Abstract
Type 1 Diabetes (T1D) is a condition requiring 24-hour management. The way in which an individual combines their 24-hour movement behaviours (24-h MBs), which is comprised of physical activity (PA), sedentary behaviour (SB), and sleep, throughout the day can have a significant impact on physical and mental health. This mixed methods systematic review aimed to investigate 24-h MBs’ relationship with glycaemic control and psychosocial outcomes in adolescents (11–18 years) with T1D. Ten databases were searched for quantitative and qualitative English language articles reporting at least one of the behaviours and their relationship with outcomes. There were no restrictions on article publication dates or study design. Articles were subjected to title and abstract screening, full text screening, data extraction and quality assessment. Data were summarised narratively, and a meta-analysis was conducted where possible. From 9922 studies, 84 were included for data extraction (quantitative (n = 76), qualitative (n = 8)). Meta-analyses revealed a significant favourable association between PA and HbA1c (−0.22 [95% CI: −0.35, −0.08; I2 = 92.7%; p = 0.001). SB had an insignificant unfavourable association with HbA1c (0.12 [95% CI: −0.06, 0.28; I2 = 86.1%; p = 0.07]) and sleep had an insignificant favourable association (−0.03 [95% CI: −0.21, 0.15; I2 = 65.9%; p = 0.34]). Importantly, no study investigated how combinations of behaviours collectively interacted and impacted on outcomes.
Keywords: 24-hour movement behaviours, physical activity, sedentary behaviour, sleep, type 1 diabetes, adolescents, glycaemic control, quality of life, review
1. Introduction
Type 1 Diabetes (T1D) is a condition characterised by insulin deficiency, resulting in blood glucose levels having to be monitored and managed over the full 24-hour day using complex patterns of insulin administration. Everyday activities can affect blood glucose levels and management decisions are constantly required to maintain stable glycemia [1,2]. Although T1D can develop at any age, it most commonly develops during childhood and adolescence and is the most common form of diabetes for these age groups. The global estimate for children and adolescents (<20 years old) diagnosed with the condition is 1,211,900 with an estimated 149,500 diagnosed each year [3].
Adolescence is recognised as a particularly challenging time for individuals with T1D due to increased ownership of their condition and navigation of the usual changes occurring throughout adolescence (e.g., changes to the social environment, exploration of different lifestyles, biological changes, academic/work pressures etc.) [4]. The deterioration of metabolic control in T1D commonly occurs during adolescence with 13–25-year-olds experiencing the worst average plasma glucose concentration (HbA1c compared to other age groups) [5]. A range of mental health issues can also occur during this period, including depression and anxiety, impacting individuals’ psychosocial functioning [6,7].
Previous systematic reviews and research examining 24-hour movement behaviours (physical activity (PA), sedentary behaviour (SB), and sleep) and their relation to physical and mental health outcomes in adolescents with T1D have investigated these behaviours in isolation, with greatest emphasis placed on PA [8]. However, there has been a recent paradigm shift in the movement science literature suggesting individual movement behaviours for health should no longer be examined in isolation [9]. Instead, an integrated approach should be adopted, where all movement behaviours within the 24-hour day exist as a continuum from no movement (e.g., sleep) through low movement (e.g., sedentary behaviour) to high movement (e.g., vigorous PA (VPA)). These behaviours are considered relative or time-dependent, which means a decrease in one behaviour will result in a change in one of the other behaviours. Importantly, the way in which an individual combines these movement behaviours throughout the day can have a significant impact on physical and mental health [10].
In addition to the lack of systematic reviews investigating movement behaviours in youth with T1D in relation to the whole 24-hour day, there is a lack of systematic reviews examining quantitative and qualitative movement behaviour data together and their impact on T1D mental and physical health outcomes in adolescents. This would provide a comprehensive synthesis of the evidence beyond what is currently offered by a single method review by bringing together the findings of effectiveness (quantitative evidence) and experience (qualitative evidence) to enhance their usefulness to decision makers. Therefore, the aim of this study is to conduct a systematic review of quantitative and qualitative studies to comprehensively investigate 24-hour movement behaviours (individual and/or combined) and their impact on primary (glycated haemoglobin (HbA1c) and continuous glucose monitoring (CGM) metrics and quality of life (QoL)), and secondary outcomes (depressive symptoms, anxiety, stress/distress, self-management, coping, diabetes self-efficacy, family functioning, social competence) in adolescents with T1D.
2. Methods
2.1. Scientific Rigor
The protocol for this systematic review was registered on the 24 March 2021 in PROSPERO, the international prospective register of systematic reviews (CRD42021232460). The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement was used within the planning, conducting, and reporting of this review [11].
2.2. Inclusion Criteria
The inclusion criteria for the quantitative and qualitative studies (Table 1) were informed by PICOS (Population, Intervention, Comparison Group, Outcome, Study Design) and SPIDER (Sample, Phenomena of Interest, Design, Evaluation, Research Type) eligibility criteria, respectively [12,13].
Table 1.
Eligibility criteria for the quantitative and qualitative studies.
| PICOS Statement (Quantitative) | SPIDER Statement (Qualitative) |
|---|---|
| Population: Adolescents (11–18 years) with researcher defined diagnosed type 1 diabetes | Sample: Adolescents (11–18 years) with researcher defined diagnosed type 1 diabetes and primary caregivers/parents of adolescents with researcher defined diagnosed type 1 diabetes |
| Intervention/Exposure: Individual or combined 24-hour movement behaviours | Phenomenon of Interest: At least one 24-hour movement behaviour theme |
| Comparisons: All control/comparison groups | Design: All qualitative methods |
| Outcomes: HbA1c, CGM metrics and QoL | Evaluation: Beliefs, experiences, attitudes, behaviours and interactions etc. |
| Study: Interventional/experimental and observational | Research Type: Qualitative |
HbA1c, haemoglobin A1c; CGM, continuous glucose monitor; QoL, quality of life.
2.3. Population/Sample
Adolescents aged 11–18 years with investigator-defined T1D of all genders, all ethnicities and all diabetes durations were included. For the qualitative studies, the primary caregivers/parents of the adolescents with diagnosed T1D were also included providing their perspectives related specifically to the adolescent.
2.4. Intervention/Exposure and Phenomenon of Interest
Any individual or combined 24-hour movement behaviours (PA, SB and sleep) were included for this systematic review. Studies were included provided they were investigating habitual/usual PA. All subcategories of SB were considered (e.g., overall sedentary time, screen time and non-screen time behaviours). For sleep, all dimensions related to sleep health were included (e.g., duration, continuity or efficiency, timing, alertness/sleepiness and satisfaction/quality) [14] (Supplementary Materials Glossary of Terms S1). All outcomes of movement behaviours and related subcategories (e.g., cardiorespiratory, metabolic, muscular, morphological or motor physical fitness) were excluded.
2.5. Comparisons and Design
For the quantitative studies, all control/comparison groups were included in this review provided they were within the identified population age bracket. For the qualitative studies, all qualitative methods were considered. Only data collected in habitual settings were gathered (e.g., sleep measured within a sleep lab and physical activity measured during an exercise test within a lab were excluded unless habitual movement behaviours were also reported).
2.6. Outcomes and Evaluation
The primary outcomes of interest for the studies were glycaemic control measured by HbA1c and/or standardised CGM metrics and QoL [15,16]. Tests/measures of QoL, Health Related Quality of Life (HRQoL) and Diabetes Specific Quality of Life providing global/total scores were reported. The secondary outcomes of interest were psychosocial (individual and family level) responses [17]. Psychosocial responses were defined as depressive symptoms, anxiety, stress/distress, self-management, coping, self-efficacy, family functioning and social competence [18] (Supplementary Materials Glossary of Terms S1). For the qualitative studies, a range of phenomena were under investigation (for example, views, behaviours, opinions, attitudes, perceptions, experiences and beliefs).
2.7. Study and Research Type
This systematic review collected quantitative, qualitative, and mixed method studies. Mixed method studies were only considered if data from the quantitative or qualitative components could be clearly extracted. Specifically, quantitative included experimental/interventional studies (e.g., randomised, and non-randomised) and any non-experimental/observational studies (e.g., cross-sectional/prevalence, cohort/longitudinal, case control/case reference). Additionally, all qualitative studies were included (e.g., ethnography, phenomenology, grounded theory, narrative inquiry, case studies and visual and participatory methodologies). All commentaries, reviews, editorials, meta-analysis, and diagnostic studies were excluded from this review.
2.8. Search Strategy
An electronic literature search was conducted on the 7th of May 2021 in the following electronic databases: MEDLINE (Ovid), EMBASE (Ovid), Web of Science (Core Collection), APAPsychINFO (EBSCOhost), SPORTDiscus (EBSCOhost), Applied Social Sciences Index and Abstracts (ProQuest), Sports Medicine and Education Index (ProQuest) Wiley Cochrane Library, OpenGrey and Open Dissertations (EBSCOhost). All published studies up until the search date were included provided they met the inclusion criteria. For each database, a search strategy consisting of keywords and synonyms was developed using the PICO and SPIDER frameworks (Supplementary Materials Search Strategy S2).
2.9. Screening Process
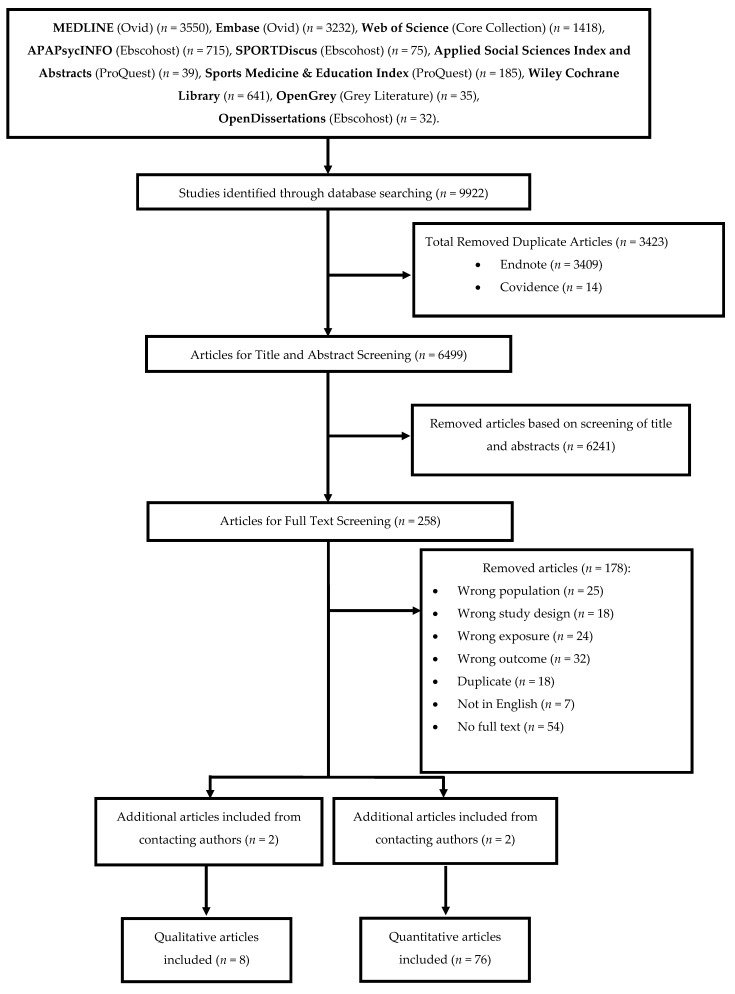
All identified citations (n = 9922) were initially compiled into EndNote reference management software (https://endnote.com (accessed on 15 May 2021)) to remove duplicates [19]. The remaining references (n = 6513) were exported to Covidence systematic review software (https://www.covidence.org (accessed on 15 May 2021)). Further duplicates identified by Covidence were removed [n = 14], resulting in a total of 6499 references for title and abstract screening.
Independent screening of the studies occurred at the title and abstract stage and the full text stage by four reviewers (M.P., S.M., E.R., W.H.). Authors were contacted for missing texts. The eligibility of each study was confirmed through completion of quantitative and qualitative inclusion/exclusion checklist. Any disagreements surrounding the exclusion or inclusion of studies at each stage were discussed until a unified decision was made. If a consensus could not be reached, a third reviewer was consulted (X.J.). Cohen’s Kappa [κ] was calculated between each reviewer pair for the title and abstract (0.60–0.85) and full text screening stages (0.62–0.69) [20] (Supplementary Materials Table S1).
2.10. Data Extraction
The PICO (quantitative) and SPIDER (qualitative) criteria were used to inform data extraction. Sample characteristics (e.g., age, diabetes duration, HbA1c and sample size), study design (e.g., experimental, cross sectional, cohort and case-control), exposure/intervention description (e.g., movement behaviour and measurement method), primary and secondary outcomes and results data were extracted from studies (Supplementary Materials Table S2). Four reviewers completed independent data extraction for the quantitative studies (M.P., S.M., E.R., W.H.) and two reviewers completed independent extraction for the qualitative studies (M.P., S.M.). Any disagreements were discussed, and a third reviewer was consulted when a consensus could not be reached (X.J.).
2.11. Quality Assessment
The Critical Appraisal Skills Programme checklists were utilised to assess the methodological quality of the cohort, case-control, randomised control trials and qualitative studies [21]. The Strengthening of Reporting of Observational Studies in Epidemiology checklist was utilised for the included cross-sectional studies [22]. Assessment of each included study was performed by M.P. No studies were excluded based on quality assessment (Supplementary Materials Table S3).
2.12. Data Synthesis
2.12.1. Narrative Synthesis of Quantitative Studies
The Excel database utilised for quantitative data extraction was initially examined by movement behaviour and respective movement behaviour constructs (Supplementary Materials Table S2). Each movement behaviour was examined in relation to the primary and secondary outcomes of interest for favourable results, unfavourable results, or results with no significance. Results were deemed favourable if the outcomes improved or indicated a trend for improvement due to higher levels of the movement behaviour. For example, for correlational results, PA’s negative correlation with HbA1c would be deemed favourable as higher PA reduced (improved) HbA1c. Additionally, for studies examining differences, results would be deemed favourable if groups with higher PA had lower (improved) HbA1c (Supplementary Materials Tables S4–S7).
2.12.2. Narrative Synthesis of Qualitative Studies
Findings/themes from each qualitative study were gathered and accompanied by illustrations (e.g., quote). Findings were then grouped by common movement behaviour categories. Textual pooling was not possible due to a low number of included qualitative studies that were also very heterogenous and were therefore presented in narrative form (Supplementary Materials Table S8).
2.12.3. Meta-Analysis Synthesis
Meta-analyses of quantitative studies were performed using the Meta-Essentials tool [https://www.erim.eur.nl/research-support/meta-essentials/ (accessed on 7 March 2022)]. The tool consists of a set of workbooks designed for Microsoft Excel that, based on the input, automatically produces all the required statistics, tables, figures, and more [23]. All the included studies for the meta-analysis included cross sectional associations between exposure and outcome which were derived from cross-sectional and longitudinal study designs only. For the longitudinal studies, the correlation statistic that was closest to the exposure was taken to highlight acute opposed to chronic effects of the exposure. Authors viewed this as more in keeping with associations present in cross-sectional studies. Correlation coefficients were extracted from studies and beta (b) coefficients were extracted and converted to correlation coefficients [24]. The I2 statistic was utilised to determine heterogeneity between studies and subsequent random-effect or fixed effect analysis (0–40% = low heterogeneity; 75–100% = significant heterogeneity) [25].
Meta-analyses were possible between the following exposure-outcome associations and their subgroups (if applicable):
PA and HbA1c (Subgroups = Light-PA (LPA), Moderate-PA (MPA), Vigorous-PA (VPA), Moderate-Vigorous-PA (MVPA) and Total-PA (TPA)).
SB and HbA1c (Subgroups = Computer, TV, Total Screen Time, Schoolwork and Total Sedentary Time).
Sleep and HbA1c (Duration dimension only due to study heterogeneity).
Unfavourable associations and favourable associations were determined based on the direction of association. For PA exposures, a negative association was deemed favourable (e.g., more PA associated with lower HbA1c) and a positive association was deemed unfavourable (e.g., more PA associated with higher HbA1c). For SB exposures, a positive association was deemed favourable (e.g., less screen time associated with lower HbA1c) and a negative association was deemed unfavourable (e.g., less screen time associated with higher HbA1c). For sleep duration, a negative association was deemed favourable (e.g., increased sleep duration was associated with lower HbA1c) and a positive association was deemed unfavourable (e.g., increased sleep duration was associated with higher HbA1c).
3. Results
3.1. Characteristics of Identified Studies
In total, 9922 articles were identified from the initial search with 84 articles included for data extraction after title, abstract and full text screening (quantitative (n = 76) [26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101] qualitative (n = 8) [102,103,104,105,106,107,108,109] (Figure 1). Included quantitative articles were published between 1990–2021 [26,53] and conducted in the USA (n = 28) [30,33,40,41,42,44,45,46,47,51,53,54,60,69,70,77,78,81,82,83,85,86,87,92,94,97], Europe (n = 25) [31,32,39,43,48,49,50,56,57,58,59,61,62,63,64,67,68,75,80,89,90,91,93,96,98] UK (n = 6) [34,37,38,66,72,88], South America) (n = 5) [35,36,71,74,84], Middle East (n = 4) [27,76,100,101], Canada (n = 3) [73,79,99], Africa (n = 2] [26,55], New Zealand (n = 1) [65] and across multiple countries (n = 2) [28,29]. There were two experimental [52,72], 10 case-control [26,36,39,57,61,63,65,82,97,99], 11 longitudinal [30,33,40,41,46,50,60,70,77,89,90] and 53 cross-sectional studies [27,28,29,31,32,34,35,37,38,42,43,44,45,47,48,49,51,53,54,55,56,58,59,62,64,66,67,68,69,71,73,74,75,76,78,79,80,81,83,84,85,86,87,88,91,92,93,94,95,96,98,100,101]. The sample of the quantitative studies included a total of 68,203 adolescents ranging from 10–23,251 [30,49] participants per study. The mean age of participants was 14.1 years (11.1–17.6 years) [54,66], with a mean diabetes duration of 5.8 years (0.5–12.5 years) and a mean HbA1c of 8.8% (73 mmol/mol) (7.2–10.2%) [47,50]. Included qualitative articles were published between 2009–2018 [102,103,105,108]. Six analysed data thematically [102,103,106,107,108,109], one used latent content analysis [105] and one used interpretive phenomenological analysis [104]. These studies were conducted in the UK (n = 5) [104,106,107,108,109], USA (n = 3) [102,103] and Europe (n = 1) [105]. The total adolescent sample of the qualitative studies was 105, ranging from 11–29 participants per study [105]. These adolescents had a mean age of 12.7 years (10.8–15.56 years) [102,109], a mean diabetes duration of 5.1 years [3.8–6.2 years] [102,106] and a mean HbA1c of 8.6% [8.3–8.9%] [102,103,104]. The total sample of parents was 92, ranging from 11–25 participants per study [102,108].
Figure 1.
Flow chart of included studies.
3.2. Movement Behaviour Composition
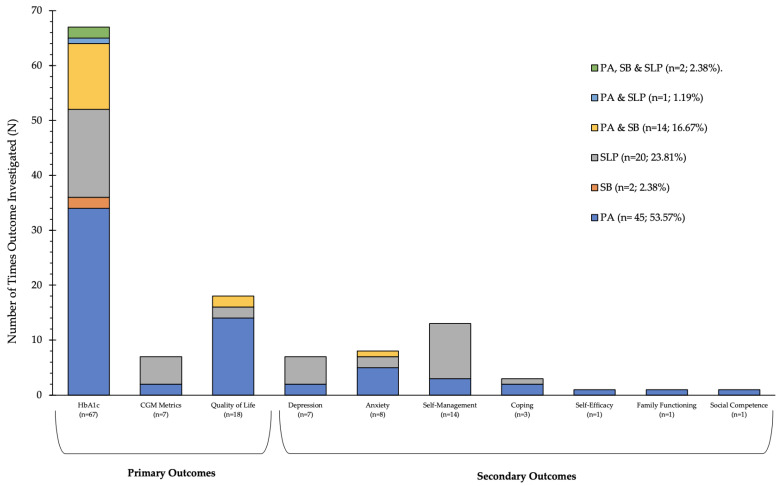
Half of all the included quantitative and qualitative studies were investigating PA individually (n = 45; 53.57%) [27,29,30,31,32,34,35,37,38,40,41,48,49,53,55,56,57,59,61,62,63,64,68,74,76,77,78,80,84,86,87,88,89,91,92,93,94,96,104,105,106,107,108,109], 2.38% (n = 2) [60,67] of studies were investigating SB individually and 23.81% (n = 20) of studies were investigating sleep individually [26,42,44,45,47,51,52,65,69,81,82,83,85,95,97,98,99,101,102,103]. A total of 16.67% (n = 14) of studies were investigating both PA and SB [28,33,39,43,50,54,58,66,70,72,73,75,90,100], 1.19% (n = 1) [46] of studies were investigating both PA and sleep and 2.38% (n = 2) of studies were investigating all three behaviours [36,71] (Figure 2).
Figure 2.
Movement Behaviours and the Addressed Primary and Secondary Outcomes of Included Studies. PA, physical activity; SB, sedentary behaviour; SLP, sleep; HbA1c, haemoglobin A1c; CGM, continuous glucose monitoring.
Specific physical activity constructs addressed in the included studies were TPA, MVPA, VPA, MPA and LPA. Sedentary behaviour constructs included total sedentary behaviour (SED), screen time (computer), screen time (television), sedentary behaviour (schoolwork) and screen time (TV and computer). Finally, sleep constructs addressed included duration, continuity/efficiency, timing, quality and alertness/sleepiness (Tables S4–S7).
3.3. Included Articles Primary and Secondary Outcome Composition
Examination of the primary outcomes showed HbA1c was addressed most frequently (n = 67), primarily by PA studies (n = 34) [27,30,31,32,34,37,38,40,41,48,49,53,55,56,61,63,64,68,74,76,78,79,80,86,88,89,92,94,96,104,106,107,108,109] followed by sleep (n = 16) [26,42,45,47,51,52,65,69,81,82,83,85,98,99,101,102] and then PA and SB studies (n = 12) [28,33,39,43,50,54,58,66,70,73,90,100]. QoL was the second most addressed outcome (n = 18), primarily by PA studies (n = 14) [29,30,35,41,55,62,63,64,76,77,93,104,106,107] followed by sleep (n = 2) [85,97] and PA and SB (n = 2) [72,75]. CGM metrics were the least frequently addressed primary outcome (n = 7), primarily addressed by sleep studies (n = 5) [44,47,65,82,85] followed by PA studies (n = 2) [84,91]. Examination of the secondary outcomes showed self-management was addressed most frequently (n = 13) [105], primarily by sleep studies (n = 10) [42,45,47,51,52,69,81,85,95,102]. Anxiety was the second most addressed (n = 8] [30,50,57,76,85,86,87,103] followed by depression (n = 7) [45,47,76,82,85,94,101]. The outcomes least frequently addressed were coping (n = 3) [85,93,109], self-efficacy (n = 1) [37], family functioning (n = 1) [106] and social competence (n = 1) [30] (Figure 2).
3.4. Physical Activity
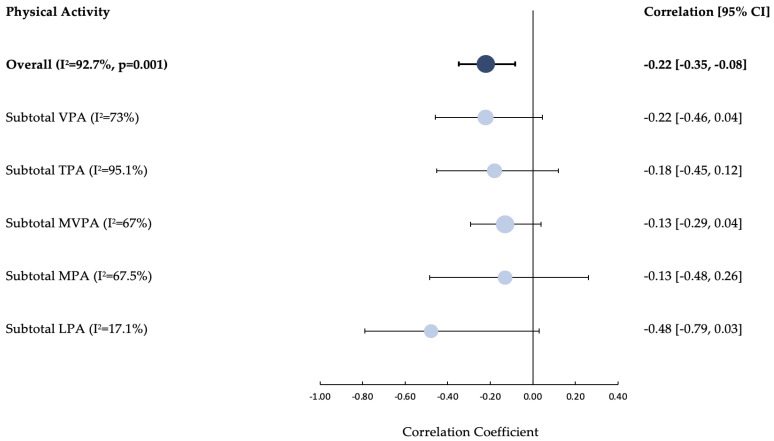
Overall, PA’s impact on HbA1c was favourable in 19/60 (32%) associations and unfavourable in 2/6 (3%), most results investigating PA and HbA1c were not significant (39/60) (60%). PA’s impact on QoL was favourable in 11/15 (73%) associations, the remainder were not significant (20%). Overall, PA had no associations with depression, self-efficacy, family functioning and social competence. A mix of favourable and unfavourable associations with anxiety and self-management were observed with no clear pattern emerging (Tables S4–S7). The random effects meta-analysis to quantify associations between PA and HbA1c highlighted a significant negative association (overall pooled correlation coefficient = −0.22 [95% CI: −0.35, −0.08; I2 = 92.7%, p = 0.001]. The subgroup analysis revealed negative associations with HbA1c for each PA construct (LPA = −0.48 [95% CI: −0.79, 0.03; I2 = 17%]; MPA = −0.13 [95% CI: −0.48, 0.26; I2 = 67.5%]; MVPA = −0.13 [95% CI: −0.29, 0.04; I2 = 67%]; TPA = −0.18 [95% CI: −0.45, 0.12; I2 = 95.1%] and VPA −0.22 [95% CI: −0.46, 0.04; I2 = 73%]) (Figure 3). Please see Figure S1 for a forest plot of individual studies.
Figure 3.
Forest Plot of the correlations between Physical Activity and Glycated Haemoglobin [HbA1c]. CI, confidence interval; LPA, light physical activity; MPA, moderate physical activity; MVPA, moderate-vigorous physical activity; TPA, total physical activity; VPA, vigorous physical activity; I2,statistic of heterogeneity.
3.5. Sedentary Behaviour
The overall impact SB had on HbA1c highlighted 12/25 (48%) unfavourable associations. The only favourable associations were present for the screen time (schoolwork) construct (3/3; 100%). There were no associations between SB and QoL or any of the secondary outcomes (Tables S4–S7).
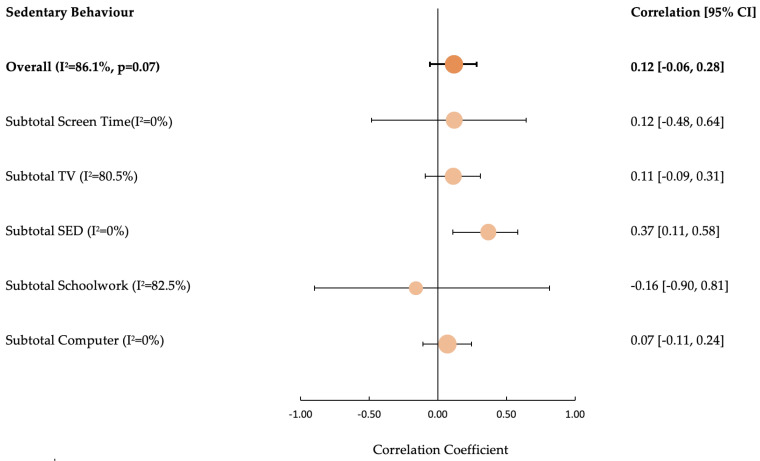
The random effects meta-analysis to quantify associations between SB and HbA1c highlighted an insignificant positive association (overall pooled correlation coefficient = 0.12 [95% CI: −0.06, 0.28; I2 = 86%; p = 0.07]). Subgroup analysis of SB constructs revealed positive association for screen time computer (0.07 [95% CI: −0.11, 0.24; I2 = 0%]), total sedentary time (0.37 [95% CI: 0.11, 0.58; I2 = 0%]), screen time television (0.11 [95% CI: −0.09, 0.31; I2 = 80.5%]) and total screen time (0.12 [95% CI: −0.48, 0.64; I2 = 0%]). However, a negative association was observed between screen time schoolwork and HbA1c (−0.16 [95% CI: −0.90, 0.81; I2 = 82.5%]) (Figure 4). Please see Figure S2 for a forest plot of individual studies.
Figure 4.
Forest Plot of the Effect of Sedentary Behaviour on Glycated Haemoglobin [HbA1c]. SED, sedentary time; CI, confidence interval; I2, statistic of heterogeneity.
3.6. Sleep
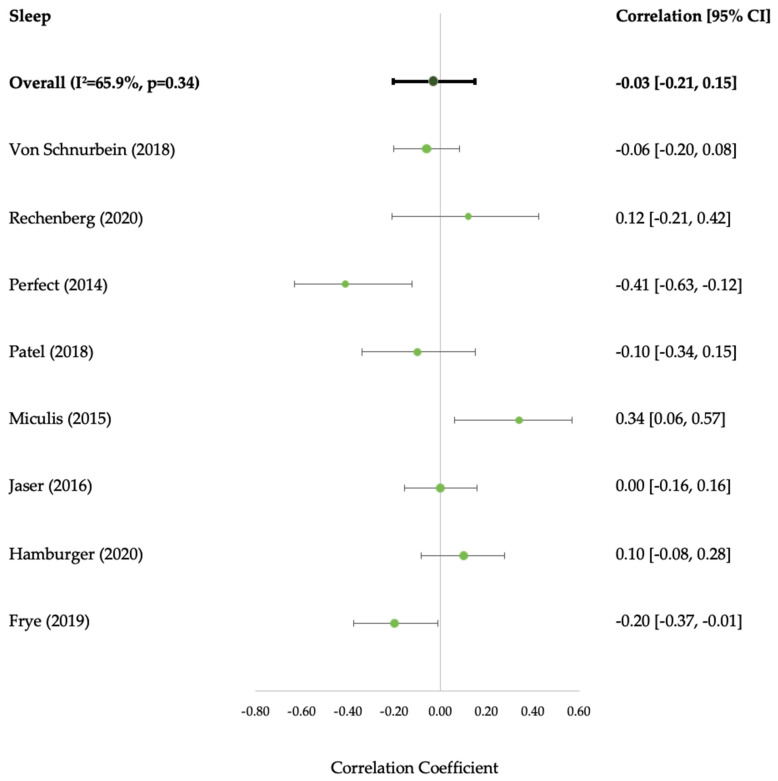
Sleep’s overall impact on HbA1c highlighted 5/32 (16%) favourable and 3/32 (9%) unfavourable associations; the majority were not associated (75%). Sleep’s impact on QoL was favourable in 0/2 associations. There was a mix of favourable (5/19) (26%) and unfavourable (3/19) (16%) associations with self-management and favourable associations with depression (5/8) (63%). No associations were observed with anxiety or coping (Tables S4–S7). It was only possible to examine sleep duration construct in the meta-analysis, and this association was not significant (−0.03 [95% CI: −0.21, 0.15; I2 = 65.9%; p = 0.34]) (Figure 5).
Figure 5.
Forest Plot of the Effect of Sleep Duration on Glycated Haemoglobin [HbA1c]. CI, confidence interval; I2, statistic of heterogeneity [42,45,51,71,81,83,85,98].
3.7. Qualitative Studies
PA was investigated most frequently (n = 6; 75%) [104,105,106,107,108,109] followed by sleep (n = 2; 25%) [102,103] with no studies focusing primarily on SB (Supplementary Materials Table S8). Adolescents with T1D and their parents discussed the positive impact PA had in relation to management of glycaemic control in 4/5 studies [104,106,108,109]. This was highlighted by both adolescents with T1D and their parents/caregivers:
‘‘It helps to sort of control. I don’t really know why but I felt that um, if I’m doing more exercise um I can normally keep my levels at a more consistent rate”.
[Adolescent, p4] [104]
“What we’ve learned is that physical activity keeps the spikes and the lows more moderate so you don’t fluctuate as much… the physical activity just makes that more stable”
[Caregiver, p4] [108]
There was discussion from the adolescents with T1D surrounding PA’s positive role for improving quality of life in all studies addressing the outcome [3/3] [104,106,107]. This was expressed through the description of positive holistic feelings:
“I feel quite satisfied”, “cheerful”, “I like walking because it really relaxes me”
[Adolescents, p7] [107]
“When you’re doing exercise you know you’re helping your body as well as yourself”
[Adolescent, p5] [104]
There was also a glimpse of discussion from parents surrounding PA role in supporting adolescents to cope and how the behaviour might strengthen family functioning [106,109]:
“a way of getting out his anger”
[Caregiver, p6] [109]
“because it’s funner with other people, like you can keep motivated, but you can also have a laugh while you’re doing it…’,
[Adolescent, p153] [106]
Both adolescents and their caregivers expressed fluctuations in glycaemic control as a barrier to obtaining sleep and the anxieties of extreme glucose fluctuations while sleeping [102,103]:
“Usually [TEEN] sleeps fine, unless his blood sugar’s high. Then he’s up every couple hours”.
[Caregiver p123] [102]
“I panic a lot because one night my blood sugar dropped in the middle of the night and I ended up like having a seizure”.
[Adolescent, p550] [103]
“My mother sets an alarm for 2:30 in the morning and she comes and checks me so she doesn’t want me to have to get up in the middle of the night”.
[Adolescent, p550] [103]
Finally, sleep was also discussed in relation to self-management behaviours by adolescents and caregivers [102]. They discuss how curtailments to sleep result in increased lethargy which has subsequent impact on management behaviours (e.g., blood glucose checks). Interestingly, one adolescent highlighted the impact reduced sleep has on their sitting levels:
“I’m not my usual self. I don’t like do things, I just kinda sit”.
[Adolescent, p546] [102]
“the lack of sleep would probably cause [her teen] to not be able to keep track of his blood sugars or test when he needs—it probably causes him to be a little lazy about it”
[Caregiver, p546] [102]
4. Discussions
To our knowledge, this is the first systematic review to examine the full spectrum of 24-hour movement behaviours in relation to glycaemic control and psychosocial outcomes in adolescents with T1D. We found no studies investigating how combinations of behaviours collectively interact and impact on any of the primary or secondary outcomes.
The results of our study confirmed previous research demonstrating isolated movement behaviours are related to HbA1c and psychosocial outcomes. Both the quantitative and qualitative findings reported higher levels of PA improved glycaemic control and quality of life [110,111,112,113]. Additionally, we identified from the qualitative findings the potential benefit of PA on family functioning that was not otherwise highlighted in the quantitative findings. Higher levels of SB worsened HbA1c in most studies, aside from screen time for schoolwork activities which indicated a trend for improved HbA1c. This could be explained by the conscientiousness personality trait associated with homework completion in adolescents which has been found to be related to positive T1D self-management behaviours and thus glycaemic control [114]. The findings from this systematic review highlighted a potential decrease in HbA1c with increased levels of sleep duration; however, the meta-analysis was insignificant and this aligns with previous research [115]. The inclusion of the qualitative studies allowed for a deeper insight into this bi-directional relationship with participants discussing experiences of glycaemic control issues that resulted in interruptions to their sleep. The mixed findings surrounding the direct association of sleep duration and HbA1c might be explained by the mediating effects of self-management activities [116]. Improvements in sleep might improve self-management activities resulting in the subsequent improvement of glycaemic control. Self-management was the most frequently investigated secondary outcome, largely in relation to sleep. We found in quantitative findings both long and short sleep duration, poor sleep quality and delayed timing (e.g., later bedtime) worsened self-management activities. This aligned with our qualitative findings where curtailments of sleep were believed to decrease self-management behaviours.
Although 20.4% of studies reported on two or more movement behaviours, these were examined separately from one another in relation to our outcomes. Most studies investigating more than one behaviour focused on examining both PA and SB. Only 2.4% of the included studies focused solely on SB, a theme consistent with findings from other systematic reviews [111]. The lack of SB focus is also present in current T1D management guidance, illustrated in the International Society for Pediatric and Adolescent Diabetes (ISPAD) PA guidelines. ISPAD recommends youth participate in mostly MVPA and VPA for 60 min/day; however, there are no specific SB guidelines. Instead, minimal SB guidance is made available within the same guidelines, simply stating: “sedentary lifestyle behaviors should be routinely screened for and discouraged in the diabetic clinic” [8]. While the research and guidance may suggest SB is deemed less important than PA, optimism lies in the fact these behaviours are often investigated in unison, indicating appreciation for the close alignment of these behaviours. This holds promise for the adoptability of a 24-hour approach by researchers and key stakeholders. Additionally, the qualitative results of this study also indicate adolescents might be willing to adopt a 24-hour approach with one adolescent recognising the interdependency of behaviours (increased levels of sitting after disruptions to sleep).
Research is beginning to investigate the levels of 24-hour movement behaviours relative to one another, rather than as individual entities in populations with chronic conditions [117]. However, no studies to our knowledge have investigated how the behaviours interact and subsequently impact outcomes in adolescents with chronic conditions, including type 1 diabetes. Evidence outside this population consistently illustrates PA, SB and sleep are linked, they impact one another, and this has consequences for physical and mental health [10]. 24-hour movement behaviour guidelines were first published in 2016 with a specific focus on children and youth [118]. These guidelines acknowledge the small percentage MVPA accounts for within the 24 h day (<5%), move away from the previous dominant focus on MVPA and additionally consider the percentage of sleep (40%), SB (40%) and LPA (15%) [119]. Adopting a 24-hour approach for adolescents with T1D is a logical step in the management of the condition for several reasons. A 24-hour approach would allow PA to remain one of the cornerstones of T1D management while also placing equal and required emphasis on the benefits of reducing SB. Additionally, although there are mixed findings surrounding the exact relationship sleep may have with glycaemic control, sleep behaviour and diabetes research is gaining traction. This is highlighted by the recent calls for T1D sleep guidance and the widely accepted difficulties both caregivers and adolescents with T1D have with sleep [65,120]. Finally, and importantly, sleep is a necessary behaviour not only to adolescents with T1D but all populations. Other T1D management tools are often prioritised over PA (e.g., diet and medication) despite the behaviour’s recognised benefits. Utilising a 24-hour approach where each behaviour is weighted and known to interact would promote the necessity and importance of PA and SB through sleep association. Essentially, PA could ‘piggyback’ on the necessity of sleep, resulting in higher prioritisation of PA and the associated improvements in outcomes.
Strengths and Limitations
This systematic review provided a comprehensive exploration of the full spectrum of movement behaviours in relation to a range of primary and secondary outcomes, creating novel and holistic findings. Throughout the review process, Covidence meta-analysis software was utilised that is designed to facilitate organisation of studies which enhanced the rigorous assessments of each stage and the reliability of this systematic review’s findings. A mixed methods approach was utilised, allowing for in depth examination of effectiveness from the quantitative studies and real-life experience from the qualitative studies. This allowed gaps between effectiveness and experience to be highlighted which exploited possible areas for future research (e.g., PA association with family functioning; the direction of relationship between sleep and glycaemic control) strengthens current findings (e.g., higher PA improves glycaemic control and quality of life) and ultimately enhances usefulness of findings to decision makers.
While the comprehensiveness of this review allowed for a holistic view of associations, there were a range of different terminologies that overlapped, creating difficulty in collating the findings. Studies examining movement behaviours should recognise the multidimensional nature of each behaviour, ensure they are reporting on the exact construct of interest and report this in a manner consistent with previous research or overarching gold standard guidance (e.g., Sedentary Behaviour Research Network or American Psychological Association Thesaurus). This uniformity of reporting would increase the overall strength of findings. While this review incorporated a range of sleep health dimensions, it did not incorporate the recent addition of the regularity dimension, and future research might investigate this dimension in relation to our outcomes. It would have been beneficial to also examine additional outcomes (e.g., hypoglycaemia, hyperglycaemia, risk factors for co-morbidities) to add a further perspective to these results. However, incorporating these outcomes in a review looking at a range of different behaviours was not feasible due to time and resource constraints.
It is important to acknowledge the limitation of HbA1c as an outcome measure. HbA1c only provides an average level of blood glucose over the past 2–3 months and little information can be ascertained regarding the frequency, duration or amplitude of intra-day (within-day) and inter-day (between-day) glycaemic excursions [16]. CGMs have increased in availability and accuracy over the last century and have facilitated diabetic glycaemic control. They produce real-time measurements of the glucose level excursions HbA1c overlooks, allowing for immediate action to address raised or lowered glucose levels that can subsequently prevent potential acute incidents (e.g., hypoglycaemia and hyperglycaemia). However, in this systematic review minimal studies investigated the movement behaviours’ impact on glycaemic control assessed via CGM metrics despite the increased availability of the device and the recommended standardised metrics that aim to guide clinicians, patients and researchers in using, analysing, and reporting CGM data.
5. Conclusions
This systematic review highlighted that no studies to date have investigated how combinations of behaviours collectively interact and impact on any of the primary or secondary outcomes in adolescents with T1D. Monitoring the full spectrum of 24-hour movement behaviours would allow for a comprehensive understanding of how the cumulation and weighting of each behaviour might interact and impact on important outcomes for adolescents with T1D. Future research should investigate the association between 24-hour movement behaviours (measured via accelerometer), glycaemic control and psychosocial outcomes. Additionally, measuring glucose control via both HbA1c and CGM glucose metrics would aid in the comprehensive investigation by providing a detailed objective and continuous pattern of glucose over the full 24-hour period. Finally, qualitative studies investigating the knowledge, awareness and feasibility of a 24-hour movement behaviour approach would aid in understanding how adolescents with T1D might adopt this type of approach.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20054363/s1, Glossary of Terms S1; Search Strategy S2; Table S1: Inter-Rater reliability between reviewers; Table S2: Characteristics of Quantitative Studies; Table S3: Quality Assessment; Tables S4–S7: Movement Behaviours Associations with Primary and Secondary Outcomes; Table S8: Characteristics of Qualitative Studies; Figure S1: Forest Plot of Physical Activity Individual Studies; Figure S2: Forest Plot of Sedentary Behaviour Individual Studies.
Author Contributions
M.P. designed the research, conducted the search, screened the studies, extracted, analysed and interpreted the data and wrote the paper. X.J., A.K. and M.C. made substantial contributions to study conception and design of the research. S.M., E.R. and W.H. screened the studies and extracted study data. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
M.C. is a consultant for Signfier Medical Technologies, but this activity is not related to the content of this article. The remaining authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
The lead researcher M.P. received a stipend for this Ph.D. project through the University of Strathclyde Student Excellence Awards. The APC was funded by the University of Strathclyde Institutional Open Access Fund (IOAF).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Atkinson M.A., Eisenbarth G.S., Michels A.W. Type 1 Diabetes. Lancet. 2014;383:69–82. doi: 10.1016/S0140-6736(13)60591-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Craig M.E., Jefferies C., Dabelea D., Balde N., Seth A., Donaghue K.C. Definition, epidemiology, and classification of diabetes in children and adolescents. Pediatr. Diabetes. 2014;15:4–17. doi: 10.1111/pedi.12186. [DOI] [PubMed] [Google Scholar]
- 3.Tuomilehto J., Ogle G.D., Lund-Blix N.A., Stene L.C. Update on Worldwide Trends in Occurrence of Childhood Type 1 Diabetes in 2020. Pediatr. Endocrinol. Rev. 2020;17:198–209. doi: 10.17458/per.vol17.2020.tol.epidemiologychildtype1diabetes. [DOI] [PubMed] [Google Scholar]
- 4.Cameron F.J., Garvey K., Hood K.K., Acerini C.L., Codner E. Ispad Clinical Practice Consensus Guidelines 2018: Diabetes in Adolescence. Pediatr. Diabetes. 2018;19:250–261. doi: 10.1111/pedi.12702. [DOI] [PubMed] [Google Scholar]
- 5.Miller K.M., Foster N.C., Beck R.W., Bergenstal R.M., DuBose S.N., DiMeglio L.A., Maahs D.M., Tamborlane W.V., T1D Exchange Clinic Network Current State of Type 1 Diabetes Treatment in the U.S.: Updated Data from the T1d Exchange Clinic Registry. Diabetes Care. 2015;38:971–978. doi: 10.2337/dc15-0078. [DOI] [PubMed] [Google Scholar]
- 6.Hislop A.L., Fegan P.G., Schlaeppi M.J., Duck M., Yeap B.B. Prevalence and associations of psychological distress in young adults with Type 1 diabetes. Diabet. Med. 2008;25:91–96. doi: 10.1111/j.1464-5491.2007.02310.x. [DOI] [PubMed] [Google Scholar]
- 7.Cameron F.J., Northam E.A., Ambler G.R., Daneman D. Routine Psychological Screening in Youth with Type 1 Diabetes and Their Parents: A Notion Whose Time Has Come? Diabetes Care. 2007;30:2716–2724. doi: 10.2337/dc07-0603. [DOI] [PubMed] [Google Scholar]
- 8.Adolfsson P., Riddell M.C., Taplin C.E., Davis E.A., Fournier P.A., Annan F., Scaramuzza A.E., Hasnani D., Hofer S.E. ISPAD Clinical Practice Consensus Guidelines 2018: Exercise in children and adolescents with diabetes. Pediatr. Diabetes. 2018;19:205–226. doi: 10.1111/pedi.12755. [DOI] [PubMed] [Google Scholar]
- 9.Pedišić Z., Dumuid D., Olds T.S. Integrating Sleep, Sedentary Behaviour and Physical Activity Research in the Emerging Field of Time-Use Epidemiology:Definitions, Concepts, Statistical Methods, Theoretical Framework, and Future Directions. Kinesiology. 2017;49:252–269. [Google Scholar]
- 10.Rollo S., Antsygina O., Tremblay M.S. The Whole Day Matters: Understanding 24-Hour Movement Guideline Adherence and Relationships with Health Indicators across the Lifespan. J. Sport Health Sci. 2020;9:493–510. doi: 10.1016/j.jshs.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., Moher D., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ. 2021;372:n160. doi: 10.1136/bmj.n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooke A., Smith D., Booth A. Beyond Pico: The Spider Tool for Qualitative Evidence Synthesis. Qual. Health Res. 2012;22:1435–1443. doi: 10.1177/1049732312452938. [DOI] [PubMed] [Google Scholar]
- 13.Schardt C., Adams M.B., Owens T., Keitz S., Fontelo P. Utilization of the Pico Framework to Improve Searching Pubmed for Clinical Questions. BMC Med. Inform. Decis. Mak. 2007;7:16. doi: 10.1186/1472-6947-7-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse D.J. Sleep Health: Can We Define It? Does It Matter? Sleep. 2014;37:9–17. doi: 10.5665/sleep.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Battelino T., Danne R.M.T., Bergenstal S.A., Amiel R., Beck T., Biester E., Bosi B.A., Buckingham W.T., Cefalu K.L., Close C., et al. Clinical Targets for Continuous Glucose Monitoring Data Interpretation: Recommendations from the International Consensus on Time in Range. Diabetes Care. 2019;42:1593–1603. doi: 10.2337/dci19-0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danne T., Nimri T.R., Battelino R.M., Bergenstal K.L., Close J.H., DeVries S., Garg L., Heinemann I., Hirsch S.A., Amiel R., et al. International Consensus on Use of Continuous Glucose Monitoring. Diabetes Care. 2017;40:1631–1640. doi: 10.2337/dc17-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Whittemore R., Jaser S., Guo J., Grey M. A conceptual model of childhood adaptation to type 1 diabetes. Nurs. Outlook. 2010;58:242–251. doi: 10.1016/j.outlook.2010.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.American Psychological Association APA Dictionary of Psychology. [(accessed on 3 January 2021)]. Available online: https://dictionary.apa.org.
- 19.Bramer W.M., Giustini D., de Jonge G.B., Holland L., Bekhuis T. De-Duplication of Database Search Results for Systematic Reviews in Endnote. J. Med. Libr. Assoc. 2016;104:240. doi: 10.3163/1536-5050.104.3.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McHugh M.L. Interrater Reliability: The Kappa Statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Critical Appraisal Skills Programme (CASP) CASP Checklists. Critical Appraisal Skills Programme (CASP); Oxford, UK: 2022. [Google Scholar]
- 22.Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Strobe Checklist. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE); Bern, Switzerland: 2022. [Google Scholar]
- 23.Suurmond R., van Rhee H., Hak T. Introduction, Comparison, and Validation of Meta-Essentials: A Free and Simple Tool for Meta-Analysis. Res. Synth. Methods. 2017;8:537–553. doi: 10.1002/jrsm.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson R.A., Brown S.P. On the Use of Beta Coefficients in Meta-Analysis. J. Appl. Psychol. 2005;90:175–181. doi: 10.1037/0021-9010.90.1.175. [DOI] [PubMed] [Google Scholar]
- 25.Higgins J., Green S., Ben Van Den A. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaborations; London, UK: 2011. [Google Scholar]
- 26.Abdelmaksoud A.A., Salah N.Y., Ali Z.M., Rashed H.R., Abido A.Y. Disturbed Sleep Quality and Architecture in Adolescents with Type 1 Diabetes Mellitus: Relation to Glycemic Control, Vascular Complications and Insulin Sensitivity. Diabetes Res. Clin. Pract. 2021;174:108774. doi: 10.1016/j.diabres.2021.108774. [DOI] [PubMed] [Google Scholar]
- 27.Al-Agha A., Ocheltree A., Hakeem A. Metabolic Control in Children and Adolescents with Insulin-Dependent Diabetes Mellitus at King Abdul-Aziz University Hospital. J. Clin. Res. Pediatr. Endocrinol. 2011;3:202–207. doi: 10.4274/jcrpe.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aman J., Skinner T.C., de Beaufort C.E., Swift P.G., Aanstoot H.J., Cameron F., Diabetes Hvidoere Study Group on Childhood Associations between Physical Activity, Sedentary Behavior, and Glycemic Control in a Large Cohort of Adolescents with Type 1 Diabetes: The Hvidoere Study Group on Childhood Diabetes. Pediatr. Diabetes. 2009;10:234–239. doi: 10.1111/j.1399-5448.2008.00495.x. [DOI] [PubMed] [Google Scholar]
- 29.Anderson B.J., Laffel L.M., Domenger C., Danne T., Phillip M., Mazza C., Hanas R., Waldron S., Beck R.W., Calvi-Gries F., et al. Factors Associated with Diabetes-Specific Health-Related Quality of Life in Youth With Type 1 Diabetes: The Global TEENs Study. Diabetes Care. 2017;40:1002–1009. doi: 10.2337/dc16-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ash G.I., Joiner K.L., Savoye M., Baker J.S., Gerosa J., Kleck E., Patel N.S., Sadler L.S., Stults-Kolehmainen M., Weinzimer S.A., et al. Feasibility and safety of a group physical activity program for youth with type 1 diabetes. Pediatr. Diabetes. 2019;20:450–459. doi: 10.1111/pedi.12841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Beraki Å., Magnuson A., Särnblad S., Åman J., Samuelsson U. Increase in physical activity is associated with lower HbA1c levels in children and adolescents with type 1 diabetes: Results from a cross-sectional study based on the Swedish pediatric diabetes quality registry (SWEDIABKIDS) Diabetes Res. Clin. Pract. 2014;105:119–125. doi: 10.1016/j.diabres.2014.01.029. [DOI] [PubMed] [Google Scholar]
- 32.Bernardini A.L., Vanelli M., Chiari G., Iovane B., Gelmetti C., Vitale R., Errico M.K. Adherence to physical activity in young people with type 1 diabetes. Acta Biomed. 2004;75:153–157. [PubMed] [Google Scholar]
- 33.Bishop F.K., Wadwa R.P., Snell-Bergeon J., Nguyen N., Maahs D.M. Changes in Diet and Physical Activity in Adolescents with and without Type 1 Diabetes over Time. Int. J. Pediatr. Endocrinol. 2014;2014:17. doi: 10.1186/1687-9856-2014-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cuenca-Garcia M., Jago R., Shield J.P., Burren C.P. How Does Physical Activity and Fitness Influence Glycaemic Control in Young People with Type 1 Diabetes? Diabet. Med. 2012;29:e369–e376. doi: 10.1111/j.1464-5491.2012.03740.x. [DOI] [PubMed] [Google Scholar]
- 35.da Costa L.M.F.C., Vieira S.E. Quality of Life of Adolescents with Type 1 Diabetes. Clinics. 2015;70:173–179. doi: 10.6061/clinics/2015(03)04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Lima V.A., Mascarenhas L.P.G., Decimo J.P., De Souza W.C., Monteiro A.L.S., Lahart I., França S.N., Leite N. Physical Activity Levels of Adolescents with Type 1 Diabetes Physical Activity in T1D. Pediatr. Exerc. Sci. 2017;29:213–219. doi: 10.1123/pes.2016-0199. [DOI] [PubMed] [Google Scholar]
- 37.Edmunds S., Roche D., Stratton G., Wallymahmed K., Glenn S.M. Physical activity and psychological well-being in children with Type 1 diabetes. Psychol. Health Med. 2007;12:353–363. doi: 10.1080/13548500600975446. [DOI] [PubMed] [Google Scholar]
- 38.Edmunds S., Roche D., Stratton G. Levels and Patterns of Physical Activity in Children and Adolescents With Type 1 Diabetes and Associated Metabolic and Physiologic Health Outcomes. J. Phys. Act. Health. 2010;7:68–77. doi: 10.1123/jpah.7.1.68. [DOI] [PubMed] [Google Scholar]
- 39.Fainardi V., Scarabello C., Cangelosi A., Fanciullo L., Mastrorilli C., Giannini C., Mohn A., Iafusco D., La Loggia A., Lombardo F., et al. Physical activity and sedentary lifestyle in children with type 1 diabetes: A multicentre Italian study. Acta Biomed. 2011;82:124–131. [PubMed] [Google Scholar]
- 40.Faulkner M.S., Michaliszyn S.F., Hepworth J.T. A personalized approach to exercise promotion in adolescents with type 1 diabetes. Pediatr. Diabetes. 2009;11:166–174. doi: 10.1111/j.1399-5448.2009.00550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Faulkner M.S., Michaliszyn S., Hepworth J.T., Wheeler M.D. Personalized Exercise for Adolescents with Diabetes or Obesity. Biol. Res. Nurs. 2014;16:46–54. doi: 10.1177/1099800413500064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frye S.S., Perfect M.M., Silva G.E. Diabetes Management Mediates the Association between Sleep Duration and Glycemic Control in Youth with Type 1 Diabetes Mellitus. Sleep Med. 2019;60:132–138. doi: 10.1016/j.sleep.2019.01.043. [DOI] [PubMed] [Google Scholar]
- 43.Galler A., Lindau M., Ernert A., Thalemann R., Raile K. Associations between Media Consumption Habits, Physical Activity, Socioeconomic Status, and Glycemic Control in Children, Adolescents, and Young Adults with Type 1 Diabetes. Diabetes Care. 2011;34:2356–2359. doi: 10.2337/dc11-0838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Griggs S., Redeker N.S., Jeon S., Grey M. Daily Variations in Sleep and Glucose in Adolescents with Type 1 Diabetes. Pediatr. Diabetes. 2020;21:1493–1501. doi: 10.1111/pedi.13117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hamburger E.R., Goethals E.R., Choudhary A., Jaser S.S. Sleep and depressive symptoms in adolescents with type 1 diabetes not meeting glycemic targets. Diabetes Res. Clin. Pract. 2020;169:108442. doi: 10.1016/j.diabres.2020.108442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hanson C.L., De Guire M.J., Schinkel A.M., Kolterman O.G., Goodman J.P., Buckingham B.A. Self-Care Behaviors in Insulin-Dependent Diabetes: Evaluative Tools and Their Associations with Glycemic Control. J. Pediatr. Psychol. 1996;21:467–482. doi: 10.1093/jpepsy/21.4.467. [DOI] [PubMed] [Google Scholar]
- 47.Hazen R.A., Fehr K.K., Fidler A., Cousino M.K., MacLeish S.A., Gubitosi-Klug R. Sleep disruption in adolescents with Type 1 diabetes mellitus: Relationships with adherence and diabetes control. Diabetes Manag. 2015;5:257–265. doi: 10.2217/dmt.15.18. [DOI] [Google Scholar]
- 48.Herbst A., Bachran R., Kapellen T., Holl R.W. Effects of Regular Physical Activity on Control of Glycemia in Pediatric Patients with Type 1 Diabetes Mellitus. Arch. Pediatr. Adolesc. Med. 2006;160:573–577. doi: 10.1001/archpedi.160.6.573. [DOI] [PubMed] [Google Scholar]
- 49.Herbst A., Kordonouri O., Schwab K.O., Schmidt F., Holl R.W., DPV Initiative of the German Working Group for Pediatric Diabetology Germany Impact of Physical Activity on Cardiovascular Risk Factors in Children with Type 1 Diabetes: A Multicenter Study of 23,251 Patients. Diabetes Care. 2007;30:2098–2100. doi: 10.2337/dc06-2636. [DOI] [PubMed] [Google Scholar]
- 50.Jabbour G. Vigorous Physical Activity Is Associated with Better Glycated Hemoglobin and Lower Fear of Hypoglycemia Scores in Youth with Type 1 Diabetes: A 2-Year Follow-Up Study. Front. Physiol. 2020;11:548417. doi: 10.3389/fphys.2020.548417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaser S.S., Ellis D. Sleep in adolescents and young adults with type 1 diabetes: Associations with diabetes management and glycemic control. Health Psychol. Behav. Med. 2016;4:49–55. doi: 10.1080/21642850.2015.1135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jaser S.S., Hamburger E.R., Bergner E.M., Williams R., Slaughter J.C., Simmons J.H., Malow B.A. Sleep Coach Intervention for Teens with Type 1 Diabetes: Randomized Pilot Study. Pediatr. Diabetes. 2020;21:473–478. doi: 10.1111/pedi.12991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johnson S.B., Freund A., Silverstein J., Hansen C.A., Malone J. Adherence-health status relationships in childhood diabetes. Health Psychol. 1990;9:606–631. doi: 10.1037/0278-6133.9.5.606. [DOI] [PubMed] [Google Scholar]
- 54.Kahkoska A.R., Nguyen C.T., Jiang X., Adair L.A., Agarwal S., Aiello A.E., Burger K.S., Buse J.B., Dabelea D., Dolan L.M., et al. Characterizing the weight-glycemia phenotypes of type 1 diabetes in youth and young adulthood. BMJ Open Diabetes Res. Care. 2020;8:e000886. doi: 10.1136/bmjdrc-2019-000886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalweit K.L., Briers N., Olorunju S.A.S. The success of various management techniques used in South African children with type 1 diabetes mellitus. South Afr. Med. J. 2015;105:400–404. doi: 10.7196/SAMJ.9334. [DOI] [PubMed] [Google Scholar]
- 56.Kokkonen J., Taanla A., Kokkonen E.-R. Diabetes in adolescence: The effect of family and psychologic factors on metabolic control. Nord. J. Psychiatry. 1997;51:165–172. doi: 10.3109/08039489709109091. [DOI] [Google Scholar]
- 57.Krzemińska K., Wieczorek D., Sitek E., Zaręba W. Anxiety of physical activity and anxiety of hypoglycaemia in adolescents with diabetes mellitus type 1. Physiotherapy. 2009;17:28–39. doi: 10.2478/v10109-010-0036-5. [DOI] [Google Scholar]
- 58.Kummer S., Stahl-Pehe K.A., Castillo C., Bachle C., Graf K., Strassburger B., Salgin E., Mayatepek G., Giani R.W., Holl T., et al. Health Behaviour in Children and Adolescents with Type 1 Diabetes Compared to a Representative Reference Population. PLoS ONE. 2014;9:e112083. doi: 10.1371/journal.pone.0112083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kyngäs H. Compliance of adolescents with diabetes. J. Pediatr. Nurs. 2000;15:260–267. doi: 10.1053/jpdn.2000.6169. [DOI] [PubMed] [Google Scholar]
- 60.Li C., Beech B., Crume T., Jr R.B.D., Dabelea D., Kaar J.L., Liese A.D., Mayer-Davis E.J., Pate R., Pettitt D.J., et al. Longitudinal association between television watching and computer use and risk markers in diabetes in the SEARCH for Diabetes in Youth Study. Pediatr. Diabetes. 2014;16:382–391. doi: 10.1111/pedi.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lukács A., Mayer K., Juhász E., Varga B., Fodor B., Barkai L. Reduced physical fitness in children and adolescents with type 1 diabetes. Pediatr. Diabetes. 2012;13:432–437. doi: 10.1111/j.1399-5448.2012.00848.x. [DOI] [PubMed] [Google Scholar]
- 62.Lukács A., Mayer K., Török A., Kiss-Toth E., Barkai L. Better cardiorespiratory fitness associated with favourable metabolic control and health-related quality of life in youths with type 1 diabetes mellitus. Acta Physiol. Hung. 2013;100:77–83. doi: 10.1556/APhysiol.100.2013.1.7. [DOI] [PubMed] [Google Scholar]
- 63.Lukacs A., Sasvari P., Torok A., Barkai L. Generic and Disease-Specific Quality of Life in Adolescents with Type 1 Diabetes: Comparison to Age-Matched Healthy Peers. J. Pediatr. Endocrinol. Metab. 2016;29:769–775. doi: 10.1515/jpem-2015-0397. [DOI] [PubMed] [Google Scholar]
- 64.Lukács A., Mayer K., Sasvári P., Barkai L. Health-Related Quality of Life of Adolescents with Type 1 Diabetes in the Context of Resilience. Pediatr. Diabetes. 2018;19:1481–1486. doi: 10.1111/pedi.12769. [DOI] [PubMed] [Google Scholar]
- 65.Macaulay G.C., Galland B.C., Boucher S.E., Wiltshire E.J., Haszard J.J., Campbell A.J., Black S.M., Smith C., Elder D., Wheeler B.J. Impact of Type 1 Diabetes Mellitus, Glucose Levels, and Glycemic Control on Sleep in Children and Adolescents: A Case-Control Study. Sleep. 2020;43:zsz226. doi: 10.1093/sleep/zsz226. [DOI] [PubMed] [Google Scholar]
- 66.MacMillan F., Kirk A., Mutrie N., Robertson K. Physical activity and sedentary behaviour in Scottish youth with type 1 diabetes. Pract. Diabetes. 2014;31:228–233c. doi: 10.1002/pdi.1874. [DOI] [Google Scholar]
- 67.Margeirsdottir H.D., Larsen J.R., Brunborg C., Sandvik L., Dahl-Jørgensen K., Diabetes Norwegian Study Group for Childhood Strong Association between Time Watching Television and Blood Glucose Control in Children and Adolescents with Type 1 Diabetes. Diabetes Care. 2007;30:1567–1570. doi: 10.2337/dc06-2112. [DOI] [PubMed] [Google Scholar]
- 68.Massin M.M., Lebrethon M.-C., Rocour D., Gérard P., Bourguignon J.-P. Patterns of physical activity determined by heart rate monitoring among diabetic children. Arch. Dis. Child. 2005;90:1223–1226. doi: 10.1136/adc.2005.075283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McDonough R.J., Clements M.A., DeLurgio S.A., Patton S.R. Sleep Duration and Its Impact on Adherence in Adolescents with Type 1 Diabetes Mellitus. Pediatr. Diabetes. 2017;18:262–270. doi: 10.1111/pedi.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Michaliszyn S.F., Faulkner M.S. Physical activity and sedentary behavior in adolescents with type 1 diabetes. Res. Nurs. Health. 2010;33:441–449. doi: 10.1002/nur.20393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Miculis C.P., De Campos W., da Silva Boguszweski M.C. Correlation between Glycemic Control and Physical Activity Level in Adolescents and Children with Type 1 Diabetes. J. Phys. Act. Health. 2015;12:232–237. doi: 10.1123/jpah.2013-0024. [DOI] [PubMed] [Google Scholar]
- 72.Mitchell F., Wilkie L., Robertson K., Reilly J.J., Kirk A. Feasibility and pilot study of an intervention to support active lifestyles in youth with type 1 diabetes: The ActivPals study. Pediatr. Diabetes. 2018;19:443–449. doi: 10.1111/pedi.12615. [DOI] [PubMed] [Google Scholar]
- 73.Mohammed J., Deda L., Clarson C.L., Stein R.I., Cuerden M.S., Mahmud F.H. Assessment of Habitual Physical Activity in Adolescents with Type 1 Diabetes. Can. J. Diabetes. 2014;38:250–255. doi: 10.1016/j.jcjd.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 74.Mosso C., Halabi V., Ortiz T., Hodgson M.I. Dietary intake, body composition, and physical activity among young patients with type 1 diabetes mellitus. J. Pediatr. Endocrinol. Metab. 2015;28:895–902. doi: 10.1515/jpem-2014-0334. [DOI] [PubMed] [Google Scholar]
- 75.Mozzillo E., Zito E., Maffeis C., De Nitto E., Maltoni G., Marigliano M., Zucchini S., Franzese A., Valerio G. Unhealthy lifestyle habits and diabetes-specific health-related quality of life in youths with type 1 diabetes. Acta Diabetol. 2017;54:1073–1080. doi: 10.1007/s00592-017-1051-5. [DOI] [PubMed] [Google Scholar]
- 76.Mutlu E.B.R.U., Mutlu C., Taskiran H., Özgen İ. Relationship between Physical Activity Level and Depression, Anxiety, Quality of Life, Self-Esteem, and Hba1c in Adolescents with Type 1 Diabetes Mellitus. Turk. J. Physiother. Rehabil. Fiz. Rehabil. 2017;28:38–46. [Google Scholar]
- 77.Naughton M.J., Yi-Frazier J.P., Morgan T.M., Seid M., Lawrence J.M., Klingensmith G.J., Waitzfelder B., Standiford D.A., Loots B., Search for Diabetes in Youth Study Group Longitudinal Associations between Sex, Diabetes Self-Care, and Health-Related Quality of Life among Youth with Type 1 or Type 2 Diabetes Mellitus. J. Pediatr. 2014;164:1376–1383. doi: 10.1016/j.jpeds.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neyman A., Woerner S., Russ M., Yarbrough A., DiMeglio L.A. Strategies That Adolescents with Type 1 Diabetes Use in Relation to Exercise. Clin. Diabetes. 2020;38:266–272. doi: 10.2337/cd19-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nguyen T., Obeid J., Walker R.G., Krause M.P., Hawke T.J., McAssey K., Vandermeulen J., Timmons B.W. Fitness and physical activity in youth with type 1 diabetes mellitus in good or poor glycemic control. Pediatr. Diabetes. 2015;16:48–57. doi: 10.1111/pedi.12117. [DOI] [PubMed] [Google Scholar]
- 80.Øverby N.C., Margeirsdottir H.D., Brunborg C., Anderssen S.A., Andersen L.F., Dahl-Jørgensen K. Norwegian Study Group for Childhood Diabetes. Physical activity and overweight in children and adolescents using intensified insulin treatment. Pediatr. Diabetes. 2009;10:135–141. doi: 10.1111/j.1399-5448.2008.00454.x. [DOI] [PubMed] [Google Scholar]
- 81.Patel N.J., Savin K.L., Kahanda S.N., Malow B.A., Williams L.A., Lochbihler G., Jaser S.S. Sleep habits in adolescents with type 1 diabetes: Variability in sleep duration linked with glycemic control. Pediatr. Diabetes. 2018;19:1100–1106. doi: 10.1111/pedi.12689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Perfect M.M., Patel P.G., Scott R.E., Wheeler M.D., Patel C., Griffin K.J., Sorensen S.T., Goodwin J.L., Quan S.F. Sleep, Glucose, and Daytime Functioning in Youth with Type 1 Diabetes. Sleep. 2012;35:81–88. doi: 10.5665/sleep.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Perfect M.M. The Relations of Sleep and Quality of Life to School Performance in Youth with Type 1 Diabetes. J. Appl. Sch. Psychol. 2014;30:7–28. doi: 10.1080/15377903.2013.853718. [DOI] [Google Scholar]
- 84.Rebesco D.B., Franca S.N., Lima V.A., Leite N., Smouter L., Souza W.C., Komatsu W.R., Mascarenhas L.P.G. Different Amounts of Moderate to Vigorous Physical Activity and Change in Glycemic Variability in Adolescents with Type 1 Diabetes: Is There Dose-Response Relationship? Arch. Endocrinol. Metab. 2020;64:312–318. doi: 10.20945/2359-3997000000254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rechenberg K., Griggs S., Jeon S., Redeker N., Yaggi H.K., Grey M. Sleep and Glycemia in Youth with Type 1 Diabetes. J. Pediatr. Health Care. 2020;34:315–324. doi: 10.1016/j.pedhc.2019.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Roberts A.J., Taplin C.E., Isom S., Divers J., Saydah S., Jensen E.T., Mayer-Davis E.J., Reid L.A., Liese A.D., Dolan L.M., et al. Association between fear of hypoglycemia and physical activity in youth with type 1 diabetes: The SEARCH for diabetes in youth study. Pediatr. Diabetes. 2020;21:1277–1284. doi: 10.1111/pedi.13092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Roberts A.J., Yi-Frazier J.P., Carlin K., Taplin C.E. Hypoglycaemia avoidance behaviour and exercise levels in active youth with type 1 diabetes. Endocrinol. Diabetes Metab. 2020;3:e00153. doi: 10.1002/edm2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Roche D.M., Edmunds S., Cable T., Didi M., Stratton G. Skin microvascular reactivity in children and adolescents with type 1 diabetes in relation to levels of physical activity and aerobic fitness. Pediatr. Exerc. Sci. 2008;20:426–438. doi: 10.1123/pes.20.4.426. [DOI] [PubMed] [Google Scholar]
- 89.Salvatoni A., Cardani R., Biasoli R., Salmaso M., De Paoli A., Nespoli L. Physical Activity and Diabetes. Acta Biomed. Atenei Parm. 2005;76:85–88. [PubMed] [Google Scholar]
- 90.Särnblad S., Ekelund U., Åman J. Physical activity and energy intake in adolescent girls with Type 1 diabetes. Diabet. Med. 2005;22:893–899. doi: 10.1111/j.1464-5491.2005.01544.x. [DOI] [PubMed] [Google Scholar]
- 91.Schiel R., Thomas A., Kaps A., Bieber G. An Innovative Telemedical Support System to Measure Physical Activity in Children and Adolescents with Type 1 Diabetes Mellitus. Exp. Clin. Endocrinol. Diabetes. 2011;119:565–568. doi: 10.1055/s-0031-1273747. [DOI] [PubMed] [Google Scholar]
- 92.Schweiger B., Klingensmith G., Snell-Bergeon J.K. Physical Activity in Adolescent Females with Type 1 Diabetes. Int. J. Pediatr. 2010;2010:328318. doi: 10.1155/2010/328318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Serrabulho M., Matos M., Raposo J. The health and lifestyles of adolescents with type 1 diabetes in Portugal. Eur. Diabetes Nurs. 2015;9:12–16a. doi: 10.1002/edn.197. [DOI] [Google Scholar]
- 94.Tercyak K.P., Beville K.W., Walker L.R., Prahlad S., Cogen F.R., Sobel D.O., Streisand R. Health Attitudes, Beliefs, and Risk Behaviors among Adolescents and Young Adults with Type 1 Diabetes. Child. Health Care. 2005;34:165–180. doi: 10.1207/s15326888chc3403_1. [DOI] [Google Scholar]
- 95.Turner S.L., Queen T.L., Butner J., Wiebe D., Berg C.A. Variations in Daily Sleep Quality and Type 1 Diabetes Management in Late Adolescents. J. Pediatr. Psychol. 2016;41:661–669. doi: 10.1093/jpepsy/jsw010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Valerio G., Spagnuolo M.I., Lombardi F., Spadaro R., Siano M., Franzese A. Physical activity and sports participation in children and adolescents with type 1 diabetes mellitus. Nutr. Metab. Cardiovasc. Dis. 2007;17:376–382. doi: 10.1016/j.numecd.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 97.Varni J.W., Limbers C.A., Bryant W.P., Wilson D.P. The Pedsql Multidimensional Fatigue Scale in Type 1 Diabetes: Feasibility, Reliability, and Validity. Pediatr. Diabetes. 2009;10:321–328. doi: 10.1111/j.1399-5448.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 98.von Schnurbein J., Boettcher C., Brandt S., Karges B., Dunstheimer D., Galler A., Denzer C., Denzer F., Vollbach H., Wabitsch M., et al. Sleep and glycemic control in adolescents with type 1 diabetes. Pediatr. Diabetes. 2018;19:143–149. doi: 10.1111/pedi.12538. [DOI] [PubMed] [Google Scholar]
- 99.Yeshayahu Y., Mahmud F.H. Altered Sleep Patterns in Adolescents with Type 1 Diabetes: Implications for Insulin Regimen. Diabetes Care. 2010;33:e142. doi: 10.2337/dc10-1536. [DOI] [PubMed] [Google Scholar]
- 100.Yetim A., Alikasifoglu M., Bas F., Eliacik K., Cig G., Erginoz E., Ercan O., Bundak R. Glycemic Control and Health Be-haviors in Adolescents with Type 1 Diabetes. Turk. J. Pediatr. 2018;60:244–254. doi: 10.24953/turkjped.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 101.Adler A., Gavan M.Y., Tauman R., Phillip M., Shalitin S. Do Children, Adolescents, and Young Adults with Type 1 Diabetes Have Increased Prevalence of Sleep Disorders? Pediatr. Diabetes. 2017;18:450–458. doi: 10.1111/pedi.12419. [DOI] [PubMed] [Google Scholar]
- 102.Bergner E.M., Williams R., Hamburger E.R., Lyttle M., Davis A.C., Malow B., Simmons J.H., Lybarger C., Capin R., Jaser S.S. Sleep in Teens with Type 1 Diabetes: Perspectives from Adolescents and Their Caregivers. Diabetes Educ. 2018;44:541–548. doi: 10.1177/0145721718799086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rechenberg K., Grey M., Sadler L. “Anxiety and Type 1 diabetes are like cousins”: The experience of anxiety symptoms in youth with Type 1 diabetes. Res. Nurs. Health. 2018;41:544–554. doi: 10.1002/nur.21913. [DOI] [PubMed] [Google Scholar]
- 104.Ryninks K., Sutton E., Thomas E., Jago R., Shield J.P.H., Burren C.P. Attitudes to Exercise and Diabetes in Young People with Type 1 Diabetes Mellitus: A Qualitative Analysis. PLoS ONE. 2015;10:e0137562. doi: 10.1371/journal.pone.0137562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wennick A., Lundqvist A., Hallström I.K. Everyday Experience of Families Three Years after Diagnosis of Type 1 Diabetes in Children: A research paper. J. Pediatr. Nurs. 2009;24:222–230. doi: 10.1016/j.pedn.2008.02.028. [DOI] [PubMed] [Google Scholar]
- 106.Wilkie L., Mitchell F., Robertson K., Kirk A. Motivations for physical activity in youth with type 1 diabetes participating in the ActivPals project: A qualitative study. Pract. Diabetes. 2017;34:151–155. doi: 10.1002/pdi.2107. [DOI] [Google Scholar]
- 107.Quirk H., Glazebrook C., Martin R., Blake H. “We Don’t Worry about Diabetes That Much”: A Qualitative Study Exploring Perceptions of Physical Activity among Children with Type 1 Diabetes. Adv. Pediatr. Res. 2016;3:2. [Google Scholar]
- 108.Blake H., da Silva L., Glazebrook C. “They Don’t See It as Priority If the Kid’s Not Sporty”: Parents’ Perceptions of Clinic Communication around Physical Activity to Children with Type 1 Diabetes and Their Families. Adv. Pediatr. Res. 2018;5:1–14. doi: 10.24105/apr.2018.5.22. [DOI] [Google Scholar]
- 109.Quirk H., Blake H., Dee B., Glazebrook C. “You Can’t Just Jump on a Bike and Go”: A Qualitative Study Exploring Parents’ Perceptions of Physical Activity in Children with Type 1 Diabetes. BMC Pediatr. 2014;14:313. doi: 10.1186/s12887-014-0313-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kennedy A., Nirantharakumar K., Chimen M., Pang T.T., Hemming K., Andrews R., Narendran P. Does Exercise Improve Glycaemic Control in Type 1 Diabetes? A Systematic Review and Meta-Analysis. PLoS ONE. 2013;8:e58861. doi: 10.1371/journal.pone.0058861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.MacMillan F., Kirk A., Mutrie N., Matthews L., Robertson K., Saunders D.H. A Systematic Review of Physical Activity and Sedentary Behavior Intervention Studies in Youth with Type 1 Diabetes: Study Characteristics, Intervention Design, and Efficacy. Pediatr. Diabetes. 2014;15:175–189. doi: 10.1111/pedi.12060. [DOI] [PubMed] [Google Scholar]
- 112.Quirk H., Blake H., Tennyson R., Randell T.L., Glazebrook C. Physical activity interventions in children and young people with Type 1 diabetes mellitus: A systematic review with meta-analysis. Diabet. Med. 2014;31:1163–1173. doi: 10.1111/dme.12531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Absil H., Baudet L., Robert A., Lysy P.A. Benefits of physical activity in children and adolescents with type 1 diabetes: A systematic review. Diabetes Res. Clin. Pract. 2019;156:107810. doi: 10.1016/j.diabres.2019.107810. [DOI] [PubMed] [Google Scholar]
- 114.Rassart J., Oris L., Prikken S., Weets I., Moons P., Luyckx K. Personality Functioning in Adolescents and Emerging Adults with Type 1 Diabetes. J. Adolesc. Health. 2018;63:792–798. doi: 10.1016/j.jadohealth.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 115.Reutrakul S., Thakkinstian A., Anothaisintawee T., Chontong S., Borel A.-L., Perfect M.M., Janovsky C.C.P.S., Kessler R., Schultes B., Harsch I.A. Sleep Characteristics in Type 1 Diabetes and Associations with Glycemic Control: Systematic Review and Meta-Analysis. Sleep Med. 2016;23:26–45. doi: 10.1016/j.sleep.2016.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ji X., Wang Y., Saylor J. Sleep and Type 1 Diabetes Mellitus Management among Children, Adolescents, and Emerging Young Adults: A Systematic Review. J. Pediatr. Nurs. 2021;61:245–253. doi: 10.1016/j.pedn.2021.06.010. [DOI] [PubMed] [Google Scholar]
- 117.Elmesmari R.A., Reilly J.J., Paton J.Y. 24-Hour Movement Behaviors in Children with Chronic Disease and Their Healthy Peers: A Case-Control Study. Int. J. Environ. Res. Public Health. 2022;19:2912. doi: 10.3390/ijerph19052912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tremblay M.S., Carson J.P.V., Chaput S., Gorber T.C., Dinh M., Duggan G., Faulkner C.E., Gray R., Gruber K., Janson I., et al. Canadian 24-Hour Movement Guidelines for Children and Youth: An Integration of Physical Activity, Sedentary Behaviour, and Sleep. Appl. Physiol. Nutr. Metab. 2016;41:S311–S327. doi: 10.1139/apnm-2016-0151. [DOI] [PubMed] [Google Scholar]
- 119.Chaput J.-P., Carson V., Gray C.E., Tremblay M.S. Importance of All Movement Behaviors in a 24 Hour Period for Overall Health. Int. J. Environ. Res. Public Health. 2014;11:12575–12581. doi: 10.3390/ijerph111212575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Perfect M.M. Sleep-related disorders in patients with type 1 diabetes mellitus: Current insights. Nat. Sci. Sleep. 2020;12:101–123. doi: 10.2147/NSS.S152555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.