Abstract
The activation of Wnt/β-catenin signalling is a prerequisite for odontogenesis. APC, a member of the AXIN-CK1-GSK3β-APC β-catenin destruction complex, functions to modulate Wnt/β-catenin signalling to establish regular teeth number and positions. APC loss-of-function mutations are associated with the over-activation of WNT/β-catenin signalling and subsequent familial adenomatous polyposis (FAP; MIM 175100) with or without multiple supernumerary teeth. The ablation of Apc function in mice also results in the constitutive activation of β-catenin in embryonic mouse epithelium and causes supernumerary tooth formation. The objective of this study was to investigate if genetic variants in the APC gene were associated with supernumerary tooth phenotypes. We clinically, radiographically, and molecularly investigated 120 Thai patients with mesiodentes or isolated supernumerary teeth. Whole exome and Sanger sequencing identified three extremely rare heterozygous variants (c.3374T>C, p.Val1125Ala; c.6127A>G, p.Ile2043Val; and c.8383G>A, p.Ala2795Thr) in APC in four patients with mesiodentes or a supernumerary premolar. An additional patient with mesiodens was compound as heterozygous for two APC variants (c.2740T>G, p.Cys914Gly, and c.5722A>T, p.Asn1908Tyr). Rare variants in APC in our patients are likely to contribute to isolated supernumerary dental phenotypes including isolated mesiodens and an isolated supernumerary tooth.
Keywords: Extra teeth, mesiodentes, Gardner syndrome, supernumerary tooth, WNT signaling, taurodontism, root maldevelopment
1. Introduction
Supernumerary teeth refer to extra teeth that exceed the usual number in deciduous or permanent dentitions [1,2,3], though they are rare in deciduous dentition [2,4]. Supernumerary teeth may occur as a single tooth or multiple teeth in any region of the maxilla, premaxilla, and mandible in the same person [1,5]. The prevalence of supernumerary teeth has been reported to be 0.04–3.8% [1,3,5,6,7]. However, supernumerary teeth located in the premaxilla region (referred to as mesiodens) are the most common form, with an estimated prevalence ranging from 0.15% to 1.9%, depending on the method of selecting the samples and the studied population [8,9,10,11]. Interestingly, supernumerary teeth are more prevalent in Asian populations [5,12,13]. However, as supernumerary teeth can remain unerupted, the true incidence is likely to be under-reported as radiography is not used in all dental assessments [6].
Wnt/β-catenin signalling is involved in almost every aspect of embryonic development and also regulates homeostatic self-renewal in tissue regeneration and repair. Wnt/β-catenin signalling plays a particularly critical role in the development of ectodermally derived organs, including skin, hair, sweat glands, nails, and teeth [14,15]. Indeed, defects in the components of the Wnt signaling pathway (eg. WNT10A and WNT10B) that lead to reduced WNT signalling strength are the most common causes of tooth agenesis [14,15,16,17,18].
Recently, mutations in LRP5, LRP6, WLS, DKK1, and LRP4 have been reported to be predisposing factors for mesiodens in humans [19,20,21,22,23]. Supernumerary incisors have also been reported in various mouse knockout lines, including mutants of Wnt pathway receptors (Lrp4) and inhibitors (Wise) [24,25]. Supernumerary teeth have also been reported to be associated with various malformation syndromes, including APC-associated familial adenomatous polyposis (FAP) [2,26,27], RUNX2-associated cleidocranial dysplasia [27,28,29], and TRPS1-associated tricho-rhino-phalangeal syndrome [27,30,31,32,33].
The APC gene (MIM 611731) encodes a multidomain tumour-suppressor protein that, as described above, has an important role in assembling the AXIN-CK1-GSK3β-APC destruction complex that modulates the level of WNT/β-catenin signalling [34,35]. APC loss-of-function mutations are associated with over-activation of WNT/β-catenin signalling and subsequent FAP (MIM 175100) or Gardner syndrome [26], characterized by adenomatous polyposis of the colon, multiple supernumerary teeth, craniofacial osteomas, epidermal cysts, congenital hypertrophy of the retinal pigmented epithelium, and desmoid tumours [26,36]. The ablation of the Apc function in mice also results in the constitutive activation of β-catenin in embryonic mouse epithelium and causes supernumerary tooth formation [37,38,39]. Intriguingly, only 11–27% of patients with familial adenomatous polyposis have supernumerary teeth with no genotype-phenotype correlation [2]. The objective of this study was to investigate if genetic variants in the APC gene were associated with supernumerary tooth phenotypes.
Here, we report five rare variants in APC in six patients with isolated mesiodentes and a patient with an isolated supernumerary premolar. To the best of our knowledge, this is the first report demonstrating that variants in APC are implicated in isolated supernumerary tooth formation.
2. Results
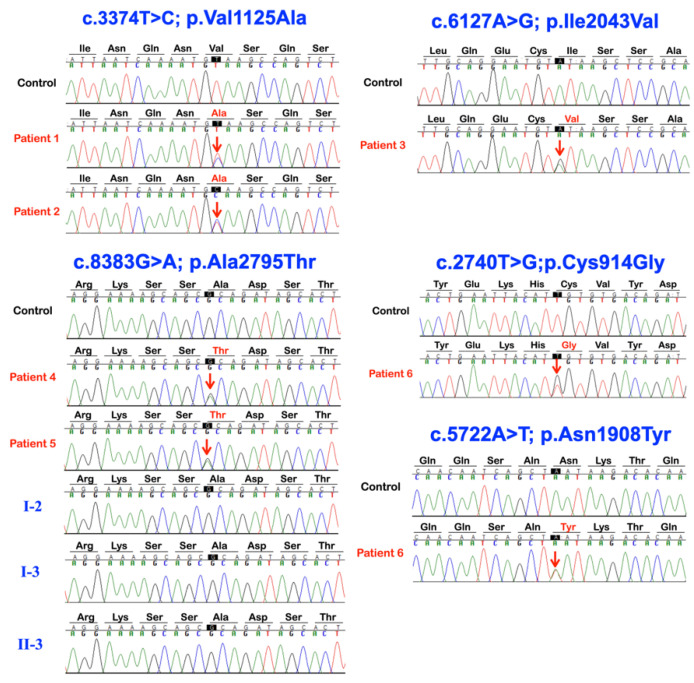
We identified five APC missense variants in five unrelated patients (plus one affected family member in family 4) presenting with either isolated mesiodentes (five cases) or an isolated supernumerary premolar (one case) (Table 1; Figure 1 and Figure 2). All the variants were validated using Sanger sequencing and, in the case of family 4, the variant was present in both the affected siblings but not in the unaffected sibling and unaffected mother (Figure 3).
Table 1.
Patients with APC variants and their dental phenotypes.
| Families | Patients | Phenotypes |
APC Variants NM_000038.6; NP_000029.2 |
Prediction/Ranking |
|---|---|---|---|---|
| 1 | 1 (Female) |
Supernumerary erupted mandibular left premolar | c.3374T>C p.Val1125Ala chr5-112174665-T-C rs377278397 MAF: 0.0005993 |
MutationTaster: Disease causing Prob = 0.944130296883564 Polyphen-2: Benign; score = 0.001 SIFT: Tolerated; score = 0.244 CADD-PHRED score = 21.7 DANN: Benign; score = 0.9507 |
| 2 | 2 (Male) |
Mesiodens (Double with normal orientation) |
||
| 3 | 3 (Female) |
Mesiodens (Single; erupted) |
c.6127A>G p.Ile2043Val chr5-112177418-A-G rs876660233 MAF: 0.000007085 |
MutationTaster: Disease causing Prob = 0.999923872840111 Polyphen-2: Probably damaging; score = 0.958 SIFT: Damaging; score = 0.011 CADD-PHRED score = 24.9 DANN score = 0.9983 |
| 4 | Normal mother | No variants | MutationTaster: Disease causing Prob = 0.999223891277048 Polyphen-2: Probably damaging; score = 0.936 SIFT: Tolerated; score = 0.068 CADD-PHRED score = 22.2 DANN score = 0.9909 |
|
| 4 (Male) |
Mesiodens (Double; unerupted & inverted) |
c.8383G>A p.Ala2795Thr chr5-112179674-G-A rs369264968 MAF:0.00004400 |
||
| 5 (Male) |
Mesiodens (Double; both were erupted) |
|||
| Normal sister | No variants | |||
| 5 | 6 (Male) |
Mesiodens (Double; unerupted) One is inverted, the other had normal orientation |
Variant 1 c.2740T>G p.Cys914Gly chr5-112174031-T-G rs1554084426 Not reported in gnomAD Variant 2 c.5722A>T p.Asn1908Tyr chr5-112177013-A-T No rs number Not reported in gnomAD |
Variant 1 MutationTaster: Disease causing Prob = 0.99929839701033 Polyphen-2: Benign score = 0.055 SIFT: Tolerated; score = 0.127 CADD-PHRED score = 21.5 DANN score = 0.8956 Variant 2 MutationTaster: Polymorphism Prob = 0.999999988244843 Polyphen-2: Benign score = 0.214 SIFT: Damaging; score = 0.046 CADD-PHRED score = 17.19 DANN score = 0.9609 |
Figure 1.
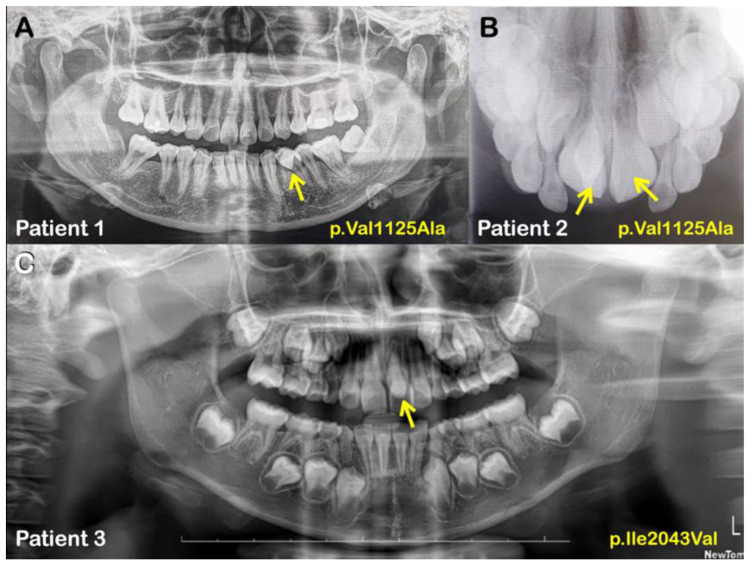
(A) Patient 1. Panoramic radiograph showing a supernumerary left mandibular premolar (arrow). (B) Patient 2. Occlusal radiograph showing double mesiodentes (arrows). (C) Patient 3. Panoramic radiograph showing a mesiodens (arrow).
Figure 2.
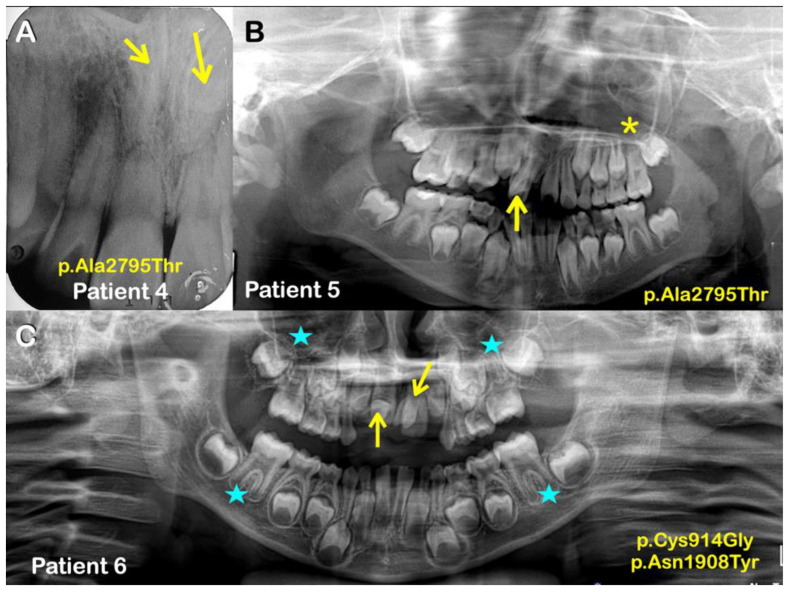
(A) Patient 4. Periapical radiograph showing double mesiodentes (inverted) (arrows). (B) Patient 5. Panoramic radiograph showing a mesiodens (arrow) and taurodontism (asterisk). (C) Patient 6. Panoramic radiograph showing double mesiodentes (arrows) and taurodontism (asterisks).
Figure 3.
Sequence chromatograms of APC variants in patients 1–6, controls, and their unaffected family members.
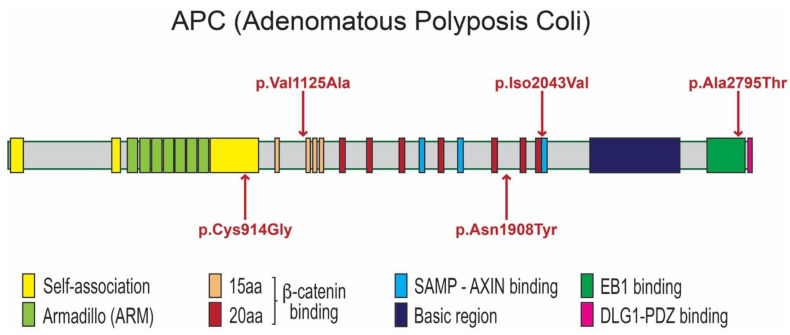
A heterozygous missense variant (c.3374T>C; p.Val1125Ala; rs377278397; MAF: 0.0005993) was found in two unrelated patients—one (patient 1) with double mesiodentes and the other (patient 2) with the extra premolar. The CADD score of the p.Val1125Ala variant is 21.7 and it is predicted to be disease causing according to MutationTaster. The amino acid residue Val1125 is located immediately before the second of the four 15-amino acid repeat motifs (Figure 4), which are required for binding to the C-terminal Binding Protein (CTBP; MIM 602618) [40].
Figure 4.
Protein domains of APC proteins. APC is comprised of regions required for oligomerization, Armadillo repeats, β-catenin-binding domain, AXIN-binding domain, Basic domain, EB1-binding domain, and HDLG-binding domain. Locations of mutations are indicated by red arrows.
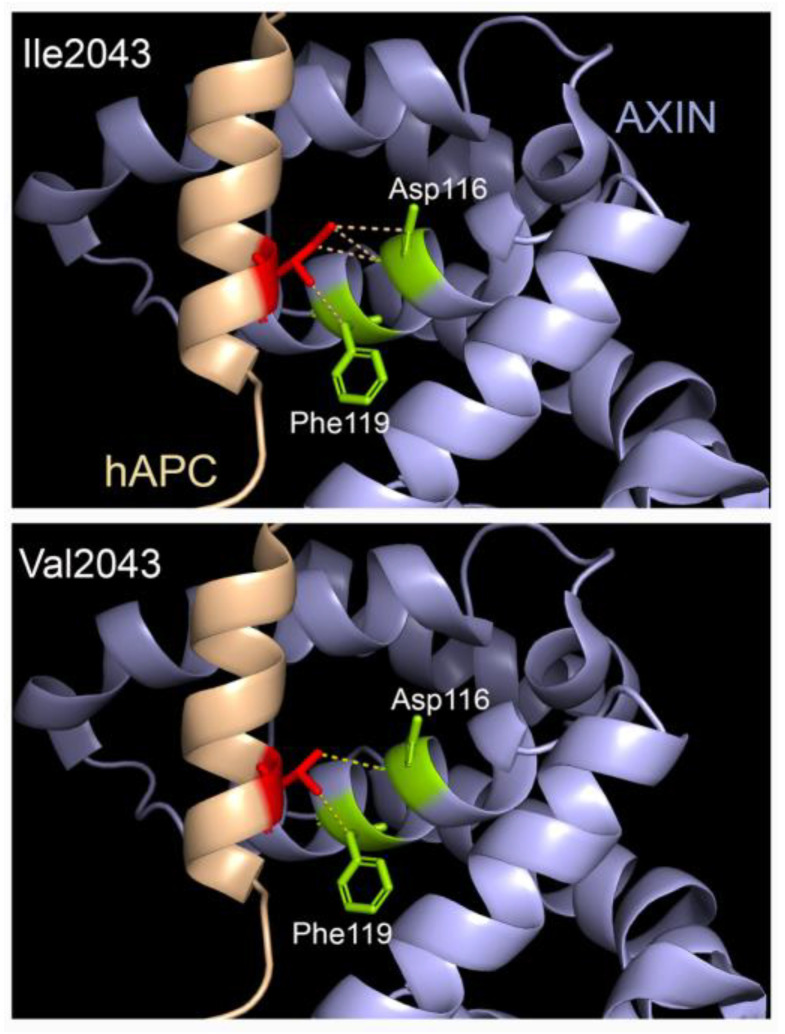
An additional rare variant, c.6127A>G; p.Ile2043Val (rs876660233; MAF: 0.000007085), was identified in the third individual (patient 3). The CADD score of p.Ile2043Val is 24.9 and it is predicted to be disease causing, probably damaging, and damaging by MutationTaster, PolyPhen-2, and SIFT, respectively. The p.Ile2043Val variant is located within the SAMP3 motif, which is critically required for interaction with the RGS domain of AXIN (Figure 4 and Figure 5). Residue Ile2043 is one of only a few invariant residues in the SAMP domains and is one of nine residues that have a direct contact with AXIN, specifically with Asp116 and Phe119 within the a4 helix of the AXIN RGS domain [41].
Figure 5.
The Ile2043Val missense variant in the SAMP3 domain of APC may impact the strength of interaction with AXIN. Human APC SAMP3 domain is shown in beige while the RGS domain of AXIN is coloured mauve. Isoleucine 2043 in wildtype human APC has four contacts with the AXIN RGS domain: three with Asp116 and one with Phe119. The valine substitution in APC is predicted to reduce the contacts with Asp116 to just one.
The third variant, c.8383G>A; p.Ala2795Thr (rs369264968; MAF: 0.00004400), was found in two siblings of family 4 (patients 4 and 5)—each with double mesiodentes—but not in the third sibling that had normal dentition (Figure 1 and Figure 2; Table 1). The CADD score of the p.Ala2795Thr variant is 22.2 and it is predicted to be disease causing and probably damaging by MutationTaster and PolyPhen-2, respectively. The p.Ala2795Thr variant is located toward the C-terminus of the APC protein in a region required for interaction with the End-Binding 1 protein (EB1; MIM 603108), which targets APC to microtubule plus-ends [42].
The sixth patient (patient 6), who had double mesiodentes, was found to be compound heterozygous for two variants: c.2740T>G; p.Cys914Gly and c.5722A>T; p.Asn1908Tyr. Neither variant was reported in gnomAD or LOVD, although the former is reported in NCBI with reference number: rs1554084426. The p.Cys914Gly variant is located between the armadillo repeat domain and the 15 amino acid actin and β-catenin binding repeats (Figure 4). This inter-repeat region is one of three segments of the APC N-terminus that has self-association properties and is thus thought to be involved in the oligomerization of APC. The CADD score of the p.Cys914Gly variant is 21.5 and it is predicted to be disease causing by MutationTaster. The p.Asn1908Tyr variant (CADD score: 17.19) is located within the overlapping region required for both b-catenin and AXIN binding (Figure 4). Specifically, the variant lies between the 20 amino acid repeat 5 and 6 that contribute to b-catenin binding and are located between the second and third SAMP domains (AXIN binding). It is predicted to be damaging by SIFT.
3. Discussion
The WNT/β-catenin, BMP, and SHH signalling pathways play important interrelated roles in the process of tooth development, with mutations in components of the Wnt/β-catenin pathway arguably being the most common cause of dental anomalies [15,19,20,21,43]. The downregulation of WNT/β-catenin is implicated in both tooth agenesis and microdontia [19,20,21,43,44], while the over-activation of WNT/β-catenin and SHH signalling is implicated in supernumerary tooth formation [7,27,31].
The activation of WNT/β-catenin signalling is initiated when a WNT ligand binds to a Frizzled (FZD) receptor and a coreceptor, LDL receptor-related protein 5 or 6 (LRP5/6), forming a WNT-FZD-LRP5/6 complex [16,45,46]. The WNT-FZD-LRP5/6 complex subsequently recruits a ‘destruction complex’ consisting of AXIN, Casein kinase 1 (CK1), Glycogen synthase kinase 3β (GSK3β), and Adenomatous polyposis coli (APC). This recruited AXIN-CK1-GSK3β-APC complex interacts with the intracellular domains of FZD-LRP5/6 at the plasma membrane through an adaptor protein Disheveled (DVL) and subsequently becomes inactivated. Once the destruction complex is inactivated, the phosphorylation of β-catenin is inhibited, leading to the accumulation of β-catenin in the cytoplasm and nucleus. The nuclear accumulation of β-catenin mediates the transcriptional activation of the TCF/LEF and WNT responsive genes [16,47]. When there is limited or no WNT ligand, β-catenin is degraded by the proteasome after being phosphorylated by a GSK3β-AXIN-CK1-APC destruction complex. Ultimately, the levels of β-catenin at the intercellular junctions and in the nucleus determine the activation of WNT responsive genes and the biological response of the cells. Complicating this further is that the level of WNT/β-catenin signalling is also precisely modulated by the binding of WNT ligands to inhibitors such as DKK1, DKK2, SOST, SOSTDC1 KREMEN1, and KREMEN2 [16], and the binding of WNT inhibitors to LRP5 and LRP6 co-receptors [16,45,46].
Here, we report the first description of heterozygous APC variants in patients with mesiodentes or an isolated supernumerary tooth. Two unrelated Thai patients (patients 1 and 2) carried the same rare missense variant (p.Val1125Ala), while one patient (patient 6) with mesiodens carried a compound heterozygous mutation (p.Cys914Gly and p.Asn1908Tyr) in APC. APC is a tumour-suppressor protein and its loss-of-function mutations have been demonstrated to result in the over-activation of WNT/β-catenin signalling, and familial adenomatous polyposis syndrome with or without supernumerary tooth formation [35,39].
APC loss-of function or constitutive β-catenin activation in adult dental tissue has previously been shown to result in new tooth formation [39,48]. The process is non-cell autonomous. Only a small number of APC-deficient cells was reportedly sufficient to induce the surrounding wild-type epithelial and mesenchymal cells to collaborate in forming the new teeth [39]. Notably, supernumerary incisors were formed in adult mouse oral tissues in response to epithelial APC loss-of-function or Wnt/β-catenin over-activation [39].
3.1. APC Protein Structure and Possible Impact of Identified Variants on APC Function
APC is a protein with multiple functionally characterized domains (Figure 4). It consists of multiple regions important for oligomerization, an armadillo repeat region, a series of 15- and 20-amino acid repeats, an AXIN-binding domain, and a C-terminus that contains a basic domain and binding sites for End-Binding protein 1 (EB1) and the human Discs Large (HDLG) protein [42,49]. Each of these domains can serve as binding sites for one or more different partner proteins, including those of the Wnt signaling complex (β-catenin, AXIN, and KIF3a), the cytoskeletal regulators including of EB1, HDLG, and IQGAP1, the Rac guanine-nucleotide-exchange factor (GEF), Asef1, and CTBP [26,42,50].
Each of the identified variants can feasibly impact APC function in various ways but with a similar impact on Wnt signaling. The p.Val1125Ala change is located in the 15-amino acid repeat region that is required for binding to CTBP. Reduced CTBP interaction strength would be predicted to result in increased β-catenin, leading to the over-activation of WNT/β-catenin signalling, thus explaining subsequent supernumerary tooth formation. The association of this rare variant in two unrelated patients with supernumerary teeth supports its pathogenicity.
Some of the other variants are also predicted to lead to increased Wnt/β-catenin signalling but through reduced interaction with other protein partners. Based on the location of the p.Ile2043Val change—in a highly conserved residue in the APC SAMP3 motif (Figure 5) that is directly involved in contact with the RGS domain of AXIN—we would predict a reduced interaction with AXIN, rather than with CTBP. The result of such a disruption would likely impact the proper nucleation of actin, with a potential indirect impact on the signalling. In contrast, the p.Ala2795Thr variant may perturb the interaction with EB1 and/or self-association (Figure 5). EB1 is a protein that binds to the plus end of microtubules and plays a critical role in microtubule dynamics [51]. Reduced the binding of APC to EB1 would be predicted to lead to increased nuclear β-catenin, and, thus, the elevation of WNT/β-catenin signalling.
In patient 6, either or both rare missense variants could contribute to the presentation of mesiodens. The p.Cys914Gly variant could have an effect on the APC multimerization and, thus, the assembly of the larger destruction complex. In contrast, the p.Asn1908Tyr variant (Figure 4)—a completely novel variant—resides in a highly charged inter-repeat region and changes from the neutral polar asparagine (Asn) to a hydrophobic non-polar residue, tyrosine (Tyr). Such a significant change might be sufficient to disrupt its binding with either AXIN or b-catenin, leading in either case to impaired β-catenin degradation and the over-activation of Wnt/β-catenin signalling.
3.2. APC Mutations, Ciliary Dysfunction, and Supernumerary Tooth Formation
Primary cilia are crucial for the integration of Hh and Wnt/β-catenin signalling and both pathways are important for tooth development [52,53,54]. The dysregulation of SHH and Wnt/β-catenin signalling pathways are implicated in the malformation of teeth [55]. The EB1 and kinesin-family-member 3A (KIF3A) are APC-binding proteins that are involved in intraflagellar transport [26]. KIF3A, an anterograde intraflagellar transport motor protein, is important for tooth development because it regulates the integrity of primary cilia and various cellular functions including the differentiation of stem cells involved in tooth development via the WNT pathway [56]. The loss of Kif3a in mice results in the over-activation of WNT signalling and subsequent dental anomalies [52]. Therefore, supernumerary tooth formation as a result of APC mutations implicates ciliary dysfunction as an underlying pathogenetic mechanism [26,52].
Lastly, we are convinced that these APC variants contributed to the supernumerary tooth phenotypes in the patients because no other rare variants in the known dental anomalies-associated genes (including WNT10A, WNT10B, LRP4, LRP5, LRP6, DKK1, PAX9, AXIN2, MSX1, WLS, BMP4, GREM2, TFAP2B, TSPEAR, PITX2, EVC, EVC2, COL1A2, ANTXR1, FGF10, SMOC2, KREMEN1, KDF1, ATF1, DUSP10, EDA, EDAR, EDARADD, and CASC8 [19,20,21,22,23] were identified in any of these patients.
4. Materials and Methods
4.1. Patient Recruitment
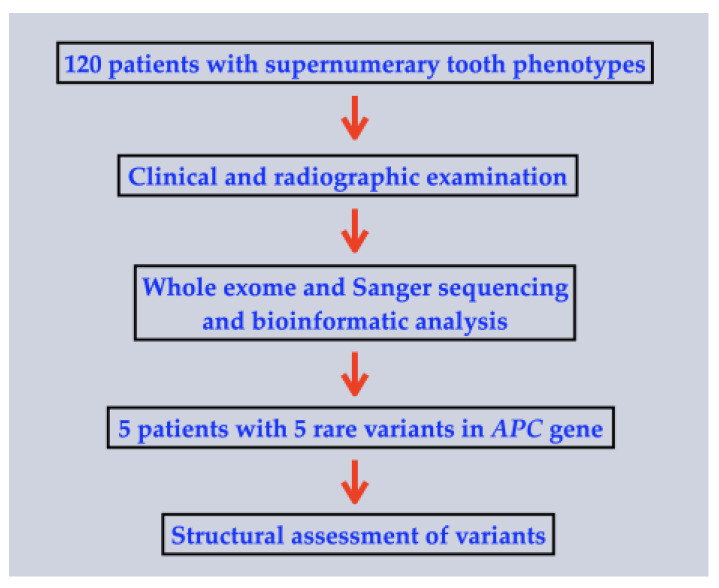
This study was conducted in accordance with the Declaration of Helsinki and national guidelines. Informed consent was obtained from the patients or the parents of the patients in accordance with the regulations of the Human Experimentation Committee of the Faculty of Dentistry, Chiang Mai University (certificate of approval number 71/2020). The inclusion criteria were patients with isolated supernumerary tooth phenotypes including, mesiodens. The exclusion criteria were patients with a normal number of teeth. Oral and radiographic examinations (panoramic or periapical radiographies) were performed on the cohort of 120 patients affected with various kinds of isolated extra tooth phenotypes, which included 93 patients with isolated mesiodentes and 27 (17 males; 10 females) patients with isolated supernumerary teeth (Figure 6).
Figure 6.
Flowchart describing the methodology of the study.
Regarding 93 patients with mesiodentes, 63 (68%) were males and 30 (32%) were females. Seventy-seven patients (82.8%) had single mesiodentes, while 16 (17.2%) of them had double mesiodentes. The orientation of the mesiodentes was noted in 63 mesiodentes of 53 patients; 42 (66.7%) of them had normal orientation, 20 (31.7%) were inverted, and 1 (1.6%) had transverse orientation. The eruption status had been noted in 59 mesiodentes of 48 patients: 31 (52.5%) erupted and 28 (47.5%) were unerupted. For the patients with isolated supernumerary teeth that were not mesiodens or mesiodentes, the information of the supernumerary teeth was not available.
4.2. Whole Exome Sequencing, Mutation Analysis, and Bioinformatic Analyses
All the consented patients’ genomic DNA was isolated from saliva using Oragene-DNA (OG-500) Kit (DNA Genotek, Ottawa, Ontario, Canada). Whole exome sequencing (WES) was performed for 120 patients with isolated mesiodentes or isolated supernumerary teeth (Macrogen Inc, Seoul, Korea). It was also performed on some of the unaffected siblings and unaffected parents (if available). The average depth of the sequencing was 80× using the targeted capture SureSelect V6 kit (PR7000-0152; Agilent Technologies, Santa Clara, CA, USA), which also captures untranslated regions. We adopted GATK3.8 best practices to identify variants for each sample; the alignment of the raw sequencing FASTQ file with the human genome reference sequence, GRCh38+decoy, was performed using BWA-mem. The variant effect predictor (VEP) and the database of nonsynonymous functional prediction (dbNSFP) were used to computationally assign effects to the resulting variants of each individual. The annotated variant calling format (VCF) files were stored in our in-house database that allows us to query pathogenic variants according to different modes of segregation. Furthermore, the variant allele frequencies were determined by comparing against public databases, including 1000 Genomes, gnomAD, GenomeAsia, and the recent Thai Reference Exome database. The prioritization of the variants was established based on multiple considerations: (1) whether the gene harbouring each variant has an established role in tooth development and/or was previously implicated in dental anomalies; (2) the allele rarity; (3) CADD score > 15; and (4) localization to or near an important functional region of the protein. The selected variants of interest were then validated and, if familial samples available, tested for segregation using PCR-based amplification and Sanger sequencing. The Sanger sequencing was performed to confirm the variants. The sequence primers used were as follows: Exon 16a, forward: 5′-TGGGCAAGACCCAAACACAT-3′; reverse: 5′-TGGATGGAGCTGATTCTGCC-3′. Exon 16b, forward: 5′-AGGGGCAGCAACTGATGAAA-3′; reverse: 5′-GCAGCAGCAGCTTGATGTAA-3′. Exon 16c, forward: 5′-GGGTAATGGCAGTGTTCCCA-3′; reverse: 5′-GTAAGACCCAGAATGGCGCT-3′. Exon 16d, forward: 5′-AGTTTGGAGAGAGAACGCGG-3′; reverse: 5′-GTCGGCTGGGTATTGACCAT-3′. Exon 16e, forward: 5′-TTCACCTCATCATTACACGCCT-3′; reverse: 5′-TCAGGGGGCTCAGTCTCTTT-3′.
4.3. Structural Assessment of Variants
An available crystal structure of the human APC SAMP3 domain in complex with AXIN (1emu.pdb; [41]) was retrieved from the Protein Data Bank (www.rcsb.org) (accessed on 8 January 2023) and investigated using PyMol visualization software (Schrödinger Inc, New York, NY, USA).
5. Study Limitation
Our cohort consisted of 120 patients with supernumerary tooth phenotypes. However, we only had DNA samples and dental information of the affected patients who came for oral and radiographic examinations. We are aware that it would have been ideal if we had the unaffected family members of each patient participate in the study in order to study the co-segregation between the phenotype and genotype. It would also have strengthened the relationship of the heterozygous variants in the APC gene and the supernumerary tooth phenotypes. In some patients, mesiodens was an incidental finding。 Most patients came for dental check-up or treatments of dental caries. Therefore, the use of cone beam computed tomography (CBCT) was not indicated. The use of CBCT would have shown the better morphology of mesiodentes. However, the use of CBCT would have been over-treatment for the patients who came to our dental clinic for a check-up. Notably, approximately 50% of mesiodentes are unerupted; therefore, the people who carried APC variants in the studied population of gnomAD, might not be dentally “normal” as they might have unerupted mesiodentes.
6. Conclusions
The activation of Wnt/β-catenin signalling is a prerequisite for odontogenesis [39]. In the normal situation, APC, a member of the AXIN-CK1-GSK3β-APC destruction complex, functions to modulate Wnt/β-catenin signalling to establish regular teeth number and positions. The presence of supernumerary teeth in the patients with APC-associated familial polyposis syndrome indicates the direct relationship of APC variants and supernumerary tooth formation. The association of rare APC variants with isolated supernumerary tooth formation in multiple individuals supports a contributory, if not, causal role in the formation of mesiodentes and a supernumerary tooth.
Acknowledgments
We thank our patients and their families for their kind cooperation and for allowing us to use their medical and dental information for the benefit of other patients.
Author Contributions
Conceptualization, C.P., S.N., K.C., N.L., W.I., B.O., S.T., P.A., C.N., T.C.C. and P.K.; methodology, C.P., S.N., K.C., N.L., W.I., B.O., S.T., P.A., C.N., T.C.C. and P.K.; validation, C.P., S.N., W.I., S.T., P.A., C.N., T.C.C. and P.K.; formal analysis, C.P., S.N., K.C., N.L., W.I., B.O., S.T., P.A., C.N., T.C.C. and P.K.; investigation, C.P., S.N., K.C., N.L., W.I., B.O., S.T., P.A., C.N., T.C.C. and P.K.; resources, P.K.; data curation, C.P., N.L., W.I., S.T., P.A., C.N., T.C.C. and P.K.; writing—original draft preparation, C.P., S.N., K.C., N.L., W.I., B.O., S.T., P.A., C.N., T.C.C. and P.K.; writing—review and editing, C.P., S.N., K.C., N.L., W.I., B.O., S.T., P.A., C.N., T.C.C. and P.K.; supervision, P.K.; project administration, P.K.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study involving human participants was approved by the Human Experimentation Committee of the Faculty of Dentistry, Chiang Mai University (No. 71/2020) and was performed in accordance with the ethical standards of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Informed Consent Statement
Written informed consent has been obtained from the patients or their parents to publish this paper.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the Genomics Thailand Research Grant of the Health Systems Research Institute (HSRI) (Grant number 64-123).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Rajab L., Hamdan M. Supernumerary teeth: Review of the literature and a survey of 152 cases. Int. J. Paediatr. Dent. 2002;12:244–254. doi: 10.1046/j.1365-263X.2002.00366.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang X.P., Fan J. Molecular genetics of supernumerary tooth formation. genesis. 2011;49:261–277. doi: 10.1002/dvg.20715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anthonappa R.P., King N.M., Rabie A.B. Aetiology of supernumerary teeth: A literature review. Eur. Arch. Paediatr. Dent. 2013;14:279–288. doi: 10.1007/s40368-013-0082-z. [DOI] [PubMed] [Google Scholar]
- 4.Shilpa G., Gokhale N., Mallineni S.K., Nuvvula S. Prevalence of dental anomalies in deciduous dentition and its association with succedaneous dentition: A cross-sectional study of 4180 South Indian children. J. Indian. Soc. Pedod. Prev. Dent. 2017;35:56–62. doi: 10.4103/0970-4388.199228. [DOI] [PubMed] [Google Scholar]
- 5.Davis P.J. Hypodontia and hyperdontia of permanent teeth in Hong Kong schoolchildren. Community Dent. Oral Epidemiol. 1987;15:218–220. doi: 10.1111/j.1600-0528.1987.tb00524.x. [DOI] [PubMed] [Google Scholar]
- 6.Hagiwara Y., Uehara T., Narita T., Tsutsumi H., Nakabayashi S., Araki M. Prevalence and distribution of anomalies of permanent dentition in 9584 Japanese high school students. Odontology. 2016;104:380–389. doi: 10.1007/s10266-015-0225-2. [DOI] [PubMed] [Google Scholar]
- 7.Lu X., Yu F., Liu J., Cai W., Zhao Y., Zhao S., Liu S. The epidemiology of supernumerary teeth and the associated molecular mechanism. Organogenesis. 2017;13:71–82. doi: 10.1080/15476278.2017.1332554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kazanci F., Celikoglu M., Miloglu O., Yildirim H., Ceylan I. The frequency and characteristics of mesiodens in a Turkish patient population. Eur. J. Dent. 2011;5:361–365. doi: 10.1055/s-0039-1698906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mukhopadhyay S. Mesiodens: A clinical and radiographic study in children. J. Indian. Soc. Pedod. Prev. Dent. 2011;29:34. doi: 10.4103/0970-4388.79928. [DOI] [PubMed] [Google Scholar]
- 10.Colak H., Uzgur R., Tan E., Hamidi M., Turkal M., Colak T. Investigation of prevalence and characteristics of mesiodens in a non-syndromic 11256 dental outpatients. Eur. Rev. Med. Pharmacol. Sci. 2013;17:2684–2689. [PubMed] [Google Scholar]
- 11.Alarcón J., Guzmán J., Masuko T.S., Cáceres P.N., Fuentes R. Non-Syndromic Familial Mesiodens: Presentation of Three Cases. Diagnostics. 2022;12:1869. doi: 10.3390/diagnostics12081869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Niswander J.D., Sujaku C. Congenital anomalies of teeth in Japanese children. Am. J. Phys. Anthropol. 1963;21:569–574. doi: 10.1002/ajpa.1330210413. [DOI] [PubMed] [Google Scholar]
- 13.Shih W.-Y., Hsieh C.-Y., Tsai T.-P. Clinical evaluation of the timing of mesiodens removal. J. Chin. Med. Assoc. 2016;79:345–350. doi: 10.1016/j.jcma.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 14.Nusse R., Clevers H. Wnt/β-catenin signaling, disease, and emerging therapeutic modalities. Cell. 2017;169:985–999. doi: 10.1016/j.cell.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 15.Doolan B., Onoufriadis A., Kantaputra P., McGrath J. WNT10A, dermatology and dentistry. Br. J. Dermatol. 2021;185:1105–1111. doi: 10.1111/bjd.20601. [DOI] [PubMed] [Google Scholar]
- 16.MacDonald B.T., He X. Frizzled and LRP5/6 receptors for Wnt/β-catenin signaling. Cold Spring Harb. Perspect. Biol. 2012;4:a007880. doi: 10.1101/cshperspect.a007880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yu F., Cai W., Jiang B., Xu L., Liu S., Zhao S. A novel mutation of adenomatous polyposis coli (APC) gene results in the formation of supernumerary teeth. J. Cell. Mol. Med. 2018;22:152–162. doi: 10.1111/jcmm.13303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang T., Li F., Cheng X., Wang J., Zhang W., Zhang B., Tang Y., Li Q., Zhou C., Tu S. Wnt inhibition sensitizes PD-L1 blockade therapy by overcoming bone marrow-derived myofibroblasts-mediated immune resistance in tumors. Front. Immunol. 2021;12:619209. doi: 10.3389/fimmu.2021.619209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kantaputra P.N., Guven Y., Tripuwabhrut K., Adisornkanj P., Hatsadaloi A., Kaewgahya M., Olsen B., Ngamphiw C., Jatooratthawichot P., Tongsima S. Mutations in LRP5 and BMP4 are associated with mesiodens, tooth agenesis, root malformation, and oral exostoses. Clin. Genet. 2022;102:333–338. doi: 10.1111/cge.14183. [DOI] [PubMed] [Google Scholar]
- 20.Kantaputra P., Jatooratthawichot P., Chintakanon K., Intachai W., Pradermdutsadeeporn P., Adisornkanj P., Tongsima S., Ngamphiw C., Olsen B., Tucker A.S. Mutations in LRP6 highlight the role of WNT signaling in oral exostoses and dental anomalies. Arch. Oral. Biol. 2022;142:105514. doi: 10.1016/j.archoralbio.2022.105514. [DOI] [PubMed] [Google Scholar]
- 21.Kantaputra P., Tripuwabhrut K., Jatooratthawichot P., Adisornkanj P., Hatsadaloi A., Porntrakoolsaree N., Kaewgaya M., Olsen B., Tongsima S., Ngamphiw C. Mutations in the WLS are associated with dental anomalies, torus palatinus, and torus mandibularis. [(accessed on 14 November 2022)];Eur. J. Orthod. 2022 :cjac068. doi: 10.1093/ejo/cjac068. Available online: https://pubmed.ncbi.nlm.nih.gov/36374649/ [DOI] [PubMed] [Google Scholar]
- 22.Kantaputra P., Jatooratthawichot P., Kottege N., Anthonappa R.P., Kaewgahya M., Tongsima S., Ngamphiw C., Ketudat Cairns J.R., Predes D., He X. DKK1 is a strong candidate for mesiodens and taurodontism. [(accessed on 4 January 2023)];Clin. Genet. 2023 doi: 10.1111/cge.14295. Available online: https://pubmed.ncbi.nlm.nih.gov/36601665/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kantaputra P.N., Jatooratthawichot P., Adisornkanj P., Kitsadayurach P., Kaewgahya M., Olsen B., Ohazama A., Ngamphiw C., Tongsima S., Cox T.C., et al. Rare Variants in LRP4 Are Associated with Mesiodens, Root Maldevelopment, and Oral Exostoses in Humans. Biology. 2023;12:220. doi: 10.3390/biology12020220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munne P.M., Tummers M., Järvinen E., Thesleff I., Jernvall J. Tinkering with the inductive mesenchyme: Sostdc1 uncovers the role of dental mesenchyme in limiting tooth induction. Development. 2009;136:393–402. doi: 10.1242/dev.025064. [DOI] [PubMed] [Google Scholar]
- 25.Ohazama A., Johnson E.B., Ota M.S., Choi H.Y., Porntaveetus T., Oommen S., Itoh N., Eto K., Gritli-Linde A., Herz J., et al. Lrp4 modulates extracellular integration of cell signaling pathways in development. PLoS ONE. 2008;3:e4092. doi: 10.1371/journal.pone.0004092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.García E.B.G., Knoers N.V. Gardner’s syndrome (familial adenomatous polyposis): A cilia-related disorder. Lancet. Oncol. 2009;10:727–735. doi: 10.1016/S1470-2045(09)70167-6. [DOI] [PubMed] [Google Scholar]
- 27.Lubinsky M., Kantaputra P.N. Syndromes with supernumerary teeth. Am. J. Med. Genet. Part A. 2016;170:2611–2616. doi: 10.1002/ajmg.a.37763. [DOI] [PubMed] [Google Scholar]
- 28.Otto F., Kanegane H., Mundlos S. Mutations in the RUNX2 gene in patients with cleidocranial dysplasia. Hum. Mutat. 2002;19:209–216. doi: 10.1002/humu.10043. [DOI] [PubMed] [Google Scholar]
- 29.Hordyjewska-Kowalczyk E., Sowińska-Seidler A., Olech E.M., Socha M., Glazar R., Kruczek A., Latos-Bieleńska A., Tylzanowski P., Jamsheer A. Functional analysis of novel RUNX2 mutations identified in patients with cleidocranial dysplasia. Clin. Genet. 2019;96:429–438. doi: 10.1111/cge.13610. [DOI] [PubMed] [Google Scholar]
- 30.Kantaputra P., Miletich I., Lüdecke H.-J., Suzuki E., Praphanphoj V., Shivdasani R., Wuelling M., Vortkamp A., Napierala D., Sharpe P. Tricho-rhino-phalangeal syndrome with supernumerary teeth. J. Dent. Res. 2008;87:1027–1031. doi: 10.1177/154405910808701102. [DOI] [PubMed] [Google Scholar]
- 31.Kunotai W., Ananpornruedee P., Lubinsky M., Pruksametanan A., Kantaputra P.N. Making extra teeth: Lessons from a TRPS1 mutation. Am. J. Med. Genet. Part A. 2017;173:99–107. doi: 10.1002/ajmg.a.37967. [DOI] [PubMed] [Google Scholar]
- 32.Kantaputra P.N., Coury S.A., Tan W.-H. Impaired dentin mineralization, supernumerary teeth, hypoplastic mandibular condyles with long condylar necks, and a TRPS1 mutation. Arch. Oral. Biol. 2020;116:104735. doi: 10.1016/j.archoralbio.2020.104735. [DOI] [PubMed] [Google Scholar]
- 33.Nik Kantaputra P., Jotikasthira D., Carlson B., Wongmaneerung T., Quarto N., Khankasikum T., Powcharoen W., Intachai W., Tripuwabhrut K. TRPS1 mutation associated with trichorhinophalangeal syndrome type 1 with 15 supernumerary teeth, hypoplastic mandibular condyles with slender condylar necks and unique hair morphology. J. Dermatol. 2020;47:774–778. doi: 10.1111/1346-8138.15360. [DOI] [PubMed] [Google Scholar]
- 34.Rusan N.M., Peifer M. Original CIN: Reviewing roles for APC in chromosome instability. J. Cell Biol. 2008;181:719–726. doi: 10.1083/jcb.200802107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 36.Gardner E.J. A genetic and clinical study of intestinal polyposis, a predisposing factor for carcinoma of the colon and rectum. Am. J. Hum. Genet. 1951;3:167. [PMC free article] [PubMed] [Google Scholar]
- 37.Järvinen E., Birchmeier W., Taketo M.M., Jernvall J., Thesleff I. Continuous tooth generation in mouse is induced by activated epithelial Wnt/β-catenin signaling. Proc. Natl. Acad. Sci. USA. 2006;103:18627–18632. doi: 10.1073/pnas.0607289103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu F., Chu E.Y., Watt B., Zhang Y., Gallant N.M., Andl T., Yang S.H., Lu M.-M., Piccolo S., Schmidt-Ullrich R. Wnt/β-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 2008;313:210–224. doi: 10.1016/j.ydbio.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X.-P., O’Connell D.J., Lund J.J., Saadi I., Kuraguchi M., Turbe-Doan A., Cavallesco R., Kim H., Park P.J., Harada H. Apc inhibition of Wnt signaling regulates supernumerary tooth formation during embryogenesis and throughout adulthood. Development. 2009;136:1939–1949. doi: 10.1242/dev.033803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hamada F., Bienz M. The APC tumor suppressor binds to C-terminal binding protein to divert nuclear beta-catenin from TCF. Dev. Cell. 2004;7:677–685. doi: 10.1016/j.devcel.2004.08.022. [DOI] [PubMed] [Google Scholar]
- 41.Spink K.E., Polakis P., Weis W.I. Structural basis of the Axin-adenomatous polyposis coli interaction. EMBO J. 2000;19:2270–2279. doi: 10.1093/emboj/19.10.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Aoki K., Taketo M.M. Adenomatous polyposis coli (APC): A multi-functional tumor suppressor gene. J. Cell. Sci. 2007;120:3327–3335. doi: 10.1242/jcs.03485. [DOI] [PubMed] [Google Scholar]
- 43.Kantaputra P., Jatooratthawichot P., Tantachamroon O., Nanekrungsan K., Intachai W., Olsen B., Tongsima S., Ngamphiw C., Cairns J.R.K. Novel Dental Anomaly–associated Mutations in WNT10A Protein Binding Sites. [(accessed on 7 May 2022)];Int. Dent. J. 2022 73:79–86. doi: 10.1016/j.identj.2022.04.006. Available online: https://www.sciencedirect.com/science/article/pii/S0020653922000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng Y., Baugh E., Akyalcin S., Letra A. Functional effects of wnt10a rare variants associated with tooth agenesis. J Dent Res. 2021;100:302–309. doi: 10.1177/0022034520962728. [DOI] [PubMed] [Google Scholar]
- 45.Chu M.L.-H., Ahn V.E., Choi H.-J., Daniels D.L., Nusse R., Weis W.I. Structural studies of Wnts and identification of an LRP6 binding site. Structure. 2013;21:1235–1242. doi: 10.1016/j.str.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tamai K., Semenov M., Kato Y., Spokony R., Liu C., Katsuyama Y., Hess F., Saint-Jeannet J.-P., He X. LDL-receptor-related proteins in Wnt signal transduction. Nature. 2000;407:530–535. doi: 10.1038/35035117. [DOI] [PubMed] [Google Scholar]
- 47.Jeong W., Jho E.-h. Regulation of the low-density lipoprotein receptor-related protein LRP6 and its association with disease: Wnt/β-catenin signaling and beyond. Front. Cell Dev. Biol. 2021;9:714330. doi: 10.3389/fcell.2021.714330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuraguchi M., Wang X.-P., Bronson R.T., Rothenberg R., Ohene-Baah N.Y., Lund J.J., Kucherlapati M., Maas R.L., Kucherlapati R. Adenomatous polyposis coli (APC) is required for normal development of skin and thymus. PLoS Genet. 2006;2:e146. doi: 10.1371/journal.pgen.0020146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fearnhead N.S., Britton M.P., Bodmer W.F. The abc of apc. Hum. Mol. Genet. 2001;10:721–733. doi: 10.1093/hmg/10.7.721. [DOI] [PubMed] [Google Scholar]
- 50.Polakis P. The adenomatous polyposis coli (APC) tumor suppressor. Biochim. Biophys. Acta. 1997;1332:F127–F147. doi: 10.1016/S0304-419X(97)00008-5. [DOI] [PubMed] [Google Scholar]
- 51.Juanes M.A., Fees C.P., Hoeprich G.J., Jaiswal R., Goode B.L. EB1 Directly Regulates APC-Mediated Actin Nucleation. Curr Biol. 2020;30:4763–4772.e4768. doi: 10.1016/j.cub.2020.08.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu B., Chen S., Cheng D., Jing W., Helms J. Primary cilia integrate hedgehog and Wnt signaling during tooth development. J. Dent. Res. 2014;93:475–482. doi: 10.1177/0022034514528211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hampl M., Cela P., Szabo-Rogers H.L., Kunova Bosakova M., Dosedelova H., Krejci P., Buchtova M. Role of Primary Cilia in Odontogenesis. J. Dent. Res. 2017;96:965–974. doi: 10.1177/0022034517713688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li S., Zhang H., Sun Y. Primary cilia in hard tissue development and diseases. Front. Med. 2021;15:657–678. doi: 10.1007/s11684-021-0829-6. [DOI] [PubMed] [Google Scholar]
- 55.Hermans F., Hemeryck L., Lambrichts I., Bronckaers A., Vankelecom H. Intertwined signaling pathways governing tooth development: A give-and-take between canonical Wnt and Shh. Front. Cell Dev. Biol. 2021;9:758203. doi: 10.3389/fcell.2021.758203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jiang S., Chen G., Feng L., Jiang Z., Yu M., Bao J., Tian W. Disruption of kif3a results in defective osteoblastic differentiation in dental mesenchymal stem/precursor cells via the Wnt signaling pathway. Mol. Med. Rep. 2016;14:1891–1900. doi: 10.3892/mmr.2016.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.