Over the past decade, studies of Neisseria gonorrhoeae have shown that in media containing glucose, lactate stimulates metabolism, and this could affect pathogenicity (7, 19). Recently, the probable mechanism of this stimulation has been identified as one that could apply to other pathogens (68). Earlier studies implicated lactate metabolism in the serum resistance of Haemophilus influenzae (30), and during the past year, the use of signature-tagged mutagenesis and an infant rat model identified a putative (i.e., homologous with the gene in Escherichia coli) lactate permease-deficient mutant of Neisseria meningitidis with diminished virulence (62; C. M. Tang, personal communication). There may be a common role for lactate in the pathogenicity of these and other pathogens. Lactate and glucose are present together in most sites where infection occurs in vivo. This review summarizes the present position on gonococci and its implications regarding other pathogens. Throughout, the phrase “stimulation of metabolism by lactate” refers to this event occurring in a medium containing glucose.
ENERGY SOURCES OF GONOCOCCI
The nutritional requirements for growth of gonococci include amino acids, purines, pyrimidines, vitamins, and an energy source (8). The last is restricted; only glucose, pyruvate, and lactate are used efficiently (39). The mechanism for lactate stimulation of gonococcal metabolism depends on interaction between it and glucose. The literature records information on the metabolism of one or the other alone but not in combination; it is summarized below.
Glucose is metabolized at pH 7.2 and 8.0 primarily (ca. 80%) via the Entner-Doudoroff pathway, with some contribution (ca. 20%) from the pentose phosphate pathway (40). At a pH above 7, most of the pyruvate and acetyl coenzyme A (CoA) generated from glucose accumulates as acetate, with only small amounts being oxidized by the tricarboxylic acid (TCA) cycle (40). However, at pH 6, the contribution of the pentose pathway increases to about 50% and more acetyl-CoA is metabolized via the TCA cycle (40).
Lactate provides energy for growth by being a substrate for electron transport when it is oxidized to pyruvate (3, 4). Gonococci contain at least three lactic dehydrogenase (LDH) enzymes. The most important are two electron transport-linked LDHs that are associated with the cytoplasmic membrane and independent of NAD+ (13, 67). Isoenzyme LDH-I utilizes lactate exclusively as its substrate and with a preference for the d-isomer, while isoenzyme LDH-II has broad substrate specificity (lactate, phenyl-lactate, and 4-hydroxy-phenyl-lactate), but it is steriospecific for l-isomers. The third LDH is a cytoplasmic, soluble NAD+-dependent LDH (25). Pyruvate produced from lactate is catabolized by the TCA cycle (24).
STIMULATION OF METABOLISM BY LACTATE
Evidence for the stimulation of metabolism by lactate comes from several sources.
Interaction of gonococci with neutrophils.
Cohen and his colleagues showed that when gonococci were phagocytosed by neutrophils, their metabolic activity increased: oxygen consumption was raised two- to threefold (7). The stimulating activity was shown by cell-free supernatants from neutrophils, and lactate was demonstrated in these supernatants (7). When gonococci grown in vitro were suspended in Hank's balanced salt solution (HBSS) which contains glucose, lactate, at levels present in the neutrophil supernatants, increased oxygen consumption twofold (7).
Stimulation of gonococci emerging from lag phase.
Stimulation of gonococci emerging from lag phase was first indicated by the discovery that lactate stimulated sialylation of gonococcal lipopolysaccharide (LPS) by host-derived cytidine 5′-monophospho-N-acetyl neuraminic acid (CMP-NANA). This sialylation is catalyzed by a gonococcal sialyltransferase and renders gonococci resistant to complement-mediated killing by fresh human serum and affects other important facets of pathogenicity (20, 60). The sialylation of LPS by CMP-NANA was increased by a second host-derived factor, which was identified as lactate (45, 46). Lactate alone does not induce serum resistance, and pretreatment experiments showed that it acts separately from CMP-NANA (47).
Lactate produces a 20 to 90% increase in sialylation by CMP-NANA. The enhancement phenomenon is evident only in organisms emerging from lag phase. It was seen when stationary-phase gonococci were incubated in shaken cultures with minute amounts of lactate (1 to 50 μM) for 1 to 1.5 h at 37°C in a defined medium containing vitamins, amino acids, and 28 mM glucose (19, 45, 47). Total counts in both control and lactate-treated cultures did not change, but the A650 values increased, indicating emergence from lag phase. Assays for 2-keto-3-deoxyoctonate showed that the LPS content of the lactate-treated gonococci was 10 to 20% higher than that for control organisms (19). Thus, an increased quantity of the sialyl receptor is one factor contributing to enhancement of sialylation and another is increased sialyltransferase activity (18).
The increase in LPS content indicated a general stimulation of metabolism during emergence from lag phase. This was confirmed by the 30 to 60% higher pentose and 10 to 20% higher protein contents of lactate-treated gonococci. Also, lactate produced other changes. Unlike control organisms, lactate-treated gonococci did not have hair-like, possibly stress-related, appendages on their surfaces, and for unknown reasons, their solutions in 1 M NaOH were more viscous (19).
Lactate enhancement of LPS sialylation had revealed a general stimulation of metabolism. What would occur under conditions more akin to those existing during infection? Short-term incubation of gonococci in the defined medium was repeated with glucose concentrations reduced (5 mM) and lactate concentrations increased (1 and 10 mM) to those found in sites relevant to infection in vivo (Table 1). Under these conditions, the gonococci divided up to two times during the 1- to 1.5-h incubation and the A650 values increased, more so for the lactate-treated gonococci (19). As for the enhancement conditions, LPS, protein, and pentose contents were higher (10 to 20%) for the lactate-treated organisms (19). The possibility that lactate was correcting oxygen stress in the shaken cultures was ruled out by showing that lactate enhanced LPS production, protein synthesis, and the viscosity of NaOH solutions for gonococci in stationary as well as shaken cultures (N. Woodcock and H. Smith, unpublished data).
TABLE 1.
Lactate, glucose, and pyruvate concentrations in blood plasma and other body fluids of humansa
| Body fluid | Approximate concn (mmol liter−1 or kg−1) of:
|
||
|---|---|---|---|
| Lactate | Glucose | Pyruvate | |
| Blood plasma | 1.5 | 4.5 | 0.03 |
| Saliva | 0.3 | 0.03 | 0.04 |
| Gastric Juice | 0.3 | INAb | 0.12 |
| Bile | 2.5 | low | 0.3 |
| Ejaculate | 4.0 | 0.35 | 3.0 |
| Vagina | 6.2 | 3.3 | Presentc |
| Urine | 0.5 | 0.4 | 0.10 |
| CNS | 1.5 | 3.5 | 0.12 |
| Tears | INA | 0.2 | 0.47 |
| Aqueous humor | 4.7 | 4.2 | INA |
| Sweat | 7.0 | 0.4 | 0.4 |
| Synovial fluid | 2.0 | 4.0 | 0.15 |
Increased growth rate.
In the defined medium containing 2 mM lactate, logarithmic growth of gonococci was much slower than with 1 mM glucose, although the rate of lactate consumption was greater (53). When lactate was added to the glucose-containing medium, the growth rate was about 20% greater than for glucose alone, and again, lactate was used far more rapidly than glucose (53). This concomitant and more-rapid use of lactate in a mixture with glucose also occurred when gonococci were grown in fluid harvested from infected subcutaneous plastic chambers in guinea pigs (21). In these chambers, gonococci interact with neutrophils of the inflammatory response which glycolyze glucose to lactate (21). Gonococci that had been subjected to oxidative stress in vitro in order to mimic conditions within neutrophils used lactate more rapidly than glucose (17).
Pyruvate had the same stimulating effect as lactate on the growth rate of gonococci in the defined medium containing glucose (53). Also, within human endocervical epithelial cells, intracellular gonococci bind host pyruvate kinase via their Opa proteins and require host pyruvate for growth (66).
MECHANISM OF METABOLIC STIMULATION
Three series of experiments revealed the probable mechanism for lactate stimulation of metabolism. In all three, gonococci were grown in the synthetic medium with either glucose alone or together with lactate at concentrations (5 and 1 mM, respectively) akin to those occurring in vivo (Table 1).
Uptake of metabolites.
Cohen and colleagues (7) suggested that, as for E. coli and Staphylococcus aureus (38, 57), stimulation of gonococcal metabolism might be due to increased uptake of essential metabolites effected by a membrane-located LDH linked to the electron transport chain. They showed that adding serum to a suspension of gonococci in HBSS increased [14C]adenine uptake and oxygen consumption three- to fourfold (6, 7, 10). Dialysis of serum eliminated both effects (6, 10). Lactate partially restored the stimulation of oxygen consumption, but its effect on the reduced [14C]adenine incorporation was not reported (6, 7, 10).
In our experiments (18), although uptake of [14C]adenine was increased by about 40% for lactate-treated gonococci compared to that for control organisms, uptake of [14C]glucose and [14C]proline was unaffected. Hence, there was no evidence for a general stimulation of nutrient transport.
14C-labelling experiments.
Gonococci grown for 1.5 h in the medium containing glucose and lactate with added [14C]lactate were examined by tricene sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (68). The most heavily radiolabelled bands were LPS components and a low-Mr component that migrated with a lipid marker. The majority of proteins stained by silver were not significantly labelled. Three of five proteins that carried label were identified by N-terminal sequencing as GroEL, porin 1B, and a peroxiredoxin protein. There was no evidence of their being produced in quantities greater than those of the other proteins.
Next, gonococci were grown with glucose alone and with added lactate but [14C]glucose was included (68). Again, by SDS-PAGE, the low-Mr component that migrated with a lipid marker and LPS were heavily radiolabelled. Many of the silver-stained protein components were radiolabelled, but no differences in them and gonococci grown with glucose alone and with lactate could be detected. This was the same for the silver-stained protein bands.
Thin-layer chromatography of homogenates of gonococci grown with glucose and lactate with added [14C]lactate confirmed that lactate carbon was incorporated into membrane lipids and showed, by saponification with NaOH, that it was present in their fatty acids (68). Then, the radiolabelling of the five proteins by [14C]lactate was shown to be due to the fatty acids in attached lipid; the areas of the dried, fluorographed, SDS-PAGE gels that contained the protein bands were excised, saponified, and extracted with chloroform for scintillation counting. The gel area containing the LPS bands was also treated in this manner with similar results.
There were two main conclusions. First, lactate carbon was preferentially incorporated into the fatty acids of membrane lipids and LPS. Second, there was no evidence for the specific induction of additional proteins by lactate in the presence of glucose. These results agreed with the findings of previous pulse-chase experiments using [35S]methionine (47). Proteomics might reveal lactate-induced proteins, but they are unlikely to be major constitutents of N. gonorrhoeae.
13C labelling and NMR spectroscopy.
The fate of lactate and glucose carbon in gonococcal lipids was examined further by 13C labelling and nuclear magnetic resonance (NMR) spectroscopy (68). These lipids are about 70% phosphatidyl ethanolamines and 20% phosphatidyl glycerols, with small amounts of other phosphatides: the fatty acids are lauric acid (C12:0), myristic acid (C14:0), a C14:1 fatty acid, palmitic acid (C16:0), palmitoleic acid (C16:1), and cis-vaccenic acid (C18:1) (51). The 13C-NMR signals from such lipids are listed in the first two columns of Table 2.
TABLE 2.
NMR signals in spectra of lipids extracted from N. gonorrhoeae grown with [13C]glucose alone, [13C]lactate and unlabelled glucose, and [13C]glucose and unlabelled lactatea
| Chemical groupb | Chemical shift (ppm)c | Presence in lipid sample with:
|
||
|---|---|---|---|---|
| [13C]glucose | [13C]lactate + glucose | [13C]glucose + lactate | ||
| Fatty acids | ||||
| CH3 and CH2 | 15–45 | + | + | + |
| CH=CH | 130 | + | + | + |
| C=O | 175 | + | + | + |
| Ethanolamine and glycerol (CH2N, CHN, CH2O, CHO) | 60–75 | + | − | + |
Data summarized from Fig. 4 and 5 of reference 68.
Lipid signals are shown by a mixture of two typical lipids: phosphatidyl ethanolamine dimyristyl and phosphatidyl glycerol dioleoyl.
ppm, parts per million.
Gonococci were grown for 4.5 to 6 h in the defined medium containing [13C]glucose (sample A), [13C]lactate and unlabelled glucose (sample B), or [13C]glucose and unlabelled lactate (sample C). The results of NMR spectroscopy on lipids extracted from gonococci in the three cultures (samples A, B, and C) are summarized in Table 2. The spectrum of sample A showed fatty acid, glycerol, and ethanolamine signals, indicating that, in the absence of lactate, 13C from glucose is incorporated into all lipid components. In contrast, the spectrum of sample B showed only fatty acid signals, indicating that, in the presence of glucose, 13C from lactate does not go into ethanolamine/glycerol components. The main features of the spectrum of sample C were similar to those of sample A, showing that, even in the presence of lactate, 13C from glucose is incorporated into all lipid components. However, there were differences between the two spectra. The spectrum of sample C showed additional small signals at chemical shifts 16, 40, and 124 ppm (see Fig. 5 of reference 68), indicating formation of one or more fatty acids different from those present in sample A. Furthermore, the ratio of the integrated areas of all 13C signals (fatty acid, ethanolamine, and glycerol) to the mass of lipid examined was significantly greater in sample C than in sample A (68). Hence, lactate apparently increases lipid synthesis from glucose, another surprising manifestation of its stimulatory role. With lactate carbon also going into fatty acid moieties, a lower incorporation of glucose carbon might have been expected.
The cardinal conclusion from the 13C-NMR spectroscopy is that, in the presence of glucose, lactate carbon is not incorporated into the ethanolamine and glycerol moieties of lipids. Recently, this conclusion has been reinforced by NMR spectroscopy of LPS, purified from the three cultures described above, which showed that, in the presence of glucose, lactate carbon is not incorporated into its sugar moieties (E. A. Yates and H. Smith, unpublished data).
Mechanism.
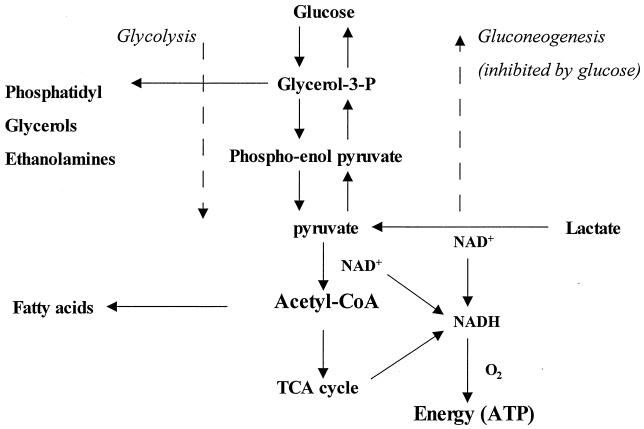
Figure 1 is a simplified diagram of lactate metabolism in bacteria. Early biochemical studies (see above) showed that gonococci possessed some of the relevant enzymes, such as LDHs and those of the TCA cycle. Also, a search of the N. gonorrhoeae Genome Database at the University of Oklahoma showed the presence of the genes for all the glycolysis/gluconeogenesis pathway and the TCA cycle enzymes.
FIG. 1.
Schematic diagram of metabolism of lactate by bacteria possessing enzymes of the glycolysis/gluconeogenesis pathway and TCA cycle.
As mentioned above, when gonococci are cultured in a defined medium with lactate alone, growth is slow despite rapid use of lactate (53). This is because lactate must fulfill two functions. First, there is gluconeogenesis to produce the sugar, glycerol, and ethanolamine moieties of gonococcal constituents. The second is formation of acetyl-CoA, the precursor of fatty acid synthesis and constituents and products of the TCA cycle. Energy is generated via NADH production from the initial oxidation to pyruvate and acetyl-CoA and from the TCA cycle (Fig. 1). The slow growth is probably due to the time needed for production of a wide range of biosynthetic precursors and products, including those generated by gluconeogenesis.
When lactate is present with glucose, NMR spectroscopy shows that gluconeogenesis from lactate does not take place because its carbon is not found in the ethanolamine and glycerol moieties of lipids or in the sugar groups of LPS. The lactate is used solely as a source of acetyl-CoA and energy. Indeed, much of its carbon is found in fatty acids which come from acetyl-CoA. By the NADH derived from its initial oxidation to pyruvate and acetyl-CoA and from the TCA cycle (24), lactate will produce more energy than is obtained from glucose alone. The latter is metabolized largely from the Entner-Doudoroff pathway, (40) which generates relatively small amounts of energy. The provision of a ready source of NADH, and hence ATP by the rapid route to acetyl-CoA, is the probable reason for the stimulation of gonococcal metabolism and oxygen uptake when lactate is added to media containing glucose. This view is supported by the fact that pyruvate also increases growth rate in the presence of glucose (53).
This mechanism, i.e., rapid production of NADH leading to energy generation, could operate when amounts of lactate are minute and a small gonococcal inoculum is emerging from lag phase, as in the conditions for demonstrating the enhancement of LPS sialylation described above. The activity of minute amounts of lactate raises the possibility of a signalling role, but the fact that pyruvate is as active as lactate under these conditions (45) supports the metabolic mechanism described above. Metabolic roles for small amounts of lactate are known in other circumstances, e.g., the glycolytic pathway in E. coli. A phosphoenol pyruvate synthetase-negative mutant (pps′) of E. coli grows in acetate, but the addition of less than 30 μM lactate stops growth, possibly due to pyruvate back up (14, 22, 34). The stimulating effect of metabolites during the lag phase is well-known (37). These metabolites might be made by the bacteria, but their rate of synthesis is too low to meet the requirements for initiating growth. α-Ketoglutaric acid and glutamate affect E. coli in this way (37). They may also provide rapidly essential intermediates which otherwise would be made slowly (37); thus, lactate could form acetyl-CoA and NADH quickly for gonococci.
POTENTIAL IMPACT ON PATHOGENICITY
The first point is that lactate stimulation of gonococcal metabolism depends on the presence of both lactate and glucose, which occurs in genital secretions, inflammatory exudates, and blood (Table 1). The second point is that lactate stimulation of metabolism could be important in the vital primary lodgement stage of infection (58). Here, relatively few invading organisms must compete with any commensals on mucous surfaces and multiply in the face of formidable host defenses present in the tissues and those mobilized by inflammation. Modest increases (10 to 20%) in virulence determinant production and growth rate could tip the scales in favor of the pathogen. The demonstration that in vitro, lactate stimulates LPS production and protein synthesis by gonococci as they emerge from lag phase and begin to multiply is very pertinent to this stage.
In transmission of gonorrhea, the majority of the gonococci from an infected host will have LPS that had been sialylated in that host; but some may contain unsialylated LPS (60). When gonococci divide in the new host, those with unsialylated LPS will be produced but this LPS will be available for sialylation by CMP-NANA present in the tissues. Hence, lactate stimulation of metabolism at this early stage will increase both unsialylated and sialylated LPS. The unsialylated LPS could contribute to gonococcal invasion of urethral epithelial cells because it is a ligand for human asialoglycoprotein on the surface of these cells, and in vitro, the linkage promotes cell entry (41). The sialylated LPS could play a dual role. Sialylation prevents binding LPS to asialoglycoprotein, and in vitro, it interfers with Opa protein-mediated entry to epithelial cells (20, 41, 60). Hence, in vivo, it could inhibit initial invasion. On the other hand, sialylation of LPS inhibits complement-mediated killing of gonococci by human serum, ingestion and killing by neutrophils, and the antibody response (20, 60); it could therefore promote infection in the early stages. Infection experiments with human volunteers indicate that this dual role operates in vivo (60).
The general increase in protein synthesis promoted by lactate should result in greater quantities of protein virulence determinants, some of which could be involved in the early stages of infection. Examples are type IV pili, gonococcal adhesins (41); Opa proteins, which promote entry to epithelial cells (41); the sialyltransferase needed for LPS sialylation (60); and porin 1B, which inserts into the membranes of neutrophils and inhibits their action (5). Specific evidence that lactate does increase production of these virulence determinants is available for sialyltransferase (18).
The more-rapid growth rate promoted by lactate in media containing glucose could also be beneficial in the primary lodgement period by providing additional replacements for gonococci removed or killed by the host defenses (58). The increased oxygen consumption caused by lactate might impair the oxygen-dependent killing mechanism of neutrophils (7), but LDH-deficient mutants survived in phagocytes as well as the wild type did (S. Edupugante, R. D. Chruckshank, S. Buragena, M. S. Cohen, and M. M. Hobbs, Abstr. 12th Int. Pathog. Neisseria Conf., abstr. no. 067, 2000).
Larger quantities of LPS and protein virulence determinants would also increase inflammation, the main harmful effect of gonorrhea. LPS is a potent stimulant of inflammatory cytokines (50). Both induction of cytokines and the toxicity of LPS depend on a full complement of fatty acids in lipid A and on their structures (54; C. D. Ellis, C. M. A. Kahn, B. Lindner, U. Zahringer, and R. Demarco de Hormaeche, Abstr. 12th Int. Pathog. Neisseria Conf., abstr. 023, 2000). As mentioned above, NMR spectroscopy of lipids indicated that lactate caused the production of small quantities of different fatty acids from glucose. If this occurred for the lipid A of LPS, it may affect cytokine production. Turning to protein virulence determinants, GroEL is produced by gonococci and can cause inflammation because it is a powerful inducer of cytokines (9, 42).
The evidence above strongly suggests that the lactate effect is relevant to gonococcal pathogenicity, but this has not been proven by showing that a mutant unable to use lactate is attenuated in a relevant animal model. Unfortunately, suitable mutants have not emerged. LDH-deficient mutants appear potentially useful but gonococci make at least three LDHs, and this has prevented the isolation of mutants that are completely unable to use lactate (13). However, a suitable mutant may become available in the future. Recently, a putative lactate permease-deficient meningococcal mutant showing markedly attenuated virulence in an infant rat model has been isolated (62; M. Tang, personal communication). The genome of N. gonorrhoeae contains a gene identical to that of meningococci, and it may be possible to transfer the meningococcal mutation to gonococci. If this is achieved, virulence comparisons with the wild type could proceed in oestradiol-treated mice (28), and ultimately, human volunteers.
WIDER IMPLICATIONS
Lactate stimulation of gonococcal metabolism and the mechanism concerned may have wider implications because in vivo, both lactate and glucose are present at sites which carry or are attacked by different pathogens, and many of the latter have the enzymes required for the processes depicted in Fig. 1.
Blood plasma and body fluids of the alimentary and urogenital tracts, the central nervous system, and other tissues contain both lactate and glucose and also pyruvate, which for gonococci has the same effect as lactate (Table 1). In the fluids of the alimentary tract, the concentrations are lower than elsewhere but there is enough present to influence small numbers of invading pathogens at the beginning of infection. With regard to respiratory infection, a literature survey to see if lactate, glucose, and pyruvate are present in nasopharangeal, bronchial, and lung secretions provided no information. However, much of the glucose in the lung circulation is processed to lactate (ca. 35%) and pyruvate (ca. 2%); in oxygenated lungs perfused with 4 to 6 mM glucose (i.e., the plasma concentration) about 50 mmol of lactate is produced per hour per kg of tissue (64).
A site of infection common to most pathogens is within the phagocytes of the inflammatory response and the fluid that surrounds them. Since glucose is continuously entering these phagocytes and being converted to lactate, some of which escapes, precise figures for the concentrations of glucose and lactate in phagocytes are not available. However, these levels must be substantial: in 1 min, 1012 human leucocytes, whose volume is about half a liter, can metabolize about 2.3 mmol of glucose and produce about 5 mmol of lactate (36). Human neutrophils (5 × 106 ml−1) incubated at 37°C for 2 h in HBSS produced 0.2 mM lactate in the cell supernatant (7). With regard to the fluid surrounding phagocytes in vivo, 4 h after gonococcal infection of subcutaneously implanted chambers in guinea pigs, when there is a massive influx of phagocytes, lactate and glucose concentrations were both about 4 mM, and by 12 h, lactate had increased and glucose had decreased about 40% (21). A literature survey on lactate and glucose in and around macrophages yielded no definite information, but the situation should be similar to that for inflammatory leukocytes.
Turning to the requirement for the enzymes of the glycolytic/gluconeogenesis pathway and the TCA cycle, the genome sequences of some representative pathogens (Table 3) show that the former pathway is ubiquitous and often the TCA cycle enzymes are complete. Hence, the mechanism for lactate stimulation of metabolism that occurs in gonococci, i.e., glucose blockage of lactate gluconeogenesis and energy production by fast channelling to acetyl-CoA, could operate in many cases.
TABLE 3.
Complements of enzymes needed for glycolysis/gluconeogenesis and the TCA cycle indicated by genome sequences of bacterial pathogens
| Pathogen | Complement of enzymes for:
|
Reference(s) or source | |
|---|---|---|---|
| Glycolysis/gluconeogenesis | TCA cycle | ||
| Escherichia coli O157:H7 | Complete | Complete | 48 |
| Borrelia burgdorferi | Complete | Absent | 16 |
| Campylobacter jejuni | Complete for gluconeogenesis | Complete | 43 |
| Haemophilus influenzae | Complete | Incomplete | 15, 26 |
| Helicobacter pylori | Complete | Incomplete | 26, 65 |
| Mycobacterium tuberculosis | Complete | Complete | 11 |
| Neisseria meningitidis | Complete | Complete | 44, 63 |
| Vibrio cholerae | Complete | Complete | 23 |
| Pseudomonas aeruginosa | Complete | Complete | 61, P. Williamsa |
| Streptococcus pneumoniae | Complete | Incomplete | T. J. Mitchella |
| Yersinia pestis | Complete | Complete | P. Williams |
| Salmonella typhi | Complete | Complete | Douglas and Holden |
Personal communications.
Information is not available about the influence of lactate on the pathogenicity of most of the bacteria listed in Table 3, but for two of them (N. meningitidis and Haemophilus influenzae) there is evidence that lactate may play a role similar to that for gonococci.
N. meningitidis.
The genome sequences of two serotypes (44, 63) show that meningococci have all the enzymes necessary for the processes summarized in Fig. 1. Earlier biochemical work had identified three LDHs (12), the pyruvate dehydrogenase system that converts pyruvate to acetyl-CoA (29), and all the TCA cycle enzymes (24). As for gonococci, all early metabolic studies of meningococci involved either glucose or lactate but not mixtures of the two. Experiments using mixtures have not been done, in particular, those on the affect of lactate on LPS production in media containing glucose. Meningococcal LPS can be sialylated either endogenously or exogenously by CMP-NANA; this affects several aspects of pathogenicity and relates to whether strains are carrier or invasive (59, 60). Also, lactate metabolism may affect production of another virulence determinant, the capsule. Recently, a putative lactate permease-deficient mutant of N. meningitidis was isolated by signature-tagged mutagenesis (62). In an infant rat model of infection, the mutant was considerably attenuated; it had a competitive index of 0.1 against the wild type (Tang, personal communication). Since meningococci can use glucose without lactate for growth, complete attenuation would not be expected. The properties of this mutant and the presence of lactate and glucose in sites relevant to meningococcal infection suggest that it would be worthwhile to investigate the role of lactate in meningococcal metabolism and pathogenicity along the same lines as for gonococci.
H. influenzae.
The genome sequence of H. influenzae Rd (15) indicates the presence of all the enzymes needed for glycolysis/gluconeogenesis and lipid synthesis. However, three enzymes of the TCA cycle, citrate synthetase, aconitase and isocitric dehydrogenase, are missing (15). Nevertheless, generation of NADH from lactate could occur from its oxidation to pyruvate and acetyl-CoA even if little NADH were available from the incomplete TCA cycle. As for gonococci and meningococci, the LPS of H. influenzae can be sialylated; this and capsular polysaccharide can contribute to serum resistance (30, 59).
Investigations by Anderson, Kuratana, and others (2, 27, 30–33, 55, 56) that were similar to those on gonococci indicated that lactate in the presence of glucose influences metabolism in relation to pathogenicity. H. influenzae in blood samples and nasal washings were more resistant to complement-mediated killing by fresh human serum than organisms grown in broth (55, 56). This resistance could be achieved in vitro by growing H. influenzae with low-Mr filtrates of serum or nasal washings (32, 55, 56). It was lost on subculture in broth (56). As for gonococci (20, 60), conversion of H. influenzae to serum resistance affected other aspects of pathogenicity. The serum-resistant organisms were less susceptibile to complement-mediated killing by antibodies to LPS and outer membrane proteins and to the opsonic activity of antibody to capsular polysaccharide (2, 27, 31–33, 55). They were also more virulent for infant rats (55).
The induction of serum resistance by incubation with serum filtrates occurred by two mechanisms (31). In one, both capsular-deficient mutants and capsulated wild types were converted to resistance and LPS content increased. In the second, only capsulated wild types were converted to resistance and capsular polysaccharide increased. The first mechanism was blocked by chloramphenicol but not the second (31). The work on gonococci by Cohen et al (7) prompted examination of the influence of lactate on the two mechanisms (30). Substitution of the serum filtrate by a buffer containing lactate, glucose, urea, and bicarbonate produced the first mechanism of resistance induction and a mixture of lactate and Ca2+ produced the second (30). In the first mechanism, where glucose is present, lactate may play a role similar to that for gonococci, i.e., a general stimulation of metabolism including LPS synthesis by energy derived from a fast track to acetyl-CoA. In the second mechanism, where glucose is absent, lactate could be partially replaced by pyruvate and completely by NADH (30). It was suggested that lactate stimulates capsular polysaccharide production by serving as a source of both carbon and reducing power (30).
Considering the relevance of these observations to infection in vivo (30), Kuratana and Anderson pointed out that in the nasopharynx where H. influenzae persists, lactate, urea, bicarbonate, glucose and Ca2+ would be available in saliva and also in plasma on the rare occasions when H. influenzae invades the blood stream. They suggested that lactate rather than glucose may be the major carbon and energy source to which H. influenzae has adapted. Further studies along the lines of those carried out for gonococci seem warranted.
Other pathogens.
All the pathogens listed in Table 3 except M. tuberculosis and S. pneumoniae have genes that code for putative lactate permeases. If the studies on meningococci and H. influenzae reveal effects of lactate similar to those demonstrated for gonococci, their extension to other pathogens should be considered.
CONCLUSION
In media containing glucose, lactate stimulates the metabolism of N. gonorrhoeae, and this result affects aspects of pathogenicity, particularly in relation to the early stages of infection. Similar observations have been made for H. influenzae. A putative lactate permease-deficient mutant of N. meningitidis is significantly attenuated compared with wild types. In vivo, lactate and glucose are present together in many sites infected by a variety of pathogens. A mechanism for the stimulating effect of lactate, glucose blockage of lactate gluconeogenesis providing a fast track to acetyl-CoA and extra energy production, has been demonstrated for N. gonorrhoeae. Genome sequences of representative bacterial pathogens show in many cases that the enzymes necessary for this mechanism are present. It seems possible that lactate in the presence of glucose could affect the pathogenicity of bacteria other than gonococci and that there may be a common mechanism.
ACKNOWLEDGMENTS
We are indebted to M. J. Gill and T. W. Overton for help in literature and genome surveys.
REFERENCES
- 1.Al-Mushrif S, Eley A, Jones B M. Inhibition of chemotaxis by organic acids from anaerobes may prevent a purulent response in bacterial vaginosis. J Med Microbiol. 2000;49:1023–1030. doi: 10.1099/0022-1317-49-11-1023. [DOI] [PubMed] [Google Scholar]
- 2.Anderson P, Flesher A, Shaw S, Harding A L, Smith D H. Phenotypic and genetic variation in the susceptibility of Haemophilus influenzae type b to antibodies to somatic antigens. J Clin Investig. 1980;65:885–891. doi: 10.1172/JCI109741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barron E S G, Hastings A B. Studies on biological oxidations. II. The oxidation of lactic acid by α-hydroxyoxidase and its mechanism. J Biol Chem. 1933;100:155–182. [Google Scholar]
- 4.Barron E S G, Miller C P., Jr Studies on biological oxidations. I. Oxidations produced by gonococci. J Biol Chem. 1932;97:691–715. [Google Scholar]
- 5.Bjerknes R, Guttormsen H, Solberg C O, Wetzler L M. Neisserial porins inhibit human neutrophil actin polymerization, degranulatation, opsonin receptor expression, and phagocytosis but prime the neutrophils to increase their oxidative burst. Infect Immun. 1995;63:160–167. doi: 10.1128/iai.63.1.160-167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Britigan B E, Chai Y, Cohen M S. Effects of human serum on the growth and metabolism of Neisseria gonorrhoeae: an alternative view of serum. Infect Immun. 1985;50:738–744. doi: 10.1128/iai.50.3.738-744.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Britigan B E, Klapper D, Svendsen T, Cohen M S. Phagocyte-derived lactate stimulates oxygen consumption by Neisseria gonorrhoeae. J Clin Investig. 1988;81:318–324. doi: 10.1172/JCI113323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Catlin B W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973;128:175–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- 9.Coates A R M, Henderson B. Chaperonins in health and disease. Ann N Y Acad Sci. 1998;851:48–53. doi: 10.1111/j.1749-6632.1998.tb08975.x. [DOI] [PubMed] [Google Scholar]
- 10.Cohen M S, Cooney M H. A bacterial respiratory burst: stimulation of the metabolism of Neisseria gonorrhoeae by human serum. J Infect Dis. 1984;150:49–56. doi: 10.1093/infdis/150.1.49. [DOI] [PubMed] [Google Scholar]
- 11.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, 3rd, Tekaia F, Badcock K, Basham D, Brown D, Chillingworth T, Connor R, Davies R, Devlin K, Feltwell T, Gentles S, Hamlin N, Holroyd S, Hornsby T, Jagels K, Barrell B G, et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 12.Erwin A L, Gotschlich E M. Oxidation of d-lactate and l-lactate by Neisseria meningitidis: purification and cloning of meningococcal d-lactate dehydrogenase. J Bacteriol. 1993;175:6382–6391. doi: 10.1128/jb.175.20.6382-6391.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fischer R S, Martin G C, Rao P, Jensen R A. Neisseria gonorrhoeae possesses two nicotinamide adenine dinucleotide-dependent lactate dehydrogenases. FEMS Microbiol Lett. 1994;115:39–44. doi: 10.1111/j.1574-6968.1994.tb06611.x. [DOI] [PubMed] [Google Scholar]
- 14.Flatgaard J E, Hoehn B, Henning U. Mutants of Escherichia coli K-12 which synthesize the pyruvated dehydrogenase complex constitutively. Arch Biochem Biophys. 1971;143:461–470. doi: 10.1016/0003-9861(71)90231-1. [DOI] [PubMed] [Google Scholar]
- 15.Fleischman R D, et al. Whole-genome random sequencing and assembly of Haemophilus influenzae pd. Science. 1995;269:496–512. doi: 10.1126/science.7542800. [DOI] [PubMed] [Google Scholar]
- 16.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter C, et al. Genomic sequence of a Lyme disease spirochaete Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 17.Fu K, Hassett D J, Cohen M S. Oxidant stress in Neisseria gonorrhoeae: adaptation and effects on l-(+)-lactate dehydrogenase activity. Infect Immun. 1989;57:2173–2178. doi: 10.1128/iai.57.7.2173-2178.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao L, Linden L, Parsons N J, Cole J A, Smith H. Uptake of metabolites by gonococci grown with lactate in a medium containing glucose; evidence for a surface location of the sialyltransferase. Microb Pathog. 2000;28:257–266. doi: 10.1006/mpat.1999.0348. [DOI] [PubMed] [Google Scholar]
- 19.Gao L, Parsons N J, Curry A, Cole J A, Smith H. Lactate causes changes in gonococci including increased lipopolysaccharide synthesis during short-term incubation in media containing glucose. FEMS Microbiol Lett. 1998;169:309–316. doi: 10.1111/j.1574-6968.1998.tb13334.x. [DOI] [PubMed] [Google Scholar]
- 20.Gill M J, McQuillen D P, van Putten J P M, Wetzler L M, Bramley J, Crooke H, Parsons N J, Cole J A, Smith H. Functional characterization of a sialyltransferase deficient mutant of Neisseria gonorrhoeae. Infect Immun. 1996;64:3374–3378. doi: 10.1128/iai.64.8.3374-3378.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldner M, Penn C W, Sanyal S C, Veale D R, Smith H. Phenotypically determined resistance of Neisseria gonorrhoeae in normal human serum; environmental factors in subcutaneous chambers in guinea pigs. J Gen Microbiol. 1979;122:235–245. doi: 10.1099/00221287-114-1-169. [DOI] [PubMed] [Google Scholar]
- 22.Haydon D J, Quail M A, Guest J R. A mutation causing constitutive synthesis of the pyruvate dehydrogenase complex in Escherichia coli is located within the pdhR gene. FEBS Lett. 1993;336:43–47. doi: 10.1016/0014-5793(93)81605-y. [DOI] [PubMed] [Google Scholar]
- 23.Heidelberg J F, Eisen J A, Nelson W C, Clayton R A, Gwinn M L, Dodson R J, Haft D H, Hickey E K, Peterson J D, Umayam L, Gill S R, Nelson K E, Read T D, Tettelin H, Richardson D, Ermolaeva M D, Vamathevan J, Bass S, Qin H, Dragoi I, Sellers P, McDonald L, Utterback T, Fleishmann R D, Nierman W C, White O, et al. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature. 2000;406:477–483. doi: 10.1038/35020000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Holten E. Radiorespirometric studies in genus Neisseria. 3. The catabolism of pyruvate and acetate. Acta Pathol Microbiol Scand Sect B. 1976;84:9–16. [PubMed] [Google Scholar]
- 25.Holten E, Jyssum K. Activities of some enzymes concerning pyruvate metabolism in Neisseria. Acta Pathol Microbiol Scand Sect B. 1974;82:843–848. doi: 10.1111/j.1699-0463.1974.tb02382.x. [DOI] [PubMed] [Google Scholar]
- 26.Huynen M A, Dandekar T, Bork P. Variation and evolution of the citric acid cycle: a genomic perspective. Trends Microbiol. 1999;7:281–291. doi: 10.1016/s0966-842x(99)01539-5. [DOI] [PubMed] [Google Scholar]
- 27.Inzana T J, Anderson P. Serum factor-dependent resistance of Haemophilus influenzae type b to antibody to lipopolysaccharide. J Infect Dis. 1985;151:869–877. doi: 10.1093/infdis/151.5.869. [DOI] [PubMed] [Google Scholar]
- 28.Jerse A E. Experimental gonococcal genital tract infection and opacity protein expression in estradiol-treated mice. Infect Immun. 1999;67:5699–5708. doi: 10.1128/iai.67.11.5699-5708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jyssum K. Intermediate reactions of the tricarboxylic acid cycle in meningococci. Acta Pathol Microbiol Scand Sect B. 1960;48:121–132. doi: 10.1111/j.1699-0463.1960.tb04748.x. [DOI] [PubMed] [Google Scholar]
- 30.Kuratana M, Anderson P. Host metabolites that phenotypically increase the resistance of Haemophilus influenzae Type b to clearance mechanisms, J. Infect Dis. 1991;163:1073–1079. doi: 10.1093/infdis/163.5.1073. [DOI] [PubMed] [Google Scholar]
- 31.Kuratana M, Hansen E J, Anderson P. Multiple mechanisms in serum factor-induced resistance of Haemophilus influenzae type b antibody. Infect Immun. 1990;58:914–917. doi: 10.1128/iai.58.4.914-919.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuratana M, Inzana T J, Anderson P. Source of low-molecular-weight host factors that phenotypically increase the resistance of Haemophilus influenzae type b to bacteriolysis: nonidentity with a factor active in gonococci. Microb Pathog. 1989;7:3–77. doi: 10.1016/0882-4010(89)90113-7. [DOI] [PubMed] [Google Scholar]
- 33.Kuratana M, Loeb M R, Hansen E J, Anderson P. The antigen specificity of a serum factor-induced phenotypic shift in Haemophilus influenzae type b, strain Eag. J Infect Dis. 1989;159:1135–1139. doi: 10.1093/infdis/159.6.1135. [DOI] [PubMed] [Google Scholar]
- 34.Langley D, Guest J R. On the location of a mutation causing constitutive synthesis of the pyruvate dehydrogenase complex of Escherichia coli K12. FEMS Microbiol Lett. 1979;5:5–8. [Google Scholar]
- 35.Lentner C. Geigy scientific tables, vol. 1. Units of measurement, body fluids, composition of the body, nutrition. Basel, Switzerland: Ciba Geigy, Ltd.; 1981. [Google Scholar]
- 36.Lentner C. Geigy scientific tables, vol. 3. Physical chemistry, composition of the blood, hematology, somatometric data. Basel, Switzerland: Ciba Geigy, Ltd.; 1984. [Google Scholar]
- 37.Lichstein H C. Symposium on initiation of bacterial growth III. Physiological aspects of growth initiation. Bacteriol Rev. 1959;23:261–266. doi: 10.1128/br.23.4.261-266.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lombardi F J, Kaback H R. Mechanism of active transport in isolated membrane vesicles. VIII. The transport of amino acids by membranes prepared from Escherichia coli. J Biol Chem. 1972;247:7844–7857. [PubMed] [Google Scholar]
- 39.Morse S A, Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974;145:1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- 40.Morse S A, Hebeler B H. Effect of pH on the growth and glucose metabolism of Neisseria gonorrhoeae. Infect Immun. 1978;21:87–95. doi: 10.1128/iai.21.1.87-95.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nassif X, Apicella M A. Gonococcal lipooligosaccharide: an adhesin for bacterial dissemination? Trends Microbiol. 2000;8:539–541. doi: 10.1016/s0966-842x(00)01879-5. [DOI] [PubMed] [Google Scholar]
- 42.Pannekoek Y, van Putten J P M, Dankert J. Identification and molecular analysis of a 63-kilodalton stress protein from Neisseria gonorrhoeae. J Bacteriol. 1992;174:6928–6937. doi: 10.1128/jb.174.21.6928-6937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parkhill J, Wren B W, Mungall K, Ketley J M, Churcher C, Basham D, Chillingworth T, Davies R M, Feltwell T, Holroyd S, Jagels K, Karlyshev A V, Moule S, Pallen M J, Penn C W, Quail M A, Rajandream M A, Rutherford K M, van Vliet A H, Whitehead S, Barrell B G, et al. The genome sequence of the food-borne pathogen Campylobacter jejuni reveals hypervariable sequences. Nature. 2000;403:665–668. doi: 10.1038/35001088. [DOI] [PubMed] [Google Scholar]
- 44.Parkhill J, Achtman M, James K D, Bentley S D, Churcher C, Klee S R, Morelli G, Basham D, Brown D, Chillingworth T, Davies R M, Davis P, Devlin K, Feltwell T, Hamlin N, Holroyd S, Jagels K, Leather S, Moule S, Mungall K, Quail M A, Rajandream M A, Rutherford K M, Simmonds M, Skelton J, Whitehead S, Spratt B G, Barrell B G, et al. Complete DNA sequence of a serogroup A strain of Neisseria meningitidis Z2491. Nature. 2000;404:502–506. doi: 10.1038/35006655. [DOI] [PubMed] [Google Scholar]
- 45.Parsons N J, Boons G J, Ashton P R, Redfern P D, Quirk P, Gao Y, Constantinidou C, Patel J, Bramley J, Cole J A, Smith H. Lactic acid is the factor in blood cell extracts which enhances the ability of CMP-NANA to sialylate gonococcal lipopolysaccharide and induce serum resistance. Microb Pathog. 1996;20:87–100. doi: 10.1006/mpat.1996.0008. [DOI] [PubMed] [Google Scholar]
- 46.Parsons N J, Constantinidou C, Cole J A, Smith H. Sialylation of lipopolysaccharide by CMP-NANA in viable gonococci is enhanced by low Mr material released from blood cell extracts but not by some UDP-sugars. Microb Pathog. 1994;16:413–421. doi: 10.1006/mpat.1994.1041. [DOI] [PubMed] [Google Scholar]
- 47.Parsons N J, Emond J P, Goldner M, Bramley J, Crooke H, Cole J A, Smith H. Lactate enhancement of sialylation of gonococcal lipopolysaccharide and induction of serum resistance by CMP-NANA is not due to direct activation of the sialyltransferase: metabolic events are involved. Microb Pathog. 1996;21:193–204. doi: 10.1006/mpat.1996.0054. [DOI] [PubMed] [Google Scholar]
- 48.Perna N T, 3rd Plunkett G, Burland V, Mau B, Glasner J D, Rose D J, Mayhew G F, Evans P S, Gregor J, Kirkpatrick H A, Posfai G, Hackett J, Klink S, Boutin A, Shao Y, Miller L, Grotbeck E J, Davis N W, Lim A, Dimalanta E T, Potamousis K D, Apodaca J, Anantharaman T S, Lin J, Yen G, Schwartz D C, Welch R A, Blattner F R, et al. Genome sequence of enterohaemorrhagic Escherichia coli O157: H7. Nature. 2001;409:529–533. doi: 10.1038/35054089. [DOI] [PubMed] [Google Scholar]
- 49.Preti G, Huggins G R. Organic constituents of vaginal secretions. In: Hafez E S E, Evans T N, editors. The human vagina. Amsterdam, The Netherlands: Elsevier/North Holland Biomedical Press; 1978. pp. 151–166. [Google Scholar]
- 50.Proctor R A, Denlinger L C, Bertics P J. Lipopolysaccharide and bacterial virulence. In: Roth J A, Bolin C A, Brogden K A, Minion F C, Wannemuehler M J, editors. Virulence mechanisms of bacterial pathogens. Washington, D.C.: American Society for Microbiology; 1995. pp. 173–194. [Google Scholar]
- 51.Rahman M M, Kolli V S K, Kahler C M, Shih G, Stephens D S, Carlson R W. The membrane phospholipids of Neisseria meningitidis and Neisseria gonorrhoeae as characterized by fast atom bombardment mass spectrometry. Microbiology. 2000;146:1901–1911. doi: 10.1099/00221287-146-8-1901. [DOI] [PubMed] [Google Scholar]
- 52.Rajan N, Cao Q, Anderson B E, Pruden D L, Sensibar J, Duncan J L, Schaeffer A J. Roles of glycoproteins and oligosaccharides found in human vaginal fluid in bacterial adherence. Infect Immun. 1999;67:5027–5032. doi: 10.1128/iai.67.10.5027-5032.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Regan T, Watts A, Smith H, Cole J A. Regulation of the lipopolysaccharide-specific sialyltransferase activity of gonococci by growth state of the bacteria, but not by carbon source, catabolite repression or oxygen supply. Antonie Leeuwenhoek. 1999;75:369–379. doi: 10.1023/a:1002019420453. [DOI] [PubMed] [Google Scholar]
- 54.Rietschel E T, Kirikae T, Schade F U, Mamat U, Schmidt G, Loppnow H, Ulmer A J, Zahringer U, Seydel U, Di Padova F, Schreier M, Brade H. Bacterial endotoxin: molecular relationships of structure to activity and function. FASEB J. 1994;8:217–225. doi: 10.1096/fasebj.8.2.8119492. [DOI] [PubMed] [Google Scholar]
- 55.Rubin L G, Moxon E R. The effect of serum-factor induced resistance to somatic antibodies on the virulence of Haemophilus influenzae type b. J Gen Microbiol. 1985;131:515–520. doi: 10.1099/00221287-131-3-515. [DOI] [PubMed] [Google Scholar]
- 56.Shaw S, Smith A L, Anderson P, Smith D H. The paradox of Haemophilus influenzae type b bacteremia in the presence of serum bactericidal activity. J Clin Investig. 1976;58:1019–1029. doi: 10.1172/JCI108525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Short S A, Kaback H R. Mechanisms of active transport in isolated bacterial membrane vesicles. Further studies on amino acid transport in Staphylococcus aureus membrane vesicles J. Biol Chem. 1974;249:4275–4281. [PubMed] [Google Scholar]
- 58.Smith H. The revival of interest in mechanisms of bacterial pathogenicity. Biol Rev. 1995;70:277–316. doi: 10.1111/j.1469-185x.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 59.Smith H. Host factors that influence the behaviour of bacterial pathogens in vivo. Int J Med Microbiol. 2000;290:207–213. doi: 10.1016/S1438-4221(00)80117-4. [DOI] [PubMed] [Google Scholar]
- 60.Smith H, Parsons N J, Cole J A. Sialylation of neisserial lipopolysaccharide: a major influence on pathogenicity. Microb Pathog. 1995;19:365–377. doi: 10.1006/mpat.1995.0071. [DOI] [PubMed] [Google Scholar]
- 61.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, Hickey M J, Brinkman F S, Hufnagle W O, Kowalik D J, Lagrou M, Garber R L, Goltry L, Tolentino E, Westbrock-Wadman S, Yuan Y, Brody L L, Coulter S N, Folger K R, Kas A, Larbig K, Lim R, Smith K, Spencer D, Wong G K, Wu Z, Paulsen I T, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunist pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 62.Sun Y, Bakshi S, Chalmers R, Tang C M. Functional genomics of Neisseria meningitidis pathogenesis. Nat Med. 2000;6:1269–1273. doi: 10.1038/81380. [DOI] [PubMed] [Google Scholar]
- 63.Tettelin H, et al. Complete genome sequence of Neisseria meningitidis serogroup B strain MC58. Science. 2000;287:1809–1815. doi: 10.1126/science.287.5459.1809. [DOI] [PubMed] [Google Scholar]
- 64.Tierney D F, Young S L. Glucose and intermediary metabolism in the lung. In: Fishman A P, Fischer A B, editors. Handbook of physiology, vol. 1: The respiratory system. Bethesda, Md: American Physiological Society; 1985. pp. 255–275. [Google Scholar]
- 65.Tomb J F. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 66.Williams J M, Chen G-C, Zhu L, Rest R F. Using the yeast two-hybrid system to identify human epithelial cell proteins that bind gonococcal Opa proteins: intracellular gonococci bind pyruvate kinase via their Opa proteins and require host pyruvate for growth. Mol Microbiol. 1998;27:171–186. doi: 10.1046/j.1365-2958.1998.00670.x. [DOI] [PubMed] [Google Scholar]
- 67.Winter D B, Morse S A. Physiology and metabolism of pathogenic Neisseria: partial characterization of the respiratory chain of Neisseria gonorrhoeae. J Bacteriol. 1975;123:631–636. doi: 10.1128/jb.123.2.631-636.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yates E, Gao L, Woodcock N, Parsons N J, Cole J, Smith H. In a medium containing glucose, lactate carbon is incorporated by gonococci predominantly into fatty acids and glucose carbon incorporation is increased; implications regarding lactate stimulation of metabolism. Int J Med Microbiol. 2000;290:627–639. doi: 10.1016/S1438-4221(00)80012-0. [DOI] [PubMed] [Google Scholar]