Abstract
The historical insights and background of the discovery, development and clinical use of deferiprone (L1) and the maltol–iron complex, which were discovered over 40 years ago, highlight the difficulties, complexities and efforts in general orphan drug development programs originating from academic centers. Deferiprone is widely used for the removal of excess iron in the treatment of iron overload diseases, but also in many other diseases associated with iron toxicity, as well as the modulation of iron metabolism pathways. The maltol–iron complex is a recently approved drug used for increasing iron intake in the treatment of iron deficiency anemia, a condition affecting one-third to one-quarter of the world’s population. Detailed insights into different aspects of drug development associated with L1 and the maltol–iron complex are revealed, including theoretical concepts of invention; drug discovery; new chemical synthesis; in vitro, in vivo and clinical screening; toxicology; pharmacology; and the optimization of dose protocols. The prospects of the application of these two drugs in many other diseases are discussed under the light of competing drugs from other academic and commercial centers and also different regulatory authorities. The underlying scientific and other strategies, as well as the many limitations in the present global scene of pharmaceuticals, are also highlighted, with an emphasis on the priorities for orphan drug and emergency medicine development, including the roles of the academic scientific community, pharmaceutical companies and patient organizations.
Keywords: deferiprone; iron-maltol; ferric maltol; feraccru; accrufer; iron overload; iron deficiency anemia; thalassemia; iron metabolism; iron toxicity; drug design, development and use; orphan drugs; orphan diseases
1. Introduction
Iron is one of the essential metal ions found in all cells of the body, as well as in cancer cells and microbes. It is generally required for many enzymes and other proteins performing vital bodily functions and is involved in many physiological and biological processes, such as oxygen transport, storage and utilization; energy transduction; growth and development; lipid metabolism; and DNA synthesis [1,2,3].
Iron is tightly controlled in the human body using different mechanisms, pathways and proteins, which are involved in its uptake, distribution, utilization, recycling and excretion. Each cell requires and utilizes different amounts of iron and also other essential metal ions such as zinc and copper for different biological functions [1,2,3,4,5,6,7]. In particular, the transfer of iron to all cells of the body is accomplished by transferrin in the blood, and is mediated through transferrin receptors present on the cell membrane, followed by its incorporation in the cells using endocytosis [8,9,10,11]. Once inside the cells, iron is released into a low molecular weight iron pool and used mainly for storage but also for the production of iron-containing enzymes. Iron storage in cells is accomplished by ferritin and hemosiderin. Ferritin contains different amounts of iron, and one molecule of ferritin can store up to 4500 molecules of iron. Hemosiderin is a cluster of ferritin molecules with a broken protein shell, which mainly predominates in iron storage over ferritin in iron overload diseases and also other conditions of focal iron deposition [2,11,12,13,14,15,16].
There is wide variation in the requirements for iron and other metals for different cell types, which in the case of iron is mainly reflected by the number of transferrin receptors on the cell membrane [8,9,10,11,17]. For example, increased transferrin receptors are observed in different types of normal cells such as hepatocytes and erythropoietic cells, and also in certain cancer cell types such as breast, prostate and bladder cancers and in leukemias [8,17,18,19]. The control of the delivery of transferrin iron and also other forms of iron into cells through the modulation of transferrin receptors can directly affect key enzymes and the production of different biomolecules, including metabolites playing important roles in health and disease. Furthermore, the role and function of such metabolites related to iron could also be used for the design of specific drugs and for therapeutic strategies in different diseases [18,19,20].
It is estimated that about 4.5–5.0 g of iron is present in a 70–75 kg normal adult man, which is distributed in the blood and different organs. Most of the iron is in the form of heme in the proteins hemoglobin (2.3–2.6 g) in red blood cells (RBC) and myoglobin (0.32–0.40 g) in muscle. Iron is also distributed in the body, mainly in the form of polynuclear ferric oxyhydroxide phosphate complexes in the iron storage proteins ferritin (0.7 g) and hemosiderin (0.3 g), which are mainly found in the liver, spleen, muscles and bone marrow. Smaller amounts of iron are also found in mitochondrial cytochromes (17 mg), catalase (5 mg), transferrin (4 mg) and non-heme iron-containing enzymes (0.1 g) [20].
At normal physiological conditions and pH levels, iron is mainly found in the ferrous (Fe2+) or ferric (Fe3+) state forms, and is always bound to different ligands containing oxygen, nitrogen and sulfur [20]. Under the same conditions, non-protein-bound ferrous iron is oxidized to ferric iron, with the latter only being found in trace detectable levels (10−18 mol/L), since it mostly precipitates by forming insoluble polymeric ferric oxyhydroxide complexes with high stability constants (log K = 38) [20]. In general, the solubility of iron and other metals increases at acidic pH levels, including in the acidic environment of the stomach.
2. Diseases of Iron Metabolism Imbalance and Their Treatment
Iron imbalance is associated with serious clinical conditions affecting many categories of patients [2,3,20,21]. In particular, iron deficiency anemia (IDA) affects about one-third to one-quarter of the world’s population, with the symptoms including increased child and maternal mortality, pregnancy complications, cardiac complications, fatigue, reduced physical and mental performance and paleness [22,23,24,25]. In many of these cases, the symptoms of iron deficiency are cured using iron supplements, which are widely available in different formulations [26,27,28,29]. In general, however, most of the iron formulations suffer from low specificity, which causes reduced iron absorption and also toxicity in the gastrointestinal tract due to the presence of excess non-absorbed iron [30,31,32]. In many clinical cases such as cancer, kidney disease and other categories of patients, intravenous iron formulations are used, which can also be effective in treating IDA, but toxic side effects have also been reported [33,34,35,36,37,38,39,40]. As in many diseases, a risk/benefit assessment is required for the selection of the appropriate iron formulation and route of administration in each IDA patient case. Additional factors may also be observed in the selection of the iron formulation, such as demographic reasons and costs [41,42].
There are many other diseases related to iron metabolism imbalances in addition to IDA, including the hemoglobinopathies and idiopathic hemochromatosis, which are the most common genetic diseases affecting humans [43,44,45,46,47,48,49]. The hemoglobinopathies include thalassemia and sickle cell disease, which are mainly found in developing countries. It is estimated that about 100,000 children are born annually with thalassemia, mainly in Southeast Asia, the Middle East and Mediterranean countries, and about the same number are born with sickle cell anemia [43,44,45]. For example, thalassemia is endemic in Cyprus, where 1 in 6 persons is an asymptomatic thalassemia heterozygote carrier and about 1 in 1000 is a thalassemia major or thalassemia intermediate patient. The prevention and treatment programs for thalassemia and especially chelation therapy impose a great financial burden to the health budget of many developing countries [43,50,51].
The major form of treatment of thalassemia major is regular red blood cell transfusions every 1–4 weeks and daily chelation therapy [45,51]. Multiple transfusions cause increased iron deposition and damage to the heart, liver, spleen and other organs unless iron chelation therapy is introduced early in life [45,51]. Iron overload in thalassemia has the highest rate of morbidity and mortality of metal-related intoxication in humans [51]. Iron chelation therapy in thalassemia and other transfusional iron loading conditions is carried out worldwide using the generic drugs deferoxamine (DF), deferiprone (L1) and deferasirox (DFRA) [52,53,54,55,56,57]. The combination of chelating drugs, and especially the L1/DF combination, is also widely used in many countries [51,52,53,54,55,56,57].
There are many other diseases related to iron and other metal imbalance and toxicity conditions, where iron-chelating drugs and iron chelator complexes could be used as originally suggested in 2003 [58]. Many of these diseases affect millions of patients, including cancer, Alzheimer’s and Parkinson’s diseases, Freidreich’s disease, AIDS, infectious diseases and aluminum and other metal intoxication conditions [58]. The priority in the selection of diseases for testing the efficacy and toxicity of chelating drugs is based on a risk/benefit assessment in comparison to other available therapeutics in each disease [55]. The repurposing of chelating drugs in non-iron-loaded diseases was facilitated following new scientific evidence and also safety findings, especially related to the number of non-iron-loaded patients and conditions used for clinical trials, which progressively has increased in the last few years [59].
3. The Design and Development of Deferiprone and the Maltol–Iron Complex
Deferiprone and the tri-maltol–iron complex, also known as ferric maltol, Feraccru or Accrufer, were originally designed, synthesized and screened in vitro and in vivo in 1981 following the initiation of an academic project in 1978 [60,61,62,63]. Eventually, this project led to the discovery of the novel alpha-ketohydroxypyrone or alpha-hydroxypyrone and alpha-ketohydroxypyridine or alpha-hdroxypyridone class of iron chelators (1978–1981), which were intended for clinical use in diseases related to iron metabolism [60,61,62,63].
The path of development and the journey through the years of each of these drugs were completely different, with many difficulties and many intriguing turns of events related to research findings and the involvement of different academic centers, pharmaceutical companies, physicians, research investigators and patient organizations, which continue today [33,63].
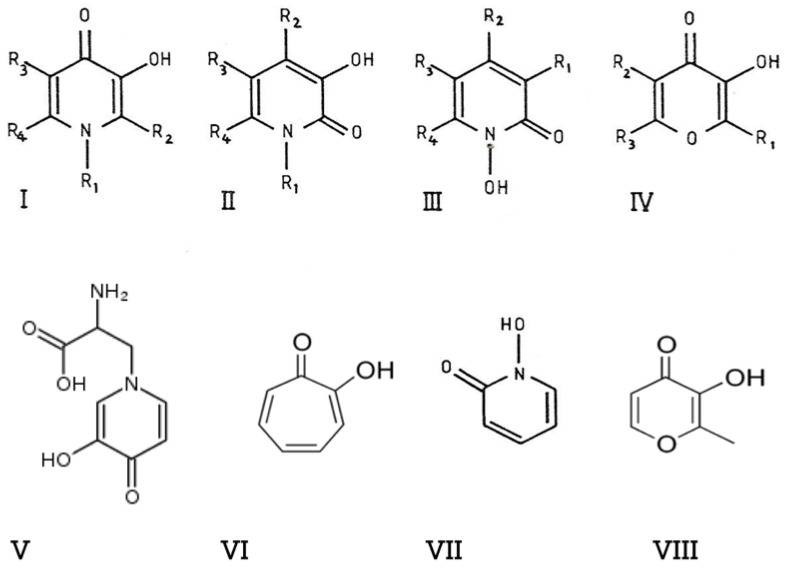
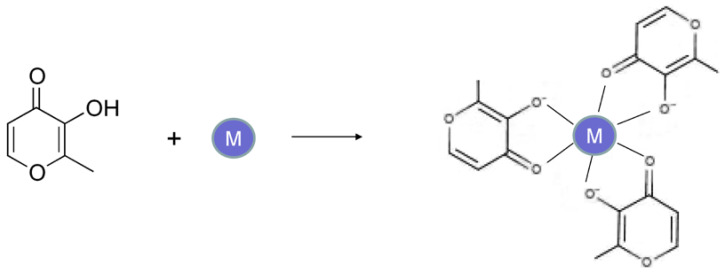
The design and development of L1 and the maltol–iron complex were initiated in the biological chemistry department of the University of Essex, United Kingdom (UK), while working on a hemoglobin project in 1979 [60,64]. The work on both drugs at this time period was partly supported by the British Technology Group (BTG), a British government organization, and the UK Thalassemia Society (UKTS), a patient charity organization [60,64]. As a result of this academic project, many different groups of iron chelators were initially screened in an in vitro system, with disappointing results until the discovery and identification towards the end of the project of the alpha-ketohydroxy (or alpha-oxohydroxy) metal-binding site on an heteroaromatic ring, which led to the discovery of the novel alpha-ketohydroxypyrone and alpha-ketohydroxypyridine class of iron chelators (1978–1981), which were intended for clinical use in diseases related to iron metabolism [60,61,62,63]. The alpha-ketohydroxy metal-binding site on an heteroaromatic ring was previously reported from natural phytochelator products such as mimosine, maltol and tropolone (Figure 1) [60,61,62,63,64,65].
Figure 1.
The design of the alpha-ketohydroxypyridine and alpha-ketohydroxypyrone iron chelators for clinical use. The chemical structures of four classes of iron chelators, namely 3-hydroxypyrid-4-ones (I), 3-hydroxypyrid-2-ones (II), 1-hydroxypyrid-2-ones (III) and 3-hydroxypyr-4-ones (IV), were identified 40 years ago, following a structure–activity simulation based on the prototype structures of the known compounds mimosine (V), tropolone (VI), 1-hydroxypyrid-2-one (VII) and maltol (VIII). Chemical synthesis and iron binding studies led to the development of the drugs deferiprone (L1) and maltol–iron for the treatment of iron overload and iron deficiency anemia, respectively (see [60]).
Based on these prototype structures of alpha-ketohydroxypyrone and alpha-ketohydroxypyridine classes of chelators, about half a dozen new compounds were initially synthesized, including L1, and about a dozen commercially available compounds including maltol were screened in vitro in different models for their iron-binding and other properties [60]. The in vitro screening system included studies on the molecular structure–activity correlation, the air and water stability and solubility of the chelator and its iron complex, the electron-releasing or -withdrawing effects of substituents present on the heteroaromatic ring with regards to iron affinity, the lipid–water partition of the chelator and chelator iron complex and stability of the iron complexes. As a result of the chemical synthesis approach that was used, new chemical synthetic routes were proposed, and also the prospect of the preparation of thousands of new alpha-ketohydroxypyrone and alpha-ketohydroxypyridine compounds with different substituents attached to the heteroaromatic ring was suggested, which could be synthesized and tested using a similar screening system [60] (Figure 1). The synthetic screening proposal included dimeric, trimeric and polymeric chelating drugs, some of which were also synthesized and tested at a later stage [60,66].
Due to lack of different screening facilities at Essex University for investigations related to the chelating compounds, including those for cell and in vivo studies, the developmental program for the iron chelators was partly moved to University College London Medical School (UCLMS) through contacts with the UKTS and associated researchers in UCLMS. In this context, new experimental screening programs were developed and showed that L1 was the most effective chelator for iron mobilization from transferrin and ferritin and also from iron-loaded mice following oral and intraperitoneal administration routes [60]. Similarly, using a different screening program, the maltol–iron complex and other lipophilic iron complexes have been shown to be effective in increasing the transport of iron into red blood cells and inverted rat intestines [60]. The results of these experiments, and especially the effects of L1 and the maltol–iron complex, were not allowed to be published for several years after the discovery, due to BTG “patent” restrictions, to the dismay of the UKTS and other patient organizations.
The situation was particularly urgent and frustrating regarding the development of orally administered L1 in thalassemia, since many patients’ lives were at risk because of complications with the 8–10 h long subcutaneous (sc) administration of DF, including compliance, toxicity and efficacy concerns [45]. In the meantime, another major invention was developed in earlier clinical studies in thalassemia patients supported by the UKTS and initiated at UCLMS using DF suppository formulations, which was published without restrictions [67]. Clinical studies at University College Hospital have shown encouraging preliminary results, with significant increases in urinary iron excretion in thalassemia patients using DF suppository formulations. Furthermore, these clinical studies have also shown the first ever evidence that DF could be absorbed from the rectum, and also that DF was not effective when it was administered orally because of molecular damage in the acid environment of the stomach [67].
3.1. The Pre-Clinical and Clinical Development of Deferiprone
The pressure by thalassemia patients and parents was mounting for the rapid development of oral chelation therapy by the early 1980s. As a result, in 1985, the UKTS sponsored the continuation of the iron chelation project at the Royal Free Hospital School of Medicine (RFHSM) of the University of London from where the first publications of the iron removal effects of L1 in comparison to parenteral DF in mice and rabbits and later in other animal species were reported [68,69,70,71,72,73]. Eventually, many other differences, including those found by academics and funding organizations for oral chelating drug selection and development processes, resulted in fierce competition for the promotion by the UCLMS with the BTG of different oral chelators, and especially lipophilic analogues of L1, in contrast to the selection of L1 at the Royal Free Hospital School of Medicine (RFHSM), which was supported by the UKTS for the treatment of iron overload [74,75,76,77].
The higher levels of safety and efficacy of L1 in different animal species in comparison to many other alpha-ketohydroxypyridines studied at the RFHSM, and several other developments such as the simple inexpensive synthesis of L1 and other alpha-ketohydroxypyridines, prompted the initiation of an application to the UK governmental regulatory authorities for clinical testing [78,79]. The application included the physicochemical characteristics and purity of L1, toxicological studies, drug formulation information and a dose protocol, RFHSM ethical committee approval and personal insurance for volunteers and patients participating in the studies, who were financially covered by the UKTS, along with many other requirements.
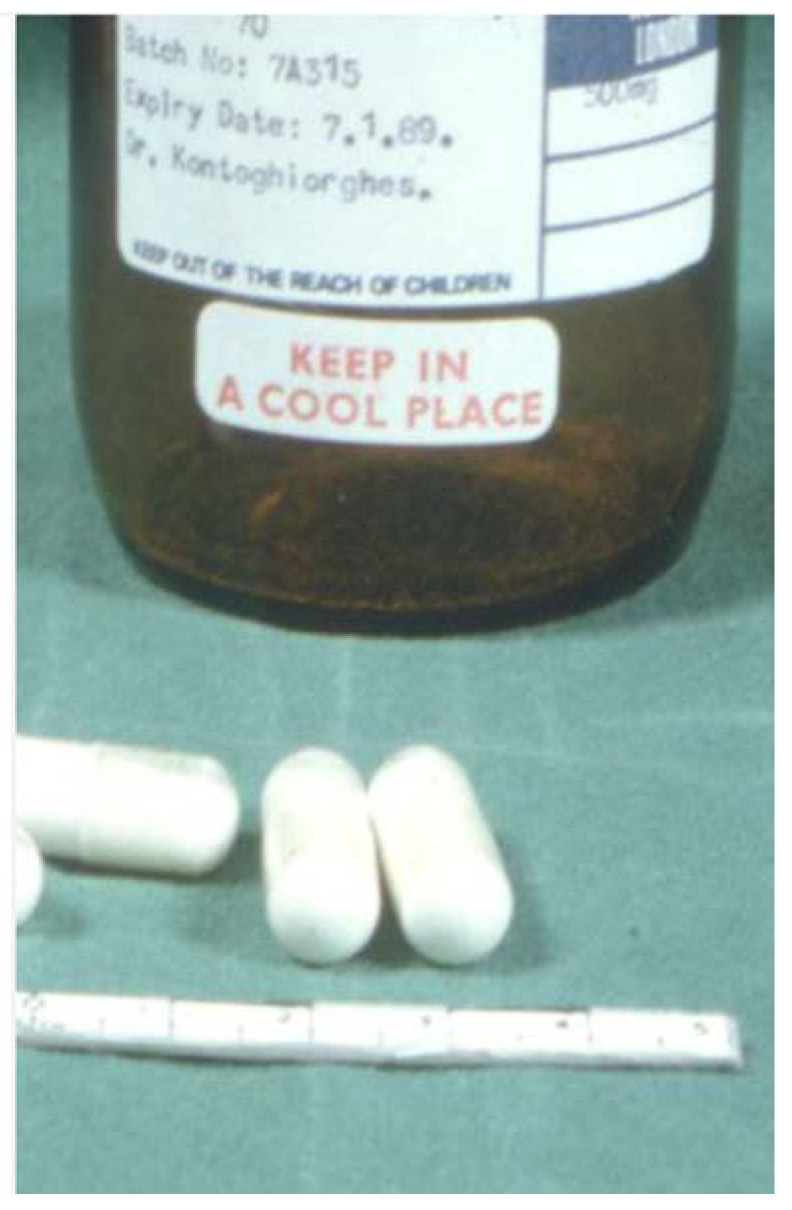
In the meantime, several kilograms of L1 were prepared using the new method of synthesis, which was encapsulated in 0.5 g quantities by the pharmacy of Royal Free Hospital using gelatin capsules (Figure 2). The encapsulation of the white crystalline solid of L1 was necessary because of its bitter taste, which was identified by the inventor [63,78]. Phase I–II clinical trials began using escalating doses of oral L1 (0.5–3.0 g) and iron balance studies in a group of iron-loaded myelodysplacia and thalassemia patients not tolerant to sc DF treatment [80,81]. The results of the clinical studies were encouraging, with increases in urinary iron excretion above the background level, even with low doses of 10 mg/kg, establishing for the first time that iron chelation in thalassemia is possible through the oral route and that L1 was the first oral iron-chelating drug in medicine [82,83]. The drug was well tolerated using different dose protocols, including the maximum dose selected at the time (110 mg/kg/day), which caused equivalent levels of iron excretion to sc DF at equivalent doses, with no increases in other essential metal ion excretion indicators [81].
Figure 2.
The first pharmaceutical formulation of deferiprone (L1) in transparent gelatin capsules, which were used for the first clinical trials in London, UK, and also in multicenter clinical trials that followed worldwide. Each capsule contained 0.5 g of L1 white solid, with no preservatives or additives (see https://www.youtube.com/watch?v=ZcvSLyIgYd8, accessed on 1 March 2023).
In the meantime, fierce competition against the development of L1 was initiated by academic and commercial groups, which began from the time of discovery and continues today for different reasons [60,63]. For example, at the initial stages, a lipophilic analogue of L1 namely 1,2-diethyl-3-hydroxypyrid-4-one (EL1NEt) was promoted by different groups of academics and supported by the BTG via studies at Essex University and UCHMS [74,75,76]. However, based on further studies and clinical trials in thalassemia patients at UCHMS, EL1NEt had a lower therapeutic index than L1 and was later abandoned [77,84,85,86]. Similarly, at an earlier stage, Ciba Geigy (now Novartis), the then manufacturers of DF, obtained all pre-clinical and clinical information on L1 from the UKTS-sponsored academic team with the prospect of its rapid development to benefit patients who were not tolerant to sc DF treatment. Following about 3 years of waiting, Ciba Geigy announced that they had carried out different animal toxicity studies with L1 and finally reported that L1 was toxic and that they cannot be involved in its development [87,88]. However, the evaluation methods used for assessing L1’s toxicity were questioned, as well as the available comparative toxicity data for DF, all of which brought into question Ciba Geigy’s assessment methods and conclusions [89,90,91,92,93]. It should be noted that the LD50 of oral L1 in mice is estimated at 2–3 g/kg and intraperitoneally administered L1 in rats at 650 mg/kg [61,77]
Following the initial clinical trials at the RFHSM with L1, many other academic hospitals caring for thalassemia and other categories of transfusional iron-loaded patients worldwide expressed a great interest in joining the clinical trials and testing L1 in their institutions using the same protocol. In most cases, the vast majority of patients selected for the clinical studies were not responding or were intolerant to sc DF therapy. In many of the external clinical studies, L1 was supplied from the RFHSM (Figure 2) to institutions in other countries, e.g., Italy, Switzerland, The Netherlands and Germany, whereas at a later stage in several other countries, e.g., for India, Switzerland, The Netherlands and Canada, L1 was prepared and supplied by local pharmaceutical companies using the published one-step method of chemical synthesis and physicochemical characterization developed at the RFHSM [78].
While the clinical trials with L1 were continuing and expanding worldwide, an incidence of agranulocytosis was reported in 1989 in a Blackfan–Diamond patient at the RFHSM, causing the postponement of the L1 trials at the RFHSM to the dismay of the UKTS’ investigating team [94]. Several other less serious toxic side effects such as neutropenia, joint and musculoskeletal pains and zinc deficiency were also reported in some patients treated with L1 in the RFHSM and other clinical centers [95,96,97]. However, the low incidence of toxic side effects of L1 reported by other clinical centers, and also the presentation of clinical trial results during the first international conference on oral chelation (ICOC) in 1989 at the RFHSM in London by clinical centers testing L1 worldwide, prompted the re-evaluation and the resumption of the clinical trials of L1 at the RFHSM [79,98,99,100,101,102,103,104].
Despite the encouraging pre-clinical and clinical results with L1, there was insufficient interest from major pharmaceutical companies for its commercial development, mainly because of financial considerations and especially the small number of thalassemia patients that require treatment in developed countries. In the meantime, many steps were taken to further advance the development of L1, including an application by the inventor proposing an international non-proprietary (INN) name for L1 in 1991, which resulted in the adoption by the World Health Organization (WHO) of the INN name deferiprone (WHO drug information list 67, volume 2 of 1992).
Unexpectedly, the major breakthrough in the pharmaceutical development of L1 came from India, which played a leading role in the pharmaceutical development and distribution of the drug in developing countries. This development came about following a collaborative project that was initiated between a parent of a thalassemia patient and member of the Thalassemia Society in Mumbay, India, and the UKTS-sponsored group at the RFHSM. The parent also worked at the Indian generic pharmaceutical company Cipla, which was one of the leading generic drug manufacturers in India [63,95,98]. Information provided by the UKTS-sponsored group working at the RFHSM to Cipla led to the chemical and pharmaceutical preparation of L1 and also the initiation of clinical trials in India [63,95,98]. Following the Indian clinical trials and application to the regulatory authorities, the world-first regulatory approval for L1 was obtained in India and L1 became available to Indian thalassemia patients in 1995, and also to many other thalassemia patients in other countries [65,95,98,105,106].
At about the same time as a BBC TV program highlighting the developments in India, the administration of the RFHSM decided to terminate all research on thalassemia supported by the UKTS, including the research on L1 and chelation therapy in general. This was a major drawback in the ongoing projects of L1, which continued elsewhere in the following years. Similarly, the BTG abandoned the development of all other chelating drugs except L1 and licensed the L1 invention to the generic pharmaceutical company Apotex, Canada, which received regulatory approval from the EU authorities for the use of L1 in 1999, followed by many other countries worldwide and also the USA FDA authorities in 2011, following the intervention of the USA Thalassemia Organization [63,105,107].
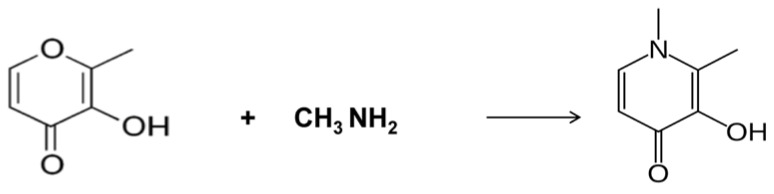
The academic and commercial conflicts regarding the use of L1 continued following the developmental stage and later introduction of DFRA in 2005 by Novartis, which was also the initial and exclusive manufacturer of DF for many years before the introduction of L1 [108,109,110,111]. It should be noted that for both the generic pharmaceutical companies Cipla and Apotex, L1 was the first non-generic drug to be manufactured and distributed in different countries. The regulatory approval obtained in both cases was similar to that of orphan or emergency drug requirements, which was based largely on published animal and clinical data obtained from the UKTS-sponsored research group at the RFHSM [63]. Deferiprone became a generic drug in 2003 and is still manufactured and supplied to thousands of patients by many generic companies worldwide using the published one-step inexpensive chemical synthesis method (Figure 3) [63,78,112].
Figure 3.
The simple one-step synthesis method of deferiprone (L1) from maltol and methylamine, which was invented in the Royal Free Hospital Medical School. The simple, inexpensive preparation of deferiprone (L1) led to the organization of clinical trials in London, UK, and multicenter clinical trials worldwide. The same synthetic method is currently used by generic pharmaceutical companies worldwide, and most importantly made deferiprone (L1) available to patients in developing countries, where thalassemia and other iron loading diseases are endemic (see [78]).
The absence of full formal animal or other pre-clinical toxicology studies in the case of L1 by either Cipla or Apotex pharmaceutical companies has put L1 at a “theoretical disadvantage”, and for that reason it was initially classified as a “second-line” iron-chelating drug in comparison to DF and DFRA [68,69,70,71,72,73,77,87,88,89,90,91,92]. However, animal toxicology data for all three drugs have shown that they all are of differential but similar toxicity levels, and in clinical practice L1 is widely used to the same extent as the other two chelating drugs in iron-loaded patients. Most importantly, L1 is regarded as the first-line iron-chelating drug in many categories of patients because of its unique properties, and especially its superior ability to remove excess cardiac iron, as shown by T2* MRI results, the increased long-term survival of transfusional iron-loaded patients and also because of its safety in patients with low iron loads [53,113,114,115,116,117,118,119]. The safety and efficacy of L1, DF and DFRA in iron-loaded patients have been recently reviewed [120]. All three iron-chelating drugs are now generic and widely used in the treatment of iron overload [121]. There is no general consensus on the use of L1, DF and DFRA or of their combinations, as well as the related protocols in transfusional iron-loaded patients. In most cases, the selection of the chelation therapy and related protocols is generally random and based on subjective criteria and with non-specific aims, and as a result their clinical application vary from country to country and from clinic to clinic [51,113].
With regards to its safety, long-term studies and continuous clinical monitoring involving thousands of thalassemia and other categories of patients for the last 30 years have confirmed the low toxicity levels of L1 [97,120]. The most serious toxic side effects of L1 reported in the last 30 years have included reversible agranulocytosis (<1%) and neutropenia (<5%), while the less serious toxic side effects have included gastric intolerance, joint pains and zinc deficiency [97,120]. Toxicity vigilance and prophylactic measures, such as weekly or fortnightly blood counts, are important factors for ensuring the safety of L1 during long-term use. Similarly, the use of zinc supplements for prophylaxis in patients on long-term treatment with L1 and DF is also recommended [120,121].
Deferiprone’s ability as a monotherapy or in combination with DF to clear all excess iron and maintain normal iron stores using personalized dose protocols in regularly transfused thalassemia major patients signifies the complete treatment of iron overload and the achievement of the major aim of iron chelation therapy (Figure 4) [119,120,121,122,123]. In addition, the continuous worldwide use of L1 in thalassemia and other iron-loaded patients 40 years after its invention and its long-term cardioprotective life-saving effects and other pharmacological properties have changed the prognosis of thalassemia major patients, and in general thalassemia is no longer considered fatal but rather a chronic disease [51,63,119,120,121,122,123].
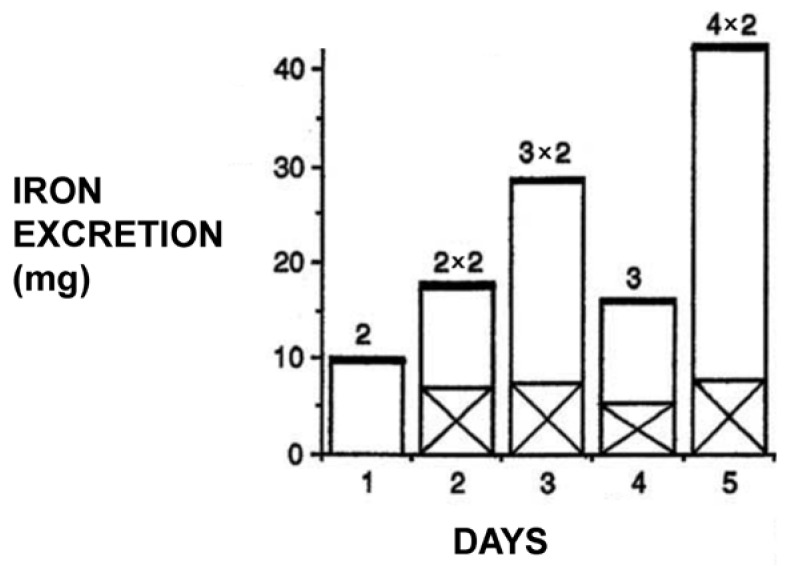
Figure 4.
Iron excretion profile in a thalassemia major patient following the daily administration of different doses of oral deferiprone (L1). Iron excretion (mg) in the urine is shown in the open columns and fecal iron excretion in the x columns. Iron excretion is dose-dependent, as shown from the doses (g) of deferiprone (L1) at the top of the column. It should be noted that almost all excess iron is excreted in the urine (see [81]).
3.2. The Pre-Clinical and Clinical Development of the Maltol–Iron Complex
Unlike the discovery and development of deferiprone, which was regarded as a life-saving drug for thousands of iron-loaded patients, especially in developing countries, the development of the maltol–iron complex was not urgently pursued by pharmaceutical companies. The delay was caused by commercial considerations and especially the availability of a large number of competing iron formulations used for the treatment of IDA, a condition generally considered non-life-threatening [23,26].
The discovery of the maltol–iron complex and its proposed use in IDA originated from the identification in 1979–1982 of the naturally occurring plant product and phytochelator maltol as an alpha-ketohydroxypyrone chelator, which was widely available and used in humans [60]. The selection of the maltol–iron complex for IDA was based on its lipophilic properties and the characterization of many other in vitro and in vivo properties, including the stability of the iron complex, the protein interactions and the ability to transfer iron in red blood cells and inverted intestinal sacs [23,60,65]. Further development of the maltol–iron complex in animal and also clinical studies continued intermittently for many years and by different groups of investigators following its discovery [60,65]. Many other studies are still ongoing for different categories of patients with IDA conditions [65].
Further developmental studies involving the in vivo confirmation of the effects of the maltol–iron complex in relation to IDA were conducted following the original studies and design phase of 1979–1982 [60]. These involved a comparative study in mice using a new screening system of the intragastric administration of different chelator iron (59-Fe) complexes and the monitoring of 59-Fe’s uptake and distribution in the body, as well as its excretion, thereby demonstrating the need for a lipophilic iron complex for IDA [60,124]. In this context, the effect of 16 natural and synthetic chelators on iron (59-Fe) absorption, in addition to maltol, have been investigated in three different experiments using single and repeated intragastric administrations of chelator iron (59-Fe) complexes [124]. Maltol and 2-hydroxy-4-methoxypyridine-1-oxide (L6), both of which form neutral, lipophilic iron complexes, were found to cause significant increases in 59-Fe absorption, while hydrophilic chelators, including DF, L1, mimosine and EDTA, caused significant decreases in iron (59-Fe) absorption in comparison to the controls. Most of the iron (59-Fe) absorbed from the chelator iron (59-Fe) mixtures was detected in red blood cells and less so in the organs, where it was mainly distributed in the liver (50–60%) as compared to the spleen (30–40%) and other organs. These findings have suggested that the iron (59-Fe) absorbed following the oral administration of chelator iron (59-Fe) complexes is diverted primarily to the bone marrow and utilized for the production of hemoglobin [50,124].
In a set of different experiments in rats, increased iron (59-Fe) uptake was observed following the intraduodenal administration of the iron (59-Fe) maltol in comparison to other hydrophilic iron (59-Fe) mixtures with fumarate, gluconate and EDTA [125]. The oral administration and dissociation of iron (59-Fe) from maltol were suggested following its entry into the intestinal wall, with the iron and maltol molecules following separate and different metabolic pathways. In this context, iron from the iron–maltol complex enters the iron metabolism pathway, involving intracellular storage in ferritin, transport in the blood by transferrin and utilization by the hemopoietic tissues for the production of hemoglobin. In the case of dissociated maltol, the metabolic pathway mostly involves glucuronidation in the liver and the formation of maltol glucorunide, which is excreted in the urine [33]. Similarly, the dissociation of maltol and iron was observed following the intravenous administration of an iron (59-Fe)–maltol mixture in rats, presumably through the donation of iron to transferrin and release of maltol in the blood stream [126]. In addition, iron (59-Fe) uptake in rats was mostly associated with hemoglobin production and less so with liver deposition and storage. The overall increased uptake of (59-Fe) iron in the presence of maltol and its utilization by the hemopoietic tissues has been observed in both mice and rats, despite the suggested dissociation of iron and maltol and the kinetic variation in iron uptake rates following the use of different iron concentrations in the mixture [124,125,126].
Despite some initial preliminary interest about the development and commercialization of the maltol–iron complex for IDA by pharmaceutical companies, further animal and clinical studies, including long-term toxicology studies, were not pursued, probably because of the overall expenditure required for a new drug’s development at the time. No further efforts for the pharmaceutical development of the maltol–iron complex were undertaken, and the interest was focused on the pharmacological research aspects of maltol and its iron complex and other metal complexes (Figure 5) [33,60,127]. However, as in many other cases of recent drug development and the introduction of new regulatory approval procedures, a loophole was used whereby the maltol–iron complex was further developed and marketed through the relaxed requirements involving an orphan drug procedure [59]. In this context, several clinical trials of the maltol–iron complex in iron-deficient patients with inflammatory bowel disease and similar diseases were initiated, considering that no effective treatments of IDA were available with other iron formulations for these categories of patients [33].
Figure 5.
The chemical structure and formation reaction of metal ion complexes of maltol. An octahedral metal ion complex of neutral charge is formed at physiological pH between maltol and trivalent metal (M) ions such as iron, gallium, aluminum and indium. Similar trivalent neutral metal ion complexes are formed using deferiprone (L1).
Many categories of patients have received the maltol–iron complex in a number of clinical studies, including for Crohn’s disease, ulcerative colitis, chronic kidney disease, pulmonary hypertension and also patients intolerant to other iron formulations [128,129,130,131,132,133,134,135,136,137,138,139]. Similarly, the maltol–iron complex was used in clinical pharmacokinetic and comparative studies with other iron formulations. The clinical and pharmacological effects of the maltol–iron complex and other metal complexes were recently reviewed (Figure 5) [33,127]. In general, the maltol–iron complex containing at least 30 mg iron could be effective if taken for a week to three months (twice daily, before breakfast and the evening meal). In such clinical cases, the serum iron, transferrin saturation, serum ferritin and reticulocyte hemoglobin contents increase significantly within a week to higher levels in comparison to other ferric formulations and are also equivalent to those of the widely used ferrous sulfate formulations. Furthermore, the level of iron absorption appears to be proportional to the dose levels of maltol–iron, and despite iron being utilized for hemoglobin synthesis and storage, maltol is cleared from the body, with no sign of accumulation in any in the patients of the different categories that have received the maltol–iron formulation [128,129,130,131,132,133,134,135,136,137,138,139].
There are several other advantages in the use of the maltol–iron complex over other iron formulations, including rapid iron absorption, low toxicity and higher selectivity in iron delivery in comparison to other iron formulations. These advantageous results, cause a decrease in the toxicity associated with the excess non-absorbable iron, which is usually observed with other iron formulations. In addition, the efficacy and low toxicity of the maltol–iron complex could benefit many patients experiencing complications and toxicity with other iron formulations, including intravenous iron and ferrous sulphate [30,36,37,38,130,131,140,141,142,143].
The level of safety of the maltol–iron complex appears in general to be satisfactory. This observation has also been reported in almost all of the clinical studies and trials, whereby the maltol–iron complex has been shown to be tolerable and effective, with significant improvements in anemia for the different categories of patients treated. In this context, the most common adverse effect during the clinical studies was mild gastrointestinal intolerance [130,131].
Overall, the maltol–iron complex may have many general advantages over other iron formulations for the treatment of IDA, which include the ease of administration, low toxicity, short timing for achieving the normalization of hemoglobin levels and low cost. In particular, the low cost of maltol–iron formulations and their general availability are major concerns affecting global health, considering that the vast majority of IDA patients live in developing countries with low health budgets. Furthermore, the low costs of maltol and iron may encourage the development of many different maltol–iron formulations, which could overturn any proprietary and other claims similar to generic pharmaceuticals [59,144,145,146]. Due to the increased availability of low-cost maltol–iron complex formulations, they have prospects of becoming the first option for the worldwide treatment of iron-deficient patients.
4. New Clinical Applications of Deferiprone and the Maltol–Iron Complex
While the major efforts in the development of L1 and the maltol–iron were based on the treatment of transfusional iron overload and IDA, respectively, their possible applications in other diseases were also suggested and tested at the early stages of their development, thereby contributing important information in the overall pharmacology and toxicology of both of these two drugs.
The additional fields of investigation related to these two drugs were broadly based on other diseases of iron metabolism, infectious diseases, conditions related to free radical pathology and metal toxicity conditions other than for iron. In the meantime, many other analogues of L1 and iron–maltol were synthesized and tested for comparison with the original findings. The investigations included in vitro, in vivo and clinical studies.
4.1. The Use of Deferiprone in Transfusional Iron Overload and Other Diseases
Thousands of patients suffering from iron overload and also other illnesses, as well as normal volunteers, have been treated in the last 40 years with L1, which is regarded as one of the safest drugs in the world per dose, e.g., 50–100 mg/kg received on a daily basis. In this context, the major category of patients receiving L1 is β-thalassemia major patients, followed by many other categories of iron-loaded patients, including β-thalassemia intermedia, HbE β-thalassemia, HbS β-thalassemia, sickle cell anemia, myelodysplastic syndrome, aplastic anemia, Fanconi’s anemia, Blackfan–Diamond anemia, pyruvate kinase deficiency, idiopathic hemochromatosis, iron overload in hemodialysis, and juvenile hemochromatosis [51,53,54,104,147,148,149,150,151,152,153,154].
Similarly, many other categories of patients with normal iron stores have also been receiving L1, mostly in short- but also in long-term clinical trials. The repurposing of L1 for diseases other than iron overload was organized based on similar dose protocols to those of iron overload and took place originally in the in the UK (RFHSM) but also other countries within a few years of initiating the clinical trials with transfusional iron-loaded thalassemia and myelodysplasia patients [80,81,82,83]. The clinical use of L1 in several other categories of patients with normal iron stores has increased with time and involved many other different dose protocols, durations of administration and numbers of patients in each category. The decision to use L1 in these conditions was in most cases related to the absence of other effective therapies. Some of the categories of diseases reported to have received L1 include renal dialysis, aluminum overload, Friedreich’s ataxia, Parkinson’s and Alzheimer’s diseases, neurodegeneration with brain iron accumulation, pantothenate kinase 2-associated neurodegeneration (PKAN), rheumatoid arthritis, aceruloplasminemia, glomerulonephritis and diabetic nephropathy, malaria and HIV [155,156,157,158,159,160,161,162,163,164,165,166].
In general, no serious toxicity was reported for clinical trials of up to 9 months duration and with a maximum dose of 100 mg/kg/day in patients with normal iron stores. Most importantly, significant clinical improvements have been reported in most of these categories of patients [155,156,157,158,159,160,161,162,163,164,165,166]. Positive outcomes have also been reported, where L1 was used in adjuvant and combination therapies, which was similar to iron-loaded cases [164,167].
However, it is unfortunate that in many of the recent clinical trials involving mainly neurodegenerative disease patients, no rationale for the selection of the L1 dose protocol nor of iron metabolism balance studies was given [158,159,160]. For example, in many such studies, L1 was used in single or repeated doses of 15 mg/kg/day with disappointing results [158,159,160]. It should be noted that previous dose escalation and iron metabolism balance studies have shown that the use of such low doses of L1 in iron-loaded and non-iron-loaded conditions was mostly ineffective in increasing iron excretion or the improvement of other hematological parameters [80,81,167,168]. The selection of posology is critical for evaluating the risk/benefit assessment of L1 and all other drugs. Furthermore, the rationale for the dose protocol selection for the use of L1 and all other drugs in any disease should be accompanied by pharmacological and therapeutic evidence such as the estimation of the pharmacokinetic, metabolic and other parameters and the critical drug concentration for optimal therapeutic activity, as previously shown for the use of L1 in thalassemia patients (Figure 6 and Figure 7) [80,81,169,170].
Figure 6.
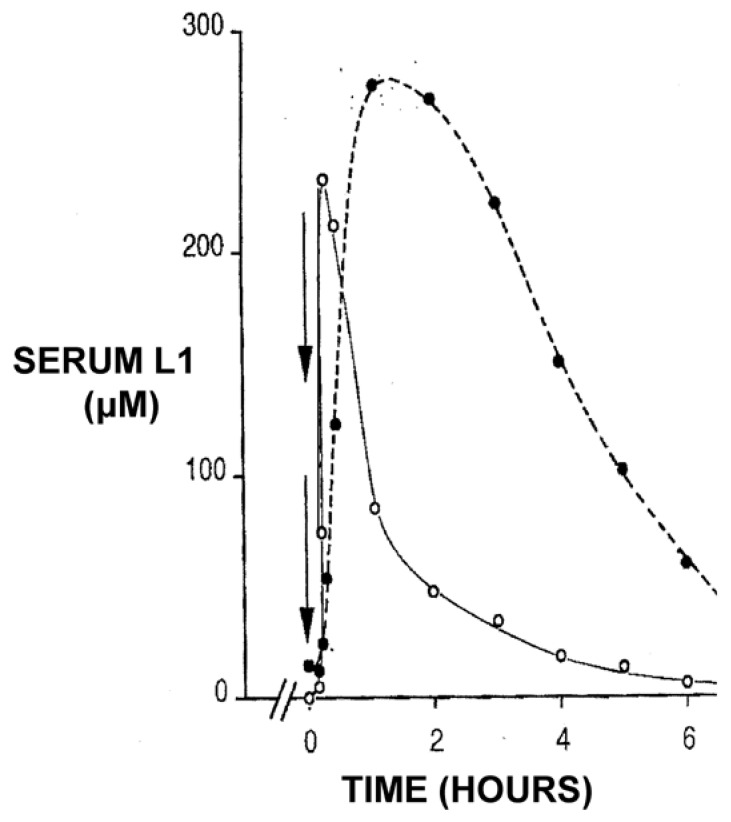
Pharmacokinetic profile of deferiprone (L1) and its glucuronide metabolite conjugate following its oral administration in a thalassemia patient. Deferiprone (L1) 3 g was administered at time 0, which was rapidly absorbed (open circles), peaked within one hour and cleared from the blood in about 6 h. The blood clearance rate of the glucuronide metabolite conjugate (black circles) was slower, as monitored by measuring the absorbance at 280 nm (see [107,170]).
Figure 7.
The sequential urinary iron excretion profile following intensive chelation therapy with deferiprone (L1) in a heavily iron-loaded thalassemia patient. Divided doses of L1 to a total of 250 mg/kg were administered in the thalassemia patient and sequential urine samples were collected over 24 h, totaling 325 mg of iron (see [169]).
It can be suggested in general that the positive results in terms of safety and the diverse categories of iron-loaded and other patients treated in clinical trials with L1 have increased the prospects of the wider use of the drug in many other diseases, including diseases related to free radical pathology and also in cancer [171,172,173].
4.2. The Use of Maltol–Metal Complexes in Diseases Other Than Iron Deficiency Anemia
The identification of the possible applications of maltol and the maltol–iron complex in medicine was proposed following their physicochemical characterization, protein and cellular interactions and in vivo and other studies [60]. Within this context, many investigations have been carried out on the possibility of the clinical use of metal complexes of maltol other than iron, such as zinc complexes as supplements for zinc deficiency, maltol–gallium complexes for the treatment of cancer, maltol complexes with diagnostic metals such as indium for use in clinical diagnosis and maltol complexes with theranostic metals such as (68-Ga) gallate (Figure 5) [33,60].
The maltol–gallium complex (gallium maltolate or maltol gallate) has attracted a lot of interest because it has reached the stage of clinical trials and veterinary use, mainly for its anticancer and antimicrobial activities [174,175,176,177,178,179,180,181]. The basic mode of action in relation to the anticancer and antimicrobial targeting of the maltol–gallium complex is the disruption of iron metabolism pathways by mimicking iron. In particular, the antimicrobial activity of the maltol–gallium complex is thought to cause the partial deprivation of iron, which is essential for the rapid growth of pathogenic microbes [178,179,180,181,182,183,184]. Similarly, the anticancer activity of the maltol–gallium complex is thought to be based on the delivery of gallium to transferrin, causing a reduction in iron uptake by the cancer cells and a reduction in their growth through the slow turnover of the iron-dependent enzyme ribonucleotide reductase, resulting in the inhibition of DNA synthesis [19,185,186,187]. This mechanism of cancer cell inhibition mainly affects cancer types such as breast, prostate and bladder cancers and leukemias, which involve an abundance of transferrin receptors and the upregulated production of ribonucleotide reductase [18,19,185,186,187,188]. A further mechanism of DNA inhibition by the maltol–gallium complex is thought to involve the transfer and incorporation or binding of gallium to nucleotide substrates and subsequently reduced DNA synthesis [19].
Another major area of application to medicine in relation to iron and other metal complexes of maltol is their use in the theranostic and diagnostic fields, which are rapidly expanding, involving many diseases [19,189,190,191,192]. The maltol complexes involving different metal ions have variable physicochemical and biochemical properties, which appear to affect the bodily distribution of the metal ions and radiotracers, similar to other metal chelator complexes [19,189,190,191,192,193,194,195]. Targeted chelator metal complexes have been identified for the diagnostic and theranostic application of radiotracer metals in cancer, inflammation and other diseases (Figure 5) [19,193,194,195]. In clinical studies in hepatocellular carcinoma patients, for example, cancer cells have been found to be highly gallium-avid in (67)Ga diagnostic scans. In this context, treatment with an oral maltol–gallium complex caused significant improvements of cancer indices and necrosis of the tumor [175].
The increased number of applications of maltol–metal complexes in medicine and veterinary medicine, as well as the pharmacological and toxicological information obtained from relevant studies, has increased the prospect of their wider use in many other fields of diagnostic and theranostic medicine [196,197,198,199]
5. Future Prospects in the Use of Deferiprone and the Maltol–Iron Complex
There are increasing prospects for the wider application of L1 and the maltol–iron complex in medicine, including for the optimization of existing therapeutic applications involving improved dose protocols, as well as combinations with other drugs or natural products, personalized medicine adjustments, the treatment of more and different clinical conditions and also more diagnostic and theranostic applications. In particular, there are many metabolic pathways and associated diseases that can further be investigated and targeted, with some involving iron interactions and free radical pathologies, many diseases related to metal metabolic pathways and toxicity other than iron, metal supplementation in essential metal ion deficiency diseases and new drug formulations and routes of administration. Many of these new applications have been suggested from relevant in vitro and in vivo studies and also following new clinical evidence [20,33,63,173,186].
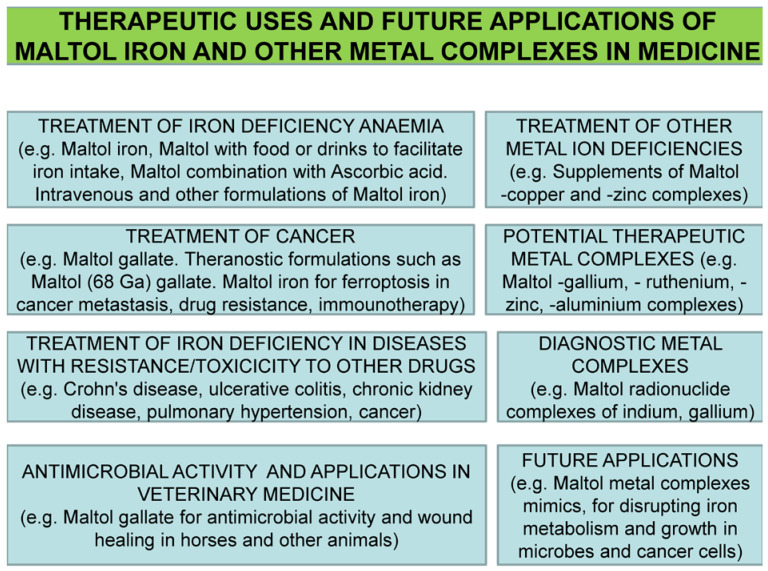
The emphasis on new clinical applications of many drugs, including both L1 and the maltol–iron complex, is usually related to orphan diseases and diseases with no effective treatments [59]. In this context, an important application for L1 and the maltol–metal complexes is the anticancer activity of newly identified cancer targets and metabolic pathways in one or more cancer types, which are in addition and not directly related to previously established and discussed targets (Figure 8) [59,173,186].
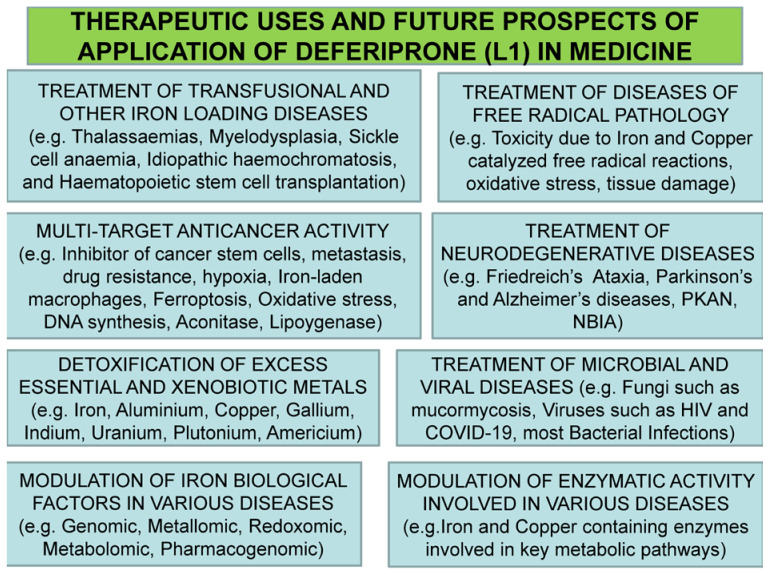
Figure 8.
Ongoing therapeutic uses and future investigations of deferiprone (L1) in medicine. Deferiprone (L1) is widely used in the treatment of iron overload worldwide and also other conditions where the present treatments appear to be ineffective. The wide range of therapeutic application prospects is related to its safety profile and also the access and distribution in almost all organs at therapeutic concentrations. PKAN: pantothenate-kinase-associated neurodegeneration; NBIA: neurodegeneration with brain iron accumulation.
Examples of previously established cancer targets include the classical restriction or reduction of the iron supply, inhibition of transferrin iron delivery and inhibition of ribonucleotide reductase in DNA synthesis, the high antioxidant potential by inhibiting free radical formation etc., [18,173,182,186,200,201,202,203,204,205,206,207]. The new anticancer strategies involving the new anticancer targets for L1 include the modulation of ferroptosis, ferritin iron removal and the control of hyperferritinemia, the inhibition of hypoxia related to the role of hypoxia-inducible factor (HIF), the modulation of the function of new molecular species such as the metalloreductase “six transmembrane epithelial antigen of prostate, family member 4 protein” (STEAP4) and the metastasis suppressor N-MYC downstream-regulated gene-1 (NDRG1), the modulation of metabolic pathways of oxidative stress damage affecting mitochondrial function and the removal of the excess iron from iron-laden macrophages [173,208,209,210,211,212,213,214,215,216,217,218,219,220,221]. In general, these iron metabolism pathways and other targets appear to affect all stages and types of cancer progression and to be modulated by L1, which was suggested as one of the most potent EMA- and FDA-approved drugs for targeting cancer stem cells and a potential anticancer drug for breast, prostate, glioblastoma and other cancers [173,222,223,224].
In contrast to the mode of action of L1 in the above targets, lipophilic chelator metal complexes similar to maltol–iron and also other more lipophilic iron complexes have been proposed for the induction of ferroptosis in cancer cells, especially in cases of refractory or recurring tumors, and also in cases of drug resistance and metastasis (Figure 9) [173,188,204]. It should be noted that about 90% of the cancer patients develop resistance to existing drugs, and also that about 90% of the mortality in cancer patients is related to metastasis [173,225,226]. Overall, opposing modes of action of anticancer activity are shown by L1 and lipophilic iron complexes, which can be utilized for targeting different stages and types of cancer [173,205,227,228,229].
Figure 9.
Ongoing therapeutic uses of maltol–iron in iron deficiency anemia and future applications of maltol–metal complexes in other fields of medicine. Many categories of patients may benefit from iron and other maltol–metal complexes. The wide range of therapeutic application prospects is related to the safety profile of the maltol–metal complexes.
The prospect of the application of L1 in infectious, inflammatory and many other diseases in addition to cancer has been previously reviewed [19,20,183,184]. However, new findings and related concepts are developing, which constitute new pharmacological targets for L1 and other chelators or chelator iron complexes, including maltol–iron. For example, common targets for chelators are hyperferritinemia and iron-laden macrophages, which are found in cancer and inflammatory and infectious diseases, including COVID-19 [173,230]. In particular, with regards to the modes of action of L1 in COVID-19, these include antiviral, antimicrobial, antioxidant, antihypoxic and antiferroptotic effects, as well as iron-mobilizing effects from ferritin, macrophages and other cells involved in the immune response and hyperinflammation. Some of the pharmacological and other characteristics of L1, including its extensive tissue distribution, could also facilitate its use in the treatment of COVID-19 through drug combinations, adjuvant therapies and disease prevention.
In general, the characteristics of the targets can impose many limitations in the selection of therapeutics, including chelating drugs for the optimization of treatments. For example, only L1 from all three iron-chelating drugs (L1, DF, DFRA) can cross the blood–brain barrier and target infections in the brain, such as meningitis caused by Neisseria meningitides or cancer types such as glioblastoma, both of which depend on iron supply for growth [19,20,173].
There are many other areas of potential development and applications of L1 and maltol–iron complexes. For example, intravenous L1 and maltol–iron complex formulations may have to be designed to benefit cancer and other categories of patients unable to receive oral formulations [34,40,185,231,232,233,234,235]. Similarly, combinations with other drugs may enhance the therapeutic efficacy in comparison to monotherapies in many diseases [81,234,235,236]. This was shown in the treatment of iron overload in thalassemia using the ICOC L1/DF combination, and similar effects are expected in terms of gastrointestinal iron uptake for the treatment of IDA using mixed chelator iron complexes of maltol and ascorbate [33,81,234,235,236,237,238,239]. Further developments are expected from the use of other metal complexes, which may result in the improvement of the diagnostic and theranostic applications, as well as in synergistic effects with other drugs or proteins in the treatment of different diseases [20,33,240,241,242,243,244,245,246,247,248,249,250,251,252,253,254,255,256,257,258,259]. In contrast, L1 can be used for the detoxification of many xenobiotic metals, including those used in diagnostic and theranostic applications [195,260,261,262]. Furthermore, improved formulations may increase the efficacy and decrease the toxicity of L1 and the maltol–iron complex in different clinical conditions [263]. In each of the above-described cases, specific conditions must be applied and specific protocols need to be designed to achieve optimal therapeutic activity and effects.
6. Conclusions
Despite the many difficulties and controversies, L1 and the maltol–iron complex are prime examples of academic drug discovery and orphan drug development medicinal products. In particular, L1 has helped in the treatment and survival of hundreds of thousands of iron-loaded and other patients worldwide in the past 30 years. Similar results are expected from the use of the recently approved maltol–iron complex for the treatment of IDA, which affects 1 in 3 or 4 individuals worldwide.
The repurposing of L1 in many categories of non-iron-loaded patients, including neurodegenerative, infectious, inflammatory, metal toxicity and other conditions, signifies the safety and multitargeting potential of the drug. Similarly, the use of the maltol–gallium complex in cancer and also of other maltol–metal complexes in other therapeutic, theranostic and diagnostic applications highlights the future routes of development and use of new therapeutic, theranostic and diagnostic maltol and other chelator metal complexes.
New advancements in the pathology of free radicals, cancers and many other diseases, including the discovery of new iron and other metal metabolic routes and proteins that can be modulated by L1, maltol–iron and also other new chelators and metal complexes, underlines the importance of chelators and chelator metal complexes in medicine. In particular, their use as monotherapies or in combinations with other drugs is essential for the improvement of the treatment of many diseases and increased prospects of patients’ survival, especially in many diseases with no effective therapies.
Acknowledgments
The study was supported from internal funds of the Postgraduate Research Institute of Science, Technology, the Environment and Medicine, a non-profit, charitable organization. I thank Christina N. Kontoghiorghe for the comments.
Abbreviations
| EMA | European Medicines Agency |
| COVID-19 | coronavirus disease 2019 |
| DF | deferoxamine |
| DFRA | deferasirox |
| EDTA | ethylenediaminetriacetic acid |
| FDA | Food and Drug Administration (USA) |
| HIF | Hypoxia-inducible factor |
| ICOC | International Committee on Chelation |
| IDA | iron deficiency anemia |
| L1 | deferiprone |
| MRI | magnetic resonance imaging |
| NDRG | 1N-MYC downstream-regulated gene-1 |
| NBIA | neurodegeneration with brain iron accumulation |
| PKAN | Pantothenate-kinase-associated neurodegeneration |
| STEAP4 | six transmembrane epithelial antigen of prostate, family member 4 protein |
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The author declares no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Katsarou A., Pantopoulos K. Basics and principles of cellular and systemic iron homeostasis. Mol. Asp. Med. 2020;75:100866. doi: 10.1016/j.mam.2020.100866. [DOI] [PubMed] [Google Scholar]
- 2.Gozzelino R., Arosio P. Iron Homeostasis in Health and Disease. Int. J. Mol. Sci. 2016;17:E130. doi: 10.3390/ijms17010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cairo G., Bernuzzi F., Recalcati S. A precious metal: Iron, an essential nutrient for all cells. Genes Nutr. 2006;1:25–39. doi: 10.1007/BF02829934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prasad A.S. Zinc: An overview. Nutrition. 1995;11:93–99. [PubMed] [Google Scholar]
- 5.Coleman J.E. Zinc proteins: Enzymes, storage proteins, transcription factors, and replication proteins. Annu. Rev. Biochem. 1992;61:897–946. doi: 10.1146/annurev.bi.61.070192.004341. [DOI] [PubMed] [Google Scholar]
- 6.Prasad A.S. Zinc deficiency. BMJ. 2003;326:409–410. doi: 10.1136/bmj.326.7386.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daniel K.G., Harbach R.H., Guida W.C., Dou Q.P. Copper storage diseases: Menkes, Wilson’s, and Cancer. Front. Biosci. 2004;9:2652–2662. doi: 10.2741/1424. [DOI] [PubMed] [Google Scholar]
- 8.Gomme P.T., McCann K.B., Bertolini J. Transferrin: Structure, function and potential therapeutic actions. Drug Discov. Today. 2005;10:267–273. doi: 10.1016/S1359-6446(04)03333-1. [DOI] [PubMed] [Google Scholar]
- 9.Pantopoulos K. TfR2 links iron metabolism and erythropoiesis. Blood. 2015;125:1055–1056. doi: 10.1182/blood-2014-12-617571. [DOI] [PubMed] [Google Scholar]
- 10.Makey D.G., Seal U.S. The detection of four molecular forms of human transferrin during the iron binding process. Biochim. Biophys. Acta Protein Struct. 1976;453:250–256. doi: 10.1016/0005-2795(76)90270-1. [DOI] [PubMed] [Google Scholar]
- 11.Sargent P.J., Farnaud S., Evans R.W. Structure/Function Overview of Proteins Involved in Iron Storage and Transport. Curr. Med. Chem. 2005;12:2683–2693. doi: 10.2174/092986705774462969. [DOI] [PubMed] [Google Scholar]
- 12.Arosio P., Elia L., Poli M. Ferritin, cellular iron storage and regulation. IUBMB Life. 2017;69:414–422. doi: 10.1002/iub.1621. [DOI] [PubMed] [Google Scholar]
- 13.Theil E.C. Ferritin: The Protein Nanocage and Iron Biomineral in Health and in Disease. Inorg. Chem. 2013;52:12223–12233. doi: 10.1021/ic400484n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Iancu T.C. Ferritin and hemosiderin in pathological tissues. Electron Microsc. Rev. 1992;5:209–229. doi: 10.1016/0892-0354(92)90011-E. [DOI] [PubMed] [Google Scholar]
- 15.Mehlenbacher M., Poli M., Arosio P., Santambrogio P., Levi S., Chasteen N.D., Bou-Abdallah F. Iron Oxidation and Core Formation in Recombinant Heteropolymeric Human Ferritins. Biochemistry. 2017;56:3900–3912. doi: 10.1021/acs.biochem.7b00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito H. Storage Iron Turnover from a New Perspective. Acta Haematol. 2019;141:201–208. doi: 10.1159/000496324. [DOI] [PubMed] [Google Scholar]
- 17.Kawabata H. Transferrin and transferrin receptors update. Free Radic. Biol. Med. 2019;133:46–54. doi: 10.1016/j.freeradbiomed.2018.06.037. [DOI] [PubMed] [Google Scholar]
- 18.Richardson D.R. Molecular Mechanisms of Iron Uptake by Cells and the Use of Iron Chelators for the Treatment of Cancer. Curr. Med. Chem. 2005;12:2711–2729. doi: 10.2174/092986705774462996. [DOI] [PubMed] [Google Scholar]
- 19.Kontoghiorghe C.N., Kolnagou A., Kontoghiorghes G.J. Potential clinical applications of chelating drugs in diseases targeting transferrin-bound iron and other metals. Expert Opin. Investig. Drugs. 2013;22:591–618. doi: 10.1517/13543784.2013.787408. [DOI] [PubMed] [Google Scholar]
- 20.Kontoghiorghes G.J., Kontoghiorghe C.N. Iron and Chelation in Biochemistry and Medicine: New Approaches to Controlling Iron Metabolism and Treating Related Diseases. Cells. 2020;9:1456. doi: 10.3390/cells9061456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999;341:1986–1995. doi: 10.1056/NEJM199912233412607. [DOI] [PubMed] [Google Scholar]
- 22.McLean E., Cogswell M., Egli I., Wojdyla D., De Benoist B. Worldwide prevalence of anaemia, WHO Vitamin and Mineral Nutrition Information System, 1993–2005. Public Health Nutr. 2009;12:444–454. doi: 10.1017/S1368980008002401. [DOI] [PubMed] [Google Scholar]
- 23.Pasricha S.-R., Tye-Din J., Muckenthaler M.U., Swinkels D.W. Iron deficiency. Lancet. 2021;397:233–248. doi: 10.1016/S0140-6736(20)32594-0. [DOI] [PubMed] [Google Scholar]
- 24.Tardy A.-L., Pouteau E., Marquez D., Yilmaz C., Scholey A. Vitamins and Minerals for Energy, Fatigue and Cognition: A Narrative Review of the Biochemical and Clinical Evidence. Nutrients. 2020;12:228. doi: 10.3390/nu12010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moustarah F., Mohiuddin S.S. Dietary Iron. StatPearls Publishing; Treasure Island, FL, USA: 2020. [Google Scholar]
- 26.Chandra J. Treating Iron Deficiency Anemia. Indian J. Pediatr. 2019;86:1085–1086. doi: 10.1007/s12098-019-03107-y. [DOI] [PubMed] [Google Scholar]
- 27.Węgier L.P., Kubiak M., Liebert A., Clavel T., Montagne A., Stennevin A., Roye S., Boudribila A. Ferrous sulfate oral solution in young children with iron deficiency anemia: An open-label trial of efficacy, safety, and acceptability. Pediatr. Int. 2020;62:820–827. doi: 10.1111/ped.14237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valenzuela C., Olivares M., Brito A., Hamilton-West C., Pizarro F. Is a 40% Absorption of Iron from a Ferrous ascorbate Reference Dose Appropriate to Assess Iron Absorption Independent of Iron Status? Biol. Trace Elem. Res. 2013;155:322–326. doi: 10.1007/s12011-013-9797-2. [DOI] [PubMed] [Google Scholar]
- 29.Patil P., Geevarghese P., Khaire P., Joshi T., Suryawanshi A., Mundada S., Pawar S., Farookh A. Comparison of Therapeutic Efficacy of Ferrous Ascorbate and Iron Polymaltose Complex in Iron Deficiency Anemia in Children: A Randomized Controlled Trial. Indian J. Pediatr. 2019;86:1112–1117. doi: 10.1007/s12098-019-03068-2. [DOI] [PubMed] [Google Scholar]
- 30.Liabeuf S., Gras V., Moragny J., Laroche M.-L., Andréjak M. Ulceration of the oral mucosa following direct contact with ferrous sulfate in elderly patients: A case report and a review of the French National Pharmacovigilance Database. Clin. Interv. Aging. 2014;9:737–740. doi: 10.2147/CIA.S58394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolkien Z., Stecher L., Mander A.P., Pereira D.I., Powell J.J. Ferrous sulfate supplementation causes significant gastro-intestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE. 2015;10:e0117383. doi: 10.1371/journal.pone.0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Campbell N.R., Hasinoff B.B. Iron supplements: A common cause of drug interactions. Br. J. Clin. Pharmacol. 1991;31:251–255. doi: 10.1111/j.1365-2125.1991.tb05525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kontoghiorghes G., Kolnagou A., Demetriou T., Neocleous M., Kontoghiorghe C. New Era in the Treatment of Iron Deficiency Anaemia Using Trimaltol Iron and Other Lipophilic Iron Chelator Complexes: Historical Perspectives of Discovery and Future Applications. Int. J. Mol. Sci. 2021;22:5546. doi: 10.3390/ijms22115546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Manz D.H., Blanchette N.L., Paul B.T., Torti F.M., Torti S.V. Iron and cancer: Recent insights. Ann. N. Y. Acad. Sci. 2016;1368:149–161. doi: 10.1111/nyas.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrari P., Nicolini A., Manca M.L., Rossi G., Anselmi L., Conte M., Carpi A., Bonino F. Treatment of mild non-chemotherapy-induced iron deficiency anemia in cancer patients: Comparison between oral ferrous bisglycinate chelate and ferrous sulfate. Biomed. Pharm. 2012;66:414–418. doi: 10.1016/j.biopha.2012.06.003. [DOI] [PubMed] [Google Scholar]
- 36.Punj S., Ghafourian K., Ardehali H. Iron deficiency and supplementation in heart failure and chronic kidney disease. Mol. Asp. Med. 2020;75:100873. doi: 10.1016/j.mam.2020.100873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kshirsagar A.V., Li X. Long-Term Risks of Intravenous Iron in End-Stage Renal Disease Patients. Adv. Chronic Kidney Dis. 2019;26:292–297. doi: 10.1053/j.ackd.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 38.Nataatmadja M.S., Francis R. Recurrent severe hypophosphatemia following intravenous iron administration. Clin. Case Rep. 2020;8:243–246. doi: 10.1002/ccr3.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rund D. Intravenous iron: Do we adequately understand the short- and long-term risks in clinical practice? Br. J. Haematol. 2020;193:466–480. doi: 10.1111/bjh.17202. [DOI] [PubMed] [Google Scholar]
- 40.Auerbach M., Gafter-Gvili A., Macdougall I.C. Intravenous iron: A framework for changing the management of iron deficiency. Lancet Haematol. 2020;7:e342–e350. doi: 10.1016/S2352-3026(19)30264-9. [DOI] [PubMed] [Google Scholar]
- 41.Mantadakis E., Chatzimichael E., Zikidou P. Iron Deficiency Anemia in Children Residing in High and Low-Income Countries: Risk Factors, Prevention, Diagnosis and Therapy. Mediterr. J. Hematol. Infect. Dis. 2020;12:e2020041. doi: 10.4084/mjhid.2020.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grover K., Kumar T., Doda A., Bhutani R., Yadav S., Kaushal P., Kapoor R., Sharma S. Prevalence of anaemia and its association with dietary habits among pregnant women in the urban area of Haryana. J. Fam. Med. Prim. Care. 2020;9:783–787. doi: 10.4103/jfmpc.jfmpc_1062_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anonymous. Community control of hereditary anaemias: Memorandum from a WHO meeting. Bull. World Health Organ. 1983;61:63–80. [PMC free article] [PubMed] [Google Scholar]
- 44.Weatherall D.J., Clegg J.B. Inherited haemoglobin disorders: An increasing global health problem. Bull. World Health Organ. 2001;79:704–712. [PMC free article] [PubMed] [Google Scholar]
- 45.Modell B., Berdoukas V. The Clinical Approach to Thalassaemia. Grune and Stratton; New York, NY, USA: 1984. pp. 165–169. [Google Scholar]
- 46.Barton J.C., Edwards C.Q., editors. Hemochromatosis: Genetics, Pathophysiology, Diagnosis and Treatment. Cambridge University Press; Cambridge, UK: 2000. [Google Scholar]
- 47.Feder J.N., Gnirke A., Thomas W., Tsuchihashi Z., Ruddy D.A., Basava A., Dormishian F., Domingo R., Jr., Ellis M.C., Fullan A., et al. A novel MHC class I–like gene is mutated in patients with hereditary haemochromatosis. Nat. Genet. 1996;13:399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 48.Pietrangelo A. Hereditary Hemochromatosis—A New Look at an Old Disease. N. Engl. J. Med. 2004;350:2383–2397. doi: 10.1056/NEJMra031573. [DOI] [PubMed] [Google Scholar]
- 49.Dubois S., Kowdley K.V. Targeted screening for hereditary haemochromatosis in high-risk groups. Aliment. Pharmacol. Ther. 2004;20:1–14. doi: 10.1111/j.1365-2036.2004.02024.x. [DOI] [PubMed] [Google Scholar]
- 50.Verma I.C. Burden of genetic disorders in india. Indian J. Pediatr. 2000;67:893–898. doi: 10.1007/BF02723953. [DOI] [PubMed] [Google Scholar]
- 51.Kolnagou A., Kontoghiorghe C.N., Kontoghiorghes G.J. Transition of Thalassaemia and Friedreich ataxia from fatal to chronic diseases. World J. Methodol. 2014;4:197–218. doi: 10.5662/wjm.v4.i4.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kontoghiorghes G.J., Eracleous E., Economides C., Kolnagou A. Advances in Iron Overload Therapies. Prospects for Effective Use of Deferiprone (L1), Deferoxamine, the New Experimental Chelators ICL670, GT56-252, L1NAll and their Combinations. Curr. Med. Chem. 2005;12:2663–2681. doi: 10.2174/092986705774463003. [DOI] [PubMed] [Google Scholar]
- 53.Telfer P.T., Warburton F., Christou S., Hadjigavriel M., Sitarou M., Kolnagou A., Angastiniotis M. Improved survival in thalassemia major patients on switching from desferrioxamine to combined chelation therapy with desferrioxamine and deferiprone. Haematologica. 2009;94:1777–1778. doi: 10.3324/haematol.2009.009118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tanner M.A., Galanello R., Dessi C., Smith G.C., Westwood M.A., Agus A., Pibiri M., Nair S.V., Walker J.M., Pennell D.J. Combined chelation therapy in thalassemia major for the treatment of severe myocardial siderosis with left ventricular dysfunction. J. Cardiovasc. Magn. Reson. 2008;10:12. doi: 10.1186/1532-429X-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pepe A., Meloni A., Pistoia L., Cuccia L., Gamberini M.R., Lisi R., D’Ascola D.G., Rosso R., Allò M., Spasiano A., et al. MRI multicentre prospective survey in thalassaemia major patients treated with defer-asirox versus deferiprone and desferrioxamine. Br. J. Haematol. 2018;183:783–795. doi: 10.1111/bjh.15595. [DOI] [PubMed] [Google Scholar]
- 56.Lin C.-H., Chen X., Wu C.-C., Wu K.-H., Song T.-S., Weng T.-F., Hsieh Y.-W., Peng C.-T. Therapeutic mechanism of combined oral chelation therapy to maximize efficacy of iron removal in transfusion-dependent thalassemia major-a pilot study. Expert Rev. Hematol. 2019;12:265–272. doi: 10.1080/17474086.2019.1593823. [DOI] [PubMed] [Google Scholar]
- 57.Eghbali A., Shokri P., Afzal R.R., Bagheri B. A 1-year randomized trial of deferasirox alone versus deferasirox and deferoxamine combination for the treatment of iron overload in thalassemia major. Transfus. Apher. Sci. 2019;58:429–433. doi: 10.1016/j.transci.2019.03.021. [DOI] [PubMed] [Google Scholar]
- 58.Kontoghiorghes G.J., Neocleous K., Kolnagou A. Benefits and risks of deferiprone in iron overload in thalassaemia and other conditions: Comparison of epidemiological and therapeutic aspects with deferoxamine. Drug Saf. 2003;26:553–584. doi: 10.2165/00002018-200326080-00003. [DOI] [PubMed] [Google Scholar]
- 59.Kontoghiorghe C.N., Andreou N., Constantinou K., Kontoghiorghes G.J. World health dilemmas: Orphan and rare diseases, orphan drugs and orphan patients. World J. Methodol. 2014;4:163–188. doi: 10.5662/wjm.v4.i3.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kontoghiorghes G.J. Ph.D. Thesis. University of Essex; Colchester, UK: 1982. [(accessed on 1 March 2023)]. The Design of Orally Active Iron Chelators for the Treatment of Thalassaemia; pp. 1–243. Available online: https://www.pri.ac.cy/files/KGJ_thesis_1982.pdf. [Google Scholar]
- 61.Kontoghiorghes G.J. Design, Properties, and Effective Use of the Oral Chelator L1 and Other Alpha-Ketohydroxypyridines in the Treatment of Transfusional Iron Overload in Thalassemia. Ann. N. Y. Acad. Sci. 1990;612:339–350. doi: 10.1111/j.1749-6632.1990.tb24321.x. [DOI] [PubMed] [Google Scholar]
- 62.Kontoghiorghes G., Pattichis K., Neocleous K., Kolnagou A. The Design and Development of Deferiprone (L1) and Other Iron Chelators for Clinical Use: Targeting Methods and Application Prospects. Curr. Med. Chem. 2004;11:2161–2183. doi: 10.2174/0929867043364685. [DOI] [PubMed] [Google Scholar]
- 63.Kontoghiorghes G.J., Kleanthous M., Kontoghiorghe C.N. The History of Deferiprone (L1) and the Paradigm of the Complete Treatment of Iron Overload in Thalassaemia. Mediterr. J. Hematol. Infect. Dis. 2020;12:e2020011. doi: 10.4084/mjhid.2020.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kontoghiorghes G.J., Wilson M.T. Structural and kinetic studies on eisenia foetida erythrocruorin. In: Lamy J., editor. Invertebrate Oxygen Binding Proteins, Structure, Active Site and Function. Marcel Dekker; New York, NY, USA: 1981. pp. 385–391. [Google Scholar]
- 65.Kontoghiorghe C.N., Kolnagou A., Kontoghiorghes G.J. Phytochelators Intended for Clinical Use in Iron Overload, Other Diseases of Iron Imbalance and Free Radical Pathology. Molecules. 2015;20:20841–20872. doi: 10.3390/molecules201119725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sheppard L.N., Kontoghiorghes G.J. Comparative iron binding studies of bis and tris (3-hydroxy-2-methylpyrid-4-ones) and desferrioxamine. Inorg. Chim. Acta. 1991;188:177–183. doi: 10.1016/S0020-1693(00)80369-2. [DOI] [Google Scholar]
- 67.Kontoghiorghes G.J., Marcus R., Huehns E. Desferrioxamine Suppositories. Lancet. 1983;322:454. doi: 10.1016/S0140-6736(83)90413-0. [DOI] [PubMed] [Google Scholar]
- 68.Kontoghiorghes G.J. New Orally Active Iron Chelators. Lancet. 1985;325:817. doi: 10.1016/S0140-6736(85)91472-2. [DOI] [PubMed] [Google Scholar]
- 69.Kontoghiorghes G.J., Sheppard L., Hoffbrand A.V., Charalambous J., Tikerpae J., Pippard M.J. Iron chelation studies using desferrioxamine and the potential oral chelator, 1,2-dimethyl-3-hydroxypyrid-4-one, in normal and iron loaded rats. J. Clin. Pathol. 1987;40:404–408. doi: 10.1136/jcp.40.4.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kontoghiorghes G.J. Dose response studies using desferrioxamine and orally active chelators in a mouse model. Scand. J. Haematol. 1986;37:63–70. doi: 10.1111/j.1600-0609.1986.tb01773.x. [DOI] [PubMed] [Google Scholar]
- 71.Kontoghiorghes G.J. Orally active alpha-ketohydroxypyridine iron chelators: Studies in mice. Mol. Pharmacol. 1986;30:670–673. [PubMed] [Google Scholar]
- 72.Kontoghiorghes G.J., Hoffbrand A.V. Orally active α-ketohydroxy pyridine iron chelators intended for clinical use: In Vivo studies in rabbits. Br. J. Haematol. 1986;62:607–613. doi: 10.1111/j.1365-2141.1986.tb04082.x. [DOI] [PubMed] [Google Scholar]
- 73.Kontoghiorghes G.J. Orally Active Alpha-Ketohydroxypyridine Iron Chelators: Effects on Iron and Other Metal Mobilisations. Acta Haematol. 1987;78:212–216. doi: 10.1159/000205877. [DOI] [PubMed] [Google Scholar]
- 74.Gyparaki M., Porter J.B., Hirani S., Streater M., Hider R.C., Huehns E.R. In Vivo Evaluation of Hydroxypyridone Iron Chelators in a Mouse Model. Acta Haematol. 1987;78:217–221. doi: 10.1159/000205878. [DOI] [PubMed] [Google Scholar]
- 75.Huehns E.R., Porter J.B., Hider R.C. Selection of Hydroxypyridin-4-Ones for the Treatment of Iron Overload Using In Vitro and In Vivo Models. Hemoglobin. 1988;12:593–600. doi: 10.3109/03630268808991649. [DOI] [PubMed] [Google Scholar]
- 76.Porter J.B., Morgan J., Hoyes K.P., Burke L.C., Huehns E.R., Hider R.C. Relative oral efficacy and acute toxicity of hydroxy-pyridin-4-one iron chelators in mice. Blood. 1990;76:2389–2396. doi: 10.1182/blood.V76.11.2389.2389. [DOI] [PubMed] [Google Scholar]
- 77.Kontoghiorghes G.J., Barr J., Nortey P., Sheppard L. Selection of a new generation of orally active α-ketohydroxypyridine iron chelators intended for use in the treatment of iron overload. Am. J. Hematol. 1993;42:340–349. doi: 10.1002/ajh.2830420403. [DOI] [PubMed] [Google Scholar]
- 78.Kontoghiorghes G.J., Sheppard L. Simple synthesis of the potent iron chelators 1-alkyl-3-hydroxy-2-methylpyrid-4-ones. Inorg. Chim. Acta. 1987;136:L11–L12. doi: 10.1016/S0020-1693(00)85549-8. [DOI] [Google Scholar]
- 79.Kontoghiorghes G.J., editor. Oral chelation in the treatment of thalassaemia and other diseases. Drugs Today. 1992;28:1–187. [Google Scholar]
- 80.Kontoghiorghes G.J., Sheppard L., Aldouri M.A., Hoffbrand A.V. 1,2-Dimethyl-3-Hydroxypyrid-4-One, an Orally Active Chelator for Treatment of Iron Overload. Lancet. 1987;329:1294–1295. doi: 10.1016/S0140-6736(87)90545-9. [DOI] [PubMed] [Google Scholar]
- 81.Kontoghiorghes G.J., Aldouri M.A., Hoffbrand A.V., Barr J., Wonke B., Kourouclaris T., Sheppard L. Effective chelation of iron in beta thalassaemia with the oral chelator 1,2-dimethyl-3-hydroxypyrid-4-one. BMJ. 1987;295:1509–1512. doi: 10.1136/bmj.295.6612.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kontoghiorghes G.J. Iron chelating drugs. BMJ. 1988;296:1672–1673. doi: 10.1136/bmj.296.6637.1672-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kontoghiorghes G.J. Oral iron chelation is here. BMJ. 1991;303:1279–1280. doi: 10.1136/bmj.303.6813.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Porter J.B., Singh S., Hoyes K.P., Epemolu O., Abeysinghe R.D., Hider R.C. Progress in Iron Research. Volume 356. Springer; Boston, MA, USA: 1994. Lessons from Preclinical and Clinical Studies with 1,2-Diethyl-3-Hydroxypyridin-4-One, CP94 and Related Compounds; pp. 361–370. [DOI] [PubMed] [Google Scholar]
- 85.Porter J.B., Abeysinghe R.D., Hoyes K.P., Barra C., Huehns E.R., Brooks P.N., Blackwell M.P., Araneta M., Brittenham G., Singh S., et al. Contrasting interspecies efficacy and toxicology of 1, 2-diethy 1–3 -hydroxypyridin-4-one, CP94, relates to differing metabolism of the iron chelating site. Br. J. Haematol. 1993;85:159–168. doi: 10.1111/j.1365-2141.1993.tb08660.x. [DOI] [PubMed] [Google Scholar]
- 86.Epemolu R.O., Ackerman R., Porter J.B., Hider R.C., Damani L.A., Singh S. HPLC determination of 1,2-diethyl-3-hydroxypyridin-4-one (CP94), its iron complex [Fe(III) (CP94)3] and glucuronide conjugate [CP94-GLUC] in serum and urine of thalassaemic patients. J. Pharm. Biomed. Anal. 1994;12:923–930. doi: 10.1016/0731-7085(94)E0027-X. [DOI] [PubMed] [Google Scholar]
- 87.Pfannkuch F., Bentley P., Schnebli H.P. Future of oral iron chelator deferiprone (L1) Lancet. 1993;341:1480. [PubMed] [Google Scholar]
- 88.Berdoukas V., Bentley P., Frost H., Schnebli H.P. Toxicity of oral iron chelator L1. Lancet. 1993;341:1088. doi: 10.1016/0140-6736(93)92443-W. [DOI] [PubMed] [Google Scholar]
- 89.Hershko C. Development of oral iron chelator L1. Lancet. 1993;341:1088–1089. doi: 10.1016/0140-6736(93)92444-X. [DOI] [PubMed] [Google Scholar]
- 90.Kontoghiorghes G.J. Misinformation about deferiprone (L1) Lancet. 1993;342:250. doi: 10.1016/0140-6736(93)92348-W. [DOI] [PubMed] [Google Scholar]
- 91.Kontoghiorghes G.J., Agarwal M.B., Grady R.W., Kolnagou A., Marx J.J. Deferiprone for thalassaemia. Lancet. 2000;356:428–429. doi: 10.1016/S0140-6736(05)73574-1. [DOI] [PubMed] [Google Scholar]
- 92.Pippard M.J., Weatherall D.J. Deferiprone for thalassaemia. Lancet. 2000;356:1444–1445. doi: 10.1016/S0140-6736(05)74089-7. [DOI] [PubMed] [Google Scholar]
- 93.Kontoghiorghes G.J., Agarwal M.B., Tondury P., Marx J.J. Deferiprone or fatal iron toxic effects? Lancet. 2001;357:882–883. doi: 10.1016/S0140-6736(05)71812-2. [DOI] [PubMed] [Google Scholar]
- 94.Hoffbrand A.V., Bartlett A.N., Veys P.A., O’Connor N.T., Kontoghiorghes G.J. Agranulocytosis and Thrombocytopenia in Patient with Blackfan-Diamond Anaemia during Oral Chelator Trial. Lancet. 1989;334:457. doi: 10.1016/S0140-6736(89)90641-7. [DOI] [PubMed] [Google Scholar]
- 95.Agarwal M.B., Gupte S.S., Viswanathan C., Vasandani D., Ramanathan J., Desai N., Puniyani R.R., Chhablani A.T. Long-term assessment of efficacy and safety of L1, an oral iron chelator, in transfusion dependent thalassaemia: Indian trial. Br. J. Haematol. 1992;82:460–466. doi: 10.1111/j.1365-2141.1992.tb06445.x. [DOI] [PubMed] [Google Scholar]
- 96.Kontoghiorghes G.J. Present status and future prospects of oral iron chelation therapy in thalassaemia and other diseases. Indian J. Pediatr. 1993;60:485–507. doi: 10.1007/BF02751425. [DOI] [PubMed] [Google Scholar]
- 97.Cohen A.R., Galanello R., Piga A., De Sanctis V., Tricta F. Safety and effectiveness of long-term therapy with the oral iron chelator deferiprone. Blood. 2003;102:1583–1587. doi: 10.1182/blood-2002-10-3280. [DOI] [PubMed] [Google Scholar]
- 98.The History of Deferiprone. [(accessed on 1 October 2019)]. Available online: https://www.youtube.com/watch?v=ZcvSLyIgYd8.
- 99.Töndury P., Kontoghiorghes G.J., Ridolfi-Lüthy A., Hirt A., Hoffbrand A.V., Lottenbach A.M., Sonderegger T., Wagner H.P., Toundury P., Ridolfi-Luuthy A. L1 (1,2-dimethyl-3-hydroxypyrid-4-one) for oral iron chelation in patients with beta-thalassaemia major. Br. J. Haematol. 1990;76:550–553. doi: 10.1111/j.1365-2141.1990.tb07915.x. [DOI] [PubMed] [Google Scholar]
- 100.Olivieri N.F., Koren G., Louis P.S., Freedman M.H., McClelland R.A., Templeton D.M. Studies of the oral chelator 1,2-dimethyl-3-hydroxypyrid-4-one in thalassemia patients. Semin. Hematol. 1990;27:101–104. [PubMed] [Google Scholar]
- 101.Goudsmit R. Long-term treatment of patients with transfusion hemosiderosis using oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) Ned. Tijdschr. Geneeskd. 1991;135:2133–2136. [PubMed] [Google Scholar]
- 102.Carnelli V., Terzoli S., Fossati G., Careddu G., Perri M., Pedrotti L., Mirra N. New therapeutic trends in thalassemia: Oral chelating agents. Pediatr. Med. Chir. 1992;14:273–275. [PubMed] [Google Scholar]
- 103.Cermák J., Brabec V. Treatment of iron overload states with oral administration of the chelator agent, L1 (Deferiprone) Vnitr. Lek. 1994;40:586–590. [PubMed] [Google Scholar]
- 104.Kersten M.J., Lange R., Smeets M.E.P., Vreugdenhil G., Roozendaal K.J., Lameijer W., Goudsmit R. Long-term treatment of transfusional iron overload with the oral iron chelator deferiprone (L1): A Dutch multicenter trial. Ann. Hematol. 1996;73:247–252. doi: 10.1007/s002770050236. [DOI] [PubMed] [Google Scholar]
- 105.Taylor D.M. Royal Society of Chemistry-sixth international symposium on applied bioinorganic chemistry. IDrugs Investig. Drugs J. 2001;4:1005–1007. [PubMed] [Google Scholar]
- 106.Rombos Y., Tzanetea R., Konstantopoulos K., Simitzis S., Zervas C., Kyriaki P., Kavouklis M., Aessopos A., Sakellaropoulos N., Karagiorga M., et al. Chelation therapy in patients with thalassemia using the orally active iron chelator deferiprone (L1) Haematologica. 2000;85:115–117. [PubMed] [Google Scholar]
- 107.Kontoghiorghes G.J. The Proceedings of the 20th International Conference on Chelation held in the USA: Advances on new and old chelation therapies. Toxicol. Mech. Methods. 2013;23:1–4. doi: 10.3109/15376516.2012.720305. [DOI] [PubMed] [Google Scholar]
- 108.Savulescu J. Thalassaemia major: The murky story of deferiprone. BMJ. 2004;328:358–359. doi: 10.1136/bmj.328.7436.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nisbet-Brown E., Olivieri N.F., Giardina P.J., Grady R.W., Neufeld E.J., Séchaud R., Krebs-Brown A.J., Anderson J.R., Alberti D., Sizer K.C., et al. Effectiveness and safety of ICL670 in iron-loaded patients with thalassaemia: A randomised, double-blind, placebo-controlled, dose-escalation trial. Lancet. 2003;361:1597–1602. doi: 10.1016/S0140-6736(03)13309-0. [DOI] [PubMed] [Google Scholar]
- 110.Neufeld E.J. Oral chelators deferasirox and deferiprone for transfusional iron overload in thalassemia major: New data, new questions. Blood. 2006;107:3436–3441. doi: 10.1182/blood-2006-02-002394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kontoghiorghes G.J. New chelation therapies and emerging chelating drugs for the treatment of iron overload. Expert Opin. Emerg. Drugs. 2006;11:1–5. doi: 10.1517/14728214.11.1.1. [DOI] [PubMed] [Google Scholar]
- 112.Viprakasit V., Nuchprayoon I., Chuansumrit A., Torcharus K., Pongtanakul B., Laothamatas J., Srichairatanakool S., Pooliam J., Supajitkasem S., Suriyaphol P., et al. Deferiprone (GPO-L-ONE®) monotherapy reduces iron overload in transfusion-dependent thalassemias: 1-year results from a multicenter prospective, single arm, open label, dose escalating phase III pediatric study (GPO-L-ONE.; A001) from Thailand. Am. J. Hematol. 2013;88:251–260. doi: 10.1002/ajh.23386. [DOI] [PubMed] [Google Scholar]
- 113.Kontoghiorghes G.J. The aim of iron chelation therapy in thalassaemia. Eur. J. Haematol. 2017;99:465–466. doi: 10.1111/ejh.12939. [DOI] [PubMed] [Google Scholar]
- 114.Maggio A., Kattamis A., Felisi M., Reggiardo G., El-Beshlawy A., Bejaoui M., Sherief L., Christou S., Cosmi C., Della Pasqua O., et al. Evaluation of the efficacy and safety of deferiprone compared with deferasirox in paediatric patients with transfusion-dependent haemoglobinopathies (DEEP-2): A multicentre, randomised, open-label, non-inferiority, phase 3 trial. Lancet Haematol. 2020;7:e469–e478. doi: 10.1016/S2352-3026(20)30100-9. [DOI] [PubMed] [Google Scholar]
- 115.Au W.Y., Lee V., Lau C.W., Yau J., Chan D., Chan E.Y.T., Cheung W.W.W., Ha S.Y., Kho B., Lee C.Y., et al. A synopsis of current care of thalassaemia major patients in Hong Kong. Hong Kong Med. J. 2011;17:261–266. [PubMed] [Google Scholar]
- 116.Maggio A., Filosa A., Vitrano A., Aloj G., Kattamis A., Ceci A., Fucharoen S., Cianciulli P., Grady R.W., Prossomariti L., et al. Iron chelation therapy in thalassemia major: A systematic review with meta-analyses of 1520 patients included on randomized clinical trials. Blood Cells Mol. Dis. 2011;47:166–175. doi: 10.1016/j.bcmd.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 117.Cohen A.R., Galanello R., Piga A., Dipalma A., Vullo C., Tricta F. Safety profile of the oral iron chelator deferiprone: A mul-ticentre study. Br. J. Haematol. 2000;108:305–312. doi: 10.1046/j.1365-2141.2000.01866.x. [DOI] [PubMed] [Google Scholar]
- 118.Maggio A., Vitrano A., Lucania G., Capra M., Cuccia L., Gagliardotto F., Pitrolo L., Prossomariti L., Filosa A., Caruso V., et al. Long-term use of deferiprone significantly enhances left-ventricular ejection function in thalassemia major patients. Am. J. Hematol. 2012;87:732–733. doi: 10.1002/ajh.23219. [DOI] [PubMed] [Google Scholar]
- 119.Kolnagou A., Kleanthous M., Kontoghiorghes G.J. Reduction of body iron stores to normal range levels in thalassaemia by using a deferiprone/deferoxamine combination and their maintenance thereafter by deferiprone monotherapy. Eur. J. Haematol. 2010;85:430–438. doi: 10.1111/j.1600-0609.2010.01499.x. [DOI] [PubMed] [Google Scholar]
- 120.Kontoghiorghes G.J., Kontoghiorghe C.N. Efficacy and safety of iron-chelation therapy with deferoxamine, deferiprone, and deferasirox for the treatment of iron-loaded patients with non-transfusion-dependent thalassemia syndromes. Drug Des. Dev. Ther. 2016;10:465–481. doi: 10.2147/DDDT.S79458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kolnagou A., Kleanthous M., Kontoghiorghes G.J. Benefits and Risks in Polypathology and Polypharmacotherapy Chal-lenges in the Era of the Transition of Thalassaemia from a Fatal to a Chronic or Curable Disease. Front. Biosci. 2022;14:18. doi: 10.31083/j.fbe1403018. [DOI] [PubMed] [Google Scholar]
- 122.Kolnagou A., Kontoghiorghe C.N., Kontoghiorghes G.J. Prevention of Iron Overload and Long Term Maintenance of Normal Iron Stores in Thalassaemia Major Patients using Deferiprone or Deferiprone Deferoxamine Combination. Drug Res. 2017;67:404–411. doi: 10.1055/s-0043-102691. [DOI] [PubMed] [Google Scholar]
- 123.Kolnagou A., Kontoghiorghes G.J. Chelation protocols for the elimination and prevention of iron overload in thalassaemia. Front. Biosci. 2018;23:1082–1098. doi: 10.2741/4634. [DOI] [PubMed] [Google Scholar]
- 124.Kontoghiorghes G.J. Chelators affecting iron absorption in mice. Arzneimittelforschung. 1990;40:1332–1335. [PubMed] [Google Scholar]
- 125.Barrand M.A., Callingham B.A., Hider R.C. Effects of the pyrones, maltol and ethyl maltol, on iron absorption from the rat small intestine. J. Pharm. Pharmacol. 1987;39:203–211. doi: 10.1111/j.2042-7158.1987.tb06249.x. [DOI] [PubMed] [Google Scholar]
- 126.Barrand M.A., Callingham B.A., Dobbin P., Hider R.C. Dissociation of a ferric maltol complex and its subsequent metabolism during absorption across the small intestine of the rat. Br. J. Pharmacol. 1991;102:723–729. doi: 10.1111/j.1476-5381.1991.tb12240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Thompson K.H., Barta C.A., Orvig C. Metal complexes of maltol and close analogues in medicinal inorganic chemistry. Chem. Soc. Rev. 2006;35:545–556. doi: 10.1039/b416256k. [DOI] [PubMed] [Google Scholar]
- 128.Harvey R.S.J., Reffitt D.M., Doig L.A., Meenan J., Ellis R.D., Thompson R.P.H., Powell J.J. Ferric trimaltol corrects iron deficiency anaemia in patients intolerant of iron. Aliment. Pharmacol. Ther. 1998;12:845–848. doi: 10.1046/j.1365-2036.1998.00380.x. [DOI] [PubMed] [Google Scholar]
- 129.Reffitt D.M., Burden T.J., Seed P.T., Wood J., Thompson R.P., Powell J.J. Assessment of iron absorption from ferric trimaltol. Ann. Clin. Biochem. 2000;37:457–466. doi: 10.1177/000456320003700405. [DOI] [PubMed] [Google Scholar]
- 130.Khoury A., Pagan K.A., Farland M.Z. Ferric Maltol: A New Oral Iron Formulation for the Treatment of Iron Deficiency in Adults. Ann. Pharmacother. 2021;55:222–229. doi: 10.1177/1060028020941014. [DOI] [PubMed] [Google Scholar]
- 131.Stallmach A., Büning C. Ferric maltol (ST10): A novel oral iron supplement for the treatment of iron deficiency anemia in inflammatory bowel disease. Expert Opin. Pharmacother. 2015;16:2859–2867. doi: 10.1517/14656566.2015.1096929. [DOI] [PubMed] [Google Scholar]
- 132.Murawska N., Fabisiak A., Fichna J. Anemia of Chronic Disease and Iron Deficiency Anemia in Inflammatory Bowel Diseases: Pathophysiology, Diagnosis, and Treatment. Inflamm. Bowel Dis. 2016;22:1198–1208. doi: 10.1097/MIB.0000000000000648. [DOI] [PubMed] [Google Scholar]
- 133.Pergola P.E., Fishbane S., Ganz T. Novel Oral Iron Therapies for Iron Deficiency Anemia in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019;26:272–291. doi: 10.1053/j.ackd.2019.05.002. [DOI] [PubMed] [Google Scholar]
- 134.Bokemeyer B., Krummenerl A., Maaser C., Howaldt S., Mroß M., Mallard N. Randomized Open-Label Phase 1 Study of the Pharmacokinetics of Ferric Maltol in Inflammatory Bowel Disease Patients with Iron Deficiency. Eur. J. Drug Metab. Pharmacokinet. 2017;42:229–238. doi: 10.1007/s13318-016-0334-5. [DOI] [PubMed] [Google Scholar]
- 135.Gasche C., Ahmad T., Tulassay Z., Baumgart D.C., Bokemeyer B., Büning C., Howaldt S., Stallmach A. AEGIS Study Group. Ferric maltol is effective in correcting iron deficiency anemia in patients with inflammatory bowel disease: Results from a phase-3 clinical trial program. Inflamm. Bowel Dis. 2015;21:579–588. doi: 10.1097/MIB.0000000000000314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schmidt C., Ahmad T., Tulassay Z., Baumgart D.C., Bokemeyer B., Howaldt S., Stallmach A., Büning C., the AEGIS Study Group Ferric maltol therapy for iron deficiency anaemia in patients with inflammatory bowel disease: Long-term extension data from a Phase 3 study. Aliment. Pharmacol. Ther. 2016;44:259–270. doi: 10.1111/apt.13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kelsey S.M., Hider R., Bloor J.R.C., Blake D.R., Gutteridge C.N., Newland A.C. Absorption of low and therapeutic doses of ferric maltol, a novel ferric iron compound, in iron deficient subjects using a single dose iron absorption test. J. Clin. Pharm. Ther. 1991;16:117–122. doi: 10.1111/j.1365-2710.1991.tb00292.x. [DOI] [PubMed] [Google Scholar]
- 138.Olsson K.M., Fuge J., Brod T., Kamp J.C., Schmitto J., Kempf T., Bauersachs J., Hoeper M.M. Oral iron supplementation with ferric maltol in patients with pulmonary hypertension. Eur. Respir. J. 2020;56:2000616. doi: 10.1183/13993003.00616-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kopyt N.P., AEGIS-CKD Study Group Efficacy and safety of oral ferric maltol (FM) in treating iron-deficiency anemia (IDA) in patients with CKD: Randomized controlled trial [FR-OR120] J. Am. Soc. Nephrol. 2018;29:70–71. [Google Scholar]
- 140.Mause S.F., Berger M., Lim H.Y., Vogt F., Brandenburg V., Stöhr R. Intravenous iron supplementation in heart failure patients induces temporary endothelial dysfunction with release of endothelial microvesicles. Front. Immunol. 2023;13:1092704. doi: 10.3389/fimmu.2022.1092704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Alexandre A.F., Morga A., Thomas C., Krucien N., Tervonen T., Jiletcovici A., Marsh K. Preferences for Anaemia Treatment Attributes among Patients with Non-Dialysis-Dependent Chronic Kidney Disease. Adv. Ther. 2023;40:641–657. doi: 10.1007/s12325-022-02367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ogawa C., Tsuchiya K., Kanemitsu M., Maeda K. Low-Dose Oral Iron Replacement Therapy Is Effective for Many Japanese Hemodialysis Patients: A Retrospective Observational Study. Nutrients. 2022;15:125. doi: 10.3390/nu15010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Xia Y., Luo Q., Huang C., Shi L., Jahangir A., Pan T., Wei X., He J., Liu W., Shi R., et al. Ferric citrate-induced colonic mucosal damage associated with oxidative stress, inflammation responses, apoptosis, and the changes of gut microbial composition. Ecotoxicol. Environ. Saf. 2023;249:114364. doi: 10.1016/j.ecoenv.2022.114364. [DOI] [PubMed] [Google Scholar]
- 144.Kontoghiorghe C.N., Kontoghiorghes G.J. New developments and controversies in iron metabolism and iron chelation therapy. World J. Methodol. 2016;6:1–19. doi: 10.5662/wjm.v6.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Sharma D.C. Patent rulings raise hope for cheap cancer drugs in India. Lancet Oncol. 2013;14:e441. doi: 10.1016/S1470-2045(13)70395-4. [DOI] [PubMed] [Google Scholar]
- 146.Luangasanatip N., Chaiyakunapruk N., Upakdee N., Wong P. Iron-chelating therapies in a transfusion-dependent thalassaemia population in Thailand: A cost-effectiveness study. Clin. Drug Investig. 2011;31:493–505. doi: 10.2165/11587120-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 147.Olivieri N.F., Matsui D., Liu P.P., Blendis L., Cameron R., A McClelland R.A., Templeton D.M., Koren G. Oral iron chelation with 1,2-dimethyl-3-hydroxypyrid-4-one (L1) in iron loaded thalassemia patients. Bone Marrow Transpl. 1993;12:9–11. [PubMed] [Google Scholar]
- 148.Morales N.P., Rodrat S., Piromkraipak P., Yamanont P., Paiboonsukwong K., Fucharoen S. Iron chelation therapy with de-feriprone improves oxidative status and red blood cell quality and reduces redox-active iron in β-thalassemia/hemoglobin E patients. Biomed. Pharmacother. 2022;145:112381. doi: 10.1016/j.biopha.2021.112381. [DOI] [PubMed] [Google Scholar]
- 149.Chang Y.-H., Shaw C.-F., Wu K.-H., Hsieh K.-H., Su Y.-N., Lu P.-J. Treatment with Deferiprone for Iron Overload Alleviates Bone Marrow Failure in a Fanconi Anemia Patient. Hemoglobin. 2009;33:346–351. doi: 10.3109/03630260903212563. [DOI] [PubMed] [Google Scholar]
- 150.Cermak J., Jonasova A., Vondrakova J., Cervinek L., Belohlavkova P., Neuwirtova R. A comparative study of deferasirox and deferiprone in the treatment of iron overload in patients with myelodysplastic syndromes. Leuk. Res. 2013;37:1612–1615. doi: 10.1016/j.leukres.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 151.Tauchenová L., Křížová B., Kubánek M., Fraňková S., Melenovský V., Tintěra J., Kautznerová D., Malušková J., Jirsa M., Kautzner J. Successful Treatment of Iron-Overload Cardiomyopathy in Hereditary Hemochromatosis with Deferoxamine and Deferiprone. Can. J. Cardiol. 2016;32:1574.e1–1574.e3. doi: 10.1016/j.cjca.2016.07.589. [DOI] [PubMed] [Google Scholar]
- 152.Kontoghiorghes G.J., Spyrou A., Kolnagou A. Iron chelation therapy in hereditary hemochromatosis and thalassemia intermedia: Regulatory and non regulatory mechanisms of increased iron absorption. Hemoglobin. 2010;34:251–264. doi: 10.3109/03630269.2010.486335. [DOI] [PubMed] [Google Scholar]
- 153.Fabio G., Minonzio F., Delbini P., Bianchi A., Cappellini M.D. Reversal of cardiac complications by deferiprone and deferoxamine combination therapy in a patient affected by a severe type of juvenile hemochromatosis (JH) Blood. 2007;109:362–364. doi: 10.1182/blood-2006-04-016949. [DOI] [PubMed] [Google Scholar]
- 154.Elalfy M.S., Hamdy M., El-Beshlawy A., Ebeid F.S.E., Badr M., Kanter J., Inusa B.P., Adly A.A.M., Williams S., Kilinc Y., et al. Deferiprone for transfusional iron overload in sickle cell disease and other anemias: Open-label study of up to 3 years. Blood Adv. 2022;7:611–619. doi: 10.1182/bloodadvances.2021006778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kontoghiorghes G.J., Barr J., Baillod R.A. Studies of aluminium mobilization in renal dialysis patients using the oral chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Arzneimittelforschung. 1994;44:522–526. [PubMed] [Google Scholar]
- 156.Kontoghiorghes G.J. Comparative efficacy and toxicity of desferrioxamine, deferiprone and other iron and aluminium chelating drugs. Toxicol. Lett. 1995;80:1–18. doi: 10.1016/0378-4274(95)03415-H. [DOI] [PubMed] [Google Scholar]
- 157.Vroegindeweij L.H.P., Boon A.J.W., Wilson J.H.P., Langendonk J.G. Effects of iron chelation therapy on the clinical course of aceruloplasminemia: An analysis of aggregated case reports. Orphanet J. Rare Dis. 2020;15:105. doi: 10.1186/s13023-020-01385-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Boddaert N., Le Quan Sang K.H., Rötig A., Leroy-Willig A., Gallet S., Brunelle F., Sidi D., Thalabard J.C., Munnich A., Cabantchik Z.I. Selective iron chelation in Friedreich ataxia: Biologic and clinical implications. Blood. 2007;110:401–408. doi: 10.1182/blood-2006-12-065433. [DOI] [PubMed] [Google Scholar]
- 159.Abbruzzese G., Cossu G., Balocco M., Marchese R., Murgia D., Melis M., Galanello R., Barella S., Matta G., Ruffinengo U., et al. A pilot trial of deferiprone for neurodegeneration with brain iron accumulation. Haematologica. 2011;96:1708–1711. doi: 10.3324/haematol.2011.043018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Martin-Bastida A., Ward R.J., Newbould R., Piccini P., Sharp D., Kabba C., Patel M.C., Spino M., Connelly J., Tricta F., et al. Brain iron chelation by deferiprone in a phase 2 randomised double-blinded placebo controlled clinical trial in Parkinson’s disease. Sci. Rep. 2017;7:1398. doi: 10.1038/s41598-017-01402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Maher P., Kontoghiorghes G.J. Characterization of the Neuroprotective Potential of Derivatives of the Iron Chelating Drug Deferiprone. Neurochem. Res. 2015;40:609–620. doi: 10.1007/s11064-014-1508-7. [DOI] [PubMed] [Google Scholar]
- 162.Romano N., Baiardi G., Pinto V.M., Quintino S., Gianesin B., Sasso R., Diociasi A., Mattioli F., Marchese R., Abbruzzese G., et al. Long-Term Neuroradiological and Clinical Evaluation of NBIA Patients Treated with a Deferiprone Based Iron-Chelation Therapy. J. Clin. Med. 2022;11:4524. doi: 10.3390/jcm11154524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rajapurkar M.M., Hegde U., Bhattacharya A., Alam M.G., Shah S.V. Effect of deferiprone, an oral iron chelator, in diabetic and non-diabetic glomerular disease. Toxicol. Mech. Methods. 2013;23:5–10. doi: 10.3109/15376516.2012.730558. [DOI] [PubMed] [Google Scholar]
- 164.Mohanty D., Ghosh K., Pathare A.V., Karnad D. Deferiprone (L1) as an adjuvant therapy for Plasmodium falciparum malaria. Indian J. Med. Res. 2002;115:17–21. [PubMed] [Google Scholar]
- 165.Saxena D., Spino M., Tricta F., Connelly J., Cracchiolo B.M., Hanauske A.R., D’Alliessi Gandolfi D., Mathews M.B., Karn J., Holland B., et al. Drug-Based Lead Discovery: The Novel Ablative An-tiretroviral Profile of Deferiprone in HIV-1-Infected Cells and in HIV-Infected Treatment-Naive Subjects of a Double-Blind, Placebo-Controlled, Randomized Exploratory Trial. PLoS ONE. 2016;11:e0154842. doi: 10.1371/journal.pone.0154842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vreugdenhil G., Swaak A.J., Kontoghiorghes G.J., Van Eijk H.G. Efficacy and Safety of Oral Iron Chelator L1 in Anaemic Rheumatoid Arthritis Patients. Lancet. 1989;334:1398–1399. doi: 10.1016/S0140-6736(89)92011-4. [DOI] [PubMed] [Google Scholar]
- 167.Arpa J., Sanz-Gallego I., Rodríguez-de-Rivera F.J., Domínguez-Melcón F.J., Prefasi D., Oliva-Navarro J., Moreno-Yangüela M. Triple therapy with deferiprone, idebenone and riboflavin in Friedreich’s ataxia-open-label trial. Acta Neurol. Scand. 2014;129:32–40. doi: 10.1111/ane.12141. [DOI] [PubMed] [Google Scholar]
- 168.Devos D., Labreuche J., Rascol O., Corvol J.-C., Duhamel A., Delannoy P.G., Poewe W., Compta Y., Pavese N., Růžička E., et al. Trial of Deferiprone in Parkinson’s Disease. N. Engl. J. Med. 2022;387:2045–2055. doi: 10.1056/NEJMoa2209254. [DOI] [PubMed] [Google Scholar]
- 169.Kontoghiorghes G.J., Bartlett A.N., Hoffbrand A.V., Goddard J.G., Sheppard L., Barr J., Nortey P. Long-term trial with the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one (L1) I. Iron Chelation and Metabolic Studies. Br. J. Haematol. 1990;76:295–300. doi: 10.1111/j.1365-2141.1990.tb07887.x. [DOI] [PubMed] [Google Scholar]
- 170.Kontoghiorghes G.J., Goddard J.G., Bartlett A.N., Sheppard L. Pharmacokinetic studies in humans with the oral iron chelator 1,2-dimethyl-3-hydroxypyrid-4-one. Clin. Pharmacol. Ther. 1990;48:255–261. doi: 10.1038/clpt.1990.147. [DOI] [PubMed] [Google Scholar]
- 171.Kontoghiorghes G.J., Kontoghiorghe C.N. Prospects for the introduction of targeted antioxidant drugs for the prevention and treatment of diseases related to free radical pathology. Expert Opin. Investig. Drugs. 2019;28:593–603. doi: 10.1080/13543784.2019.1631284. [DOI] [PubMed] [Google Scholar]
- 172.Kontoghiorghe C.N., Kolnagou A., Kontoghiorghes G.J. Antioxidant targeting by deferiprone in diseases related to oxidative damage. Front. Biosci. 2014;19:862–885. doi: 10.2741/4253. [DOI] [PubMed] [Google Scholar]
- 173.Kontoghiorghes G.J. New Iron Metabolic Pathways and Chelation Targeting Strategies Affecting the Treatment of All Types and Stages of Cancer. Int. J. Mol. Sci. 2022;23:13990. doi: 10.3390/ijms232213990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Chitambar C.R., Al-Gizawiy M.M., Alhajala H.S., Pechman K.R., Wereley J.P., Wujek R., Clark P.A., Kuo J.S., Antholine W.E., Schmainda K.M. Gallium Maltolate Disrupts Tumor Iron Metabolism and Retards the Growth of Glioblastoma by Inhibiting Mitochondrial Function and Ribonucleotide Reductase. Mol. Cancer Ther. 2018;17:1240–1250. doi: 10.1158/1535-7163.MCT-17-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Bernstein L.R., Van Der Hoeven J.J., Boer R.O. Hepatocellular carcinoma detection by gallium scan and subsequent treatment by gallium maltolate: Rationale and case study. Anti Cancer Agents Med. Chem. 2011;11:585–590. doi: 10.2174/187152011796011046. [DOI] [PubMed] [Google Scholar]
- 176.Wu X., Wang T.W., Lessmann G.M., Saleh J., Liu X., Chitambar C.R., Hwang S.T. Gallium Maltolate Inhibits Human Cutaneous T-Cell Lymphoma Tumor Development in Mice. J. Investig. Dermatol. 2015;135:877–884. doi: 10.1038/jid.2014.476. [DOI] [PubMed] [Google Scholar]
- 177.Merli D., Profumo A., Bloise N., Risi G., Momentè S., Cucca L., Visai L. Indium/Gallium Maltolate Effects on Human Breast Carcinoma Cells: In Vitro Investigation on Cytotoxicity and Synergism with Mitoxantrone. ACS Omega. 2018;3:4631–4640. doi: 10.1021/acsomega.7b02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Arnold C.E., Bordin A., Lawhon S.D., Libal M.C., Bernstein L.R., Cohen N.D. Antimicrobial activity of gallium maltolate against Staphylococcus aureus and methicillin-resistant S. aureus and Staphylococcus pseudintermedius: An in vitro study. Vet. Microbiol. 2012;155:389–394. doi: 10.1016/j.vetmic.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 179.Fecteau M.-E., Aceto H.W., Bernstein L.R., Sweeney R.W. Comparison of the antimicrobial activities of gallium nitrate and gallium maltolate against Mycobacterium avium subsp. paratuberculosis in vitro. Vet. J. 2014;202:195–197. doi: 10.1016/j.tvjl.2014.06.023. [DOI] [PubMed] [Google Scholar]
- 180.Hijazi S., Visaggio D., Pirolo M., Frangipani E., Bernstein L., Visca P. Antimicrobial Activity of Gallium Compounds on ESKAPE Pathogens. Front. Cell. Infect. Microbiol. 2018;8:316. doi: 10.3389/fcimb.2018.00316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ball K.R., Sampieri F., Chirino M., Hamilton D.L., Blyth R.I.R., Sham T.-K., Dowling P.M., Thompson J. Synchrotron X-ray Fluorescence Microscopy of Gallium in Bladder Tissue following Gallium Maltolate Administration during Urinary Tract Infection. Antimicrob. Agents Chemother. 2013;57:5197–5201. doi: 10.1128/AAC.00616-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Weinberg E.D. Iron depletion: A defense against intracellular infection and neoplasm. Life Sci. 1992;50:1289–1297. doi: 10.1016/0024-3205(92)90279-X. [DOI] [PubMed] [Google Scholar]
- 183.Kontoghiorghes G.J., Weinberg E.D. Iron: Mammalian defense systems, mechanisms of disease, and chelation therapy approaches. Blood Rev. 1995;9:33–45. doi: 10.1016/0268-960X(95)90038-1. [DOI] [PubMed] [Google Scholar]
- 184.Kontoghiorghes G.J., Kolnagou A., Skiada A., Petrikkos G. The Role of Iron and Chelators on Infections in Iron Overload and Non Iron Loaded Conditions: Prospects for the Design of New Antimicrobial Therapies. Hemoglobin. 2010;34:227–239. doi: 10.3109/03630269.2010.483662. [DOI] [PubMed] [Google Scholar]
- 185.Kontoghiorghes G.J. Advances on Chelation and Chelator Metal Complexes in Medicine. Int. J. Mol. Sci. 2020;21:2499. doi: 10.3390/ijms21072499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Kontoghiorghes G.J., Efstathiou A., Ioannou-Loucaides S., Kolnagou A. Chelators Controlling Metal Metabolism and Toxicity Pathways: Applications in Cancer Prevention, Diagnosis and Treatment. Hemoglobin. 2008;32:217–227. doi: 10.1080/03630260701727119. [DOI] [PubMed] [Google Scholar]
- 187.Chitambar C.R. The therapeutic potential of iron-targeting gallium compounds in human disease: From basic research to clinical application. Pharmacol. Res. 2017;115:56–64. doi: 10.1016/j.phrs.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 188.Merlot A.M., Kalinowski D.S., Kovacevic Z., Jansson P.J., Sahni S., Huang M.L., Lok H., Richardson D.R., Lane D.J.R. Exploiting Cancer Metal Metabolism using Anti-Cancer Metal-Binding Agents. Curr. Med. Chem. 2019;26:302–322. doi: 10.2174/0929867324666170705120809. [DOI] [PubMed] [Google Scholar]
- 189.Yasumoto E., Nakano K., Nakayachi T., Morshed S.R., Hashimoto K., Kikuchi H., Nishikawa H., Kawase M., Sakagami H. Cytotoxic activity of deferiprone, maltol and related hydroxyketones against human tumor cell lines. Anticancer Res. 2004;24:755–762. [PubMed] [Google Scholar]
- 190.Chitambar C.R. Gallium and its competing roles with iron in biological systems. Biochim. Biophys. Acta. 2016;1863:2044–2053. doi: 10.1016/j.bbamcr.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 191.Pan B., Li Y., Zhang J., Zhou Y., Li L., Xue X., Li H., Niu Q. Role of mGluR 1 in synaptic plasticity impairment induced by maltol aluminium in rats. Environ. Toxicol. Pharmacol. 2020;78:103406. doi: 10.1016/j.etap.2020.103406. [DOI] [PubMed] [Google Scholar]
- 192.Satoh E., Yasuda I., Yamada T., Suzuki Y., Ohyashiki T. Involvement of NO generation in aluminum-induced cell death. Biol. Pharm. Bull. 2007;30:1390–1394. doi: 10.1248/bpb.30.1390. [DOI] [PubMed] [Google Scholar]
- 193.Markowicz-Piasecka M., Skupień A., Mikiciuk-Olasik E., Sikora J. Biocompatibility Studies of Gadolinium Complexes with Iminodiacetic Acid Derivatives. Biol. Trace Elem. Res. 2019;189:426–436. doi: 10.1007/s12011-018-1496-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Parghane R.V., Basu S. Bilateral Orbital Soft-Tissue Metastases from Renal Neuroendocrine Tumor: Successful Theranostic Application of 68Ga/177Lu-DOTATATE with Improvement of Vision. J. Nucl. Med. Technol. 2019;47:171–172. doi: 10.2967/jnmt.118.217455. [DOI] [PubMed] [Google Scholar]
- 195.Imberti C., Adumeau P., Blower J.E., Al Salemee F., Torres J.B., Lewis J.S., Zeglis B.M., Terry S.Y.A., Blower P.J. Manipu-lating the In Vivo Behaviour of 68Ga with Tris (Hydroxypyridinone) Chelators: Pretargeting and Blood Clearance. Int. J. Mol. Sci. 2020;21:1496. doi: 10.3390/ijms21041496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Martens R.J., Mealey K., Cohen N.D., Harrington J.R., Chaffin M.K., Taylor R.J., Bernstein L.R. Pharmacokinetics of gallium maltolate after intragastric administration in neonatal foals. Am. J. Vet. Res. 2007;68:1041–1044. doi: 10.2460/ajvr.68.10.1041. [DOI] [PubMed] [Google Scholar]
- 197.Monk C.S., Sweeney R.W., Bernstein L.R., Fecteau M.E. Serum and tissue concentrations of gallium after oral ad-ministration of gallium nitrate and gallium maltolate to neonatal calves. Am. J. Vet. Res. 2016;77:151–155. doi: 10.2460/ajvr.77.2.151. [DOI] [PubMed] [Google Scholar]
- 198.Pollina G.F., Pepe M., Dean A., Di Marco V., Marton D. Reduction in absorption of gallium maltolate in adult horses following oral administration with food: Chemistry and pharmacokinetics. J. Vet. Pharmacol. Ther. 2013;36:456–461. doi: 10.1111/jvp.12022. [DOI] [PubMed] [Google Scholar]
- 199.Sampieri F., Alcorn J., Allen A.L., Clark C.R., Vannucci F.A., Pusterla N., Mapes S., Ball K.R., Dowling P.M., Thompson J., et al. Pharmacokinetics of gallium maltolate in Lawsonia intracellularis-infected and uninfected rabbits. J. Vet. Pharmacol. Ther. 2014;37:486–499. doi: 10.1111/jvp.12114. [DOI] [PubMed] [Google Scholar]
- 200.Gaur K., Vázquez-Salgado A.M., Duran-Camacho G., Dominguez-Martinez I., Benjamín-Rivera J.A., Fernández-Vega L., Carmona Sarabia L., Cruz García A., Pérez-Deliz F., Méndez Román J.A., et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics. 2018;6:126. doi: 10.3390/inorganics6040126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mannargudi M.B., Deb S. Clinical Pharmacology and Clinical Trials of Ribonucleotide Reductase Inhibitors: Is It a Viable Cancer Therapy? J. Cancer Res. Clin. Oncol. 2017;143:1499–1529. doi: 10.1007/s00432-017-2457-8. [DOI] [PubMed] [Google Scholar]
- 202.Thelander L., Gräslund A. Mechanism of inhibition of mammalian ribonucleotide reductase by the iron chelate of 1-formylisoquinoline thiosemicarbazone. Destruction of the tyrosine free radical of the enzyme in an oxygen-requiring reaction. J. Biol. Chem. 1983;258:4063–4066. doi: 10.1016/S0021-9258(18)32582-1. [DOI] [PubMed] [Google Scholar]
- 203.Ganeshaguru K., Lally J.M., Piga A., Hoffbrand A.V., Kontoghiorghes G.J. Cytotoxic Mechanisms of Iron Chelators. Drugs Today. 1992;28:29–34. [Google Scholar]
- 204.Lui G.Y., Kovacevic Z., Richardson V., Merlot A.M., Kalinowski D.S., Richardson D.R. Targeting cancer by binding iron: Dissecting cellular signaling pathways. Oncotarget. 2015;6:18748–18779. doi: 10.18632/oncotarget.4349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Blatt J., Taylor S., Kontoghiorghes G.J. Comparison of antineuroblastoma activity of desferrioxamine with that of oral iron chelators. Cancer Res. 1989;49:2925–2927. [PubMed] [Google Scholar]
- 206.Kontoghiorghes G.J. Iron Mobilization from Transferrin and Non-Transferrin-Bound-Iron by Deferiprone. Implications in the Treatment of Thalassemia, Anemia of Chronic Disease, Cancer and Other Conditions. Hemoglobin. 2006;30:183–200. doi: 10.1080/03630260600642450. [DOI] [PubMed] [Google Scholar]
- 207.Weinberg E.D. The development of awareness of the carcinogenic hazard of inhaled iron. Oncol. Res. 1999;11:109–113. [PubMed] [Google Scholar]
- 208.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E., Patel D.N., Bauer A.J., Cantley A.M., Yang W.S., et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cao J.Y., Dixon S.J. Mechanisms of ferroptosis. Cell. Mol. Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Gao W., Wang X., Zhou Y., Wang X., Yu Y. Autophagy, ferroptosis, pyroptosis, and necroptosis in tumor immuno-therapy. Signal Transduct. Target. Ther. 2022;7:196. doi: 10.1038/s41392-022-01046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lei G., Zhuang L., Gan B. Targeting ferroptosis as a vulnerability in cancer. Nat. Rev. Cancer. 2022;22:381–396. doi: 10.1038/s41568-022-00459-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wang D., Le Tang L., Zhang Y., Ge G., Jiang X., Mo Y., Wu P., Deng X., Li L., Zuo S., et al. Regulatory pathways and drugs associated with ferroptosis in tumors. Cell Death Dis. 2022;13:544. doi: 10.1038/s41419-022-04927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yang Y., Wang Y., Guo L., Gao W., Tang T.-L., Yan M. Interaction between macrophages and ferroptosis. Cell Death Dis. 2022;13:355. doi: 10.1038/s41419-022-04775-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Zhou Z., Xu B., Hu N., Guo Z., Bao W., Shao B., Yang W. Targeting the Macrophage-Ferroptosis Crosstalk: A Novel Insight into Tumor Immunotherapy. Front. Biosci. 2022;27:203. doi: 10.31083/j.fbl2707203. [DOI] [PubMed] [Google Scholar]
- 215.Miao M., Wu M., Li Y., Zhang L., Jin Q., Fan J., Xu X., Gu R., Hao H., Zhang A., et al. Clinical Potential of Hypoxia Inducible Factors Prolyl Hydroxylase Inhibitors in Treating Nonanemic Diseases. Front. Pharmacol. 2022;13:837249. doi: 10.3389/fphar.2022.837249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Tsui K.-H., Chung L.-C., Wang S.-W., Feng T.-H., Chang P.-L., Juang H.-H. Hypoxia upregulates the gene expression of mitochondrial aconitase in prostate carcinoma cells. J. Mol. Endocrinol. 2013;51:131–141. doi: 10.1530/JME-13-0090. [DOI] [PubMed] [Google Scholar]
- 217.Singh K.K., Desouki M.M., Franklin R.B., Costello L.C. Mitochondrial aconitase and citrate metabolism in malignant and nonmalignant human prostate tissues. Mol. Cancer. 2006;5:14. doi: 10.1186/1476-4598-5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Vyas S., Zaganjor E., Haigis M.C. Mitochondria and Cancer. Cell. 2016;166:555–566. doi: 10.1016/j.cell.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Orfanou I.-M., Argyros O., Papapetropoulos A., Tseleni-Balafouta S., Vougas K., Tamvakopoulos C. Discovery and Pharmacological Evaluation of STEAP4 as a Novel Target for HER2 Overexpressing Breast Cancer. Front. Oncol. 2021;11:608201. doi: 10.3389/fonc.2021.608201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chekmarev J., Azad M.G., Richardson D.R. The Oncogenic Signaling Disruptor, NDRG1: Molecular and Cellular Mechanisms of Activity. Cells. 2021;10:2382. doi: 10.3390/cells10092382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang P., Tchou-Wong K.M., Costa M. Egr-1 mediates hypoxia-inducible transcription of the NDRG1 gene through an overlapping Egr-1/Sp1 binding site in the promoter. Cancer Res. 2007;67:9125–9133. doi: 10.1158/0008-5472.CAN-07-1525. [DOI] [PubMed] [Google Scholar]
- 222.Simões R.V., Veeraperumal S., Serganova I.S., Kruchevsky N., Varshavsky J., Blasberg R.G., Ackerstaff E., Koutcher J.A. Inhibition of prostate cancer proliferation by Deferiprone. NMR Biomed. 2017;30:e3712. doi: 10.1002/nbm.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Fiorillo M., Tóth F., Brindisi M., Sotgia F., Lisanti M.P. Deferiprone (DFP) Targets Cancer Stem cell (CSC) Propagation by Inhibiting Mitochondrial Metabolism and Inducing ROS Production. Cells. 2020;9:1529. doi: 10.3390/cells9061529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Donfrancesco A., Deb G., Domicini C., Angioni A., Caniglia M., De Sio L., Fidani P., Amici A., Helson L. Deferoxamine, cyclophosphamide, etoposide, carboplatin and thotepa (DCECaT): A new cytoreductive chelation-chemotherapy regimen in patients with advanced neuroblastoma. Am. J. Clin. Oncol. 1992;15:319–322. doi: 10.1097/00000421-199208000-00009. [DOI] [PubMed] [Google Scholar]
- 225.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics, 2022. CA Cancer J. Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 226.Ramaswamy S., Ross K.N., Lander E.S., Golub T.R. A molecular signature of metastasis in primary solid tumours. Nature Genet. 2003;33:49–54. doi: 10.1038/ng1060. [DOI] [PubMed] [Google Scholar]
- 227.Kontoghiorghes G.J., Piga A., Hoffbrand A. Cytotoxic effects of the lipophilic iron chelator omadine. FEBS Lett. 1986;204:208–212. doi: 10.1016/0014-5793(86)80813-4. [DOI] [PubMed] [Google Scholar]
- 228.Cheng R., Gadde R., Fan Y., Kulkarni N., Shevale N., Bao K., Choi H.S., Betharia S., Kim J. Reversal of genetic brain iron accumulation by N,N′-bis(2-mercaptoethyl)isophthalamide, a lipophilic metal chelator, in mice. Arch. Toxicol. 2022;96:1951–1962. doi: 10.1007/s00204-022-03287-1. [DOI] [PubMed] [Google Scholar]
- 229.Kontoghiorghes G.J., Piga A., Hoffbrand A.V. Cytotoxic and DNA-inhibitory effects of iron chelators on human leukaemic cell lines. Hematol. Oncol. 1986;4:195–204. doi: 10.1002/hon.2900040303. [DOI] [PubMed] [Google Scholar]
- 230.Kontoghiorghes G.J. Deferiprone: A Forty-Year-Old Multi-Targeting Drug with Possible Activity against COVID-19 and Diseases of Similar Symptomatology. Int. J. Mol. Sci. 2022;23:6735. doi: 10.3390/ijms23126735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Park D., Lobbous M., Nabors L.B., Markert J.M., Kim J. Undesired impact of iron supplement on MRI assessment of post-treatment glioblastoma. CNS Oncol. 2022;11:CNS90. doi: 10.2217/cns-2021-0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Talboom K., Borstlap W.A.A., Roodbeen S.X., Bruns E.R.J., Buskens C.J., Hompes R., Tytgat K.M.A.J., Tuynman J.B., Consten E.C.J., Heuff G., et al. FIT collaborative group. Ferric carboxymaltose infusion versus oral iron supplementation for preoperative iron deficiency anaemia in patients with colorectal cancer (FIT): A multicentre, open-label, randomised, controlled trial. Lancet Haematol. 2023. [DOI] [PubMed]
- 233.Kontoghiorghes G.J. How to manage iron toxicity in post-allogeneic hematopoietic stem cell transplantation? Expert Rev. Hematol. 2020;13:299–302. doi: 10.1080/17474086.2020.1719359. [DOI] [PubMed] [Google Scholar]
- 234.Kontoghiorghes G.J. Advances in oral iron chelation in man. Int. J. Hematol. 1992;55:27–38. [PubMed] [Google Scholar]
- 235.Kontoghiorghes G.J., Kolnagou A. Effective new treatments of iron overload in thalassaemia using the ICOC combination therapy protocol of deferiprone (L1) and deferoxamine and of new chelating drugs. Haematologica. 2006;91:ELT04. [PubMed] [Google Scholar]
- 236.Kolnagou A., Economides C., Eracleous E., Kontoghiorghes G.J. Long Term Comparative Studies in Thalassemia Patients Treated with Deferoxamine or a Deferoxamine/Deferiprone Combination. Identification of Effective Chelation Therapy Protocols. Hemoglobin. 2008;32:41–47. doi: 10.1080/03630260701727085. [DOI] [PubMed] [Google Scholar]
- 237.Kontoghiorghes G.J., Kolnagou A., Kontoghiorghe C.N., Mourouzidis L., Timoshnikov V.A., Polyakov N.E. Trying to Solve the Puzzle of the Interaction of Ascorbic Acid and Iron: Redox, Chelation and Therapeutic Implications. Medicines. 2020;7:45. doi: 10.3390/medicines7080045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Timoshnikov V.A., Kobzeva T.V., Polyakov N.E., Kontoghiorghes G.J. Redox Interactions of Vitamin C and Iron: Inhibition of the Pro-Oxidant Activity by Deferiprone. Int. J. Mol. Sci. 2020;21:3967. doi: 10.3390/ijms21113967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Timoshnikov V.A., Selyutina O.Y., Polyakov N.E., Didichenko V., Kontoghiorghes G.J. Mechanistic Insights of Chelator Complexes with Essential Transition Metals: Antioxidant/Pro-Oxidant Activity and Applications in Medicine. Int. J. Mol. Sci. 2022;23:1247. doi: 10.3390/ijms23031247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Konstantinou E., Pashalidis I., Kolnagou A., Kontoghiorghes G.J. Interactions of Hydroxycarbamide (Hydroxyurea) with Iron and Copper: Implications on Toxicity and Therapeutic Strategies. Hemoglobin. 2011;35:237–246. doi: 10.3109/03630269.2011.578950. [DOI] [PubMed] [Google Scholar]
- 241.Voest E.E., Vreugdenhil G., Marx J.J.M. Iron-Chelating Agents in Non-Iron Overload Conditions. Ann. Intern. Med. 1994;120:490–499. doi: 10.7326/0003-4819-120-6-199403150-00008. [DOI] [PubMed] [Google Scholar]
- 242.Fernandes K.E., Weeks K., Carter D.A. Lactoferrin Is Broadly Active against Yeasts and Highly Synergistic with Amphotericin B. Antimicrob. Agents Chemother. 2020;64:e02284-19. doi: 10.1128/AAC.02284-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Rosa L., Cutone A., Lepanto M.S., Paesano R., Valenti P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017;18:1985. doi: 10.3390/ijms18091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.González-Chávez S.A., Arévalo-Gallegos S., Rascón-Cruz Q. Lactoferrin: Structure, function and applications. Int. J. Antimicrob. Agents. 2009;33:301.e1–8. doi: 10.1016/j.ijantimicag.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 245.Lan X., Zhao C., Chen X., Zhang P., Zang D., Wu J., Chen J., Long H., Yang L., Huang H., et al. Platinum pyrithione induces apoptosis in chronic myeloid leukemia cells resistant to imatinib via DUB inhibition-dependent caspase activation and Bcr-Abl downregulation. Cell Death Dis. 2017;8:e2913. doi: 10.1038/cddis.2017.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Huang H., Ni Liu N., Liao Y., Liu N., Cai J., Xia X., Guo Z., Li Y., Wen Q., Yin Q., et al. Platinum-containing compound platinum pyrithione suppresses ovarian tumor proliferation through proteasome inhibition. J. Exp. Clin. Cancer Res. 2017;36:79. doi: 10.1186/s13046-017-0547-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Muthyala R., Shin W.S., Xie J., Sham Y.Y. Discovery of 1-hydroxypyridine-2-thiones as selective histone deacetylase inhibitors and their potential application for treating leukemia. Bioorg. Med. Chem. Lett. 2015;25:4320–4324. doi: 10.1016/j.bmcl.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 248.Naves M.A., Graminha A.E., Vegas L.C., Luna-Dulcey L., Honorato J., Menezes A.C.S., Batista A.A., Cominetti M.R. Transport of the Ruthenium Complex [Ru(GA)(dppe)2]PF6 into Triple-Negative Breast Cancer Cells Is Facilitated by Transferrin Receptors. Mol. Pharm. 2019;16:1167–1183. doi: 10.1021/acs.molpharmaceut.8b01154. [DOI] [PubMed] [Google Scholar]
- 249.Peng H., Jin H., Zhuo H., Huang H. Enhanced antitumor efficacy of cisplatin for treating ovarian cancer in vitro and in vivo via transferrin binding. Oncotarget. 2017;8:45597–45611. doi: 10.18632/oncotarget.17316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Kontoghiorghes G.J., Kolnagou A. Molecular factors and mechanisms affecting iron and other metal excretion or absorption in health and disease. The role of natural and synthetic chelators. Curr. Med. Chem. 2005;12:2695–2709. doi: 10.2174/092986705774463030. [DOI] [PubMed] [Google Scholar]
- 251.Heppner D.G., Hallaway P.E., Kontoghiorghes G.J., Eaton J.W. Antimalarial properties of orally active iron chelators. Blood. 1988;72:358–361. doi: 10.1182/blood.V72.1.358.358. [DOI] [PubMed] [Google Scholar]
- 252.Baldari S., Di Rocco G., Toietta G. Current Biomedical Use of Copper Chelation Therapy. Int. J. Mol. Sci. 2020;21:1069. doi: 10.3390/ijms21031069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Barve A., Kumbhar A., Bhat M., Joshi B., Butcher R., Sonawane U., Joshi R. Mixed-ligand copper (II) maltolate complexes: Synthesis, characterization, DNA binding and cleavage, and cytotoxicity. Inorg. Chem. 2009;48:9120–9132. doi: 10.1021/ic9004642. [DOI] [PubMed] [Google Scholar]
- 254.Mehtab S., Goncalves G., Roy S., Tomaz A.I., Santos-Silva T., Santos M.F.A., Romao M.J., Jakusch T., Kiss T., Pessoa J.C. Interaction of vanadium(IV) with human serum apo-transferrin. J. Inorg. Biochem. 2013;121:187–195. doi: 10.1016/j.jinorgbio.2012.12.020. [DOI] [PubMed] [Google Scholar]
- 255.Levina A., Lay P.A. Transferrin Cycle and Clinical Roles of Citrate and Ascorbate in Improved Iron Metabolism. ACS Chem. Biol. 2019;14:893–900. doi: 10.1021/acschembio.8b01100. [DOI] [PubMed] [Google Scholar]
- 256.Page M.G.P. The Role of Iron and Siderophores in Infection, and the Development of Siderophore Antibiotics. Clin. Infect. Dis. 2019;69:S529–S537. doi: 10.1093/cid/ciz825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wilson B.R., Bogdan A.R., Miyazawa M., Hashimoto K., Tsuji Y. Siderophores in Iron Metabolism: From Mechanism to Therapy Potential. Trends Mol. Med. 2016;22:1077–1090. doi: 10.1016/j.molmed.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kojima Y., Yoshikawa Y., Ueda E., Kondo M., Takahashi S., Matsukura T., Sakurai H., Hiroi T., Imaoka S., Funae Y. Blood glucose lowering and toxicological effects of zinc (II) complexes with maltol, threonine, and picolinic acid. Res. Commun. Mol. Pathol. Pharmacol. 2002;112:91–104. [PubMed] [Google Scholar]
- 259.Ibrahim A.S., Edwards J.J.E., Fu Y., Spellberg B. Deferiprone iron chelation as a novel therapy for experimental mucormycosis. J. Antimicrob. Chemother. 2006;58:1070–1073. doi: 10.1093/jac/dkl350. [DOI] [PubMed] [Google Scholar]
- 260.Fukuda S., Ikeda M., Nakamura M., Katoh A., Yan X., Xie Y., Kontoghiorghes G.J. The Effects of Bicarbonate and its Combination with Chelating Agents Used for the Removal of Depleted Uranium in Rats. Hemoglobin. 2008;32:191–198. doi: 10.1080/03630260701727093. [DOI] [PubMed] [Google Scholar]
- 261.Fukuda S., Ikeda M., Anzai K., Suzuki M., Katoh A., Kontoghiorghes G.J. Radiation Protection by Deferiprone in Animal Models. Hemoglobin. 2006;30:201–208. doi: 10.1080/03630260600642484. [DOI] [PubMed] [Google Scholar]
- 262.Eybl V., Svihovcova P., Koutensky J., Kontoghiorghes G.J. Interaction of L1, L1NAll and deferoxamine with gallium in vivo. Drugs Today. 1992;28:173–175. [Google Scholar]
- 263.Badawy S.M., Kattamis A., Ezzat H., Deschamps B., Sicard E., Fradette C., Zhao F., Tricta F., Tsang Y.C., Sheth S., et al. The safety and acceptability of twice-daily deferiprone for transfusional iron overload: A multicentre, open-label, phase 2 study. Br. J. Haematol. 2022;197:e12–e15. doi: 10.1111/bjh.17999. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.