Abstract
Bladder cancer is the most common tumor of the urinary system, with a high incidence in the male population. Surgery and intravesical instillations can eradicate it, although recurrences are very common, with possible progression. For this reason, adjuvant therapy should be considered in all patients. Resveratrol displays a biphasic dose response both in vitro and in vivo (intravesical application) with an antiproliferative effect at high concentrations and antiangiogenic action in vivo (intraperitoneal application) at a low concentration, suggesting a potential role for it in clinical management as an adjuvant to conventional therapy. In this review, we examine the standard therapeutical approach to bladder cancer and the preclinical studies that have investigated resveratrol in xenotransplantation models of bladder cancer. Molecular signals are also discussed, with a particular focus on the STAT3 pathway and angiogenic growth factor modulation.
Keywords: apoptosis, bladder cancer, angiogenesis, proliferation, resveratrol
1. Introduction
Resveratrol (trans-3,4′,5′-trihydroxystilbene) is a stilbene that consists of two aromatic rings joined together by an ethylene bridge. It is found in several plants and fruits, such as grapes (Vitis vinifera), mulberries (Morus spp.), and peanuts (Arachis hypogaea) [1]. Resveratrol is also a phytoalexin, a compound with an antibiotic function that is produced by higher plants in response to infections or other stressors [2]. Therefore, the concentration of resveratrol in plants increases in response to environmental stress, heavy metals, and UV light [3]. Resveratrol was isolated for the first time from the roots of Veratrum grandiflorum in 1940 and, subsequently, in 1963 from the roots of Polygonum cuspidatum, a plant used in traditional Chinese and Japanese medicine [2], rich in resveratrol 3-O-β-d-glucoside (piceid) [4].
Resveratrol has shown several beneficial properties for human health, such as anti-aging [5], anti-inflammatory [6], neuroprotective [7], hepatoprotective [8], cardioprotective [9], antidiabetic [10], and antioxidant activity [8]. Resveratrol displays chemopreventive action and anticancer action in several types of neoplasia [11]. Recently, resveratrol has attracted great interest for application in bladder cancer therapy [12]. The main obstacle in understanding the health potential of resveratrol is the difficulty of comparing the effects observed in vitro with those observed in vivo as the concentrations reached in the latter are lower than those that can be studied in vitro. With these limitations and the pharmacokinetics of resveratrol in mind, we critically reviewed in vivo studies (animal models) that have sought to evaluate the uses of resveratrol in the treatment of bladder cancer.
2. Bladder Cancer
Bladder cancer is the seventh most frequent cancer in the male population worldwide, with 9.5 cases per 100.000 people/year in men and 2.4 cases per 100.000 people/year in women. The incidence of bladder cancer in Europe is significantly higher than in the rest of the world, with 20 cases in men and 4.6 cases in women per 100.000 people/year [13]. The incidence in the world changes significantly in relation to the various risk factors to which the population is exposed, as well as due to the different diagnostic techniques and the availability of treatments [14]. At least 75% of the patients present, at the first diagnosis, non-muscle-invasive bladder cancer (NMBC) at different stages (Ta, carcinoma in situ, or T1), with a higher percentage in younger patients [15]. Smoking is the major risk factor in the pathogenesis of urothelial bladder cancer and the main causative agent in at least 50% of cases of bladder cancer. The risk increases progressively with the intensity and duration of smoke exposure.
Bladder cancer is classified using the TNM Classification of Malignant Tumours (TNM), approved by the Union for International Cancer Control (UICC). This classification is essential to define the appropriate treatment for each individual case of bladder cancer [16]. When the bladder tumor is removed endoscopically, it is possible to establish the “T” of the TNM, i.e., to define whether the tumor is non-muscle invasive (Ta and T1, which indicate whether it invades the lamina propria or not, respectively) or T2 (which indicates that it is muscle-invasive). The so-called “CIS” (carcinoma in situ), a flat, non-invasive, high-grade cancer, deserves special mention. It can be invisible to cystoscopy and, therefore, may not be diagnosed or may be interpreted as a simple inflammatory area due to its usually reddish appearance [17]. Therefore, the “T” of the TNM, together with the histology of the bladder tumor, is essential to define the therapeutic or follow-up procedure to adopt. Regarding histology, over 90% of bladder tumors are urothelial, and they are classified on the basis of the risk of progression (but not of recurrence) with a double classification: WHO 1973, which divides bladder tumors into G1, G2, or G3; WHO 2004/2016, which divides them into papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade, and high-grade [18,19]. Cancer is mostly non-muscle-invasive in the early stages, and up to 15% will eventually progress to muscle-invasive bladder urothelial carcinoma [20]. Superficial bladder cancers, such as stages Ta (superficial), Tis (in situ), and T1 (tumor invades subepithelial connective tissue) account for 75–85% of neoplasms at clinical presentation, while the remaining 15–25% are invasive (T2, T3, and T4) or have metastasized at the time of diagnosis [21].
As already mentioned above, the management of bladder cancer requires correct staging using the TNM system. The “T” of TNM is obtained by transurethral resection surgery. Transurethral resection of bladder tumor (TURBT) can stage up to T2, and it alone is capable of eradicating completely a Ta or T1 tumor. However, recurrences in bladder cancer are very common, with a possible progression. More than 70% of all patients treated for superficial bladder cancer will subsequently develop one or more recurrent tumors, and about one-third of these patients will progress to cancer that invades the surrounding muscle [22,23].
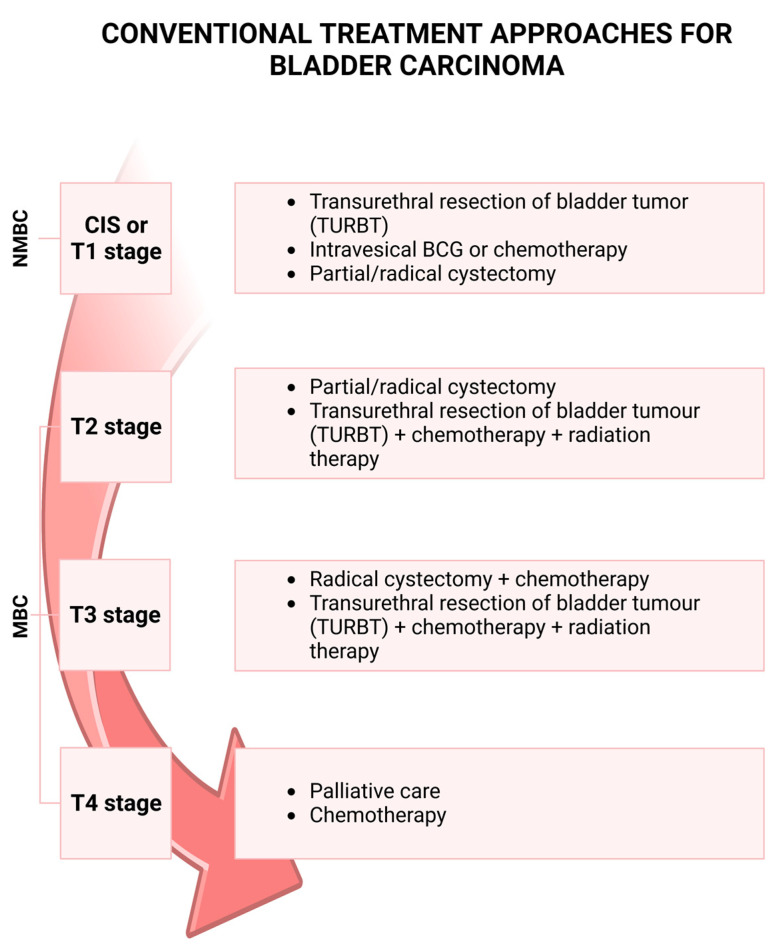
Adjuvant therapy should be considered in all patients (Figure 1): in low-grade bladder tumors, a single dose of intravesical chemotherapy (mitomycin C, epirubicin, or pyrarubicin) is suggested within 24 h of resection to prevent recurrence [24,25,26,27,28]. More intravesical instillations of chemotherapy may be necessary and suggested depending on the risk of progression and recurrence: for those patients with low-grade, low-risk tumors, only a single instillation post-surgical resection may be advised. Conversely, high-risk patients benefit from repeated treatment over time [29]. Patients with histological evidence of high-grade bladder cancer should undergo bladder instillations with bacillus Calmette–Guérin (BCG), which has a higher efficacy than chemotherapy in preventing the recurrence of non-muscle-invasive high-grade urothelial cancer [30,31,32,33,34]. CIS deserves a separate mention also regarding the treatment: in fact, it cannot be treated only with an endoscopic resection procedure. When a histological diagnosis of CIS is present, it is mandatory to perform intravesical instillations of BCG or, in certain cases, even to propose a radical cystectomy. There are some cases where the bladder tumor does not respond to BCG instillations and “relapsed tumors” or “recurrent tumors” are reported. In these cases, radical cystectomy must be proposed due to the high risk of progression and metastasis that BCG-unresponsive cancer has [35].
Figure 1.
Bladder carcinoma progression and therapy. Non-muscle-invasive bladder cancer (NMBC), including the carcinoma in situ (CIS) or T1 stages, is treated with transurethral resection of bladder tumor (TURBT), intravesical instillation of bacillus Calmette–Guérin (BCG) or chemotherapy. Treatment for muscle-invasive bladder cancer (MBC) can be cystectomy with chemotherapy or TURBT with chemotherapy and radiation therapy. The conventional treatment for the T4 stage is chemotherapy or palliative care. Created with BioRender.com.
3. Resveratrol and Bladder Cancer
The in vitro and in vivo effects of resveratrol as an antineoplastic agent in bladder cancer have recently been reviewed [11,12]. While several researchers have studied the in vitro effects of resveratrol in bladder cancer biology, only two studies have considered its potential use in in vivo models. Notably, clinical studies on the use of resveratrol in bladder cancer therapy protocols are completely missing (evaluated in https://clinicaltrials.gov/ website by using keywords such as “cancer bladder” and “resveratrol”, accessed on 4 January 2023). In this review, we focus on the possible applications of resveratrol in bladder cancer treatment by considering the evidence derived mainly from in vivo models.
A resveratrol dose of 20 mg/kg body weight, with daily intraperitoneal (i.p.) administration, inhibited the growth of subcutaneous (s.c.) xenografted bladder cancer [36]. Other investigators used resveratrol i.p. administration to evaluate the efficacy of resveratrol to inhibit tumor cell growth different from bladder cancer. In s.c. xenografted ovarian cancer using Balb/c nu/nu mice, i.p. injection at concentrations of 50 and 100 mg/kg body weight for 4 weeks reduced tumor growth [37]. Similarly, concentrations of 20 and 40 mg/kg body weight reduced the s.c. growth of tumors from Erlich’s ascites [38]. In addition, in an s.c. neuroblastoma tumor model, 5 mg of resveratrol decreased cancer growth [39]. Surprisingly, 10 mg/kg body weight of i.p. resveratrol was not able to reduce cancer growth and survival in NOD.CB17-Prkdcscid/J mice engrafted with the human t(4;11) acute lymphoblastic leukemia (ALL) cell line [40].
The lack of anticancer effects of 10 mg/kg daily of i.p. resveratrol can be associated with the low dose used since, in other studies, the relationship between anticancer effects and dose has been observed. For example, in glioblastoma s.c. syngeneic rat xenotransplants models, the i.p. injection of resveratrol (10 or 40 mg/kg daily for 4 weeks) reduced tumor mass only at the highest concentration through an antiangiogenic action [41]. Interestingly, an antiangiogenic action of resveratrol (reduction of VEGF and FGF-2) was suggested to be involved in the tumor reduction of the s.c. xenotransplated human T24 bladder cancer model [36]. Overall, i.p. resveratrol displays anticancer effects in bladder tumor xenotransplantation similar to other neoplasia at an i.p. dose higher than 10 mg/kg.
A pharmacokinetic study of i.p. administration of 10 mg/kg of resveratrol in a single dose in mice displayed a sub-micromolar plasma concentration after 1 h from the administration (the serum concentrations of the total resveratrol were ~4 ± 2 μM, roughly distributed at a 1:3:1 ratio of resveratrol/resveratrol glucuronide/resveratrol sulfate [40]), indicating that this value represents the threshold to observe the cancer growth reduction in vivo [41]. Unfortunately, scant information was reported in in vitro studies of the effects of a low micromolar concentration of resveratrol on bladder cancer biology. This last consideration made it difficult to compare and use the data obtained from the in vitro experiment where resveratrol was tested in bladder cancer cell lines at concentrations higher than 10 μM and up to 200 μM [12].
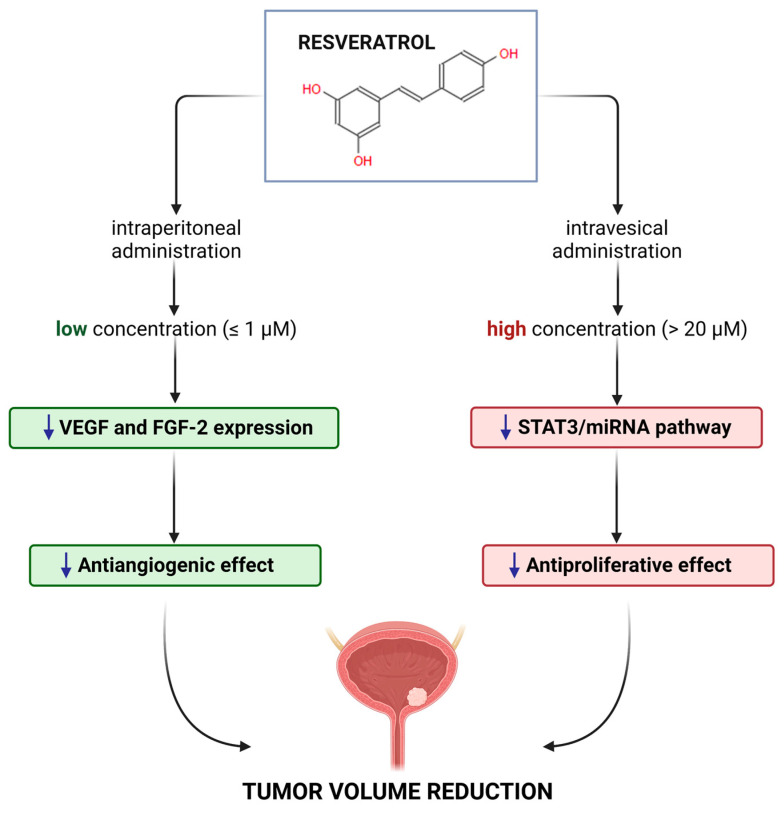
Resveratrol showed a biphasic effect on proliferation, which was related to the concentration when it was applied to a bladder cancer cell line. Specifically, at low concentrations (lower or equal to 20 μM), it had no antiproliferative effects, while at high concentrations (greater or equal to 20 μM), it exhibited an antiproliferative effect by inducing apoptosis [42]. This outcome was confirmed in several studies, as in the bladder cancer cell line T24, BTT739, Pumc-91/ADM, where an antiproliferative effect (i.e., cell cycle blockade or apoptosis) was observed at concentrations higher than 20 μM [36,43,44,45,46,47,48]. In a few studies, the antiproliferative action was observed to be time-dependent [49], whereas, in others, it depended on the status of the tumor protein p53 (TP53) [47]. At low concentrations (2.5 μM), resveratrol did not show any antiproliferative activity but retained the ability to induce the mitochondrial BCL2 apoptosis regulator (BCL2) protein and BCL2-associated agonist of cell death (BAD) after 48 h of treatment, without a significant change in the BAD/BCL2 ratio [42]. Although the mechanism is still unclear, resveratrol reduced the growth of the tumor mass in vivo when treated via i.p. (low systemic concentration), by modulating processes such as angiogenesis [36], with a reduction of the vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2). Therefore, we can hypothesize (Figure 2) that at low concentrations (obtained through a systemic administration, for example, i.p.) resveratrol has an antiangiogenic effect, reducing FGF-2 and VEGF, while at high concentrations (obtained through a local intravesical administration [44]) it has an antiproliferative effect as a consequence either of the reduction of miRNA21 [49], the signal transducer and activator of transcription 3 (STAT3), or its downstream genes, such as c-Myc, cyclinD1, survivin, and VEGF [44].
Figure 2.
Effects of resveratrol on bladder cancer in in vivo models. At low concentrations (achievable through an intraperitoneal administration, i.p., lower or equal to about 1 μM [40]), resveratrol exhibits an antiangiogenic effect, reducing vascular endothelial growth factor (VEGF) and fibroblast growth factor 2 (FGF-2). On the other hand, at high concentrations (achievable through an intravesical administration, i.v., higher than 200 μM [44]), it shows an antiproliferative effect, reducing miRNA21, the signal transducer and activator of transcription 3 (STAT3) and its downstream genes. Created with BioRender.com.
4. Other Mechanisms of Action of Resveratrol
The beneficial effects of resveratrol were associated with antioxidant properties and the ability to activate the sirtuins and protein kinase AMP-activated (AMPK) pathway. Because of the presence of more than one phenolic group, resveratrol belongs to the category of polyphenols and shows strong antioxidant properties, since it reacts with free radicals, resulting in more stable adducts, which are therefore less reactive and less toxic than the radicals themself [1], enhancing the cellular antioxidant activity. For example, resveratrol upregulates the tumor suppressor phosphatase and tensin homolog (PTEN), the major antagonist of phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K), by blocking AKT serine/threonine kinase 1 (Akt) activation, which leads to an upregulation of the mRNA levels of antioxidant enzymes such as catalase (CAT) and superoxide dismutase (SOD) [50]. Resveratrol stimulates the nuclear factor erythroid 2-related factor 2 (Nrf2), which initiates the transcription of many antioxidant genes such as SOD and CAT to reduce oxidative stress. Resveratrol could also improve the antioxidant defense system by modulating the action of antioxidant enzymes through the downregulation of the extracellular signal-regulated kinase (ERK) kinases that are activated by the reactive oxygen species (ROS). Neoplastic progression is associated with the alteration or mutation of genes that can occur spontaneously or following exposure to carcinogens. Oxidative stress plays a crucial role in the carcinogenesis process; ROS can react with DNA, causing serious damage, such as mutations [51]. Resveratrol, as a radical scavenger, appears as an anticancer agent by limiting the genotoxic impact of ROS and attenuating the processes of transformation into neoplastic cells [52]. In general, the anticancer effects have been correlated with other mechanisms of action independent from its radical scavenger properties [53]. In bladder cancer cell lines, resveratrol exerts its anticancer activity by inducing cell cycle arrest, apoptosis, differentiation, and inhibition of the proliferation of tumor cells.
It was demonstrated that resveratrol can activate the silent mating type information regulation 2 homolog 1 (SIRT1) and mimic the same beneficial effects induced by caloric restriction [54]. SIRT1 is a nicotinamide adenine dinucleotide (NAD)-dependent protein, one of the seven members of sirtuins, and belongs to the large family of the mammalian class III histone deacetylases [55]. It is mainly localized in the nucleus [56], and it is encoded by Sir2 (silent information regulator 2) [57], a highly preserved gene; homologs of Sir2 have also been found in lower organisms, such as the yeast Saccharomyces cerevisiae, the nematode Caenorhabditis elegans, and the dipterous Drosophila melanogaster [58,59]. The stimulation of SIRT1 leads to the deacetylation of lysine, coupled with the breakdown of NAD+ into nicotinamide adenine mononucleotide (NAM) and 1′-O-acetyl-ADP-ribose [59] or 1′- and 2′-O-acetyl-ADP-ribose [60], resulting in the modulation of the activity of the peroxisome proliferator-activated receptor gamma coactivator 1 alpha, (PPARGC1A, or PGC-1α) and other transcriptional factors related to aging and life span [47,61], including mitochondrial biogenesis [62].
However, even if the effects of resveratrol on SIRT1 and PGC-1α are well-known, the mechanism behind the regulation is still controversial [63]. Some studies have reported an indirect activation of SIRT1 by resveratrol, which firstly acts on AMPK, leading to an increase in NAD+ levels and, as a consequence, increases in SIRT1 and PGC-1α [64]. On the other hand, other studies have found a direct stimulation of SIRT1 by resveratrol, followed by the activation of AMPK through the deacetylation and activation of serine/threonine kinase 11 (LKB1) [65]. Data from in vitro and in vivo studies indicate a dose-dependent mechanism of resveratrol: when the dose of resveratrol was moderate (25 μM), the activation of AMPK was SIRT1-dependent, similar to that which occurs during caloric restriction, whereas a 2-fold concentration (50 μM) of resveratrol resulted in a SIRT1-independent activation of AMPK activation [63]. Furthermore, murine models lacking SIRT1 showed no differences in mitochondrial activity following treatment with both doses.
In osteoporosis rats, treatment with a high dose of resveratrol revealed a downregulation of Akt phosphorylation and a mechanistic target of rapamycin kinase (mTOR) phosphorylation, suggesting an involvement of the Akt/mTOR pathway in the bone cell autophagy activation induced by resveratrol [66] and the upregulation of the insulin signaling pathway through the phosphorylation of insulin receptor substrate 1 (IRS-1), PI3K, pyruvate dehydrogenase kinase 1 (PDK-1), Akt, and glycogen synthase kinase 3 (GSK-3) [67]. A double-blind randomized trial showed an improvement in the insulin sensitivity in T2D patients after 3 g/die of resveratrol per 12 weeks, with a significant increase in the SIRT1 and AMPK expressions in the skeletal muscle, which also determined an upregulation of the solute carrier family 2 member 4 (GLUT4) [68].
5. Pharmacokinetics of Resveratrol
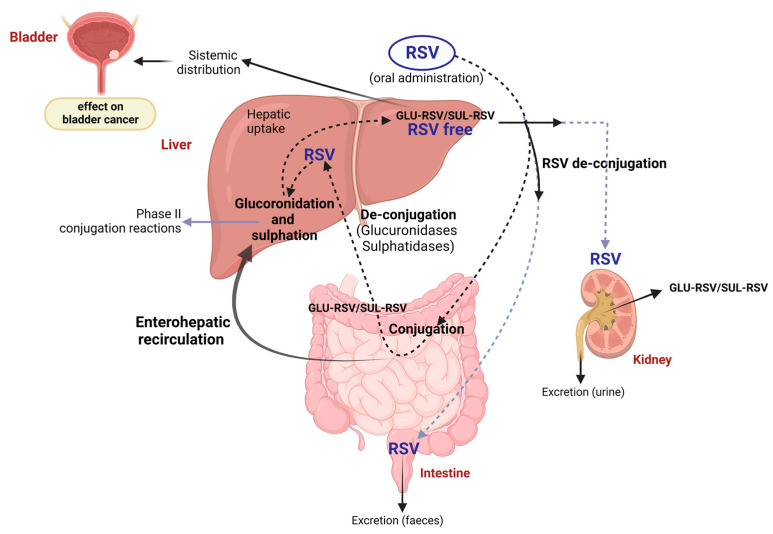
Because of its low solubility in water, resveratrol shows a limited bioavailability which complicates the possibility of replicating in vivo what has been demonstrated by in vitro studies [47,69]. Once ingested through food or as a food supplement, resveratrol is rapidly assimilated in the small intestine [70,71] by passive diffusion [72] or by carrier [73]. In the enterocyte, it undergoes conjugation with uridine diphosphoglucuronic acid (UDP-GA) or 3′-phosphoadenosine-5′-phosphosulphate (PAPS), in reactions respectively mediated by different enzymatic isoforms of UDP-glucuronyltransferase (UGT) and cytosolic sulphotransferase (SULT) [74,75,76]. The conjugated resveratrol moves from the enterocyte through the transporters breast cancer resistance protein (BCRP) and multidrug resistance-associated protein 2 (MRP2) located on the apical membrane or through MRP3 on the basolateral side, entering the portal vein system until it reaches the liver. After that, the conjugation can further be catalyzed by UGT1A1, UGT9A, and SULT1A1—the same isoforms mainly expressed in the intestine [77,78]. From the hepatocyte, free resveratrol or its metabolites, including resveratrol-3-O-sulphate, resveratrol-3-O-4’-O-disulphate, and resveratrol-3-O-glucuronide, undergo enterohepatic recirculation and are re-absorbed in the small intestine before entering the portal system and reaching the liver. Here, they can go through new reactions or head into the systemic circulation to be distributed to the whole body and definitively eliminated through the urine (Figure 3). Alternatively, resveratrol can be absorbed in the large intestine, specifically in the colon, where it is metabolized by the intestinal microbiota with the formation of dehydro-resveratrol, lunularin, or 3,4’-dihydroxy-trans-stilbene [77], or eliminated through the feces [70]. Otherwise, similarly to what is described above, free resveratrol or its metabolites can reach the liver and undergo enterohepatic recirculation or enter the systemic circulation and reach the kidneys, to be excreted [77,78].
Figure 3.
Metabolism and biotransformation of resveratrol in different organs. Resveratrol undergoes conjugation with uridine diphosphoglucuronic acid (UDP-GA, indicated as GLU-RSV in the figure) or 3′-phosphoadenosine-5′-phosphosulphate (PAPS, SUL-RSV indicated as SUL-RSV in the figure) in the enterocytes, before reaching the liver through the portal bloodstream. Here, it can be further conjugated (phase II reactions) and then absorbed by peripheral tissues. Part of the resveratrol intake can reach the plasma as free resveratrol (RES free), and another part can come from the de-conjugation reaction. In addition, conjugated resveratrol and its metabolites undergo enterohepatic circulation, where it can be metabolized by the intestinal microbiota. In the end, its excretion can occur through urine or feces. Created with BioRender.com.
The pharmacokinetics of trans-resveratrol can change according to the type of administration, dosages, and protocol treatment [79], while its plasma concentration depends on the ingested dose [77]. Despite this, a study revealed a low plasma level concentration of trans-resveratrol following a high dose intake and a short dosing interval, with a circadian variation that was correlated with higher bioavailability after morning administration [80]. Resveratrol was well-absorbed when administered orally in 500 mg tablets, and its plasma concentrations or metabolites corresponded to the concentrations of its in vitro efficacy [81]. In addition, another study reported a plasma concentration of 1 μM of resveratrol and much higher concentrations of its glucuronide and sulfate conjugates [82].
Several approaches to improve resveratrol’s bioavailability and pharmacokinetics profile have provided promising results, such as nanocrystals [83], casein nanoparticles [84], liquid micellar formulations [85], self-emulsifying drug delivery systems [86], oat protein–shellac nanoparticles [87], and layer-by-layer nano-formulations [88]. Selective organ targeting is also possible with trans-resveratrol-loaded mixed micelles, which target the brain [89], and resveratrol-loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles, which target the liver [90]. Recently, we developed a solid dispersion of resveratrol supported on magnesium dihydroxide (Resv@MDH) with better water solubility in simulated gastric fluids and an improved pharmacokinetic profile and bioavailability. Our investigation demonstrated that Resv@MDH increased resveratrol bioavailability by three times after oral administration in rabbit compared to pure resveratrol [91]. A clinical study also demonstrated that Resv@MDH improved the pharmacokinetic profile in humans [92], with a peak of plasma concentration in the micromolar range.
6. Combination of Resveratrol with Other Compounds
The use of resveratrol in combination with chemotherapeutic agents could allow for avoiding the development of drug resistance, which is a potential risk to not underestimate. Resveratrol can modulate and re-sensitize cancer cells to chemotherapeutic agents [37,93] when applied in combination with drugs in clinical therapy (Table 1).
Table 1.
Effects of treatment with resveratrol in combination with different drugs in bladder cancer cell lines. The table describes the effects of the combination between resveratrol and other compounds in the treatment of bladder cancer. DCK = deoxycytidine kinase; TK1 = thymidine kinase 1; TK2 = thymidine kinase 2; ABCC2 = ATP binding cassette subfamily C member 2; PARP = poly (ADP-ribose) polymerase; mTOR = rapamycin kinase; Akt = AKT serine/threonine kinase 1; TOP2 = DNA topoisomerase II; GST = glutathione S-transferase; LRP = LDL receptor-related protein; BCL2 = BCL2 apoptosis regulator; MRP1 = multidrug resistance-associated protein 1.
| Combination | Cell Line | Effect | Reference |
|---|---|---|---|
| Resveratrol + Gemcitabine | T24-GCB |
|
[94] |
|
Resveratrol +
Rapamycin |
TSC1-null MEFs WTMEFs 639V HCV29 MGH-U1 |
|
[95] |
| Resveratrol + Adriamycin | Pumc-91 |
|
[45] |
|
Resveratrol +
Doxorubicin |
5637 T24 |
|
[96] |
The combination of resveratrol (75 and 150 μM) with gemcitabine (10 μM) in the T24-GCB cell line revealed an additive effect by reducing the cytoplasmic levels of deoxycytidine kinase (DCK), thymidine kinase 1 (TK1), and thymidine kinase 2 (TK2), while ATP binding cassette subfamily C member 2 (ABCC2) was increased [93,94]. Poly (ADP-ribose) polymerase (PARP) cleavage and apoptosis were also increased by the combined therapy. In addition, relatively low doses of resveratrol (10 μM) reduced the migratory ability of T24-GCB cells [94]. All these findings confirm the ability of resveratrol to reverse the drug resistance of T24-GCB cells to gemcitabine.
On the other hand, in vitro treatment with rapamycin (20 nM) and resveratrol (100 μM) in different cell lines (TSC1-null MEFS, WTMEFs, 639V, HCV29, and MGH-U1 cells) unveiled the efficacy of the combination in maintaining rapamycin-induced inhibition of mTOR and resveratrol-induced inhibition of Akt activation. In addition, the combined therapy of resveratrol and rapamycin upregulated PARP and caspase 3, inducing apoptosis and preventing cell migration and colony formation in TSC1-null MEFs but not in WTMEFs, suggesting a TSC1-dependent mechanism of action [95]. These data confirm the potential of combined therapy with resveratrol and rapamycin to inhibit bladder cancer cell growth and induce cancer cell death, which could be specifically fitted for bladder cancer patients with tumors characterized by TSC1 mutations or activating PI3K/mTORC1 pathway mutations.
Another study investigated the role of resveratrol (0, 10, 50, and 100 µM) on pumc-91/ADM cells, showing a decrease in the resistance to adriamycin but an increase in the cytotoxicity of the drug by upregulating the expression levels of DNA topoisomerase II (TOP2) and downregulating the expression levels of glutathione S-transferase (GST), LDL receptor-related protein (LRP), BCL2, and MRP1 [45].
Combined therapy with doxorubicin at a low dose (2 µM) and resveratrol at high doses (150, 200, and 250 µM) in the 5637 and T24 bladder cancer cell lines showed an additive effect between the molecules, which caused enhanced cytotoxicity in both cell lines. Moreover, the combination of doxorubicin and resveratrol was more effective on the oxidative stress, cell colony formation, cell morphology, cell migration, and nuclear division index (NDI) assay [96] compared to the treatment with doxorubicin and resveratrol alone.
7. Final Remarks and Perspectives
Resveratrol displays potential anticancer activity in vivo, but data for synergetic effects with anticancer agents are missing. In glioblastoma, the combined action of resveratrol with anticancer agents needs pre-incubation [97]. In bladder cancer, this has not yet been studied, but it should be considered in order to be able to schedule the administration times in association with other chemo and radio-therapeutic agents. Another aspect of the future of the use of resveratrol for bladder cancer is the development of new formulations with better bioavailability and plasma concentrations. In fact, resveratrol has a plasma peak and low bioavailability that limit its efficacy as an anticancer agent since the plasma levels necessary for therapeutical effects are difficult to reach with common formulations [98,99]. Thus, research on a better resveratrol formulation with an increased pharmacokinetic profile represents a challenge for its potential use as adjuvant therapy in bladder cancer.
The outcomes from the combination therapy of resveratrol and drugs such as gemcitabine and rapamycin suggest a key role of resveratrol as an adjuvant in clinical therapy. The high concentrations (from 75 μM to 250 μM) indicated in the studies may be obtained using intravesical administration. However, the potential cytotoxic effect should be considered not only on the cancer cells but for the whole surrounding environment too. Therefore, we propose the instillation of resveratrol through intravesical injection after or before treatment, especially with gemcitabine, adriamycin, and doxorubicin, as rapamycin is cytostatic and not cytotoxic, in order to reduce the cytotoxicity of the combination.
Another crucial aspect regarding resveratrol is its action on miRNA21. As already highlighted, resveratrol can downregulate miRNA21 expression, especially when high doses are injected through intravesical administration. The expression of miRNA21 is high in bladder cancer and stromal cells and strictly related to cancer development [100], as it increases cancer progression by polarizing tumor-associated macrophages (TAMs) [101]. These macrophages are similar to M2 macrophage phenotypes and inhibit the function of cytotoxic T-lymphocytes (CTL). Moreover, the inhibition of miRNA21 was also correlated with the suppression of the Warburg effect in the osteosarcoma MG-63 cell line, through the reduction of the levels of lactic acid, adenosine triphosphate (ATP), and glucose uptake, and the downregulation of those proteins involved in the Warburg effect, such as GLUT1, lactate dehydrogenase A (LDHA), hexokinase 2 (HK2), and pyruvate kinase M1/2 (PKM) [102]. A similar effect is also plausible in bladder cancer, as indicated by the literature available so far.
Further studies should be carried out to better understand how these molecules work together and enhance the effectiveness of the standard clinical approach. These results could pave the way for new opportunities, such as the use of this novel resveratrol formulation in clinical applications as an adjuvant therapy for bladder cancer.
Acknowledgments
The authors thank Florinda Apone for critical suggestions and advice.
Author Contributions
Conceptualization, A.Z. and B.F.; methodology, A.Z. and B.F.; software, A.B. and L.S.; validation, A.Z. and B.F.; formal analysis, F.P.; investigation, A.P., F.C., A.L.P., A.B., L.S., C.C., R.G. and F.P.; resources, A.P., F.C., A.L.P., A.B., L.S., C.C., R.G. and F.P.; data curation, A.P., F.C., A.L.P., A.B., L.S., C.C., R.G. and F.P.; writing—original draft preparation, A.Z., B.F. A.B. and L.S.; writing—review and editing, A.Z., B.F. A.B., R.G. and L.S.; visualization, A.B. and L.S.; supervision, A.Z. and B.F.; project administration, A.Z. and B.F.; funding acquisition, A.Z. and B.F. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Fondo Integrativo Speciale per la Ricerca (FISR2020) of the Italian MUR (Ministry of University and Research) to B.F.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kiskova T., Kubatka P., Büsselberg D., Kassayova M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules. 2020;10:161. doi: 10.3390/biom10010161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug. Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 3.Marques F.Z., Markus M.A., Morris B.J. Resveratrol: Cellular actions of a potent natural chemical that confers a diversity of health benefits. Int. J. Biochem. Cell Biol. 2009;41:2125–2128. doi: 10.1016/j.biocel.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Romero-Pérez A.I., Ibern-Gómez M., Lamuela-Raventós R.M., de La Torre-Boronat M.C. Piceid, the major resveratrol derivative in grape juices. J. Agric. Food Chem. 1999;47:1533–1536. doi: 10.1021/jf981024g. [DOI] [PubMed] [Google Scholar]
- 5.Zhou D.D., Luo M., Huang S.Y., Saimaiti A., Shang A., Gan R.Y., Li H.B. Effects and Mechanisms of Resveratrol on Aging and Age-Related Diseases. Oxidative Med. Cell. Longev. 2021;2021:9932218. doi: 10.1155/2021/9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X., Song X., Zhao X., Zhang Y., Wang Y., Jia R., Zou Y., Li L., Yin Z. Insights into the Anti-inflammatory and Antiviral Mechanisms of Resveratrol. Mediat. Inflamm. 2022;2022:7138756. doi: 10.1155/2022/7138756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Islam F., Nafady M.H., Islam M.R., Saha S., Rashid S., Akter A., Or-Rashid M.H., Akhtar M.F., Perveen A., Md Ashraf G., et al. Resveratrol and neuroprotection: An insight into prospective therapeutic approaches against Alzheimer’s disease from bench to bedside. Mol. Neurobiol. 2022;59:4384–4404. doi: 10.1007/s12035-022-02859-7. [DOI] [PubMed] [Google Scholar]
- 8.Chupradit S., Bokov D., Zamanian M.Y., Heidari M., Hakimizadeh E. Hepatoprotective and therapeutic effects of resveratrol: A focus on anti-inflammatory and antioxidative activities. Fundam. Clin. Pharmacol. 2022;36:468–485. doi: 10.1111/fcp.12746. [DOI] [PubMed] [Google Scholar]
- 9.Gal R., Deres L., Toth K., Halmosi R., Habon T. The Effect of Resveratrol on the Cardiovascular System from Molecular Mechanisms to Clinical Results. Int. J. Mol. Sci. 2021;22:10152. doi: 10.3390/ijms221810152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su M., Zhao W., Xu S., Weng J. Resveratrol in Treating Diabetes and Its Cardiovascular Complications: A Review of Its Mechanisms of Action. Antioxidants. 2022;11:1085. doi: 10.3390/antiox11061085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ren B., Kwah M.X., Liu C., Ma Z., Shanmugam M.K., Ding L., Xiang X., Ho P.C., Wang L., Ong P.S., et al. Resveratrol for cancer therapy: Challenges and future perspectives. Cancer Lett. 2021;515:63–72. doi: 10.1016/j.canlet.2021.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Almeida T.C., Silva G.N.D. Resveratrol effects in bladder cancer: A mini review. Genet. Mol. Biol. 2021;44:e20200371. doi: 10.1590/1678-4685-gmb-2020-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.IARC Cancer Today. Estimated Number of New Cases in 2020, Worldwide, Both Sexes, All Ages. 2021. 2022. [(accessed on 4 January 2023)]. Available online: https://gco.iarc.fr/today/online-analysis-table.
- 14.Burger M., Catto J.W., Dalbagni G., Grossman H.B., Herr H., Karakiewicz P., Kassouf W., Kiemeney L.A., La Vecchia C., Shariat S., et al. Epidemiology and risk factors of urothelial bladder cancer. Eur. Urol. 2013;63:234–241. doi: 10.1016/j.eururo.2012.07.033. [DOI] [PubMed] [Google Scholar]
- 15.Compérat E., Larré S., Roupret M., Neuzillet Y., Pignot G., Quintens H., Houéde N., Roy C., Durand X., Varinot J., et al. Clinicopathological characteristics of urothelial bladder cancer in patients less than 40 years old. Virchows Arch. 2015;466:589–594. doi: 10.1007/s00428-015-1739-2. [DOI] [PubMed] [Google Scholar]
- 16.Brierley J.D., Gospodarowicz M.K., Wittekind C. TNM Classification of Malignant Tumours. 8th ed. Wiley-Blackwell and UICC; New York, NY, USA: 2017. UICC International Union against Cancer. [Google Scholar]
- 17.Andersson M., Berger M., Zieger K., Malmström P.U., Bläckberg M. The diagnostic challenge of suspicious or positive malignant urine cytology findings when cystoscopy findings are normal: An outpatient blue-light flexible cystoscopy may solve the problem. Scand. J. Urol. 2021;55:263–267. doi: 10.1080/21681805.2021.1928746. [DOI] [PubMed] [Google Scholar]
- 18.Sylvester R.J., Rodríguez O., Hernández V., Turturica D., Bauerová L., Bruins H.M., Bründl J., van der Kwast T.H., Brisuda A., Rubio-Briones J., et al. European Association of Urology (EAU) Prognostic Factor Risk Groups for Non-muscle-invasive Bladder Cancer (NMIBC) Incorporating the WHO 2004/2016 and WHO 1973 Classification Systems for Grade: An Update from the EAU NMIBC Guidelines Panel. Eur. Urol. 2021;79:480–488. doi: 10.1016/j.eururo.2020.12.033. [DOI] [PubMed] [Google Scholar]
- 19.MacLennan G.T., Kirkali Z., Cheng L. Histologic grading of noninvasive papillary urothelial neoplasms. Eur. Urol. 2007;51:889–898. doi: 10.1016/j.eururo.2006.10.037. [DOI] [PubMed] [Google Scholar]
- 20.Smaldone M.C., Jacobs B.L., Smaldone A.M., Hrebinko R.L., Jr. Long-term results of selective partial cystectomy for invasive urothelial bladder carcinoma. Urology. 2008;72:613–616. doi: 10.1016/j.urology.2008.04.052. [DOI] [PubMed] [Google Scholar]
- 21.Prout G.R., Jr. Bladder carcinoma and a TNM system of classification. J. Urol. 1977;117:583–590. doi: 10.1016/S0022-5347(17)58544-4. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Beltran A. Bladder cancer: Clinical and pathological profile. Scand. J. Urol. Nephrol. 2008;42:95–109. doi: 10.1080/03008880802325226. [DOI] [PubMed] [Google Scholar]
- 23.Brausi M., Collette L., Kurth K., van der Meijden A.P., Oosterlinck W., Witjes J.A., Newling D., Bouffioux C., Sylvester R.J., EORTC Genito-Urinary Tract Cancer Collaborative Group Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: A combined analysis of seven EORTC studies. Eur. Urol. 2002;41:523–531. doi: 10.1016/S0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 24.Sharma S., Ksheersagar P., Sharma P. Diagnosis and treatment of bladder cancer. Am. Fam. Physician. 2009;80:717–723. [PubMed] [Google Scholar]
- 25.Sylvester R.J., Oosterlinck W., Holmang S., Sydes M.R., Birtle A., Gudjonsson S., De Nunzio C., Okamura K., Kaasinen E., Solsona E., et al. Systematic Review and Individual Patient Data Meta-analysis of Randomized Trials Comparing a Single Immediate Instillation of Chemotherapy After Transurethral Resection with Transurethral Resection Alone in Patients with Stage pTa-pT1 Urothelial Carcinoma of the Bladder: Which Patients Benefit from the Instillation? Eur. Urol. 2016;69:231–244. doi: 10.1016/j.eururo.2015.05.050. [DOI] [PubMed] [Google Scholar]
- 26.Sylvester R.J., Oosterlinck W., van der Meijden A.P. A single immediate postoperative instillation of chemotherapy decreases the risk of recurrence in patients with stage Ta T1 bladder cancer: A meta-analysis of published results of randomized clinical trials. J. Urol. 2004;171:2186–2435. doi: 10.1097/01.ju.0000125486.92260.b2. [DOI] [PubMed] [Google Scholar]
- 27.Abern M.R., Owusu R.A., Anderson M.R., Rampersaud E.N., Inman B.A. Perioperative intravesical chemotherapy in non-muscle-invasive bladder cancer: A systematic review and meta-analysis. J. Natl. Compr. Cancer Netw. 2013;11:477–484. doi: 10.6004/jnccn.2013.0060. [DOI] [PubMed] [Google Scholar]
- 28.Perlis N., Zlotta A.R., Beyene J., Finelli A., Fleshner N.E., Kulkarni G.S. Immediate post-transurethral resection of bladder tumor intravesical chemotherapy prevents non-muscle-invasive bladder cancer recurrences: An updated meta-analysis on 2548 patients and quality-of-evidence review. Eur. Urol. 2013;64:421–430. doi: 10.1016/j.eururo.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 29.Tolley D.A., Parmar M.K., Grigor K.M., Lallemand G., Benyon L.L., Fellows J., Freedman L.S., Grigor K.M., Hall R.R., Hargreave T.B., et al. The effect of intravesical mitomycin C on recurrence of newly diagnosed superficial bladder cancer: A further report with 7 years of follow up. J. Urol. 1996;155:1233–1238. doi: 10.1016/S0022-5347(01)66226-8. [DOI] [PubMed] [Google Scholar]
- 30.Malmström P., Sylvester R.J.U., Crawford D.E., Friedrich M., Krege S., Rintala E., Solsona E., Di Stasi S.M., Witjes J.A. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur. Urol. 2009;56:247–256. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 31.Shelley M.D., Kynaston H., Court J., Wilt T.J., Coles B., Burgon K., Mason M.D. A systematic review of intravesical bacillus Calmette-Guérin plus transurethral resection vs transurethral resection alone in Ta and T1 bladder cancer. BJU Int. 2001;88:209–216. doi: 10.1046/j.1464-410x.2001.02306.x. [DOI] [PubMed] [Google Scholar]
- 32.Han R.F., Pan J.G. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216–1223. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Shelley M.D., Wilt T.J., Court J., Coles B., Kynaston H., Mason M.D. Intravesical bacillus Calmette-Guérin is superior to mitomycin C in reducing tumour recurrence in high-risk superficial bladder cancer: A meta-analysis of randomized trials. BJU Int. 2004;93:485–490. doi: 10.1111/j.1464-410X.2003.04655.x. [DOI] [PubMed] [Google Scholar]
- 34.Böhle A., Jocham D., Bock P.R. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: A formal meta-analysis of comparative studies on recurrence and toxicity. J. Urol. 2003;169:90–95. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 35.Raj G.V., Herr H., Serio A.M., Donat S.M., Bochner B.H., Vickers A.J., Dalbagni G. Treatment paradigm shift may improve survival of patients with high risk superficial bladder cancer. J. Urol. 2007;177:1283–1286. doi: 10.1016/j.juro.2006.11.090. [DOI] [PubMed] [Google Scholar]
- 36.Bai Y., Mao Q.Q., Qin J., Zheng X.Y., Wang Y.B., Yang K., Shen H.F., Xie L.P. Resveratrol induces apoptosis and cell cycle arrest of human T24 bladder cancer cells in vitro and inhibits tumor growth in vivo. Cancer Sci. 2010;101:488–493. doi: 10.1111/j.1349-7006.2009.01415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lee M.H., Choi B.Y., Kundu J.K., Shin Y.K., Na H.K., Surh Y.J. Resveratrol suppresses growth of human ovarian cancer cells in culture and in a murine xenograft model: Eukaryotic elongation factor 1A2 as a potential target. Cancer Res. 2009;69:7449–7458. doi: 10.1158/0008-5472.CAN-09-1266. [DOI] [PubMed] [Google Scholar]
- 38.El-Mowafy A.M., El-Mesery M.E., Salem H.A., Al-Gayyar M.M., Darweish M.M. Prominent chemopreventive and chemoenhancing effects for resveratrol: Unraveling molecular targets and the role of C-reactive protein. Chemotherapy. 2010;56:60–65. doi: 10.1159/000298821. [DOI] [PubMed] [Google Scholar]
- 39.Kenealey J.D., Subramanian L., Van Ginkel P.R., Darjatmoko S., Lindstrom M.J., Somoza V., Ghosh S.K., Song Z., Hsung R.P., Kwon G.S., et al. Resveratrol metabolites do not elicit early pro-apoptotic mechanisms in neuroblastoma cells. J. Agric. Food. Chem. 2011;59:4979–4986. doi: 10.1021/jf104901g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zunino S.J., Storms D.H., Newman J.W., Pedersen T.L., Keen C.L., Ducore J.M. Resveratrol given intraperitoneally does not inhibit the growth of high-risk t(4;11) acute lymphoblastic leukemia cells in a NOD/SCID mouse model. Int. J. Oncol. 2012;40:1277–1284. doi: 10.3892/ijo.2011.1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng S.H., Lin S.M., Chen J.C., Su Y.H., Huang H.Y., Chen C.K., Lin P.Y., Chen Y. Resveratrol suppresses the angiogenesis and tumor growth of gliomas in rats. Clin. Cancer Res. 2004;10:2190–2202. doi: 10.1158/1078-0432.CCR-03-0105. [DOI] [PubMed] [Google Scholar]
- 42.Stocco B., Toledo K., Salvador M., Paulo M., Koyama N., Torqueti Toloi M.R. Dose-dependent effect of resveratrol on bladder cancer cells: Chemoprevention and oxidative stress. Maturitas. 2012;72:72–78. doi: 10.1016/j.maturitas.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 43.Lin X., Wu G., Huo W.Q., Zhang Y., Jin F.S. Resveratrol induces apoptosis associated with mitochondrial dysfunction in bladder carcinoma cells. Int. J. Urol. 2012;19:757–764. doi: 10.1111/j.1442-2042.2012.03024.x. [DOI] [PubMed] [Google Scholar]
- 44.Wu M.L., Li H., Yu L.J., Chen X.Y., Kong Q.Y., Song X., Shu X.H., Liu J. Short-term resveratrol exposure causes in vitro and in vivo growth inhibition and apoptosis of bladder cancer cells. PLoS ONE. 2014;9:e89806. doi: 10.1371/journal.pone.0089806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang S., Meng Q., Xie Q., Zhang M. Effect and mechanism of resveratrol on drug resistance in human bladder cancer cells. Mol. Med. Rep. 2017;15:1179–1187. doi: 10.3892/mmr.2017.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y., Li C., Li H., Wu M., Ren C., Zhen Y., Ma X., Diao Y., Ma X., Deng S., et al. Differential sensitivities of bladder cancer cell lines to resveratol are unrelated to its metabolic profile. Oncotarget. 2017;8:40289–40304. doi: 10.18632/oncotarget.15041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Almeida T.C., Guerra C.C.C., De Assis B.L.G., de Oliveira Aguiar Soares R.D., Garcia C.C.M., Lima A.A., da Silva G.N. Antiproliferative and toxicogenomic effects of resveratrol in bladder cancer cells with different TP53 status. Environ. Mol. Mutagen. 2019;60:740–751. doi: 10.1002/em.22297. [DOI] [PubMed] [Google Scholar]
- 48.Yang Y., Zhang G., Li C., Wang S., Zhu M., Wang J., Yue H., Ma X., Zhen Y., Shu X. Metabolic profile and structure-activity relationship of resveratrol and its analogs in human bladder cancer cells. Cancer Manag. Res. 2019;11:4631–4642. doi: 10.2147/CMAR.S206748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhou C., Ding J., Wu Y. Resveratrol induces apoptosis of bladder cancer cells via miR 21 regulation of the Akt/Bcl 2 signaling pathway. Mol. Med. Rep. 2014;9:1467–1473. doi: 10.3892/mmr.2014.1950. [DOI] [PubMed] [Google Scholar]
- 50.Meng X., Zhou J., Zhao C.N., Gan R.Y., Li H.B. Health Benefits and Molecular Mechanisms of Resveratrol: A Narrative Review. Foods. 2020;9:340. doi: 10.3390/foods9030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ko J.H., Sethi G., Um J.Y., Shanmugam M.K., Arfuso F., Kumar A.P., Bishayee A., Ahn K.S. The Role of Resveratrol in Cancer Therapy. Int. J. Mol. Sci. 2017;18:2589. doi: 10.3390/ijms18122589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Delmas D., Lançon A., Colin D., Jannin B., Latruffe N. Resveratrol as a chemopreventive agent: A promising molecule for fighting cancer. Curr. Drug Targets. 2006;7:423–442. doi: 10.2174/138945006776359331. [DOI] [PubMed] [Google Scholar]
- 53.Bhaskara V.K., Mittal B., Mysorekar V.V., Amaresh N., Simal-Gandara J. Resveratrol, cancer and cancer stem cells: A review on past to future. Curr. Res. Food Sci. 2020;3:284–295. doi: 10.1016/j.crfs.2020.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Deng Z., Li Y., Liu H., Xiao S., Li L., Tian J., Cheng C., Zhang G., Zhang F. The role of sirtuin 1 and its activator, resveratrol in osteoarthritis. Biosci. Rep. 2019;39:BSR20190189. doi: 10.1042/BSR20190189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tang B.L. Sirt1 and the Mitochondria. Mol. Cells. 2016;39:87–95. doi: 10.14348/molcells.2016.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Michishita E., Park J.Y., Burneskis J.M., Barrett J.C., Horikawa I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol. Biol. Cell. 2005;16:4623–4635. doi: 10.1091/mbc.e05-01-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tanner K.G., Landry J., Sternglanz R., Denu J.M. Silent information regulator 2 family of NAD-dependent histone/protein deacetylases generates a unique product, 1-O-acetyl-ADP-ribose. Proc. Natl. Acad. Sci. USA. 2000;97:14178–14182. doi: 10.1073/pnas.250422697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brachmann C.B., Sherman J.M., Devine S.E., Cameron E.E., Pillus L., Boeke J.D. The SIR2 gene family, conserved from bacteria to humans, functions in silencing, cell cycle progression, and chromosome stability. Genes Dev. 1995;9:2888–2902. doi: 10.1101/gad.9.23.2888. [DOI] [PubMed] [Google Scholar]
- 59.Guarente L. Sirtuins, aging, and metabolism. Cold Spring Harb. Symp. Quant. Biol. 2011;76:81–90. doi: 10.1101/sqb.2011.76.010629. [DOI] [PubMed] [Google Scholar]
- 60.Jackson M.D., Denu J.M. Structural identification of 2′- and 3′-O-acetyl-ADP-ribose as novel metabolites derived from the Sir2 family of beta -NAD+-dependent histone/protein deacetylases. J. Biol. Chem. 2002;277:18535–18544. doi: 10.1074/jbc.M200671200. [DOI] [PubMed] [Google Scholar]
- 61.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Civitarese A.E., Carling S., Heilbronn L.K., Hulver M.H., Ukropcova B., Deutsch W.A., Smith S.R., Ravussin E., CALERIE Pennington Team Calorie restriction increases muscle mitochondrial biogenesis in healthy humans. PLoS Med. 2007;4:e76. doi: 10.1371/journal.pmed.0040076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Price N.L., Gomes A.P., Ling A.J., Duarte F.V., Martin-Montalvo A., North B.J., Agarwal B., Ye L., Ramadori G., Teodoro J.S., et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15:675–690. doi: 10.1016/j.cmet.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cantó C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou X., Xu S., Maitland-Toolan K.A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T.J., et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMPactivated protein kinase. J. Biol. Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang X., Jiang T., Wang Y., Guo L. The Role and Mechanism of SIRT1 in Resveratrol-regulated Osteoblast Autophagy in Osteoporosis Rats. Sci. Rep. 2019;9:18424. doi: 10.1038/s41598-019-44766-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hong H.J., Kang W., Kim D.G., Lee D.H., Lee Y., Han C.H. Effects of resveratrol on the insulin signaling pathway of obese mice. J. Vet. Sci. 2014;15:179–185. doi: 10.4142/jvs.2014.15.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Goh K.P., Lee H.Y., Lau D.P., Supaat W., Chan Y.H., Koh A.F.Y. Effects of Resveratrol in Patients with Type 2 Diabetes Mellitus on Skeletal Muscle SIRT1 Expression and Energy Expenditure. Int. J. Sport. Nutr. Exerc. Metab. 2014;24:2–13. doi: 10.1123/ijsnem.2013-0045. [DOI] [PubMed] [Google Scholar]
- 69.Walle T., Hsieh F., DeLegge M.H., Oatis J.E., Walle U.K. High absorption but very low bioavailability of oral resveratrol in humans. Drug Metab. Dispos. 2004;32:1377–1382. doi: 10.1124/dmd.104.000885. [DOI] [PubMed] [Google Scholar]
- 70.Wenzel E., Somoza V. Metabolism and bioavailability of trans-resveratrol. Mol. Nutr. Food Res. 2005;49:472–481. doi: 10.1002/mnfr.200500010. [DOI] [PubMed] [Google Scholar]
- 71.Yu C., Geun Shin Y., Chow A., Li Y., Kosmeder J.W., Sup Lee Y., Hirschelman W.H., Pezzuto J.M., Mehta R.G., Van Breemen R.B. Human, Rat, And Mouse Metabolism Of Resveratrol. Pharm. Res. 2002;19:1907–1914. doi: 10.1023/A:1021414129280. [DOI] [PubMed] [Google Scholar]
- 72.Henry C., Vitrac X., Decendit A., Ennamany R., Krisa S., Mérillon J.-M. Cellular Uptake And Efflux Of Trans -Piceid And Its Aglycone Trans -Resveratrol On The Apical Membrane Of Human Intestinal Caco-2 Cells. J. Agric. Food Chem. 2005;53:798–803. doi: 10.1021/jf048909e. [DOI] [PubMed] [Google Scholar]
- 73.Planas J.M., Alfaras I., Colom H., Juan M.E. The Bioavailability And Distribution Of Trans-Resveratrol Are Constrained By Abc Transporters. Arch. Biochem. Biophys. 2012;527:67–73. doi: 10.1016/j.abb.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 74.Böhmdorfer M., Szakmary A., Schiestl R., Vaquero J., Riha J., Brenner S., Thalhammer T., Szekeres T., Jäger W. Involvement of UDP-Glucuronosyltransferases and Sulfotransferases in the Excretion and Tissue Distribution of Resveratrol in Mice. Nutrients. 2017;9:1347. doi: 10.3390/nu9121347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Brill S.S., Furimsky A.M., Ho M.N., Furniss M.J., Li Y., Green A.G., Bradford W.W., Green C.E., Kapetanovic I.M., Iyer L.V. Glucuronidation of trans-resveratrol by human liver and intestinal microsomes and UGT isoforms. J. Pharm. Pharmacol. 2006;58:469–479. doi: 10.1211/jpp.58.4.0006. [DOI] [PubMed] [Google Scholar]
- 76.Miksits M., Maier-Salamon A., Aust S., Thalhammer T., Reznicek G., Kunert O., Haslinger E., Szekeres T., Jaeger W. Sulfation of resveratrol in human liver: Evidence of a major role for the sulfotransferases SULT1A1 and SULT1E1. Xenobiotica. 2005;35:1101–1119. doi: 10.1080/00498250500354253. [DOI] [PubMed] [Google Scholar]
- 77.Bode L.M., Bunzel D., Huch M., Cho G., Ruhland D., Bunzel M., Bub A., Franz C.M., Kulling S.E. In Vivo And In Vitro Metabolism Of Trans-Resveratrol By Human Gut Microbiota. Am. J. Clin. Nutr. 2013;97:295–309. doi: 10.3945/ajcn.112.049379. [DOI] [PubMed] [Google Scholar]
- 78.Springer M., Moco S. Resveratrol and Its Human Metabolites-Effects on Metabolic Health and Obesity. Nutrients. 2019;11:143. doi: 10.3390/nu11010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang P., Sang S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. BioFactors. 2018;44:16–25. doi: 10.1002/biof.1410. [DOI] [PubMed] [Google Scholar]
- 80.Almeida L., Vaz-Da-Silva M., Falcão A., Soares E., Costa R., Loureiro A.I., Fernandes-Lopes C., Rocha J.-F., Nunes T., Wright L., et al. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009;53:S7–S15. doi: 10.1002/mnfr.200800177. [DOI] [PubMed] [Google Scholar]
- 81.Sergides C., Chirilă M., Silvestro L., Pitta D., Pittas A. Bioavailability and safety study of resveratrol 500 mg tablets in healthy male and female volunteers. Exp. Ther. Med. 2016;11:164–170. doi: 10.3892/etm.2015.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Brown V.A., Patel K.R., Viskaduraki M., Crowell J.A., Perloff M., Booth T.D., Vasilinin G., Sen A., Schinas A.M., Piccirilli G., et al. Repeat Dose Study of the Cancer Chemopreventive Agent Resveratrol in Healthy Volunteers: Safety, Pharmacokinetics, and Effect on the Insulin-like Growth Factor Axis. Cancer Res. 2010;70:9003–9011. doi: 10.1158/0008-5472.CAN-10-2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh S.K., Makadia V., Sharma S., Rashid M., Shahi S., Mishra P.R., Wahajuddin M., Gayen J.R. Preparation and in-vitro/in-vivo characterization of trans-resveratrol nanocrystals for oral administration. Drug Deliv. Transl. Res. 2017;7:395–407. doi: 10.1007/s13346-017-0362-y. [DOI] [PubMed] [Google Scholar]
- 84.Peñalva R., Morales J., González-Navarro C.J., Larrañeta E., Quincooces G., Peñuelas I., Irache J. Increased Oral Bioavailability of Resveratrol by Its Encapsulation in Casein Nanoparticles. Int. J. Mol. Sci. 2018;19:2816. doi: 10.3390/ijms19092816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Calvo-Castro L.A., Schiborr C., David F., Ehrt H., Voggel J., Sus N., Behnam D., Bosy-Westphal A., Frank J. The Oral Bioavailability of Trans-Resveratrol from a Grapevine-Shoot Extract in Healthy Humans is Significantly Increased by Micellar Solubilization. Mol. Nutr. Food Res. 2018;62:e1701057. doi: 10.1002/mnfr.201701057. [DOI] [PubMed] [Google Scholar]
- 86.Vasconcelos T., Araújo F., Lopes C., Loureiro A., Das Neves J., Marques S., Sarmento B. Multicomponent self-nano emulsifying delivery systems of resveratrol with enhanced pharmacokinetics profile. Eur. J. Pharm. Sci. 2019;137:105011. doi: 10.1016/j.ejps.2019.105011. [DOI] [PubMed] [Google Scholar]
- 87.Yang C., Wang Y., Xie Y., Liu G., Lu Y., Wu W., Chen L. Oat protein-shellac nanoparticles as a delivery vehicle for resveratrol to improve bioavailability in vitro and in vivo. Nanomedicine. 2019;14:2853–2871. doi: 10.2217/nnm-2019-0244. [DOI] [PubMed] [Google Scholar]
- 88.Santos A.C., Veiga F., Sequeira J.A.D., Fortuna A., Falcão A., Souto E.B., Pattekari P., Ribeiro C.F., Ribeiro A.J. First-time oral administration of resveratrol-loaded layer-by-layer nanoparticles to rats—A pharmacokinetics study. Analyst. 2019;144:2062–2079. doi: 10.1039/C8AN01998C. [DOI] [PubMed] [Google Scholar]
- 89.Katekar R., Thombre G., Riyazuddin M., Husain A., Rani H., Praveena K.S., Gayen J.R. Pharmacokinetics and brain targeting of trans-resveratrol loaded mixed micelles in rats following intravenous administration. Pharm. Dev. Technol. 2019;25:300–307. doi: 10.1080/10837450.2019.1680690. [DOI] [PubMed] [Google Scholar]
- 90.Wu M., Zhong C., Deng Y., Zhang Q., Zhang X., Zhao X. Resveratrol loaded glycyrrhizic acid-conjugated human serum albumin nanoparticles for tail vein injection II: Pharmacokinetics, tissue distribution and bioavailability. Drug Deliv. 2020;27:81–90. doi: 10.1080/10717544.2019.1704944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spogli R., Bastianini M., Ragonese F., Iannitti R.G., Monarca L., Bastioli F., Nakashidze I., Brecchia G., Menchetti L., Codini M., et al. Solid Dispersion of Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Microparticles Improves Oral Bioavailability. Nutrients. 2018;10:1925. doi: 10.3390/nu10121925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Iannitti R.G., Floridi A., Lazzarini A., Tantucci A., Russo R., Ragonese F., Monarca L., Caglioti C., Spogli R., Leonardi L., et al. Resveratrol Supported on Magnesium DiHydroxide (Resv@MDH) Represents an Oral Formulation of Resveratrol With Better Gastric Absorption and Bioavailability Respect to Pure Resveratrol. Front. Nutr. 2020;7:570047. doi: 10.3389/fnut.2020.570047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cho C.J., Yang C.W., Wu C.L., Ho J.Y., Yu C.P., Wu S.T., Yu D.S. The modulation study of multiple drug resistance in bladder cancer by curcumin and resveratrol. Oncol. Lett. 2019;18:6869–6876. doi: 10.3892/ol.2019.11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cho C.J., Yu C.P., Wu C.L., Ho J.Y., Yang C.W., Yu D.S. Decreased drug resistance of bladder cancer using phytochemicals treatment. Kaohsiung J. Med. Sci. 2021;37:128–135. doi: 10.1002/kjm2.12306. [DOI] [PubMed] [Google Scholar]
- 95.Alayev A., Salamon R.S., Schwartz N.S., Berman A.Y., Wiener S.L., Holz M.K. Combination of Rapamycin and Resveratrol for Treatment of Bladder Cancer. J. Cell Physiol. 2017;232:436–446. doi: 10.1002/jcp.25443. [DOI] [PubMed] [Google Scholar]
- 96.Soares L.B.M., Lima A.P.B., Melo A.S., Almeida T.C., de Medeiros Teixeira L.F., da Silva G.N. Additive effects of resveratrol and doxorubicin on bladder cancer cells. Anticancer Drugs. 2022;33:e389–e397. doi: 10.1097/CAD.0000000000001218. [DOI] [PubMed] [Google Scholar]
- 97.Manna S.K., Mukhopadhyay A., Aggarwal B.B. Resveratrol suppresses TNF-induced activation of nuclear transcription factors NF-kappa B, activator protein-1, and apoptosis: Potential role of reactive oxygen intermediates and lipid peroxidation. J. Immunol. 2000;164:6509–6519. doi: 10.4049/jimmunol.164.12.6509. [DOI] [PubMed] [Google Scholar]
- 98.Amri A., Chaumeil J.C., Sfar S., Charrueau C. Administration of resveratrol: What formulation solutions to bioavailability limitations? J. Control. Release. 2012;158:182–193. doi: 10.1016/j.jconrel.2011.09.083. [DOI] [PubMed] [Google Scholar]
- 99.Chimento A., De Amicis F., Sirianni R., Sincropi M.S., Puoci F., Casaburi I., Saturmino C., Pezzi V. Progress to Improve Oral Bioavailability and Beneficial Effects of Resveratrol. Int. J. Mol. Sci. 2019;20:1381–1408. doi: 10.3390/ijms20061381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lin F., Yin H.B., Li X.Y., Zhu G.M., He W.Y., Gou X. Bladder cancer cell-secreted exosomal miR-21 activates the PI3K/AKT pathway in macrophages to promote cancer progression. Int. J. Oncol. 2020;56:151–164. doi: 10.3892/ijo.2019.4933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ohno R., Uozaki H., Kikuchi Y., Kumagai A., Aso T., Watanabe M., Watabe S., Muto S., Yamaguchi R. Both cancerous miR-21 and stromal miR-21 in urothelial carcinoma are related to tumour progression. Histopathology. 2016;69:993–999. doi: 10.1111/his.13032. [DOI] [PubMed] [Google Scholar]
- 102.Wu Z., Zhou Z., Zhang W., Yu Y. MiR-21-5p inhibition attenuates Warburg effect and stemness maintenance in osteosarcoma cells via inactivation of Wnt/β-catenin signaling. Acta Biochim. Pol. 2021;68:725–732. doi: 10.18388/abp.2020_5631. [DOI] [PubMed] [Google Scholar]