Abstract
Mycobacterium tuberculosis alone induces small, donor-variable amounts of tumor necrosis factor alpha (TNF-α) from primary human monocytes in vitro. However, TNF-α release is increased 5- to 500-fold when fixed activated T cells (FAT) or their isolated, unfixed membranes are added to this system. This FAT-induced synergy was at least as potent as that induced by gamma interferon (IFN-γ) at 100 U/ml. FAT-enhanced TNF-α production is at least in part transcriptionally mediated, as reflected by quantitative changes in TNF-α mRNA between 2 and 6 h poststimulation. Unlike IFN-γ-cocultured cells, FAT-treated monocytes appeared not to have enhanced TNF-α message stability, suggesting that de novo transcription may be involved in this effect. Furthermore, M. tuberculosis alone induced only minimal DNA binding of monocyte NF-κB, but cells treated with M. tuberculosis and FAT potentiated NF-κB activity more effectively. It is therefore possible that one mechanism by which FAT synergize with M. tuberculosis to stimulate TNF-α production is via NF-κB-enhanced transcription. These data strongly suggest that in the interaction of cells involved in the immune response to M. tuberculosis, T-cell stimulation of monocyte TNF-α production involves a surface membrane interaction(s) as well as soluble mediators.
Tumor necrosis factor alpha (TNF-α) has been implicated in both the pathophysiology and protective host response to Mycobacterium tuberculosis and other mycobacterial infections (7, 16, 42). Elevated levels of TNF-α are present at the site of M. tuberculosis infection, and monocytes from tuberculosis patients produce more TNF-α in vitro than monocytes from uninfected donors (2, 40). However, in the absence of costimuli, isolated human monocytes produce only moderate amounts of TNF-α in vitro in response to M. tuberculosis compared to other TNF-α-eliciting agents (28). Coculture with the Th1-derived cytokine gamma interferon (IFN-γ) markedly enhances the amount of TNF-α released in response to M. tuberculosis in vitro (28), but in vivo there appear to be other, IFNγ-independent mechanisms for increasing TNF-α production in response to M. tuberculosis (15).
Secreted products are not the only pathway of cognitive T cell-monocyte interaction. Direct contact with T cells in vitro, even in the presence of anticytokine antibodies or emetine, stimulates macrophage antimycobacterial functions, while supernatants of these cells have little effect (36, 39). Other monocyte functions, including TNF-α secretion, can be stimulated, in the absence of cofactors, by activated T cells which have been washed and then fixed in paraformaldehyde (fixed activated T cells [FAT]) (47, 48). Direct cell-cell contact is required for such stimulation, and in sites of inflammation, especially in the granuloma, such intercellular contact is common. Thus, it is important to assess the potential contribution of such membrane-mediated interaction to monokine production in the presence of M. tuberculosis.
TNF-α production in monocytes is regulated at multiple intracellular levels, beginning with transcription (13, 27, 30, 37). Increased amounts of tumor necrosis factor (TNF) mRNA (17) and activation of a relevant transcription factor, NF-κB (41, 44, 52), have been reported in monocytic cells treated with M. tuberculosis. Adhesive substrates such as fibronection and cross-linkage of specific monocyte surface molecules (e.g., CD44, CD45, CD58, and CD40) also stimulate these events (12, 18, 19, 21, 51). Furthermore, IFN-γ, which synergistically stimulates TNF-α production from M. tuberculosis-treated monocytes (7), not only increases transcription of TNF-α in some systems but it also promotes mRNA stability and translation (3, 7, 38). We therefore sought to compare and contrast the effects of T cell-monocyte contact and the effects of IFN-γ at the level of transcription and NF-κB activation.
MATERIALS AND METHODS
Materials.
Escherichia coli lipopolysaccharide O127:B8 was purchased from Sigma (Poole, England). All other materials in tissue culture had a final endotoxin content of ≤10 pg/ml, as calculated from the manufacturer's specifications. Recombinant human IFN-γ and monoclonal anti-human IFN-γ were purchased from R&D Systems (Abingdon, England). Unless otherwise stated, all other reagents for tissue culture were purchased from Sigma. Anti-CD18, CD11b and isotype control immunoglobulin G1 (IgG1) antibodies were purchased from Dakopatt (Copenhagen, Denmark), dialyzed, and incubated with polymyxin B before being added to cultures at a final concentration of 10 μg/ml; control IgG, did not stimulate TNF-α production.
Preparation of fixed T cells or T-cell membranes.
The primary source of fixed activated T cells was the HUT78 cell line, which was obtained from the National Institute of Biological Standards and Controls AIDS Reagent Programme (South Mimms, England) and was tested for mycoplasma using the Genprobe (San Diego, Calif.) Mycoplasma detection kit. Autologous T cells were elutriated from peripheral blood at 16 to 18 ml/min (see below) and then passed through a nylon wool column (Novamed, Jerusalem, Israel). Purified protein derivative (PPD)-specific T cells were generated from PPD-stimulated peripheral blood mononuclear cells (PBMC) by bimonthly interleukin-2 (IL-2) starvation followed by stimulation with irradiated, PPD-pulsed autologous feeders; during intervening passages, cells received 2 to 5 U of IL-2 per ml every 3 to 4 days. After 10 weeks the cell lines were screened for specificity by [3H]thymidine incorporation.
T cells were activated with 1 μg of phytohemagglutinin (PHA) and 5 ng of phorbol myristate acetate (PMA) for 20 h, washed, fixed in 1% paraformaldehyde, and washed again as previously described (47). Crude membrane fractions were prepared as previously described (20, 47) from HUTs activated as above, washed, and mechanically lysed in a sucrose buffer, followed by differential centrifugation.
Bacterial stocks, infective inoculum, and assay of bacterial viability.
Mouse-passaged M. tuberculosis H37Rv (a gift from J. Dhillon, St. George's Hospital Medical School, London), grown in broth as described below, was used unless otherwise specified. A gamma-irradiated stock for use in selected experiments was produced from these broth cultures by exposure to 2.5 Mrad; cultures were then counted and frozen, and aliquots were checked for viability in Kirschner's medium and 7H9 broth and on 7H10 agar (Difco, Detroit, Mich.). The 7416 H37Rv NCTC reference strain ATCC 9360 was obtained from the Public Health Laboratory Service (London). Clinical M. tuberculosis isolates were a gift from B. Allen (St. George's Medical School). All M. tuberculosis samples were grown in 7H9 broth supplemented with 10% albumin-dextrose-citrate (Difco) and polymyxin B (20 μg/ml), and without Tween. Inocula were washed and sonicated as previously described (50), and the resulting almost entirely single-cell suspension was counted in a Helber chamber and resuspended to the desired concentration in warm RPMI. An aliquot was plated on 7H10 to retrospectively assess viability. TNF-α results obtained with our stocks were verified in triplicate experiments with the NTCC H37Rv reference strain, another mouse-passaged H37Rv lab stock, and two M. tuberculosis clinical isolates. An infective ratio of 1:1 was used throughout because, although a 10:1 ratio of M. tuberculosis to monocytes induced the largest amount of TNF-α (2.3 to 3.5 ng/ml, n = 4), the viability of the monocyte population suffered considerably at this inoculum.
Viability of cell-associated bacteria was assayed by CFU as previously described (50). Briefly, monocytes were lysed, and bacterial clumps were dispersed by three 3-s sonications in 0.2% sodium dodecyl sulfate, then samples were serially diluted and plated on 7H10, and the number of CFU was counted after 4 weeks of incubation.
Isolation, culture, and stimulation of human monocytes.
Monocytes were isolated from pooled buffy coats (South Thames Blood Transfusion Service, London, England) or venous blood from laboratory volunteers in 10 mM EDTA as previously described (31). Briefly, cells were separated from plasma by centrifugation at 300 × g, and then erythrocytes were sedimented in 0.6% dextran (Pharmacia, Uppsala, Sweden). The resulting leukocyte-rich plasma was harvested, washed, resuspended in elutriation buffer (Hanks' balanced salt solution without Ca2+ or Mg2+, containing 1 mM EDTA and 1% autologous platelet-poor plasma) and loaded on a Beckman J6/JE-5.0 elutriator (running at 600 × g continuously) at a flow rate of 14 ml per min. Monocytes (87 to 95% pure by subsequent Giemsa staining) were eluted at between 20 and 22 ml per min as previously described (10), washed, and resuspended to 1.5 × 106/ml in warm RPMI 1640. In most cases, they were allowed to adhere to tissue culture plastic; in selected experiments, elutriated monocytes were added to Teflon (Savillex, Minnitonka, Minn.) or fibronectin-coated (Becton Dickinson, Franklin Lakes, N.J.) inserts. After 90 min, nonadherent cells were washed off (except in the Teflon wells) and the medium was replaced with RPMI 1640 containing 2 mM l-glutamine and 2% autologous serum. This medium was used throughout the subsequent experiments unless otherwise indicated. These cultures were allowed to quiesce overnight before the supernatant was replaced with fresh medium with or without the indicated mediators and/or bacteria. The cultures were allowed to incubate for a further 1 to 72 h before harvest. Apart from small differences dependent on the day of infection, the release of TNF-α was not appreciably altered by manipulations of culture conditions which included adherence substrate, fresh blood versus buffy coat, live versus γ-irradiated M. tuberculosis, culture and/or pretreatment of M. tuberculosis with polymyxin B (20 μg/ml), addition or subtraction of contaminating cell populations, or harvesting supernatants at later time points up to 72 h. In selected experiments (n = 3), monocyte cultures were treated with antibodies to CD11b, CD18, or an IgG1 isotype control for 30 min prior to the addition of FAT with or without M. tuberculosis.
Assay for immunoreactive TNF-α.
Monocytes were separated and cultured overnight prior to stimulation as described above. At the indicated time point poststimulation (usually 24 h), culture supernatants were harvested and centrifuged at 12,000 × g for 5 min. The resulting supernatants were frozen at −70°C and subsequently assayed by sandwich enzyme-linked immunosorbent assay (ELISA) using R&D Systems protocol AXMAB610, anti-human TNF-α antibodies (which detect both free and receptor-bound TNF-α), and control recombinant human TNF-α. Results were analyzed by three-way or four-way analysis of variance, followed by t test to compare specific conditions.
RNA extraction and RNase protection assay.
Between 1 and 8 h poststimulation, cultures of 106 monocytes were washed in RPMI, harvested, and lysed in 1 ml of RNAsol (phenol-and guanidium thiocyanate-based compound; Genosys, Cambridge, England) containing 4 μg of carrrier rRNA (Pharmacia). Lysates were immediately frozen at −70°C. RNA was later extracted in chloroform and precipitated according to the manufacturer's (Genosys) protocol. The RNA pellet was resuspended in 20 μl of Tris-EDTA and stored at −70°C until assay.
DNA templates for TNF-α and the housekeeping gene γ-actin were gifts from S. Goodbourne (St. George's Hospital Medical School). RNA probes were generated from these templates using the Maxiscript kit (Ambion, Austin, Tex.) and subsequently gel purified. The T7 RNA polymerase with 3.3 μM (50 μCi) [α-32P]UTP was used to produce the TNF-α probe, and γ-actin RNA was made with SP6 and 1.4 μM (14 μCi) [α-32P]UTP diluted 25-fold with unlabeled UTP.
RNase protection assays were performed using the RPAIII kit (Ambion) according to the manufacturer's protocol except that a phenol-choloroform extraction step was added following digestion of unhybridized RNA. The final products were electrophoresed on a 6% reducing gel and quantitated by phosphoimaging. Results are shown as a ratio of TNF-α (234 nucleotides [nt]) to γ-actin (155 nt), expressed as a percentage of the control, for each sample. (Nuclear run-on assays were precluded on the grounds of safety.)
Preparation of nuclear extracts and EMSA.
For the electrophoretic mobility shift assay (EMSA), flasks containing 3 × 106 monocytes, separated and cultured as described above, were stimulated for 60 to 90 min before being washed and gently scraped into ice-cold phosphate-buffered saline (PBS). For most experiments, cells from two donors were stimulated in parallel and then pooled when they were harvested in ice-cold PBS. Cells were pelleted at 400 × g for 10 min, lysed in nuclear isolation buffer (11) containing 0.5% NP-40, and centrifuged at 500 × g for 5 min. The resulting nuclear pellet was gently washed in nuclear isolation buffer without NP-40, recentrifuged, and then gradually resuspended in a 0.32 M high-salt buffer as previously described (49). Nuclei were incubated at +4°C for 1 h and then centrifuged at 12,000 × g for 15 min, and the supernatant was frozen at −70°C.
EMSA was performed as previously described (49) using 10 μg of nuclear extract and an [α-32P]ATP end-labeled probe, GATCGTGGGAAATTGATC, containing consensus sequences for both NF-κB and RBPJκ (recombinant binding protein Jκ, a constitutive DNA binding protein; a gift of S. Goodbourne, St. George's). The ratio of NFκB-bound probe to RBPJκ-bound probe was quantitated on a Storm phosphoimager. Anti-p50 antiserum (donated by Ron Hay to the Medical Research Council AIDS Reagent Program, National Institute of Biological Standards and Controls) was used to confirm the identity of the complex by supershifting.
RESULTS
Induction of TNF-α by M. tuberculosis.
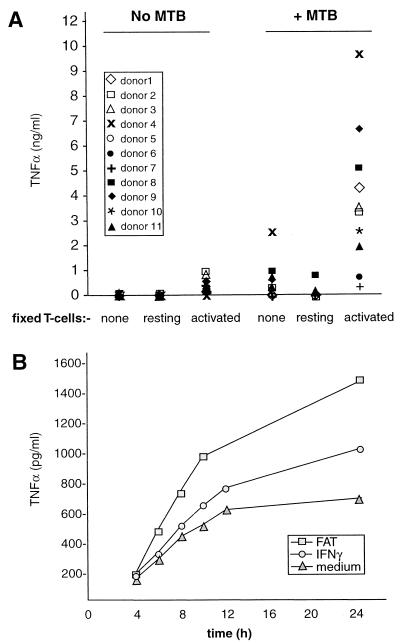
Peripheral blood monocytes purified by elutriation to 90% and then by plastic adherence to a final purity of 98% were cultured overnight to quiesce. Single-cell suspensions of M. tuberculosis H37Rv were added to these cultures in an infective ratio of 1:1, which induced detectable TNF-α (Fig. 1A) without significant loss of monocyte viability.
FIG. 1.
FAT synergize with M. tuberculosis for enhanced TNF-α production. (A) Primary monocytes were cultured in the absence or presence of M. tuberculosis (MTB; 1 bacterium per monocyte) as indicated. Some of these cultures also received 1 fixed HUT T cell (resting or activated) per monocyte, as indicated, at the time of infection. Twenty-four hours later, supernatants were collected and assayed for TNF-α by ELISA. Each symbol represents 1 of 11 donors and indicates the mean of triplicate cultures from an individual donor. M. tuberculosis and FAT individually stimulated (P < 0.001) TNF-α production compared to unstimulated monocytes. However, TNF-α production from cultures receiving both FAT and M. tuberculosis was significantly higher (P < 0.001) compared to cultures receiving either stimulus alone. Time course of TNF-α release is shown in panel B. Monocytes were stimulated as above with M. tuberculosis in the absence or presence of either FAT or IFN-γ (100 U/ml) as shown. Supernatants were harvested at the indicated time points and assayed for immunoreactive TNF-α. Results shown are from one representative of three experiments.
T-cell membranes synergize with M. tuberculosis for enhanced TNF-α production.
Addition of fixed, activated HUT-78 T cells (FAT; 1 T cell per monocyte) to monocyte cultures stimulated TNF-α release which was synergistically enhanced in the presence of an equal number of M. tuberculosis (Fig. 1). The greatest rate of TNF-α release was between 4 and 12 h (Fig. 1B), and total TNF-α in the culture medium did not increase appreciably between 24 and 72 h (not shown). The viability of M. tuberculosis was not affected up to 72 h under these conditions (n = 2, not shown), and transcription of inducible nitric oxide synthase (iNOS) from the infected human monocytes, as measured by nested PCR, was minimal (n = 3; not shown).
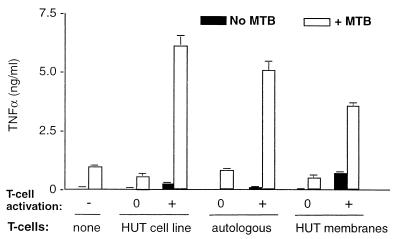
This phenomenon is unlikely to be an artifact of transformation, as fixed autologous primary T cells stimulated a similar synergistic enhancement of TNF-α as the activated HUT T-cell line (Fig. 2). In both cases enhancement appears to be dependent upon T-cell activation, as resting T-cell populations were incapable of stimulating TNF-α release (Fig. 1A and 2). However, the antigen specificity of the stimulating T cells may not be important: e.g., when antigen-specific (PPD-specific T-cell line) and non-antigen-specific T cells from the same donor were each activated in an identical manner (with PHA and PMA) prior to fixation, they stimulated similar amounts of TNF-α release from autologous monocytes (not shown).
FIG. 2.
Comparison of TNF-α-inducing effects of fixed HUTs with fixed autologous T cells or isolated membranes (prepared as described in the text). T cells were unactivated (0) or activated (+) prior to either fixation or membrane isolation. Primary monocytes were cultured in the absence (solid bars) or presence (open bars) of M. tuberculosis (MTB; 1 bacterium per monocyte) and the indicated T-cell preparation (1 T cell or equivelent per monocyte) for 24 h before supernants were collected and assayed for TNF-α by ELISA. Each bar represents the mean ± standard deviation (SD) from triplicate cultures. Results shown are from one representative of three individual experiments.
Adherence per se is also unlikely to account for either TNF-α produced in response to FAT alone or in synergy with M. tuberculosis, because monocytes adhered to plastic (standard conditions) produced similar amounts (per cell) to nonadherent (Teflon-cultured) or fibronectin-adherent cells (n = 3, not shown), although fibronectin alone induced TNF-α (≤0.2 ng/ml), consistent with previous reports (18). TNF-α production in response to FAT was, however, partially inhibited in cultures containing anti-CD18 in the presence or absence of M. tuberculosis by 39.7% (± 23.7% standard error of the mean [SEM]) and by 74.0% (± 26.5%), respectively; anti-CD11b inhibited TNF-α production in FAT- and M. tuberculosis-stimulated cultures by 37.7% (±17.1%) and FAT-only-treated wells by 58.7% (± 36.0%). These results confirm the results of others showing the involvement, but not sole responsibility, of CR3 in this process.
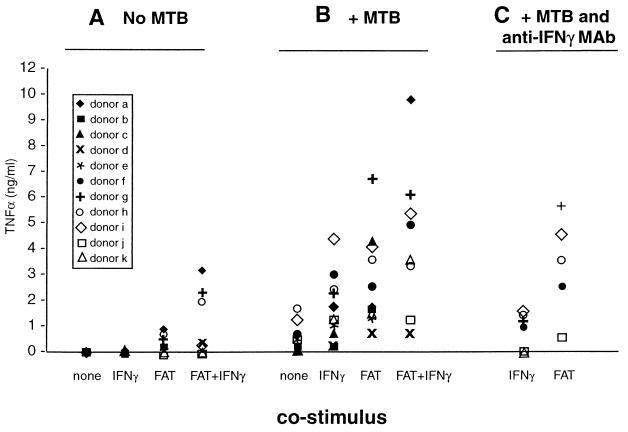
The majority of stimulatory and synergistic effects of whole fixed cells can be mimicked by membranes prepared from HUTs (20, 48), activated in parallel to those fixed and used whole (Fig. 2), further suggesting the participation of a T-cell membrane molecule in this phenomenon. Part of the reason that whole cells stimulate better than isolated membranes may be because of loss of material in membrane preparation; by increasing the amount of membrane fraction added, TNF-α release comparable to that by whole cells can be induced (not shown). The possibility that soluble factors may leak from FAT and contribute to monocyte stimulation has been previously excluded in similar systems (20, 47), in this system, coculture (rather than preincubation) of a neutralizing anti-IFN-γ antibody in cultures containing FAT plus M. tuberculosis did not significantly affect TNF-α release into the medium, while in parallel cultures stimulated with IFN-γ (100 U/ml) and M. tuberculosis, anti-IFN-γ monoclonal antibody (MAb) reduced TNF-α levels to those in cultures which received M. tuberculosis alone (Fig. 3).
FIG. 3.
FAT are at least as effective as IFN-γ in synergizing with M. tuberculosis to induce TNF-α release from monocytes. Primary monocytes were cultured in the absence (A) or presence (B and C) of M. tuberculosis (MTB; 1 bacterium per monocyte) with the indicated costimulus (FAT, 1 T cell per monocyte, or IFN-γ [100 U/ml]) for 24 h before supernants were collected and assayed for TNF-α by ELISA. The cultures from a subgroup of donors represented in C also received a monoclonal antibody against IFN-γ at the time of stimulation. Each symbol represents the release of TNF-α from the cells of an individual donor; each point represents the mean of triplicate cultures from an individual donor (points overlap on or near the baseline for unstimulated or IFN-γ-treated cells). Monocyte TNF-α release induced by FAT was higher than that stimulated by IFN-γ (P < 0.05). IFN-γ plus FAT treatment stimulated more TNF-α production in the presence or absence of M. tuberculosis than did IFN-γ alone (P = 0.01). In a separate analysis, anti-IFN-γ MAb inhibited IFN-γ (P < 0.05) but not FAT coinduction of TNF-α in the subgroup of donors (f to k) tested.
Comparison of effects of FAT with those of IFN-γ.
Many monocyte functions in response to a variety of stimuli are synergistically enhanced by IFN-γ, including the release of TNF-α from M. tuberculosis-infected cells (7, 25, 28). The ability of FAT to enhance TNF-α production relative to IFN-γ is compared in Fig. 3. Although there is variation in the response of normal human donors to all three stimuli—and more so to combinations of stimuli—FAT-costimulated TNF-α release from M. tuberculosis-infected cells is similar to (or, for most donors, better than) an optimal dose (100 U/ml) of IFN-γ (Fig. 3B). These relative amounts of elicited TNF-α remain the same regardless of experimental kinetics (not shown).
In contrast to IFN-γ, FAT are capable of stimulating secretion of TNF-α in the absence of costimuli (47, 48) (Fig. 3A). Monocytes stimulated with both FAT and IFN-γ also produced more TNF-α in the presence or absence of M. tuberculosis than IFN-γ alone. Autologous T cells (primary or PPD-specific lines) which were activated and washed but added to monocyte cultures unfixed (therefore secreting IFN-γ and other cytokines) also enhanced TNF-α production to a greater extent than did the same cells when fixed (n = 2; not shown).
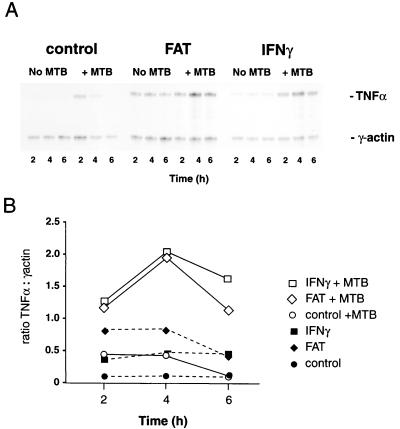
FAT and M. tuberculosis stimulate transcription of TNF-α mRNA.
mRNA was harvested from parallel cultures at earlier time points between 1 and 8 h poststimulation and assayed by an RNase protection assay. Differences in the levels of TNF-α mRNA were detectable as early as 2 h after stimulation (Fig. 4) and persisted until 8 h (n = 2; not shown). Peak increase in TNF-α mRNA was seen at 4 h (Fig. 4), and the differences in subsequent TNF-α protein release (Fig. 1B and 3) were crudely reflected in the relative amounts of TNF-α transcribed, i.e., FAT or IFN-γ synergistically augmented TNF-α release in the presence of M. tuberculosis (Fig. 4). However, in both the presence and absence of M. tuberculosis, the relative amount of RNA to protein was higher in the IFN-γ-treated cells than in FAT-treated cells (Fig. 3 and 4). mRNA from M. tuberculosis-infected cells cocultured with FAT appeared to decay earlier than that from those cocultured with IFN-γ (Fig. 4), an observation which was maintained when the transcriptional inhibitor actinomycin D was added to parallel cultures 2 h poststimulation (n = 2, not shown). Although indirect, the data suggest that FAT- and M. tuberculosis-treated cells increase TNF-α production by an increase in de novo transcription.
FIG. 4.
Effect of M. tuberculosis, FAT, and IFN-γ on TNF-α mRNA levels in primary monocytes. (A) Monocytes were treated for the indicated number of hours in the absence (left-hand lanes) or presence (right-hand lanes) of M. tuberculosis (MTB;1 bacterium per monocyte), without (top panel) or with FAT (1 cell per monocyte; middle panel) or IFN-γ (100 U/ml; lower panel) as indicated before RNA was harvested and assayed by RNase protection (as described in Materials and Methods). RNA for TNF-α (upper band, 234 nt) and γ-actin (lower band, 155 nt) is shown from one representative of three experiments. (B) Phosphoimage quantitation of A. Results shown were derived from ratios of TNF-α to γ-actin in monocyte RNA from cells treated for the indicated number of hours in the absence (solid symbols) or presence (open symbols) of M. tuberculosis without (O) or with FAT (◊) or with IFN-γ (□) as indicated.
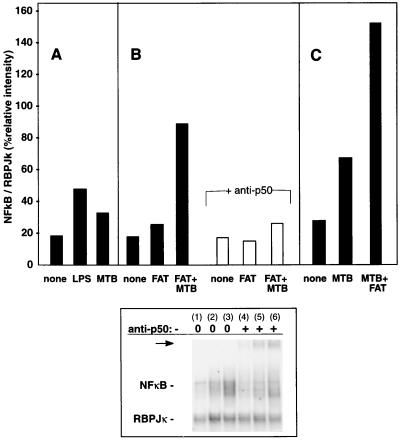
FAT and M. tuberculosis synergistically activate NF-κB.
Transcription of monocyte TNF-α in response to many stimuli involves at least some degree of NF-κB activation (13, 37, 43, 44, 45, 52). Nuclear extracts from monocytes treated with M. tuberculosis and/or FAT for 90 min were assayed for NF-κB binding activity by gel retardation. Although a physiological inoculum of M. tuberculosis does not activate NF-κB to the same level as maximal stimulation with LPS does (Fig. 5A), and FAT alone stimulated minimal monocyte NF-κB activity (Fig. 5B), together M. tuberculosis and FAT synergistically stimulated increased NF-κB binding (Fig. 5B and C). The identity of the κB binding band was confirmed by its dimunition (Fig. 5B) and supershifting (Fig. 5, top panel, arrow) with anti-NF-κB p50 antiserum. These results point to one potential mechanism for upregulation of TNF-α transcription in costimulated cells.
FIG. 5.
NF-κB activation by M. tuberculosis in the absence or presence of FAT membranes. Monocytes were stimulated with LPS (1 μg/ml) or M. tuberculosis (MTB; 3 γ-irradiated bacteria per monocyte) and/or FAT membranes (the equivalent of 3 T cells per monocyte) as indicated for 90 min before nuclear proteins were extracted and assayed by EMSA. Results shown are the percentage of radiolabeled probe binding to NF-κB relative to a constitutive internal control (RBPJκ) from one representative of two experiments for each of A, B, and C (solid bars). In addition, the identity of the NF-κB was confirmed by gel retardation with anti-NF-κB p50 antiserum (B; open bars; arrow, bottom panel). The lanes in the lower panel correspond to the bars shown in B as follows: 1 and 4, unstimulated; 2 and 5, FAT; 3 and 6, FAT plus M. tuberculosis; 1 to 3, no antibody; 4 to 6, plus anti-p50.
DISCUSSION
These results are the first formal demonstration that T-cell membranes can synergistically stimulate TNF-α from primary human monocytes infected with live M. tuberculosis. Although it has been demonstrated previously that adhesion per se (18), ligation of specific adhesion molecules (12, 51), and stabilization of the cytoskeleton by taxol (9) all stimulate monokine release by macrophages or macrophage-like cells, to date most studies of the mechanism of TNF-α release in tuberculosis have focused on soluble mediators, such as IFN-γ, as regulatory factors (7, 28). In this study we demonstrate that FAT costimulate M. tuberculosis-induced TNF-α as well as if not better than IFN-γ (Fig. 3).
These results are unlikely to be an artifact of fixation, as unfixed membrane preparations could substitute for whole fixed T cells, nor is this phenomenon likely to be a result of residual PHA-PMA carryover, because many other T-cell lines, as well as cycloheximide-treated HUTs activated and fixed as FAT used in Fig. 1, do not stimulate monokine secretion (8, 20, 47). Conversely, physiological stimuli, including those likely to be present at the site of M. tuberculosis infection, including cytokines (32) and mycobacterial antigens (5), activate T cells (subsequently washed and fixed) to stimulate monokine secretion in the absence of PHA and PMA
The T-cell ligand(s) and cognate macrophage receptors responsible for this phenomenon have not been identified. The ability of crude or partially purified membrane protein preparations or of fixed T cells to stimulate TNF-α production suggests that it is a membrane molecule or group of molecules (38) (Fig. 2). Previous studies have demonstrated that FAT prepared from HUT or primary T cells alone stimulate a low but significant level of monokine production (47, 48). Our studies confirm that while a 1:1 ratio of T cells to monocytes triggers only minimal TNF-α release in the absence of M. tuberculosis, in the presence of M. tuberculosis it potentiates monocyte TNF-α release severalfold (Fig. 1 to 3). Several membrane-associated candidate molecules modify monokine release in other systems, including CD11a,b,c, CD18, CD40L, CD69, and CD2, yet none of these molecules appears to be acting in isolation upon human monocytes (8, 14, 23, 29, 35, 47, 51). For example, FAT with undetectable levels of CD40L are capable of stimulating monokine secretion (32), and ligation of monocyte CD11/CD18 in the absence of costimulation produces no TNF-α (14, 51). These beta-integrins are involved both in intercellular adhesion and in phagocyte-M. tuberculosis binding, yet our preliminary attempts to block FAT stimulation with anti-CD11b and anti-CD18 antibodies achieved only partial inhibition of TNF-α secretion. These results were subsequently supported when neither anti-CD18, anti-CD40, nor anti-CD69 was able to completely abrogate FAT-stimulated TNF-α release from the monocytes of reactional leprosy patients (29). Antibody blocking of FAT-mediated stimulation with anti-CD69 or anti-CD2 has also achieved only partial inhibition (8, 20, 23), despite the ability of its immobilized ligand, CD58, to stimulate TNF secretion (51). In addition, antibodies to the macrophage adhesion molecules I-CAM-1 (CD54), which is significantly upregulated during M. tuberculosis infection, and the alpha integrin common chain CD29 had no effect (8, 20, 47, 51), even though adherence to fibronectin stimulates monocyte TNF-α secretion (12). Immobilized antibodies to CD45 and to the extracellular matrix III receptor CD44 stimulate monocytes to secrete modest amounts of TNF-α (19, 51), but the effect of blocking potential T-cell ligands for these molecules have not yet been examined. However, although these and other studies reinforce the notion of redundancy and/or synergy, at least in humans, among known T-cell membrane molecules that stimulate monokine secretion, they do not preclude an as-yet-unidentified molecule acting in isolation. The identification and isolation of such a molecule(s) is the subject of ongoing work.
Previous studies have demonstrated that human monocyte TNF-α is regulated primarily at the transcriptional level, with a minor translational component (13, 27, 30, 37). TNF-α mRNA accumulation in M. tuberculosis- and FAT-treated monocytes (Fig. 4) preceded protein secretion (Fig. 1B) with the appropriate kinetics to suggest a large degree of transcriptional regulation. Changes in transcript stability and/or transcriptional repression mediated by 3′ AU sequences (3, 4, 6, 30, 37, 38) vary with the stimulus and cell type studied. Although FAT- and M. tuberculosis-treated monocytes, like FAT-treated THP-1 cells (48) and like LPS-treated human monocytes (13, 27), showed no sign of enhanced TNF-α message stability in the presence of actinomycin D, these additional regulatory influences are not excluded.
Definition of the mechanism for any enhanced TNF-α transcription awaits identification of the promoter elements used in human macrophages. Three main groups of transcription factors have been implicated in the human macrophage TNF-α gene: AP-1, C/EBP, and NF-κB (22, 26, 37, 43, 45). Depending on the stimulus and system used, a role can be demonstrated for all of these transcription factors, yet none of them seems to be absolutely required for TNF-α production in human macrophages (19, 22). In addition, proteins from all three families promiscuously interact with each other in many systems to inhibit or promote transcription, depending both on the proteins and on the context of the binding sites (1). Whether these transcription factors, separately or together, are required for M. tuberculosis-stimulated human TNF-α transcription remains to be determined (22, 52).
NF-κB plays an important role in the murine TNF-α promoter (33) and is activated in human monocytes in response to many stimuli (30, 36, 37), including M. tuberculosis (41, 44, 52) (Fig. 5). The addition of FAT to M. tuberculosis-stimulated monocyte cultures synergistically increased the binding of nuclear protein extracts to κB sequences (Fig. 5). M. tuberculosis binds many macrophage surface receptors, including the toll-like receptors TLR-2 and TLR-4, leading to a series of intracellular events resembling IL-1 and LPS signaling pathways, which culminate in NF-κB activation in murine cells (24, 46). How FAT, which alone induce only minimal changes in NF-κB binding activity, could influence these pathways and result in increased NF-κB binding is not yet known. One possibility is via enhanced MEKK1 activity as a result of a cytoskeletal response to adhesive interactions with T cells (53). Another may involve interactions of NF-κB family members with other transcription factors, such as C/EBP proteins (1) activated in response to M. tuberculosis (52) as discussed above, although C/EBPβ activation in response to adherence has not yet been examined. Whatever the preceding upstream events, increased transcription factor binding to κB sites provides one mechanism by which M. tuberculosis plus FAT could stimulate de novo transcription of TNF-α mRNA.
Lymphocyte contact-dependent stimulation in the presence of M. tuberculosis may also be relevant to other aspects of macrophage biology. Although we found no difference in the viability of M. tuberculosis associated with FAT-treated monocytes versus M. tuberculosis in untreated monocytes 48 to 72 h postinfection, Silver et al. reported a reduction in M. tuberculosis CFU during the first 96 h of coculture of monocytes with unfixed lymphocytes (36). Whether there is overlap in the contact-dependent mechanisms responsible for TNF-α release or whether other mechanisms (e.g., cytotoxic T cells) operate in these latter studies (36) requires further clarification. However, as enhancement of TNF-α production by lymphocytes appears to have a strict requirement for activation (Fig. 1 and 2), while naive (prior to culture) nonadherent cells from PPD-negative donors are able to stimulate M. tuberculosis killing (36), it is likely that some differences exist.
Many other cytokine genes, adhesion molecules, and human immunodeficiency virus type 1 (HIV-1) contain promoter elements similar to the TNF-α gene and are influenced by similar stimuli, including M. tuberculosis, opening the possibility of contact-mediated modulation of these processes. It is therefore possible that T-cell contact may augment M. tuberculosis-induced modulation of HIV-1 transcription in macrophages. Indeed, engagement of CD11a, CD18, CD44, CD45, and CD58 stimulates HIV transcription in the chronically infected myelomonocytic cell line, OM10.1, although this is largely dependent on autocrine TNF-α (34). However, in the inflammatory foci, not all of the heterogeneous macrophage population may react the same to these stimuli. In fact, maturing monocytes become, at least transiently, refractory to stimuli inducing TNF production, including M. tuberculosis (28), while in some mature macrophage populations, M. tuberculosis inhibits HIV-1 transcription, possibly by inducing the expression of dominant-negative C/EBPβ isoforms (52).
In mycobacterial infection, where TNF-α appears to contribute to both the containment and pathology of the disease, novel therapeutic approaches are being proposed based on modulation of TNF-α release (7, 42). However, in our in vitro system, which was essentially free from any potential contaminating cofactors, M. tuberculosis on its own elicited only moderate amounts (0 to 0.5 ng/ml) of TNF-α from monocytes from the majority of donors. T cells were also needed for substantial TNF-α release; in this respect, surface contact was as least as potent as the secreted stimulus IFN-γ. The data presented in this paper clearly demonstrate a major role for direct cellular interaction in the production of TNF-α from monocytes. Such interaction is likely to be of significance in the host response to M. tuberculosis and should be considered in any therapeutic approach via TNF-α modulation.
ACKNOWLEDGMENTS
We thank Steve Goodbourn for providing valuable advice and reagents and Martin Bland for statistical advice and analysis.
REFERENCES
- 1.Akira S, Kiishimoto T. NF-IL6 and NF-κB in cytokine gene regulation. Adv Immunol. 1997;65:1–46. [PubMed] [Google Scholar]
- 2.Barnes P F, Fong S-J, Brennan P J, Twomey P E, Mazumder A, Modlin R L. Local production of tumor necrosis factor and IFN-γ in tuberculous pleuritis. J Immunol. 1990;145:149–154. [PubMed] [Google Scholar]
- 3.Beutler B, Tkacenko V, Milsark I, Krochin N, Cerami A. Effect of γ-interferon on cachectin expression by mononuclear phagocytes. J Exp Med. 1986;164:1791–1796. doi: 10.1084/jem.164.5.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caput D, Beutler B, Hartog K. Identification of a common nucleotide sequence in the 3′-untranslated region of GM-CSF mRNA molecules specifying inflammatory mediators. Proc Natl Acad Sci USA. 1986;83:1670–1674. doi: 10.1073/pnas.83.6.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chizzolini C, Chicheportiche R, Burger D, Dayer J M. Human Th1 cells preferentially induce interleukin (IL)-1β while Th2 cells induce IL-1 receptor antagonist production upon cell/cell contact with monocytes. Eur J Immunol. 1997;27:171–177. doi: 10.1002/eji.1830270125. [DOI] [PubMed] [Google Scholar]
- 6.Collart M A, Belin D, Vassalli J-D, de Kossodo S, Vassalli P. γ Interferon enhances macrophage transcription of the tumor necrosis factor/cachectin, interleukin 1, and urokinase genes, which are controlled by short-lived repressors. J Exp Med. 1986;164:2113–2118. doi: 10.1084/jem.164.6.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooper A, Orme I. Cytokines in immunity to tuberculosis. In: Raledge C, Dale J, editors. Mycobacteria: molecular biology and virulence. Oxford, England: Blackwell Science, Inc.; 1999. pp. 389–397. [Google Scholar]
- 8.Dayer J M, Isler P, Nicod L P. Adhesion molecules and cytokine production. Am Rev Respir Dis. 1993;148:S70–S74. doi: 10.1164/ajrccm/148.6_Pt_2.S70. [DOI] [PubMed] [Google Scholar]
- 9.Ding A H, Porteu F, Sanchez E, Nathan C F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990;248:370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- 10.Doherty D E, Haslett C, Tonnensen M G, Henson P M. Human monocyte adherence: a primary effect of chemotactic factors on the monocyte to stimulate adherence to human endothelium. J Immunol. 1987;138:1762–1771. [PubMed] [Google Scholar]
- 11.Dyer R B, Herzog N K. Isolation of intact nuclei for nuclear extract preparation from a fragile B-lymphocyte cell line. Biotechniques. 1995;19:193–195. [PubMed] [Google Scholar]
- 12.Eierman D F, Johnson C E, Haskill J S. Human monocyte inflammatory mediator gene expression is selectively regulated by adherence substrate. J Immunol. 1989;142:1970–1976. [PubMed] [Google Scholar]
- 13.Espel E, Garcia-Sanz J A, Aubert V, Menoud V, Sperisen P, Fernandez N, Spertini F. Transcriptional and translational control of TNFα gene expression in human monocytes by major histocompatibility complex class II ligands. Eur J Immunol. 1996;26:2417–2424. doi: 10.1002/eji.1830261023. [DOI] [PubMed] [Google Scholar]
- 14.Fan S T, Edgington T S. Integrin regulation of leukocyte inflammatory functions—CD11b/CD18 enhancement of the tumor-necrosis-factor-alpha responses of moncytes. J Immunol. 1993;150:2972–2980. [PubMed] [Google Scholar]
- 15.Flynn J L, Chan J, Triebold K J, Dalton D K, Stewart T A, Bloom B R. An essential role for interferon-gamma in resistance to Mycobacterium tuberculosis infection. J Exp Med. 1993;178:2249–2254. doi: 10.1084/jem.178.6.2249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flynn J L, Goldstein M M, Chan J, Triebold K J, Pfeffer K, Lowenstein C J, Schreiber R, Mak T W, Bloom B R. Tumor necrosis factor-alpha is required in the protective immune response against Mycobacterium tuberculosis in mice. Immunity. 1995;2:561–572. doi: 10.1016/1074-7613(95)90001-2. [DOI] [PubMed] [Google Scholar]
- 17.Friedland J S, Remick D G, Shattock R, Griffin G E. Secretion of interleukin-8 following phagocytosis of Mycobacterium tuberculosis by human monocyte cell lines. Eur J Immunol. 1992;22:1373–1378. doi: 10.1002/eji.1830220607. [DOI] [PubMed] [Google Scholar]
- 18.Haskill S, Johnson C, Eierman D, Becker S, Warren K. Adherence induces selective mRNA expression of monocyte mediators and proto-oncogenes. J Immunol. 1988;140:1690–1694. [PubMed] [Google Scholar]
- 19.Hayes A L, Smith C, Foxwell B M J, Brennan F M. CD45-induced tumor necrosis factor alpha production in monocytes is phosphotidylinositol 3-kinase-dependent and nuclear factor-kappa B-independent. J Biol Chem. 1999;274:33455–33461. doi: 10.1074/jbc.274.47.33455. [DOI] [PubMed] [Google Scholar]
- 20.Isler P, Vey E, Zhang J H, Dayer J M. Cell surface glycoproteins expressed on activated human T cells induce production of interleukin-1 beta by monocytic cells: a possible role of CD69. Eur Cytokine Network. 1993;4:15–23. [PubMed] [Google Scholar]
- 21.Kiener P A, Moran-Davis P, Rankin B M, Wahl A F, Aruffo A, Hollenbaugh D. Stimulation of CD40 with purified soluble GP39 induces proinflammatory responses in human monocytes. J Immunol. 1995;155:4917–4925. [PubMed] [Google Scholar]
- 22.Liu H T, Sidiropoulos P, Song G B, Pagliari L J, Birrer M J, Stein B, Anrather J, Pope R M. TNF-α gene expression in macrophages: Regulation by NF-κB is independent of c-Jun or C/EBPβ. J Immunol. 2000;164:4277–4285. doi: 10.4049/jimmunol.164.8.4277. [DOI] [PubMed] [Google Scholar]
- 23.McAllister P T, Ellis T M. CD2 regulates T cell-dependent induction of monocyte IL-1β mRNA during anti-CD3 mitogenesis. Cell Immunol. 1996;170:120–126. doi: 10.1006/cimm.1996.0141. [DOI] [PubMed] [Google Scholar]
- 24.Means T K, Jones B W, Schromm A B, Shrutleff B A, Smith J A, Keane J, Golenbock D T, Vogel S N, Fenton M J. Differential effects of a toll-like receptor antagonist on Mycobacterium tuberculosis-induced macrophage responses. J Immunol. 2001;166:4074–4082. doi: 10.4049/jimmunol.166.6.4074. [DOI] [PubMed] [Google Scholar]
- 25.Murray H W. Current and future clinical applications of interferon-gamma in host antimicrobial defense. Intensive Care Med. 1996;22:S456–S461. doi: 10.1007/BF01743724. [DOI] [PubMed] [Google Scholar]
- 26.Pope R, Mungre S, Liu H T, Thimmapaya B. Regulation of TNF-α expression in normal macrophages: the role of C/EBPβ. Cytokine. 2000;12:1171–1181. doi: 10.1006/cyto.2000.0691. [DOI] [PubMed] [Google Scholar]
- 27.Raabe T, Bukrinsky M, Currie R A. Relative contribution of transcription and translation to the induction of tumor necrosis factor-α by lipopolysaccharide. J Biol Chem. 1998;273:974–980. doi: 10.1074/jbc.273.2.974. [DOI] [PubMed] [Google Scholar]
- 28.Rook G A W, Taverne J, Leveton C, Steele J. The role of gamma-interferon, vitamin D3 metabolites and tumor necrosis factor in the pathogenesis of tuberculosis. Immunology. 1987;62:229–234. [PMC free article] [PubMed] [Google Scholar]
- 29.Sampaio E P, Oliveira R B, Warwick-Davies J, Neto R B F, Griffin G E, Shattock R J. T cell-monocyte contact enhances tumor necrosis factor-α production in response to Mycobacterium leprae. J Clin Investing. 2000;182:1463–1472. doi: 10.1086/315902. [DOI] [PubMed] [Google Scholar]
- 30.Sariban E, Imamura K, Luebbers R, Kufe D. Transcriptional and posttranscriptional regulation of tumor necrosis factor gene expression in human monocytes. J Clin Investig. 1988;81:1506–1510. doi: 10.1172/JCI113482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savill J S, Wyllie A J, Henson J E, Walport M J, Henson P M, Haslett C. Macrophage phagocytosis of aging neutrophils in inflammation. J Clin Investig. 1989;83:865–875. doi: 10.1172/JCI113970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sebbag M, Parry S L, Brennan F M, Feldman M. Cytokine stimulation of T lymphocytes regulates their capacity to induce monocyte production of tumor necrosis factor-α, but not interleukin-10: possible relevance to pathophysiology of rheumatoid arthritis. Eur J Immunol. 1997;27:624–632. doi: 10.1002/eji.1830270308. [DOI] [PubMed] [Google Scholar]
- 33.Shahkov A N, Collart M A, Vassalli P, Nedospasov S A, Jongeneel C V. κB-type enhancers are involved in lipopolysaccharide-mediated transcriptional activation of the tumor necrosis factor α gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shattock R J, Rizzardi G P, Hayes P, Griffin G. Engagement of adhesion molecules (CD18, CD11a, CD45, CD44 and CD58) enhances human immunodeficiency virus type I replication in monocytic cells through a tumor necrosis factor-modulated pathway. J Infect Dis. 1996;174:54–62. doi: 10.1093/infdis/174.1.54. [DOI] [PubMed] [Google Scholar]
- 35.Shu U, Kiniwa M, Wu C Y. Activated T cells induce interleukin-12 production by monocytes via CD40-CD40 ligand interaction. Eur J Immunol. 1995;25:1125–1128. doi: 10.1002/eji.1830250442. [DOI] [PubMed] [Google Scholar]
- 36.Silver R F, Li Q, Boom W H, Ellner J J. Lymphocyte-dependent inhibition of growth of virulent Mycobacterium tuberculosis H37Rv within human monocytes: Requirement for CD4(+) T cells in purified protein derivative-positive, but not in purified protein derivative-negative subjects. J Immunol. 1998;160:2408–2417. [PubMed] [Google Scholar]
- 37.Sung S-S J, Walters J A, Hudson J, Gimble J M. Tumor necrosis factor-α mRNA accumulation in human myelomonocytic cell lines. J Immunol. 1991;147:2047–2054. [PubMed] [Google Scholar]
- 38.Suk K, Erickson K L. Differential regulation of tumor necrosis factor-α mRNA degradation in macrophages by interleukin-4 and interferon-γ. Immunology. 1996;87:551–558. doi: 10.1046/j.1365-2567.1996.500561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sypek J P, Jacobson S, Vorys A, Wyler D J. Comparison of gamma-interferon, tumor necrosis factor, and direct cell contact in activation of antimycobacterial defense in murine macrophages. Infect Immun. 1993;61:3901–3906. doi: 10.1128/iai.61.9.3901-3906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takashima T, Ueta C, Tsuyuguchi I, Kishimoto S. Production of tumor necrosis factor alpha by monocytes from patients with pulmonary tuberculosis. Infect Immun. 1990;58:3286–3292. doi: 10.1128/iai.58.10.3286-3292.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Toossi Z, Hamilton B D, Phillips M H, Averill L E, Ellner J J, Salveker A. Regulation of nucelar factor-κB and its inhibitor IκB-α/MAD-3 in monocytes by Mycobacterium tuberculosis and during human tuberculosis. J Immunol. 1997;159:4109–4116. [PubMed] [Google Scholar]
- 42.Tramontana J M, Utaipat U, Molloy A, Akarasewi P, Burroughs M, Makonkawkeyoon S, Johnson B, Klausner J D, Rom W, Kaplan G. Thalidomide treatment reduces tumor necrosis factor-alpha production and enhances weight gain in patients with pulmonary tuberculosis. Mol Med. 1995;1:384–397. [PMC free article] [PubMed] [Google Scholar]
- 43.Trede N S, Tsytsykova A V, Chatila T, Goldfeld A E, Gehas R S. Transcriptional activation of the human TNF-α promoter by superantigen in human monocytic cells: role of NF-κB. J Immunol. 1995;155:902–908. [PubMed] [Google Scholar]
- 44.Tschou-Wong K M, Tanabe O, Chi C, Yie T A, Rom W N. Activation of NFκB in Mycobacterium tuberculosis-induced interleukin-2 receptor expression in mononuclear phagocytes. Am J Respir Crit Care Med. 1999;159:1323–1329. doi: 10.1164/ajrccm.159.4.9710105. [DOI] [PubMed] [Google Scholar]
- 45.Udalova I A, Knight J C, Vidal V, Nedospasov S A, Kwiatkowski D. Complex NF-κB interactions at the distal tumor necrosis factor promoter region in human monocytes. J Biol Chem. 1998;273:21178–21186. doi: 10.1074/jbc.273.33.21178. [DOI] [PubMed] [Google Scholar]
- 46.Underhill D M, Ozinsky A, Smith K D, Aderem A. Toll-like receptor-2 mediates mycobacteria-induced proinflammatory signaling in macrophages. Proc Natl Acad Sci USA. 1999;96:14459–14463. doi: 10.1073/pnas.96.25.14459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vey E, Zhang J H, Dayer J M. IFN-gamma and 1,25 (OH)2D3 induce on THP-1 cells distinct patterns of cell surface antigen expression, cytokine production, and responsiveness to contact with activated T cells. J Immunol. 1992;149:2040–2046. [PubMed] [Google Scholar]
- 48.Vey E, Burger D, Dayer J M. Expression and cleavage of tumor necrosis factor-alpha and tumor necrosis factor receptors by human monocytic cell lines upon direct contact with stimulated T cells. Eur J Immunol. 1996;26:2404–2409. doi: 10.1002/eji.1830261021. [DOI] [PubMed] [Google Scholar]
- 49.Visvanathan K V, Goodbourn S. Double-stranded RNA activates binding of NFκB an inducible element in the human γ interferon promoter. EMBO J. 1989;8:1129–1138. doi: 10.1002/j.1460-2075.1989.tb03483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Warwick-Davies J, Lowrie D B, Cole P J. Selective deactivation of human monocyte functions by TGF-β. J Immunol. 1995;155:3186–3193. [PubMed] [Google Scholar]
- 51.Webb D S A, Shimuzu Y, Van Seventer G A, Shaw S, Gerrard T L. LFA-3, CD44 and CD45: Physiologic triggers of human monocyte TNF and IL-1 release. Science. 1990;249:1295–1297. doi: 10.1126/science.1697984. [DOI] [PubMed] [Google Scholar]
- 52.Weiden M, Tanaka N, Quiao Y, Zhao B Y, Honda Y, Nakata K, Canova A, Levy D E, Rom W M, Pine R. Differentiation of monocytes to macrophages switches the Mycobacterium tuberculosis effect on HIV-1 replication from stimulation to inhibition: modulation of interferon response and CCAAT/enhancer binding protein β expression. J Immunol. 2000;165:2028–2039. doi: 10.4049/jimmunol.165.4.2028. [DOI] [PubMed] [Google Scholar]
- 53.Yujiri T, Fanger G R, Garrington T P, Schlesinger T K, Gibson S, Johnson G L. MEK kinase 1 (MEKK1) transduces c-Jun NH2-terminal kinase activation in response to changes in the microtubule. J Biol Chem. 1999;274:12605–12610. doi: 10.1074/jbc.274.18.12605. [DOI] [PubMed] [Google Scholar]