Abstract
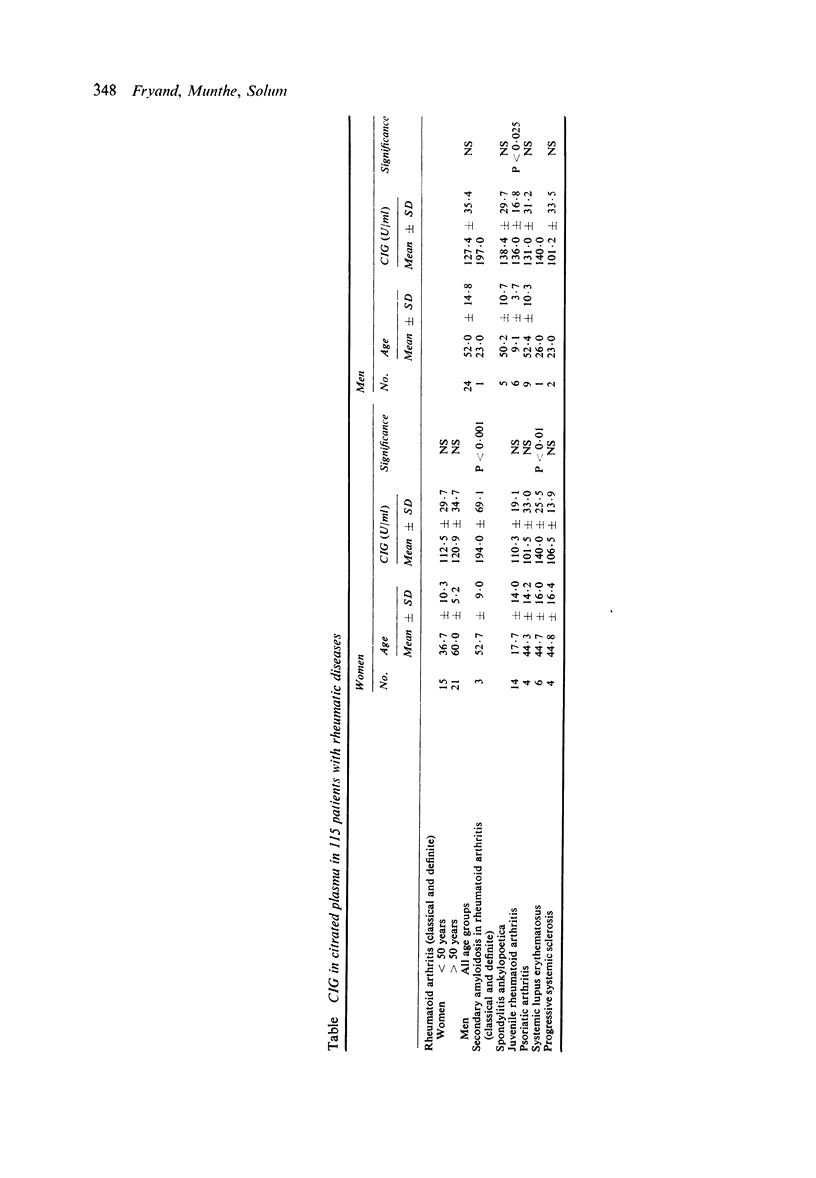
Cold insoluble globulin (CIG) is a normal glycoprotein of human serum and plasma. The physiological significance of this protein is unknown, but is shows a temperature-dependent relation to fibrinogen and fibrin. It is possible that it represents a substrate for activated fibrin-stabilising factor in the polymerisation of fibrin. CIG is found on the surface of fibroblasts. In the present study CIG was estimated in citrated plasma in 115 patients with rheumatic diseases. Increased amounts were found in patients with systemic lupus erythematosus, secondary amyloidosis in classical and definite rheumatoid arthritis, and in male patients with juvenile rheumatoid arthritis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronsen K. F., Ekelund G., Kindmark C. O., Laurell C. B. Sequential changes of plasma proteins after surgical trauma. Scand J Clin Lab Invest Suppl. 1972;124:127–136. doi: 10.3109/00365517209102760. [DOI] [PubMed] [Google Scholar]
- Fyrand O., Solum N. L. Studies on cold insoluble globulin in dermatological patients. I. Immunochemical quantitation in citrated plasma from patients with increased amounts of heparin precipitable fraction (HPF). Thromb Res. 1976 Nov;9(5):447–455. doi: 10.1016/0049-3848(76)90200-0. [DOI] [PubMed] [Google Scholar]
- Fyrand O., Solum N. O. Heparin precipitable fraction (HPF) from dermatological patients. II. Studies on the non-clottable proteins. Identification of cold insoluble globulin as the main non-clottable component. Thromb Res. 1976 May;8(5):659–672. doi: 10.1016/0049-3848(76)90246-2. [DOI] [PubMed] [Google Scholar]
- Fyrand O. Studies on cold insoluble globulin in dermatological patients. II. Its immunochemical quantitation in citrated plasma in a group of unselected dermatological inpatients. Acta Derm Venereol. 1977;57(3):227–231. [PubMed] [Google Scholar]
- Ganrot P. O. Variation of the concentrations of some plasma proteins in normal adults, in pregnant women and in newborns. Scand J Clin Lab Invest Suppl. 1972;124:83–88. doi: 10.3109/00365517209102755. [DOI] [PubMed] [Google Scholar]
- Hällén J., Laurell C. B. Plasma protein pattern in cirrhosis of the liver. Scand J Clin Lab Invest Suppl. 1972;124:97–103. doi: 10.3109/00365517209102757. [DOI] [PubMed] [Google Scholar]
- Johansson B. G., Kindmark C. O., Trell E. Y., Wollheim F. A. Sequential changes of plasma proteins after myocardial infarction. Scand J Clin Lab Invest Suppl. 1972;124:117–126. doi: 10.3109/00365517209102759. [DOI] [PubMed] [Google Scholar]
- Laurell C. B. Composition and variation of the gel electrophoretic fractions of plasma, cerebrosinal fluid and urine. Scand J Clin Lab Invest Suppl. 1972;124:71–82. doi: 10.3109/00365517209102754. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Chen A. B., Huseby R. M. The cold-insoluble globulin of human plasma: studies of its essential structural features. Biochim Biophys Acta. 1975 Apr 29;386(2):509–524. doi: 10.1016/0005-2795(75)90294-9. [DOI] [PubMed] [Google Scholar]
- Mosesson M. W., Umfleet R. A. The cold-insoluble globulin of human plasma. I. Purification, primary characterization, and relationship to fibrinogen and other cold-insoluble fraction components. J Biol Chem. 1970 Nov 10;245(21):5728–5736. [PubMed] [Google Scholar]
- Runne U., Orfanos C. E. Amyloid production by dermal fibroblasts. Electron microscopic studies on the origin of amyloid in various dermatoses and skin tumours. Br J Dermatol. 1977 Aug;97(2):155–166. doi: 10.1111/j.1365-2133.1977.tb15061.x. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Vaheri A. Interaction of soluble fibroblast surface antigen with fribrinogen and fibrin. J Exp Med. 1975 Feb 1;141(2):497–501. doi: 10.1084/jem.141.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaheri A., Ruoslahti E. Disappearance of a major cell-type specific surface glycoprotein antigen (SF) after transformation of fibroblasts by Rous sarcoma virus. Int J Cancer. 1974 May 15;13(5):579–586. doi: 10.1002/ijc.2910130502. [DOI] [PubMed] [Google Scholar]


