Abstract
While Hepatitis B virus (HBV) and the human immunodeficiency virus (HIV) are endemic in West Africa, the prevalence of HBV/HIV coinfection and their associated risk factors in children remains unclear. In this review, we sought to assess HBsAg seroprevalence among 0- to 16-year-olds with and without HIV in West African countries and the risk factors associated with HBV infection in this population. Research articles between 2000 and 2021 that reported the prevalence of HBV and associated risk factors in children in West Africa were retrieved from the literature using the Africa Journals Online (AJOL), PubMed, Google Scholar, and Web of Science databases as search tools. StatsDirect, a statistical software, was used to perform a meta-analysis of the retained studies. HBV prevalence and heterogeneity were then assessed with a 95% confidence interval (CI). Publication bias was evaluated using funnel plot asymmetry and Egger’s test. Twenty-seven articles conducted across seven West African countries were included in this review. HBV prevalence among persons aged 0 to 16 years was 5%, based on the random analysis, given the great heterogeneity of the studies. By country, the highest prevalence was observed in Benin (10%), followed by Nigeria (7%), and Ivory Coast (5%), with Togo (1%) having the lowest. HBV prevalence in an HIV-infected population of children was (9%). Vaccinated children had lower HBV prevalence (2%) than unvaccinated children (6%). HBV prevalence with a defined risk factor such as HIV co-infection, maternal HBsAg positivity, undergoing surgery, scarification, or being unvaccinated ranged from 3–9%. The study highlights the need to reinforce vaccination of newborns, screening for HBV, and HBV prophylaxis among pregnant women in Africa, particularly in West Africa, to achieve the WHO goal of HBV elimination, particularly in children.
Keywords: prevalence, hepatitis B virus, West Africa, risk factors, HIV, children
1. Introduction
Hepatitis B virus (HBV) infection remains a major cause of acute and chronic liver disease with significant associated morbidity and mortality, worldwide. The World Health Organization (WHO) estimated that 296 million people were living with chronic HBV infection in 2019, with 1.5 million new infections each year and 887,000 deaths due to chronic HBV infection (CHB) [1]. The risk of chronic infection after exposure to HBV depends on age at time of infection, with a 90% risk when infection occurs in infancy and <10% risk when infection occurs in immunocompetent adolescents and adults [2]. Sub-Saharan Africa (SSA) is one of the highest endemic regions with an estimated HBV surface antigen (HBsAg) sero-prevalence (heretofore referred to as HBV prevalence) of more than 8% [3]. In SSA countries, including those in West Africa, the main sources of transmission are mother-to-child during delivery and horizontal during early childhood through close interaction with infected household contacts [4]. Globally, there are 2 million estimated new HBV infections in children < 5 years of age [1], resulting in HBV prevalence of 5–8% in children in SSA, with, for example, a reported 5.8% of five-year-old West African children being infected [5].
HBV burden is particularly high in HIV-endemic areas. HBV prevalence in the people living with Human Immunodeficiency Virus/Acquired ImmunoDeficiency Syndrome, HIV/AIDS is estimated to be more than 7% [6,7,8]. Given similar transmission mechanisms of both HBV and HIV, HBV/HIV co-infection is relatively common in HIV-endemic areas in SSA, in both adults and children. The Joint United Nations Program on HIV/AIDS (UNAIDS) estimated, in 2019, that 1.7 million children were living with HIV worldwide [9]. Few studies, however, have focused on HIV/HBV co-infection in the pediatric population. Although, HBV infection is usually asymptomatic during childhood, co-infection poses a particular challenge as the disease progresses rapidly towards chronicity with increased risk of mortality from cirrhosis or hepatocellular carcinoma (HCC) [10,11,12]. In addition, children are highly vulnerable to HIV infection and are at higher risk of anti-retroviral therapy (ART) failure compared to adults, especially in resource-limited settings [13,14].
Although HIV treatment significantly reduces vertical transmission, some residual risk exists when the status of HIV and HBV are not known during pregnancy. In West Africa, the average childbearing age ranges from 18.8 to 21.8 years and HBV viral load levels are often high in this age group due to immune tolerance [8,15]. In addition, hepatitis B e-antigen (HBeAg) status and high HBV viral load during pregnancy are established risk factors for perinatal transmission [14,15,16]. However, HBV is a vaccine-preventable disease with 95% of properly vaccinated children being well protected. The hepatitis B vaccine (HepB) is recommended for all infants at birth, and for children and adults at high risk [17].
Population-based HBsAg sero-surveys have been recommended as a monitoring tool for the impact of HepB vaccination programs in areas of high and moderate endemicity. However, data on chronic HBV prevalence, HIV co-infection, and other associated risk factors in children in West Africa are limited, particularly following the introduction of HBV vaccination programs. The absolute number of children chronically infected with HBV is not known. Yet, for evaluating national vaccination programs and national disease prevention and control efforts in West Africa and SSA, it is critical to understand the current prevalence of HBV infection.
We, therefore, conducted a systematic review of published studies, between 2000 and 2021, to assess the prevalence in West Africa of HBsAg sero-positivity among persons aged 0 to 16 years, with and without HIV infection, to determine the prevalence of HBV and HIV/HBV co-infection and other risk factors associated with HBV infection in this population.
2. Materials and Methods
2.1. Inclusion Criteria and Exclusion Reasons
Original research articles in peer-reviewed journals with full-text in English and available online, published between 2000 and 2021, with a clear and concise description of the sample types, size, and methods used to assess HBV sero-prevalence were included. We focused on full-text articles that reported the study design of HBsAg testing to assess HBV sero-prevalence in persons with or without HIV co-infection, conducted in West African countries, and included children, aged 0 to 16 years, in the sample.
Studies conducted among West African populations residing outside Africa were excluded. Systematic reviews, commentaries and editorials, case report studies, surveillance reports, conference abstracts, animal studies, and articles describing the sero-prevalence of HBV only among adult populations were excluded. Studies with insufficient or inaccessible data, pre-prints, studies with a sample size < 100, and studies that did not describe their sampling technique or that used purposive sampling were excluded.
2.2. Data Sources and Study Screening Strategies
A systematic search, using key search words, was conducted on Africa Journals Online (AJOL), PubMed, Google Scholar, and Web of Science databases, supplemented by a manual search of retrieved references. The keywords used were HBV prevalence, children, HBV, HBV risk factors, HIV co-infection, and the names of the 14 countries of West Africa (Benin, Burkina Faso, Côte d’Ivoire or Ivory Coast, Cape Verde or Capo Verde, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Senegal, Sierra Leone, and Togo). Prior to screening, all identified articles were imported into Covidence, a systematic review management tool [18]. Two reviewers (D.B.F. and A.M.S.) independently screened the titles and abstracts for study eligibility and full-text review. Reviewers then independently read the full text and selected studies for inclusion. After each step of the review, the two reviewers met and reached consensus on the final selection of studies for inclusion.
2.3. Description of the Study Area
West Africa is comprised of 14 countries (see above) and has one of the world’s fastest-growing populations, with more than 420 million people, equivalent to 5% of the total world population and with a median age of 18 years [19].
2.4. Data Extraction
After full-text review, the two reviewers, individually, extracted data from all eligible and retained articles, using the Covidence tool. Any differences observed by a reviewer in data extractio, were reconciled prior to conducting the meta-analysis. In the event of disagreement, a third reviewer (MIK) was consulted to establish consensus. Information extracted from the articles included: sociodemographic characteristics, sample size, the prevalence of HBsAg, risk factors including HIV co-infection, mother’s HBsAg status, HBV contact(s) in the family, blood transfusion, circumcision, or other scarification, surgery, and receipt of HepB vaccine, including a birth dose.
2.5. Quality Assessment
The Newcastle–Ottawa scale (NOS) for cross-sectional studies quality assessment tool was used to assess the quality of each study [20]. The tool includes multiple sections. The first section focuses on the sample selection of each study. The second section deals with the comparability of the study including type of study, diagnostic method, and year of publication. The last section focuses on the statistical analysis of each study. A total NOS score ranges from 0 to 10. Study scores of 9–10 points are considered very good, 7–8 as good and 5–6 as satisfactory.
2.6. Data Analysis
StatsDirect, a statistical software (Version 3.0.0, StatsDirect Ltd., Cheshire, UK) was used to conduct the meta-analysis of the proportions of HBsAg in the retained studies [21]. Individual study proportions were assessed with a 95% confidence interval (CI) and the pooled effect. Sources of variation among studies were assessed by sub-group analysis, using the following grouping variables: study setting (hospital/other health care setting versus community setting), year of study, year of publication, and the prevalence of HBsAg among children, aged 0–16 years, stratified by “with” group risk factors, “without” group risk factors, and individual risk factors including HIV status, with HBsAg positive mother, and HBV vaccination including birth dose status.
We also assessed the study type, country, sample type, and testing method. Heterogeneity across the studies was assessed by the Quoran (Q) statistic test and the I2 statistic. High heterogeneity (I2 > 73% and p het < 0.05) represents the percentage of total variation across studies, attributable to heterogeneity rather than to chance [22]. Sources of heterogeneity were analyzed through sub-group analysis and one sensitivity analysis of HIV/HBV co-infection.
Publication bias was evaluated using funnel plot asymmetry and Egger’s test [23]. In this test, p > 0.05 indicates an absence of evidence of publication bias (not significant). A random-effects model (REM) was used to pool HBV prevalence. Some representative results of the REM are presented graphically using forest plots.
We also assessed publication bias analysis in different subgroups. A sensitivity analysis was carried out by excluding 2 studies likely to strongly bias the results of HBV prevalence. Analyses of HBV prevalence were assessed with a 95% CI and p < 0.05 was considered as significant. All calculations were done using the StatsDirect software version 3.
3. Results
3.1. Study Selection
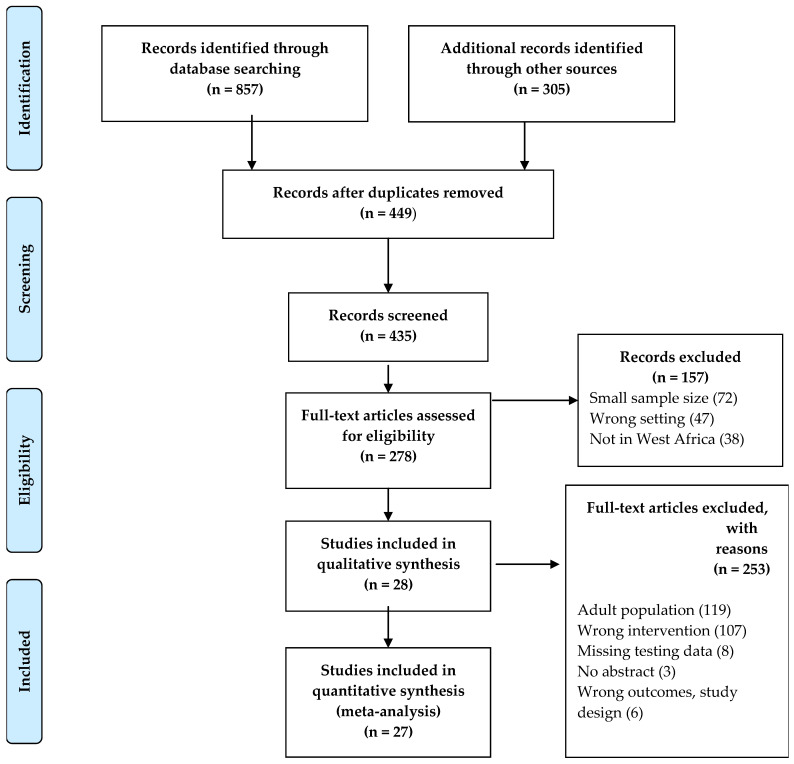
The databases and other literature search tools yielded a total of 1162 articles. Figure 1 summarizes the flow chart for the selection of studies. Duplicate articles (n = 713), due to the diverse search tools used, were removed. Titles and/or abstracts were first used to screen the articles, according to the systematic review and meta-analysis criteria. After screening, 157 articles were further excluded because of a sample size < 100 and/or not within the publication period. However, data were extracted from four general population studies with more than 100 children and adolescents in each of the studies [24,25,26,27]. Of the 278 articles fully screened, 253 were excluded, based on the inclusion and exclusion criteria, as described in the Methods section. A total of 27 articles met the eligibility criteria for the meta-analysis. The included studies were conducted in seven of the fourteen West African countries, Nigeria (thirteen articles), Senegal (five articles), Burkina Faso and Ghana (three articles each), Benin, Togo, and Ivory Coast (one article each), as shown in Table 1.
Figure 1.
Study Eligibility Flow Diagram.
Table 1.
Characteristics of Systematic Review Studies and Meta-Analysis of HBV Prevalence in Children in West Africa.
| 1st Author [Reference] |
Study Setting |
Study Year | Publication Year | Country | Study Type | Sample Size | Number AgHBs+ | Prevalence of AgHB+ (%) | Diagnostic Method | Sample Type | Score Quality |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Studies in Hospital Settings | |||||||||||
| Adeoye OA [28] | Urban | 2012 | 2021 | Nigeria | Cross-sectional | 261 | 3 | 1.1 | ELISA | SERA | 7 |
| Anigilaje EA [29] | Urban | 2008–2012 | 2013 | Nigeria | Cross-sectional | 395 | 31 | 7.8 | ELISA | SERA | 10 |
| Barro M [30] | Urban | 2013 | 2019 | Burkina Faso | Cohort | 2015 | 53 | 2.6 | ELISA | PLASMA | 9 |
| d’Almeida M [31] | Urban | 2014 | 2015 | Benin | Cross-sectional | 104 | 10 | 9.6 | RDT | PLASMA | 9 |
| Ezeilo MC [32] | Urban | 2017 | 2018 | Nigeria | Cross-sectional | 270 | 31 | 22.5 | ELISA | PLASMA | 7 |
| Ikpeme EE [33] | Urban | 2010–2011 | 2013 | Nigeria | Cohort | 166 | 10 | 6.0 | ELISA | SERA | 9 |
| Bukbuk DN [27] | Urban | 2009–2010 | 2016 | Nigeria | Cohort | 177 | 44 | 24.9 | ELISA | SERA | 6 |
| LO G [34] | Urban | 2016 | 2019 | Senegal | Cross-sectional | 295 | 3 | 1.1 | ELISA | SERA | 9 |
| Edward AD [35] | Urban | 2004 | 2007 | Nigeria | Retrospective | 251 | 31 | 12 | ELISA | SERA | 7 |
| Ekouevi DK [36] | Urban | 2017 | 2020 | Togo | Cross-sectional | 210 | 3 | 1.3 | RDT | SERA | 7 |
| Nacro B [37] | Urban | 2001 | 2001 | Burkina Faso | Cross-sectional | 103 | 41 | 39.8 | RDT | SERA | 8 |
| Nwolisa E [38] | Urban | 2010 | 2013 | Nigeria | Cross-sectional | 139 | 8 | 5.8 | RDT | SERA | 6 |
| Toyé RM [39] | Urban | 2015 | 2021 | Senegal | Retrospective | 613 | 25 | 4.1 | RDT | SERA | 8 |
| Ashir GM [40] | Urban | 2007 | 2009 | Nigeria | Cross-sectional | 284 | 54 | 2.8 | ELISA | SERA | 7 |
| Apiung T [41] | Urban | 2012–2013 | 2017 | Ghana | Cross-sectional | 424 | 3 | 0.05 | ELISA | PLASMA | 7 |
| Sadoh AE [42] | Urban | 2011 | 2014 | Nigeria | Cross-sectional | 150 | 21 | 13.9 | ELISA | SERA | 8 |
| Hagan OCK [43] | Urban | 2012–2013 | 2018 | Ghana | Cross-sectional | 387 | 11 | 2.8 | ELISA | SERA | 7 |
| Ba A [44] | Urban | 2013–2015 | 2018 | Senegal | Cross-sectional | 252 | 7 | 2.8 | ELISA | SERA | 7 |
| Quaye T [45] | Urban | 2019 | 2021 | Ghana | Cross-sectional | 350 | 5 | 1.4 | ELISA | SERA | 8 |
| Studies in Community Settings | |||||||||||
| Gueye SB [46] | Urban | 2007–2012 | 2016 | Senegal | Retrospective | 930 | 28 | 3.0 | ELISA | TOTAL BLOOD | 7 |
| Omeje KN [26] | Urban | 2010–2011 | 2017 | Nigeria | Cross-sectional | 208 | 6 | 2.8 | RDT | SERA | 5 |
| Sanou AM [47] | Rural | 2015 | 2018 | Burkina Faso | Cross-sectional | 265 | 9 | 3.4 | RDT | SERA | 6 |
| Périères L [48] | Rural | 2018–2019 | 2021 | Senegal | Cross-sectional | 1327 | 17 | 1.2 | ELISA | SERA | 10 |
| Ouattara A [25] | Urban | 2006 | 2019 | Cote d’Ivoire | Cross-sectional | 282 | 15 | 5.3 | ELISA | SERA | 9 |
| Odusanya OO [49] | Rural/Urban | 2001 | 2005 | Nigeria | Case control | 223 | 3 | 1.2 | ELISA | SERA | 8 |
| Odusanya OO [49] | Rural/Urban | 2001 | 2005 | Nigeria | Case control | 219 | 10 | 4.6 | ELISA | SERA | 8 |
| Ikobah J [50] | Urban | 2014 | 2016 | Nigeria | Cross-sectional | 595 | 6 | 0.6 | RDT | SERA | 7 |
| Adoga, MP [24] | Urban | 2008–2009 | 2010 | Nigeria | Cohort | 409 | 12 | 2.9 | ELISA | SERA/PLASMA | 8 |
3.2. Characteristics of the Systematic Review Studies
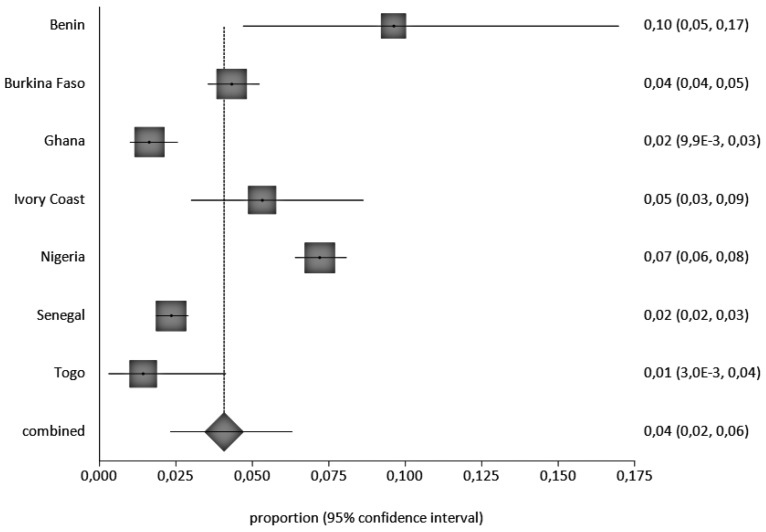
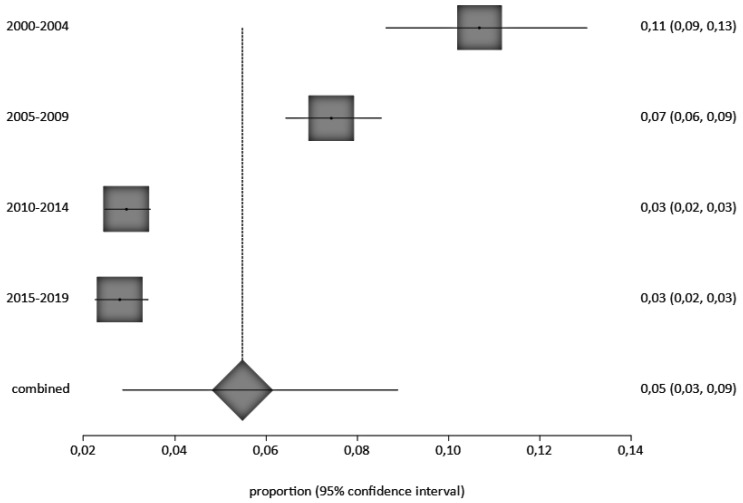
Four were cohort studies, nineteen were cross-sectional, three were retrospective cohort studies, and one was case-control. A total of 11,304 children, aged 0 to 16 years, were included in the retained studies, with studies from Nigeria accounting for most children (3747), of which, 270 were HbsAg positive (270/3747 = 7.2%), followed by Senegal with 3417 children and 80 HBsAg positive, (80/3417 = 2.3%), Burkina Faso, 2383 children (103/2383 = 4.3%), Ghana, 1661 (19/1161 = 1.6%), Ivory coast, 285, (15/2825.3%), Togo, 210 (3/210 = 1.4%) and Benin 103 (10/103 = 9.7%), (Supplementary Data S1). We observed a decrease in the prevalence of HBV between 2000 and 2021. HBV prevalence by country and study periods calculated by the StatsDirect software are presented in Figure 2 and Figure 3, respectively.
Figure 2.
Overall HBV Prevalence in Children, 0–16 Years Old, in West Africa, Pooled by Country. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence.
Figure 3.
Forest Plot of Pooled HBV Prevalence by Year of Study Interval. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence.
Multiple techniques were reported to detect HBsAg, with direct enzyme-linked immunoassay test (ELISA) being the most common, followed by rapid diagnostic assays (RDT) using serum or plasma serological markers of HBV.
Nineteen (n = 19), 70% of the studies were conducted in hospital settings and eight (30%) in community settings. Most of the studies involved children only, although we extracted data from four studies of the general population that included >100 children, 0–16 years old [24,25,26,27].
Nine (=9), 33% of studies included persons living with HIV (PLWH), including a total of 2317 children, and three studies included high-risk children born to HBsAg-positive mothers. Most studies were conducted in urban areas, with only four studies being in mixed urban and rural areas. Seven (n = 7), 26% of studies included HepB vaccine status; however, most studies were missing vaccination information (Supplementary Data S2).
3.3. Overall Pooled HBV Prevalence in Children in West Africa and Publication Bias
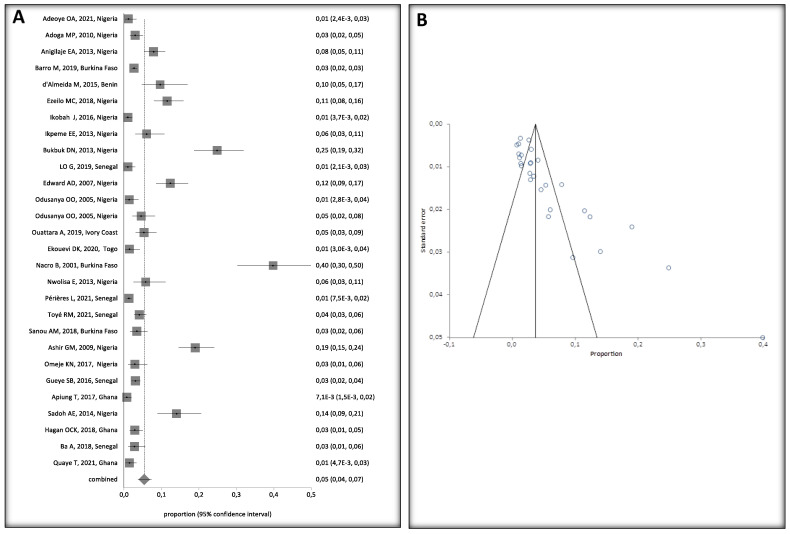
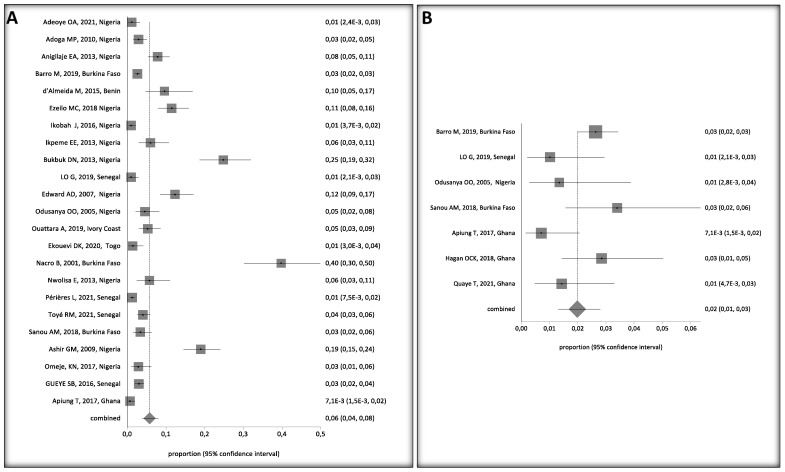
HBV prevalence among children, 0–16 years old, varied widely in West Africa (Figure 4A). Crude overall HBV prevalence in the pooled sample of 11,304 children was 5% (95%, CI 4–7%). The seroprevalence data are presented in the random effect model because of the substantial heterogeneity of HBV prevalence across the studies (I2 > 94.2%, 95% CI = 93% to 95.1%). Egger’s test was significant (p < 0.001) for HBV prevalence, suggesting publication bias in the studies (Figure 4B).
Figure 4.
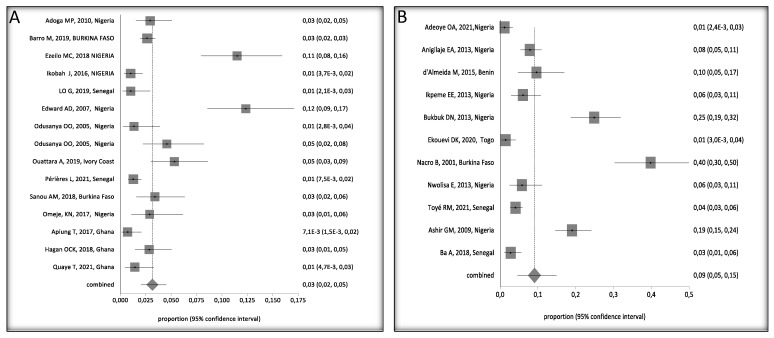
(A) Forest Plot of Global HBV Prevalence in Children in West Africa, 2000 and 2021. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence in each study in the meta-analysis [24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50]. (B) Bias Assessment Plot.
3.4. Sensitivity Analysis
To further assess the strength of the HBV prevalence results, we conducted a leave-two-out sensitivity analysis by removing two studies that included two high-risk populations: a study of HBV-HIV co-infected children with high HBV prevalence [37] and a study of infants born to HBsAg positive mothers [27]. The overall HBsAg positive pooled prevalence rate was 5% with heterogeneity prior to the exclusion of these two studies (I2 of 94.2%, 95% CI = 93% to 95.1%, p < 0.0001). However, after the exclusion, the pooled prevalence rate only dropped to 4% (95% CI 3% to 6%) with heterogeneity (I2 of 91.2%, 95% CI = 88.8% to 92.9%), indicating that the pooled HBV prevalence was not affected by the two studies and that the results are robust (Supplementary Data S3).
3.5. HBV Prevalence in Children with or without Risk Factors
Overall, HBV prevalence in children ranged from 4% (95%CI: 0.03 to 0.06) in children without risk factors, (Figure 5A) to 9% (95%CI: 5 to 15%) in children with at least one risk factor, such as HIV/HBV co-infection or being born to an HbsAg positive mother (Figure 5B).
Figure 5.
Forest Plots of HBV Prevalence in Children 0–16 Years Old in West Africa, (A) Without Risk Factors and (B) With Risk Factors [24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,41,43,44,45,47,48,49,50]. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence in each study in the meta-analysis.
A funnel plot of HBV prevalence in children with and without HIV shows a not strictly symmetrical display of the prevalence reported by the individual studies (Figure 3). However, the random effects model (DerSimonian Laird) suggests that there is evidence of publication bias, as revealed by the Egger’s test, with a bias = 4.447819 (95% CI = 3.043189 to 5.852449) and p < 0.0001.
3.6. Risk Factors of HBV Infection in Children
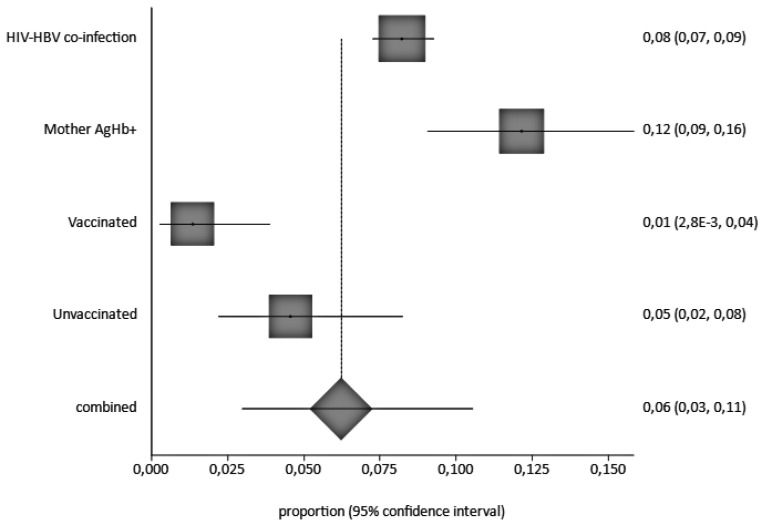
An analysis of children by individual risk factor shows HBV prevalences of 12%, 8%, 5%, and 1% in children born to HBsAg positive mothers, HBV/HIV co-infected, unvaccinated with a birth dose, and vaccinated, respectively, as shown in Figure 6.
Figure 6.
Pooled HBV Infection Prevalence by Risk Factor A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence.
3.7. HBV Prevalence in Hospital and Community Settings
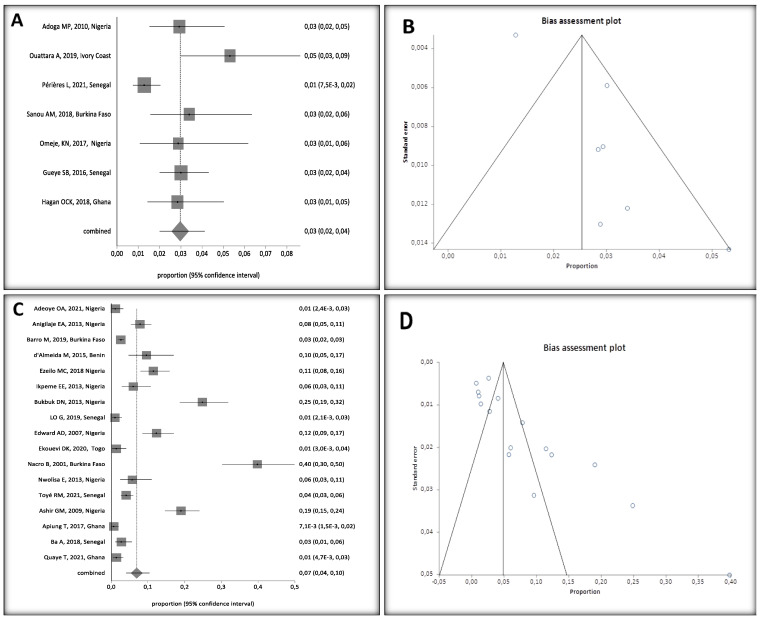
Subgroup analysis showed higher HBV prevalence in studies conducted in hospital settings [7% (95% CI. 4 to 11%)] compared to studies conducted in community settings among children without any risk factors [3% (95%CI: 2 to 4%)] (Figure 7A,C).
Figure 7.
(A) Forest Plots of HBV Prevalence in Children, 0–16 Years Old, in West Africa, Conducted in Community Settings, (B) Heterogeneity in Community Setting Studies, (C) Forest Plots of HBV Prevalence in Hospital Settings, and (D) Heterogeneity in Hospital Setting Studies [24,25,26,28,29,30,31,32,33,34,36,37,38,39,40,41,43,44,45,46,47,48]. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence in each study in the meta-analysis.
Community setting studies had an Egger Bias of 2.685631 (95% CI = 1.00063 to 4.370632) (p = 0.0094), corresponding to low heterogeneity between studies, compared to an Egger Bias of 5.116854 (95% CI = 2.916234 to 7.317473) (p = 0.0002) for hospital setting studies, corresponding to a high heterogeneity (Figure 7B,D).
3.8. HBV Prevalence by HIV Status in Children
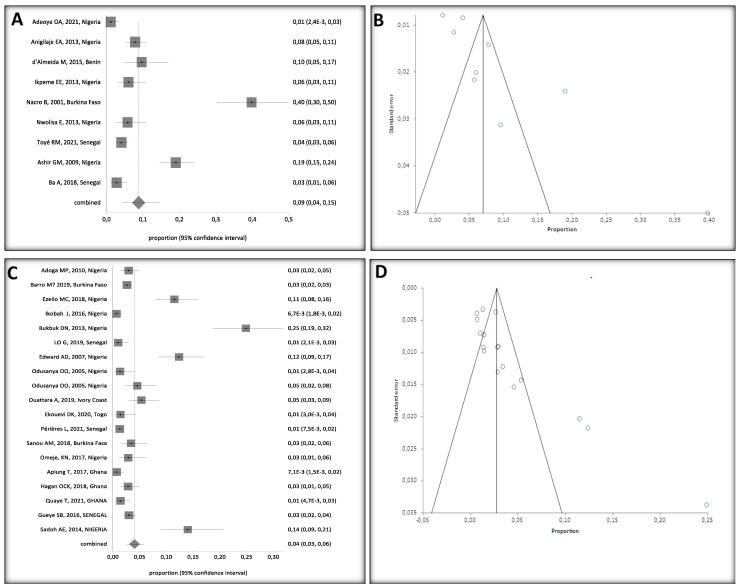
HBV/HIV co-infected children had an overall HBV prevalence of 9% (95% CI: 4 to 15%), with an Egger bias of 6.253247 (95% CI = 2.533005–9.97349; p = 0.0054), corresponding to low heterogeneity between studies, as opposed to studies of HBV mono-infected children who had an overall HBV prevalence of 4% (95% CI, 2 to 6%) and an Egger bias of 3.7865 (Figure 8).
Figure 8.
(A) Forest Plots of HBV/HIV Co-Infected Children, (B) Bias Assessment of Co-Infected Children, (C) Forest Plots of HBV Mono-Infected Children, (D) Bias Assessment of HBV Mono-Infected Children [24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,42,43,44,45,47,48,49,50]. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence in each study in the meta-analysis.
HBV Prevalence among Vaccinated and Unvaccinated Children
HBV prevalence was still high in studies of unvaccinated children [6% (95%, CI: 4–8%)] (Figure 9A) compared to studies of vaccinated children [2% (95%, CI: 1–3%)] (Figure 9B). Due to a lack vaccination status data in most studies, HBV prevalence caused by natural infection (anti-HBs + anti-HBc) could not be evaluated.
Figure 9.
(A) HBV Prevalence Among Unvaccinated and (B) Vaccinated Children [24,25,26,27,28,29,30,31,32,33,34,36,37,38,39,40,41,43,45,46,47,49]. A black square represents the HBV prevalence in the forest plot. The square position represents the prevalence in each study in the meta-analysis.
4. Discussion
A systematic review of HBV prevalence in children in West Africa aged 0–16 years yielded 27 studies meeting inclusion criteria for a meta-analysis. The studies, however, only included data from seven of West Africa’s fourteen countries. Most studies were cross-sectional; three-quarters used the Enzyme-Linked Immunosorbent Assay (ELISA) as the HBV diagnostic tool, and most were conducted in a hospital setting (18/27) in an urban area. Indeed, most healthcare facilities in West Africa are in urban areas.
Overall, HBV prevalence was 5%, based on the random analysis, given the heterogeneity of the studies. This meta-analysis revealed that HBV prevalence in children in West Africa is moderate, according to the WHO’s criteria for HBV endemicity [51]. HBV prevalence by country ranged from 1% in Togo to 10% in Benin. The prevalence was also variable inside the same country, such as in Nigeria or Ghana, for which there are a high number of published data available. Differences in health system resources across West African countries, although already generally limited in all of West Africa, may still explain some of the discrepancies. In fact, some countries such as Mali and Benin lack resources to screen pregnant women or provide vaccine doses at birth for newborns. Findings from a study with timely dose of hepatitis B Birth dose (HepB-BD) in Africa demonstrated the variability of birth dose implementation and the challenges countries face in immunizing babies [52]. In the absence of efforts for preventing mother-to-child transmission, it is anticipated that HBV prevalence in children in these countries would stay higher and fall short of the WHO targets.
Due to the significant burden of HBV-related diseases in SSA, where >8% of the general population is chronically infected, HBV infection is still ubiquitous across West Africa. This can impact HBV prevalence in these countries [53] and the prevalence is around 5% among pregnant HBV/HIV co-infected women [54]. There has been a significant decrease in the prevalence of HBsAg among children in West Africa since the year 2000. This is clearly demonstrated by comparing the 12% HBsAg prevalence in children and adolescents < 19 years old in West African SSA in 1990 [51], and the prevalence of HBV infection in children at 2.53% in 2019, amounting to 360,000 infected children each year [55]. We believe that the decrease may be related to the introduction of infant HBV vaccination programs in most African countries and including the HepB-BD in a few countries.
Studies conducted in hospital settings showed higher prevalence of HBV infection (7%) and this is likely related to the studies being conducted on sick children, who are also tested at higher rates than non-hospitalized children. By contrast, healthy children in the community setting had an HBV prevalence of only 3% and may represent early success of the newborn HepB vaccination programs. Prevalence varied from 3% to 9% among infants with HBV/HIV co-infection, those born to mothers who tested positive for HBsAg, and those who had other risk factors, such as having undergone surgery, scarification, or not receiving vaccinations. Other risk factors were not available in many studies. In one Nigerian study, risk factors such as previous history of jaundice (p = 0.26), blood transfusion (p = 0.24), past history of surgery (p = 0.47), or scarification marks (p = 0.17) were not associated with HBV prevalence [26].
HIV and HBV infections have many similarities, such as common routes of transmission, high prevalence in certain geographical regions, same at-risk groups, and the risk of mother-to-child transmission. All these factors contribute to a significant association between HBV and HIV co-infection in the pediatric population [56,57]. We found an overall HIV/HBV co-infected prevalence of 9%. However, in a sensitivity analysis, we removed one study from Burkina Faso (2001) that reported a co-infection rate of 40%, and yielded a prevalence of 6%, ranging from 1.15% in Nigeria (2021) to 10% in Benin (2015) (Figure 3). The finding from this study is consistent with prior studies. In studies from South Africa, HIV/HBV co-infection prevalence in children ranged from 5–17%, with the higher prevalence occurring in the industrialized towns where mining activity is associated with increased sexual activity. In Nigerian studies, HBV/HIV co-infection prevalence varied by geopolitical region, ranging from 5.8 to 19% [13,29,38,40,58]. Finally, a Tanzanian study of HBV/HIV co-infected children reported 1.2% prevalence [59]. Reported HBV/HIV co-infection prevalence in children has been somewhat higher in Nigeria (7.8%) [29], 10.4% in Zambia [60], and 12.1% in Ivory Coast [61]. The generational effect benefits of HepB vaccination may be a factor in the relatively lower HBV-HIV co-infection in later studies compared to earlier studies. It is also important to note that HIV/HBV co-infection considerably increases the risk of mother-to-child transmission, if the mother is infected and untreated.
Perinatal and childhood acquisition of HBV not only leads to increased risk of chronicity but also strongly predict worse long-term outcomes for liver cirrhosis and hepatocellular carcinoma [16]. HIV/HBV co-infection in childhood further places children at high risk for associated morbidity and mortality, similar to adults, hence the urgent need to implement HepB vaccination at birth, routine screening, and follow-up. In fact, despite more than two decades since the introduction of HepB vaccination programs, the overall prevalence of HBV infection still remains high in many settings in SSA [53,62,63].
The difference in HBV prevalence infection between vaccinated and unvaccinated children is still substantial (2% versus 6%). While there is a residual possibility of HBV infection despite newborn HepB vaccination, often, it is unclear if infection occurred before or after completion of the HepB vaccine series [41]. In SSA, horizontal transmission in children, aged 6 months to 5 years, is common due to close interactions with infected household contacts and playmates. We examined the impact of HepB newborn vaccination compared to the evolution of the epidemic in each country. In Nigeria, overall HBV prevalence was 7% and a case-control study found that HBsAg prevalence was significantly lower among vaccinated (1.4%) compared with unvaccinated (4.8%) children [49]. In Senegal and Ghana, where all the studies were conducted after newborn HepB vaccination had been introduced, overall HBV prevalence was only 2%. In Togo, one in ten women of childbearing age was infected with HBV, but less than 2% of infants under five years of age who received the HepB vaccine at birth were infected with HBV. These results are similar to other studies that found significant reductions in HBsAg positivity post-vaccination with a protective efficacy of between 67–94% [64,65]. Such a high level of vaccine efficacy is likely to positively impact and prevent community transmission of HBV.
In 1992, the World Health Assembly adopted a resolution recommending the introduction of HepB into national immunization programs [66]. In 2016, the WHO and the World Health Assembly established the target of controlling and eliminating HBV worldwide by 2030. The WHO recommends that all infants receive their first dose of HepB as soon as possible after birth, ideally within 24 h, followed by two or three doses of HepB at least 4 weeks apart to complete the vaccination series [67]. Despite this recommendation, HepB vaccination at birth has not been widely implemented in most national vaccination programs in SSA, particularly in West Africa, despite the Expanded Program of Immunization (EPI) [68]. Indeed, in most West African countries, parents must still pay for the first HepB dose, which, at ~$US 8 per dose, is prohibitive, given that 85% of the population in SSA live on less than $5.50/day [69]. In 2021, coverage with timely HepB-BD was 42% globally and only 17% for infants in the WHO African region [70]. A total of 114 countries worldwide had introduced HepB-BD in their routine immunization schedule [71]. Yet, this number includes only 14 (30%) of 47 countries in the WHO African region [72]. Limited countries have data on Studies of the Effectiveness of HepB-BD Vaccination in Africa with only two studies published to date. In 2001–2002, Ekra and colleagues conducted a nonrandomized controlled trial in four health centers in Abidjan, Cote d’Ivoire [73] and a second effectiveness study was conducted from 2009–2016 in a single center in Tokombéré district, Cameroon [74]. Based on the results of those published studies, the authors assumed a high-level transmission rate in the absence of vaccination and highlighted the benefit of the addition of HepB-BD to the three-dose HepB vaccination schedule for infants. In addition, a residual risk of mother-to-child transmission of hepatitis B virus infection despite timely birth-dose vaccination in Cameroon has been reported. In addition, studies from Hawaii, Taiwan, and China provide assurance that routine infant HepB immunization beginning with HepB-BD vaccination is a highly effective public health strategy that will progressively protect generations to come from HBV-related liver disease, HCC, and premature mortality [75,76].
The universal vaccination strategy implemented in Thailand provides evidence of the effect of newborn HepB vaccination (HepB-BD) in eliminating HBV infection [77]. As more African countries seek to implement HepB-BD, attention to disparities in implementation need to be addressed particularly in rural and underprivileged settings [63]. Maternal education and community engagement are essential to scale up HepB-BD in SSA through the Global Alliance for Vaccines and Immunisation (GAVI). A study in Nigeria demonstrated that missed doses were largely avoided when staff completed a vaccine checklist before releasing mother–child pairs [78]. There must also be a focus on offsetting the costs of distributing the HepB-BD throughout SSA.
In endemic regions of Africa and Asia, in-utero infection of the fetus, vertical transmission, constitutes the main mode of HBV transmission [79]. A meta-analysis indicated that maternal viral load was an important risk factor for mother-to-child transmission (MTCT) in HBeAg-positive mothers, and maternal viral load was dose-dependent with HBV MTCT incidence [80]. Other studies have shown that a high viral load in HBsAg-positive mothers can lead to vaccination failure in the newborn, even if combined immunoglobin treatment and vaccination at birth are delivered [81].
HBsAg screening for all pregnant women is critical. Focusing on HBsAg positive women and providing prophylactic treatment to women with high viral loads can be an effective approach to reduce transmission to the infant [82]. A Senegalese study of HBV in children born to HIV-positive mothers showed a low rate of HBsAg (2.6%) if the mother was treated with lamivudine (3TC) or tenofovir (TDF), compared to 7.9% if the mother was untreated [46]. Another Senegalese study showed low HBV prevalence, but only 56% of children had a sero-protective level >10 UI/L [34]. It is, therefore, strongly recommended to vaccinate children and to adhere to the necessary doses to protect them against HBV infection.
Limitations
This meta-analysis has several limitations, particularly the substantial heterogeneity of eligible studies. Prior HBV prevalence meta-analyses in Africa have also had high levels of heterogeneity [83,84]. Data about children, aged 0–16 years, from four general population studies were used and were the only data available from several countries. However, it was challenging to analyze risk factors for children separately from adults in these studies. The quality of the studies varied between countries, as well as within the same country. Studies showed that protective levels of HbsAb antibodies decrease with age after vaccine introduction [30,43]; however, most of the studies included in this review did not report prevalence by age group.
5. Conclusions
In conclusion, this study using the most recent data available estimated HBV prevalence in children aged 0–16 years in West Africa, revealing a decrease in prevalence over the past two decades; yet, a persistently high prevalence in high-risk child populations still exists. The studies are robust and cover periods before and after the introduction of HepB vaccination in different West African countries. The meta-analysis identified wide variation in HBV prevalence, depending on the country, as well as in subgroups of HBV/HIV co-infected children, children born to HBsAg-positive mothers, and vaccinated or unvaccinated children. Overall, pooled prevalence and pooled subgroup prevalence remain moderate in West Africa and reaffirm the likely impact of newborn HepB vaccination. The study reinforces the need to implement HepB-BD and screening and prophylaxis of HBV in pregnant women. These interventions are essential to prevent mother-to-child transmission in order to achieve the WHO goal of targeted HBV elimination.
Acknowledgments
The authors are grateful to Northwestern University’s Institute for Global Health program, the National Institutes of Health and the HBNU Consortium, the Agence Nationale de la Recherche sur le SIDA et les Maladies Infectieuses Emergentes (ANRS MIE) for her support through the Laboratory of virology Saint-Antoine Hospital in Paris, France and the University of Sciences, Techniques and Technologies of Bamako (USTTB), Mali.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijerph20054142/s1. Supplementary Data S1 HBV Prevalence in Children, 0–16 Years Old, in West Africa; Supplementary Data S2 Nine studies included persons living with HIV (PLWH); Supplementary Data S3 Before and after sensititve analysis.
Author Contributions
Conceptualization, D.B.F.; methodology, D.B.F., M.I.K. and A.M.S.; software, D.B.F., M.I.K. and Y.C.; validation, D.B.F., A.M.S. and J.L.H.; formal analysis, D.B.F.; investigation, D.B.F., A.M.S. and B.D.; data curation, D.B.F., A.M.S. and M.I.K.; writing—original draft preparation, D.B.F., A.M.S., M.I.K., J.L.H., R.L.M. and C.A.H.; writing—review and editing, D.B.F., A.M.S., M.M., M.I.K., B.D., Y.C., S.M.M., C.A.H., A.I.M., M.S., J.G., M.H.E.-S., L.M.-J., R.L.M., M.D. and J.L.H.; visualization, D.B.F., A.M.S., M.M., M.I.K., B.D., Y.C., S.M.M., C.A.H., A.I.M., M.S., J.G., M.H.E.-S., L.M.-J., R.L.M., M.D. and J.L.H.; supervision, J.L.H., S.M.M., M.M., A.I.M., M.S., J.G., L.M.-J., M.H.E.-S. and M.D.; project administration, D.B.F.; funding acquisition, D.B.F. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Fogarty International Center, grant number: K43TW011957. Fogarty International Center (D43CA260658, D43TW010350, D43TW010543), and ANRS-MIE22295, The content is solely the responsibility of the authors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.World Health Organization . Global Progress Report on HIV, Viral Hepatitis and Sexually Transmitted Infections, 2021: Accountability for the Global Health Sector Strategies 2016–2021: Actions for Impact: Web Annex 2: Data Methods. WHO; Geneva, Switzerland: 2021. [Google Scholar]
- 2.Edmunds W.J., Medley G.F., Nokes D.J., Hall A.J., Whittle H.C. The Influence of Age on the Development of the Hepatitis B Carrier State. Proc. Biol. Sci. 1993;253:197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 3.Hussein M.K., Dorothy N., Ponsiano O., Ali K., Abdul W., Hakim S. Prevalence and Predictors of Hepatitis B Virus (HBV) Infection in East Africa: Evidence from a Systematic Review and Meta-Analysis of Epidemiological Studies Published from 2005 to 2020. Arch. Public Health. 2021;79:167. doi: 10.1186/s13690-021-00686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumberger S. Master’s Thesis. UiT Norges Arktiske Universitet; Tromsø, Norway: 2016. Prevention of Mother to Child Transmission of Hepatitis B: A Global Challenge. [Google Scholar]
- 5.Razavi-Shearer D., Gamkrelidze I., Nguyen M.H., Chen D.-S., Van Damme P., Abbas Z., Abdulla M., Abou Rached A., Adda D., Aho I., et al. Global Prevalence, Treatment, and Prevention of Hepatitis B Virus Infection in 2016: A Modelling Study. Lancet Gastroenterol. Hepatol. 2018;3:383–403. doi: 10.1016/S2468-1253(18)30056-6. [DOI] [PubMed] [Google Scholar]
- 6.Platt L., French C.E., McGowan C.R., Sabin K., Gower E., Trickey A., McDonald B., Ong J., Stone J., Easterbrook P., et al. Prevalence and Burden of HBV Co-Infection among People Living with HIV: A Global Systematic Review and Meta-Analysis. J. Viral Hepat. 2020;27:294–315. doi: 10.1111/jvh.13217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffie P.A., Patassi A., Doumbia A., Bado G., Messou E., Minga A., Allah-Kouadio E., Zannou D.M., Seydi M., Kakou A.R., et al. Changes in Viral Hepatitis B Screening Practices over Time in West African HIV Clinics. Med. Mal. Infect. 2017;47:394–400. doi: 10.1016/j.medmal.2017.04.011. [DOI] [PubMed] [Google Scholar]
- 8.Zampino R., Boemio A., Sagnelli C., Alessio L., Adinolfi L.E., Sagnelli E., Coppola N. Hepatitis B Virus Burden in Developing Countries. World J. Gastroenterol. 2015;21:11941–11953. doi: 10.3748/wjg.v21.i42.11941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.HIV/AIDS (UNAIDS), J.U.N.P. on Global HIV & AIDS Statistics-2019 Fact Sheet, 2019. [(accessed on 28 December 2022)]. Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf.
- 10.McMahon B.J. The Natural History of Chronic Hepatitis B Virus Infection. Hepatology. 2009;49:S45–S55. doi: 10.1002/hep.22898. [DOI] [PubMed] [Google Scholar]
- 11.Shimakawa Y., Yan H.-J., Tsuchiya N., Bottomley C., Hall A.J. Association of Early Age at Establishment of Chronic Hepatitis B Infection with Persistent Viral Replication, Liver Cirrhosis and Hepatocellular Carcinoma: A Systematic Review. PLoS ONE. 2013;8:e69430. doi: 10.1371/journal.pone.0069430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larouze B., Saimot G., Lustbader E.D., London W.T., Werner B.G., Payet M., Blumberg B.S. Host Responses to Hepatitis-B Infection in Patients with Primary Hepatic Carcinoma and Their Families: A Case/Control Study in Senegal, West Africa. Lancet. 1976;308:534–538. doi: 10.1016/S0140-6736(76)91792-X. [DOI] [PubMed] [Google Scholar]
- 13.Sadoh A.E., Sadoh W.E., Iduoriyekemwen N.J. HIV Co-Infection with Hepatitis B and C Viruses among Nigerian Children in an Antiretroviral Treatment Programme. S. Afr. J. Child Health. 2011;5:7–10. [Google Scholar]
- 14.Wen W.-H., Lai M.-W., Chang M.-H. A Review of Strategies to Prevent Mother-to-Infant Transmission of Hepatitis B Virus Infection. Expert Rev. Gastroenterol. Hepatol. 2016;10:317–330. doi: 10.1586/17474124.2016.1120667. [DOI] [PubMed] [Google Scholar]
- 15.Beasley R.P., Trepo C., Stevens C.E., Szmuness W. The e Antigen and Vertical Transmission of Hepatitis B Surface Antigen. Am. J. Epidemiol. 1977;105:94–98. doi: 10.1093/oxfordjournals.aje.a112370. [DOI] [PubMed] [Google Scholar]
- 16.Kao J.-H. Hepatitis B Vaccination and Prevention of Hepatocellular Carcinoma. Best Pract. Res. Clin. Gastroenterol. 2015;29:907–917. doi: 10.1016/j.bpg.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 17.Hepatitis B Vaccination|CDC. [(accessed on 4 December 2022)]; Available online: https://www.cdc.gov/vaccines/vpd/hepb/index.html.
- 18.Covidence—Better Systematic Review Management. [(accessed on 4 December 2022)]. Available online: https://www.covidence.org/
- 19.World Population to Reach 8 Billion This Year, as Growth Rate Slows. [(accessed on 28 December 2022)]. Available online: https://news.un.org/en/story/2022/07/1122272.
- 20.Abesig J., Chen Y., Wang H., Sompo F.M., Wu I.X.Y. Prevalence of Viral Hepatitis B in Ghana between 2015 and 2019: A Systematic Review and Meta-Analysis. PLoS ONE. 2020;15:e0234348. doi: 10.1371/journal.pone.0234348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Proportion Meta-Analysis—StatsDirect. [(accessed on 4 December 2022)]. Available online: https://www.statsdirect.com/help/default.htm#meta_analysis/proportion.htm.
- 22.Higgins J.P.T., Thompson S.G., Deeks J.J., Altman D.G. Measuring Inconsistency in Meta-Analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Egger M., Davey Smith G., Schneider M., Minder C. Bias in Meta-Analysis Detected by a Simple, Graphical Test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adoga M.P., Gyar S.D., Pechulano S., Bashayi O.D., Emiasegen S.E., Zungwe T., Iperepolu O.H., Agupugo C., Agwale S.M. Hepatitis B Virus Infections in Apparently Healthy Urban Nigerians: Data from Pre-Vaccination Tests. J. Infect Dev. Ctries. 2010;4:397–400. doi: 10.3855/jidc.591. [DOI] [PubMed] [Google Scholar]
- 25.Ouattara A., Assi C., Soro D., Allah-Kouadio E., Lohouès-Kouacou M.J., Camara B.M. Seroprevalence of Viral Hepatitis Markers B in Secondary School in Abidjan: Advocacy for a Catch-up Vaccination. Open J. Gastroenterol. 2019;9:7. doi: 10.4236/ojgas.2019.91002. [DOI] [Google Scholar]
- 26.Omeje K.N., Ibekwe R.C., Ojukwu J.O., Una A.F., Ibe B.C. Risk Factors for Hepatitis B Surface Antigenaemia among Secondary School Students in Abakaliki, South Eastern Nigeria. Niger. J. Paediatr. 2017;44:14–21. doi: 10.4314/njp.v44i1.3. [DOI] [Google Scholar]
- 27.Bukbuk D.N., Denue B.A., Ngoshe I., Dawurung J., Oderinde S. Hepatitis B Surface Antigenaemia among High Risk Groups in Northeastern Nigeria. Niger. Med. Pract. 2016;69:77–82. doi: 10.4314/nmp.v69i6. [DOI] [Google Scholar]
- 28.Adeoye O.A., Oniyangi O., Ojuawo I.A. Prevalence and Risk Factors of Hepatitis b Infection in HIV Infected Children Seen at National Hospital Abuja. Niger. J. Paediatr. 2021;48:62–65. doi: 10.4314/njp.v48i2.1. [DOI] [Google Scholar]
- 29.Anigilaje E.A., Olutola A. Prevalence and Clinical and Immunoviralogical Profile of Human Immunodeficiency Virus-Hepatitis B Coinfection among Children in an Antiretroviral Therapy Programme in Benue State, Nigeria. Hindawi. 2013;2013:7. doi: 10.1155/2013/932697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barro M., Valea D., Ouermi S.A., Sessouma S., Sanogo B., Ouattara I.A.B., Ouedraogo A.S., Nacro B., Moyen G. Serological Profile of Hepatitis B in Children after the Introduction of Its Vaccination in Burkina Faso. Pediatr. Rep. 2019;11:75–77. doi: 10.4081/pr.2019.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.d’Almeida M., Adedemy J.D., Agossou J., Noudamadjo A., Agossou C., Agossou R., Koumakpai-Adeothy S. Frequency of HIV and Viral Hepatitis B Co-Infection in Children Aged 1 to 15 Years Attended in a Hospital Environment in Parakou (Benin) Curr. Pediatr. Res. 2015;19:81–89. [Google Scholar]
- 32.Ezeilo M.C., Engwa G.A., Iroha R.I., Odimegwu D.C. Seroprevalence and Associated Risk Factors of Hepatitis B Virus Infection Among Children in Enugu Metropolis. Virol. Res. Treat. 2018;9:1–7. doi: 10.1177/1178122X18792859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ikpeme E.E., Etukudo O.M., Ekrikpo U.E. Seroprevalence of HBV and HIV Co-Infection in Children and Outcomes Following Highly Active Antiretroviral Therapy (HAART) in Uyo, South-South Nigeria. Afr. Health Sci. 2013;13:955–961. doi: 10.4314/ahs.v13i4.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lô G., Sow-Sall A., Diop-Ndiaye H., Babacar N., Diouf N.N., Daffé S.M., Ndao B., Thiam M., Mbow M., Soumboundou M.B., et al. Hepatitis B Virus (HBV) Infection amongst Children in Senegal: Current Prevalence and Seroprotection Level. Pan. Afr. Med. J. 2019;32:140. doi: 10.11604/pamj.2019.32.140.14485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alikor E.A., Erharbor O.N. Seroprevalences of Hepatitus B Surface Antigenaemia in Children in a Tertiary Health Institution in the Niger Delta of Nigeria. Niger. J. Med. 2007;16:326–329. doi: 10.4314/njm.v16i4.37331. [DOI] [PubMed] [Google Scholar]
- 36.Ekouevi D.K., Larrouy L., Gbeasor-Komlanvi F.A., Mackiewicz V., Tchankoni M.K., Bitty-Anderson A.M., Gnatou G.Y.-S., Sadio A., Salou M., Dagnra C.A., et al. Prevalence of Hepatitis B among Childbearing Women and Infant Born to HBV-Positive Mothers in Togo. BMC Infect. Dis. 2020;20:839. doi: 10.1186/s12879-020-05574-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nacro B., Dao B., Dahourou H. HBs Antigen in Children with Suspicion of HIV Infection. J. Trop. Pediatr. 2001;47:303–304. doi: 10.1093/tropej/47.5.303. [DOI] [PubMed] [Google Scholar]
- 38.Nwolisa E., Mbanefo F., Ezeogu J., Amadi P. Prevalence of Hepatitis B Co-Infection amongst HIV Infected Children Attending a Care and Treatment Centre in Owerri, South-Eastern Nigeria. Pan. Afr. Med. J. 2013;14:89. doi: 10.11604/pamj.2013.14.89.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyé R.M., Cohen D., Pujol F.H., Sow-Sall A., Lô G., Hoshino K., Mizokami M., Zoulim F., Lemoine M., Touré-Kane C., et al. Hepatitis B Virus Genotype Study in West Africa Reveals an Expanding Clade of Subgenotype A4. Microorganisms. 2021;9:623. doi: 10.3390/microorganisms9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ashir G.M., Rabasa A.I., Gofama M.M., Bukbuk D., Abubakar H., Farouk G.A. Study of Hepatic Functions and Prevalence of Hepatitis B Surface Antigenaemia in Nigerian Children with Human Immunodeficiency Virus Infection. Niger. J. Med. 2009;18:260–262. doi: 10.4314/njm.v18i3.51171. [DOI] [PubMed] [Google Scholar]
- 41.Apiung T., Ndanu T.A., Mingle J.A., Sagoe K.W. Hepatitis B Virus Surface Antigen and Antibody Markers in Children at a Major Paediatric Hospital after the Pentavalent DTP-HBV-Hib Vaccination. Ghana Med. J. 2017;51:13–19. doi: 10.4314/gmj.v51i1.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sadoh A.E., Ofili A. Hepatitis B Infection among Nigerian Children Admitted to a Children’s Emergency Room. Afr. Health Sci. 2014;14:377–383. doi: 10.4314/ahs.v14i2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hagan O.C.K., Nsiah P., Obiri-Yeboah D., Yirdong F., Annan I., Eliason S., Nuvor S.V. Impact of Universal Childhood Vaccination against Hepatitis B in Ghana: A Pilot Study. J. Public Health Afr. 2018;9:721. doi: 10.4081/jphia.2018.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ba A., Ndiaye F.K., Djeng Y.J., Cames C., Diack A., N’diaye O. Impact of Highly Active Antiretroviral Therapy on Chronic Hepatitis B Serological Markers among Senegalese HIV Co-Infected Children. Int. J. MCH AIDS. 2019;8:131–137. doi: 10.21106/ijma.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quaye T., Narkwa P.W., Domfeh S.A., Kattah G., Mutocheluh M. Immunosurveillance and Molecular Detection of Hepatitis B Virus Infection amongst Vaccinated Children in the West Gonja District in Savanna Region of Ghana. PLoS ONE. 2021;16:e0257103. doi: 10.1371/journal.pone.0257103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gueye S.B., Diop-Ndiaye H., Lo G., Mintsa S., Guindo I., Dia A., Sow-Sall A., Gaye-Diallo A., Mboup S., Touré-Kane C. HBV Carriage in Children Born from HIV-Seropositive Mothers in Senegal: The Need of Birth-Dose HBV Vaccination. J. Med. Virol. 2016;88:815–819. doi: 10.1002/jmv.24409. [DOI] [PubMed] [Google Scholar]
- 47.Sanou A.M., Ilboudo A.K., Meda C.Z., Togozia A., Coulibaly A., Cisse A., Sagna T., Kania D., Tarnagda Z. Hepatitis B Vaccination in Burkina Faso: Prevalence of HBsAg Carriage and Immune Response in Children in the Western Region. J. Infect. Dev. Ctries. 2018;12:1002–1008. doi: 10.3855/jidc.10433. [DOI] [PubMed] [Google Scholar]
- 48.Périères L., Protopopescu C., Lo G., Marcellin F., Ba E.H., Coste M., Touré Kane C., Diallo A., Sokhna C., Boyer S., et al. Sibling Status, Home Birth, Tattoos and Stitches Are Risk Factors for Chronic Hepatitis B Virus Infection in Senegalese Children: A Cross-Sectional Survey. J. Viral. Hepat. 2021;28:1515–1525. doi: 10.1111/jvh.13589. [DOI] [PubMed] [Google Scholar]
- 49.Odusanya O.O., Alufohai F.E., Meurice F.P., Wellens R., Weil J., Ahonkhai V.I. Prevalence of Hepatitis B Surface Antigen in Vaccinated Children and Controls in Rural Nigeria. Int. J. Infect. Dis. 2005;9:139–143. doi: 10.1016/j.ijid.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 50.Ikobah J., Okpara H., Elemi I., Ogarepe Y., Udoh E., Ekanem E. The Prevalence of Hepatitis B Virus Infection in Nigerian Children Prior to Vaccine Introduction into the National Programme on Immunization Schedule. Pan. Afr. Med. J. 2016;23:128. doi: 10.11604/pamj.2016.23.128.8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ott J.J., Stevens G.A., Groeger J., Wiersma S.T. Global Epidemiology of Hepatitis B Virus Infection: New Estimates of Age-Specific HBsAg Seroprevalence and Endemicity. Vaccine. 2012;30:2212–2219. doi: 10.1016/j.vaccine.2011.12.116. [DOI] [PubMed] [Google Scholar]
- 52.Moturi E., Tevi-Benissan C., Hagan J.E., Shendale S., Mayenga D., Murokora D., Patel M., Hennessey K., Mihigo R. Implementing a Birth Dose of Hepatitis B Vaccine in Africa: Findings from Assessments in 5 Countries. J. Immunol. Sci. 2018;5:31–40. doi: 10.29245/2578-3009/2018/si.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schweitzer A., Horn J., Mikolajczyk R.T., Krause G., Ott J.J. Estimations of Worldwide Prevalence of Chronic Hepatitis B Virus Infection: A Systematic Review of Data Published between 1965 and 2013. Lancet. 2015;386:1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 54.Kafeero H.M., Ndagire D., Ocama P., Walusansa A., Sendagire H. Sero-Prevalence of Human Immunodeficiency Virus-Hepatitis B Virus (HIV-HBV) Co-Infection among Pregnant Women Attending Antenatal Care (ANC) in Sub-Saharan Africa (SSA) and the Associated Risk Factors: A Systematic Review and Meta-Analysis. Virol. J. 2020;17:170. doi: 10.1186/s12985-020-01443-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.World Health Organization . World Health Statistics 2021. World Health Organization; Geneva, Switzerland: 2021. [Google Scholar]
- 56.Gupta S., Singh S. Hepatitis B and C Virus Co-Infections in Human Immunodeficiency Virus Positive North Indian Patients. World J. Gastroenterol. 2006;12:6879–6883. doi: 10.3748/wjg.v12.i42.6879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mustapha S.K., Jibrin Y.B. The Prevalence of Hepatitis B Surface Antigenaemia in Patients with Human Immunodeficiency Virus (HIV) Infection in Gombe, Nigeria. Ann. Afr. Med. 2004;3:10–12. [Google Scholar]
- 58.Otegbayo J.A., Taiwo B.O., Akingbola T.S., Odaibo G.N., Adedapo K.S., Penugonda S., Adewole I.F., Olaleye D.O., Murphy R., Kanki P. Prevalence of Hepatitis B and C Seropositivity in a Nigerian Cohort of HIV-Infected Patients. Ann. Hepatol. 2008;7:152–156. doi: 10.1016/S1665-2681(19)31872-1. [DOI] [PubMed] [Google Scholar]
- 59.Telatela S.P., Matee M.I., Munubhi E.K. Seroprevalence of Hepatitis B and C Viral Co-Infections among Children Infected with Human Immunodeficiency Virus Attending the Paediatric HIV Care and Treatment Center at Muhimbili National Hospital in Dar-Es-Salaam, Tanzania. BMC Public Health. 2007;7:338. doi: 10.1186/1471-2458-7-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Peebles K., Nchimba L., Chilengi R., Bolton Moore C., Mubiana-Mbewe M., Vinikoor M.J. Pediatric HIV–HBV Coinfection in Lusaka, Zambia: Prevalence and Short-Term Treatment Outcomes. J. Trop. Pediatr. 2015;61:464–467. doi: 10.1093/tropej/fmv058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rouet F., Chaix M.-L., Inwoley A., Anaky M.-F., Fassinou P., Kpozehouen A., Rouzioux C., Blanche S., Msellati P., Programme Enfant Yopougon (Agence Nationale de Recherches sur le SIDA et les Hépatites Virales B et C 1244/1278) Frequent Occurrence of Chronic Hepatitis B Virus Infection among West African HIV Type-1-Infected Children. Clin. Infect. Dis. 2008;46:361–366. doi: 10.1086/525531. [DOI] [PubMed] [Google Scholar]
- 62.Ott J.J., Horn J., Krause G., Mikolajczyk R.T. Time Trends of Chronic HBV Infection over Prior Decades—A Global Analysis. J. Hepatol. 2017;66:48–54. doi: 10.1016/j.jhep.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 63.Boisson A., Goel V., Yotebieng M., Parr J.B., Fried B., Thompson P. Implementation Approaches for Introducing and Overcoming Barriers to Hepatitis B Birth-Dose Vaccine in Sub-Saharan Africa. Glob. Health Sci Pract. 2022;10:e2100277. doi: 10.9745/GHSP-D-21-00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Whittle H., Jaffar S., Wansbrough M., Mendy M., Dumpis U., Collinson A., Hall A. Observational Study of Vaccine Efficacy 14 Years after Trial of Hepatitis B Vaccination in Gambian Children. BMJ. 2002;325:569. doi: 10.1136/bmj.325.7364.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Reda A.A., Arafa M.A., Youssry A.A., Wandan E.H., Ab de Ati M., Daebees H. Epidemiologic Evaluation of the Immunity against Hepatitis B in Alexandria, Egypt. Eur. J. Epidemiol. 2003;18:1007–1011. doi: 10.1023/A:1025805817101. [DOI] [PubMed] [Google Scholar]
- 66.Sellier P.O., Maylin S., Berçot B., Chopin D., Lopes A., Simoneau G., Evans J., Delcey V., Bénifla J.-L., Simon F. Prospective Interventional Study of Tenofovir in Pregnancy to Prevent Vertical Transmission of Hepatitis B in Highly Viremic Women. Eur. J. Gastroenterol. Hepatol. 2017;29:259–263. doi: 10.1097/MEG.0000000000000793. [DOI] [PubMed] [Google Scholar]
- 67.World Health Organization Hepatitis B Vaccines: WHO Position Paper—July 2017. Wkly. Epidemiol. Rec. 2017;92:369–392. [Google Scholar]
- 68.Howell J., Lemoine M., Thursz M. Prevention of Materno-Foetal Transmission of Hepatitis B in Sub-Saharan Africa: The Evidence, Current Practice and Future Challenges. J. Viral. Hepat. 2014;21:381–396. doi: 10.1111/jvh.12263. [DOI] [PubMed] [Google Scholar]
- 69.85% of Africans Live on Less than $5.50 per Day. [(accessed on 4 December 2022)]. Available online: https://blogs.worldbank.org/opendata/85-africans-live-less-550-day.
- 70.Immunization Coverage. [(accessed on 17 February 2023)]. Available online: https://www.who.int/news-room/fact-sheets/detail/immunization-coverage.
- 71.Muhoza P. Routine Vaccination Coverage—Worldwide, 2020. MMWR Morb. Mortal. Wkly. Rep. 2021;70:1495. doi: 10.15585/mmwr.mm7043a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Introduction of HepB Birth Dose. [(accessed on 17 February 2023)]. Available online: https://immunizationdata.who.int/pages/vaccine-intro-by-antigen/hepb_bd.html?ISO_3_CODE=&YEAR=
- 73.Ekra D., Herbinger K.H., Konate S., Leblond A., Fretz C., Cilote V., Douai C., Da Silva A., Gessner B.D., Chauvin P. A Non-Randomized Vaccine Effectiveness Trial of Accelerated Infant Hepatitis B Immunization Schedules with a First Dose at Birth or Age 6 Weeks in Côte d’Ivoire. Vaccine. 2008;26:2753–2761. doi: 10.1016/j.vaccine.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 74.Shimakawa Y., Veillon P., Birguel J., Pivert A., Sauvage V., Guillou-Guillemette H.L., Roger S., Njouom R., Ducancelle A., Amta P., et al. Residual Risk of Mother-to-Child Transmission of Hepatitis B Virus Infection despite Timely Birth-Dose Vaccination in Cameroon (ANRS 12303): A Single-Centre, Longitudinal Observational Study. Lancet Glob Health. 2022;10:e521–e529. doi: 10.1016/S2214-109X(22)00026-2. [DOI] [PubMed] [Google Scholar]
- 75.Ni Y.-H., Chen D.-S. Hepatitis B Vaccination in Children: The Taiwan Experience. Pathol. Biol. 2010;58:296–300. doi: 10.1016/j.patbio.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 76.Liu Z., Li M., Hutton D.W., Wagner A.L., Yao Y., Zhu W., Cao L., Tang S., Pan J., Wang Y., et al. Impact of the National Hepatitis B Immunization Program in China: A Modeling Study. Infect. Dis. Poverty. 2022;11:106. doi: 10.1186/s40249-022-01032-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Posuwan N., Wanlapakorn N., Sa-nguanmoo P., Wasitthankasem R., Vichaiwattana P., Klinfueng S., Vuthitanachot V., Sae-lao S., Foonoi M., Fakthongyoo A., et al. The Success of a Universal Hepatitis B Immunization Program as Part of Thailand’s EPI after 22 Years’ Implementation. PLoS ONE. 2016;11:e0150499. doi: 10.1371/journal.pone.0150499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Charles R., Vallée J., Tissot C., Lucht F., Botelho-Nevers E. Vaccination Errors in General Practice: Creation of a Preventive Checklist Based on a Multimodal Analysis of Declared Errors. Fam. Pract. 2016;33:432–438. doi: 10.1093/fampra/cmw026. [DOI] [PubMed] [Google Scholar]
- 79.Elsheikh R.M., Daak A.A., Elsheikh M.A., Karsany M.S., Adam I. Hepatitis B Virus and Hepatitis C Virus in Pregnant Sudanese Women. Virol. J. 2007;4:104. doi: 10.1186/1743-422X-4-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen H.-L., Zha M.-L., Cai J.-Y., Qin G. Maternal Viral Load and Hepatitis B Virus Mother-to-Child Transmission Risk: A Systematic Review and Meta-Analysis. Hepatol. Res. 2018;48:788–801. doi: 10.1111/hepr.13072. [DOI] [PubMed] [Google Scholar]
- 81.Akinbodewa A.A., Gbadegesin B.A., Adejumo O.A., Ahmed S.D., Uwameiye O., Dada S.A., Okunola O., Osho P.O. A Multicentre Study of Awareness and Practice of Vaccination Against Infectious Diseases Among Haemo-Dialysis Subjects in Nigeria. West Afr. J. Med. 2019;36:239–245. [PubMed] [Google Scholar]
- 82.Pan C.Q., Duan Z., Dai E., Zhang S., Han G., Wang Y., Zhang H., Zou H., Zhu B., Zhao W., et al. Tenofovir to Prevent Hepatitis B Transmission in Mothers with High Viral Load. N. Engl. J. Med. 2016;374:2324–2334. doi: 10.1056/NEJMoa1508660. [DOI] [PubMed] [Google Scholar]
- 83.Yazie T.D., Tebeje M.G. An Updated Systematic Review and Meta-Analysis of the Prevalence of Hepatitis B Virus in Ethiopia. BMC Infect. Dis. 2019;19:917. doi: 10.1186/s12879-019-4486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang H., Men P., Xiao Y., Gao P., Lv M., Yuan Q., Chen W., Bai S., Wu J. Hepatitis B Infection in the General Population of China: A Systematic Review and Meta-Analysis. BMC Infect. Dis. 2019;19:811. doi: 10.1186/s12879-019-4428-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding author.