Abstract
Background: Myocarditis, diagnosed by symptoms and troponin elevation, has been well-described with COVID-19 infection, as well as shortly after COVID-19 vaccination. The literature has characterized the outcomes of myocarditis following COVID-19 infection and vaccination, but clinicopathologic, hemodynamic, and pathologic features following fulminant myocarditis have not been well-characterized. We aimed to compare clinical and pathological features of fulminant myocarditis requiring hemodynamic support with vasopressors/inotropes and mechanical circulatory support (MCS), in these two conditions. Methods: We analyzed the literature on fulminant myocarditis and cardiogenic shock associated with COVID-19 and COVID-19 vaccination and systematically reviewed all cases and case series where individual patient data were presented. We searched PubMed, EMBASE, and Google Scholar for “COVID”, “COVID-19”, and “coronavirus” in combination with “vaccine”, “fulminant myocarditis”, “acute heart failure”, and “cardiogenic shock”. The Student’s t-test was used for continuous variables and the χ2 statistic was used for categorical variables. For non-normal data distributions, the Wilcoxon Rank Sum Test was used for statistical comparisons. Results: We identified 73 cases and 27 cases of fulminant myocarditis associated with COVID-19 infection (COVID-19 FM) and COVID-19 vaccination (COVID-19 vaccine FM), respectively. Fever, shortness of breath, and chest pain were common presentations, but shortness of breath and pulmonary infiltrates were more often present in COVID-19 FM. Tachycardia, hypotension, leukocytosis, and lactic acidosis were seen in both cohorts, but patients with COVID-19 FM were more tachycardic and hypotensive. Histologically, lymphocytic myocarditis dominated both subsets, with some cases of eosinophilic myocarditis in both cohorts. Cellular necrosis was seen in 44.0% and 47.8% of COVID-19 FM and COVID-19 vaccine FM, respectively. Vasopressors and inotropes were used in 69.9% of COVID-19 FM and in 63.0% of the COVID-19 vaccine FM. Cardiac arrest was observed more in COVID-19 FM (p = 0.008). Venoarterial extracorporeal membrane oxygenation (VA-ECMO) support for cardiogenic shock was also used more commonly in the COVID-19 fulminant myocarditis group (p = 0.0293). Reported mortality was similar (27.7%) and 27.8%, respectively) but was likely worse for COVID-19 FM as the outcome was still unknown in 11% of cases. Conclusions: In the first series to retrospectively assess fulminant myocarditis associated with COVID-19 infection versus COVID-19 vaccination, we found that both conditions had a similarly high mortality rate, while COVID-19 FM had a more malignant course with more symptoms on presentation, more profound hemodynamic decompensation (higher heart rate, lower blood pressure), more cardiac arrests, and higher temporary MCS requirements including VA-ECMO. In terms of pathology, there was no difference in most biopsies/autopsies that demonstrated lymphocytic infiltrates and some eosinophilic or mixed infiltrates. There was no predominance of young males in COVID-19 vaccine FM cases, with male patients representing only 40.9% of the cohort.
Keywords: fulminant myocarditis, cardiogenic shock, COVID-19
1. Introduction
Myocarditis has been reported in association with COVID-19 infections and shortly after COVID-19 mRNA vaccination. We previously summarized all individual case reports in the English-language literature published in 2020 and found that myocardial involvement in COVID patients may present as a new and rapid decrease in left ventricular ejection fraction (LVEF), typically in the setting of bilateral lung damage. Moderate/large pericardial effusion was common, and cardiac tamponade occurred in 12.5% of patients. Cardiogenic shock developed in one third of the patients and mortality appeared to be high at 26% [1]. Later, we reported on acute myocarditis cases occurring within 60 days of mRNA COVID-19 vaccination that were reported in the English-language literature published in 2021 [2] and found that majority (87.1%) of patients were male, presenting typically with chest pain and elevated troponins. Cardiogenic shock was present in 11%, and mortality was 1.7% [2]. The outcomes of fulminant myocarditis with cardiogenic shock following COVID-19 infection and COVID-19 vaccination specifically in terms of inotrope use, mechanical circulatory support (MCS) use, and survival have not been well-characterized.
The pathophysiology of myocardial injury in both COVID-19 infection and post COVID-19 vaccination is not well understood. In the case of COVID-19, it is thought that both direct viral damage and hyperimmune response may play a role [3]. Because COVID-19 virus was rarely found in the cardiomyocytes [4], an exaggerated immune response is a plausible mechanism. In the case of mRNA vaccine, which does not contain the virus, immune response also may play a role. It was previously suggested that COVID-induced and COVID vaccine-induced myocarditis may share a common pathology [5].
We therefore wanted to compare clinical and pathological features of myocarditis in both entities. To avoid diagnostic ambiguity, we focused only on cases of fulminant myocarditis with a significant drop in left ventricular ejection fraction (LVEF) and hemodynamic compromise requiring pharmacological support (vasopressors, inotropes), or MCS, or both.
2. Methods
2.1. Study Design
This study was a systematic review of the literature conducted following PRISMA guidelines [6] to retrieve publications containing data regarding clinical presentations, course, and outcomes of fulminant myocarditis +/− cardiogenic shock following COVID-19 infection and COVID-19 vaccination. Registration of a review protocol was unnecessary because data contained in the published literature were used to conduct this study.
2.2. Eligibility Criteria
The publications included were full-length manuscripts retrieved with our search that contained data on one or more patients, who were 18 years old or older, with either a positive test for COVID-19 or COVID-19 vaccination with fulminant myocarditis diagnosed by all of the following characteristics: (1) new systolic dysfunction reported as a decrease in left ventricular ejection fraction (LVEF); (2) hemodynamic instability requiring pharmacological support (vasopressors, inotropes) or mechanical circulatory support (MCS), or both. Publications were excluded if these were written in languages other than English, contained pediatric population data (patients younger than 18 years old), or had insufficient data on individual patients.
2.3. Search Method
Following PRISMA guidelines [6], a systematic search of the literature was conducted using PubMed, Google Scholar, and EMBASE. The keywords used were “COVID”, “COVID-19”, and “coronavirus” in combination with “vaccine”, “fulminant myocarditis”, “acute heart failure”, and “cardiogenic shock”. In relevant papers, the list of references was manually searched as well. The search was limited to the articles published in English from 1 December 2020–31 August 2022. All the identified publications were screened to exclude duplicates by comparing titles, authors, and digital object identifiers. After removing duplicates, all the remaining publications were screened for the exclusion criteria by reading titles and abstracts. After removing publications that met the exclusion criteria, the remaining publications were further screened for the inclusion and exclusion criteria by reading the full-text publications.
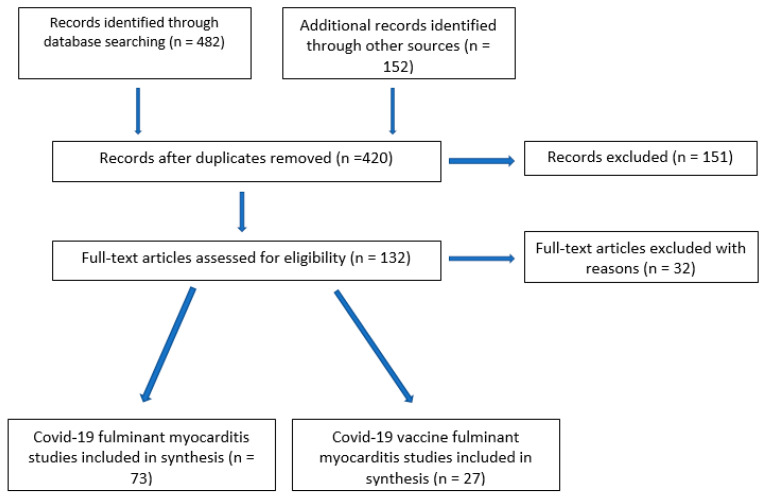
The list of references for each relevant publication was manually examined. Through this searched method, the publications to be included in the analysis were identified (Figure 1). To assess for reproducibility, the search was conducted independently by two members of the working group, and results were manually compared.
Figure 1.
The literature search: the flow diagram.
2.4. Data Extraction Process
The included publications were analyzed in a qualitative manner for authors’ names, date of publication, medical center where they were treated, country, and the timeline of the events. These publications were used to identify our subjects of interest. Once identified, subjects were labeled and their data extracted. These data were used to perform quantitative analyses of demographics, comorbidities, biomarkers, symptoms on presentation, imaging studies, type of vaccine (where applicable), changes of LVEF, wall motion abnormalities, presence of cardiogenic shock, use of temporary mechanical circulatory support devices (MCS), and outcomes (survival to discharge). The time of the onset of symptoms was limited to 60 days from either COVID-19 positive test or COVID-19 vaccine.
2.5. Statistical Analysis
Data extracted from the publications were grouped into continuous or categorical variables for analysis. Prior to choosing the appropriate test, the distribution of the data was analyzed, particularly for continuous variables. For continuous variables, the Student’s t-test was used when appropriate, with attention given to equality of variances. However, the data were not normally distributed for most continuous variables, so the Wilcoxon Rank Sum Test was used for statistical comparisons. For categorical variables, the χ2 statistic was used to make statistical comparisons. When sample size for entries within specific subgroups was less than 10, the Fisher’s exact test was utilized. Statistical significance was determined at alpha levels of 0.05 and 0.01. All statistical analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC, USA).
3. Results
3.1. Literature Search
The search identified 634 (482 + 152) publications. After removing duplicates and screening for exclusion and inclusion criteria, 100 publications were included in the analysis (Figure 1).
3.2. Patient Characteristics
3.2.1. Fulminant Myocarditis in COVID-19 Positive Patients
There were 73 cases of fulminant myocarditis in COVID-19 positive patients, comprising 40 (54.8%) males and a mean age of 45.1 ± 15.8 years (Table 1). On presentation, fever (n = 37, 50.7%), shortness of breath (n = 35, 47.9%), and chest pain (n = 10, 13.7%) were the commonest symptoms. On physical examination, tachycardia and hypotension were common with a mean heart rate of 125.1 ± 21.9 beats per minute, a mean systolic blood pressure of 86.7 ± 18.8 mmHg, and a mean diastolic blood pressure of 54.6 ± 14.9 mmHg. Leukocytosis and lactic acidosis were common with a mean white blood count of 19.200 ± 980/mm3 and serum lactate level of 7.3 ± 4.2 mmol/L. Diffuse bilateral pulmonary infiltrates, characteristic of COVID-19 infection, were present in 31 (42.5%) patients.
Table 1.
Cases of COVID-19 fulminant myocarditis.
| Authors | Country | Age, Years | Sex (M = 1; F = 2) | Vasoactive Agents (Yes = 1; No or not Reported = 2) |
Intra-Aortic Balloon Pump (Yes = 1; No = 2) | Impella (Yes = 1; No = 2) | ECMO (Yes = 1; No = 2) | Survival to Discharge (Yes = 1; No = 2) |
Treatment | Biopsy/Infiltrate |
|---|---|---|---|---|---|---|---|---|---|---|
| Afriyie [7] | USA | 27 | 1 | 1 | 2 | Remdesivir, Steroids | ||||
| Albert [4] | USA | 49 | 1 | 2 | 1 | 1 | Tocilizumab, Steroids, IVIG | Lymphocytes | ||
| Aldeghaither [8] | Canada | 39 | 2 | 1 | 1 | 1 | Steroids | Eosinophils | ||
| Aldeghaither [8] | Canada | 25 | 1 | 1 | 2 | 1 | Steroids, IVIG, Anakinra (IL-antagonist) | Eosinophils | ||
| Aldeghaither [8] | Canada | 21 | 1 | 1 | 1 | 1 | Steroids, Anakinra, IVIG | Lymphocytes | ||
| Almanza [9] | Colombia | 75 | 2 | 1 | 2 | Tocilizumab | Lymphocytes | |||
| Anupama [10] | USA | 66 | 1 | 1 | 1 | Hydroxy-chloroquine, IV diuretics, IVIG, convalescent plasma | ||||
| Bergman [11] | USA | 53 | 1 | 1 | 1 | 1 | Hydroxy-chloroquine, Sarilumab (IL-6 inhibitor) | |||
| Bernal-Torres [12] | Colombia | 38 | 2 | 1 | 1 | Steroids, IVIG, Hydroxy-chloroquine, Lopinavir/Ritonavir | ||||
| Bhardwaj [13] | USA | 22 | 1 | 2 | 1 | 1 | Steroids, Remdesivir | |||
| Bhardwaj [13] | USA | 53 | 2 | 2 | 1 | 1 | Steroids, Remdesivir, Convalescent Plasma | |||
| Bhardwaj [13] | USA | 28 | 2 | 2 | 1 | 2 | Steroids, Remdesivir, Tocilizumab, IVIG | |||
| Bhardwaj [13] | USA | 27 | 2 | 2 | 1 | 1 | Steroids, Remdesivir, IVIG | |||
| Bhardwaj [13] | USA | 46 | 1 | 2 | 1 | 1 | Steroids, Remdesivir, IVIG | |||
| Bhardwaj [13] | USA | 68 | 1 | 2 | 1 | 2 | Steroids | |||
| Bhardwaj [13] | USA | 26 | 2 | 2 | 1 | 1 | Steroids | |||
| Bhardwaj [13] | USA | 66 | 1 | 2 | 1 | 1 | Steroids | |||
| Bhardwaj [13] | USA | 24 | 1 | 2 | 1 | 1 | Steroids | |||
| Bulbul [14] | Qatar | 49 | 2 | 1 | 1 | 1 | Hydroxy-chloroquine, Oseltamivir, Teicoplanin, Steroids, Tocilizumab, Lopinavir, Ritonavir | |||
| Chitturi [15] | USA | 65 | 2 | 1 | 2 | 1 | Remdesivir, Steroids, Tocilizumab | |||
| Coll [16] | USA | 39 | 1 | 1 | 2 | 2 | 2 | 1 | Steroids, IVIG | |
| Coyle [17] | USA | 57 | 1 | 1 | 1 | Hydroxy-chloroquine, Steroids, Colchicine, Tocilizumab | ||||
| Craver [18] | USA | 18 | 1 | 2 | 2 | Eosinophils | ||||
| Fiore [19] | Italy | 45 | 1 | 1 | 1 | 2 | 2 | 1 | Hydroxy-chloroquine, Anakinra, | Lymphocytes |
| Fried [20] | USA | 38 | 1 | 1 | 1 | Hydroxy-chloroquine | ||||
| Fried [20] | USA | 64 | 2 | 1 | 2 | |||||
| Garau [21] | Belgium | 18 | 2 | 1 | 1 | 1 | 1 | Steroids, Hydroxy-chloroquine, IVIG | Lymphocytes | |
| Garot [22] | France | 18 | 1 | 1 | 1 | Hydroxy-chloroquine | ||||
| Gauchotte [23] | France | 69 | 1 | 1 | 1 | 2 | Lymphocytes | |||
| Gaudriot [24] | France | 38 | 1 | 1 | 1 | 2 | 1 | 1 | Steroids, Mycophenolate Mofetil, Cyclosporine | Lymphocytes |
| Gay [25] | USA | 56 | 1 | 1 | 2 | 1 | 1 | Steroids, Tocilizumab | ||
| Ghafoor [26] | USA | 54 | 2 | 1 | 2 | 2 | 1 | 2 | ||
| Gill [27] | USA | 65 | 2 | 1 | 1 | 2 | 2 | |||
| Gill [27] | USA | 34 | 2 | 2 | 1 | 1 | Colchicine, Steroids | |||
| Gomez [28] | Spain | 53 | 1 | 1 | 2 | |||||
| Hamdan [29] | UAE | 45 | 2 | 1 | 1 | 1 | Steroids | |||
| Hu [30] | China | 37 | 1 | 1 | 1 | Steroids, IVIG. | ||||
| Hussain [31] | USA | 51 | 1 | 1 | Hydroxy-chloroquine, Azithromycin, Colchicine, Steroids, Remdesivir | |||||
| Irabien-Ortiz [32] | Spain | 59 | 2 | 1 | 1 | 1 | Lopinavir/Ritonavir, IVIG, Steroids | |||
| Ishikura [33] | Japan | 35 | 1 | 1 | 1 | 1 | 1 | IVIG, Steroids | Lymphocytes | |
| Jacobs [34] | Belgium | 48 | 1 | 1 | 1 | 2 | Hydroxy-chloroquine | Lymphocytes | ||
| Kallel [35] | Morocco | 26 | 1 | 1 | 2 | 2 | 2 | 1 | ||
| Khatri [36] | USA | 50 | 1 | 1 | 2 | 2 | Hydroxy-chloroquine, IVIG, Steroids | |||
| Li [37] | USA | 60 | 1 | 1 | 1 | Hydroxy-chloroquine, Steroids, IVIG | ||||
| Mansoor [38] | USA | 72 | 2 | 1 | 2 | Chloroquine, IVIG, Steroids | ||||
| Menter [39] | Switzer-land | 47 | 2 | 1 | 2 | 2 | 2 | 2 | Lymphocytes, Neutrophils | |
| Milla-Godoy [40] | USA | 45 | 2 | 1 | 2 | Hydroxy-chloroquine | ||||
| Nakatani [41] | Japan | 49 | 1 | 1 | 2 | IVIG, Steroids | Lymphocytes | |||
| Naneishvili [42] | UK | 44 | 2 | 1 | 1 | Steroids | ||||
| Nwaejike [43] | UK | 39 | 1 | 1 | 1 | 1 | ||||
| Okor [44] | USA | 72 | 2 | 1 | 2 | 2 | 2 | 2 | Steroids | |
| Othenin-Girard [45] | Switzer-land | 22 | 1 | 2 | 1 | 1 | Steroids, Tocilizumab, IVIG, Cyclo-phosphamide, Rituximab | Lymphocytes | ||
| Papageorgiou [46] | Sweden | 43 | 1 | 1 | 1 | 1 | Steroids, Colchicine | None | ||
| Purdy [47] | USA | 53 | 1 | 1 | 1 | Steroids, Hydroxy-chloroquine | ||||
| Rajpal [48] | USA | 60 | 2 | 1 | 1 | 1 | Steroids | Lymphocytes | ||
| Richard [49] | USA | 28 | 2 | 1 | 1 | 1 | Steroids | |||
| Ruiz [50] | USA | 35 | 2 | 2 | 1 | 1 | IVIG, Remdesivir, Steroids | |||
| Salamanca [51] | Spain | 44 | 1 | 1 | 1 | 1 | Steroids, Tocilizumab, Hydroxy-chloroquine, Lopinavir/Ritonavir | Lymphocytes | ||
| Sampaio [52] | Brazil | 45 | 2 | 1 | 1 | 1 | Tocilizumab, IVIG, Convalescent Plasma, Steroids | |||
| Sanchez [53] | Spain | 42 | 2 | 2 | 1 | 1 | 2 | |||
| Shah [54] | Qatar | 19 | 1 | 1 | Steroids, Hydroxy-chloroquine, Tocilizumab, IVIG | |||||
| Shahrami [55] | Iran | 78 | 1 | 1 | 2 | Steroids, IVIG, Hydroxy-chloroquine | ||||
| Shen [56] | USA | 43 | 1 | 1 | 1 | 2 | 2 | 1 | IVIG | |
| Tavazzi [57] | Italy | 69 | 1 | 1 | 1 | 1 | 2 | Lopinavir/Ritonavir, Hydroxy-chloroquine, Steroids | Lymphocytes | |
| Thaker [58] | USA | 42 | 2 | 2 | 1 | 2 | 2 | 1 | Tocilizumab | |
| Thaker [58] | USA | 42 | 2 | 2 | 1 | Steroids, Tocilizumab, IVIG | ||||
| Thomson [59] | Australia | 39 | 2 | 1 | 1 | 2 | Steroids, IVIG | Macrophages | ||
| Trpkov [60] | Canada | 62 | 2 | 2 | 1 | Steroids, Anakinra | ||||
| Verma [61] | USA | 48 | 2 | 2 | 1 | 1 | 1 | Steroids, IVIG, Remdesivir, Tocilizumab | Macrophages | |
| Veronese [62] | Italy | 51 | 2 | 2 | 1 | 1 | 1 | Steroids | Lymphocytes | |
| Yalcinkaya [63] | Turkey | 29 | 1 | 1 | Eosinophils | |||||
| Yeleti [64] | USA | 25 | 2 | 2 | 2 | 1 | 1 | Steroids Remdesivir, Convalescent Plasma | Lymphocytes | |
| Zeng [65] | China | 63 | 1 | 2 | 2 | Antiviral |
The lowest recorded LVEF was 19.2 ± 8.9%, and it recovered to 56.5 ± 8.0%. Pericardial effusion on echocardiography was reported in 26 (35.6%) patients, including tamponade in 9 (12.3%) cases. In 25 (34.3%) cases, coronary angiogram was performed and was negative for obstructive lesions in all cases. Cardiac magnetic resonance imaging was done in 13 (17.8%) patients and was consistent with myocarditis. In terms of management, 68.5% received steroids, 26.0% received antiviral agents, and 37.0% received antibiotics.
Vasopressors and inotropes were used in 51 (69.9%) patients and MCS in 55 (75.3%), including intra-aortic balloon pump (12/16.4%), Impella® devices (Abiomed, Danvers, MA, USA) (5/6.8%), and venoarterial extracorporeal membrane oxygenation (VA ECMO) in (38) 52.1% of cases. In the course of the disease, cardiac arrest occurred in 18 (24.7%) patients. Out of this cohort, 44 (60.3%) survived to discharge, 21 (28.8%) died, and in 8 (11.0%), the outcome was still unknown at the time of publication.
Pathology findings were available in 25 (34.2%) patients who had either undergone biopsy or autopsy. In 17 patients, the infiltrates in the myocardium were predominantly lymphocytic; in four patients, the infiltrates were eosinophilic; and in two patients, the infiltrates were dominated by macrophages. In one patient, the biopsy was negative. Cellular necrosis was present in 11 patients (44.0%). In four cases, the COVID-19 viral particles were identified in either the interstitial cells or in the cardiomyocytes themselves, while in seven patients, the presence of COVID RNA was confirmed by genomic studies. In four cases, there were microthrombi reported in the myocardium.
3.2.2. Fulminant Myocarditis after COVID-19 Vaccination
There were 27 cases of fulminant myocarditis in patients within 60 days of COVID-19 vaccination, comprising 11 (40.7%) males and a mean age of 50.3 ± 16.4 years (Table 2). The Pfizer vaccine was used in 15 (55.5%) cases, of which the myocarditis developed after the first dose in eight (53.3% of vaccinated with the Pfizer product), after the second dose in seven (25.9%), after the booster in one, and were unreported in the rest of the patients. The Moderna vaccine was used in six (22.2%) patients, and myocarditis developed in two patients after the first dose, three patients after the second dose, and one after the booster. Vero cell, AstraZeneca, and Janssen vaccines were used in two patients each. Although we limited the timing from the last dose of the vaccine to the onset of symptoms to 60 days, the longest interval between the vaccine and the symptoms in our cohort was 24 days, with a mean of 6.8 ± 5.4 days.
Table 2.
Cases of COVID-19 vaccine fulminant myocarditis.
| Authors | Country | Age, Years | Sex (M = 1; F = 2) | Type of Vaccine (Pfizer =1; Moderna = 2; Vero Cell =3; AstraZeneca = 4; Janssen = 5) | Dose to Symptoms (Days) |
Number of Doses | Vasoactive Agents (Yes = 1; No or not Reported = 2) |
Intra-Aortic Balloon Pump (Yes = 1; No = 2) |
Impella (Yes = 1; No = 2) |
ECMO (Yes = 1; No = 2) |
Survival to Discharge (Yes = 1; No = 2) |
Treatment | Biopsy/ Infiltrate |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbate [66] | USA | 27 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 | Steroids | |
| Abbate [66] | USA | 34 | 2 | 1 | 9 | 1 | 1 | 2 | 2 | 1 | 1 | Steroids | Lymphocytes |
| Agdamag [67] | USA | 80 | 2 | 1 | 7 | 1 | 1 | 2 | 1 | 2 | 1 | Steroids | Eosinophils |
| Ameratunga [68] | New Zealand | 57 | 2 | 1 | 1 | 1 | 2 | 2 | Eosinophils | ||||
| Araki [69] | Japan | 53 | 2 | 1 | 4 | 2 | 1 | 1 | 1 | 1 | 1 | Steroids | None |
| Brage [70] | Spain | 62 | 2 | 2 | 1 | 3 | 1 | 2 | 1 | 2 | 1 | Steroids | |
| Choi [71] | Korea | 22 | 1 | 1 | 5 | 1 | 2 | 2 | 2 | 2 | 2 | Neutrophils, Histiocytes | |
| Cui [72] | China | 57 | 2 | 3 | 4 | 1 | 1 | 2 | 2 | 1 | Steroids | Lymphocytes | |
| Cui [72] | China | 63 | 1 | 3 | 4 | 1 | 2 | 1 | 2 | 2 | 1 | Steroids | Lymphocytes |
| Hoshino [73] | Japan | 27 | 1 | 2 | 8 | 1 | 1 | 1 | 1 | 2 | Steroids, IVIG | Lymphocytes | |
| Kadwalwala [74] | USA | 38 | 1 | 2 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | Steroids | Lymphocytes |
| Kawano [75] | Japan | 60 | 2 | 1 | 24 | 2 | 1 | 2 | 1 | 1 | 1 | Steroids | Lymphocytes |
| Kazama [76] | Japan | 48 | 2 | 2 | 7 | 2 | 1 | 1 | 1 | 1 | 1 | Lymphocytes | |
| Kim [77] | Korea | 63 | 2 | 4 | 1 | 1 | 2 | 2 | 2 | 1 | 2 | Lymphocytes | |
| Kimura [78] | Japan | 69 | 1 | 1 | 7 | 1 | 1 | 1 | 1 | 1 | 1 | Steroids | Lymphocytes, Eosinophils |
| Koiwaya [79] | Japan | 77 | 1 | 1 | 8 | 1 | 2 | 1 | 2 | 1 | 1 | Lymphocytes | |
| Lim [80] | South Korea | 38 | 2 | 1 | 7 | 2 | 2 | 1 | 1 | Lymphocytes | |||
| Nassar [81] | USA | 70 | 2 | 5 | 2 | 1 | 2 | 2 | 2 | 2 | |||
| Oka [82] | Japan | 50 | 1 | 1 | 10 | 2 | 1 | 2 | 2 | 2 | 1 | Steroids | Lymphocytes |
| Olmos [83] | Canada | 49 | 2 | 1 | 6 | 2 | 1 | 1 | 1 | Steroids | Lymphocytes, Eosinophils | ||
| Sung [84] | USA | 63 | 1 | 1 | 7 | 2 | 1 | 1 | 1 | Steroids | Giant cells | ||
| Ujieta [85] | USA | 62 | 2 | 5 | 4 | 1 | 2 | Steroids | Lymphocytes | ||||
| Verma [86] | USA | 45 | 2 | 1 | 10 | 1 | 1 | 2 | 2 | 2 | 1 | Lymphocytes, Eosinophils | |
| Verma [86] | USA | 42 | 1 | 2 | 14 | 2 | 2 | 2 | Lymphocytes, Eosinophils | ||||
| Wu [87] | Taiwan | 44 | 2 | 4 | 2 | 1 | 2 | 2 | 2 | 1 | |||
| Yamamoto [88] | Japan | 41 | 1 | 2 | 19 | 2 | 2 | 1 | 1 | 1 | Steroids | Lymphocytes | |
| Yamamoto [88] | Japan | 18 | 2 | 1 | 9 | 1 | 2 | 1 | 2 | 2 | 1 | Steroids | Lymphocytes |
Fever (n = 14, 51.9%), chest pain (n = 11, 40.7%), and shortness of breath (n = 10, 37.0%) were the most common symptoms. On physical examination, tachycardia with a mean heart rate of 111.3 ± 24.7 beats per minute and hypotension with a systolic blood pressure of 90.1 ± 17.0 mmHg and a diastolic blood pressure of 62.9 ± 12.7 mmHg were common. Leukocytosis and lactic acidosis were common with a mean white blood count of 14.300 ± 1020/mm3, and serum lactate level was 9.6 ± 8.4 mmol/L. Pulmonary edema or signs of congestion on chest X-ray were present in five (18.5%) patients.
The lowest recorded LVEF was 20.8 ± 9.7%, and it recovered to 56.1 ± 7.1%. Pericardial effusion on echocardiography was reported in 12 (44.4%) patients. In 21 (77.8%) patients, coronary angiogram was performed, and it was negative for obstructive lesions in all cases. Cardiac magnetic resonance imaging was done in nine cases (33.3%) and was consistent with myocarditis in eight of them. In one case, wherein cardiac MRI was negative for myocarditis, characteristic changes of myocarditis were seen on biopsy [69].
In terms of management, 66.7% received steroids. Vasopressors and inotropes were used in 17 (63.0%) cases and MCS in 29 (>100%), because more than one device was used in seven cases, including intra-aortic balloon pump (8/30.0%), Impella® (Abiomed, Danvers, MA, USA) (9/33.3%), and venoarterial extracorporeal membrane oxygenation (VA ECMO) in (12) 44.4% of cases. In the course of the disease, cardiac arrest occurred in seven (25.9%) patients. Out of this cohort, 19 (70.3%) survived to discharge, and 21 (28.7%) died.
Pathological diagnosis was available in 23 (85.2%) cases. Infiltrates were lymphocytic in 14 cases, mixed lymphocytic and eosinophilic in 3 cases, predominantly eosinophilic in 3 cases, and giant cells were present in one case. In one case, infiltrates consisted of neutrophils and histiocytes, and in one case, biopsy was negative. Cellular necrosis was present in 11/23 (47.8%) cases. There were no reports of microthrombotic injuries in this cohort.
3.2.3. Comparison of COVID-19 FM with COVID-vaccine FM
In comparison of COVID-19 and COVID vaccine related FM, patients with COVID myocarditis had a higher heart rate (125.1 ± 21.9 beats per minute vs. 106.8 ± 30.7 beats per minute, p = 0.021), lower diastolic and mean blood pressures (54.6 ± 14.8 mmHg vs. 62.9 ± 12.7 mmHg, p = 0.031; and 62.1 ± 17.0 mmHg vs. 69.7 ± 17.mmHg, p = 0.049, respectively), a trend towards higher brain natriuretic peptide concentration (11,468.3 ± 18,237.7 pg/dL vs. 947.0 ± 6144.8 pg/dL, p = 0.056), and a more frequent occurrence of pulmonary infiltrates on chest X-ray, while patients with recent vaccination presented more often with generalized symptoms of malaise and fatigue (Table 3). COVID-19 FM patients more often experienced cardiac arrest and used VA ECMO support, Nevertheless, there was no mortality difference (Table 3).
Table 3.
Comparison of clinical features and outcomes of fulminant myocarditis and cardiogenic shock following COVID-19 and COVID vaccination.
| COVID-19 Fulminant Myocarditis | COVID-19 Vaccine Fulminant Myocarditis | p-Value | |||||
|---|---|---|---|---|---|---|---|
| Continuous Variables | |||||||
| n | Mean | SD | n | Mean | SD | p-Value | |
| Heart rate, beats per minute | 29 | 125.1 | 21.9 | 18 | 106.8 | 30.7 | 0.0211 |
| Diastolic blood pressure, mmHg | 28 | 54.6 | 14.8 | 17 | 62.9 | 12.7 | 0.0312 |
| Mean arterial pressure, mmHg | 33 | 62.1 | 17.0 | 18 | 69.7 | 17.6 | 0.0497 |
| BNP, pg/dL | 16 | 11,468.3 | 18,237.7 | 9 | 947.0 | 614.8 | 0.0558 |
| Age | 73 | 45.1 | 15.8 | 27 | 50.3 | 16.4 | 0.1490 |
| Systolic blood pressure, mmHg | 30 | 86.7 | 18.8 | 18 | 90.2 | 17.0 | 0.5287 |
| White blood cells, peak | 33 | 19.2 | 9.8 | 12 | 14.3 | 10.2 | 0.1457 |
| Lactate | 33 | 7.3 | 4.2 | 8 | 9.6 | 8.4 | 0.7174 |
| C-reactive protein | 55 | 94.2 | 350.8 | 14 | 33.6 | 68.1 | 0.0552 |
| Peak troponin I | 20 | 5.7 × 108 | 2.5 × 109 | 13 | 6.4 × 108 | 2.3 × 109 | 0.5311 |
| LVEF at admission | 63 | 19.3 | 8.9 | 24 | 20.8 | 9.7 | 0.5513 |
| Length of stay total | 41 | 26.9 | 20.0 | 15 | 27.1 | 25.4 | 0.5661 |
| Categorical variables | |||||||
| n | Yes | No | n | Yes | No | ||
| Fever | 48 | 38 | 10 | 26 | 14 | 12 | 0.0229 |
| Shortness of breath | 42 | 35 | 7 | 24 | 10 | 14 | 0.0005 |
| Pulmonary infiltrates on chest X-ray | 73 | 34 | 39 | 27 | 5 | 22 | 0.0117 |
| General symptoms | 73 | 10 | 63 | 27 | 13 | 14 | 0.0008 |
| Cardiac arrest | 22 | 18 | 4 | 23 | 7 | 16 | 0.0008 |
| VA-ECMO | 49 | 38 | 11 | 23 | 12 | 11 | 0.0293 |
| Gender | 73 | 40 (M) | 33 (F) | 27 | 11 (M) | 16 (F) | 0.2120 |
| Intubation | 73 | 30 | 43 | 27 | 9 | 18 | 0.4798 |
| Right ventricular dysfunction | 73 | 26 | 47 | 27 | 3 | 24 | 0.0165 |
| Chest pain | 73 | 10 | 63 | 27 | 11 | 16 | 0.0032 |
| Pericardial effusion | 73 | 15 | 58 | 27 | 12 | 15 | 0.0169 |
| Intra-aortic balloon pump | 73 | 12 | 61 | 27 | 8 | 19 | 0.1432 |
| Survival | 73 | 44 | 29 | 27 | 19 | 8 | 0.3532 |
| Gastrointestinal symptoms | 73 | 16 | 57 | 27 | 4 | 23 | 0.4305 |
BNP—brain natriuretic peptide, VA-ECMO—venoarterial extracorporeal membrane oxygenation, SD—standard deviation.
4. Discussion
In this systematic review, we summarized the features, including demographics, symptoms, clinical characteristics, treatment, and outcomes of fulminant myocarditis occurring in the setting of COVID infection versus recent vaccination against COVID. While there were several papers trying to compare COVID versus COVID-vaccine related myocarditis, this is the first analysis, to our knowledge, focusing on the most severe forms of myocarditis with low LVEF and the need for vasopressors with or without MCS, suggestive of severe fulminant myocarditis with cardiogenic shock.
We found that:
Patients with COVID-19 FM, in comparison with COVID-19 vaccine FM, had higher a heart rate, lower diastolic and mean blood pressures, and a higher prevalence of pulmonary infiltrates on chest X-ray, and they presented more commonly with fever and shortness of breath and less commonly with generalized symptoms.
Despite differences in presentation, mortality in COVID-19 FM myocarditis and COVID-19 vaccine FM was similar (27.7% and 27.8%, respectively).
There was no difference in pathology between COVID-19 FM myocarditis and COVID-19 vaccine FM, with most biopsies/autopsies demonstrating lymphocytic infiltrates and some eosinophilic or mixed infiltrates.
There was no predominance of young males in COVID-19 vaccine FM cases. Females represented 59.3% of this cohort.
Patients with COVID-19 FM more frequently presented with fever and shortness of breath. Heart rate was also higher, while diastolic and mean blood pressures were lower, in COVID-19 FM. Patients in the COVID cohort experienced cardiac arrest and required ECMO support more commonly than patients with recent vaccination. They also had more pulmonary infiltrates on chest radiogram. The difference in presentation therefore can be explained by concomitant lung disease in the setting of acute respiratory infection.
Multiple papers describing vaccine-induced myocarditis noticed that affected individuals were predominantly young males in their teens or early 20s [2,89]. Surprisingly, we did not see that in the case of fulminant myocarditis as 59.3% of our cohort were females. In published reports on COVID-19 vaccine induced myocarditis, males constituted over 65% [90]; male to female ratio was reported as 3:2 [91] or even 14:1 [92]. According to another report, male sex increased the odds of myocarditis 4.4 times [93]. In our review, only 40.1% of patients were male, which also suggests a principally different mechanism in FM compared to that in usual clinically mild myocarditis.
This observation may mean that patients’ predisposition and not the virus or the vaccine determines catastrophic events with rapid and profound deterioration of the contractile function of the myocardium. This may explain similar outcomes. Differences in clinical presentation may be due to concomitant respiratory infection in COVID patients. Besides, the true mortality rate in the COVID-19 FM remains unknown. Because of the urge to publish any new evidence at the height of the pandemic, the outcomes are unknown in 11% of cases we collected. They were published while the patients still remained hospitalized. At the same time, in the COVID-19 vaccine FM, outcomes were reported in all cases. This allows the speculation that true mortality in COVID-19 FM may be higher.
Histologically, the data showed predominantly lymphocytic myocarditis in the majority of cases of fulminant myocarditis and cardiogenic shock of both etiologies. There was evidence of COVID-19 viral particle or viral genome seen on myocardial samples in some patients with COVID-19 FM. We did not see any cases with the evidence of severe acute respiratory syndrome coronavirus 2 spike protein in COVID-19 vaccine FM although it was reported after the vaccination before [75]. Microthrombi in the setting of COVID-19 myocarditis were quite rare but were seen in four cases. Further, some very unusual platelet thrombi have been reported [19].
The differences between COVID-induced and COVID-vaccine induced myocarditis may be explained by: (1) different pathophysiology or (2) same pathophysiology; however, patients were more symptomatic and hemodynamically unstable because they may have other manifestations of acute respiratory infection (they present with fever and shortness of breath more commonly than in the case of a vaccine). The mechanism of myocardial damage in either case is not well established. Direct viral infection of cardiomyocytes is one potential mechanism [94]. The COVID-19 virus is a large, enveloped, single-stranded RNA virus that binds to the angiotensin-converting enzyme (ACE)2 receptor and enters the cell. ACE2 receptor is present in multiple organs, but the expression in different tissues is different, and the cardiac muscle is rich with ACE2 receptors [95]. Nevertheless, in a few cases, the viral particles were found in the myocardium [57,96], but in other cases they were not. Moreover, in the case we reported [64], parvovirus was found in the cardiac muscle. Potentially, COVID-19 may make patients more susceptible to myocarditis caused by other pathogens. Shah et al. noticed a high (22%) co-infection rate in patients with coronavirus [54,97].
The other possibility involves the virus triggering immune response in the form of cytokine storm releasing IL-6, IL-10, and TNF-alpha, causing myocarditis. The intensity of the storm is linked to the severity of infection [98] or represent an exaggerated hyperimmune response of the host. Further, there is no unity behind the understanding of pathophysiology of vaccine-induced myocarditis. Since the vaccines do not contain the virus, the mechanisms may include hypersensitivity reaction and genetic susceptibility (variants in genes encoding human leukocyte antigen, desmosomal, cytoskeletal, or sarcomeric proteins, making an individual more susceptible to myocardial damage). Spike protein is present in mRNA vaccines. The viral spike protein, produced by the cell after mRNA- vaccine entry, induces an adaptive immune response to identify and destroy viruses that express the spike protein. Vaccine-induced spike-protein IgG antibodies thereby neutralize the virus. The antibodies to SARS-CoV-2 spike glycoproteins may cross-react with myocardial contractile proteins, including myocardial α- myosin heavy chain [99]. Spike S protein was detectable 2 weeks after the first dose of the BNT162b2 (Pfizer BioNTech) vaccine, with a significant increase 2 weeks after the second dose. Further, antibodies to the SARS-CoV-2 spike S protein were increased on day 14 after the second dose [100]. Moreover, the Pfizer vaccine stimulates the spike S protein specific T cell responses, which were detectable up to 4 weeks after the second dose. Therefore, the vaccine can produce both cellular and humoral immune response. The amplified immune response to the spike protein seems to be the most likely pathophysiology behind both COVID-induced and COVID vaccine-induced fulminant myocarditis.
It remains unclear why most individuals developing post-vaccine myocarditis experienced self-limited symptoms of chest pain and demonstrated mild troponin elevation, while others presented with overwhelming hemodynamic compromise or cardiac arrest. The similarity of manifestations between COVID-19 FM and COVID-19 vaccine FM suggests that such hyperreaction to either infection or the vaccine characterizes the individual reactivity more than the trigger. The devastating nature of fulminant myocarditis of either origin justifies future clinical, immunological, and genetic study in order to understand, treat, and ultimately prevent such response in the future.
Pathology was available only in some reports on COVID-19 FM, but in almost all cases of COVID-19 vaccine FM.
5. Conclusions
In the first series to retrospectively assess fulminant myocarditis associated with COVID-19 infection versus COVID-19 vaccination, we found that COVID-19 FM has a more malignant course with more symptoms on presentation, more profound hemodynamic decompensation (higher heart rate and lower blood pressure), more cardiac arrests, and more temporary MCS requirements including VA-ECMO. Nevertheless, mortality was similarly high at 27.7% and 27.8%, respectively. In terms of pathology, there was no difference in most biopsies/autopsies that demonstrated lymphocytic infiltrates and some eosinophilic or mixed infiltrates.
There was no predominance of young males in COVID-19 vaccine FM cases. Males represented only 40.1% of this cohort.
6. Limitations
The retrospective and descriptive nature of our study was a limitation. Since we limited our literature search from 1 December 2021–31 August 2022, several cases published in 2020 and later in 2022 were not included in the analysis. Definition of myocarditis was also based on local clinical expertise and diagnostic modalities. The criteria for cardiogenic were limited to any inotrope/vasopressor use or use of MCS, and we may have overestimated the incidence of cardiogenic shock. Due to inadequate data in some patients, some may have had concomitant pericarditis (myopericarditis), which may have a variable presentation from frank myocarditis. Some patients included in this series may have had an alternative diagnosis, although the severe fulminant myocarditis suggests otherwise. Pathology was available only in some reports on COVID-19 FM, but in almost all cases of COVID-19 vaccine FM.
Abbreviations
| COVID-19 FM | COVID-19 fulminant myocarditis |
| COVID-19 vaccine FM | COVID-19 vaccine associated fulminant myocarditis |
| CS | Cardiogenic Shock |
| MCS | Mechanical Circulatory Support |
| VA-ECMO | Venoarterial Extracorporeal Membrane Oxygenation |
| IABP | Intraortic Balloon Pump |
| LVEF | Left Ventricular Ejection Fraction |
| ACE2 | Angiotensin-Converting Enzyme-2 |
Author Contributions
Methodology, C.S.; formal analysis, M.E.G.; data curation, M.E.G. and C.S.; writing—original draft, M.E.G. and A.E.; writing—review & editing, O.J.I. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Because it was the review of published literature, no IRB approval was required.
Informed Consent Statement
Because there were no human participants, no consent was required.
Data Availability Statement
All studies are published and publicly available.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The research received no funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guglin M., Ballut K., Ilonze O., Jones M., Rao R. Clinical variants of myocardial involvement in COVID-19-positive patients: A cumulative experience of 2020. Hearth Fail. Rev. 2021;27:1341–1353. doi: 10.1007/s10741-021-10129-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ilonze O.J., Guglin M.E. Myocarditis following COVID-19 vaccination in adolescents and adults: A cumulative experience of 2021. Hearth Fail. Rev. 2022;27:2033–2043. doi: 10.1007/s10741-022-10243-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inciardi R.M., Lupi L., Zaccone G., Italia L., Raffo M., Tomasoni D., Cani D.S., Cerini M., Farina D., Gavazzi E., et al. Cardiac Involvement in a Patient with Coronavirus Disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albert C.L., Carmona-Rubio A.E., Weiss A.J., Procop G.G., Starling R.C., Rodriguez E.R. The Enemy within: Sudden-Onset Reversible Cardiogenic Shock with Biopsy-Proven Cardiac Myocyte Infection by Severe Acute Respiratory Syndrome Coronavirus 2. Circulation. 2020;142:1865–1870. doi: 10.1161/CIRCULATIONAHA.120.050097. [DOI] [PubMed] [Google Scholar]
- 5.Altman N.L., Berning A.A., Saxon C.E., Adamek K.E., Wagner J.A., Slavov D., Quaife R.A., Gill E.A., Minobe W.A., Jonas E.R., et al. Myocardial Injury and Altered Gene Expression Associated with SARS-CoV-2 Infection or mRNA Vaccination. JACC Basic Transl. Sci. 2022 doi: 10.1016/j.jacbts.2022.08.005. in press . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liberati M., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Afriyie F., Fohle E., Dekowski S.S., Kumar S. A Case of Isolated SARS-CoV-2 Fulminant Myopericarditis without Respiratory Failure. Cureus. 2021;13:e14003. doi: 10.7759/cureus.14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldeghaither S.M., Qutob R.M., Assanangkornchai N.M., Issa-Chergui B., Tam M.C., Larotondo R.R., Samoukovic G.M. Clinical and Histopathologic Features of Myocarditis in Multisystem Inflammatory Syndrome (Adult)—Associated COVID-19. Crit. Care Explor. 2022;10:e0630. doi: 10.1097/CCE.0000000000000630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Almanza-Hurtado A., Martínez-Ávila M.C., Rodríguez-Yánez T., Paternina-Mendoza M.C., Gutiérrez-Ariza J.C., Gómez-Arroyo G. Viral Cardiomyopathies Associated with SARS-CoV-2 Infection. Clin. Med. Insights Case Rep. 2022;15:11795476221088140. doi: 10.1177/11795476221088140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anupama B.K., Thapa S.S., Amzuta I. Transient Cardiomyopathy in a Patient with Coronavirus Disease-2019. J. Investig. Med. High Impact Case Rep. 2020;8:2324709620947577. doi: 10.1177/2324709620947577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bergman Z.R., Prathibha S., Bauman B.D., Yannopoulos D., Brunsvold M.E. Venoarteriovenous ECMO in Concomitant Acute Respiratory Distress Syndrome and Cardiomyopathy Associated with COVID-19 Infection. Case Rep. Crit. Care. 2021;2021:8848013. doi: 10.1155/2021/8848013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernal-Torres W., Herrera-Escandón Á., Hurtado-Rivera M., Plata-Mosquera C.A. COVID-19 fulminant myocarditis: A case report. Eur. Hearth J. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bhardwaj A., Kirincich J., Rampersad P., Soltesz E., Krishnan S. Fulminant myocarditis in COVID-19 and favorable outcomes with VA-ECMO. Resuscitation. 2022;175:75–76. doi: 10.1016/j.resuscitation.2022.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bulbul R.F., Suwaidi J.A., Al-Hijji M., Tamimi H.A., Fawzi I. COVID-19 Complicated By Acute Respiratory Distress Syndrome, Myocarditis, and Pulmonary Embolism. A Case Report. J. Crit. Care Med. 2021;7:123–129. doi: 10.2478/jccm-2020-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chitturi K.R., Thacker S., Al-Saadi M.A., Kassi M. Successful treatment of acute heart failure in COVID-19-induced cytokine storm with tocilizumab: A case report. Eur. Hearth J. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Coll M.D., Yanamandala M., Ferro E.G., Nutt C.T., Wei E.Q., Wang C.J., Mehra M.R. Early Immunosuppression and Rapid Recovery of Cardiogenic Shock in Multisystem Inflammatory Syndrome from Convalescent COVID-19. JACC: Case Rep. 2021;3:1403–1408. doi: 10.1016/j.jaccas.2021.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coyle J., Igbinomwanhia E., Sanchez-Nadales A., Danciu S., Chu C., Shah N. A Recovered Case of COVID-19 Myocarditis and ARDS Treated with Corticosteroids, Tocilizumab, and Experimental AT-001. JACC Case Rep. 2020;2:1331–1336. doi: 10.1016/j.jaccas.2020.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Craver R., Huber S., Sandomirsky M., McKenna D., Schieffelin J., Finger L. Fatal Eosinophilic Myocarditis in a Healthy 17-Year-Old Male with Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2c) Fetal Pediatr. Pathol. 2020;39:263–268. doi: 10.1080/15513815.2020.1761491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiore G., Sanvito F., Fragasso G., Spoladore R. Case report of cardiogenic shock in COVID-19 myocarditis: Peculiarities on diagnosis, histology, and treatment. Eur. Hearth J. 2021;5:ytab357. doi: 10.1093/ehjcr/ytab357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fried J.A., Ramasubbu K., Bhatt R., Topkara V.K., Clerkin K.J., Horn E., Rabbani L., Brodie D., Jain S.S., Kirtane A.J., et al. The Variety of Cardiovascular Presentations of COVID-19. Circulation. 2020;141:1930–1936. doi: 10.1161/CIRCULATIONAHA.120.047164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garau G., Joachim S., Duliere G., Melissopoulou M., Boccar S., Fraipont V., Dugauquier C., Troisfontaines P., Hougrand O., Delvenne P., et al. Sudden cardiogenic shock mimicking fulminant myocarditis in a surviving teenager affected by severe acute respiratory syndrome coronavirus 2 infection. ESC Hearth Fail. 2020;8:766–773. doi: 10.1002/ehf2.13049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garot J., Amour J., Pezel T., Dermoch F., Messadaa K., Felten M.L., Raymond V., Baubillier E., Sanguineti F., Garot P. SARS-CoV-2 Fulminant Myocarditis. JACC Case Rep. 2020;2:1342–1346. doi: 10.1016/j.jaccas.2020.05.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gauchotte G., Venard V., Segondy M., Cadoz C., Esposito-Fava A., Barraud D., Louis G. SARS-Cov-2 fulminant myocarditis: An autopsy and histopathological case study. Int. J. Leg. Med. 2021;135:577–581. doi: 10.1007/s00414-020-02500-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gaudriot B., Mansour A., Thibault V., Lederlin M., Cauchois A., Lelong B., Ross J.T., Leurent G., Tadié J.M., Revest M., et al. Successful heart transplantation for COVID-19-associated post-infectious fulminant myocarditis. ESC Heart Fail. 2021;8:2625–2630. doi: 10.1002/ehf2.13326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gay H.C., Sinha A., Michel E., Mozer A.B., Budd A., Feinstein M.J., Benzuly K.H., Al-Qamari A., Pawale A.A., Vorovich E.E. Fulminant myocarditis in a patient with coronavirus disease 2019 and rapid myocardial recovery following treatment. ESC Hearth Fail. 2020;7:4367–4370. doi: 10.1002/ehf2.13041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ghafoor K., Ahmed A., Abbas M. Fulminant Myocarditis with ST Elevation and Cardiogenic Shock in a SARS-CoV-2 Patient. Cureus. 2021;13:e16149. doi: 10.7759/cureus.16149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill G.S., Vlacancich R., Mehta N., Chaturvedi M., Papolos A. Spectrum of Cardiac Involvement in COVID-19. Cureus. 2020;12:e8638. doi: 10.7759/cureus.8638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gómez H.L., Bielsa A.P., Banzo M.J.A. Fulminant myocarditis and cardiogenic shock during SARS-CoV-2 infection. Med. Clin. 2020;155:463–464. doi: 10.1016/j.medcli.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamdan R., E Nassef M., Khan J., Cheriyan A., Yaseen N., Singer N.A.H.M., Kadri Z., Al Nooryani A. Reverse TakoTsubo or Fulminant myocarditis? Life saving VA ECMO in a COVID 19 patient. Ann. Cardiol. D′angéiologie. 2022;71:228–231. doi: 10.1016/j.ancard.2022.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu H., Ma F., Wei X., Fang Y. Coronavirus fulminant myocarditis saved with glucocorticoid and human immunoglobulin. Eur. Heart J. 2021;42:206. doi: 10.1093/eurheartj/ehaa190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hussain H., Fadel A., Alwaeli H., Guardiola V. Coronavirus (COVID-19) Fulminant Myopericarditis and Acute Respiratory Distress Syndrome (ARDS) in a Middle-Aged Male Patient. Cureus. 2020;12:e8808. doi: 10.7759/cureus.8808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Irabien-Ortiz Á., Carreras-Mora J., Sionis A., Pàmies J., Montiel J., Tauron M. Fulminant myocarditis due to COVID-19. Rev. Esp. Cardiol. 2020;73:503–504. doi: 10.1016/j.recesp.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ishikura H., Maruyama J., Hoshino K., Matsuoka Y., Yano M., Arimura T., Katano H., Kato S., Kitamura T., Nakamura Y. Coronavirus disease (COVID-19) associated delayed-onset fulminant myocarditis in patient with a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. J. Infect. Chemother. 2021;27:1760–1764. doi: 10.1016/j.jiac.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jacobs W., Lammens M., Kerckhofs A., Voets E., Van San E., Van Coillie S., Peleman C., Mergeay M., Sirimsi S., Matheeussen V., et al. Fatal lymphocytic cardiac damage in coronavirus disease 2019 (COVID-19): Autopsy reveals a ferroptosis signature. ESC Hearth Fail. 2020;7:3772–3781. doi: 10.1002/ehf2.12958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kallel O., Bourouis I., Bougrine R., Housni B., El Ouafi N., Ismaili N. Acute myocarditis related to Covid-19 infection: 2 cases report. Ann. Med. Surg. 2021;66:102431. doi: 10.1016/j.amsu.2021.102431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khatri A., Wallach F. Coronavirus disease 2019 (Covid-19) presenting as purulent fulminant myopericarditis and cardiac tamponade: A case report and literature review. Hearth Lung. 2020;49:858–863. doi: 10.1016/j.hrtlng.2020.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li A., Garcia-Bengochea Y., Stechel R., Azari B.M. Management of COVID-19 myopericarditis with reversal of cardiac dysfunction after blunting of cytokine storm: A case report. Eur. Hearth J. 2020;4:1–6. doi: 10.1093/ehjcr/ytaa224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mansour J., Short R.G., Bhalla S., Woodard P.K., Verma A., Robinson X., Raptis D.A. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: A report of two cases. Clin. Imaging. 2021;78:247–249. doi: 10.1016/j.clinimag.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Menter T., Cueni N., Gebhard E.C., Tzankov A. Case Report: Co-occurrence of Myocarditis and Thrombotic Microangiopathy Limited to the Heart in a COVID-19 Patient. Front. Cardiovasc. Med. 2021;8:695010. doi: 10.3389/fcvm.2021.695010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Milla-Godoy G.C., Park R., Jiang W., Hartkopf M.W., Treadwell T. Fulminant COVID-19-Associated Myocarditis in an Otherwise Healthy Female. Cureus. 2021;13:12736. doi: 10.7759/cureus.12736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakatani S., Ohta-Ogo K., Nishio M., Amemiya K., Sato S., Sawano H., Hatakeyama K., Katano H., Suzuki T., Hirooka K. Microthrombosis as a cause of fulminant myocarditis-like presentation with COVID-19 proven by endomyocardial biopsy. Cardiovasc. Pathol. 2022;60:107435. doi: 10.1016/j.carpath.2022.107435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Naneishvili T., Khalil A., O’Leary R., Prasad N. Fulminant myocarditis as an early presentation of SARS-CoV-2. BMJ Case Rep. 2020;13:e237553. doi: 10.1136/bcr-2020-237553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nwaejike N., Strang T., Garcia M., Charlesworth M., Shaw S.M., Barnard J.B. Emergency biventricular assist device implantation in a patient with suspected COVID-19 disease. Anaesth. Rep. 2020;8:196–199. doi: 10.1002/anr3.12079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okor I., Sleem A., Zhang A., Kadakia R., Bob-Manuel T., Krim S.R. Suspected COVID-19–Induced Myopericarditis. Ochsner J. 2021;21:181–186. doi: 10.31486/toj.20.0090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Othenin-Girard A., Regamey J., Lamoth F., Horisberger A., Glampedakis E., Epiney J.B., Kuntzer T., de Leval L., Carballares M., Hurni C.A., et al. Multisystem inflammatory syndrome with refractory cardiogenic shock due to acute myocarditis and mononeuritis multiplex after SARS-CoV-2 infection in an adult. Swiss Med. Wkly. 2020;150:w20387. doi: 10.4414/smw.2020.20387. [DOI] [PubMed] [Google Scholar]
- 46.Papageorgiou J.-M., Almroth H., Törnudd M., van der Wal H., Varelogianni G., Lawesson S.S. Fulminant myocarditis in a COVID-19 positive patient treated with mechanical circulatory support—A case report. Eur. Hearth J. 2020;5:ytaa523. doi: 10.1093/ehjcr/ytaa523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Purdy A., Ido F., Sterner S., Tesoriero E., Matthews T., Singh A. Myocarditis in COVID-19 presenting with cardiogenic shock: A case series. Eur. Hearth J. 2021;5:ytab028. doi: 10.1093/ehjcr/ytab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rajpal S., Kahwash R., Tong M.S., Paschke K., Satoskar A.A., Foreman B., Fuster V. Fulminant Myocarditis Following SARS-CoV-2 Infection: JACC Patient Care Pathways. JACC Case Rep. 2022;4:567–575. doi: 10.1016/j.jaccas.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richard I., Robinson B., Dawson A., Aya A., Ali R. An Atypical Presentation of Fulminant Myocarditis Secondary to COVID-19 Infection. Cureus. 2020;12:e9179. doi: 10.7759/cureus.9179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz J.G., Kandah F., Dhruva P., Ganji M., Goswami R. Case of Coronavirus Disease 2019 Myocarditis Managed with Biventricular Impella Support. Cureus. 2021;13:13197. doi: 10.7759/cureus.13197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Salamanca J., Díez-Villanueva P., Martínez P., Cecconi A., de Marcos B.G., Reyes G., Salas C., Segovia J., Jiménez-Borreguero L.J., Alfonso F. COVID-19 “Fulminant Myocarditis” Successfully Treated with Temporary Mechanical Circulatory Support. JACC: Cardiovasc. Imaging. 2020;13:2457–2459. doi: 10.1016/j.jcmg.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sampaio P.P.N., Ferreira R.M., de Albuquerque F.N., Colafranceschi A.S., Almeida A.C., Nunes M.A.V., Filho J.M., Lima R.A.C. Rescue Venoarterial Extracorporeal Membrane Oxygenation After Cardiac Arrest in COVID-19 Myopericarditis: A Case Report. Cardiovasc. Revascul. Med. 2020;28:57–60. doi: 10.1016/j.carrev.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sánchez-Recalde Á., Solano-López J., Miguelena-Hycka J., Martín-Pinacho J.J., Sanmartín M., Zamorano J.L. COVID-19 and cardiogenic shock. Different cardiovascular presentations with high mortality. Rev. Esp. Cardiol. 2020;73:669–672. doi: 10.1016/j.recesp.2020.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shah J.Z., Kumar S.A., Patel A.A. Myocarditis and Pericarditis in Patients with COVID-19. Heart Views. 2020;21:209–214. doi: 10.4103/HEARTVIEWS.HEARTVIEWS_154_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shahrami B., Davoudi-Monfared E., Rezaei Z., Gheibi S., Ardabili A.V., Arabzadeh A.A., Talebi A., Mojtahedzadeh M. Management of a critically ill patient with COVID-19-related fulminant myocarditis: A case report. Respir. Med. Case Rep. 2022;36:101611. doi: 10.1016/j.rmcr.2022.101611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shen M., Milner A., Foppiano Palacios C., Ahmad T. Multisystem inflammatory syndrome in adults (MIS-A) associated with SARS-CoV-2 infection with delayed-onset myocarditis: Case report. Eur. Heart J. Case Rep. 2021;5:ytab470. doi: 10.1093/ehjcr/ytab470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tavazzi G., Pellegrini C., Maurelli M., Belliato M., Sciutti F., Bottazzi A., Sepe P.A., Resasco T., Camporotondo R., Bruno R., et al. Myocardial localization of coronavirus in COVID-19 cardiogenic shock. Eur. J. Heart Fail. 2020;22:911–915. doi: 10.1002/ejhf.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thaker R., Shah A., Kim J., Kassi M. Acute Circulatory Collapse and Advanced Therapies in Patients with COVID-19 Infection. Methodist DeBakey Cardiovasc. J. 2021;17:43–52. doi: 10.14797/mdcvj.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thomson A., Totaro R., Cooper W., Dennis M. Fulminant Delta COVID-19 myocarditis: A case report of fatal primary cardiac dysfunction. Eur. Hearth J. 2022;6:ytac142. doi: 10.1093/ehjcr/ytac142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Trpkov C., MacMullan P., Feuchter P., Kachra R., Heydari B., Merchant N., Bristow M.S., White J.A. Rapid Response to Cytokine Storm Inhibition Using Anakinra in a Patient with COVID-19 Myocarditis. CJC Open. 2020;3:210–213. doi: 10.1016/j.cjco.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verma A.K., Olagoke O., Moreno J.D., Rezaee N., Ma P., Liu J., Javaheri A., Lavine K., Lin C.-Y. SARS-CoV-2-Associated Myocarditis: A Case of Direct Myocardial Injury. Circ. Heart Fail. 2022;15:e008273. doi: 10.1161/CIRCHEARTFAILURE.120.008273. [DOI] [PubMed] [Google Scholar]
- 62.Veronese G., Cipriani M., Bottiroli M., Garascia A., Mondino M., Pedrotti P., Pini D., Cozzi O., Messina A., Droandi G., et al. Fulminant myocarditis triggered by OC43 subtype coronavirus: A disease deserving evidence-based care bundles. J. Cardiovasc. Med. 2020;21:529–531. doi: 10.2459/JCM.0000000000000989. [DOI] [PubMed] [Google Scholar]
- 63.Yalcinkaya D., Yarlioglues M., Yigit H., Caydere M., Murat S.N. Cardiac mobile thrombi formation in an unvaccinated young man with SARS-CoV-2 associated fulminant eosinophilic myocarditis. Eur. Heart J. Case Rep. 2022;6:ytac036. doi: 10.1093/ehjcr/ytac036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeleti R., Guglin M., Saleem K., Adigopula S.V., Sinha A., Upadhyay S., Everett J.E., Ballut K., Uppuluri S., Rao R.A. Fulminant myocarditis: COVID or not COVID? Reinfection or co-infection? Future Cardiol. 2021;17:1307–1311. doi: 10.2217/fca-2020-0237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zeng J.-H., Liu Y.-X., Yuan J., Wang F.-X., Wu W.-B., Li J.-X., Wang L.-F., Gao H., Wang Y., Dong C.-F., et al. First case of COVID-19 complicated with fulminant myocarditis: A case report and insights. Infection. 2020;48:773–777. doi: 10.1007/s15010-020-01424-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Abbate A., Gavin J., Madanchi N., Kim C., Shah P.R., Klein K., Boatman J., Roberts C., Patel S., Danielides S. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int. J. Cardiol. 2021;340:119–121. doi: 10.1016/j.ijcard.2021.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Agdamag A.C.C., Gonzalez D., Carlson K., Konety S., McDonald W.C., Martin C.M., Maharaj V., Alexy T. Fulminant myocarditis following coronavirus disease 2019 vaccination: A case report. Eur. Hearth J. 2022;6:ytac007. doi: 10.1093/ehjcr/ytac007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ameratunga R., Woon S.T., Sheppard M.N., Garland J., Ondruschka B., Wong C.X., Stewart R.A., Tatley M., Stables S.R., Tse R.D. First Identified Case of Fatal Fulminant Necrotizing Eosinophilic Myocarditis Following the Initial Dose of the Pfizer-BioNTech mRNA COVID-19 Vaccine (BNT162b2, Comirnaty): An Extremely Rare Idiosyncratic Hypersensitivity Reaction. J. Clin. Immunol. 2022;42:441–447. doi: 10.1007/s10875-021-01187-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Araki T., Morimoto R., Ito R., Mizutani T., Kimura Y., Kazama S., Oishi H., Kuwayama T., Hiraiwa H., Kondo T., et al. A Case of Systemic Capillary Leak Syndrome with Severe Cardiac Dysfunction After mRNA Vaccination for COVID-19. CJC Open. 2022;4:656–659. doi: 10.1016/j.cjco.2022.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brage E.T., Ruíz J.R., Martín J.G., Rodríguez J.D.O., Tocino R.V., Diego S.R., Hernández P.L.S., Hernández L.B., Sánchez E.F. Fulminant myocarditis in a patient with a lung adenocarcinoma after the third dose of modern COVID-19 vaccine. A case report and literature review. Curr. Probl. Cancer. 2022;6:100153. doi: 10.1016/j.cpccr.2022.100153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi S., Lee S., Seo J.-W., Kim M.-J., Jeon Y.H., Park J.H., Lee J.K., Yeo N.S. Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J. Korean Med. Sci. 2021;36:e286. doi: 10.3346/jkms.2021.36.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui G., Li R., Zhao C., Wang D.W. Case Report: COVID-19 Vaccination Associated Fulminant Myocarditis. Front. Cardiovasc. Med. 2022;8:769616. doi: 10.3389/fcvm.2021.769616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hoshino N., Yanase M., Ichiyasu T., Kuwahara K., Kawai H., Muramatsu T., Ishii H., Tsukamoto T., Morimoto S.-I., Izawa H. An autopsy case report of fulminant myocarditis: Following mRNA COVID-19 vaccination. J. Cardiol. Cases. 2022;26:391–394. doi: 10.1016/j.jccase.2022.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadwalwala M., Chadha B., Ortoleva J., Joyce M. Multimodality imaging and histopathology in a young man presenting with fulminant lymphocytic myocarditis and cardiogenic shock after mRNA-1273 vaccination. BMJ Case Rep. 2021;14:e246059. doi: 10.1136/bcr-2021-246059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kawano H., Motokawa T., Kurohama H., Okano S., Akashi R., Yonekura T., Ikeda S., Izumikawa K., Maemura K. Fulminant Myocarditis 24 Days after Coronavirus Disease Messenger Ribonucleic Acid Vaccination: A Case Report. Intern. Med. 2022;61:2319–2325. doi: 10.2169/internalmedicine.9800-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kazama S., Okumura T., Kimura Y., Ito R., Araki T., Mizutani T., Oishi H., Kuwayama T., Hiraiwa H., Kondo T., et al. Biopsy-Proven Fulminant Myocarditis Requiring Mechanical Circulatory Support Following COVID-19 mRNA Vaccination. CJC Open. 2022;4:501–505. doi: 10.1016/j.cjco.2022.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim D., Choi J.H., Jang J.Y., So O., Cho E., Choi H., Hong K.S., Park K.T. A Case Report for Myopericarditis after BNT162b2 COVID-19 mRNA Vaccination in a Korean Young Male. J. Korean Med. Sci. 2021;36:e277. doi: 10.3346/jkms.2021.36.e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kimura M., Hashimoto T., Noda E., Ishikawa Y., Ishikita A., Fujino T., Matsushima S., Ide T., Kinugawa S., Nagaoka K., et al. Fulminant necrotizing eosinophilic myocarditis after COVID-19 vaccination survived with mechanical circulatory support. ESC Hearth Fail. 2022;9:2732–2737. doi: 10.1002/ehf2.13962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Koiwaya H., Nishihira K., Tomozoe K., Shibata Y. Serial histopathologic assessment of fulminant myocarditis after the first mRNA COVID-19 vaccine dose. Eur. Hearth J. 2022;43:1995. doi: 10.1093/eurheartj/ehac083. [DOI] [PubMed] [Google Scholar]
- 80.Lim Y., Kim M.C., Kim K.H., Jeong I.-S., Cho Y.S., Choi Y.D., Lee J.E. Case Report: Acute Fulminant Myocarditis and Cardiogenic Shock After Messenger RNA Coronavirus Disease 2019 Vaccination Requiring Extracorporeal Cardiopulmonary Resuscitation. Front. Cardiovasc. Med. 2021;8:758996. doi: 10.3389/fcvm.2021.758996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nassar M., Nso N., Gonzalez C., Lakhdar S., Alshamam M., Elshafey M., Abdalazeem Y., Nyein A., Punzalan B., Durrance R.J., et al. COVID-19 vaccine-induced myocarditis case report with literature review. Diabetes Metab. Syndr. Clin. Res. Rev. 2021;15:102205. doi: 10.1016/j.dsx.2021.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Oka A., Sudo Y., Miyoshi T., Ozaki M., Kimura Y., Takagi W., Ugawa S., Okada T., Nosaka K., Doi M. Fulminant myocarditis after the second dose of COVID-19 mRNA vaccination. Clin. Case Rep. 2022;10:e05378. doi: 10.1002/ccr3.5378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Olmos C.V., Trahan S., Rochon A., Ducharme A. Severe myocarditis after SARS-CoV-2 vaccination in a 49-year-old woman. CMAJ. 2022;194:E581–E584. doi: 10.1503/cmaj.211687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sung J.G., Sobieszczyk P.S., Bhatt D.L. Acute Myocardial Infarction within 24 Hours After COVID-19 Vaccination. Am. J. Cardiol. 2021;156:129–131. doi: 10.1016/j.amjcard.2021.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ujueta F., Azimi R., Lozier M.R., Poppiti R., Ciment A. Lymphohistocytic myocarditis after Ad26.COV2.S viral vector COVID-19 vaccination. IJC Hearth Vasc. 2021;36:100869. doi: 10.1016/j.ijcha.2021.100869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verma A.K., Lavine K.J., Lin C.-Y. Myocarditis after Covid-19 mRNA Vaccination. N. Engl. J. Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu L., Baylan U., van der Leeden B., Schurink B., Roos E., Schalkwijk C.G., Bugiani M., van der Valk P., van Rossum A.C., Zeerleder S.S., et al. Cardiac inflammation and microvascular procoagulant changes are decreased in second wave compared to first wave deceased COVID-19 patients. Int. J. Cardiol. 2021;349:157–165. doi: 10.1016/j.ijcard.2021.11.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yamamoto M., Tajiri K., Ayuzawa S., Ieda M. Pathological findings of clinically suspected myocarditis temporally associated with COVID -19 vaccination. Eur. J. Hearth Fail. 2022;24:1132–1138. doi: 10.1002/ejhf.2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Naveed Z., Li J., Spencer M., Wilton J., Naus M., García H.A.V., Otterstatter M., Janjua N.Z. Observed versus expected rates of myocarditis after SARS-CoV-2 vaccination: A population-based cohort study. CMAJ. 2022;194:E1529–E1536. doi: 10.1503/cmaj.220676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roh D.-E., Na H., Kwon J.-E., Choi I., Kim Y.-H., Cho H.-J. Chest Pain and Suspected Myocarditis Related to COVID-19 Vaccination in Adolescents—A Case Series. Children. 2022;9:693. doi: 10.3390/children9050693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Park H., Yun K.W., Kim K.-R., Song S.H., Ahn B., Kim D.R., Kim G.B., Huh J., Choi E.H., Kim Y.-J. Epidemiology and Clinical Features of Myocarditis/Pericarditis before the Introduction of mRNA COVID-19 Vaccine in Korean Children: A Multicenter Study. J. Korean Med Sci. 2021;36:e232. doi: 10.3346/jkms.2021.36.e232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lane S., Yeomans A., Shakir S. Reports of myocarditis and pericarditis following mRNA COVID-19 vaccination: A systematic review of spontaneously reported data from the UK, Europe and the USA and of the scientific literature. BMJ Open. 2022;12:e059223. doi: 10.1136/bmjopen-2021-059223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tuvali O., Tshori S., Derazne E., Hannuna R.R., Afek A., Haberman D., Sella G., George J. The Incidence of Myocarditis and Pericarditis in Post COVID-19 Unvaccinated Patients—A Large Population-Based Study. J. Clin. Med. 2022;11:2219. doi: 10.3390/jcm11082219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Turner A.J., Hiscox J.A., Hooper N.M. ACE2, from vasopeptidase to SARS virus receptor. Trends Pharmacol. Sci. 2004;25:291–294. doi: 10.1016/j.tips.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zou X., Chen K., Zou J., Han P., Hao J., Han Z. Single-cell RNA-seq data analysis on the receptor ACE2 expression reveals the potential risk of different human organs vulnerable to 2019-nCoV infection. Front. Med. 2020;14:185–192. doi: 10.1007/s11684-020-0754-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dolhnikoff M., Ferreira Ferranti J., de Almeida Monteiro R.A., Duarte-Neto A.N., Soares Gomes-Gouvêa M., Viu Degaspare N. SARS-CoV-2 in cardiac tissue of a child with COVID-19-related multisystem inflammatory syndrome. Lancet Child Adolesc. Health. 2020;4:790–794. doi: 10.1016/S2352-4642(20)30257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim D., Quinn J., Pinsky B., Shah N.H., Brown I. Rates of Co-infection Between SARS-CoV-2 and Other Respiratory Pathogens. JAMA. 2020;323:2085–2086. doi: 10.1001/jama.2020.6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jone P.N., John A., Oster M.E., Allen K., Tremoulet A.H., Saarel E.V., Lambert L.M., Miyamoto S.D., De Ferranti S.D. American Heart Association Leadership Committee and Congenital Cardiac Defects Committee of the Council on Lifelong Congenital Heart Disease and Heart Health in the Young. SARS-CoV-2 Infection and Associated Cardiovascular Manifestations and Complications in Children and Young Adults: A Scientific Statement from the American Heart Association. Circulation. 2022;145:e1037–e1052. doi: 10.1161/CIR.0000000000001064. [DOI] [PubMed] [Google Scholar]
- 99.Heymans S., Cooper L.T. Myocarditis after COVID-19 mRNA vaccination: Clinical observations and potential mechanisms. Nat. Rev. Cardiol. 2021;19:75–77. doi: 10.1038/s41569-021-00662-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bansal S., Perincheri S., Fleming T., Poulson C., Tiffany B., Bremner R.M., Mohanakumar T. Cutting Edge: Circulating Exosomes with COVID Spike Protein Are Induced by BNT162b2 (Pfizer-BioNTech) Vaccination prior to Development of Antibodies: A Novel Mechanism for Immune Activation by mRNA Vaccines. J. Immunol. 2021;207:2405–2410. doi: 10.4049/jimmunol.2100637. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All studies are published and publicly available.