Abstract
AMSH, a molecule that associates with STAM1, is involved in the in vitro cell growth signaling mediated by interleukin 2 and granulocyte-macrophage colony-stimulating factor. To investigate the in vivo functional role of AMSH, we have generated AMSH-deficient mice by gene targeting. The AMSH-deficient mice were morphologically indistinguishable from their littermates at birth, and histopathological examinations revealed normal morphogenesis in all tissues tested. However, all the AMSH-deficient mice exhibited postnatal growth retardation and died between postnatal day 19 (P19) and P23. Examination of brain sections at P6 demonstrated significant loss of neurons and apoptotic cells in the CA1 subfield of the hippocampus. Brain atrophy developed by P16 and was accompanied by complete loss of the CA1 neurons in the hippocampus and marked atrophy of the cerebral cortex. Furthermore, AMSH-deficient hippocampal neuronal cells were unable to survive in vitro, even in the presence of several stimulatory cytokines, while AMSH-deficient cerebellar neurons, thymocytes, and embryonic fibroblasts survived normally. Taken together, these observations indicate that AMSH is an essential molecule for the survival of neuronal cells in early postnatal mice.
Cytokines are essential factors for cell activation, differentiation, survival, and apoptosis in many biological events. The effects of cytokines on target cells are mediated by their interactions with specific receptors, which transduce the intracellular signals in the target cells. Among the many cytokines, interleukin 2 (IL-2) is known to be a critical soluble ligand for activating T cells in immune responses (12, 34, 35). During the search for signaling molecules involved in the IL-2-mediated signaling pathway, we identified the STAM family of molecules, STAM1 and STAM2, both of which are associated with Jak2 and Jak3 tyrosine kinases (9, 36, 37). We subsequently isolated a novel adapter molecule from human T cells that we named AMSH, for associated molecule with the SH3 domain of STAM1 (39). Recently, a novel SH3-binding motif (SBM) of AMSH that interacts with the SH3 domain of both STAM1 and STAM2 was identified (17, 39). SH3 deletion mutants of the STAMs act as dominant-negative forms in signaling that induces cell growth, and the wild-type, but not the mutant, STAMs enhance c-myc induction that is mediated by IL-2 and granulocyte-macrophage colony-stimulating factor (GM-CSF) (37). Hence, we hypothesized that AMSH might be involved in the cytokine-mediated signaling through its interaction with the STAMs. In this context, we have already demonstrated that an AMSH mutant with its C-terminal half deleted and retaining its STAM1-binding ability confers a dominant-negative effect on IL-2- and GM-CSF-mediated signaling pathways that induce DNA synthesis and c-myc transcription (39). The deleted C terminus of AMSH contained an Mov34/MPN domain (3), which is conserved in several subunits of the COP9 signalsome, although the function of this domain is still unknown. Collectively, these observations suggest that AMSH may be involved in cytokine signaling, particularly in the signaling that occurs downstream of the Jaks and STAMs.
Recently, we generated STAM1-deficient mice, which showed a loss of hippocampal CA3 pyramidal neurons, suggesting that STAM1 is critically involved in neuronal cell survival in vivo (43). In spite of the functional significance of STAM1 in in vitro cytokine signaling, the STAM1 deficiency had little effect on lymphocyte development or proliferative responses to IL-2 and GM-CSF in vivo (43). The discrepancy between the in vitro and in vivo functional roles of STAM1 may be accounted for by the compensating effect of STAM2. The neuronal abnormalities observed in STAM1-deficient mice suggested that AMSH might also be involved in neuronal cell survival.
We previously identified a novel Grb2 family molecule, Gads/Grf40, as a molecule that is associated with AMSH (1). Gads is involved in T-cell receptor (TCR) signaling through its interactions with SLP76 and LAT (1, 20). Mice with a knockout for Gads and mice transgenic for a mutant Gads with its SH2 domain deleted showed impairments in pre-T-cell development (18, 44), suggesting that Gads is indispensable in T-cell development. Although the biological significance of the interaction between Gads and AMSH is still unclear, we expected that AMSH might contribute to the regulation of T-cell development through pre-TCR and TCR signaling.
To elucidate the in vivo functional significance of AMSH, we report here the generation of AMSH-deficient mice by gene targeting and demonstrate that AMSH is essential for the survival of neurons of the hippocampal CA1 and cerebral cortex but dispensable for the development of lymphocytes and for their intracellular signal transduction that is mediated by cytokines and TCR ligation.
MATERIALS AND METHODS
Targeted disruption of AMSH.
The targeting vector was constructed using a phosphoglycerate kinase (PGK)-neo cassette flanked by a pair of loxP sequences for positive selections and a diphtheria toxin A-chain gene cassette without a polyadenylation site for negative selection (Fig. 1B). This targeting construct replaced a 0.6-kb HindIII-BamHI genomic fragment in the fourth intron flanked by 4.1-kb (SmaI-ScaI) and 4.8-kb (BamHI-BamHI) genomic sequences derived from the 129/Sv genomic library (Fig. 1B). The construct was linearized and electroporated into 129/Sv-derived J1 embryonic stem (ES) cells, and G418-resistant colonies were selected (21, 22, 25). Homologous recombination events were assessed by Southern blot hybridization. The targeted ES clones were then transiently transfected with pCXN2-Cre, an expression vector for a recombinase Cre, and G418-sensitive colonies were selected. Removal of the 3.7-kb fragment of the PGK-neo cassette between the two loxP sequences was checked by Southern blot hybridization and PCR. The obtained ES clones were injected into C57BL/6 blastocysts and transferred to foster mothers to obtain chimeric mice. The chimeric male mice were mated with C57BL/6 females. The F1 heterozygous mice carrying the AMSH mutation were identified by Southern blot hybridization and intercrossed to produce F2 homozygous offspring. The F2 mice were genotyped by Southern blot hybridization and PCR with DNA from tail biopsy specimens. The following oligonucleotide primers were used in the PCR: AMSH-5AM, TCCCACCTCCTCTTGCTATTTCATACCC, and AMSH-3L, ACTTGACAGACTTTAGAATCACCCAGAA (Fig. 1B).
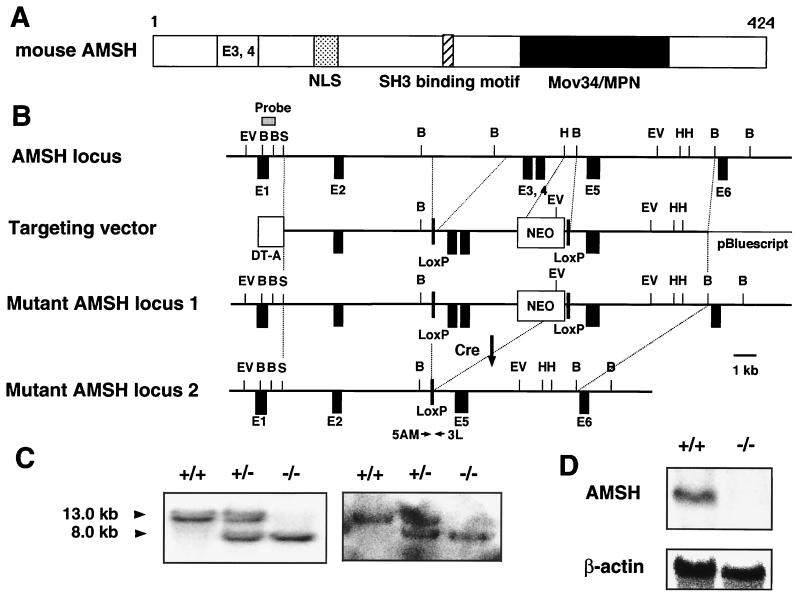
FIG. 1.
Generation of AMSH-deficient mice. (A) Schematic structure of mouse AMSH. We cloned murine AMSH cDNA (data not shown; DDBJ accession no. AB010123). The deduced amino acid sequence of AMSH consists of 424 residues and contains three characteristic regions as follows: NLS (Arg112-Lys127) is a putative bipartite nuclear localization signal, SBM (Pro231-Pro239) is an SH3 domain-binding motif (PX[V/I][D/N]RXXKP) (16), and Mov34/MPN (Glu252-Glu361) is an MPR1–PAD1–N-terminal domain (3). (B) Schematics of the genomic, targeted, recombined, and Cre-treated mutant alleles of AMSH. The AMSH mutation was engineered by replacing a genomic fragment with a PGK-neo cassette including exons 3 and 4 flanked by a pair of loxP sequences. The targeted ES clones were transiently transfected with a Cre recombinase expression vector, and G418-susceptible colonies were selected. Removal of the 3.7-kb fragment including the neo cassette between the two loxP sequences was checked by Southern blot hybridization and PCR. B, BamHI; EV, EcoRV; H, HindIII; S, SalI; DT-A, diphtheria toxin A. (C) Southern blot analysis of DNA prepared from mouse tails. DNA was digested with EcoRV, and the blot was probed with the flanking 5′ probe as shown in panel B. (D) Northern blot analysis of total RNA from newborn brains. Twenty micrograms of total RNA prepared from brains of newborn wild-type mice and homozygotes for the AMSH mutant mice was blotted onto a nylon membrane. The blot was hybridized with the AMSH cDNA probe and then rehybridized with the β-actin probe.
Northern blot analysis.
Total RNA of each tissue derived from an adult C57BL/6 mouse was extracted by using TRIzol (Life Technologies, Inc., Rockville, Md.). Twenty micrograms of the RNA from several tissues indicated was electrophoresed and blotted onto a Hybond-N nylon membrane (Amersham Pharmacia Biotech, Little Chalfont, Buckinghamshire, United Kingdom). A 1.6-kbp fragment of AMSH cDNA was used as a probe. The glyceraldehyde-3-phosphate dehydrogenase and β-actin probes were described previously (13, 39). The probes were labeled with [α-32P]dCTP (Amersham Pharmacia Biotech) by using the Random primer DNA labeling kit, version 2 (Takara Biomedicals, Tokyo, Japan). Hybridization was performed for 20 h at 42°C in 50% formamide–5× Denhardt's solution–5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% sodium dodecyl sulfate–20 mM Tris-HCl (pH 7.5)–200 μg of sonicated and denatured salmon sperm DNA/ml. The membranes were washed in 0.2× SSC and 0.1% sodium dodecyl sulfate three times at 65°C. Radioactivity was analyzed with a Bio-Image MacBAS1500 analyzer (Fuji Film, Tokyo, Japan).
Proliferation assay.
Single-cell suspensions of spleen cells or thymus in RPMI 1640 medium supplemented with 10% fetal calf serum, 50 μM 2-mercaptomethanol, penicillin, and streptomycin were plated in 96-well plates at a density of 2 × 105 to 5 × 105 cells per well in 200 μl of medium. Stimuli were added as indicated and cultured for 42 h. The stimuli were recombinant human IL-2 (Ajinomoto, Tokyo, Japan), recombinant murine IL-4 (PeproTech, Rocky Hill, N.J.), anti-CD3 monoclonal antibody (MAb; 145.2C11), and concanavalin A (ConA). The cells were then pulsed with [3H]thymidine and harvested after 6 h. Incorporated [3H]thymidine was counted with a MicroBeta liquid scintillation counter (Amersham Pharmacia Biotech).
Flow cytometry.
Thymocytes and splenic cells were suspended in phosphate-buffered saline supplemented with 3% fetal calf serum. They were preincubated in normal mouse serum to prevent labeled MAbs from nonspecifically binding to the cell surface. They were then stained with MAbs conjugated with fluorescein isothiocyanate or phycoerythrin for 30 min at 4°C. All the MAbs used were purchased from Pharmingen. The surface stainings with MAbs were analyzed with a FACSCalibur flow cytometer (Becton Dickinson Immunocytometry Systems, Inc., Mountain View, Calif.) in two- or three-color mode using CellQuest software.
Histopathological analyses.
Brains of wild-type and AMSH-deficient mice were removed, fixed in 4% paraformaldehyde–0.1 M phosphate buffer, and embedded in paraffin. Paraffin sections of 5 μm in thickness were prepared and stained with toluidine blue.
TUNEL staining.
Terminal deoxynucleotidyltransferase (TdT)-mediated dUTP-biotin nick end labeling (TUNEL) was performed by following protocols attached to a commercial kit (TACS2 TdT-Blue Label in situ apoptosis detection kit; Trevigen Instructions, Gaithersburg, Md.). Briefly, after deparaffinization and rehydration, brain sections were digested for 15 min in proteinase K. The reaction was terminated with tap H2O, and the tissue sections were treated with 1× TdT labeling buffer for 5 min. The sections were incubated at 37°C with labeling reaction mix containing TdT, biotinylated dUTP, and manganese chloride in 1× TdT labeling buffer for 1 h in a humidified chamber. The reaction was terminated with 1× TdT stop buffer. The DNA was visualized by treating the sections with streptavidin-conjugated horseradish peroxidase and TACS Blue Label (Trevigen). The sections were counterstained with nuclear fast red (Sigma).
In situ hybridization.
In situ hybridization for mouse AMSH was performed according to the modified method described previously (27, 29). Fresh frozen whole bodies of mouse embryos at various embryonic stages or brain tissues extracted from mice at various postnatal stages were sectioned with a thickness of 30 μm on a cryostat. After fixation in 4% paraformaldehyde–0.1 M sodium phosphate buffer (pH 7.2), the sections were acetylated with 0.25% acetic anhydride in 0.1 M triethanolamine (pH 8.0) and prehybridized for 1 h in a buffer containing 50% deionized formamide, 4× SSC, 0.02% Ficoll, 1% sodium N-lauroyl sarcosinate (Sarkosyl), 0.1 M phosphate buffer, and 100 μg of tRNA/ml. Hybridization was performed overnight at 42°C in the prehybridization buffer supplemented with 10% dextran sulfate, 100 mM dithiothreitol, and the 35S-labeled AMSH oligonucleotide probe. The sections were washed with 0.1× SSC–0.1% Sarkosyl at 50°C four times for 30 min. They were exposed to Hyperfilm β-max (Amersham, Arlington, Ill.) for 2 weeks at room temperature. They were subsequently autoradiographed using NTB2 nuclear track emulsion (Eastman Kodak, Rochester, N.Y.) for 3 weeks at 4°C.
Primary culture of embryonic hippocampal neurons.
The preparation of primary culture of embryonic hippocampal or cerebellar neurons was previously described (43). In brief, primary hippocampal or cerebellar neurons were isolated from wild-type and AMSH−/− embryos on embryonic day 18.5 (E18.5). Fetal hippocampi or cerebellar cortex was dissected and minced with scissors. Individual cells were mechanically isolated by trituration in calcium–magnesium-free Hanks' balanced salt solution with a 9-in. siliconized Pasteur pipette. The cells were plated on poly-d-lysine-coated plates (Falcon, Lincoln Park, N.J.) and cultured in neurobasal medium (Life Technologies, Inc.) containing 0.5 mM l-glutamine and B27 supplement (Life Technologies, Inc.) at 37°C in a humidified atmosphere of 5% CO2 and 95% room air. In the case of hippocampal neurons, 20 μM l-glutamate was added during the first 3 days of culture. Plating densities ranged from 600 to 800 cells/mm2.
Cell survival assay in primary neuronal cell culture.
Neuronal cells isolated from the hippocampus or cerebellar cortex were cultured as described above in the absence or presence of natural murine nerve growth factor (NGF) (Life Technologies, Inc.), recombinant human brain-derived neurotrophic factor (BDNF; PeproTech, Inc.), recombinant human transforming growth factor β1 (TGF-β1; PeproTech, Inc.), and recombinant murine tumor necrosis factor alpha (TNF-α; PeproTech, Inc.). To estimate the cell survival, Alamar blue fluorescent dye (Alamar Biosciences, Sacramento, Calif.), which is a redox indicator to assess viability and mitochondrial activity of the cells (40, 41), was used by following a protocol manual for Fluoroskan Ascent (Labsystems). The culture method that we used induces the differentiation of neurons, during which mitochondrial numbers and activity in the individual neuronal cells increase. The Alamar blue assay detects not only live cells but also differentiated neuronal cells as increased mitochondrial activity occurs. Because this method does not require cell washing before the measurement, both viable attached and detached cells in the same well can be estimated. In brief, at the indicated time after culture of the neuronal cells, Alamar blue dye was added into each culture well at a 10% concentration, and the cells were incubated at 37°C for exactly 120 min in the dark. The fluorescence intensity (590 nm) of each well, which was excited by 544-nm light, was measured with Fluoroskan Ascent (Labsystems). The viability index (mean intensity of test well − mean intensity of blank) was expressed at 590 nm. The survival index was defined as a ratio of (viability index at the indicated day)/(viability index at day 0) × 100%.
RESULTS
Generation of AMSH-deficient mice.
To generate a targeted disruption of AMSH, we first screened a mouse 129/Sv genomic library using a human AMSH cDNA fragment as the probe. The genomic sequences of the three isolated clones overlapped in part and encompassed at least six exons, including a 5′ noncoding exon of AMSH. A targeting vector for the mouse AMSH gene was created by inserting a loxP-flanked neomycin resistance cassette, which included exons 3 and 4, upstream of exon 5 (Fig. 1A and B). The targeting vector was introduced into ES cells through homologous recombination, and homologous recombination events were identified using Southern blot analysis. Three ES clones carrying the mutant allele were selected and then transiently transfected with a plasmid that carries Cre recombinase driven by the β-actin promoter to eliminate the neomycin resistance cassette. Two independent ES clones were obtained and microinjected into C57BL/6 mouse blastocysts to generate chimeric mice. Chimeric mice derived from one of the two ES clones were found to transmit the mutant allele to their offspring. Wild-type, homozygous, and heterozygous mutant genotypes were determined by Southern blot analysis of DNA from the progeny obtained by interbreeding the heterozygous mice; 8.0- and 13.0-kb bands were detected as the mutant and wild-type alleles, respectively (Fig. 1C).
To examine the expression of AMSH, we performed Northern blot analyses using the total RNA of brains from wild-type and homozygous mice. As with gene knockout mice, no significant band was detected in the AMSH−/− lane, probably due to an instability of the mutated AMSH mRNA (Fig. 1D). These results clearly demonstrated that the homologous mutation of the AMSH gene led to an AMSH deficiency in the mice.
Phenotypes of AMSH-deficient mice.
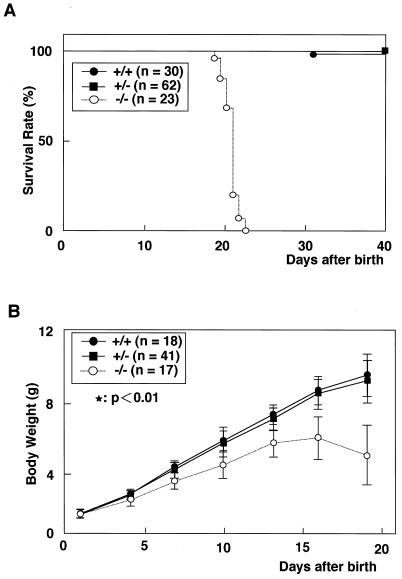
Newborn AMSH-deficient mice showed no morphological abnormality, compared with their littermates at birth. Genotypic analysis of neonatal offspring (n = 237) from AMSH+/− matings revealed an expected Mendelian ratio of AMSH+/+ (24.0%), AMSH+/− (52.3%), and AMSH−/− (23.6%) animals, indicating that AMSH is not crucial for embryonic development. However, all the AMSH−/− mice died between postnatal day 19 (P19) and P23 (20.8 ± 1.1) (Fig. 2A). They appeared to die of starvation, as their stomachs were found to be empty upon dissection (data not shown). The AMSH−/− mice showed significant growth retardation by P7, and their body weights began to fall after P16 (Fig. 2B). At P15, the AMSH−/− mice exhibited neurological abnormalities of their hind limbs, which retracted toward the trunk when the animals were lifted by their legs (data not shown). Drooping of the upper eyelid (blepharoptosis) was seen in a third of AMSH−/− mice at P16 (data not shown). Histopathological analyses of 12-day-old AMSH−/− mice showed no abnormality in any of the tissues tested (thymus, spleen, liver, lung, heart, kidney, intestinal tracts, colon, and stomach) except the brain, which is described below. AMSH+/− mice showed no distinguishable differences in survival and growth rates from their wild-type littermates (Fig. 2).
FIG. 2.
Life span and growth characteristics of the AMSH mutant mice. (A) Survival curve of homozygotes, heterozygotes, and wild-type mice derived from heterozygous intercrosses. (B) Average body weights of the AMSH mutant mice.
Because AMSH was initially cloned as a possible signaling molecule downstream of the cytokine receptors for IL-2 and GM-CSF (39), we investigated whether the AMSH deficiency affected the development of T and B lymphocytes and the proliferative responses of T cells to stimulation with cytokines or an anti-CD3 antibody. Thymocytes and splenic cells derived from 15-day-old AMSH−/− mice were analyzed by flow cytometry to detect the expression of CD4, CD8, B220, and CD3. There were no differences in the T-cell subpopulations in the thymus and the splenic B-cell population between the AMSH−/− and AMSH+/+ mice (Fig. 3A). The splenic cells and thymocytes derived from AMSH−/− mice responded equally as well as those from wild-type mice to various stimuli, including ConA, IL-4, IL-2, anti-CD3, and combinations of these agents (Fig. 3B to D). These results indicate that AMSH is dispensable for T- and B-cell development and for T-cell proliferative responses upon stimulation with cytokines or an anti-CD3 antibody.
FIG. 3.
Comparison of T-cell development and proliferative responses to cytokines between STAM1+/+ and STAM1−/− mice. (A) Flow cytometric analysis of AMSH-deficient mice. Thymic lymphocytes derived from 15-day-old AMSH+/+ (n = 8) and AMSH−/− (n = 8) mice were doubly stained with anti-CD4 and anti-CD8 MAbs. Splenic lymphocytes derived from 15-day-old AMSH+/+ and AMSH−/− mice were doubly stained with anti-CD3 and anti-B220 MAbs. Numbers indicate the average percentages of the gated cellular subpopulations within the lymphocyte population. (B) Proliferative responses of spleen cells. Total splenocytes (2 × 105 per well) were stimulated with indicated ligands: 10 μg of ConA/ml, 10 nM IL-4, 10 nM IL-2, and 5 μg of anti-CD3 MAb/ml. They were cultured for 42 h, then pulsed with [3H]thymidine, and harvested after 6 h. (C) Proliferative responses of splenic T cells to recombinant IL-2. ConA-activated splenic T cells (2 × 105 per well) were stimulated with IL-2. [3H]thymidine incorporation was measured as described above. (D) Proliferative responses of thymocytes to CD3 stimulation. Total thymocytes (5 × 105 per well) were stimulated with anti-CD3 MAb or anti-CD3 plus anti-CD28 MAb.
Histopathological abnormalities of the AMSH−/− brain.
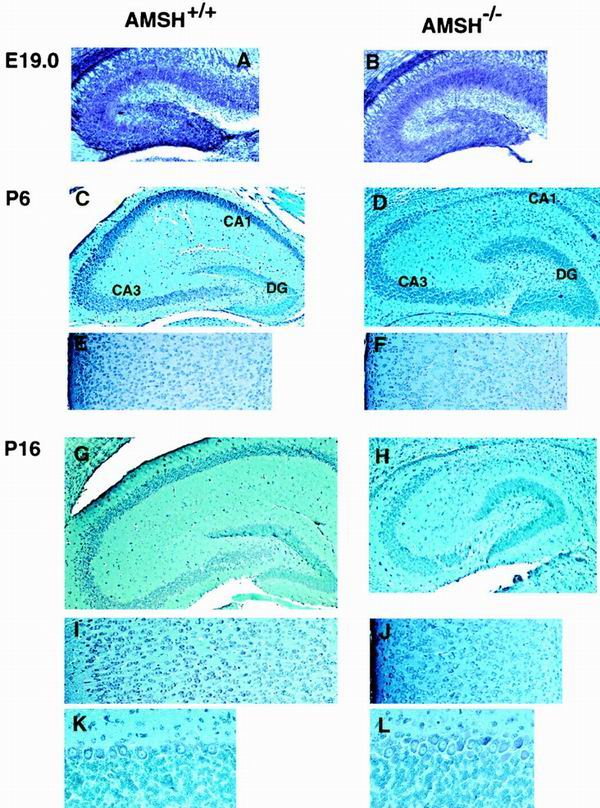
Brain samples from AMSH−/− mice were examined histopathologically and compared with samples from AMSH+/+ mice. We found no differences in the various brain regions, including the hippocampus and cerebral cortex, between AMSH−/− and AMSH+/+ embryos at E19.0 (Fig. 4A and B) or mice at P1 (data not shown). However, by P6 the AMSH−/− mice exhibited appreciable degradation of the hippocampal CA1 neurons, although their cerebral cortices seemed to be normal (Fig. 4C to F). The extents of hippocampal CA1 neuronal loss in caudal, middle, and rostral sections were similar (data not shown). At day 11 of age, AMSH−/− hippocampal CA1 subfields were markedly diminished, and surprisingly, the cellularity of AMSH−/− cerebral cortex was clearly reduced (data not shown). In 16-day-old AMSH−/− mice, the hippocampal CA1 subfield was completely abolished. The number of neurons of the cerebral cortex was markedly reduced, and the layers of the cortex became undefined (Fig. 4G to J). In contrast to the hippocampus and cerebral cortex at P16, we could not detect histopathological abnormalities in any other brain region, including the cerebellar cortex (Fig. 4K and L) and the olfactory bulb (data not shown), although AMSH was abundantly expressed in these regions (see below).
FIG. 4.
Abnormalities in hippocampal CA1 subfields and cerebral cortex in AMSH-deficient mice. The figure shows toluidine blue staining of anterior coronal hippocampus sections (A to D, G, and H), coronal sections of cerebral cortex (E, F, I, and J), and coronal sections of cerebellar cortex (K and L). The figure shows AMSH+/+ mice (A, C, E, G, I, and K) and AMSH−/− mice (B, D, F, H, J, and L) at E19.0 (A and B), at 6 days old (C to F), and at 16 days old (G to L). Magnifications, approximately ×50 (A and B), approximately ×30 (C, D, G, and H), approximately ×60 (E, F, I, and J), and approximately ×200 (K and L).
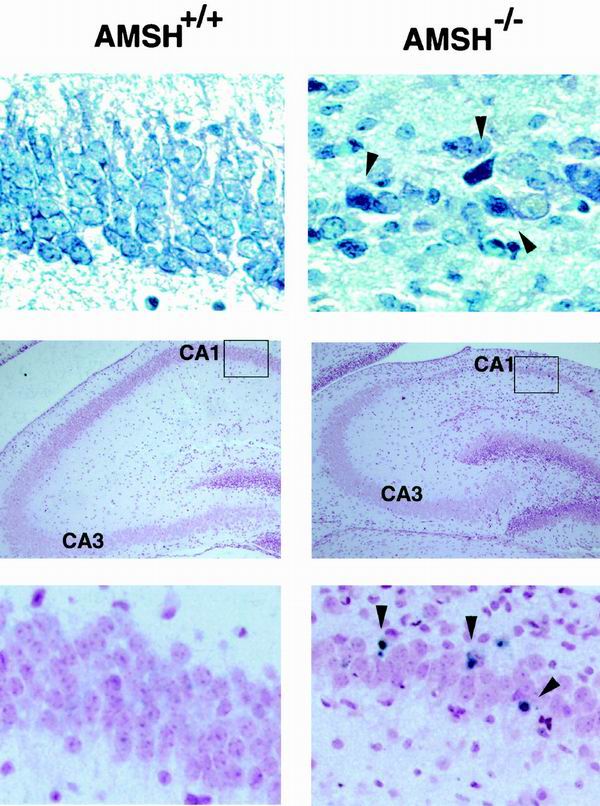
Examination of the hippocampal CA1 subfield of 6-day-old AMSH−/− mice at higher magnifications clearly revealed several pyknotic neurons (Fig. 5). To investigate the mechanism underlying the degradation of the neurons, we performed TUNEL staining for the hippocampal CA1 subfield. TUNEL-positive cells were detected in the hippocampal CA1 subfield of the AMSH−/− mice but not of wild-type mice (Fig. 5), suggesting that the degradation and loss of neurons in the AMSH−/− mice were caused by apoptosis.
FIG. 5.
Appotosis of the AMSH-deficient hippocampal neurons in vivo. The top two panels show toluidine blue staining of anterior coronal hippocampus sections; the four lower panels show TUNEL staining of anterior coronal hippocampus sections. The figure shows AMSH+/+ mice (left panels) and AMSH−/− mice (right panels) at 6 days old. The bottom two panels represent the magnified views of the areas in the boxes as shown in the middle two panels (left and right, respectively). Arrowheads in the top right and bottom right panels indicate pyknotic and TUNEL-positive cells, respectively. Magnifications, approximately ×300 (top and bottom panels) and approximately ×60 (middle panels).
Expression of AMSH mRNA in brain of wild-type mice.
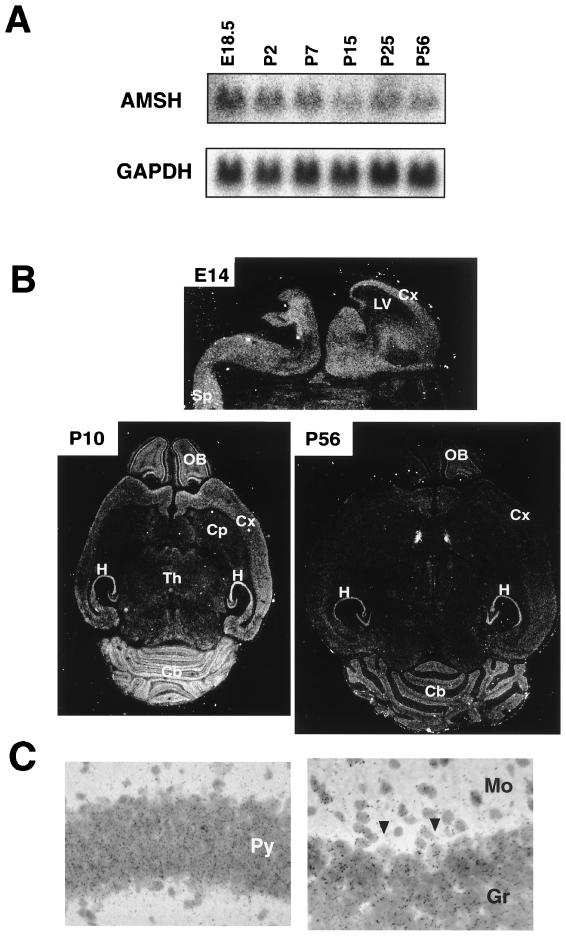
To examine the expression of AMSH mRNA in brain, Northern blot analysis was carried out using whole brains dissected from C57BL/6 mice at various ages from E18.5 to P56. The expression level of AMSH mRNA was highest at E18.5, gradually decreased with age until P15, and remained constant in the adult brain at P56 (Fig. 6A). These results suggest that AMSH may play a role in the embryonic and neonatal stages. Furthermore, in situ hybridization was performed to examine the expression and localization of the AMSH transcript in the brain. AMSH mRNA was expressed diffusely in both mantle and ventricular layers throughout the brain at E14 (Fig. 6B). By P10, its expression was localized to the olfactory bulb, cerebral cortex, hippocampus, and cerebellum (Fig. 6B). AMSH mRNA was clearly expressed in both granule and Purkinje cells in the cerebellar cortex as well as the pyramidal cells in the hippocampal CA1 subfield (Fig. 6C). Although the localization of the AMSH transcript in the P56 adult brain was similar to that seen in the P10 brain, the expression level was markedly reduced in the adult (Fig. 6B). The localization of the AMSH transcript was compatible with that of the neuronal loss seen in the AMSH-deficient brain, suggesting that AMSH is involved in the survival of the neonatal neurons in the hippocampus and cerebrum. However, in situ hybridization analysis was unable to exclude the possibility that glial cells also express AMSH. Thus, the cellular specificity of AMSH expression remains to be examined.
FIG. 6.
Expression of AMSH mRNA in brains. (A) Northern blot analysis of AMSH mRNA expression at different ages. Twenty micrograms of total RNA prepared from brains of C57BL/6 mice at several ages as indicated was subjected to Northern blot analysis. The blot was hybridized with the AMSH cDNA probe and then rehybridized with the glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. (B) In situ hybridization of AMSH probe with brain sections. A whole-embryo section at 14 days and brain sections at 10 and 56 days of age were used for in situ hybridization of AMSH mRNA. (C) Expression of AMSH mRNA in the hippocampus and cerebellar cortex. A bright-field micrograph shows the gene expression for AMSH in the hippocampus (right) and cerebellum (left) on P10. Note that the autoradiographic silver grains were accumulated on the granule cells and Purkinje's cells (arrows) in the left panel. LV, lateral ventricle; Cx, cerebral cortex; Sp, spinal cord; OB, olfactory bulb; H, hippocampus; Cp, caudate putamen; Th, thalamus; Cb, cerebellar cortex; Py, pyramidal layer; Gr, granule cell layer; Mo, molecular layer.
Impaired survival activity of primary neurons derived from AMSH-deficient mice.
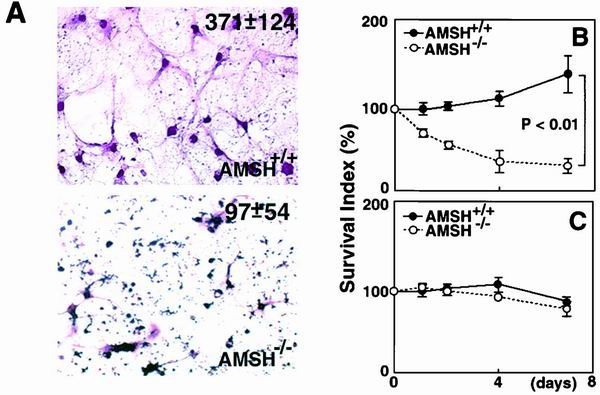
Hippocampal CA1 neurons are known to be susceptible to hypoglycemia, anoxia, and metabolic stresses, which lead to the induction of apoptotic cell death (7, 8, 10, 11, 14, 24). To exclude the possibility that the loss of AMSH-deficient hippocampal neurons was mediated by such stresses, we prepared in vitro primary cultures of hippocampal neurons isolated from AMSH+/+ and AMSH−/− embryos at E18.5. The AMSH-deficient hippocampal neurons died immediately after in vitro cultivation, while the wild-type hippocampal neurons survived to differentiate into typical neuronal cells during cultivation for at least 8 days (Fig. 7A and B). Cell culture of AMSH-deficient hippocampal neurons demonstrated many pyknotic and dead cells (Fig. 7A). In the culture, even the differentiated neuronal cells with typical dendrites also showed pyknotic features (Fig. 7A). These results clearly suggest that AMSH is required for survival of the hippocampal neurons in vitro. To address the selective degeneration of AMSH-deficient neurons as observed in the in vivo experiments, we examined whether AMSH-deficient neurons from the cerebellar cortex survived in primary cultures, as AMSH mRNA expression was detected in the neurons of the cerebellar cortex (Fig. 6B and C). As expected, AMSH−/− neurons from the cerebellar cortices showed a survival rate comparable to that of the AMSH+/+ neurons (Fig. 7C). These results suggest that AMSH plays a critical role in the survival of specific neuronal cells.
FIG. 7.
Defective survival of AMSH-deficient hippocampal neurons in vitro. (A) Primary culture of hippocampal neurons prepared from the AMSH+/+ and AMSH−/− embryos. Primary hippocampal neurons were prepared from the AMSH+/+ and AMSH−/− embryos at E18.5. The cells were cultured in complete medium as described in Materials and Methods for 8 days and then stained with hematoxylin and eosin. The numbers represent absolute counts (per square millimeter) of the developed neuron cells after 8 days of culture. (B and C) Defective survival of the AMSH−/− hippocampal neurons in primary culture. When the cells were prepared, three embryonic hippocampi (B) or cerebellar cortices (C) with the same genotype were combined to provide sufficient cell numbers. The cells were plated in triplicate wells and cultured for the indicated days. Next, the cells were incubated with Alamar blue and the viability of the cells was estimated by measuring the fluorescence intensity of each well with Fluoroskan Ascent. The viability was expressed as the difference between the mean intensity of the test well and the mean intensity of the blank at 590 nm. The survival index was defined as the ratio (viability at the indicated day)/(viability at day 0) × 100%. The results are average survival indices among four independent experiments. Error bars represent standard deviations.
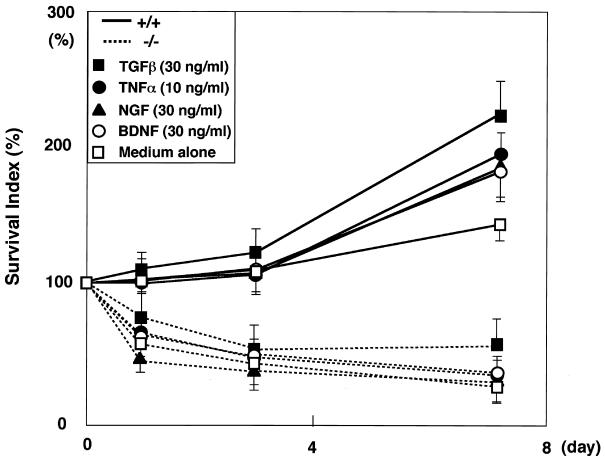
Cytokines, including NGF (16, 30, 32), TGF-β (2, 19, 28), TNF-α (4, 38, 42), and BDNF (32), are known to play important roles in the survival and differentiation of neuronal cells in vivo and in vitro. To elucidate the mechanism underlying the neuronal cell survival associated with AMSH function, we investigated whether these cytokines could rescue the death of the AMSH-deficient neurons in vitro. The viability and mitochondrial activity of the neural cells were measured by Alamar blue fluorescent dye staining. Stimulation of AMSH+/+ hippocampal neurons with TGF-β, TNF-α, NGF, or BDNF induced significant increases in their survival indices, whereas the survival indices of AMSH−/− hippocampal neurons were not significantly increased by stimulation with these cytokines (Fig. 8). These results indicate that none of these cytokines could rescue the hippocampal neurons from cell death in the AMSH−/− mice.
FIG. 8.
Effects of several cytokines on survival of AMSH-deficient neurons. Primary hippocampal neuron cultures and cell survival assays were performed as described for Fig. 7B. TGF-β, TNF-α, NGF, or BDNF was added to the primary culture. Survival indices at the indicated days were plotted. The results are average survival indices of four independent experiments in the presence of the cytokines indicated. Error bars represent standard deviations.
DISCUSSION
We confirmed that murine AMSH possesses a putative nuclear localization signal, an SH3 domain-binding motif (PX[V/I][D/N]RXXKP [SBM]), and an Mov34/MPN domain (3), all of which are conserved in both murine and human AMSH homologues (data not shown; GenBank accession no. AB010123) (Fig. 1A). To investigate the in vivo functional role of AMSH, we generated AMSH-deficient mice, which we report here. Our previous study suggested a possible involvement of AMSH in the in vitro signaling mediated by IL-2 and GM-CSF (39). Despite a possible in vitro functional significance of AMSH, the present study showed that deficiency in AMSH had little effect on cellular responsiveness to IL-2 in vivo. Nevertheless, we did demonstrate here that AMSH plays an essential role in the in vivo survival of immature neurons of the hippocampal CA1 subfield and cerebral cortex.
Intact cytokine signaling but loss of neuronal cells in AMSH-deficient mice.
The AMSH-deficient mice showed normal development of hematopoietic cell populations, including T cells, B cells, and others, and their lymphocytes responded to IL-2 in the same way as did the lymphocytes of wild-type mice. These results seem to be incompatible with our previous observation that the overexpression of the C-terminal deletion mutant of AMSH induces a suppression of IL-2- and GM-CSF-mediated signaling in BAF-B03 cells (39). This discrepancy may be explained by another finding. We have identified a molecule that is homologous to AMSH, named AMSH-LP, which is, like AMSH, ubiquitously expressed among various tissues and is structurally similar to AMSH (our unpublished data). Hence, we suspect that AMSH-LP may compensate for the loss of AMSH in the cytokine-mediated signaling in AMSH-deficient mice. Our previous observation could be explained if the AMSH mutant overexpressed in vitro acted as a dominant-negative form of both AMSH and AMSH-LP.
Although lymphocytes derived from the AMSH-deficient mice showed a normal response to IL-2, the mice had neuronal cell defects in the hippocampal CA1 subfield and cerebral cortex. Interestingly, we had already demonstrated that loss of hippocampal neurons also occurs in STAM1-deficient mice after birth. However, the defective subfields of the hippocampus were different between the AMSH-deficient and STAM1-deficient mice: neurons of the CA1 subfield were primarily lost in the AMSH-deficient mice, while the loss of neurons was restricted to the CA3 subfield in the STAM1-deficient mice (43). It is possible that the expression levels of AMSH, AMSH-LP, STAM1, and STAM2 vary among the subfields of the hippocampus, and their normal expression levels may be reflected in the regional differences in neuronal loss seen when these molecules are deleted.
Because the hippocampi and cerebral cortices of AMSH−/− embryos did not show the abnormalities seen in the postnatal animals, the neuronal cell defects must have occurred as postnatal events, after the normal hippocampus and cerebral cortex had developed. To investigate the postnatal events that affected the neurons, we examined the blood pH and sugar levels in the AMSH−/− mice. There was no significant difference in the pH levels between AMSH−/− mice and their wild-type littermates, suggesting that systemic anoxia or metabolic acidosis is probably not involved in the neuronal death observed in the AMSH−/− mice. The blood sugar levels were also indistinguishable at P12; however, at P16, the AMSH−/− mice showed a significantly lower blood sugar level (80.0 ± 11.9 mg/dl, n = 8) than did their wild-type littermates (107.4 ± 10.5 mg/dl, n = 8). Because the neuronal loss in the hippocampus occurred at P6, hypoglycemia may not be primarily responsible for the hippocampal damage. We cannot, however, exclude the possibility that hypoglycemia may be partially involved in the brain atrophy and neuronal loss seen in the AMSH-deficient cerebral cortex. Collectively, these results strongly suggest that AMSH is directly implicated in neonatal neuronal survival in vivo.
Involvement of AMSH in neuronal cell survival.
The in vitro survival assays revealed that the AMSH-deficient neurons were impaired in their ability to survive, suggesting that AMSH plays an important role in the survival of neurons. Because cells positive by the TUNEL reaction were detected in the CA1 subfield of the hippocampus of AMSH−/− mice (Fig. 5), at first glance it may seem that AMSH could be involved in antiapoptotic signaling. However, the long-term in vitro growth and survival of embryonic fibroblasts upon apoptotic stimulation with UV, X-rays, or hydrogen peroxide were indistinguishable between the AMSH-deficient and wild-type mice, and the dexamethasone-induced apoptosis of AMSH-deficient thymocytes was comparable to that of the wild-type thymocytes (data not shown). Furthermore, although AMSH was appreciably expressed in neurons of the olfactory bulb and cerebellum of the wild-type mice, no clear changes in histological architecture were detected in the brain loci of the AMSH-deficient mice. All these observations suggest that the AMSH function in cell survival may be restricted to neurons in the hippocampus and cerebral cortex. The mechanisms of this restricted occurrence of the neuron defects in the AMSH-deficient mice remain to be resolved.
To explain the defective survival of the AMSH-deficient neurons, we propose that AMSH may participate in signal transduction pathways mediated by neurotrophic or neurotropic factors that are effective for the survival of the hippocampal and cerebral neurons. NGF and BDNF, both neurotrophic factors, were unable to rescue the impaired survival of AMSH-deficient neurons in vitro (Fig. 8), leading us to speculate that AMSH might be implicated in the signaling pathways through these neurotrophic factors. In addition, the AMSH-deficient mice showed a pattern of survival and body weight curves that were similar to data obtained for several mice with gene knockouts for neurotrophic factors or their receptors, such as NGF, BDNF, and trkA (5, 6, 15, 31). In this context, our preliminary experiments demonstrated an interaction between AMSH and Grb2 (data not shown), and Grb2 is known to be critically involved in the Ras/mitogen-activated protein kinase signaling pathway, which is important for the survival and differentiation of neurons upon stimulation with NGF and BDNF (23, 33). It will be interesting to determine whether AMSH functions in the survival or differentiation of neurons through its interaction with Grb2 upstream of the Ras/mitogen-activated protein kinase pathway. Nevertheless, NGF or BDNF knockout mice showed no significant loss of the hippocampal and cerebral neurons (6, 15), which is distinct from the abnormality exhibited in the AMSH-deficient mice. Further study of the possible involvement of AMSH in the signaling pathway mediated by NGF or BDNF is required.
Selective degeneration of the AMSH-deficient CA1 neurons in the hippocampus.
Experimental hypoxia, hypoglycemia, or drug-induced metabolic acidosis can induce selective degeneration of the hippocampal CA1 neurons and cerebral cortex (7, 8, 10, 11, 14, 24). This is characterized by rapid loss of neurons accompanied by massive apoptosis. The histology of the CA1 subfield in mice treated with these artificial stresses is very similar to that observed in the AMSH-deficient mice, although the time course of the neuronal loss was different. Therefore, AMSH-deficient mice may be useful for elucidating the regulatory mechanisms underlying the susceptibility of these neurons to stress. Mutant mice exhibiting spontaneous loss of the hippocampal CA1 neurons during the neonatal period have not been reported thus far. It is worth noting, however, that an accelerated senescence-prone mouse strain, SAPM8, is known to show selective reduction of the CA1 neurons with age. Furthermore, the loss of the CA1 neurons is mild (26), in contrast to the severe loss of the hippocampal CA1 neurons seen in the AMSH-deficient mice. In this case, a different mechanism may be responsible for the loss of CA1 neurons seen in these two different mouse strains.
ACKNOWLEDGMENTS
We thank T. Noda for providing the J1 ES cell line and pLox-neo plasmids and L. C. Ndhlovu for critically reading the manuscript.
This work was supported in part by CREST (Core Research for Evolutional Science and Technology) of the Japan Science and Technology Corporation (JST), a grant-in-aid for scientific research on priority areas from the Ministry of Education, Science, Sports and Culture of Japan, and the Inamori Foundation.
REFERENCES
- 1.Asada H, Ishii N, Sasaki Y, Endo K, Kasai H, Tanaka N, Takeshita T, Tsuchiya S, Konno T, Sugamura K. Grf40, a novel Grb2 family member, is involved in T cell signaling through interaction with SLP-76 and LAT. J Exp Med. 1999;189:1383–1390. doi: 10.1084/jem.189.9.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Böttner M, Krieglstein K, Unsicker K. The transforming growth factor-betas: structure, signaling, and roles in nervous system development and functions. J Neurochem. 2000;75:2227–2240. doi: 10.1046/j.1471-4159.2000.0752227.x. [DOI] [PubMed] [Google Scholar]
- 3.Chamovitz D, Segal D. JAB1/CSN5 and the COP9 signalosome. EMBO Rep. 2001;2:96–101. doi: 10.1093/embo-reports/kve028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng B, Christakos S, Mattson M P. Tumor necrosis factors protect neurons against metabolic-excitotoxic insults and promote maintenance of calcium homeostasis. Neuron. 1994;12:139–153. doi: 10.1016/0896-6273(94)90159-7. [DOI] [PubMed] [Google Scholar]
- 5.Conover J C, Erickson J T, Katz D M, Bianchi L M, Poueymirou W T, McClain J, Pan L, Helgren M, Ip N Y, Boland P, Friedman B, Wiegand S, Vejsada R, Kato A C T, deChiara M, Yancopoulos G D. Neuronal deficits, not involving motor neurons, in mice lacking BDNF and/or NT4. Nature. 1995;375:235–238. doi: 10.1038/375235a0. [DOI] [PubMed] [Google Scholar]
- 6.Crowley C, Spencer S D, Nishimura M C, Chen K S, Pitts-Meek S, Armanini M P, Ling L H, MacMahon S B, Shelton D L, Levinson A D. Mice lacking nerve growth factor display perinatal loss of sensory and sympathetic neurons yet develop basal forebrain cholinergic neurons. Cell. 1994;76:1001–1011. doi: 10.1016/0092-8674(94)90378-6. [DOI] [PubMed] [Google Scholar]
- 7.Deshpande J, Bergstedt K, Linden T, Kalimo H, Wieloch T. Ultrastructural changes in the hippocampal CA1 region following transient cerebral ischemia: evidence against programmed cell death. Exp Brain Res. 1992;88:91–105. doi: 10.1007/BF02259131. [DOI] [PubMed] [Google Scholar]
- 8.Ding D, Moskowitz S I, Li R, Lee S B, Esteban M, Tomaselli K, Chan J, Bergold P J. Acidosis induces necrosis and apoptosis of cultured hippocampal neurons. Exp Neurol. 2000;162:1–12. doi: 10.1006/exnr.2000.7226. [DOI] [PubMed] [Google Scholar]
- 9.Endo K, Takeshita T, Kasai H, Sasaki Y, Tanaka N, Asao H, Kikuchi K, Yamada M, Chen M, O'Shea J J, Sugamura K. STAM2, a new member of the STAM family, binding to the Janus kinases. FEBS Lett. 2000;477:55–61. doi: 10.1016/s0014-5793(00)01760-9. [DOI] [PubMed] [Google Scholar]
- 10.Ferrand-Drake M, Friberg H, Wieloch T. Mitochondrial permeability transition induced DNA-fragmentation in the rat hippocampus following hypoglycemia. Neuroscience. 1999;90:1325–1338. doi: 10.1016/s0306-4522(98)00559-4. [DOI] [PubMed] [Google Scholar]
- 11.Friberg H, Ferrand-Drake M, Bengtsson F, Halestrap A P, Wieloch T. Cyclosporin A, but not FK 506, protects mitochondria and neurons against hypoglycemic damage and implicates the mitochondrial permeability transition in cell death. J Neurosci. 1998;18:5151–5159. doi: 10.1523/JNEUROSCI.18-14-05151.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillis S, Smith K A. Long term culture of tumour-specific cytotoxic T cells. Nature. 1977;268:154–156. doi: 10.1038/268154a0. [DOI] [PubMed] [Google Scholar]
- 13.Ishii N, Asao H, Kimura Y, Takeshita T, Nakamura M, Tsuchiya S, Konno T, Maeda M, Uchiyama T, Sugamura K. Impairment of ligand binding and growth signaling of mutant IL-2 receptor γ-chains in patients with X-linked severe combined immunodeficiency. J Immunol. 1994;153:1310–1317. [PubMed] [Google Scholar]
- 14.Iwai T, Hara A, Niwa M, Nozaki M, Uematsu T, Sakai N, Yamada H. Temporal profile of nuclear DNA fragmentation in situ in gerbil hippocampus following transient forebrain ischemia. Brain Res. 1995;671:305–308. doi: 10.1016/0006-8993(94)01363-m. [DOI] [PubMed] [Google Scholar]
- 15.Jones K R, Farinas I, Backus C, Reichardt L F. Targeted disruption of the BDNF gene perturbs brain and sensory neuron development but not motor neuron development. Cell. 1994;76:989–999. doi: 10.1016/0092-8674(94)90377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaplan D R, Miller F D. Neurotrophin signal transduction in the nervous system. Curr Opin Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- 17.Kato M, Miyazawa K, Kitamura N. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J Biol Chem. 2000;275:37481–37487. doi: 10.1074/jbc.M007251200. [DOI] [PubMed] [Google Scholar]
- 18.Kikuchi K, Kawasaki Y, Ishii N, Sasaki Y, Asao H, Takeshita T, Miyoshi I, Kasai N, Sugamura K. Suppression of thymic development by the dominant-negative form of Gads. Int Immunol. 2001;13:777–783. doi: 10.1093/intimm/13.6.777. [DOI] [PubMed] [Google Scholar]
- 19.Krieglstein K, Richter S, Farkas L, Schuster N, Dunker N, Oppenheim R W, Unsicker K. Reduction of endogenous transforming growth factors beta prevents ontogenetic neuron death. Nat Neurosci. 2000;3:1085–1090. doi: 10.1038/80598. [DOI] [PubMed] [Google Scholar]
- 20.Law C L, Ewings M K, Chaudhary P M, Solow S A, Yun T J, Marshall A J, Hood L, Clark E A. GrpL, a Grb2-related adaptor protein, interacts with SLP-76 to regulate nuclear factor of activated T cell activation. J Exp Med. 1999;189:1243–1253. doi: 10.1084/jem.189.8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miura S, Takeshita T, Asao H, Kimura Y, Murata K, Sasaki Y, Hanai J I, Beppu H, Tsukazaki T, Wrana J L, Miyazono K, Sugamura K. Hgs (Hrs), a FYVE domain protein, is involved in Smad signaling through cooperation with SARA. Mol Cell Biol. 2000;20:9346–9355. doi: 10.1128/mcb.20.24.9346-9355.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata K, Ishii N, Takano H, Miura S, Ndhlovu L C, Nose M, Noda T, Sugamura K. Impairment of antigen-presenting cell function in mice lacking expression of OX40 ligand. J Exp Med. 2000;191:365–374. doi: 10.1084/jem.191.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakamura T, Sanokawa R, Sasaki Y, Ayusawa D, Oishi M, Mori N. N-Shc: a neural-specific adapter molecule that mediates signaling from neurotrophin/Trk to Ras/MAPK pathway. Oncogene. 1996;13:1111–1121. [PubMed] [Google Scholar]
- 24.Nitatori T, Sato N, Waguri S, Karasawa Y, Araki H, Shibanai K, Kominami E, Uchiyama Y. Delayed neuronal death in the CA1 pyramidal cell layer of the gerbil hippocampus following transient ischemia is apoptosis. J Neurosci. 1995;15:1001–1011. doi: 10.1523/JNEUROSCI.15-02-01001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohbo K, Suda T, Hashiyama M, Mantani A, Ikebe M, Miyakawa K, Moriyama M, Nakamura M, Katsuki M, Takahashi K, Yamamura K, Sugamura K. Modulation of hematopoiesis in mice with a truncated mutant of the interleukin-2 receptor γ chain. Blood. 1996;87:956–967. [PubMed] [Google Scholar]
- 26.Onozuka M, Watanabe K, Mirbo S M, Ozono S, Nishiyama K, Karasawa N, Nagatsu I. Reduced mastication stimulates impairment of spatial memory and degeneration of hippocampal neurons in aged SAMP8 mice. Brain Res. 1999;24:148–153. doi: 10.1016/s0006-8993(99)01255-x. [DOI] [PubMed] [Google Scholar]
- 27.Owada Y, Tominaga T, Yoshimoto T, Kondo H. Molecular cloning of rat cDNA for cytosolic phospholipase A2 and the increased gene expression in the dentate gyrus following transient forebrain ischemia. Brain Res Mol Brain Res. 1994;25:364–368. doi: 10.1016/0169-328x(94)90174-0. [DOI] [PubMed] [Google Scholar]
- 28.Prehn J H M, Bindokas V P, Marcuccilli C J, Krajewski S, Reed J C, Miller R J. Regulation of neuronal Bcl2 protein expression and calcium homeostasis by transforming growth factor type β confers wide-ranging protection on rat hippocampal neurons. Proc Natl Acad Sci USA. 1994;91:12599–12603. doi: 10.1073/pnas.91.26.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sakagami H, Saito S, Kitani T, Okuno S, Fujisawa H, Kondo H. Localization of the mRNAs for two isoforms of Ca2+/calmodulin-dependent protein kinase kinases in the adult rat brain. Brain Res Mol Brain Res. 1998;54:311–315. doi: 10.1016/s0169-328x(97)00362-8. [DOI] [PubMed] [Google Scholar]
- 30.Semkova I, Krieglstein J. Neuroprotection mediated via neurotrophic factors and induction of neurotrophic factors. Brain Res Brain Res Rev. 1999;30:176–188. doi: 10.1016/s0165-0173(99)00013-2. [DOI] [PubMed] [Google Scholar]
- 31.Smeyne R J, Klein R, Schnapp A, Long L K, Bryant S, Lewin A, Lira S A, Barbacid M. Severe sensory and sympathetic neuropathies in mice carrying a disrupted Trk/NGF receptor gene. Nature. 1994;368:246–249. doi: 10.1038/368246a0. [DOI] [PubMed] [Google Scholar]
- 32.Snider W D. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 33.Suen K L, Bustelo X R, Pawson T, Barbacid M. Molecular cloning of the mouse grb2 gene: differential interaction of the Grb2 adaptor protein with epidermal growth factor and nerve growth factor receptors. Mol Cell Biol. 1993;13:5500–5512. doi: 10.1128/mcb.13.9.5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Nakamura M, Takeshita T. The common γ-chain for multiple cytokine receptors. Adv Immunol. 1995;59:225–277. doi: 10.1016/s0065-2776(08)60632-x. [DOI] [PubMed] [Google Scholar]
- 35.Sugamura K, Asao H, Kondo M, Tanaka N, Ishii N, Ohbo K, Nakamura M, Takeshita T. The interleukin-2 receptor γ chain: its role in the multiple cytokine receptor complexes and T cell development in XSCID. Annu Rev Immunol. 1996;14:179–205. doi: 10.1146/annurev.immunol.14.1.179. [DOI] [PubMed] [Google Scholar]
- 36.Takeshita T, Arita T, Asao H, Tanaka N, Higuchi M, Kuroda H, Kaneko K, Munakata H, Endo Y, Fujita T, Sugamura K. Cloning of a novel signal-transducing adaptor molecule containing an SH3 domain and ITAM. Biochem Biophys Res Commun. 1996;225:1035–1039. doi: 10.1006/bbrc.1996.1290. [DOI] [PubMed] [Google Scholar]
- 37.Takeshita T, Arita T, Higuchi M, Asao H, Endo K, Kuroda H, Tanaka N, Murata K, Ishii N, Sugamura K. STAM, signal transducing adaptor molecule, is associated with Janus kinases and involved in signaling for cell growth and c-myc induction. Immunity. 1997;6:449–457. doi: 10.1016/s1074-7613(00)80288-5. [DOI] [PubMed] [Google Scholar]
- 38.Tamatani M, Che Y H, Matsuzaki H, Ogawa S, Okado H, Miyake S, Mizuno T, Tohyama M. Tumor necrosis factor induces Bcl-2 and Bcl-x expression through NFkappaB activation in primary hippocampal neurons. J Biol Chem. 1999;274:8531–8538. doi: 10.1074/jbc.274.13.8531. [DOI] [PubMed] [Google Scholar]
- 39.Tanaka N, Kaneko K, Asao H, Kasai H, Endo Y, Fujita T, Takeshita T, Sugamura K. Possible involvement of a novel STAM-associated molecule “AMSH” in intracellular signal transduction mediated by cytokines. J Biol Chem. 1999;274:19129–19135. doi: 10.1074/jbc.274.27.19129. [DOI] [PubMed] [Google Scholar]
- 40.Toku K, Tanaka J, Yano H, Desaki J, Zhang B, Yang L, Ishihara K, Sakanaka M, Maeda N. Microglial cells prevent nitric oxide-induced neuronal apoptosis in vitro. J Neurosci Res. 1998;53:415–425. doi: 10.1002/(SICI)1097-4547(19980815)53:4<415::AID-JNR3>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 41.White M J, Dicaprio M J, Greenberg D A. Assessment of neuronal viability with Alamar blue in cortical and granule cell cultures. J Neurosci Methods. 1996;70:195–200. doi: 10.1016/s0165-0270(96)00118-5. [DOI] [PubMed] [Google Scholar]
- 42.Wilde G J, Pringle A K, Sundstrom L E, Mann D A, Iannotti F. Attenuation and augmentation of ischaemia-related neuronal death by tumour necrosis factor-alpha in vitro. Eur J Neurosci. 2000;12:3863–3870. doi: 10.1046/j.1460-9568.2000.00273.x. [DOI] [PubMed] [Google Scholar]
- 43.Yamada M, Takeshita T, Miura S, Murata K, Kimura Y, Ishii N, Nose M, Sakagami H, Kondo H, Tashiro F, Miyazaki J, Sasaki H, Sugamura K. Loss of hippocampal CA3 pyramidal neurons in mice lacking STAM1. Mol Cell Biol. 2001;21:3807–3819. doi: 10.1128/MCB.21.11.3807-3819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoder J, Pham C, Iizuka Y M, Kanagawa O, Liu S K, McGlade J, Cheng A M. Requirement for the SLP-76 adaptor GADS in T cell development. Science. 2001;291:1987–1991. doi: 10.1126/science.1057176. [DOI] [PubMed] [Google Scholar]