Abstract
While neurotransmitter identity was once considered singular and immutable for mature neurons, it is now appreciated that one neuron can release multiple neuroactive substances (co-transmission) whose identities can even change over time. To explore the mechanisms that tune the suite of transmitters a neuron releases, we developed transcriptional and translational reporters for cholinergic, glutamatergic, and GABAergic signaling in Drosophila. We show that many glutamatergic and GABAergic cells also transcribe cholinergic genes, but fail to accumulate cholinergic effector proteins. Suppression of cholinergic signaling involves posttranscriptional regulation of cholinergic transcripts by the microRNA miR-190; chronic loss of miR-190 function allows expression of cholinergic machinery, reducing and fragmenting sleep. Using a “translation-trap” strategy we show that neurons in these populations have episodes of transient translation of cholinergic proteins, demonstrating that suppression of co-transmission is actively modulated. Posttranscriptional restriction of fast transmitter co-transmission provides a mechanism allowing reversible tuning of neuronal output.
One-Sentence Summary:
Cholinergic co-transmission in large populations of glutamatergic and GABAergic neurons in the Drosophila adult brain is controlled by miR-190.
Small molecule chemicals mediating neuronal communication are packaged into vesicles for release by vesicular neurotransmitter transporter proteins (vNTs). The most common fast-acting neurotransmitters in both vertebrates and invertebrates each have a cognate vNT (or vNT family): VAChT for acetylcholine (ACh), VGAT for gamma-amino butyric acid (GABA) and VGluT for glutamate (Glu) (1). Co-transmission, release of multiple neuroactive molecules from a single cell, has been reported in many animals, and usually involves release of a bioamine or peptide neuromodulator with a fast transmitter (2, 3). This type of modulation can be regulated by changes in environment or neuronal activity (4). Interestingly, co-transmission between multiple fast-acting neurotransmitters has only been seen functionally in a few cases (5, 6), though some studies have reported the co-expression of multiple vNT mRNAs (7–9). Such co-transmission can have profound effects on circuit dynamics (10, 11). Using new genetic tools to study transcription and translation of vNTs for fast neurotransmitters, we demonstrate here that there are large populations of fully differentiated glutamatergic and GABAergic neurons in the adult fly brain that transcribe genes specifying synthesis and release of ACh but block accumulation of protein products via microRNA (miR) repression. This suggests a widespread but tightly-regulated potential for co-transmission.
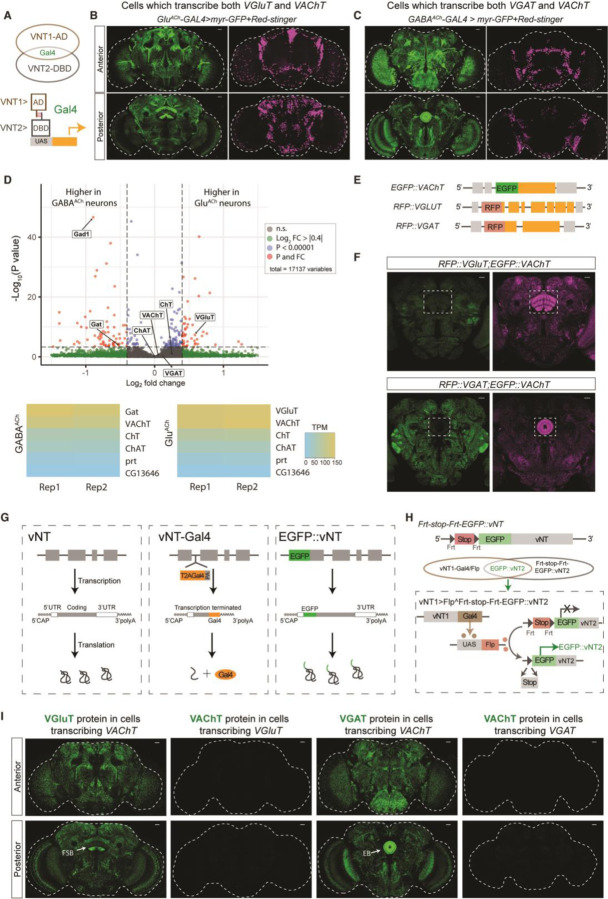
To map the extent of co-transcription of vNTs, we used a split-Gal4 strategy (12) in which Gal4-DBD or AD sequences are inserted into the endogenous loci of VAChT, VGluT and VGAT genes to put them under control of NT-specific transcriptional programs (Fig. 1A and fig. S1A). Both the VGluT-AD:VAChT-DBD (Fig. 1B) and VAChT-AD:VGluT-DBD (fig. S1C) split-Gal4s show broad expression with the strongest signal in fan-shape body (FSB) neurons. As expected for intersectional drivers, VAChT:VGluT split-Gal4 labels fewer neurons than either VAChT- or VGluT-Gal4 drivers (fig. S1B). We will refer to the cell subset labeled by this intersectional tool as “GluACh” neurons and the split-Gal4 as GluACh-Gal4. Similarly, both the VGAT-AD:VAChT-DBD (Fig. 1C) and VAChT-AD:VGAT-DBD (fig. S1D) split-Gal4s had a broad, but distinct expression profile with the strongest EGFP signal in ellipsoid body (EB) neurons; we call these cells “GABAACh” neurons. VGAT:VGluT split-GAL4 brains showed little consistent co-expression (data not shown). These results suggested potential co-expression of VAChT with both the VGluT and VGAT genes and possible co-transmission.
Fig. 1. Transcription of VAChT in VGluT/VGAT positive neurons.
(A) Schematic diagram of split-Gal4 strategy. Expression of AD and DBD in the same neurons reconstitutes Gal4 protein to initiate expression. (B-C) Expression patterns of VGluT-AD;VAChT-DBD split-Gal4 and (B) VGAT-AD;VAChT-DBD split-Gal4 (C): Anterior (top) and posterior (bottom). Green indicates neuronal membrane, while magenta shows nuclei. Dashed white lines outline the brain. (D) Nuclear RNAseq demonstrates high cholinergic mRNA levels in GluACh and GABAACh neurons. Volcano plot (top) shows statistically significant enrichment of VGluT in GluACh cells (adjusted P value (Padj) < 0.05; log2 fold change (FC) = 0.58) and Gat (Padj < 0.05; log2FC = − 0.52) and Gad1 (Padj < 0.05; log2FC = −0.89) in GABAACh neurons. VAChT, ChaT and ChT mRNAs were not differentially expressed (Padj > 0.05). Heat maps (bottom) for each cell type show that cholinergic markers are present in both cell types at levels (TPM, transcripts per million) comparable to VGluT and VGAT respectively, while control vesicular transporters are not expressed. (E) Schematic diagram of N-terminal genomic fusion lines EGFP::VAChT, RFP::VGluT and RFP::VGAT. (F) Representative single-slice pictures of adult brains of RFP::VGluT;EGFP::VAChT (left) and RFP::VGAT; EGFP::VAChT (right) flies. Green indicates EGFP expression while magenta indicates RFP expression. The dashed box outlines EGFP and RFP signals in fan-shape body (left) and ellipsoid body (right). (G) Schematic diagrams showing the transcription and translation processing of vNT mRNA in wildtype (left), T2A Gal4 alleles (middle) and EGFP::vNT fusion alleles (right). For vNT-Gal4 alleles, transcription of vNT is terminated at the Gal4 insertion site, inducing loss of vNT 3’UTR information, with production of separate terminated vNT and GAL4 proteins. For EGFP::vNT alleles, EGFP is transcribed and translated within the intact vNT mRNA, making EGFP::vNT fusion protein. (H) Schematic diagram showing the flip-out stop gene strategy. vNT1-Gal4 drives FLP recombinase expression which excises the FRT-flanked stop cassette preceding EGFP::vNT2, allowing EGFP::vNT2 expression. Thus, the EGFP signals indicate transcription of vNT1 with transcription and translation of vNT2 in the same neuron. (I) VAChT-Gal4 flip-out derepression of EGFP::VGluT shows EGFP in FSB, while VAChT-Gal4 flip-out of EGFP::VGAT shows EGFP in EB. Flip-out derepression ECFP::VAChT shows no signal. Dashed white lines indicate the whole brain. Scale bars = 20µm.
To verify co-transcription of the native vNT genes in these cells, we analyzed nuclear polyA-containing RNA from INTACT-sorted GluACh and GABAACh nuclei (13) (Fig. 1D), a technique which minimizes the effects of cytoplasmic posttranscriptional processes on mRNA levels (14). GABAACh nuclei express high levels of GAD1 and GAT mRNA, while GluACh nuclei express high levels of VGluT as expected. VAChT, ChaT and ChT mRNA are also expressed strongly in both cell types. Surprisingly, nuclear VGAT mRNA was also found in both cell types. Portabella and CG13646, vNTs related respectively to VAChT/VGluT and VGAT, were not found at significant levels in either population.
To be co-transmitting, GluACh and GABAACh neurons would need to express the protein products of both vNT genes. To directly visualize the vNT proteins we fused fluorescent proteins (FPs) to the N-termini of the endogenous coding sequences using CRISPR/Cas9 (Fig. 1E). These fusion alleles faithfully recapitulate the native protein distribution as assessed by immunostaining of heterozygotes (fig. S2). Co-staining for EGFP and RFP in RFP::VGluT;EGFP::VAChT fly brains, we found strong RFP::VGluT protein expression in FSB neurons, but no EGFP::VAChT protein at the same level of the confocal stack (Fig. 1F). Similarly, in RFP::VGAT;EGFP::VAChT fly brains, strong RFP::VGAT staining is present in EB neurons, but EGFP::VAChT protein is not (Fig. 1F).
While split-Gal4 expressed from the VAChT locus is clearly present in FSB and EB, the lack of EGFP::VAChT indicates that the protein does not accumulate in these regions. We hypothesized that difference may be a function of the structure of the VAChT transcripts produced in these two different CRISPR-engineered animals. In split-GAL4 lines (and the T2A-Gal4 lines used below), GAL4 coding sequence(s), followed by a polyadenylation site, are inserted into a vNT intron, producing a truncated transcript that lacks the vNT gene’s 3’UTR, a region which can contain cis regulatory sequences regulating translation and/or RNA stability (15). For FP::vNT fusion alleles, the FP coding sequence is fused in-frame to form a functional chimeric vNT protein, meaning the FP::vNT mRNA has all the regulatory information native to the wildtype vNT mRNA (Fig. 1G). This suggests that while both the VAChT split-Gal4 and EGFP::VAChT mRNA are transcribed in GluACh and GABAACh neurons but that mRNA containing native 3’UTR sequences is not translated.
To test this idea, we created conditional FP fusion alleles containing an Frt-stop-Frt-FP cassette downstream of the start codon of each vNT gene (Fig. 1H). In these animals, FP::vNT transcription is blocked until FLP recombinase is expressed, excising the stop cassette. GAL4+ cells then become competent to generate a FP::vNT mRNA containing all the endogenous UTR information. Frt-stop-Frt-ECFP::VAChT flies were validated by driving FLP expression with VT030559-Gal4 in cholinergic mushroom body cells. ECFP::VAChT was present in mushroom body as expected and dependent on GAL4 (fig. S3A). Similarly, EGFP::VGluT in FSB neurons and EGFP::VGAT signals in the anterior paired lateral (APL) neurons demonstrate the specificity of these lines (fig. S3B and C).
Fig. 1H shows the strategy used to test for posttranscriptional suppression of VAChT protein expression in GluACh and GABAACh neurons. FLP recombinase, driven in cells which transcribe vNT1, catalyzes excision of the stop cassette for FP::vNT2. Only if the cells which transcribe vNT1 are also competent to both transcribe and translate vNT2, is an FP signal is seen. Using VAChT-Gal4 to flip out the stop cassette for EGFP::VGluT results in strong protein signal in the same pattern observed for GluACh-Gal4, indicating that VGluT is both transcribed and translated in this subset of VAChT-transcribing neurons (Fig. 1I). However, FLP-derepression of ECFP:: VAChT with VGluT-Gal4 produces no detectable protein in adult brain (Fig. 1I), suggesting either degradation or translational suppression of VAChT mRNA in GluACh cells. GABAACh neurons behaved similarly: EGFP::VGAT expression confirmed transcription and translation of VGAT mRNA while the absence of ECFP::VAChT protein shows there is no translation of VAChT mRNA in GABAACh neurons. Thus, VGluT/VGAT are transcribed and translated in GluACh/GABAACh neurons while VAChT mRNA is transcribed, but either degraded or untranslated, in both groups.
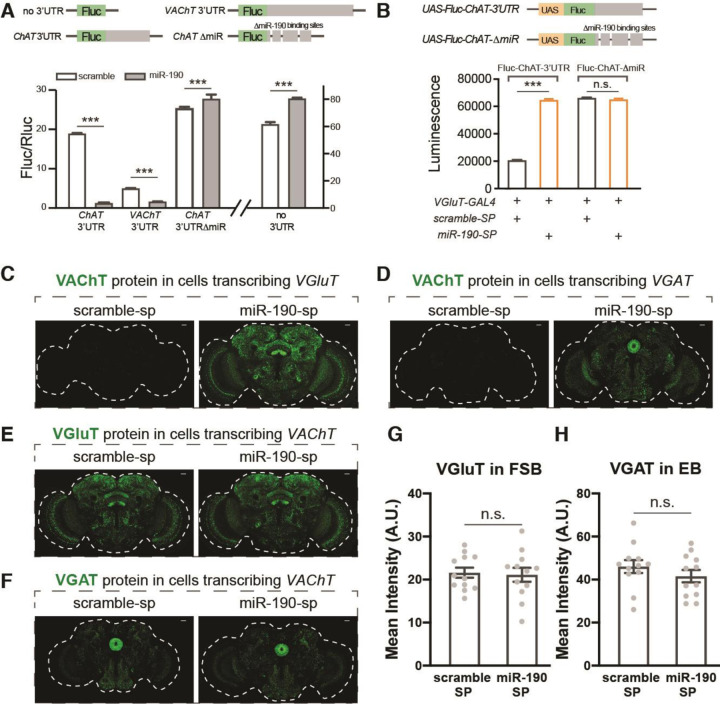
Control of protein synthesis by microRNAs, small non-coding RNAs, which bind to mRNA to initiate degradation or inhibit translation, is widespread (16). In silico evaluation of the VAChT 3’UTR (www.targetscan.org/fly_72/) identified multiple high-confidence binding sites for miR-190, a microRNA which also targets several other cholinergic mRNAs, including ChAT, acetylcholine’s synthetic enzyme and ChT, the choline transporter. To determine whether miR-190 regulates production of these cholinergic effector proteins, we assayed its ability to suppress expression of a Firefly luciferase (Fluc) gene which had either the VAChT or ChAT 3’UTR. Co-transfection of S2 cells with miR-190 and a Renilla luciferase (Rluc) control plasmid induces a significant decrease in the Fluc/Rluc ratio with both 3’UTRs compared to a scrambled miR, but no miR-190-dependent decrease is found when the three putative miR-190 binding sites of the ChAT 3’UTR are deleted or there is no 3’UTR (Fig. 2A). These results suggest that miR-190 can suppress the expression of cholinergic proteins by directly binding to their 3’UTRs.
Fig. 2. MiR-190 blocks VAChT protein accumulation.
(A) S2 cells were co-transfected with Fluc-UTR, Rluc and scramble or miR-190 plasmids. For the no 3’UTR plasmid, only Fluc is included; for the VAChT-3’UTR and ChAT-3’UTR plasmids, Fluc is followed by 3’UTR of VAChT or ChAT; for the ChAT-del plasmid, the three predicted miR-190 binding sites in the ChAT 3’UTR are deleted. Firefly luciferase (Fluc) activity was normalized to Renilla luciferase (Rluc) activity. n=6 for each group. Co-expression of miR-190 blocks Fluc expression only when plasmids contain VAChT or ChAT 3’UTRs. Deletion of predicted miR-190 binding sites in the ChAT 3’UTR blocks miR-190 suppression. (B) Expression of miR-190 sponge in VGluT positive neurons up-regulates luciferase activity when the Fluc transgene has a ChAT 3’UTR, indicating that miR-190 is expressed in these adult neurons. When miR-190 binding sites are deleted from the transgene’s ChAT 3’UTR, luciferase activity is no longer responsive to miR-190 sponge. n=6 for each group. (C-D) Representative pictures of VGluT-Gal4 (C) or VGAT-Gal4 (D) driving flip-out derepression of ECFP::VAChT flies, with scramble or miR-190 sponge expressed in the same neurons. (E-F) Representative pictures of VAChT-Gal4 driving flip-out derepression of EGFP::VGluT (E) or EGFP::VGAT (F) while expressing scramble or miR-190 sponge in the same neurons. (G) Quantification of EGFP::VGluT protein in FSB neurons from panel E. (H) Quantification of EGFP::VGAT protein in EB neurons from panel F. n=12 for each group in panels G and H. Dashed white lines indicate the whole brain. Scale bars = 20µm. Data are shown as mean ± SEM, and analyzed by Student’s t-test. n.s. indicated no difference, *** indicates p<0.001. Gray dots show individual values in panels G and H.
To test the idea that miR-190 is responsible for in vivo suppression of cholinergic transmission in GluACh and GABAACh cells, we suppressed miR-190 function in specific neurons using miR-190 sponge lines (17). To validate the specificity and efficacy of the sponges and to test for the presence of miR-190 in adult glutamatergic cells, we created UAS-driven Fluc reporter lines that had either the ChAT 3’UTR or a mutant ChAT 3’UTR with miR-190 sites deleted. Co-expression of the reporters under control of VGluT-Gal4 with the miR-190 sponge or scramble control demonstrates that miR-190 is present in adult glutamatergic neurons and that its function can be inhibited in vivo by the sponge (Fig. 2B).
To explore the role of miR-190 in regulation of endogenous VAChT translation, we asked if expression of the miR-190 sponge would result in ECFP::VAChT protein expression in glutamatergic or GABAergic neurons. For both GluACh and GABAACh cells, miR-190 sponge produced strong ECFP::VAChT protein signal in the expected patterns (Fig. 2C and D and fig. S4). These results indicate that miR-190 suppresses accumulation of VAChT protein in GluACh and GABAACh cells in adult heads. Interestingly, expression of miR-190 sponge does not change the expression pattern or intensity of EGFP::VGluT or EGFP::VGAT protein in FSB or EB neurons (Fig. 2E to H), suggesting that miR-190 has no role in the regulation of VGluT or VGAT protein levels.
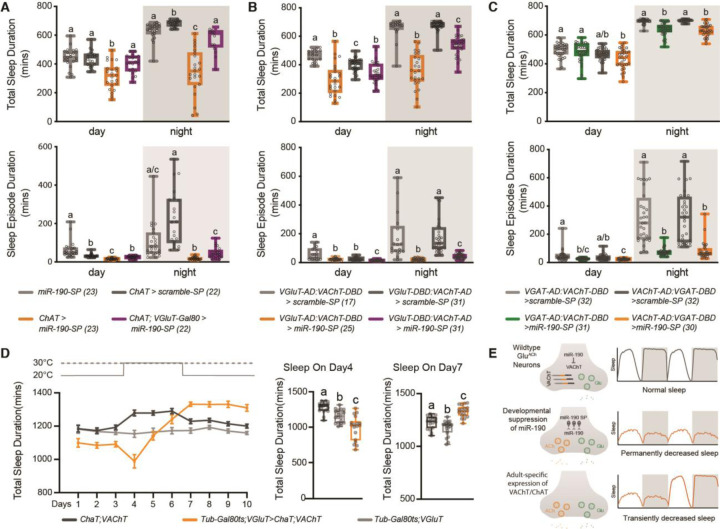
The circuitry controlling sleep in Drosophila includes regions that contain GluACh (dFSB) and GABAACh (EB) neurons (18). Suppression of miR-190 function pan-neuronally, as well as in either glutamatergic or cholinergic neurons, reduces daytime and nighttime sleep significantly (fig. S5A to C) compared to expression of a scrambled control sponge. To ask if this is the result of reducing miR-190 in GluACh neurons, we expressed the sponge under control of ChAT-GAL4 with VGluT-GAL80 to block GAL4 action in GluACh cells, and found the sleep reduction was rescued (Fig. 3A). Indeed, suppression of miR-190 function in GluACh neurons using GluACh-GAL4 split drivers also leads to a large reduction in total sleep (Fig. 3B). Taken together, this demonstrates that loss of miR-190 in GluACh cells decreases sleep. Suppression of miR-190 function in GABAergic neurons (fig. S5D) or specifically in GABAACh cells (Fig. 3C) also decreases nighttime sleep, but to a lesser extent than GluACh manipulations. However, there is significant sleep fragmentation with miR-190 sponge in both populations (Fig. 3A to C and fig. S6 and fig. S7). Locomotor activity while awake is unaffected or reduced (fig. S8), indicating the decrease of sleep is not due to hyperactivity.
Fig. 3. MiR-190 regulates sleep by controlling cholinergic co-transmission in glutamatergic neurons.
(A) Reduction and fragmentation of sleep by suppression of miR-190 function in cholinergic neurons maps to neurons also expressing VGluT. (B) In VGluT:VAChT split-Gal4 neurons, miR-190 suppression reduces and fragments daytime and nighttime sleep. (C) In VGAT:VAChT split-Gal4 neurons, miR-190 suppression reduces and fragments daytime and nighttime sleep. (D) Temporally-controlled overexpression of ChAT and VAChT from transgenes lacking cognate 3’UTRs in VGluT+ neurons decreases sleep acutely and triggers fast compensation. n=18–20. Data are shown as mean ± SEM, gray circles show individual values. Statistical differences are indicated by letters, with genotypes that are not significantly different having the same letter. (E) Summary model. In GluACh neurons, suppression of miR-190 function during development induces ACh co-transmission and alters adult sleep circuits. Adult-specific expression of VAChT/ChAT decreases sleep acutely and triggers strong homeostatic compensation.
We reasoned that if the sleep effects of miR-190 suppression were due to cholinergic transmission in glutamatergic neurons, expressing both ChAT and VAChT in these neurons should phenocopy the miR-190 sponge. Although GluACh-GAL4-driven expression of ChAT/VAChT transgenes lacking 3’UTR sequences was completely lethal (supporting the importance of the miR-190 suppression mechanism), limiting expression to adulthood with TubGAL80ts rescued viability and was sufficient to immediately both decrease and severely fragment sleep (Fig. 3D and fig. S9A and B). But in contrast to the suppression of miR-190 function using drivers that express during development, total sleep recovers rapidly, even before the end of protein induction (Fig. 3D and fig. S9C). These data suggest that the adult sleep phenotype seen with temporally-uncontrolled expression of miR-190 sponge may be due to developmental rewiring of the sleep homeostat circuit; limiting sponge expression using Tub-GAL80ts supports this (Rivera-Rodriguez and Adel et al., in preparation). We hypothesize that without developmental suppression of miR-190 function, the homeostat is intact and the sleep loss due to adult expression of VAChT/ChAT is subject to strong compensation (Fig. 3E).
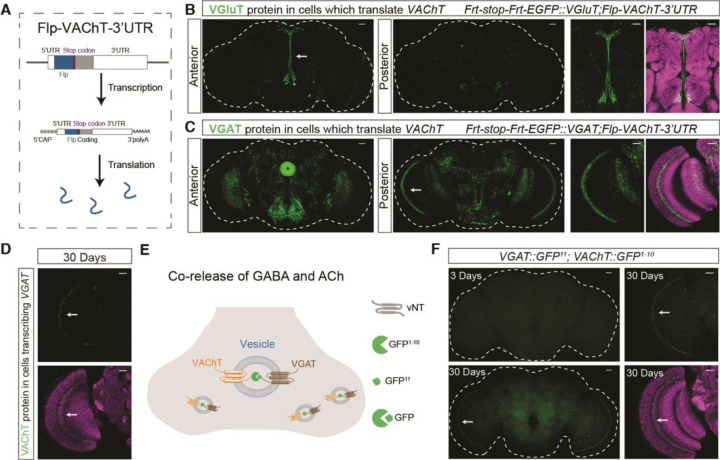
The adult persistence of the miR-190 mechanism for suppression of cholinergic transmission raises the question of whether there are situations where co-transmission is permitted as a form of plasticity. While VAChT accumulation in normal GluACh or GABAACh cells is not detectable (Fig. 1I), limited local or transient expression would be difficult to visualize. To capture transient events, we designed a “translation-trap” (Fig. 4A). The FLP recombinase coding region was inserted into the VAChT locus. FLP-encoding mRNA is translated only under conditions permissive for VAChT mRNA translation. Combining this allele with FRT-stop-FRT-EGFP::VGluT and VGAT alleles allows permanent marking of GluACh and GABAACh neurons that have at some point translated VAChT mRNA. Fig. 4B shows EGFP::VGluT staining indicating there are GluACh neurons in the pars intercerebralis and ventral areas of the brain which have translated VAChT mRNA. Similarly, EGFP::VGAT signals in EB, medulla and several other central brain regions demonstrate translation of VChAT in GABAACh neurons (Fig. 4C). These data show that miR-190 function is transiently suppressed in multiple neuron groups.
Fig. 4. VAChT repression is released in specific cell types and VAChT traffics to VGAT vesicles.
(A) Schematic diagram showing the translation-trap strategy. In Flp-VAChT-3’UTR flies. Flp is transcribed and translated as part of the full 3’UTR-containing VAChT mRNA. (B) Flp-VAChT-3’UTR flip-out derepression of EGFP::VGluT marks central brain neurons. White arrow shows the region enlarged at right. (C) Flp-VAChT-3’UTR flip-out derepression of EGFP::VGAT medulla and central brain neurons. White arrow shows the region enlarge at right. (D) In 30 day old flies, VGAT-Gal4 flip-out derepression of ECFP::VAChT (strategy as in Fig. 1H) generates ECFP signal in medulla (white arrow). (E) Schematic of strategy to visualize VAChT localization. VAChT and VGAT alleles were generated with lumenal split GFP fusions. GFP reconstitution only occurs if VAChT and VGAT are in the same vesicle. (F) In 30 day old flies, reconstituted GFP signal is visible without staining in medulla neurons. In 3 day old flies, no GFP is detected. For panels B to D and panel F, green shows EGFP or ECFP, while magenta is Brp staining. Scale bars = 20µm.
Because our translation-trap is an irreversible mark, it does not indicate when or for how long VAChT translation occurred, or whether it is responsive to physiological state. To ask whether VAChT translation was occurring in adults, we returned to animals in which VAChT in GABAACh cells is tagged with ECFP (Fig. 1H). While ECFP::VAChT was undetectable in young animals (Fig. 1I), it appears in GABAACh medulla neurons in 30-day old brains, consistent with results from the translation-trap showing these cells translate VAChT (Fig. 4D). VGAT expression in GABAACh neurons did not change with age (fig. S10). These data demonstrate that VAChT translation occurs in mature GABAACh neurons and is stimulated by physiological changes associated with aging.
Though the appearance of VAChT protein in nerve terminals is consistent with ability to package ACh, we sought to determine if the protein was in synaptic vesicles. We knocked GFP1−10 and GFP11 into the VAChT gene, and GFP11 into the VGAT gene (fig. S11A and B), such that the split GFP would be on the luminal face of the vNT (fig. S11C). Notably, in 30-day-old flies, we found clear reconstitution of live GFP signals between VAChT-GFP1−10 with VGAT-GFP11 in medulla neurons; no signal was found 3-day-old animals (Fig. 4E and F). These results indicate that VAChT and VGAT are present in the same vesicles, suggesting ACh and GABA co-release in aging flies. While the functional effect of this co-release has yet to be determined, it is notable that aging in flies, like in humans, is associated with significant increases in sleep fragmentation (19).
Co-transmission is now recognized as a common and important mode of neuronal communication, and it can be dynamic. NT plasticity involving replacement of one transmitter with another, either developmentally (20) or in the context of a few neurons in a mature circuit (4, 21), has been shown in multiple species. In cases where the molecular mechanism is known, these switching events have ultimately required transcriptional changes (22, 23). In this study we describe a mechanistically-distinct phenomenon in which the transcription of cholinergic genes is already active in thousands of GABAergic and glutamatergic neurons in the adult fly brain, and functional expression is controlled by a reversable microRNA switch. Since GABA and glutamate are generally inhibitory transmitters in the central brain of Drosophila, this gives GluACh and GABAACh neurons the ability to rapidly and transiently alter the magnitude or even the sign of their output by scaling miR-190 levels. These neurons may be akin to the reserve pool neurons of the adult zebrafish spinal cord that can reversibly acquire and release glutamate to enhance neuromuscular junction function acutely after locomotor stress (24).
While the extent and the full range of triggers controlling the potential for transmitter plasticity in these cells are unknown, we show that there are certain cells populations that reliably turn on VAChT translation (Fig. 4B and C), some in response to aging (Fig. 4D to F). How this is accomplished will require further study; but there are many examples of regulated miR degradation (25), one of which has been shown to control miR-190 levels (26). It is also interesting to consider whether posttranscriptional processes may provide a more general mode of fast but transient control of transmission. We note that there are high levels of VGAT transcription in GluACh neurons (Fig. 1D). Transient modulation of co-transmission provides a powerful mechanism for sculpting behavior in response to external and internal signals.
Supplementary Material
Acknowledgments:
We thank Ed Dougherty in the Brandeis Imaging Facility for assistance. We also thank Paul Garrity, Piali Sengupta and Sebastian Kadener for critical comments on this manuscript.
Funding:
This work was supported by NIH R01067284, NIH R21NS096414 and NIH P01NS090994 to LCG. MH and EJRR were supported by NIH T32NS019929 and EJRR was supported by NIH F31NS110273. Stocks obtained from the Bloomington Drosophila Stock Center (NIH P40OD018537) were used in this study.
Footnotes
References and Notes
- 1.Ayala-Lopez N., Watts S. W., Physiology and Pharmacology of Neurotransmitter Transporters. Compr Physiol 11, 2279–2295 (2021). [DOI] [PubMed] [Google Scholar]
- 2.Silm K. et al. , Synaptic Vesicle Recycling Pathway Determines Neurotransmitter Content and Release Properties. Neuron 102, 786–800 e785 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherer L. M. et al. , Octopamine neuron dependent aggression requires dVGLUT from dual-transmitting neurons. PLoS Genet 16, e1008609 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spitzer N. C., Neurotransmitter Switching in the Developing and Adult Brain. Annu Rev Neurosci 40, 1–19 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Granger A. J., Wallace M. L., Sabatini B. L., Multi-transmitter neurons in the mammalian central nervous system. Curr Opin Neurobiol 45, 85–91 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim S., Wallace M. L., El-Rifai M., Knudsen A. R., Sabatini B. L., Co-packaging of opposing neurotransmitters in individual synaptic vesicles in the central nervous system. Neuron 110, 1371–1384 e1377 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pankova K., Borst A., RNA-Seq Transcriptome Analysis of Direction-Selective T4/T5 Neurons in Drosophila. PLoS ONE 11, e0163986 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Croset V., Treiber C. D., Waddell S., Cellular diversity in the Drosophila midbrain revealed by single-cell transcriptomics. eLife 7, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lacin H. et al. , Neurotransmitter identity is acquired in a lineage-restricted manner in the Drosophila CNS. eLife 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nusbaum M. P., Blitz D. M., Marder E., Functional consequences of neuropeptide and small-molecule co-transmission. Nat Rev Neurosci 18, 389–403 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Granger A. J., Mulder N., Saunders A., Sabatini B. L., Cotransmission of acetylcholine and GABA. Neuropharmacology 100, 40–46 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luan H., Peabody N. C., Vinson C. R., White B. H., Refined spatial manipulation of neuronal function by combinatorial restriction of transgene expression. Neuron 52, 425–436 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ma J., Weake V. M., Affinity-based isolation of tagged nuclei from Drosophila tissues for gene expression analysis. J Vis Exp, (2014). [DOI] [PMC free article] [PubMed]
- 14.Solnestam B. W. et al. , Comparison of total and cytoplasmic mRNA reveals global regulation by nuclear retention and miRNAs. BMC Genomics 13, 574 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mayr C., Regulation by 3’-Untranslated Regions. Annu Rev Genet, (2017). [DOI] [PubMed]
- 16.Jonas S., Izaurralde E., Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet 16, 421–433 (2015). [DOI] [PubMed] [Google Scholar]
- 17.Fulga T. A. et al. , A transgenic resource for conditional competitive inhibition of conserved Drosophila microRNAs. Nature communications 6, 7279 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Artiushin G., Sehgal A., The Drosophila circuitry of sleep-wake regulation. Curr Opin Neurobiol 44, 243–250 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koh K., Evans J. M., Hendricks J. C., Sehgal A., A Drosophila model for age-associated changes in sleep:wake cycles. Proc Natl Acad Sci U S A 103, 13843–13847 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furshpan E. J., MacLeish P. R., O’Lague P. H., Potter D. D., Chemical transmission between rat sympathetic neurons and cardiac myocytes developing in microcultures: evidence for cholinergic, adrenergic, and dual-function neurons. Proc Natl Acad Sci U S A 73, 4225–4229 (1976). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dulcis D., Jamshidi P., Leutgeb S., Spitzer N. C., Neurotransmitter switching in the adult brain regulates behavior. Science 340, 449–453 (2013). [DOI] [PubMed] [Google Scholar]
- 22.Dulcis D. et al. , Neurotransmitter Switching Regulated by miRNAs Controls Changes in Social Preference. Neuron 95, 1319–1333 e1315 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dulcis D., Spitzer N. C., Reserve pool neuron transmitter respecification: Novel neuroplasticity. Developmental neurobiology 72, 465–474 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bertuzzi M., Chang W., Ampatzis K., Adult spinal motoneurons change their neurotransmitter phenotype to control locomotion. Proc Natl Acad Sci U S A 115, E9926–E9933 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu X., Shah A., Baraban J. M., Rapid reversal of translational silencing: Emerging role of microRNA degradation pathways in neuronal plasticity. Neurobiol Learn Mem 133, 225–232 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi C. Y. et al. , The ZSWIM8 ubiquitin ligase mediates target-directed microRNA degradation. Science 370, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Daniels R. W., Rossano A. J., Macleod G. T., Ganetzky B., Expression of multiple transgenes from a single construct using viral 2A peptides in Drosophila. PLoS ONE 9, e100637 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Daniels R. W. et al. , A single vesicular glutamate transporter is sufficient to fill a synaptic vesicle. Neuron 49, 11–16 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fei H. et al. , Mutation of the Drosophila vesicular GABA transporter disrupts visual figure detection. J Exp Biol 213, 1717–1730 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J. et al. , Fiji: an open-source platform for biological-image analysis. Nature methods 9, 676–682 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Donelson N. C. et al. , High-resolution positional tracking for long-term analysis of Drosophila sleep and locomotion using the “tracker” program. PLoS ONE 7, e37250 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.et al H. J. C., Rest in Drosophila is a Sleep-like State. Neuron 25, 129–138 (2000). [DOI] [PubMed] [Google Scholar]
- 33.Lim C. et al. , The novel gene twenty-four defines a critical translational step in the Drosophila clock. Nature 470, 399–403 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.