Abstract
Objectives:
To investigate the influence of maternal physiological and psychological factors during pregnancy and after birth on infant and children’s sleep outcomes.
Methods:
Six databases were searched from inception to April 2021. Longitudinal studies that investigated the association of risk factors during and after pregnancy and children’s sleep-related outcomes were included. Hedge’s g and odds ratio were pooled as effect size with random-effects model, respectively.
Results:
A total of 32 papers were included. Both prenatal maternal alcohol use (OR = 1.85, 95% CI: 1.04, 3.28) and tobacco smoking (OR = 1.28, 95% CI: 1.01, 1.62) were associated with shorter child sleep duration. Pre- and postnatal maternal depression symptoms were associated with increased child sleep problems at six months of age (OR = 1.97, 95% CI: 1.19, 3.24, and 2.05, 95% CI: 1.37, 3.07, respectively). Pre- and postnatal maternal major depression disorder were associated with shorter sleep duration (Hedge’s g = −0.97, 95% CI: −1.57, −0.37) and lower sleep efficiency (Hedge’s g = −1.44, 95% CI: −1.93, −0.95). Prenatal anxiety had no impact on child sleep problems (OR = 1.34, 95% CI: 0.86, 2.10).
Conclusion:
Maternal pregnancy and obstetric factors, and psychological factors are potential risk factors of poor child sleep health. Future research is warranted to better understand the impact of these risk factors on long-term child sleep outcomes and their potential mediating mechanisms.
Keywords: Child, Sleep, Perinatal period, Risk factor, Systematic review and Meta-analysis
INTRODUCTION
Sleep is essential for maintaining human daily functioning and good health and wellbeing. It is estimated that poor sleep health affects 10–28.3% of infants and school-aged children 1,2. Poor sleep health is associated with disrupted glucose metabolism 3 and obesity in children 4. It also has negative effects on cognition, including decreased alertness and attention 5 and poor school performance in children 6. The high prevalence of poor sleep health in children and the wide negative consequences of poor sleep health on children’s physical and psychological well-being has made children’s sleep health a public concern. Identifying risk factors for children’s poor sleep health is warranted.
From the socioecological perspective, a wide array of individual-level factors, family and community-level factors, and more upstream social and societal factors contribute to sleep health 7,8. Among these factors, perinatal factors are of particular importance and warrant further investigation, since human sleep development starts from fetal life 9 and greatly changes during the first years of life 10. Maternal physiological factors, including pregnancy and obstetric factors (e.g., born with small for gestational age, and cerebral hemorrhage) 11, preterm birth 9, and maternal substance use during pregnancy 12, are associated with compromised child sleep. However, the relationship between pregnancy and obstetric factors and child sleep, together with its underlying mechanisms, remains an under-investigated area of study 9.
Regarding psychological factors, both prenatal 13 and postnatal maternal depressive symptoms 14 were correlated with child shorter sleep duration. Postnatal maternal depression can also predict increased infant night waking and problematic sleep patterns 15. However, prenatal and postnatal depression are highly correlated 16 and thus may have a potential mediating relationship. Yet, few studies have investigated both pre- and postnatal maternal depression and their implication in the prediction of child sleep. Other psychological factors such as maternal anxiety and stress may also play a role in child sleep health. A recent study found that maternal prenatal anxiety comorbid with depression were associated with shorter total sleep duration, longer settling time, and higher sleep problems in toddlers 17. Another study reported that maternal prenatal psychological stress was associated with parent-reported sleep problems and increasing variability in actigraphy-measured circadian power in toddlers 18. However, the relationship between maternal prenatal anxiety, stress, and child sleep remains under investigated.
Beyond influencing children’s macro sleep architecture (e.g., sleep duration and sleep efficiency) and subjective-reported sleep problems, physiological factors such as preterm birth 19 and psychological factors such as psychological stress during pregnancy 18 were also found to be associated with alterations in children’s micro sleep architecture. Sleep micro-architecture such as δ power and σ power can be quantified by power spectral analysis of the electroencephalogram and reflect nuanced deep sleep status and homeostatic regulation that cannot be captured by macro-architecture metrics 19. Thus, micro-architecture changes may indicate further changes within children’s sleep health.
Recently two systematic reviews found that family context 20 and paternal parenting factors 21 were associated with early childhood sleep health. Specifically, household chaos and poor marital relationships were associated with children’s sleep problems and sleep timing 20. Paternal postnatal depression and parenting stress were positively linked to children’s sleep problems, bedtime difficulties, and sleep consolidation, while more paternal involvement in children’s bedtime caring and interaction was associated with children’s higher sleep quality and sleep-wake self-regulation 21. To our knowledge, no systematic review has explored multiple domains of early risk factors of infant and children’s sleep health. This systematic review aimed to examine the relationship among a wide array of perinatal physiological and psychological risk factors before, during, and after birth and children’s sleep architecture as well as parent-reported sleep problems.
METHODS
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) 22 (see Figure 1).
Figure 1.
PRISMA flow diagram
Data sources and search strategy
Six databases including PubMed, EMBASE, Cochrane Library, CINAHL, Scopus, and APA PsychInfo were searched from inception to April 2021. The search strategy for PubMed was ((((((child[MeSH Terms]) OR (children[MeSH Terms])) OR (pediatrics[MeSH Terms])) OR (infant[MeSH Terms])) AND (((sleep[MeSH Terms]) OR (circadian rhythm[MeSH Terms])) OR (sleep*))) AND (((((depression[MeSH Terms]) OR (anxiety[MeSH Terms])) OR (Risk Factors[MeSH Terms])) OR (Life Change Events[MeSH Terms])) OR (Smoking[MeSH Terms]))) AND ((((((maternal[MeSH Terms]) OR (Mothers[MeSH Terms])) OR (Obstetric Labor Complications[MeSH Terms])) OR (Obstetric Complication*)) OR (Maternal Exposure[MeSH Terms])) OR (preterm birth[MeSH Terms])). These keywords were chosen based on our preliminary reading of relevant articles and the consultation of a librarian. The search strategy was adapted according to the indexing systems of other databases. Language and study design filters were used in the initial search to enhance the specificity of the literature search. Regarding grey literature, four sleep society websites (i.e. British Sleep Society, American Academy of Sleep Medicine, World Sleep Society, and Sleep Research Society) and the websites of three maternal child health organizations (i.e. Center on Developing Child, Bill &Melinda Gates foundation, and National Partnership for Women & Families) were searched. Two relevant studies were found from the above websites and were included for screening. Two rounds of screening were conducted by two reviewers independently. First, the titles and abstracts of acquired articles were screened for their relevance and eligibility for the current systematic review. Then relevant articles that were in line with the inclusion criteria were included for full-text screening. Articles evaluated as relevant after two rounds of screening were included for data extraction and quality appraisal.
Inclusion criteria
Table 1 showed the inclusion criteria based on the population, intervention/exposure, comparison, outcome, and study design (PICOS) framework. Specifically, observational studies, including outcomes of case-control and longitudinal studies that investigate the association between perinatal physiological and psychological factors and children’s sleep health, were included. Only longitudinal studies and case control studies were included since they provide more insights in the direction of the associations between predictors and outcomes 23. Multiple papers generated from the same data source were reviewed as a single study and only relevant data were included.
Table 1.
PICOS criteria for inclusion of studies
| Parameter domain | Inclusion criteria |
|---|---|
|
| |
| Population | Pregnant women regardless of health status or diseases, and their children of this pregnancy |
| Exposures |
Maternal risk factors within the pre-, peri-, and postnatal period: ● Physiological factors: pregnancy and obstetric factors, and substance use during pregnancy ● Psychological factors: ● Negative psychological factors: major depressive disorder, depression symptoms, major anxiety disorder, anxiety symptoms, and maternal stress. ● Positive psychological factors: happiness and satisfaction towards life, etc. |
| Comparators | No exposure |
| Outcomes | Macro sleep architecture measured with objective devices or questionnaires; or micro sleep architecture quantified by power spectral analysis of the electroencephalogram; or parent or child self-reported child sleep outcomes including sleep problems, sleep disturbances, etc. No longest follow-up cut-off time point was set. |
| Study design | Case-control or longitudinal study |
Exclusion criteria
Reviews, conference proceedings, and abstracts were excluded since this study focused primarily on peer-reviewed original studies. Non-English language papers were also excluded due to lack of time and funding to use professional translations. Studies that did not contain a control group were excluded. Studies focused on sleep disordered breathing, sudden infant death, and familial and environmental risk factors of child sleep were excluded due to the scope of the current review. Studies with focus on paternal parenting factors were excluded to avoid duplication with a recently published systematic review 21. Studies exploring the exposure factors beyond the first six months post-delivery were excluded since the first six months after birth was defined as the postpartum period 24.
Data extraction
A standardized data extraction form was developed and included the following information: year of publication, country, aim of study, study design, population characteristics, perinatal exposure and their measurement tools, children’s sleep outcome and measurement tools, timepoints and duration of follow-up, main results including effect size and confidence intervals, and adjusted variables.
Study quality
All of the included studies used a longitudinal design. The quality of these studies was evaluated by the Newcastle-Ottawa Scale (NOS) with three dimensions: selection, comparability, and exposure 25. The total possible score for the NOS is 9, with higher scores indicating higher quality 25. Since potential bias in poor quality studies can influence the conclusion of a systematic review, only studies evaluated as having high or moderate quality were included for our data synthesis.
Data synthesis
Meta-analysis was only conducted when studies reported the same exposure and same sleep outcomes. Additionally, meta-analysis was only feasible when the sleep outcomes were all reported in the same format (i.e., all reported in continuous format, or all reported in dichotomous format). The effect sizes were pooled according to the categories of risk exposures and sleep outcome variables. Regarding continuous variables, Hedge’s g was pooled as the effect size since each sleep outcome was generally measured by different tools across the included studies. For dichotomous outcome variables, the odds ratio (OR) was pooled as the effect size. Following the recommended good practice of meta-analysis of OR 26, the unadjusted ORs were directly computed when the number of events and frequencies were provided in the original studies. Random-effects models were used in all analyses since it was more appropriate in combining effect sizes from heterogeneous populations 27. Heterogeneity and variation in the pooled estimations were computed by Cochrane’s Q test and I2 respectively, with a p value < 0.1 indicating significant heterogeneity, and I2 values of 25%, 50%, and 75% indicating minor, moderate, and high heterogeneity respectively 28. Due to the small number of included studies in each comparison (n < 3), subgroup analysis and publication bias were not appropriate and thus were not conducted.
The statistical synthesis was conducted with R (version 4.0.2). When a sleep outcome was measured at multiple timepoints, only the outcomes measured at the same or adjacent child developmental stage were combined during data comparison. The population characteristics and the name of the cohort study (when provided) were carefully checked to identify papers from the same study to prevent the result of the same participants being included more than once in the same comparison. Narrative summary was conducted to synthesize the findings of included studies when statistical synthesis was not appropriate.
RESULTS
Characteristics of included studies
As shown in Figure 1, a total of 1711 papers were retrieved from the literature search. A total of 32 papers reporting the results of 30 studies were included for quality appraisal and data synthesis. The included studies were conducted in the US (n = 3), Canada (n = 1), Brazil (n = 2), Europe (n = 14), Asia (n = 3), and Oceania (n = 7). Three studies employed retrospective longitudinal study design and asked parents to recall perinatal risk exposure 29–31, while other studies employed the prospective longitudinal design. Nine studies explicitly reported a population-based participant recruitment 16,32–39. The follow-up frequency ranged from 1–6 times, with the earliest postnatal follow-up started from 10 days after birth 40 and the longest follow-up happened at 24 years of the children’s age 34. Regarding study quality appraisal, 96.67% (29/30) of included studies had the NOS score ranging from 6 to 8, indicating moderate to high quality. Detailed characteristics of included studies were listed in Table 2 to Table 3.
Table 2.
Characteristics of included studies focusing on maternal physiological risk and child sleep outcomes
| No. | Author/year | Study design | Country/Region | Population | Exposure | Exposure measure | Sleep outcomea | Sleep outcome measure | Follow up time points | Adjusted variables | Study quality (NOSb) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Physiological risks - Pregnancy and obstetric factors as main exposure | |||||||||||
| 1 | Björkqvist et al. 2018 34 | Prospective longitudinal study | Finland | Participants from two cohort study: The Preterm Birth and Early-life Programming of Adult Health and Disease (ESTER) study and the Arvo Ylppö Longitudinal Study (AYLS) | Preterm birth | Medical record; | Bedtime ×, Get up time ×, Actual sleep time ×, WASO ×, Sleep midpoint weekday/weekend ×; Maternal circadian preference ×. | Actigraph; MEQ | Once: 24 years of child age | Child age, sex, cohort, birth weight, parity, maternal gestational hypertension and diabetes, smoking during pregnancy, maternal BMI before pregnancy, and workload. | 8 |
| 2 | Caravale et al. 2017* 42 | Prospective longitudinal study | Italy | Preterm children born at a NICU of a public hospital in Rome, and healthy full-term peers from the same geographic area | Preterm birth | Medical record | Bedtime difficulties ×, sleep difficulties (+); Bedtime ×, get up time ×, TSD ×; FNW ×; nocturnal wakefulness ×. | SDSC; BISQ | Once: 2 years of child age | NA | 6 |
| 3 | Pierrehumbert et al. 2003 56 | Prospective longitudinal study | Switzerland | All preterm infants (< 33 gestation weeks) admitted to the neonatal intensive care unit of the Lausanne University Hospital over a 12-month period (January to December 1998), and healthy children born at the same hospital | Perinatal risk severity caused by prematurity; Parental traumatic memories about birth | Perinatal Risk Inventory; Perinatal PTSD questionnaire | Sleep problems (+) | SDQ | Once: 18 months of child age | NA | 6 |
| 4 | Shang et al. 2006 29 | Retrospective longitudinal study | Taiwan China | Children from preschool, kindergarten and grades 1–3 at elementary schools randomly chosen from 12 school districts of Taipei city. | Prenatal alcohol, coffee, and drug intake, and prenatal and obstetric complication; Current parental mental distress; current child behavior | SDQ; The Chinese Health Questionnaire; CBCL | Early insomnia (+); bed time (+); FNW (+); sleep talking (+), nightmare (+), enuresis ×, bruxism (+) and snoring × | SHQ | Once: 4–9 years of child age (mean age: 7.37 years) | Child age and sex | 5 |
| 5 | Yiallourou et al. 2018* 31 | Retrospective longitudinal study | Australia | Children with preterm birth and/or fetal growth restriction history born at Monash Medical Center, and children with birthweight appropriate for gestational age recruited from the community | Preterm birth; Fetal grown restriction | Medical record | Macro sleep architecture: TSD (−), WASO% (+), TIB ×, SE (−), SL ×, NREM (−), REM ×; NREM% ×, REM% ×. Micro sleep architecture: the quantification of electroencephalogram waveforms: δ (+), θ (+), α (+), σ (−), and β (−) |
Polysomnography + electroencephalogram | Once: 9 years of child age on average | Child age at investigation | 7 |
| Physiological risks – Substance use | |||||||||||
| 1 | Santos et al. 2012 36 and Xavier et al. 2020 57 were from the same wave of cohort study | Prospective longitudinal study | Brazil | Participants from the 2004 Pelotas Birth Cohort Study (Pregnant women from a birth cohort started in 2004 in the city of Pelotas and all live births between January 1 and December 31) | Maternal pre- and postnatal heavy caffeine consumption (>=300 mg/day); | SDQ | FNW × | SDQ | Once: 3 months of child age | Maternal age, skin color, schooling, parity, alcohol consumption, child’s gender, and family income. | 7 |
| 2 | Gillioen et al. 2017* 62 | Prospective longitudinal study | France | Participants in the Autonomic Baby Evaluation (AuBE) cohort study | Prenatal maternal tobacco smoking | SDQ | TSD (−); SE ×; QS% ×; AS% ×; IS ×; Arousals × | Polysomnography | Twice: 0 and 6 months of child age | NA | 7 |
| 3 | Eiden et al. 2018* 46 | Prospective longitudinal study | US | Women who presented for prenatal care at a large city hospital with no illicit drug use other than cannabis, and their children | Pre- and postnatal tobacco and cannabis use | Self report; Maternal saliva sample and infant meconium test | Sleep problems (+) | CBCL sleep composite score | Twice: 2 and 3 years of child age | Child sex, breastfeeding days. | 7 |
| 4 | O’Callaghan et al. 2019* 47 | Prospective longitudinal study | Australia | Pregnant women from the Mater-University of Queensland Study of Pregnancy (MUSP) who delivered a singleton child in Brisbane, and their children | Maternal pre and postnatal smoking | SDQ | Sleep problems (+) | CBCL and YSR sleep composite score | Three times: 5, 14, and 21 years of child age | Pregnancy lifestyle exposures including alcohol, tea and coffee intake, social and maternal factors including maternal age, education, family income, planned pregnancy, breastfeeding status at 6 months. | 8 |
| 5 | Pesonen et al. 2009* 51 | Prospective longitudinal study | Finland | Women and full-term children from an urban cohort | Prenatal maternal alcohol and tobacco use; birth weight, length at birth, Ponderal index at birth | Birth records; SDQ | Short sleep (+); SE (−); SD (+) | Actigraph; SDSC | Once: eight years of child age | “Maternal licorice consumption, child’s current BMI, atopic eczema or other allergies, and asthma | 7 |
| 6 | Sarfi et al. 2009* 63 | Prospective longitudinal study | Norway | Pregnant women who received either methadone or buprenorphine as opioid maintenance treatment (OMT) at a Norwegian OMT program, and their infants. A comparison group of healthy children was recruited from local health care centers in different parts of Oslo. | Maternal substance use and OMT treatment | Clinical interview; the European Addiction Severity Index; Infant meconium analysis | Bedtime ×; total time awake during daytime ×; TSD × | Sleep diary | Once: 3 months of child age | NA | 6 |
| 7 | Alvik et al. 2011* 32 | Prospective longitudinal study | Norway | Pregnant women in Oslo aged 26–35 years and their infants | Maternal alcohol use, smoking during pregnancy, prenatal MDS, prenatal anxiety, relation satisfaction, birth weight, medical problems, and Apgar score | SDQ | Sleep problems (+) | SDQ | Once: 6 months of child age | NA | 8 |
| 8 | Chandler-Mather et al. 2021 61 | Prospective longitudinal study | Australia | A subsample of participants from the Longitudinal Study of Australian Children (LSAC) Birth Cohort (specifically, mother who gave birth in 2004, and their children) | Maternal alcohol use during pregnancy | SDQ | Sleep problems (+) | SDQ | Four times: 2–3, 4–5, 6–7, and 8–9 years of child age | Maternal age, education, marital status, family income, cigarette use during pregnancy, maternal stress during pregnancy, child sex, birthweight, and gestational weeks | 8 |
Note:
Study name followed by a indicates the study was included for statistical synthesis.
Sleep outcome followed by a “+” indicates the results of the exposure group were significantly longer or stronger than the results of the control group; a “-” indicates the results of the exposure group were significantly shorter or weaker than the results of the control group, and a “×” indicates no differences between exposure and control groups.
NOS: the Newcastle-Ottawa Scale, with a NOS score of 0–4, 5–6, and 7–9 indicating low, medium, and high quality respectively.
Abbreviation: AS: Active sleep; BDI-II: Beck depression inventory; BISQ: Brief infant sleep questionnaire; CBCL: Child Behavior Checklist; FNW: Frequency of night waking; IS: indeterminate sleep; ISQ: Infant sleep questionnaire; ITQ: Infant temperament questionnaire; MDS: Maternal depression symptoms; MEQ: Morningness–Eveningness Questionnaire; NA: not available; NREM: Non-rapid eye movement; PTSD: post-traumatic stress disorder; QS: Quiet sleep; TIB: Time in bed; REM: Rapid eye movement; SD: sleep disturbance; SDQ: Self-developed questionnaire; SDSC: Sleep disturbance for children; SE: sleep efficiency; SHQ: Sleep habit questionnaire; SL: Sleep latency; SPQ: Sleep practices questionnaire; SQ: sleep quality; SWT: sleep-wake transition; TSD: Total sleep duration; WASO: Wake after sleep onset; YSR: Youth Self-Report.
Table 3.
Characteristics of included studies focusing on psychological risk and child sleep outcomes
| No. | Author/year | Study design | Country/Region | Population | Exposure | Exposure measure | Sleep outcome | Sleep measure | Follow up time points | Adjusted variables | Study quality (NOSa) |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| 1 | Armitage et al. 2009* 65 | Prospective longitudinal study | US | Pregnant women went into perinatal mood disorders or obstetrics clinics at the University of Michigan, and their infants | Pre-, and/or postnatal MDD | Structured clinical interviews for DSM-IV (SCID); EPDS; BDI-II. | SL (+), TSD ×, Nocturnal total sleep time (−), daytime sleep episodes (+); SE (−), and FNW (+) | Sleep diary; Actigraph | Twice: 2 weeks and 6 months of child age | Child sex and maternal medication use during pregnancy | 8 |
| 2 | Baird et al. 2009 33 | Prospective longitudinal study | UK | Pregnant women at Southampton who were participants of the Southampton Women’s Survey (SWS) study, and their infants | Preconceptional psychological distress; | GHQ-12 | FNW (+) | SDQ | Twice: 6 and 12 months of child age | The following variables were included as confounders: maternal age, possible postnatal depression, educational attainment, receipt of benefits, smoking and alcohol consumption during pregnancy, infant sex, birthweight-for-gestational age Z-score, breastfeeding, and bedroom sharing | 7 |
| 3 | Bat-Pitault et al. 2017* 66 | Prospective longitudinal study | France | Participants in the Autonomic Baby Evaluation (AuBE) cohort study | Pre- and postnatal MDD | HAD; Structured clinical interviews for DSM-IV (SCID); | Macro sleep architecture: TSD (−); NREM (−); REM (−); SE (−); Arousal (−); Awake time (+); Micro sleep architecture: slow-wave activity (+); δ (+), lower spindle density (−). |
Polysomnography | Twice: 0 and 6 months of child age | NA | 7 |
| 4 | Brandlistuen et al. 2015 67 and Ystrom et al. 2017 68 were the same cohort | Prospective longitudinal study | Norway | Participants from the Norwegian Mother and Child Cohort Study (MoBa) | Prenatal antidepressants use (Brandlistuen et al. 2015); Postnatal maternal symptoms of anxiety and depression (Ystrom et al. 2017) | Self-report; SCL; DSM-III-R; | Sleep problems ×; FNW × | CBCL sleep composite score | Three times: 6, 18, and 36 months of child age | Maternal concomitant use of other drugs, smoking and alcohol use during pregnancy, maternal age at delivery and parity, child sex, birthweight, and gestational age | 7 |
| 5 | Chuang et al. 2011 45 | Prospective longitudinal study | Taiwan, China | Pregnant women attending the National Taiwan University Hospital for delivery and postpartum care from April 2004 to January 2005, and their children. | Maternal postnatal mental health; work stress | MHI-5; SF-36 | Sleep problems × | CBCL sleep composite score | Once: 24 months of child age | Parental age, education, and occupation; maternal smoking, passive smoking, drinking during pregnancy; Child sex, birth order, Apgar score, neonatal jaundice birthweight, gestational age | 7 |
| 6 | Dias et a. 2020 52 | Prospective longitudinal study | Portugal | Pregnant women who gave birth in two public hospitals in Northern Portugal, and their infants | Pre- and postnatal MDS | EPDS | Sleep problems (+) | CSHQ | Once: 6 months of infant age | Maternal education | 7 |
| 7 | Galbally et al. 2018* 43 | Prospective longitudinal study | Australia | Pregnant women from the Mercy Pregnancy and Emotional Wellbeing Study, and their infants | Prenatal MDD and antidepressants use | Structured clinical review for SCD-IV; EPDS; SDQ; Medical record; Maternal blood and umbilical cord blood test. | TSD ×; FNW ×; Wakefulness duration ×; SL × | BISQ | Twice: 6 and 12 months of infant age | NA | 7 |
| 8 | Garthus-Niegel et al. 2018 39 | Prospective longitudinal study | Norway | Pregnant women from the Norwegian Akershus Birth Cohort (ABC), and their children | Postpartum PTSD | IES | TSD ×; Nocturnal sleep duration ×; FNW (+); Wakefulness duration (+); Settling time (+);Sleep problems (+) | SDQ; BISQ | Twice: 8 weeks and 24 months of child age | Postpartum depression, anxiety, prenatal PTSD, maternal age, education, employment status, parity, obstetric complications, infant sex, birth weight, prematurity, and breastfeed method. | 7 |
| 9 | Goldberg et al. 2013 54 | Prospective longitudinal study | US | Pregnant women from a prenatal stress longitudinal study conducted between 2003 and 2009 at a university medical center in Southern California, and their infants | Postnatal MDS; postnatal anxiety symptom | CES-D; STAI | Bedtime distress (+); Nighttime sleep issues ×; Bothered by nighttime sleep issues × | SPQ; SDQ | Twice: 6 and 12 months of child age | Ethnicity/culture, infant sex and temperament | 7 |
| 10 | Halal et al. 2021 85 | Prospective longitudinal study | Brazil | Participant from the 2015 Pelotas Birth Cohort Study (Pregnant women in Pelotas city who gave birth between January 1 and December 31, 2015, and their infants) | Pre- and postnatal MDS | EPDS | Mother-reported TSD ×, SL (+), TSD (−), FNW (+), and SP (+); Actigraph-measured TSD ×, SL ×, SE ×, nocturnal wakefulness ×, and number of night waking × | BISQ; Actigraph | Once: 12 months of child age | Maternal age, maternal education, ethnicity, presence of partner of spouse, parity, abortion history, number of relatives, offspring living in the same household, planned pregnancy, antenatal care appointments, intragestational morbidities, physical activity during pregnancy, substance use during pregnancy, perceived spouse support, etc. | 7 |
| 11 | Kim et al. 2020* 14 | Prospective longitudinal study | New Zealand | Pregnant women in the Growing Up in New Zealand prebirth cohort study, and their children | Pre- and postnatal MDS; Infant temperament | EPDS; IBQ-R VSF | Short night sleep duration (+); FNW (+) | SDQ | Once: 24 months of child age | Maternal and household demographics; maternal health and employment; parenting role expectations and child factors including sex, birth weight, gestation, parent-reported health or developmental problems, feeding method | 7 |
| 12 | Liu et al. 2020 30 | Retrospective longitudinal study | China | Participants of the China Jintan Child Cohort Study | Pre- and postnatal MDS; Prenatal maternal happiness | SDQ | Postnatal MDS and Sleep problems (+); Prenatal happiness and Sleep problems (−) | CBCL sleep composite score | Once: 5–6 years of child age | Sociodemographic information collected included the child’s sex, age, residence (e.g., urban, suburban, or rural), cesarean birth complications (e.g. preterm) and maternal and paternal education level. | 7 |
| 13 | Matenchuk et al. 2019 16 | Prospective longitudinal study | Canada | A subsample of 619 Canadian infants from the Edmonton site of the Canadian Healthy Infant Longitudinal Development (CHILD) birth cohort. | Maternal education level; Pre- and postnatal MDS | SDQ; CES-D | TSD (−); | BISQ | Once: 3 months of infant age | Maternal factors including maternal age, race, siblings at home, maternal prenatal smoking, and child factors including sex, gestational age at birth, birth mode, breastfeeding status, solids, and colic. | 7 |
| 14 | Petzoldt et al. 2016* 40 | Prospective longitudinal study | Germany | Pregnant women from the Maternal Anxiety in Relation to Infant Development (MARI) study and their infant | Pre- and postnatal MDD and/or anxiety disorder | Diagnosed by the CIDI-V | Sleep problems (+) | SDQ | Four times: 10 days, 2, 4, and 16 months of child age | Sociodemographic features maternal age, marital status, years of education and employment, infant birth weight, week of gestation at birth, mode of delivery and sex of infant) and maternal report breastfeeding at 4 months. | 6 |
| 15 | Simcock et al. 2019 48 | Prospective longitudinal study | Australia | Women at a major tertiary hospital in a flood-affected area of Brisbane who were attending antenatal clinics or who were already enrolled in another unrelated study (Midwives @ New Group Practice Options, M@NGO) | Disaster-related prenatal maternal stress | SDQ | Sleep problems (+) | CBCL sleep composite score | Once: 2.5 years of child age | maternal marital status, SES, perinatal maternal depression, current maternal mood (anxiety, depression, and stress) infant gestational age and birth weight | 7 |
| 16 | Toffol et al. 2019* 37 | Prospective longitudinal study | Finland | Participants come from the Prediction and Prevention of Pre-eclampsia and Intrauterine Growth Restriction (PREDO) study | Pre- and postnatal MDS | CESD; BDI | TSD (−); SL (+); FNW (+); SD (+) | BISQ; SDSC | Once: 3.5 years of child age | Maternal age at delivery, BMI, parity, smoking during pregnancy, hypertensive and diabetic factors, child sex, gestational age, birth weight, child psychiatric symptoms | 8 |
| 17 | Cook et al. 2020* 70 | Prospective longitudinal study | Australia | Participants from the Maternal Health Study, who were registered to give birth at one of the six public hospitals in Melbourne, and their infants. | Pre- and postnatal MDS, anxiety or panic attacks, maternal physical health and well-being, and IPV | EPDS; CAS; SF-36 | FNW (+);Sleep problems (+) | SDQ | Four times: 3, 6, 9, and 12 months of child age | Maternal age at the time of birth, relationship status, country of birth, and infant sex | 6 |
Note:
Study name followed by a indicates the study was included for statistical synthesis.
NOS: the Newcastle-Ottawa Scale, with a NOS score of 0–4, 5–6, and 7–9 indicating low, medium, and high quality respectively.
Abbreviation: BDI-II: Beck depression inventory; BISQ: Brief infant sleep questionnaire; CAS: Composite Abuse Scale; CES-D: Center for Epidemiological Studies Depression Inventory; CIDI-V: Composite International Diagnostic Interview for Women; EPDS: Edinburgh Postnatal Depression Scale; FNW: Frequency of night waking; GHQ-12: General health questionnaire; HAD: Hospital anxiety depression; IBQ-R VSF: the very short form of Infant behavior questionnaire-revised; IES: Impact of event scale; IPV: intimate partner violence; ISQ: Infant sleep questionnaire; MDD: Major depressive disorders; MDS: Maternal depression symptoms; MHI-5: Mental health index – five item; NA: not available; NREM: Non-rapid eye movement; PTSD: post traumatic stress disorder; REM: Rapid eye movement; SD: Sleep disturbances; SDQ: Self-developed questionnaire; SDSC: Sleep disturbance for children; SE: sleep efficiency; SES: Social economic status; SF-36: The 36-item Short Form; SHQ: Sleep habit questionnaire; SL: Sleep latency; SPQ: Sleep practices questionnaire; STAI: State-trait anxiety inventory; TSD: total sleep duration.
Characteristics of child sleep outcome measurements
Sleep outcomes were assessed using a variety of methods, including self-report questionnaires only (n = 23), objective measures such as actigraphy or polysomnography only (n = 3), and using both subjective and objective measures together (n = 4). The majority of the included studies used validated questionnaires, self-developed questions, and sleep diary to ask parents to report child sleep outcomes. Validated questionnaires included the Infant Toddler Social Emotional Assessment (ITSEA) 41, the Brief Infant Sleep Questionnaire (BISQ) 7,16,39,42–44, the Child Behavior Checklist (CBCL) 30,35,45–49, the Sleep Disturbance Scale for Children (SDSC) 37,42,50,51, the modified Sleep Habit Questionnaire (SHQ) 29, the Child Sleep Habit Questionnaire (CSHQ) 52,53, and the Sleep Practices Questionnaire (SPQ) 54. Figueiredo and colleagues (2017) used the Infant Sleep Chronogram to ask parents to draw the children’s sleep and wake duration 55.
Physiological risk factors
For the purposes of this study, physiological risk factors were operationally divided into pregnant and obstetric factors, and substance use.
Pregnant and obstetric factors
Four studies investigated preterm birth as a main risk factor for child sleep problems and its larger relationship with child macro sleep architecture, sleep habits, and sleep problems 31,34,42,56. The relationship between preterm birth and child macro sleep architecture remains inconsistent. Two studies found no differences in most macro sleep architecture variables including bedtime, get up time, total sleep duration, nocturnal wakefulness, sleep midpoint, and circadian preference between preterm and full term children at 2 years of age 42 and in early adulthood 34. However, in another study of 5-to-12 year-olds that employed polysomnography to measure and compare sleep in preterm children with fetal growth restrictions, preterm children with appropriate birth weight for gestational age, and full term children, the preterm children with appropriate birth weight for their gestational age had a significant decrease in total sleep duration, sleep efficiency, and non-rapid eye movement sleep, and a significant increase in wake after sleep onset compared to the other two groups 31. This study also investigated micro sleep architecture and found that preterm children had altered microarchitecture compared to full-term children. Specifically, preterm children with fetal growth restriction had the highest δ and α power (p <0.01), while preterm children with appropriate birth weight for gestational age had the lowest θ and β power (p < 0.01) 31.
Although the relationship between preterm birth and child macro sleep architecture remains inconsistent, there is a consistent upward trend in parent-reported sleep problems in preterm children. Specifically, preterm children experienced increased parent-reported sleep difficulties (e.g., nocturnal movement, restlessness during the night, etc., with the Hedge’s g = 0.42, 95% CI: 0.04, 0.80, p = 0.03) 42, and parent-reported sleeping problems at children’s 18 months of age (Hedge’s g = 1.14, 95% CI: 0.64, 1.65, p < 0.001) 56. Two studies investigated a wide range of maternal and child risk factors and found no significant relationship between preterm birth history, child sleep duration trajectory, (AOR = 1.22, 95% CI: 0.89, 1.67) 57 and frequent night waking trajectory (AOR = 1.11, 95% CI: 0.64, 1.92) 58.
Other prenatal and obstetric factors were also found to be potential risk factors for poor sleep in children. Shang et al. (2006) reported that vaginal bleeding during pregnancy was correlated with child sleep talking (OR = 2.0, 95% CI: 1.2, 3.2) and nightmares (OR = 1.9, 95% CI: 1.1, 3.5) at 4–9 years of age, while being first-born was associated with both child sleep talking (OR = 1.9, 95% CI: 1.4, 2.6) and bruxism (OR = 1.8, 95% CI: 1.3, 2.5) 29. However, two studies found no correlation between birth order and child night waking trajectories from 2–6 years of age 58 and child sleep duration at age 18 59. Additionally, lower birth weight (OR = 1.7, 95% CI: 1.1, 2.7) and shorter birth length (OR = 2.2, 95% CI: 1.3, 3.7) were associated with low sleep efficiency at age eight 51.
Substance use
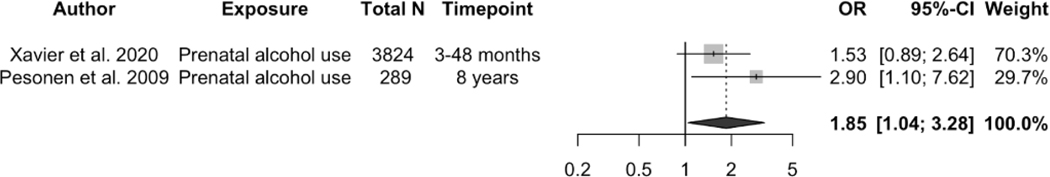
A total of 12 studies reported the association of maternal prenatal substance use including alcohol 32,51,53,57,60,61, tobacco smoking 13,16,47,51,53,62, tobacco and cannabis use 46, and drug use and opioid maintenance treatment 63 with child sleep outcomes. Prenatal maternal alcohol drinking was associated with shorter child sleep duration (pooled OR = 1.85, 95% CI: 1.04, 3.28, p = 0.0352, I2 = 21.6%, see Figure 2a), lower sleep efficiency (OR = 3.6, 95% CI: 1.3, 10.0, n = 289) 51, and increasing sleep problems at 6 months of age (OR = 6.4, 95% CI: 2.69, 15.23, n = 1303) 32.
Figure 2.
Effects of the association between prenatal alcohol drinking and child short sleep duration (yes/no). A random effects model was used to calculate the pooled estimate of the odds ratio (OR) and its 95% confidence interval (CI). The area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect (OR 5 1.85, 95% CI: 1.04–3.28, p 5 0.0352, I2 5 21.6%).
In addition, another study found that maternal heavy alcohol intake during pregnancy may have long-term impact on child sleep problems. One study found that maternal heavy alcohol intake (>=1 glasses of alcohol per day) during pregnancy was associated with child sleep problems at seven years of age (β = 2.55, 95% CI: 0.21, 4.89, n = 2746) 53. Another study also reported that heavy alcohol drinking during pregnancy (drinking > 7 standard drinks per week on average, or drinking >= 5 drinks per occasion on more than 2 occasions per week, or drinking >=11 drinks per occasion) was predictive of sleep problems across 2–9 years of age (adjusted β = 0.557, 95% CI: 0.127, 0.988, n = 3447) 61.
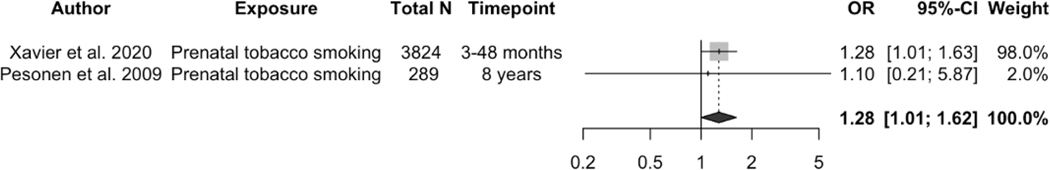
Regarding maternal tobacco smoking, Gillioen and colleagues (2017) employed polysomnography to measure child sleep outcomes and found that maternal tobacco smoking during the whole pregnancy was associated with decreased child total sleep duration by 30.6 minutes at birth (p < 0.001) 62. Tobacco use can also increase the risk of short sleep duration at later age (pooled OR = 1.28, 95% CI: 1.01, 1.62, p = 0.0437, I2 = 0, see Figure 2b). Prenatal tobacco smoking was consistently found by four studies to be associated with child sleep problems 46,47,53,60. Compared to postnatal maternal smoking, prenatal tobacco smoking was more predictive of children’s sleep problems at 14 years of age reported both by their mother (OR: 1.63, 95% CI: 1.30, 2.05, n = 3421), and by themselves (OR: 1.29, 95% CI: 1.05, 1.60, n = 3405) 47. Higher frequency of tobacco and cannabis use during pregnancy was associated with higher sleep problem scores at three years of age in children and the association was stronger for girls 46.
One study employed opioid maintenance treatment targeting women who used drugs before pregnancy and the impact of this treatment on infants’ sleep-wake cycles and distress and found that there was no statistically significant difference in these infants’ total sleep time, awake time, and distress bouts (all p > 0.05) compared to children whose mothers did not report any pre-pregnancy drug use 63. One study investigated maternal pre- and postnatal heavy caffeine intake (>= 300 mg/day) and found that caffeine intake had no relationship with children’s frequent night waking at three months of age (Prevalence Ratio = 1.65, 95% CI: 0.86, 3.17, p = 0.135) 36.
Psychological factors
Psychological risk factors investigated in the included studies were categorized into negative emotions and positive emotions. Regarding negative emotions, maternal depression symptoms were the most commonly investigated risk factor (n = 10), followed by anxiety symptoms (n = 4), major depression disorder (n = 3), distress (n = 3), anxiety disorder (n = 1), postnatal general mental health (n = 1), and postpartum PTSD (n =1). Positive emotions included happiness during pregnancy (n =1), and satisfaction with life during pregnancy (n = 1).
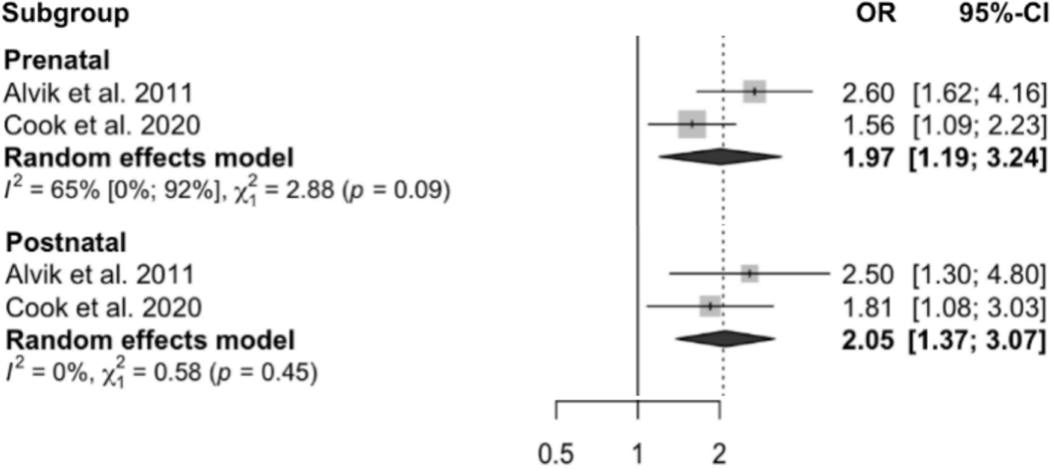
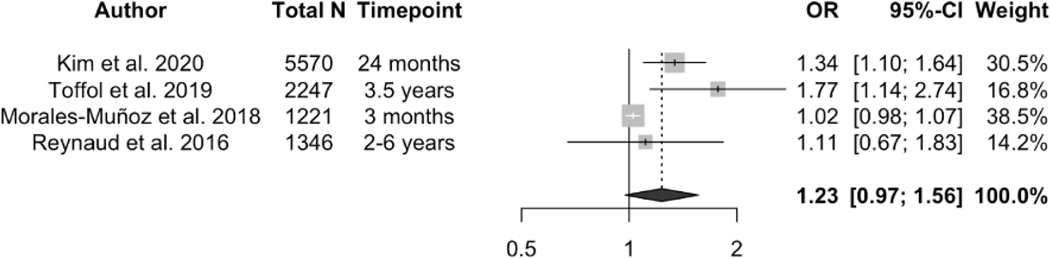
Regarding maternal depression symptoms, two studies reported that compared to children whose mother did not experience prenatal depressive symptoms, children with prenatal maternal depressive symptoms exposure had significantly shorter 24-hour total sleep duration at 3 months (Hedge’s g = −0.27, 95% CI: −0.48, −0.06, n = 554) 16, 6 months (β = −0.61, 95% CI: −0.96, −0.26), 12 months (β = −0.39, 95% CI: −0.72, −0.06), and 24 months of age (β = −0.70, 95% CI: −0.94, −0.45, n = 1676) 13, respectively. Moreover, both pre- and postnatal maternal depression symptoms were associated with increased child sleep problems at 6 months of age, with pooled OR = 1.97 (95% CI: 1.19, 3.24) and 2.05 (95% CI: 1.37, 3.07) respectively (see Figure 3). Random effects models found no association between prenatal maternal depression symptoms and children’s frequent night waking (pooled OR = 1.23, 95% CI: 0.97, 1.56, p = 0.082, I2 = 75%, see Figure 4) or sleep latency (pooled Hedge’s g = 0.97, 95% CI: −0.90, 2.84, p = 0.390, I2 = 89.6%, see supplementary Figure 1).
Figure 3.
Effects of the association between prenatal maternal tobacco smoking and child short sleep duration (yes/no). A random effects model was used to calculate the pooled estimate of the odds ratio (OR) and its 95% confidence interval (CI). The area of each symbol is proportional to the weight of the study. The diamond represents the pooled effect (OR 5 1.28, 95% CI: 1.01–1.62, p 5 0.0437, I2 5 0).
Figure 4.
Effect size of the association between prenatal and postnatal maternal depression symptoms and child sleep problems (yes/no). A random effects model was used to calculate the pooled estimate of the odds ratio (OR) and its 95% confidence interval. The area of each symbol is proportional to the weight of the study.
Compared to prenatal depression symptoms, postnatal maternal depression symptoms were more predictive of child sleep problems at a later age. One study found that compared to prenatal depression symptoms, maternal postpartum depression symptoms were more predictive of infants’ overall sleep problems scoring (β = 0.25, p = 0.002) and bedtime resistance (β = 0.20, p = 0.014, n = 164) at 6 months of age 52. Liu and colleagues (2020) employed a self-developed questionnaire to evaluate maternal depressive emotions and found that postpartum maternal depressive emotions were predictive of preschool children’s sleep problems (B = 3.41, standard error = 0.72, p = 0.04, n = 1257) 30. Maternal depressive symptoms may have long-term impacts on child sleep. One study found that postpartum maternal depressive symptoms were associated with children’s sleep problems at 18 years of age (OR = 1.26 95% CI: 1.15, 1.39, p < 0.001, n = 2913) after adjusting for prenatal maternal depressive symptom and adolescents’ concurrent depression 64.
Two studies investigated pre- and postnatal maternal major depression disorder and its relationship with child macro sleep architecture 65,66. Random-effects model showed that pre- and postnatal maternal depression disorder was associated with shortened child total sleep duration (pooled Hedge’s g = −0.97, 95% CI: −1.57, −0.37, p = 0.002, I2 = 28.5%, see Supplementary Figure 2a) and lower sleep efficiency (pooled Hedge’s g = −1.44, 95% CI: −1.93, −0.95, p < 0.001, I2 = 0, see Supplementary Figure 2b). Pre- and postnatal maternal depression disorder was also predictive of longer nocturnal sleep latency in children, more daytime sleep episodes, and increased night waking (all p < 0.05) 65,66. In addition, two studies investigated maternal pre- and postnatal use of antidepressants 35,43. Both studies adjusted for pre- and postnatal maternal depression symptom and found no association between maternal antidepressant use during pregnancy and infants’ sleep duration, nocturnal wakefulness, sleep onset, or parent-reported infant sleep problems 43,67.
Mediating relationships between maternal depressive symptoms and child sleep health have also been found in three studies. Toffol and colleagues (2019) found that postpartum maternal depressive symptoms partially mediated the associations between prenatal maternal depressive symptoms, child sleep latency, and total sleep disorders, and fully mediated the associations between prenatal maternal depressive symptoms, child nocturnal sleep duration, and night waking 37. Infant temperament 14 and child behavior 30 were also found as mediators between maternal depressive symptoms and child sleep respectively. Specifically, the indirect effect of maternal prenatal depression on child night waking frequencies through infant negative affectivity remained statistically significant (z = 0.0011, 95% bootstrapping CI: 0.0004 – 0.0018) 14. Child behavior problems mediated the association between maternal pre- and postnatal depressive symptoms and child sleep problems 30.
Several studies explored other maternal negative psychological factors and their relationship with child sleep 13,39,40,45,48,53,68–70. Specifically, three studies reported an inconsistent relationship between maternal prenatal anxiety and child sleep problems 40,69,70. Our pooled results found no predictive effect of maternal anxiety on child sleep problems, pooled OR = 1.34 (95% CI: 0.86, 2.10), p = 0.194, I2 = 62.5% (see Supplementary Figure 3). Other negative maternal psychological factors were also found to be associated with poor child sleep health. Postpartum PTSD seemed to predict child night waking frequency and duration (β = 0.10 and β = 0.08, respectively) and sleep problems (β = 0.12), all p < 0.01 39. Another psychological factor, disaster-related prenatal maternal stress, predicted higher child sleep problem scores (β=0.249, p = 0.003) 48. However, Chuang and colleagues (2011) reported that maternal postnatal general mental health was not associated with child sleep problems (β = 0.011, p = 0.484) 45.
While most studies focused on negative emotions, there are emerging studies reporting the influence of positive emotions during pregnancy. They found a protective influence of positive emotions on parent-reported child sleep problems. Women’s self-reported happiness during the first (β = −1.71, p < 0.001), second (β = −1.91, p = 0.04) and third trimesters (β = −2.27, p = 0.001) were associated with lower child sleep problem scores 30. Additionally, satisfaction with life during pregnancy was associated with fewer child sleep problems (OR = 0.50, 95% CI: 0.27, 0.92) 32.
DISCUSSION
Child sleep disturbance is a major public health concern given its negative consequences for physical, cognitive, and emotional/behavioral outcomes 71,72. An increasing number of studies have recognized the contributing role of perinatal factors in children’s sleep outcomes. To our knowledge, this is the first systematic review and meta-analysis investigating the associations of a wide array of physiological and psychological factors and child sleep health outcome. The overall results showed the following were potential risk factors of child sleep health: prenatal physiological factors (e.g., preterm birth, obstetric complications, and maternal alcohol or tobacco use), psychological factors (e.g. pre- and postnatal maternal depression symptoms, major depression disorder, general stress, disaster-related prenatal stress, and postnatal PTSD). On the other hand, happiness and satisfaction with life during pregnancy were protective factors of parent-reported child sleep problems and sleep onset difficulty.
Physiological risk factors
Pregnancy and obstetric factors
Preterm birth was the most frequently reported obstetric exposure. However, the findings of these studies reported inconsistent relationships between preterm birth and child sleep macro architecture including bedtime, morning wake time, sleep duration, night awakenings, and sleep midpoint. One potential reason for the inconsistency in these studies was the employment of different sleep measures such as actigraph, polysomnography, and subjective reports. However, preterm birth was consistently found as a predictor of child sleep problems in early childhood. Additionally, preterm children have also been reported to have altered micro sleep architecture including δ, α, θ, and β power. A possible mechanism for the predictive effect of preterm birth on poor child micro-sleep architecture and parent-reported sleep problems is that preterm infants have a higher risk of impaired neurodevelopment and circadian development 9. Since preterm neonates usually are admitted in the neonatal intensive care unit (NICU), extrinsic factors such as clinical treatments, light, and noise in the NICU may impair sleep quality and continued development of neurofunction 73. The common morbidities preterm neonates experience such as hypoxia and cerebral ischemia may also play a role in altering sleep health 9. Regarding full-term children with small body size at birth, this often indicates intrauterine growth restriction (IUGR). Children with IUGR have a greater reduction in brain structure, organization of neural connections between brain regions, and less neural myelination 74. Since sleep can be considered as a complex phenotype of brain and neural plasticity development 75, alteration in brain and neural function are likely to be presented through sleep.
Substance use
Consistent with the larger body of evidence, prenatal alcohol use (at least once a week) and tobacco and cannabis use in the included studies were all found as predictors of a wide range of factors associated with worse quality of child sleep, including shorter sleep duration, lower sleep efficiency, and increasing sleep problems, with a dose-response relationship in both alcohol 32 and tobacco and cannabis use 46. A potential mechanism for this relationship is that alcohol exposure is detrimental to fetus central neural system development 76. Tobacco smoking and cannabis use during pregnancy may activate fetal nicotinic acetylcholine receptors, resulting in changes in intrinsic modulating function of neurotransmitters 77. Moreover, a bidirectional relationship was found between child sleep/behavior problems and maternal pre- and postnatal alcohol use 32 and tobacco and cannabis use 46. One possible reason for the association between child sleep/behavior problems and subsequent maternal substance use is that when facing child sleep problems, mothers with substance use history may find the problem challenging and not feel they have enough competence to deal with the problem. Under this stressful situation, the lure to continue substance use out-competes the drive to fulfill the parenting role 78.
Psychological factors
Meta-analytic pooled results in the current study showed that pre- and postnatal maternal depressive symptoms were associated with significant shorter sleep duration and increased sleep problems during early childhood. Similarly, maternal pre- and postnatal major depressive disorder was associated with shorter sleep duration, lower sleep efficiency, lower sleep latency, increased daytime sleep episodes, and increased night waking in children. The relationship between maternal depression and poor child sleep health is in line with the findings of other research. In a recent population-based cross-sectional study, moderate/severe maternal depressive symptoms were associated with less than 10 hour/day of sleep duration in preschool children 79. Similarly, another study also reported that higher number of infant sleep bouts (i.e., infant going to sleep and waking up) per 24 hours were associated with maternal depressive symptoms at 2 and 6 weeks postpartum 80. Other negative psychological factors, including postpartum PTSD and prenatal disaster-related stress, were found to be predictive of child night waking 39 and sleep problems 48.
The mechanism underlying maternal negative psychological factors and child poor sleep health remains unclear. One potential mechanism is that infant temperament and children’s behavior problems mediate the pathway from maternal depression to child sleep night waking 14,30, and thus highlights the potential of enhancing sleep and behavior regulation strategies as interventions to improve children’s sleep health. From the physiological perspective, maternal prenatal alterations in the hypothalamic-pituitary-adrenal (HPA) axis function may play a role in this relationship 81. Specifically, prenatal depression and stress activate maternal HPA axis through the paraventricular nucleus of the hypothalamus, resulting in maternal increasing production of glucocorticoids 82, which further reset the phase of fetus circadian clock system 83. Due to the circadian rhythm and HPA axis development during early infancy, the effect of increased cortisol level during pregnancy may become evident during late life 82. Moreover, maternal negative psychological signals such as depression and stress can interfere with the programming of fetus circadian clock genes and have lasting impact on offspring’s sleep 82. Another potential reason is that depressed mothers are more inclined to dysfunctional perceptions about child sleep and are more likely to have suboptimal interaction and mitigation strategies to deal with children’s poor sleep behavior 84. However, since most included studies that investigated prenatal depression and child sleep did not control for maternal concurrent depression level when child sleep outcome was measured, they may miss important changes in maternal depression during the perinatal period and fail to detect the dynamic effects of perinatal maternal depression on child sleep 85. Future research should consider including both pre- and postnatal maternal depression in analysis.
It is noteworthy that maternal depressive symptoms did not contribute to every aspect of children’s poor sleep outcome. The pooled results of the current study suggested that pre- and postnatal maternal depressive symptoms were not predictive of child frequent night waking and delayed sleep latency. Several reasons may potentially explain this result. First, the included studies in this comparison all used subjective sleep measures, which may include recall bias that does not reflect the true night waking, duration of bedtime, and the time it took to fall asleep. Recently, Halal and colleagues (2021) employed both subjective sleep measure and actigraphy to detect child night waking. They found that children of perinatal depressed mothers had 0.44 more times of mother-reported night waking, however, no significant association was found between actigraph-captured night waking and postnatal maternal depressive symptoms 86. Another possible reason is the threshold for frequent night waking was different among different studies. One study defined waking up more than three times per night as frequent night waking 87, while the other two studies qualified two or more times per night as frequent night waking 14,37.
Regarding maternal anxiety, our pooled results found a non-significant relationship between prenatal maternal anxiety and child sleep problems. The following reasons can help explain this finding. First, only three studies were included in our statistical synthesis, and all of them explored both maternal anxiety and depression. The small number of included studies may not have enough power to differentiate and detect the effect size of maternal anxiety apart from depression. Second, maternal anxiety during and after pregnancy are more common than expected but are often underestimated. The latest meta-analysis using multivariate Bayesian approach estimated the prevalence of women having at least one or more anxiety disorders during the prenatal and postpartum period is 20.7% (95% CI: 16.7 – 25.4%) 88. The subclinical anxiety symptoms were less likely to be reported and treated. Since prenatal maternal anxiety is associated with alteration in children’s certain brain structure and function in frontal, temporal, and limbic areas 89 and thus potentially influence sleep architecture, our finding should be considered with caution and future research in this area is warranted.
Two of the included studies found that positive emotions (i.e., happiness and satisfaction with life) during pregnancy were protective factors of child sleep problems 30,32. A possible mechanism for this association is that positive emotions during pregnancy may improve maternal resilience to later parenting and settling their children 30. Another potential reason is that mothers would have experienced more positive emotions during pregnancy and would have had lower levels of stress hormones and thus would have had decreased sympathoadrenal activation.
Strengths and Limitations
This systematic review has several limitations. Despite using six databases and several sleep research society websites, our literature search may not be exhaustive. Due to the small number of included studies in each meta-analytic comparison, subgroup analysis and publication bias were not feasible to investigate whether the relationship between identified risk and protective factors would be moderated by potential covariates/confounders such as age and sleep measure tools. Moreover, a few of the included studies did not specify which covariates were controlled. A significant number of the included studies reported heterogeneous formats of data, preventing the data from being statistically synthesized. Thus, the conclusions drawn from narrative summaries need additional studies to confirm their validity. Another limitation is that only papers written in English were included, so the current study is subjective to language bias. Lastly, due to the scope of the review we did not include upstream social and environmental determinants of sleep health in our analysis. Despite these limitations, this study’s strengths lie in the comprehensive literature search, investigation of a wide array of risk factors, and inclusion of only moderate to high quality longitudinal studies.
CONCLUSION
Child sleep health remains a public health concern given its negative consequences for child development and family wellbeing. A better understanding of potential early-stage risk factors could inform more proactive prevention and mitigating efforts. This study identified preterm birth and small body size at birth, perinatal substance use, and maternal pre- and postnatal negative emotions as risk factors for negative child sleep outcomes. Public health policies that advocate prenatal care and maternal mental health during the perinatal period not only benefit pregnant women and their offspring’s physical well-being but also these children’s sleep health. Future research is warranted to confirm the impact of less studied risk factors and mechanisms of bidirectional and mediating relationships among multiple risk factors.
Supplementary Material
Funding:
This work is supported in part by the National Institute of Health (R01HD087485). The funding source had no role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Statement of approval: All authors have seen and approved the manuscript.
Author disclosure statement: All authors declare that they have no relevant or material financial interests that related to the research described in this paper.
Conflict of interest: None.
References
- 1.Byars KC, Yolton K, Rausch J, et al. Prevalence, patterns, and persistence of sleep problems in the first 3 years of life. Pediatrics. 2012;129(2):e276–e284. doi: 10.1542/peds.2011-0372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fatima Y, Doi SAR, Najman JM, et al. Continuity of sleep problems from adolescence to young adulthood: results from a longitudinal study. Sleep Health. 2017; 3(4):290–295. doi: 10.1016/j.sleh.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 3.Kay DB, Karim HT, Soehner AM, et al. Sleep-wake differences in relative regional cerebral metabolic rate for glucose among patients with insomnia compared with good sleepers. Sleep. 2016;39(10):1779–1794. doi: 10.5665/sleep.6154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang F, Liu H, Wan Y, et al. Sleep duration and overweight/obesity in preschool-aged children: a prospective study of up to 48,922 children of the Jiaxing birth cohort. Sleep. 2016;39(11):2013–2019. doi: 10.5665/sleep.6234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Louca M, Short MA. The effect of one night’s sleep deprivation on adolescent neurobehavioral performance. Sleep. 2014;37(11):1799–1807. doi: 10.5665/sleep.4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu J, Liu X, Ji X, et al. Sleep disordered breathing symptoms and daytime sleepiness are associated with emotional problems and poor school performance in children. Psychiatry Res. 2016;242:218–225. doi: 10.1016/j.psychres.2016.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hale L, Troxel W, Buysse DJ. Sleep health: an opportunity for public health to address health equity. Annu Rev Public Health. Apr 2 2020;41:81–99. doi: 10.1146/annurev-publhealth-040119-094412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandner MA, Fernandez FX. The translational neuroscience of sleep: a contextual framework. Science. Oct 29 2021;374(6567):568–573. doi: 10.1126/science.abj8188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bennet L, Walker DW, Horne RSC. Waking up too early – the consequences of preterm birth on sleep development. J Physiol. 2018;596(23):5687–5708. doi: 10.1113/JP274950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang F. Sleep and early brain development. Ann Nutr Metab. 2019;75(1):44–54. doi: 10.1159/000508055 [DOI] [PubMed] [Google Scholar]
- 11.Stangenes KM, Hysing M, Fevang SK, et al. Prenatal and neonatal factors predicting sleep problems in children born extremely preterm or with extremely low birthweight. Front Pediatr. 2018;6:178–178. doi: 10.3389/fped.2018.00178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Winiger EA, Hewitt JK. Prenatal cannabis exposure and sleep outcomes in children 9–10 years of age in the adolescent brain cognitive ddevelopment study. Sleep health. 2020;6(6):787–789. doi: 10.1016/j.sleh.2020.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nevarez MD, Rifas-Shiman SL, Kleinman KP, et al. Associations of early life risk factors with infant sleep duration. Article. Acad Pediatr. 2010;10(3):187–193. doi: 10.1016/j.acap.2010.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim Y, Bird A, Peterson E, et al. Maternal antenatal depression and early childhood sleep: potential pathways through infant temperament. Article. J Pediatr Psychol. 2020;45(2):203–217. doi: 10.1093/jpepsy/jsaa001 [DOI] [PubMed] [Google Scholar]
- 15.Slomian J, Honvo G, Emonts P, et al. Consequences of maternal postpartum depression: a systematic review of maternal and infant outcomes. Womens Health (Lond). Jan-Dec 2019;15:1745506519844044. doi: 10.1177/1745506519844044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matenchuk BA, Tamana SK, Lou WYW, et al. Prenatal depression and birth mode sequentially mediate maternal education’s influence on infant sleep duration. Article. Sleep Med. 2019;59:24–32. doi: 10.1016/j.sleep.2019.01.015 [DOI] [PubMed] [Google Scholar]
- 17.Ma S, Yin X, Tao R, et al. Association of maternal prenatal depression and anxiety with toddler sleep: the China-Anhui Birth Cohort study. Arch Womens Ment Health. Apr 2022;25(2):431–439. doi: 10.1007/s00737-021-01200-w [DOI] [PubMed] [Google Scholar]
- 18.van den Heuvel MI, Hect JL, Smarr BL, et al. Maternal stress during pregnancy alters fetal cortico-cerebellar connectivity in utero and increases child sleep problems after birth. Sci Rep. Jan 26 2021;11(1):2228. doi: 10.1038/s41598-021-81681-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan M, Wong TCH, Weichard A, et al. Sleep macro-architecture and micro-architecture in children born preterm with sleep disordered breathing. Pediatric Research. 2020/03/01 2020;87(4):703–710. doi: 10.1038/s41390-019-0453-1 [DOI] [PubMed] [Google Scholar]
- 20.Covington LB, Patterson F, Hale LE, et al. The contributory role of the family context in early childhood sleep health: a systematic review. Sleep Health. Apr 2021;7(2):254–265. doi: 10.1016/j.sleh.2020.11.010 [DOI] [PubMed] [Google Scholar]
- 21.Ragni B, De Stasio S, Barni D. Fathers and sleep: a systematic literature review of bidirectional links between paternal factors and children’s sleep in the first three years of life. Clin Neuropsychiatry. 2020;17(6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati AF, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. 2009;(1549–1676 (Electronic)) [PMC free article] [PubMed] [Google Scholar]
- 23.VanderWeele TJ, Jackson JW, Li S. Causal inference and longitudinal data: a case study of religion and mental health. Social Psychiatry and Psychiatric Epidemiology. 2016/11/01 2016;51(11):1457–1466. doi: 10.1007/s00127-016-1281-9 [DOI] [PubMed] [Google Scholar]
- 24.Romano M, Cacciatore A, Giordano R, et al. Postpartum period: three distinct but continuous phases. J Prenat Med. 2010;4(2):22–25. [PMC free article] [PubMed] [Google Scholar]
- 25.Wells G, Shea B, O’connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2015. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 26.Chang BH, Hoaglin DC. Meta-Analysis of Odds Ratios: Current Good Practices. Med Care. Apr 2017;55(4):328–335. doi: 10.1097/mlr.0000000000000696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borenstein M, Hedges LV, Higgins JPT, et al. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1(2):97–111. doi: 10.1002/jrsm.12 [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Altman Dg Fau - Gotzsche PC, Gotzsche Pc Fau - Juni P, et al. Cochrane handbook for systematic reviews of interventions. 2011;343(1756–1833 (Electronic))doi: 10.1136/bmj.d5928 [DOI] [Google Scholar]
- 29.Shang CY, Gau SSF, Soong WT. Association between childhood sleep problems and perinatal factors, parental mental distress and behavioral problems. Article. J Sleep Res. 2006;15(1):63–73. doi: 10.1111/j.1365-2869.2006.00492.x [DOI] [PubMed] [Google Scholar]
- 30.Liu J, Ji X, Wang G, et al. Maternal emotions during the pre/postnatal periods and children’s sleep behaviors: the mediating role of children’s behavior. J Affect Disord. Aug 1 2020;273:138–145. doi: 10.1016/j.jad.2020.03.178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yiallourou SR, Arena BC, Wallace EM, et al. Being born too small and too early may alter sleep in childhood. Sleep. 2018;41(2):1. doi: 10.1093/sleep/zsx193 [DOI] [PubMed] [Google Scholar]
- 32.Alvik A, Torgersen AM, Aalen OO, et al. Binge alcohol exposure once a week in early pregnancy predicts temperament and sleeping problems in the infant. Article. Early Hum Dev. 2011;87(12):827–833. doi: 10.1016/j.earlhumdev.2011.06.009 [DOI] [PubMed] [Google Scholar]
- 33.Baird J, Hill CM, Kendrick T, et al. Infant sleep disturbance is associated with preconceptional psychological distress: findings from the Southampton Women’s Survey. Sleep. 2009;32(4):566–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bjorkqvist J, Pesonen AK, Kuula L, et al. Premature birth and circadian preference in young adulthood: evidence from two birth cohorts. Chronobiol Int. Apr 2018;35(4):555–564. doi: 10.1080/07420528.2017.1420078 [DOI] [PubMed] [Google Scholar]
- 35.Brandlistuen RE, Ystrom E, Eberhard-Gran M, et al. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Article. International Journal of Epidemiology. 2015;44(4):1397–1407. doi: 10.1093/ije/dyv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santos IS, Matijasevich A, Domingues MR. Maternal caffeine consumption and infant nighttime waking: prospective cohort study. Pediatrics. 2012;129(5):860–868. doi: 10.1542/peds.2011-1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Toffol E, Lahti-Pulkkinen M, Lahti J, et al. Maternal depressive symptoms during and after pregnancy are associated with poorer sleep quantity and quality and sleep disorders in 3.5-year-old offspring. Article. Sleep Med. 2019;56:201–210. doi: 10.1016/j.sleep.2018.10.042 [DOI] [PubMed] [Google Scholar]
- 38.Williamson AA, Mindell JA, Hiscock H, et al. Sleep problem trajectories and cumulative socio-ecological risks: birth to school-age. Conference Paper. J Pediatr. 2019;215:229–237.e4. doi: 10.1016/j.jpeds.2019.07.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garthus-Niegel S, Horsch A, Bickle Graz M, et al. The prospective relationship between postpartum PTSD and child sleep: A 2-year follow-up study. Article. J Affect Disord. 2018;241:71–79. doi: 10.1016/j.jad.2018.07.067 [DOI] [PubMed] [Google Scholar]
- 40.Petzoldt J, Wittchen HU, Einsle F, et al. Maternal anxiety versus depressive disorders: specific relations to infants’ crying, feeding and sleeping problems. Article. Child: Care Health Dev. 2016;42(2):231–245. doi: 10.1111/cch.12292 [DOI] [PubMed] [Google Scholar]
- 41.Agrawal A, Ickovics J, Lewis JB, et al. Postpartum intimate partner violence and health risks among young mothers in the United States: a prospective study. Journal Article; Multicenter Study; Randomized Controlled Trial; Research Support, N.I.H., Extramural. Matern Child Health J. 2014;18(8):1985–1992. doi: 10.1007/s10995-014-1444-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caravale B, Sette S, Cannoni E, et al. Sleep characteristics and temperament in preterm children at two years of age. J Clin Sleep Med. Sep 15 2017;13(9):1081–1088. doi: 10.5664/jcsm.6728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Galbally M, Watson SJ, Teti D, et al. Perinatal maternal depression, antidepressant use and infant sleep outcomes: exploring cross-lagged associations in a pregnancy cohort study. Article. J Affect Disord. 2018;238:218–225. doi: 10.1016/j.jad.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 44.Morales-Muñoz I, Partonen T, Saarenpää-Heikkilä O, et al. The role of parental circadian preference in the onset of sleep difficulties in early childhood. Article. Sleep Med. 2019;54:223–230. doi: 10.1016/j.sleep.2018.10.039 [DOI] [PubMed] [Google Scholar]
- 45.Chuang CH, Jeng SF, Hsieh WS, et al. Maternal psychosocial factors around delivery, and the behavior of 2-year-old children. Article. Pediatr Int. 2011;53(5):656–661. doi: 10.1111/j.1442-200X.2010.03315.x [DOI] [PubMed] [Google Scholar]
- 46.Eiden RD, Zhao J, Casey M, et al. Pre- and postnatal tobacco and cannabis exposure and child behavior problems: bidirectional associations, joint effects, and sex differences. Article. Drug Alcohol Depend. 2018;185:82–92. doi: 10.1016/j.drugalcdep.2017.11.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Callaghan F, O’Callaghan M, Scott JG, et al. Effect of maternal smoking in pregnancy and childhood on child and adolescent sleep outcomes to 21 years: a birth cohort study. Article. BMC Pediatr. 2019;19(1)doi: 10.1186/s12887-019-1439-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simcock G, Cobham VE, Laplante DP, et al. A cross-lagged panel analysis of children’s sleep, attention, and mood in a prenatally stressed cohort: The QF2011 Queensland flood study. J Affect Disord. 2019;255:96–104. doi: 10.1016/j.jad.2019.05.041 [DOI] [PubMed] [Google Scholar]
- 49.Weinraub M, Friedman SL, Knoke B, et al. Patterns of developmental change in infants’ nighttime sleep awakenings from 6 through 36 months of age. Article. Dev Psychol. 2012;48(6):1511–1528. doi: 10.1037/a0027680 [DOI] [PubMed] [Google Scholar]
- 50.Northerner L, Trentacosta C, McLear C. Negative affectivity moderates associations between cumulative risk and at-risk toddlers’ behavior problems. J Child Fam Stud. 2016;25(2):691–699. doi: 10.1007/s10826-015-0248-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pesonen A-K, Räikkönen K, Matthews K, et al. Prenatal origins of poor sleep in children. Sleep. 2009;32(8):1086–1092. doi: 10.1093/sleep/32.8.1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dias CC, Figueiredo B. Mother’s prenatal and postpartum depression symptoms and infant’s sleep problems at 6 months. Infant Ment Health J. Sep 2020;41(5):614–627. doi: 10.1002/imhj.21869 [DOI] [PubMed] [Google Scholar]
- 53.Harskamp-van Ginkel MW, Kool RE, van Houtum L, et al. Potential determinants during ‘the first 1000 days of life’ of sleep problems in school-aged children. Sleep Med. May 2020;69:135–144. doi: 10.1016/j.sleep.2019.12.020 [DOI] [PubMed] [Google Scholar]
- 54.Goldberg WA, Lucas-Thompson RG, Germo GR, et al. Eye of the beholder? Maternal mental health and the quality of infant sleep. Social Science & Medicine. 2013;79:101–108. doi: 10.1016/j.socscimed.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Figueiredo B, Dias CC, Pinto TM, et al. Exclusive breastfeeding at three months and infant sleep-wake behaviors at two weeks, three and six months. Infant Behav Dev. 2017;49:62–69. doi: 10.1016/j.infbeh.2017.06.006 [DOI] [PubMed] [Google Scholar]
- 56.Pierrehumbert B, Nicole A, Muller-Nix C, et al. Parental post-traumatic reactions after premature birth: implications for sleeping and eating problems in the infant. Arch Dis Child Fetal Neonatal Ed. Sep 2003;88(5):F400–4. doi: 10.1136/fn.88.5.f400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xavier MO, Bielemann RM, Carpena MX, et al. Sleep duration trajectories from age 3 to 48 months in The Pelotas (Brazil) 2004 birth cohort study. Article. Paediatr Perinat Epidemiol. 2020;34(1):60–69. doi: 10.1111/ppe.12628 [DOI] [PubMed] [Google Scholar]
- 58.Reynaud E, Forhan A, Heude B, et al. Night-waking trajectories and associated factors in French preschoolers from the EDEN birth-cohort. Article. Sleep Med. 2016;27–28:59–65. doi: 10.1016/j.sleep.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 59.Schäfer AA, Domingues MR, Dahly DL, et al. Correlates of self-reported weekday sleep duration in adolescents: the 18-year follow-up of the 1993 Pelotas (Brazil) birth cohort study. Sleep Med. 2016;23:81–88. doi: 10.1016/j.sleep.2016.02.013 [DOI] [PubMed] [Google Scholar]
- 60.O’Connor TG, Caprariello P, Blackmore ER, et al. Prenatal mood disturbance predicts sleep problems in infancy and toddlerhood. Article. Early Hum Dev. 2007;83(7):451–458. doi: 10.1016/j.earlhumdev.2006.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chandler-Mather N, Occhipinti S, Donovan C, et al. An investigation of the link between prenatal alcohol exposure and sleep problems across childhood. Drug Alcohol Depend. 2021;218:108412–108412. doi: 10.1016/j.drugalcdep.2020.108412 [DOI] [PubMed] [Google Scholar]
- 62.Gillioen B, Plancoulaine S, Montemitro E, et al. Maturation of arousals during day and night in infants with non-smoking and smoking mothers. Article. Early Hum Dev. 2017;115:46–50. doi: 10.1016/j.earlhumdev.2017.08.006 [DOI] [PubMed] [Google Scholar]
- 63.Sarfi M, Martinsen H, Bakstad B, et al. Patterns in sleep-wakefulness in three-month old infants exposed to methadone or buprenorphine. Article. Early Hum Dev. 2009;85(12):773–778. doi: 10.1016/j.earlhumdev.2009.10.006 [DOI] [PubMed] [Google Scholar]
- 64.Taylor AK, Netsi E, O’Mahen H, et al. The association between maternal postnatal depressive symptoms and offspring sleep problems in adolescence. Psychol Med. 2017;47(3):451–459. doi: 10.1017/S0033291716002427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Armitage R, Flynn H, Hoffmann R, et al. Early developmental changes in sleep in infants: The impact of maternal depression. Sleep. 2009;32(5):693–696. doi: 10.1093/sleep/32.5.693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bat-Pitault F, Sesso G, Deruelle C, et al. Altered sleep architecture during the first months of life in infants born to depressed mothers. Sleep Med. 2017;30:195–203. doi: 10.1016/j.sleep.2016.11.018 [DOI] [PubMed] [Google Scholar]
- 67.Brandlistuen RE, Ystrom E, Eberhard-Gran M, et al. Behavioural effects of fetal antidepressant exposure in a Norwegian cohort of discordant siblings. Int J Epidemiol. 2015;44(4):1397–1407. doi: 10.1093/ije/dyv030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ystrom E, Hysing M, Torgersen L, et al. Maternal symptoms of anxiety and depression and child nocturnal awakenings at 6 and 18 months. Article. J Pediatr Psychol. 2017;42(10):1156–1164. doi: 10.1093/jpepsy/jsx066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barazzetta M, Ghislandi S. Family Income and Material Deprivation: Do They Matter for Sleep Quality and Quantity in Early Life? Evidence From a Longitudinal Study. Sleep. 2017;40(3):zsw066. doi: 10.1093/sleep/zsw066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cook F, Conway L, Gartland D, et al. Profiles and predictors of infant sleep problems across the first year. Article. J Dev Behav Pediatr. 2020;41(2):104–116. doi: 10.1097/DBP.0000000000000733 [DOI] [PubMed] [Google Scholar]
- 71.Liu J, Zhou G, Wang Y, et al. Sleep problems, fatigue, and cognitive performance in Chinese kindergarten children. J Pediatr. 2012;161(3):520–525.e2. doi: 10.1016/j.jpeds.2012.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Z, Dai Y, Liu X, et al. Early childhood co-sleeping predicts behavior problems in preadolescence: a prospective cohort study. Behav Sleep Med. 2020:1–14. doi: 10.1080/15402002.2020.1818564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van den Hoogen A, Teunis CJ, Shellhaas RA, et al. How to improve sleep in a neonatal intensive care unit: a systematic review. Early Hum Dev. 2017;113:78–86. doi: 10.1016/j.earlhumdev.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 74.Muñoz-Moreno E, Fischi-Gomez E, Batalle D, et al. Structural brain network reorganization and social cognition related to adverse perinatal condition from infancy to early adolescence. Front Neurosci. 2016;10:560. doi: 10.3389/fnins.2016.00560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Scher MS, Loparo KA. Neonatal EEG/sleep state analyses: a complex phenotype of developmental neural plasticity. Dev Neurosci. 2009;31(4):259–75. doi: 10.1159/000216537 [DOI] [PubMed] [Google Scholar]
- 76.Testa M, Quigley BM, Eiden RD. The effects of prenatal alcohol exposure on infant mental development: a meta-analytical review. Alcohol Alcohol. Jul-Aug 2003;38(4):295–304. doi: 10.1093/alcalc/agg087 [DOI] [PubMed] [Google Scholar]
- 77.Smith AM, Dwoskin LP, Pauly JR. Early exposure to nicotine during critical periods of brain development: mechanisms and consequences. J Pediatr Biochem. 2010;1(2):125–141. doi: 10.3233/jpb-2010-0012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Neger EN, Prinz RJ. Interventions to address parenting and parental substance abuse: conceptual and methodological considerations. Clin Psychol Rev. 2015;39:71–82. doi: 10.1016/j.cpr.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schultz LF, Kroll C, Constantino B, et al. Association of Maternal Depression and Anxiety Symptoms with Sleep Duration in Children at Preschool Age. Matern Child Health J. 2020;24(1):62–72. doi: 10.1007/s10995-019-02843-z [DOI] [PubMed] [Google Scholar]
- 80.Sharkey K, Iko I, Machan J, et al. Infant sleep and feeding patterns are associated with maternal sleep, stress, and depressed mood in women with a history of major depressive disorder (MDD). Archives of Women’s Mental Health. 2016;19(2):209–218. doi: 10.1007/s00737-015-0557-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Field T. Prenatal depression effects on early development: a review. Infant behavior and development. 2011;34(1):1–14. [DOI] [PubMed] [Google Scholar]
- 82.Astiz M, Oster H. Perinatal Programming of Circadian Clock-Stress Crosstalk. Neural Plast. 2018;2018:5689165. doi: 10.1155/2018/5689165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tahara Y, Aoyama S, Shibata S. The mammalian circadian clock and its entrainment by stress and exercise. J Physiol Sci. 2017;67(1):1–10. doi: 10.1007/s12576-016-0450-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teti DM, Crosby B, Teti DM, et al. Maternal depressive symptoms, dysfunctional cognitions, and infant night waking: the role of maternal nighttime behavior. Child Dev. 2012;83(3):939–953. doi: 10.1111/j.1467-8624.2012.01760.x [DOI] [PubMed] [Google Scholar]
- 85.Swanson LM, Kalmbach DA, Raglan GB, et al. Perinatal Insomnia and Mental Health: a Review of Recent Literature. Curr Psychiatry Rep. Oct 26 2020;22(12):73. doi: 10.1007/s11920-020-01198-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Halal CS, Bassani DG, Santos IS, et al. Maternal perinatal depression and infant sleep problems at 1 year of age: subjective and actigraphy data from a population-based birth cohort study. Article. J Sleep Res. 2021;30(2)doi: 10.1111/jsr.13047 [DOI] [PubMed] [Google Scholar]
- 87.Morales-Muñoz I, Saarenpää-Heikkilä O, Kylliäinen A, et al. The effects of maternal risk factors during pregnancy on the onset of sleep difficulties in infants at 3 months old. Article. J Sleep Res. 2018;27(5)doi: 10.1111/jsr.12696 [DOI] [PubMed] [Google Scholar]
- 88.Fawcett EJ, Fairbrother N, Cox ML, et al. The Prevalence of Anxiety Disorders During Pregnancy and the Postpartum Period: A Multivariate Bayesian Meta-Analysis. J Clin Psychiatry. 2019;80(4)doi: 10.4088/JCP.18r12527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Adamson B, Letourneau N, Lebel C. Prenatal maternal anxiety and children’s brain structure and function: A systematic review of neuroimaging studies. Journal of Affective Disorders. 2018;241:117–126. doi: 10.1016/j.jad.2018.08.029 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.