Abstract
Homocysteine (Hcy) is a vascular risk factor associated with cognitive impairment and cerebrovascular disease but has also been implicated in Alzheimer’s disease (AD). Using multivariate Scaled Subprofile Model (SSM) analysis, we sought to identify a network pattern in structural neuroimaging reflecting the regionally distributed association of plasma Hcy with subcortical gray matter (SGM) volumes and its relation to other health risk factors and cognition in 160 healthy older adults, ages 50–89. We identified an SSM Hcy-SGM pattern that was characterized by bilateral hippocampal and nucleus accumbens volume reductions with relative volume increases in bilateral caudate, pallidum, and putamen. Greater Hcy-SGM pattern expression was associated with greater white matter hyperintensity (WMH) volume, older age, and male sex, but not with other vascular and AD-related risk factors. Mediation analyses revealed that age predicted WMH volume, which predicted Hcy-SGM pattern expression, which, in turn, predicted cognitive processing speed performance. These findings suggest that the multivariate SSM Hcy-SGM pattern may be indicative of cognitive aging, reflecting a potential link between vascular health and cognitive dysfunction in healthy older adults.
Keywords: cognitive aging, vascular risk, Scaled Subprofile Model, white matter hyperintensity, gray matter atrophy
1. Introduction
Elevated plasma homocysteine (Hcy) levels have been implicated as a vascular risk factor in healthy aging and have been related to increasing risk for cerebrovascular disease (CVD; Hankey and Eikelboom, 2001). Hcy has also been associated with an increased risk for Alzheimer’s disease (AD; Clarke et al., 1998; Hooshmand et al., 2013). As a sulfhydryl-containing amino acid, Hcy is produced via the metabolism of the dietary essential amino acid methionine. Disturbances in metabolism related to increasing age may lead to excess Hcy concentrations in plasma (Hankey and Eikelboom, 2001). Having a high level of plasma Hcy has been associated with greater risk for stroke (Hankey and Eikelboom, 2001) and brain atrophy (Den Heijer et al., 2003; Tan et al., 2018; Williams et al., 2002) in structural neuroimaging studies, especially involving subcortical brain structures preferentially affected by age-related CVD and AD pathology (Nie et al., 2017; Werden et al., 2017; Yi et al., 2016).
Higher Hcy levels have been related to medial temporal lobe atrophy in AD patients (Hooshmand et al., 2013) and to hippocampal atrophy more specifically in non-demented older adults (Den Heijer et al., 2003; Williams et al., 2002), supporting its role as a potential risk factor for AD. A recent study of non-demented older adults, however, observed that less gray matter volumes in subcortical structures of the nucleus accumbens, thalamus, and brain stem, but not hippocampus, were associated with higher levels of Hcy (Tan et al., 2018). Furthermore, the pathway by which elevated Hcy contributes to subcortical brain atrophy and the risk for both CVD and AD is not fully understood.
Hcy is recognized as pro-thrombotic and pro-atherogenic (Hankey and Eikelboom, 2001) and has been implicated in small vessel CVD, as reflected by greater white matter hyperintensity (WMH) lesion load in several studies of middle-to-older-aged adults (Raz et al., 2012; Wright et al., 2005). Moreover, considerable attention has been directed toward both plasma Hcy levels and associated WMH burden as biomarkers indicating increased risk for CVD, as well as a potential vascular linkage to increased AD risk (Hooshmand et al., 2013).
Converging evidence suggests that age-related vascular changes associated with WMH burden contribute to AD-like patterns of brain atrophy, showing spatial overlap of the associated atrophy with the brain-based effects of Hcy (Habes et al., 2016). It has been reported that increased WMH burden is associated with smaller volumes in brain regions typically impacted by AD, such as the medial temporal lobe and more specifically the hippocampus (Crane et al., 2015; Van Der Flier et al., 2005), which, in turn, may lead to diminished cognitive function in old age (Van Der Flier et al., 2005). Together, these findings suggest the possibility that WMH burden may be an important link in the association between elevated plasma Hcy and brain atrophy in key subcortical structures that have been implicated in the development of both CVD and AD in aging.
Previous studies have typically applied univariate analysis techniques, focusing on individual brain regions without considering individual differences in Hcy in relation to regional covariance patterns across multiple subcortical brain structures. The Scaled Subprofile Model (SSM; Alexander and Moeller, 1994) provides a multivariate analysis method based on a modified principal component analysis (PCA) to test differences in the covariance across brain regions, detecting regionally distributed patterns of brain structural volumes without the need for correction of multiple comparisons (Alexander and Moeller, 1994).
The SSM has been applied in numerous structural neuroimaging studies of healthy aging (Alexander et al., 2006; Bergfield et al., 2010; Brickman et al., 2007), the association with apolipoprotein E ε4 (APOE) genetic risk for AD (Alexander et al., 2012b), and the relation to blood pressure control (Kern et al., 2017). It has also been applied in structural brain imaging studies of aging with non-human primate (Alexander et al., 2008) and rodent (Alexander et al., 2020) models. Applying the SSM to subcortical gray matter (SGM) volumes provides the opportunity to identify a regional network covariance pattern of SGM associated with Hcy and to evaluate whether Hcy-related SGM volume pattern differences are mediated by WMH volume and related to cognitive function in the context of healthy aging.
In the present study, we sought (1) to investigate how plasma levels of total Hcy are associated with differences in volumes of subcortical brain structures often implicated in the risk for CVD and AD in a cohort of healthy older adults, 50 to 89 years of age, by applying the SSM to volumes of SGM to create a Hcy-related network pattern of regional covariance; (2) to evaluate its relation to total WMH volume, as well as other vascular health and AD-related risk factors; and (3) to investigate its potential contribution as a mediator of poorer cognitive function in healthy aging. We hypothesized that multivariate SSM network covariance analysis would identify a regional network pattern of Hcy-related SGM volumes, including reductions in hippocampal and nucleus accumbens volumes, that shows greater pattern expression related to aging, and would be exacerbated by greater WMH lesion load, leading to diminished cognitive performance in this healthy older adult cohort.
2. Material and Methods
2.1. Participants
Participants were 160 community-dwelling healthy volunteers, 50–89 years of age (mean age = 69.66 ± 9.95 years, 53.8% women), who completed blood tests, brain MRI scans, a graded exercise test, and neuropsychological tests as part of a study of healthy cognitive aging. The sample was mostly White (95.0%), with a mean education level of 15.98 ± 2.57 years (Table 1). Participants were screened to exclude significant medical, psychiatric, and neurological disorders through an extensive medical screen combined with a neurological examination by an experienced specialist in geriatric neurology (GAH). Exclusion criteria also included a Hamilton Depression Rating Scale (HAM-D) score greater than 9 as well as a Mini-Mental State Exam (MMSE) score less than 26. The mean score of the MMSE in the sample was 29.01 ± 1.25. The demographic characteristics are shown in Table 1. After the study procedures and possible risks were explained, all participants provided informed and written consent. The study was reviewed and approved by the Institutional Review Board (IRB) at the University of Arizona.
Table 1.
Characteristics of the Study Sample (n = 160)
| Variable | M | SD |
|---|---|---|
|
| ||
| Age (years) | 69.66 | 9.95 |
| Sex (Female, %) | 53.8 | |
| Race/ethnicitya | ||
| White (%) | 95.0 | |
| Asian (%) | 1.9 | |
| American Indian/Alaska Native (%) | 0.6 | |
| Multiracialb (%) | 2.5 | |
| Education (years) | 15.98 | 2.57 |
| MMSE Score | 29.01 | 1.25 |
| Plasma Total Hcy (μMol/L) | 10.65 | 2.99 |
| Total WMH Volume (mL) | 6.57 | 10.53 |
| VO2max (mL/kg/min) | 24.30 | 5.72 |
| APOE ε4 Carriers (%) | 30.0 | |
| Hypertensionc (%) | 33.1 | |
| Diabetesd (%) | 3.8 | |
| Smoking History (current or past, %) | 41.9 | |
| Serum Vitamin B12e (pg/mL) | 662.49 | 240.75 |
| GDSf | 0.95 | 1.48 |
| TMT-A (seconds) | 32.18 | 10.37 |
| TMT-B (seconds) | 76.14 | 30.50 |
| SRT CLTR (words) | 64.62 | 37.07 |
Note. M and SD are used to represent mean and standard deviation, respectively.
MMSE, Mini-Mental Status Exam; Hcy, homocysteine; WMH, white matter hyperintensity; VO2max, volume of maximum oxygen consumption; APOE, apolipoprotein E; GDS, Geriatric Depression Scale; TMT, Trail Making Test; SRT, Buschke Selective Reminding Test; CLTR, consistent long-term retrieval.
Self-reported race and ethnicity.
Four participants (2.5%) self-reported as multiracial, which included two identifying as White, Black or African American, and American Indian or Alaska Native; and two as White and American Indian or Alaska Native.
Self-reported hypertension status.
Self-report of a diagnosis of diabetes.
n = 158.
n = 159.
2.2. Laboratory Assessments
Blood samples were collected during the imaging visit after at least 12 hours of fasting, placed on ice immediately, and then prepared for testing in the Clinical Chemistry Laboratory of the Department of Pathology at the University of Arizona Medical Center, Tucson, Arizona according to standard laboratory procedures following the manufacturer’s instructions. Plasma levels of total Hcy were measured by fluorescence polarization immunoassay on the Axsym analyzer or chemiluminescence microparticle immunoassay on the ci8200 analyzer (Abbott Laboratories, Chicago, IL). Validation between these two analyzers was confirmed by the Clinical Chemistry Laboratory with a Deming regression coefficient of > 0.999. Serum vitamin B12 levels were measured using enhanced chemiluminescence immunoassay on the Vitros ECi Immunodiagnostic System (Ortho Clinical Diagnostics, Raritan, NJ).
APOE alleles were determined in the Neurogenomics Division of the Translational Genomics Research Institute (TGen), Phoenix, Arizona from genomic DNA isolated from blood samples by the polymerase chain reaction/restriction enzyme method, as previously described (Van Etten et al., 2021). In brief, extracted DNA was assayed by restriction fragment length polymorphism to determine the APOE genotype for each participant. DNA amplification was performed using AmpliTaq Gold Fast PCR Master Mix (Applied Biosystems: Thermo Fisher Scientific, Waltham, MA) using primer sequences (FWD 5’-ACA-GAA-TTG-GCC-CCG-GCC-TGG-TAC-3’ and REV 5’-TAA-GCT-TGG-CAC-GGC-TGT-CCA-AGG-A-3’). Each sample was then incubated with 10 units of HhaI restriction enzyme (New England BioLabs, Ipswich, MA) at 37 °C for 16 hours and evaluated with a 4% agarose gel for banding patterns consistent with one of the six common APOE genotypes. In this cohort, there were 48 APOE ε4 carriers (30.0%) and 112 ε4 non-carriers (70.0%).
2.3. Cardiorespiratory Fitness Assessment
During a treadmill graded exercise test conducted at the Pulmonary Function Laboratory at the University of Arizona Medical Center, Tucson, Arizona, oxygen uptake (VO2) was measured using indirect calorimetry. Measurements of expiratory gases were determined by standard techniques of open-circuit spirometry using a metabolic cart. The treadmill session began at a low intensity with a 1 mph speed and a 0% grade, but speed and grade were increased based on the modified Naughton treadmill protocol during exercise (Berry et al., 1996; Strzelczyk et al., 2001). Maximal oxygen consumption (VO2max) to measure cardiorespiratory fitness was considered achieved when two of the following three criteria were satisfied: (1) a plateau in VO2 with an increase in workload; (2) a respiratory exchange ratio of 1.1 or higher; and (3) a heart rate within ten beats of the age-predicted maximum. Termination criteria were consistent with the guidelines of the American College of Sports Medicine (ACSM, 2000).
2.4. Magnetic Resonance Imaging
Scan acquisition was performed on a 3T GE Signa scanner (HD Signa Excite; General Electric, Milwaukee, WI). Volumetric T1-weighted MRI scans were acquired with a spoiled gradient echo (SPGR) sequence (TR = 5.3 ms, TE = 2.0 ms, TI = 500 ms, flip Angle = 15°, matrix = 256 × 256, field of view (FOV) = 25.6 cm, slice thickness = 1.0 mm). We also obtained fluid attenuated inverse recovery (FLAIR) T2-weighted scans (TR = 11,000 ms, TE = 120 ms, TI = 2,250 ms, flip angle = 90°, matrix = 256 × 256, FOV = 25.0 cm, slice thickness = 2.6 mm).
T1-weighted MRI scans were processed using the automated recon-all pipeline in FreeSurfer v5.3 (http://surfer.nmr.mgh.harvard.edu). Details of the FreeSurfer processing stages for the automated subcortical segmentation have been described (Fischl et al., 2004, 2002). Briefly, the processing pipeline involved automated Talairach transformation, intensity normalization, removal of non-brain tissues, subcortical gray/white matter segmentation, tessellation of the gray/white matter boundary, automated topology correction, and surface deformation. Results of the processing steps were visually inspected for segmentation accuracy and re-processed, as needed. The processing stream provided bilateral SGM volumes of seven structures for each hemisphere: thalamus, caudate, putamen, pallidum, hippocampus, amygdala, and nucleus accumbens. Figure 1 illustrates the SGM volumes averaged over the 160 participants.
Fig. 1.
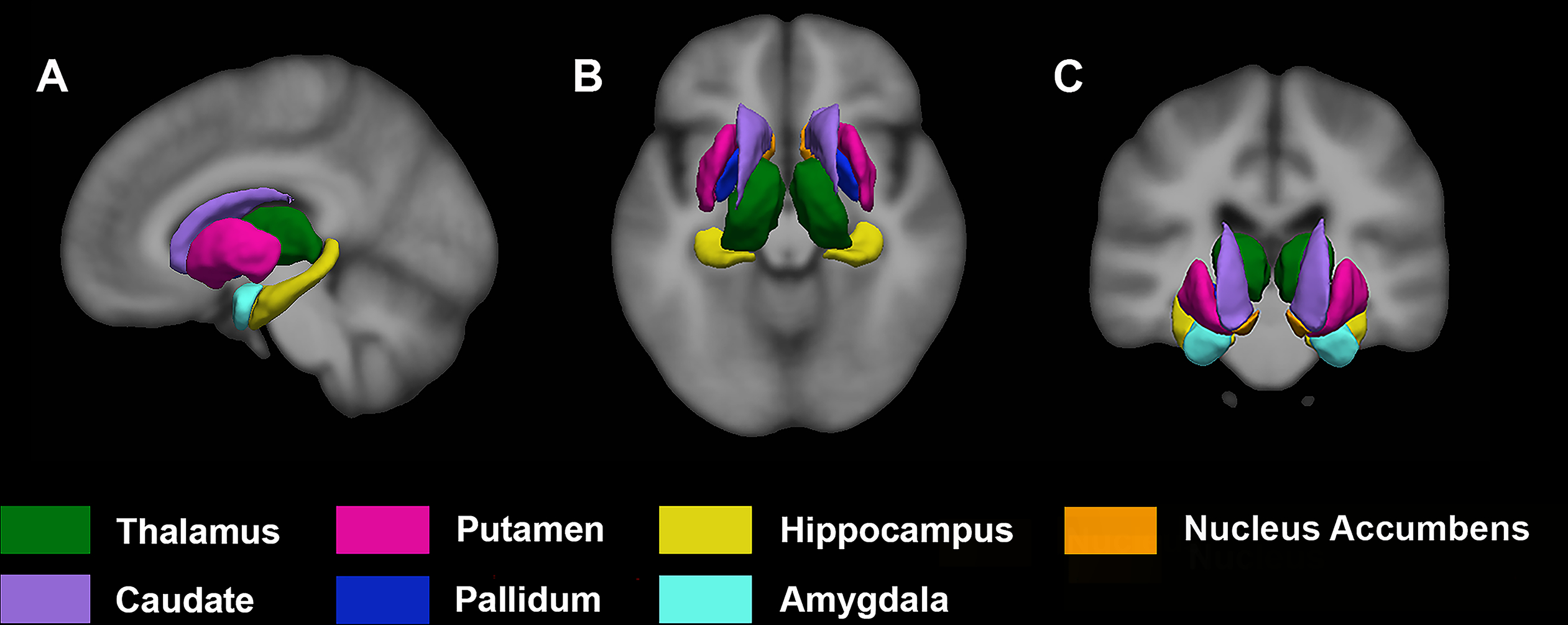
An illustration of SGM structural volumes averaged over the 160 study participants overlaid on a section of the average T1 image. (A) Sagittal, (B) Axial, and (C) Coronal sections. Left hemisphere appears on the right side of the image. SGM, subcortical gray matter.
WMH volume was automatically segmented using a combination of T1 and FLAIR images with the lesion growth algorithm (LGA) of the lesion segmentation toolbox (LST) (Schmidt et al., 2012) with Statistical Parametric Mapping (SPM12; Wellcome Trust Center for Neuroimaging, London, UK). The processing steps for the segmentation of WMH in this healthy older adult cohort have been described (Franchetti et al., 2020; Van Etten et al., 2021).
Briefly, manually segmented WMH maps were generated for a subset of 35 participants using ITK-SNAP (www.itksnap.org) following consensus review by two expert raters (PKB and GEA) and a specialist in geriatric neurology (GAH), and served as the reference images to assess the LGA segmentation accuracy across a range of optimization kappa values (0.05 – 1.00). Finally, mean spatial overlap with reference WMH maps using dice coefficients was computed at each kappa value to determine an optimal threshold of 0.35 (Van Etten et al., 2021).
Lesion probability maps for all study participants were then generated using LGA at the optimal kappa value of 0.35 and visually inspected for segmentation quality, which was followed by computing total WMH volume (mL) by summing voxel volumes. The WMH volume values were natural log-transformed for analyses. We obtained estimates of total intracranial volume (TIV) in native brain space from each of the T1 images using SPM12 by calculating the sum of the total gray matter, white matter, and cerebrospinal fluid segments (Alexander et al., 2012a).
2.5. Neuropsychological Outcomes
Neuropsychological tests were administered to assess performance on cognitive domains that are particularly vulnerable to the effects of aging: speed of processing, executive function, and verbal memory (Alexander et al., 2012b). For this study, we focused on three measures obtained from the Trail Making Test, Parts A and B (TMT) and the 12-trial version of the Buschke Selective Reminding Test using a 12-word list (SRT). The total time to completion of TMT-A was natural-log transformed and used as a measure of processing speed with higher values representing poorer processing speed performance (Crowe, 1998). We used the standardized residual of TMT-B, after statistically removing the processing speed performance on TMT-A, as a measure of cognitive flexibility and shifting within the executive functioning domain (Crowe, 1998). For memory, we used the number of words recalled without interruption on at least three successive trials, scored as consistent long-term retrieval (CLTR) on the SRT, a measure highly sensitive to age-related differences in verbal episodic memory performance (Sliwinski et al., 1997).
2.6. Statistical Analyses
We performed regional network analysis using a multivariate model of regional covariance (Alexander and Moeller, 1994) in Matlab R2020a (MathWorks, Natick, MA). We applied the SSM to volumetric measures of 14 regions of interest (ROI) (i.e., bilateral volumes of seven SGM structures) to identify subcortical regional covariance related to plasma total Hcy.
As previously described (Alexander and Moeller, 1994), after natural-log transformation, the mean values across the ROIs for each subject and across subjects for each ROI were subtracted. PCA was subsequently performed to obtain a set of regional covariance patterns and to produce corresponding subject scores, which reflect the extent to which each subject expresses the identified regional covariance patterns. We used Akaike information criteria (AIC; Burnham and Anderson, 2002) for model selection, which seeks the optimization of a bias-variance trade-off (Habeck et al., 2005) to select the best set of components to predict Hcy. Based on the lowest AIC value in the model, the first set of sequential components was chosen as the best set.
Bootstrap resampling (Alexander et al., 2012a; Efron and Tibshirani, 1994; Habeck et al., 2005) was applied to the SSM analysis with 10,000 iterations to provide reliability estimates at each ROI for the resulting pattern weights associated with Hcy. The bootstrap distributions provided confidence intervals (CI) for the observed linear combination of network pattern weights. This network analysis was followed by univariate regression for the individual SGM volumes in relation to Hcy, adjusted for TIV, to test how each ROI contributed to the Hcy-related network pattern, with false discovery rate (FDR) correction for multiple comparisons.
Block-wise multiple regression analysis was subsequently performed to investigate the effects of WMH burden, demographic characteristics, and vascular and AD-related risk factors on the Hcy-related SGM network pattern. With the model adjusted for TIV (block 1), total WMH volume was initially entered (block 2), followed by age and sex (block 3). We subsequently tested the SSM pattern prediction by vascular and AD risk factors (block 4), including APOE ε4 status, self-reported hypertension status, a self-reported history of current or past smoking, and VO2max. Finally, we repeated the multiple regression analysis above, including levels of vitamin B12 (block 5) as an added covariate (Wright et al., 2005).
We subsequently performed mediation model analyses to test whether the influence of age on the Hcy-SGM pattern was mediated by WMH burden while accounting for sex differences and with TIV, APOE ε4 status, hypertension status, smoking history, VO2max, and vitamin B12 levels further added as covariates. Serial multiple mediation models were then used to evaluate how the Hcy-related SGM pattern related to cognitive aging and whether WMH load mediated the pattern’s association with age-related cognitive function.
In these models, we tested age as a predictor and separately included cognitive outcomes in three domains—processing speed, executive function, and verbal memory—as a dependent variable, with WMH volume and the Hcy-SGM pattern tested as mediators. We focused on three selected cognitive measures expected to be vulnerable to aging (Alexander et al., 2012b) to limit Type I error across multiple mediation models. We initially included TIV and the time interval between MRI scans and administration of the neuropsychological tests (Mean ± SD = 58.56 ± 47.35 days) as covariates, with additional adjustments for sex and years of education as covariates to control their potential influence on cognitive performance. APOE ε4 status, hypertension status, smoking history, VO2max, and vitamin B12 levels were subsequently added to account for their relation to increased risk of WMH burden, volume reductions, and cognitive function. Depression ratings on the Geriatric Depression Scale (Yesavage et al., 1982) were included as an additional covariate in a follow-up analysis to control its potential association with processing speed performance.
A bootstrapping procedure with 10,000 iterations was applied using the PROCESS macro (v3.4; Hayes, 2018) to construct percentile CIs for indirect effects. We report completely standardized indirect effects as effect size measures (Preacher and Kelley, 2011). The 95% percentile bootstrap CIs that do not include zero indicate significant mediation effects. Statistical analyses were all performed using SPSS 26.0 (IBM Corp., Armonk, NY).
3. Results
Using SSM analysis of the 14 SGM volumes with AIC, we identified the best linear combination of components associated with plasma levels of Hcy in this healthy aging cohort. This model included the first component and accounted for 9.4% of the variance in the levels of plasma total Hcy, with higher levels of Hcy associated with greater expression of the network pattern (F(1,158) = 16.30, p < .0001; Fig. 2A). The regional pattern was characterized by bilateral volumetric reductions in the hippocampus and nucleus accumbens with relative bilateral volumetric increases in the caudate, putamen, and pallidum. Figure 2B presents the Hcy-related SSM loadings for the 14 subcortical ROIs.
Fig. 2.
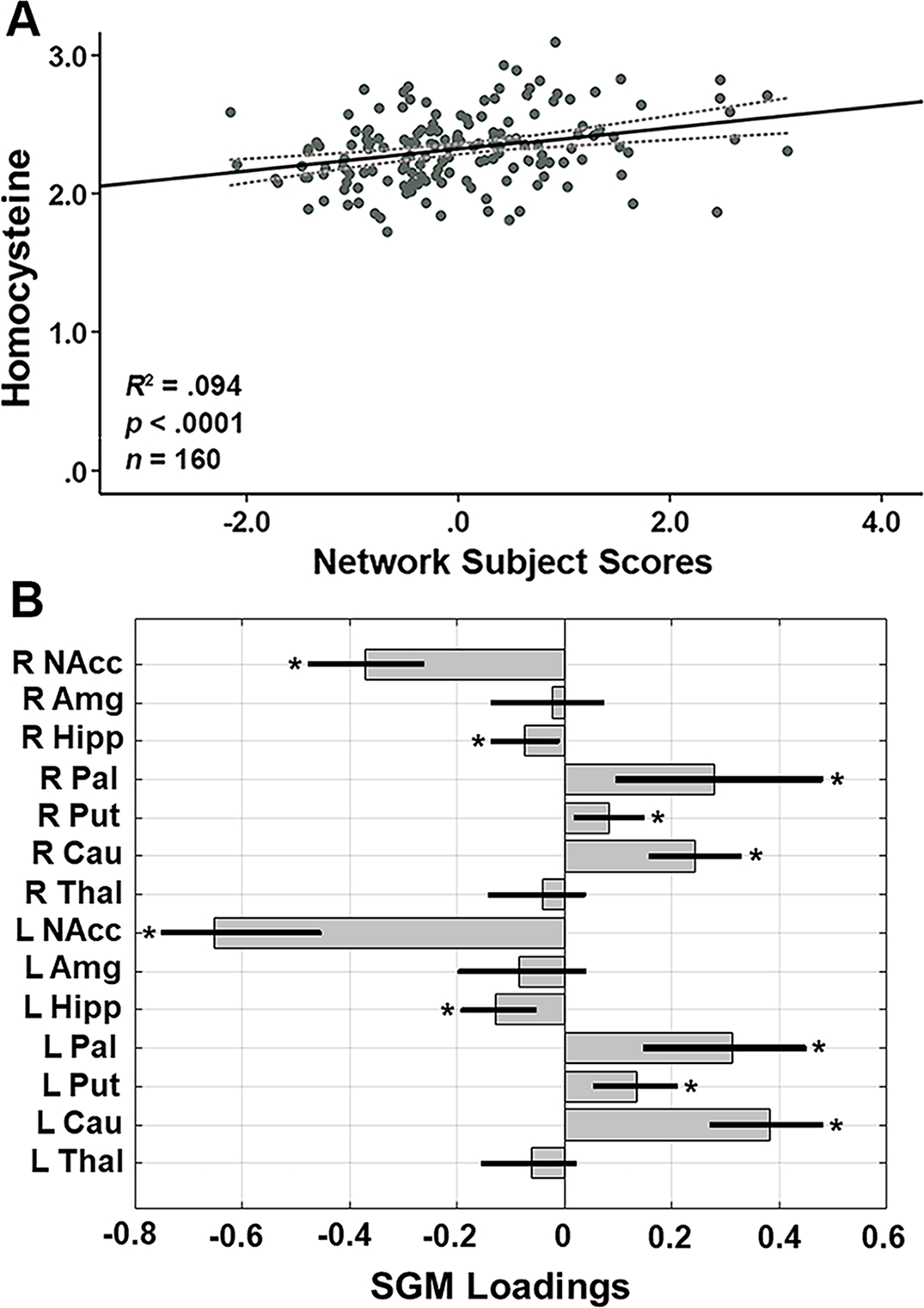
(A) SGM network subject scores and total Hcy levels. The subject scores of the Hcy-related SGM network pattern were derived from the first SSM component. Total Hcy levels were natural log transformed. The gray dotted lines represent 95% confidence intervals. The scatterplot shows that higher total Hcy levels were associated with greater expression of the SGM network pattern (R2 = .094, p < .0001, n = 160). (B) Hcy-related loadings for the SSM network pattern of the 14 SGM regions. The gray bars indicate point estimates for the loadings, black lines indicate the 95% confidence intervals from the bootstrap resampling with 10,000 iterations, and asterisks represent statistically significant regions contributing to the covariance pattern. L = left; R = right; NAcc = nucleus accumbens; Amg = amygdala; Hipp = hippocampus; Pal = pallidum; Put = putamen; Cau = caudate; Thal = thalamus; Hcy, homocysteine; SGM, subcortical gray matter; SSM, Scaled Subprofile Model.
Follow-up univariate regional analyses with adjustment for TIV confirmed that each of the bilateral hippocampus (β = −.295, p-FDR = .003 for left; β = −.259, p-FDR = .008 for right) and bilateral nucleus accumbens (β = −.247, p-FDR = .008 for left; β = −.204, p-FDR = .028 for right) volumes were inversely associated with levels of Hcy. Each of bilateral caudate (β = .162, p-FDR = .170 for left; β = .065, p-FDR = .560 for right), bilateral putamen (β = −.000, p-FDR = .999 for left; β = −.100, p-FDR = .301 for right), and bilateral pallidum (β = .114, p-FDR = .220 for left; β = −.080, p-FDR = .374 for right) volumes, however, did not show significant univariate positive associations with Hcy after adjusting for TIV, suggesting that the regions showing increases in the SSM pattern reflect relative preservation for these subcortical volumes.
With TIV added to a multiple regression model, we tested the Hcy-SGM network pattern prediction by total WMH volume, age, sex, and vascular health and AD-related risk factors of APOE ε4 status, hypertension status, smoking history, and VO2max to further evaluate their association with the SSM pattern. As shown in Table 2, after adjusting for TIV, there was a significant association between total WMH volume and the Hcy-SGM network pattern (β = .427, p = 9.0903E-9). After additionally entering age and sex, each emerged as statistically significant predictors (β = .339, p < .0001 for age; β = .188, p = .041 for sex) and the effect of WMH remained significant (β = .245, p = .002).
Table 2.
Summary of Multiple Regression Analyses for Variables Predicting the Hcy-SGM Network Pattern
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| β | B | SE | β | B | SE | β | B | SE | β | B | SE | |
|
| ||||||||||||
| TIV | .20* | 1.39 | .54 | .20** | 1.39 | .49 | .06 | .39 | .64 | .06 | .41 | .65 |
| WMH | .43*** | .43 | .07 | .25** | .25 | .08 | .23 ** | .23 | .08 | |||
| Age | .34*** | .03 | .01 | .30 ** | .03 | .01 | ||||||
| Sex | .19* | .38 | .18 | .22 * | .43 | .19 | ||||||
| APOE ε4 status | .02 | .05 | .15 | |||||||||
| Hypertension | .06 | .12 | .16 | |||||||||
| Smoking | −.07 | −.13 | .14 | |||||||||
| VO2max | −.07 | −.01 | .02 | |||||||||
| Adjusted R2 | .03 | .21 | .31 | .30 | ||||||||
| F for change in R2 | 6.52* | 36.89*** | 11.44*** | .71 | ||||||||
Note. β represents the standardized coefficient. B and SE indicate the unstandardized coefficient and standard error of B, respectively. WMH indicates total WMH volume natural log-transformed and TIV-adjusted. The n is the same for all columns. In a follow-up analysis with serum vitamin B12 included as an added covariate, vitamin B12 levels were not related to the network pattern (β = .01, t(148) = .140, p = .889), after controlling for TIV, APOE ε4 status, hypertension status, smoking history, and VO2max, which were all non-significant covariates (all p’s > .05). The effects that remained significant after further adjustments for vitamin B12 are bolded in the final model. Sex, APOE ε4 status, hypertension status, and smoking history were dummy coded 1 for males, APOE ε4 carriers, self-reported hypertensives, and current or past smokers, respectively.
Hcy, homocysteine; SGM, subcortical gray matter; TIV, total intracranial volume; WMH, white matter hyperintensity; APOE, apolipoprotein E; VO2max, volume of maximum oxygen consumption as the index of cardiorespiratory fitness.
p < .05
p < .01
p < .001
Subsequently adding vascular and AD-related risk factors to the model, we found that APOE ε4 status (p = .729), hypertension (p = .441), smoking history (p = .345), and VO2max (p = .437) did not additionally contribute, whereas greater WMH volume, increasing age, and male sex remained associated with greater expression of the Hcy-SGM pattern (Table 2). Specifically, age was a highly significant predictor of the network pattern (β = .297, p = .002), followed by WMH load (β = .231, p = .004) and sex (β = .217, p = .027).
In a follow-up analysis with serum vitamin B12 as an additional covariate, the associations of age, WMH volume, and sex with the Hcy-related SGM pattern remained significant (β = .299, p = .002 for age; β = .224, p = .007 for WMH volume; β = .211, p = .034 for sex, respectively); whereas vitamin B12 levels were not (p = .889), after we controlled for initial covariates in the model, which were all non-significant covariates (all p’s > .05).
We then sought to examine whether the association of age with the Hcy-SGM pattern was mediated by WMH lesion load. The mediation model showed that the influence of age on the Hcy-SGM pattern was mediated by WMH volume after controlling for TIV, sex, APOE ε4 status, hypertension status, smoking history, VO2max, and further vitamin B12 levels (Effect = .119, SE = .048, 95% CI [.030; .219]), with age having a reduced, but still significant direct effect on the Hcy-related SGM pattern (Total effect = .418, SE = .009, 95% CI [.025; .059]; Direct effect = .299, SE = .010, 95% CI [.011; .049]). As shown in Figure 3A, the individual associations indicated that increasing age predicted greater total WMH volume (β = .505, B = .055, SE = .009, p < .0001), which then predicted greater expression of the Hcy-SGM network pattern (β = .236, B = .218, SE = .080, p = .007).
Fig. 3.
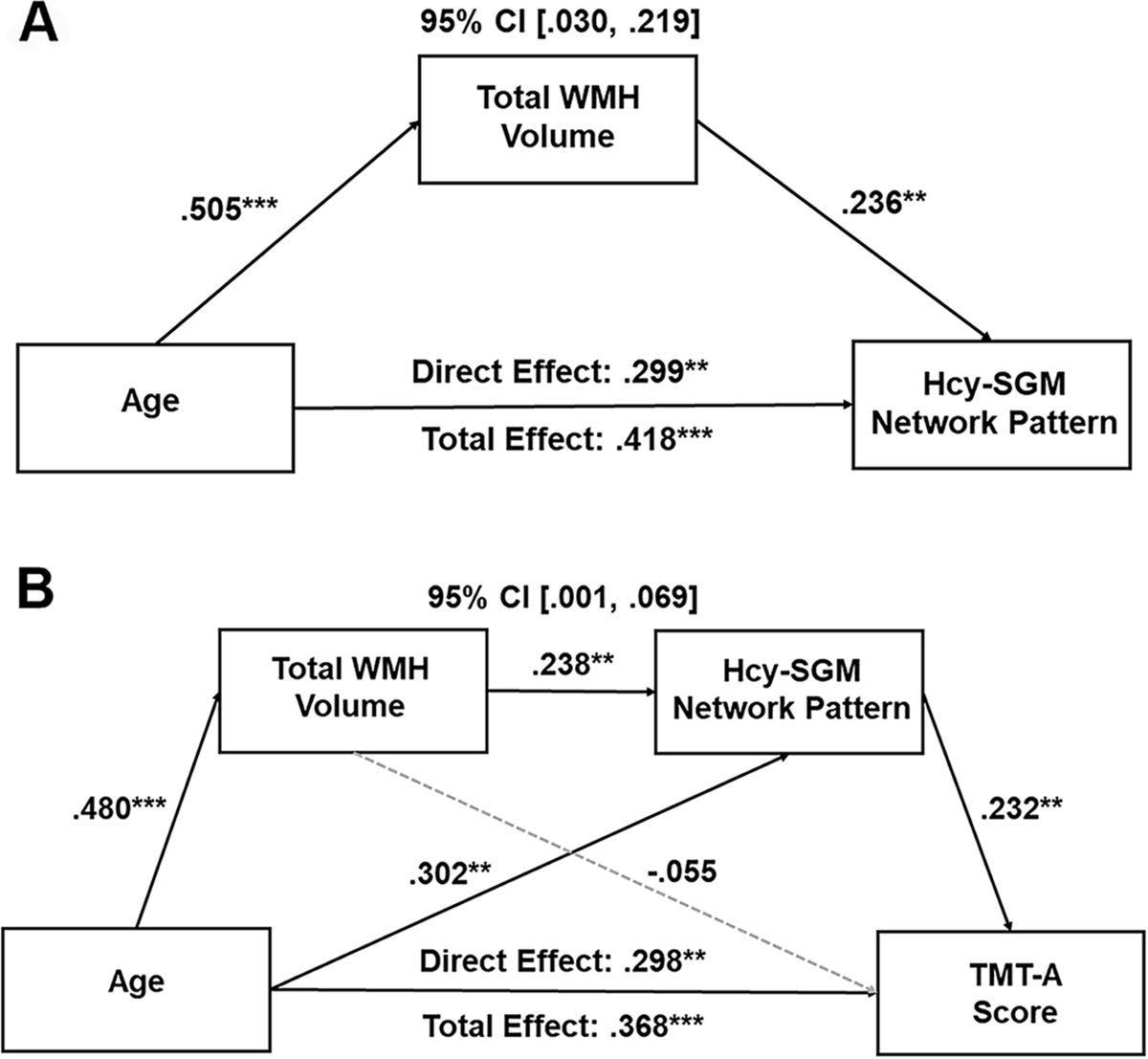
(A) Mediation model of total WMH volume on the association between age and the Hcy-related SGM network SSM pattern. Boxes and paths indicate hypothesized variables and their associations. Black solid lines indicate statistically significant paths. Standardized path coefficients with TIV, sex, APOE ε4 status, hypertension status, smoking history, VO2max, and vitamin B12 levels as covariates are presented. In this model, zero did not lie within the 95% CIs, indicating a significant mediation effect. (B) Total WMH volume and Hcy-related SGM network SSM pattern sequentially mediating the relationship between age and TMT-A score. Boxes and paths indicate hypothesized variables and associations. Black solid lines indicate statistically significant paths, whereas gray dotted lines indicate non-significant paths. Standardized path coefficients with TIV, the time interval between MRI scans and neuropsychological tests, sex, years of education, APOE ε4 status, hypertension status, smoking history, VO2max, and vitamin B12 levels as covariates are presented. This sequential mediation effect remained significant after additionally adjusting for depression ratings.
CI, confidence interval; WMH, white matter hyperintensity; Hcy, homocysteine; SGM, subcortical gray matter; TMT, Trail Making Test; SSM, Scaled Subprofile Model; TIV, total intracranial volume; APOE, apolipoprotein E, VO2max, volume of maximal oxygen consumption. *p < .05, **p < .01, ***p < .001
We then extended this mediation analysis to include cognition by testing serial mediation models to examine how the Hcy-related SGM pattern impacts cognitive aging. The serial mediation model revealed that the relationship between age and processing speed was serially mediated through WMH lesion load and the Hcy-related SGM pattern after controlling for TIV, the time interval between MRI scans and neuropsychological tests, sex, years of education, APOE ε4 status, hypertension status, smoking history, VO2max, and vitamin B12 levels (Effect = .026, SE = .018, 95% CI [.001; .069]; Fig. 3B). Additionally, adjusting for depression ratings did not diminish this mediation effect (Effect = .027, SE = .018, 95% CI [.001; .069]).
As presented in Figure 3B, the serial mediation showed the following pattern: increasing age predicted greater total WMH volume, which predicted greater expression of the Hcy-SGM network pattern, leading to poorer processing speed performance. Specifically, we found a significant direct effect (Effect = .298, SE = .003, 95% CI [.003; .015]), as well as total effect (Effect = .368, SE = .003, 95% CI [.006; .017]) of age on processing speed performance. There was also a significant relationship between age and WMH load (β = .480, B = .052, SE = .009, p < .0001) and between WMH load and the Hcy-SGM network pattern (β = .238, B = .220, SE = .082, p = .008), followed by greater expression of the network pattern associated with poorer processing speed performance (β = .232, B = .071, SE = .027, p = .008).
In contrast, serial mediation models of age predicting cognitive performance through WMH load and the Hcy-related SGM pattern, using the same covariates, showed no significant indirect effects for the residualized TMT-B value (Effect = −.013, SE = .015, 95% CI [−.050; .010]), with non-significant total and direct effects (Total effect = .155, SE = .010, 95% CI [−.004; .035]; Direct effect = .160, SE = .012, 95% CI [−.007; .039]); and for the SRT CLTR score (Effect = .005, SE = .010, 95% CI [−.014; .027]), with significant total and direct effects (Total effect = −.403, SE = .315, 95% CI [−2.129; −.883]; Direct effect = −.461, SE = .367, 95% CI [−2.447; −.999]).
4. Discussion
Using multivariate network analysis, we identified a Hcy-related SGM network covariance pattern reflecting the association of a peripheral plasma biomarker of vascular health, Hcy, with subcortical volumes of brain atrophy in healthy aging. This pattern was characterized by reductions in bilateral nucleus accumbens and hippocampus volumes with relative preservation in bilateral putamen, pallidum, and caudate. Greater expression of the Hcy-SGM network pattern was associated with increasing age, greater total WMH volume, and male sex, but not with APOE ε4 status, hypertension status, smoking history, VO2max, and serum vitamin B12 levels in this healthy aging cohort.
We further found that the influence of age on the Hcy-SGM pattern was mediated by WMH lesion load. Additionally, when this model was extended to include cognition, age was associated with increased WMH load, leading to greater expression of the Hcy-SGM network pattern, which, in turn, led to poorer processing speed performance. That these results of the serial mediation were not influenced by differences in TIV, sex, educational level, APOE ε4 status, hypertension status, smoking history, VO2max, vitamin B12 levels, and ratings of depressed mood in our sample supports the robustness of our observed findings. Notably, the direct effects of age on the Hcy-related SGM pattern in the simple mediation model and on processing speed performance in the serial mediation model remained significant, indicating that the mediators partially explain the influence of age on these outcomes.
Our study sought to investigate the effects of total plasma Hcy levels on the brain and cognitive aging in healthy older adults, identifying a regionally specific network pattern of decreased SGM volumes. This Hcy-related pattern was characterized by reductions in the bilateral hippocampus and nucleus accumbens. These findings are consistent with prior studies that have shown hippocampal brain atrophy in relation to high levels of plasma Hcy in community-dwelling non-demented older adults (Den Heijer et al., 2003; Williams et al., 2002). In line with our findings, Tan et al. (2018) also observed an association of higher Hcy levels with a smaller volume of nucleus accumbens. The authors, however, did not find hippocampal volume reductions related to Hcy levels in non-demented older adults (Tan et al., 2018). Taking a multivariate network approach, our study indicated that both volumes of the hippocampus and nucleus accumbens may be particularly vulnerable to the brain-based effects of Hcy in the context of healthy aging.
Consistent with higher Hcy levels generally observed in men compared to women (Hankey and Eikelboom, 2001), we also found greater expression of the Hcy-SGM network pattern in men, suggesting that the impact of high Hcy levels on the brain may differ based on sex, with men exposed to a greater risk of structural brain-based effects of Hcy. Future work with larger samples is needed to examine the question of sex-specific vulnerability to morphological differences in subcortical areas by elevated Hcy levels and the associated development of CVD and AD in the context of healthy aging.
We also observed relative increases in gray matter volume associated with Hcy in the bilateral pallidum, caudate, and putamen. Although our follow-up univariate analyses confirmed significant reductions in the bilateral nucleus accumbens and hippocampus volumes, the basal ganglia structural volumes showing relative increases in the pattern were not significant, suggesting that they may be regions of relative preservation with increasing levels of Hcy. Such regional pattern increases may reflect subcortical brain volumes relatively less affected by Hcy-related adverse effects. Future longitudinal studies of SGM morphological changes with Hcy in the context of healthy aging would be important in further clarifying the physiological implications of the structural brain-based effects of Hcy in subcortical brain regions.
Importantly, the subcortical areas where gray matter volumes were preferentially impacted by Hcy correspond with those found in previous studies of AD, as well as CVD (Nie et al., 2017; Werden et al., 2017; Yi et al., 2016). Prior research has shown that in addition to hippocampal atrophy, nucleus accumbens volume loss may also predict conversion from mild cognitive impairment to AD and was associated with severe cognitive impairment regardless of conversion status (Yi et al., 2016). This involvement of both the hippocampus and nucleus accumbens was also supported in a study reporting that AD patients had severe atrophy in the bilateral hippocampus and nucleus accumbens compared with healthy controls, which was further associated with global clinical severity scores (Nie et al., 2017). Our results suggest that greater expression of a regionally specific Hcy-related pattern of SGM mainly characterized by volume reductions in the hippocampus and nucleus accumbens could provide a potential mechanism linking higher levels of plasma Hcy during aging to increased vulnerability to the development of subsequent dementia (Seshadri et al., 2002). Future work, however, is needed to directly assess the potential role of the SSM Hcy-SGM pattern as an early indicator of dementia risk related to CVD and the possibility of additive or synergistic effects on developing AD-related neuropathology.
The inverse relationship of Hcy with hippocampal and nucleus accumbens volumes may be due to neurotoxic effects of Hcy, which might be potentiated by a disruption of the blood-brain barrier as well as toxic effects on brain micro-vessels (Kamath et al., 2006). Evidence from laboratory investigations in rodents has suggested hippocampal neurons are sensitive to excitotoxic and oxidative injury, and cell death induced by Hcy (Kruman et al., 2000). It has also been suggested that prominent glutamate input from the ventral hippocampus, amygdala, and prefrontal cortex to the nucleus accumbens contributes to selective vulnerability of the involved structures to Hcy-induced glutamate excitotoxicity (Britt et al., 2012). While we found decreased SGM volumes of both the hippocampus and nucleus accumbens with Hcy, marked vulnerability of the hippocampus to neurotoxic effects of Hcy has been shown in previous studies (Den Heijer et al., 2006; Kruman et al., 2000). Additionally, evidence from animal models has suggested that Hcy may differentially affect hippocampal subfields (Ataie et al., 2010), which has also been suggested for CVD and AD in the context of human aging (de Flores et al., 2015; Pin et al., 2021). Thus, future work on hippocampal regional vulnerability to Hcy neurotoxicity is needed to provide further insights into the structural brain-based effects of Hcy and its implications for the associated development of CVD and AD.
Prior studies have additionally suggested that Hcy may lead to brain atrophy by increasing the development of atherosclerosis and thrombosis through damage to the vessel walls, increased oxidative stress and inflammation, and enhanced endothelial cell injury (Hankey and Eikelboom, 2001; Lazzerini et al., 2007). Support for higher plasma Hcy concentrations contributing to brain lesions through vascular pathology has also been suggested, showing a relationship between high levels of Hcy and increased WMH volume (Raz et al., 2012; Wright et al., 2005).
We found that the SSM Hcy-SGM pattern was associated with increased WMH volume and adjusting for other vascular health factors did not appreciably attenuate this relationship. These associations between the network pattern and total WMH volume also remained after controlling for serum levels of vitamin B12. Moreover, age was associated with increased WMH load, which then predicted greater Hcy-SGM pattern expression. These results provide support for the view that Hcy may influence SGM volume through the accumulation of small vessel vascular pathology. Our findings suggest that WMH burden related to aging may, in part, drive Hcy effects on subcortical brain structures, in a manner that likely exacerbates morphological differences in subcortical areas through a pathway of common CVD impacts. In this case, older age may lead to greater vulnerability to hippocampal and nucleus accumbens atrophy associated with increased levels of Hcy contributing to brain lesions with vascular pathology, through the accumulation of vascular impacts of WMH burden such as ischemic damage (Gouw et al., 2008; Habes et al., 2016; Raz et al., 2012).
That the association between Hcy-related subcortical volumetric differences and cognitive functioning was observed only for performance on a cognitive test of processing speed is also consistent with a vascular origin of the Hcy-SGM pattern, as previous studies have suggested decrements in processing speed may be preferentially associated with CVD (Righart et al., 2013). These results also remained significant after we controlled for depressive symptoms, indicating that slower processing speed could not be explained by depressed mood in our cohort. Together, these findings suggest two pathways through which Hcy impacts processing speed performance: a process leading to morphological differences in SGM driven by Hcy and an additional process related to WMH lesion load, which may exacerbate Hcy-related impacts on subcortical brain structures.
Consistent with our findings, previous studies have linked high plasma concentrations of Hcy to cognitive dysfunction with an inverse association most noticeable for processing speed assessed by TMT-A (Feng et al., 2013). Vascular white matter lesions have also been shown to affect structural cortical brain alterations, particularly in the frontal lobes (Habes et al., 2016), leading to the disruption of frontal-subcortical circuits by subcortical ischemic lesions (Righart et al., 2013). Our study was limited to investigating how Hcy levels related to the volumes of subcortical brain structures. To gain a full picture of the influence of Hcy on structural brain aging, further research is needed to investigate how frontal lobe and other cortical brain volumes relate to Hcy-related subcortical volumetric differences and cognitive outcomes observed in the present study.
Additionally, a recent study using viral vector-mediated gene transfer has demonstrated that in non-human primates, the mesoaccumbal circuit underlies high-effortful motivation (Vancraeyenest et al., 2020). Building on findings from non-human animal models, it was also shown in a recent human neuroimaging study that encoding of effort-related information engages a sub-region of the ventral striatum including the nucleus accumbens (Suzuki et al., 2021). Thus, deficits in processing speed related to hippocampal and nucleus accumbens volume reductions driven by higher Hcy levels could be related to a disruption of the mesoaccumbal pathway by vascular white matter lesions in regions involved in motivational processing for effortful task responses.
Further research with larger and more ethnically diverse cohorts of healthy older adults is needed to evaluate the generalizability of our findings. It is possible that associations between Hcy and deficits in other cognitive domains might be evident in larger samples. Although we tested a hypothesized pathway using mediation models with bootstrap resampling that showed significant indirect effects, these findings do not permit clear conclusions regarding causal relationships. Intervention and longitudinal neuroimaging studies would be important to extend our findings and to help clarify whether reductions in volumes of the hippocampus and nucleus accumbens by Hcy are on the causal pathway, leading to cognitive decline and the early development and progression of CVD, as well as the potential for linkages to the risk for AD.
5. Conclusions
Together, our findings indicate that with the use of a multivariate SSM of regional covariance, we were able to identify a Hcy-SGM pattern, mainly characterized by brain volume reductions of hippocampus and nucleus accumbens, that may provide an important early brain-based indicator of cognitive aging, suggesting a potential link between a peripheral fluid biomarker of vascular health, CVD, and cognitive dysfunction in healthy older adults.
Acknowledgments
We thank the study participants for their valuable contributions to this research.
Funding:
The authors would like to acknowledge support from the National Institutes of Health (grant numbers AG025526, AG019610, AG072980, AG049464, AG067200, AG0720445); the state of Arizona and Arizona Department of Health Services; and McKnight Brain Research Foundation.
Footnotes
Disclosure statement
The authors have no actual or potential conflicts of interest to disclose.
References
- ACSM, 2000. American College of Sports Medicine (ACSM’s) guidelines for exercise testing and prescription, 6th ed. Baltimore, MD: Lippincott Williams and Wilkins. [Google Scholar]
- Alexander GE, Bergfield KL, Chen K, Reiman EM, Hanson KD, Lin L, Bandy D, Caselli RJ, Moeller JR, 2012a. Gray matter network associated with risk for Alzheimer’s disease in young to middle-aged adults. Neurobiol Aging 33, 2723–2732. 10.1016/j.neurobiolaging.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Aschenbrenner M, Merkley TL, Santerre-Lemmon LE, Shamy JL, Skaggs WE, Buonocore MH, Rapp PR, Barnes CA, 2008. Age-related regional network of magnetic resonance imaging gray matter in the rhesus macaque. J Neurosci 28, 2710–2718. 10.1523/JNEUROSCI.1852-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Chen K, Merkley TL, Reiman EM, Caselli RJ, Aschenbrenner M, Santerre-Lemmon L, Lewis DJ, Pietrini P, Teipel SJ, 2006. Regional network of magnetic resonance imaging gray matter volume in healthy aging. Neuroreport 17, 951–956. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Lin L, Yoshimaru ES, Bharadwaj PK, Bergfield KL, Hoang LT, Chawla MK, Chen K, Moeller JR, Barnes CA, Trouard TP, 2020. Age-Related Regional Network Covariance of Magnetic Resonance Imaging Gray Matter in the Rat. Front Aging Neurosci 12, 267. 10.3389/fnagi.2020.00267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander GE, Moeller JR, 1994. Application of the scaled subprofile model to functional imaging in neuropsychiatric disorders: A principal component approach to modeling brain function in disease. Hum Brain Mapp 2, 79–94. 10.1002/hbm.460020108 [DOI] [Google Scholar]
- Alexander GE, Ryan L, Bowers D, Foster TC, Bizon JL, Geldmacher DS, Glisky EL, 2012b. Characterizing cognitive aging in humans with links to animal models. Front Aging Neurosci 4, 21. 10.3389/fnagi.2012.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataie A, Sabetkasaei M, Haghparast A, Moghaddam AH, Kazeminezhad B, 2010. Neuroprotective effects of the polyphenolic antioxidant agent, Curcumin, against homocysteine-induced cognitive impairment and oxidative stress in the rat. Pharmacol Biochem Behav 96, 378–385. 10.1016/j.pbb.2010.06.009 [DOI] [PubMed] [Google Scholar]
- Bergfield KL, Hanson KD, Chen K, Teipel SJ, Hampel H, Rapoport SI, Moeller JR, Alexander GE, 2010. Age-related networks of regional covariance in MRI gray matter: Reproducible multivariate patterns in healthy aging. Neuroimage 49, 1750–1759. 10.1016/j.neuroimage.2009.09.051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berry MJ, Brubaker PH, O’Toole ML, Rejeski WJ, Soberman J, Ribisl PM, Miller HS, Afable RF, Applegate W, Ettinger WH, 1996. Estimation of VO2 in older individuals with osteoarthritis of the knee and cardiovascular disease. Med Sci Sports Exerc 28, 808–814. 10.1097/00005768-199607000-00006 [DOI] [PubMed] [Google Scholar]
- Brickman AM, Habeck C, Zarahn E, Flynn J, Stern Y, 2007. Structural MRI covariance patterns associated with normal aging and neuropsychological functioning. Neurobiol Aging 28, 284–295. 10.1016/j.neurobiolaging.2005.12.016 [DOI] [PubMed] [Google Scholar]
- Britt JP, Benaliouad F, McDevitt RA, Stuber GD, Wise RA, Bonci A, 2012. Synaptic and Behavioral Profile of Multiple Glutamatergic Inputs to the Nucleus Accumbens. Neuron 76, 790–803. 10.1016/j.neuron.2012.09.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnham KP, Anderson DR, 2002. Model selection and multimodel inference: A practical information-theoretic approach, 2nd ed. New York, NY: Springer. [Google Scholar]
- Clarke R, Smith AD, Jobst KA, Refsum H, Sutton L, Ueland PM, 1998. Folate, vitamin B12, and serum total homocysteine levels in confirmed Alzheimer disease. Arch Neurol 55, 1449–1455. 10.1001/archneur.55.11.1449 [DOI] [PubMed] [Google Scholar]
- Crane DE, Black SE, Ganda A, Mikulis DJ, Nestor SM, Donahue MJ, MacIntosh BJ, 2015. Grey matter blood flow and volume are reduced in association with white matter hyperintensity lesion burden: A cross-sectional MRI study. Front Aging Neurosci 7, 131. 10.3389/fnagi.2015.00131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe SF, 1998. The differential contribution of mental tracking, cognitive flexibility, visual search, and motor speed to performance on parts A and B of the trail making test. J Clin Psychol 54, 585–591. [DOI] [PubMed] [Google Scholar]
- de Flores R, La Joie R, Chételat G, 2015. Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience 309, 29–50. 10.1016/j.neuroscience.2015.08.033 [DOI] [PubMed] [Google Scholar]
- Den Heijer T, Vermeer SE, Clarke R, Oudkerk M, Koudstaal PJ, Hofman A, Breteler MMB, 2003. Homocysteine and brain atrophy on MRI of non-demented elderly. Brain 126, 170–175. 10.1093/brain/awg006 [DOI] [PubMed] [Google Scholar]
- Efron BT, Tibshirani RJ, 1994. An Introduction to the Bootstrap. Boca Raton, FL: CRC Press. [Google Scholar]
- Feng L, Isaac V, Sim S, Ng TP, Krishnan KRR, Chee MWL, 2013. Associations between elevated homocysteine, cognitive impairment, and reduced white matter volume in healthy old adults. Am J Geriatr Psychiatry 21, 164–172. 10.1016/j.jagp.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, Van Der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM, 2002. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33, 341–355. 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, VanDerKouwe AJW, Makris N, Ségonne F, Quinn BT, Dale AM, 2004. Sequence-independent segmentation of magnetic resonance images. Neuroimage 23, S69–S84. 10.1016/j.neuroimage.2004.07.016 [DOI] [PubMed] [Google Scholar]
- Franchetti MK, Bharadwaj PK, Nguyen LA, VanEtten EJ, Klimentidis YC, Hishaw GA, Trouard TP, Raichlen DA, Alexander GE, 2020. Interaction of Age and Self-reported Physical Sports Activity on White Matter Hyperintensity Volume in Healthy Older Adults. Front Aging Neurosci 12, 346. 10.3389/fnagi.2020.576025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouw AA, Seewann A, Vrenken H, Van Der Flier WM, Rozemuller JM, Barkhof F, Scheltens P, Geurts JJG, 2008. Heterogeneity of white matter hyperintensities in Alzheimer’s disease: Post-mortem quantitative MRI and neuropathology. Brain 131, 3286–3298. 10.1093/brain/awn265 [DOI] [PubMed] [Google Scholar]
- Habeck C, Krakauer JW, Ghez C, Sackeim HA, Eidelberg D, Stern Y, Moeller JR, 2005. A new approach to spatial covariance modeling of functional brain imaging data: Ordinal trend analysis. Neural Comput 17, 1602–1645. 10.1162/0899766053723023 [DOI] [PubMed] [Google Scholar]
- Habes M, Erus G, Toledo JB, Zhang T, Bryan N, Launer LJ, Rosseel Y, Janowitz D, Doshi J, Van Der Auwera S, Von Sarnowski B, Hegenscheid K, Hosten N, Homuth G, Völzke H, Schminke U, Hoffmann W, Grabe HJ, Davatzikos C, 2016. White matter hyperintensities and imaging patterns of brain ageing in the general population. Brain 139, 1164–1179. 10.1093/brain/aww008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankey GJ, Eikelboom JW, 2001. Homocysteine and stroke. Curr Opin Neurol 14, 95–102. [DOI] [PubMed] [Google Scholar]
- Hayes AF, 2018. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York, NY: Guilford publications. [Google Scholar]
- Hooshmand B, Polvikoski T, Kivipelto M, Tanskanen M, Myllykangas L, Erkinjuntti T, Mäkelä M, Oinas M, Paetau A, Scheltens P, Van Straaten ECW, Sulkava R, Solomon A, 2013. Plasma homocysteine, Alzheimer and cerebrovascular pathology: a population-based autopsy study. Brain 136, 2707–2716. 10.1093/brain/awt206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath AF, Chauhan AK, Kisucka J, Dole VS, Loscalzo J, Handy DE, Wagner DD, 2006. Elevated levels of homocysteine compromise blood-brain barrier integrity in mice. Blood 107, 591–593. 10.1182/blood-2005-06-2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kern KC, Wright CB, Bergfield KL, Fitzhugh MC, Chen K, Moeller JR, Nabizadeh N, Elkind MSV, Sacco RL, Stern Y, DeCarli CS, Alexander GE, 2017. Blood pressure control in aging predicts cerebral atrophy related to small-vessel white matter lesions. Front Aging Neurosci 9, 132. 10.3389/fnagi.2017.00132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix LR, Mattson MP, 2000. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. J Neurosci 20, 6920–6926. 10.1523/jneurosci.20-18-06920.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzerini PE, Capecchi PL, Selvi E, Lorenzini S, Bisogno S, Galeazzi M, Laghi Pasini F, 2007. Hyperhomocysteinemia, inflammation and autoimmunity. Autoimmun Rev 6, 503–509. 10.1016/j.autrev.2007.03.008 [DOI] [PubMed] [Google Scholar]
- Nie X, Sun Y, Wan S, Zhao H, Liu R, Li X, Wu S, Nedelska Z, Hort J, Qing Z, Xu Y, Zhang B, 2017. Subregional structural alterations in hippocampus and nucleus accumbens correlate with the clinical impairment in patients with Alzheimer’s Disease clinical spectrum: Parallel combining volume and vertex-based approach. Front Neurol 8, 15. 10.3389/fneur.2017.00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin G, Coupé P, Nadal L, Manjon JV, Helmer C, Amieva H, Mazoyer B, Dartigues JF, Catheline G, Planche V, 2021. Distinct Hippocampal Subfields Atrophy in Older People with Vascular Brain Injuries. Stroke 52, 1741–1750. 10.1161/STROKEAHA.120.031743 [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Kelley K, 2011. Supplemental Material for Effect Size Measures for Mediation Models: Quantitative Strategies for Communicating Indirect Effects. Psychol Methods 16, 93–115. 10.1037/a0022658.supp [DOI] [PubMed] [Google Scholar]
- Raz N, Yang Y, Dahle CL, Land S, 2012. Volume of white matter hyperintensities in healthy adults: Contribution of age, vascular risk factors, and inflammation-related genetic variants. Biochim Biophys Acta - Mol Basis Dis 1822, 361–369. 10.1016/j.bbadis.2011.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righart R, Duering M, Gonik M, Jouvent E, Reyes S, Hervé D, Chabriat H, Dichgans M, 2013. Impact of regional cortical and subcortical changes on processing speed in cerebral small vessel disease. NeuroImage Clin 2, 854–861. 10.1016/j.nicl.2013.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt P, Gaser C, Arsic M, Buck D, Förschler A, Berthele A, Hoshi M, Ilg R, Schmid VJ, Zimmer C, Hemmer B, Mühlau M, 2012. An automated tool for detection of FLAIR-hyperintense white-matter lesions in Multiple Sclerosis. Neuroimage 59, 3774–3783. 10.1016/j.neuroimage.2011.11.032 [DOI] [PubMed] [Google Scholar]
- Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PWF, Wolf PA, 2002. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N Engl J Med 346, 476–483. 10.1056/NEJMoa011613 [DOI] [PubMed] [Google Scholar]
- Sliwinski M, Buschke H, Stewart WF, Masur D, Lipton RB, 1997. The effect of dementia risk factors on comparative and diagnostic selective reminding norms. J Int Neuropsychol Soc 3, 317–326. 10.1017/s1355617797003172 [DOI] [PubMed] [Google Scholar]
- Strzelczyk TA, Cusick DA, Pfeifer PB, Bondmass MD, Quigg RJ, 2001. Value of the bruce protocol to determine peak exercise oxygen consumption in patients evaluated for cardiac transplantation. Am Heart J 142, 466–475. 10.1067/mhj.2001.117508 [DOI] [PubMed] [Google Scholar]
- Suzuki S, Lawlor VM, Cooper JA, Arulpragasam AR, Treadway MT, 2021. Distinct regions of the striatum underlying effort, movement initiation and effort discounting. Nat Hum Behav 5, 378–388. 10.1038/s41562-020-00972-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B, Venketasubramanian N, Vrooman H, Cheng CY, Wong TY, Ikram MK, Chen C, Hilal S, 2018. Homocysteine and Cerebral Atrophy: The Epidemiology of Dementia in Singapore Study. J Alzheimer’s Dis 62, 877–885. 10.3233/JAD-170796 [DOI] [PubMed] [Google Scholar]
- Van Der Flier WM, Van Straaten ECW, Barkhof F, Ferro JM, Pantoni L, Basile AM, Inzitari D, Erkinjuntti T, Wahlund LO, Rostrup E, Schmidt R, Fazekas F, Scheltens P, 2005. Medial temporal lobe atrophy and white matter hyperintensities are associated with mild cognitive deficits in non-disabled elderly people: The LADIS study. J Neurol Neurosurg Psychiatry 76, 1497–1500. 10.1136/jnnp.2005.064998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Etten EJ, Bharadwaj PK, Hishaw GA, Huentelman MJ, Trouard TP, Grilli MD, Alexander GE, 2021. Influence of regional white matter hyperintensity volume and apolipoprotein E ε4 status on hippocampal volume in healthy older adults. Hippocampus 31, 469–480. 10.1002/hipo.23308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancraeyenest P, Arsenault JT, Li X, Zhu Q, Kobayashi K, Isa K, Isa T, Vanduffel W, 2020. Selective Mesoaccumbal Pathway Inactivation Affects Motivation but Not Reinforcement-Based Learning in Macaques. Neuron 108, 568–581. 10.1016/j.neuron.2020.07.013 [DOI] [PubMed] [Google Scholar]
- Werden E, Cumming T, Li Q, Bird L, Veldsman M, Pardoe HR, Jackson G, Donnan GA, Brodtmann A, 2017. Structural MRI markers of brain aging early after ischemic stroke. Neurology 89, 116–124. 10.1212/WNL.0000000000004086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JH, Pereira EAC, Budge MM, Bradley KM, 2002. Minimal hippocampal width relates to plasma homocysteine in community-dwelling older people. Age Ageing 31, 440–444. 10.1093/ageing/31.6.440 [DOI] [PubMed] [Google Scholar]
- Wright CB, Paik MC, Brown TR, Stabler SP, Allen RH, Sacco RL, DeCarli C, 2005. Total homocysteine is associated with white matter hyperintensity volume: The Northern Manhattan study. Stroke 36, 1207–1211. 10.1161/01.STR.0000165923.02318.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, Leirer VO, 1982. Development and validation of a geriatric depression screening scale: A preliminary report. J Psychiatr Res 17, 37–49. 10.1016/0022-3956(82)90033-4 [DOI] [PubMed] [Google Scholar]
- Yi HA, Möller C, Dieleman N, Bouwman FH, Barkhof F, Scheltens P, Van Der Flier WM, Vrenken H, 2016. Relation between subcortical grey matter atrophy and conversion from mild cognitive impairment to Alzheimer’s disease. J Neurol Neurosurg Psychiatry 87, 425–432. 10.1136/jnnp-2014-309105 [DOI] [PubMed] [Google Scholar]


