotypes, such as the loss of the wing margin (Fig. 7b), not normally associated with Trl loss of function. Since neither of these phenotypic effects becomes more severe when the transgenic flies are heterozygous for the TrlR67 mutation, it would appear that the hsp83:GAGA-LacZ transgene mimics a neomorphic allele of Trl.
One plausible explanation for the neomorphic activity of the GAGA-LacZ protein is that it interacts with novel sites in the genome, interfering with the regulation of genes located near these ectopic sites. To test this possibility, we double labeled polytene chromosomes using antibodies against β-galactosidase and the GAGA-581 protein. In most, but not all cases, we were able to detect the GAGA-LacZ fusion at sites that have the GAGA-581 protein (Fig. 7c). Since the signal produced by the β-galactosidase antibody is significantly fainter than that from the GAGA-581 antibody, it is possible that the few sites for the endogenous GAGA protein that are not labeled with the anti-LacZ antibody may actually have the GAGA-LacZ protein. On the other hand, we found several sites that have the GAGA-LacZ fusion but not the GAGA-581 protein. Although this result indicates that the in vivo specificity of the GAGA-LacZ fusion differs from that of the normal GAGA protein, we cannot rule out the possibility that some other mechanism is responsible for the neomorphic activity of the fusion protein.
DISCUSSION
The Drosophila GAGA factor functions in the establishment and/or maintenance of nucleosome-free regions of chromatin and has been implicated in processes as diverse as chromosome condensation and segregation, gene regulation, Polycomb-group silencing, and insulator activity (4, 7, 18, 21–23, 32). There are two major GAGA isoforms in D. melanogaster, GAGA-519 and GAGA-581, which have completely different C-terminal Q domains. In spite of this difference, the two isoforms behave identically in in vitro DNA binding experiments and in tissue culture transient-transfection assays (3). The two proteins also appear to colocalize completely on polytene chromosomes (3). These findings have raised the possibility that these two GAGA isoforms may be functionally equivalent.
In the studies reported here, we have tested this hypothesis by examining how the isoforms function in vivo. We find that they have overlapping but not identical activities and, consequently, are not interchangeable. Although both isoforms can rescue the zygotic lethality of Trl mutations, the GAGA-581 isoform is more effective. On the other hand, with respect to most other known Trl phenotypes, the GAGA-519 protein seems to be more active. Thus, the homeotic transformation of segment A5 to A6, which is likely due to Abd-B misexpression, can be rescued by the GAGA-519 protein, but not by the GAGA-581 protein. Similarly, both female sterility and maternal-effect lethality are rescued more effectively by the GAGA-519 protein. At least two factors account for the maternal-effect lethality of Trl mutations (4). The initial defect in the offspring of Trl13C mothers is the disruption of the nuclear division cycles at the syncytial blastoderm stage. Subsequently, genes that have GAGA binding sites in their regulatory regions are not properly expressed. Interestingly, the GAGA-581 protein, like the GAGA-519 protein, appears to fully rescue the nuclear division defects. Moreover, as is observed for the GAGA-519 protein, it preferentially localizes to centromeric heterochromatin in blastoderm nuclei. Since little if any GAGA-581 is present at this stage in wild-type embryos, we cannot say whether it normally functions at centromeres or is involved in chromosome mechanics at other stages of development. However, our results do suggest that centromere binding and GAGA function during mitosis do not depend upon the exact sequence and length of the Q domain. Thus, the GAGA-581 protein must be unable to substitute for the GAGA-519 protein in some other vital process during embryogenesis. At this point the most likely process is transcription. However, since both isoforms are able to rescue defects in ftz transcription, we presume that the GAGA-519 protein would have to have some other gene-specific function, perhaps equivalent to its role in Abd-B expression.
We can envision two possible explanations for the differences in the biological activities of the GAGA-519 and GAGA-581 proteins. Since the Q domains have distinct sequences, they may not be able to mediate the same spectrum of protein-protein interactions. Although this seems very likely to be the case, it is also possible that the two proteins cannot bind or do not bind with equal efficiency to all of the same target sites. The idea that the Q domains influence or modulate site selection in vivo is supported by the finding that the GAGA-LacZ fusion binds to novel sites (and may not bind to all of the normal GAGA sites) in polytene chromosomes. While this idea is contrary to the conclusions from previous studies (3), it seems possible that the in vitro binding assays were not sensitive enough to detect subtle differences in sequence preference that could be important in vivo, especially in an environment that has many other proteins that could potentially compete for the same sequences. Similarly, although GAGA-581 and GAGA-519 appear to colocalize on polytene chromosomes (3), the resolution of this experiment is not sufficient to conclude that the two proteins are binding to precisely the same sequences rather than nearby or adjacent sequences, or that they are binding with the same avidity. Since the Q domain of GAGA-519 appears to influence the topology of DNA upon GAGA factor binding in vitro (37), it is not unreasonable to suppose that this domain could influence the affinity of the protein for some sites.
It is somewhat surprising that the GAGA-LacZ fusion is able to rescue the zygotic lethality of Trl mutations almost as well as the GAGA-581 isoform does. It could be argued that the GAGA-519 Q domain sequence included in the GAGA-LacZ fusion is responsible for its rescuing activity. However, the activity profile of the GAGA-LacZ fusion more closely resembles that of GAGA-581 than that of GAGA-519. An alternative possibility is suggested by the finding that the GAGA-519 Q domain promotes the formation of tetrameric protein complexes (37). Not only does β-galactosidase form tetramers but also tetramerization is required for enzymatic activity (13). Since the GAGA-LacZ fusion protein has enzymatic activity (see Fig. 7a), it would appear that it assembles into tetrameric complexes. Hence, a quite plausible hypothesis is that β-galactosidase is able to partially substitute for the Q domain because of its ability to tetramerize. Conversely, the fact that the GAGA-LacZ fusion can rescue zygotic lethality would imply that (the postulated) protein-protein interactions mediated specifically by the fully intact Q domain are not critical for some of the general GAGA functions in the zygote such as chromatin remodeling and the formation of nucleosome free regions of chromatin. On the other hand, since the GAGA-LacZ protein lacks some of the activities of the GAGA-519 protein, it would appear that the C-terminal sequences deleted in the fusion are important for the special functions of this isoform. This is, of course, consistent with the finding that the activities of the GAGA-519 and GAGA-581 isoforms are not identical.
ACKNOWLEDGMENTS
We thank C. Benyajati for the GAGA isoform-specific antibodies and the GAGA-581 cDNA, K. Bhat for the GAGA-519 cDNA, and S. C. R. Elgin for sharing materials and unpublished results. The mouse monoclonal antibody against β-galactosidase was obtained from the Developmental Studies Hybridoma Bank, University of Iowa.
This work was supported by an NIH grant to P.S. and an NIH training grant to A.J.G.
REFERENCES
- 1.Agianian B, Leonard K, Bonte E, Van der Zandt H, Becker P B, Tucker P A. The glutamine-rich domain of the Drosophila GAGA factor is necessary for amyloid fibre formation in vitro, but not for chromatin remodelling. J Mol Biol. 1999;285:527–544. doi: 10.1006/jmbi.1998.2355. [DOI] [PubMed] [Google Scholar]
- 2.Bardwell V J, Treisman R. The POZ domain: a conserved protein-protein interaction motif. Genes Dev. 1994;8:1664–1677. doi: 10.1101/gad.8.14.1664. [DOI] [PubMed] [Google Scholar]
- 3.Benyajati C, Mueller L, Xu N, Pappano M, Gao J, Mosammaparast M, Conklin D, Granok H, Craig C, Elgin S. Multiple isoforms of GAGA factor, a critical component of chromatin structure. Nucleic Acids Res. 1997;25:3345–3353. doi: 10.1093/nar/25.16.3345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhat K M, Farkas G, Karch F, Gyurkovics H, Gausz J, Schedl P. The GAGA factor is required in the early Drosophila embryo not only for transcriptional regulation but also for nuclear division. Development. 1996;122:1113–1124. doi: 10.1242/dev.122.4.1113. [DOI] [PubMed] [Google Scholar]
- 5.Bhat K M, Schedl P. The Drosophila miti-mere gene, a member of the POU family, is required for the specification of the RP2/sibling lineage during neurogenesis. Development. 1994;120:1483–1501. doi: 10.1242/dev.120.6.1483. [DOI] [PubMed] [Google Scholar]
- 6.Biggin M D, Tjian R. Transcription factors that activate the Ultrabithorax promoter in developmentally staged extracts. Cell. 1988;53:699–711. doi: 10.1016/0092-8674(88)90088-8. [DOI] [PubMed] [Google Scholar]
- 7.Cavalli G, Paro R. The Drosophila Fab-7 chromosomal element conveys epigenetic inheritance during mitosis and meiosis. Cell. 1998;93:505–518. doi: 10.1016/s0092-8674(00)81181-2. [DOI] [PubMed] [Google Scholar]
- 8.Deshpande G, Samuels M E, Schedl P D. Sex-lethal interacts with splicing factors in vitro and in vivo. Mol Cell Biol. 1996;16:5036–5047. doi: 10.1128/mcb.16.9.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Deshpande G, Stukey J, Schedl P. scute (sis-b) function in Drosophila sex determination. Mol Cell Biol. 1995;15:4430–4440. doi: 10.1128/mcb.15.8.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DiBello P R, Withers D A, Bayer C A, Fristrom J W, Guild G M. The Drosophila Broad-Complex encodes a family of related proteins containing zinc fingers. Genetics. 1991;129:385–397. doi: 10.1093/genetics/129.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elgin S C. Chromatin structure and gene activity. Curr Opin Cell Biol. 1990;2:437–445. doi: 10.1016/0955-0674(90)90125-x. [DOI] [PubMed] [Google Scholar]
- 12.Farkas G, Gausz J, Galloni M, Reuter G, Gyurkovics H, Karch F. The Trithorax-like gene encodes the Drosophila GAGA factor. Nature. 1994;371:806–808. doi: 10.1038/371806a0. [DOI] [PubMed] [Google Scholar]
- 13.Fowler A V, Zabin I. The amino acid sequence of beta galactosidase. I. Isolation and composition of tryptic peptides. J Biol Chem. 1970;245:5032–5041. [PubMed] [Google Scholar]
- 14.Gaszner M, Vazquez J, Schedl P. The Zw5 protein, a component of the scs chromatin domain boundary, is able to block enhancer-promoter interaction. Genes Dev. 1999;13:2098–2107. doi: 10.1101/gad.13.16.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Godt D, Couderc J L, Cramton S E, Laski F A. Pattern formation in the limbs of Drosophila: bric a brac is expressed in both a gradient and a wave-like pattern and is required for specification and proper segmentation of the tarsus. Development. 1993;119:799–812. doi: 10.1242/dev.119.3.799. [DOI] [PubMed] [Google Scholar]
- 16.Granok H, Leibovitch B A, Shaffer C D, Elgin S C. Chromatin. Ga-ga over GAGA factor. Curr Biol. 1995;5:238–241. doi: 10.1016/s0960-9822(95)00048-0. [DOI] [PubMed] [Google Scholar]
- 17.Gross D S, Garrard W T. Nuclease hypersensitive sites in chromatin. Annu Rev Biochem. 1988;57:159–197. doi: 10.1146/annurev.bi.57.070188.001111. [DOI] [PubMed] [Google Scholar]
- 18.Hagstrom K, Muller M, Schedl P. A Polycomb and GAGA dependent silencer adjoins the Fab-7 boundary in the Drosophila bithorax complex. Genetics. 1997;146:1365–1380. doi: 10.1093/genetics/146.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horard B, Tatout C, Poux S, Pirrotta V. Structure of a Polycomb response element and in vitro binding of polycomb group complexes containing GAGA factor. Mol Cell Biol. 2000;20:3187–3197. doi: 10.1128/mcb.20.9.3187-3197.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lintermann K G, Roth G E, King-Jones K, Korge G, Lehmann M. Comparison of the GAGA factor genes of Drosophila melanogaster and Drosophila virilis reveals high conservation of GAGA factor structure beyond the BTB/POZ and DNA-binding domains. Dev Genes Evol. 1998;208:447–456. doi: 10.1007/s004270050202. [DOI] [PubMed] [Google Scholar]
- 21.Lu Q, Wallrath L L, Granok H, Elgin S C. (CT)n · (GA)n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophila hsp26 gene. Mol Cell Biol. 1993;13:2802–2814. doi: 10.1128/mcb.13.5.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mishra R K, Mihaly J, Barges S, Spierer A, Karch F, Hagstrom K, Schweinsberg S E, Schedl P. The iab-7 Polycomb response element maps to a nucleosome-free region of chromatin and requires both GAGA and pleiohomeotic for silencing activity. Mol Cell Biol. 2001;21:1311–1318. doi: 10.1128/MCB.21.4.1311-1318.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohtsuki S, Levine M. GAGA mediates the enhancer blocking activity of the eve promoter in the Drosophila embryo. Genes Dev. 1998;12:3325–3330. doi: 10.1101/gad.12.21.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omichinski J G, Pedone P V, Felsenfeld G, Gronenborn A M, Clore G M. The solution structure of a specific GAGA factor-DNA complex reveals a modular binding mode. Nat Struct Biol. 1997;4:122–132. doi: 10.1038/nsb0297-122. [DOI] [PubMed] [Google Scholar]
- 25.Pedone P V, Ghirlando R, Clore G M, Gronenborn A M, Felsenfeld G, Omichinski J G. The single Cys2-His2 zinc finger domain of the GAGA protein flanked by basic residues is sufficient for high-affinity specific DNA binding. Proc Natl Acad Sci USA. 1996;93:2822–2826. doi: 10.1073/pnas.93.7.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Platero J S, Csink A K, Quintanilla A, Henikoff S. Changes in chromosomal localization of heterochromatin-binding proteins during the cell cycle in Drosophila. J Cell Biol. 1998;140:1297–1306. doi: 10.1083/jcb.140.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Raff J W, Kellum R, Alberts B. The Drosophila GAGA transcription factor is associated with specific regions of heterochromatin throughout the cell cycle. EMBO J. 1994;13:5977–5983. doi: 10.1002/j.1460-2075.1994.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Read D, Manley J L. Alternatively spliced transcripts of the Drosophila tramtrack gene encode zinc finger proteins with distinct DNA binding specificities. EMBO J. 1992;11:1035–1044. doi: 10.1002/j.1460-2075.1992.tb05142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shopland L S, Hirayoshi K, Fernandes M, Lis J T. HSF access to heat shock elements in vivo depends critically on promoter architecture defined by GAGA factor, TFIID, and RNA polymerase II binding sites. Genes Dev. 1995;9:2756–2769. doi: 10.1101/gad.9.22.2756. [DOI] [PubMed] [Google Scholar]
- 30.Soeller W C, Oh C E, Kornberg T B. Isolation of cDNAs encoding the Drosophila GAGA transcription factor. Mol Cell Biol. 1993;13:7961–7970. doi: 10.1128/mcb.13.12.7961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soeller W C, Poole S J, Kornberg T. In vitro transcription of the Drosophila engrailed gene. Genes Dev. 1988;2:68–81. doi: 10.1101/gad.2.1.68. [DOI] [PubMed] [Google Scholar]
- 32.Strutt H, Cavalli G, Paro R. Co-localization of Polycomb protein and GAGA factor on regulatory elements responsible for the maintenance of homeotic gene expression. EMBO J. 1997;16:3621–3632. doi: 10.1093/emboj/16.12.3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaquero A, Espinás M L, Azorín F, Bernués J. Functional mapping of the GAGA factor assigns its transcriptional activity to the C-terminal glutamine-rich domain. J Biol Chem. 2000;275:19461–19468. doi: 10.1074/jbc.M000967200. [DOI] [PubMed] [Google Scholar]
- 34.Waterbury J A, Horabin J I, Bopp D, Schedl P. Sex determination in the Drosophila germline is dictated by the sexual identity of the surrounding soma. Genetics. 2000;155:1741–1756. doi: 10.1093/genetics/155.4.1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waterbury J A, Jackson L L, Schedl P. Analysis of the doublesex female protein in Drosophila melanogaster: role in sexual differentiation and behavior and dependence on intersex. Genetics. 1999;152:1653–1667. doi: 10.1093/genetics/152.4.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wieschaus E, Nüsslein-Volhard C. Looking at embryos. In: Roberts D B, editor. Drosophila: a practical approach. Oxford, United Kingdom: IRL Press; 1986. pp. 199–227. [Google Scholar]
- 37.Wilkins R C, Lis J T. DNA distortion and multimerization: novel functions of the glutamine-rich domain of the GAGA factor. J Mol Biol. 1999;285:515–525. doi: 10.1006/jmbi.1998.2356. [DOI] [PubMed] [Google Scholar]
- 38.Wilkins R C, Lis J T. Dynamics of potentiation and activation: GAGA factor and its role in heat shock gene regulation. Nucleic Acids Res. 1997;25:3963–3968. doi: 10.1093/nar/25.20.3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wu C, Bingham P M, Livak K J, Holmgren R, Elgin S C. The chromatin structure of specific genes. I. Evidence for higher order domains of defined DNA sequence. Cell. 1979;16:797–806. doi: 10.1016/0092-8674(79)90095-3. [DOI] [PubMed] [Google Scholar]