Abstract
Background:
Rotary and ultrasonic instruments are not appreciable in the complete removal of Biodentine from root canals. Therefore, organic solvents can be used as an adjunct for its retrieval.
Aim:
The aim of this study was to compare the efficacy of 10% citric acid, 2% acetic acid, and 20% tartaric acid on the microhardness of Biodentine and radicular dentin.
Materials and Methods:
Forty single-rooted extracted teeth were decoronated at the cementoenamel junction and root canals were prepared with peeso reamers. Two-millimeter sections were obtained and restored with Biodentine. All samples were subjected to Vicker's microhardness test to record the microhardness of Biodentine and radicular dentin. Samples were randomly divided into four experimental groups and treated with (n = 20) – distilled water, 10% citric acid, 2% acetic acid, and 20% tartaric acid groups for 10 min, after which specimens were again subjected to the same microhardness test.
Statistical Analysis:
The data were subjected to Kruskal–Wallis ANOVA test, followed by Wilcoxon signed-rank test with a level of significance set at P ≤ 0.05.
Results:
10% citric acid followed by 2% acetic acid exhibited the lowest mean microhardness values after immersing in respective solutions, whereas 20% tartaric acid exhibited the highest mean microhardness values on Biodentine and radicular dentin.
Conclusion:
10% citric acid can be used for retrieving Biodentine from root canals for a limited time without adversely affecting the physical and chemical composition of radicular dentin.
Keywords: Acetic acid, Biodentine, Citric acid, Radicular dentin, Tartaric acid
INTRODUCTION
A variety of calcium silicate-based materials have shown immense advancements in endodontics for applications such as vital pulp therapy, root end fillings, perforation repair, and repair of resorptive lesions.[1] Newer advancements such as bioceramic materials in endodontics are being introduced to optimize the care of dental patients, influencing the prognosis of many cases which were once considered next to impossible.[2] Biodentine is one such widely used calcium silicate-based cement in endodontic clinical situations for the repair of root perforations, apexification, root-end restoration, as dentin substitute for pulp capping and posterior restorations.[3] Powder is composed of tricalcium silicate, calcium carbonate, and zirconium oxide, whereas the liquid consists of calcium chloride which acts as an accelerator, water, and hydrosoluble polymer which acts as a water-reducing agent. It has been used as an endodontic root filling material because of its ability for tissue repair, biomimetic mineralization, antibacterial properties, good compressive strength, short setting time, better handling properties, biocompatibility, and bioactivity properties in contrast to other silicate cement.[4]
Although the inceptive steps of root canal therapy is a predictable procedure with a high 5-year survival rate of 90.85%, sometimes failures can commence after treatment.[5] Therefore, the retrievability of the root filling material through a nonsurgical approach is an important concern in dentistry today. The rotary and ultrasonic instruments have not shown substantial thorough removal of mineral trioxide aggregate (MTA) from root canals and the need for organic solvents for its retrieval has been mentioned in several studies.[6,7] MTA is commonly used because of its biocompatibility, ability to attain hermetic seal with root canal, and external surface of root along with the ability to set in the presence of blood. Its major disadvantages are difficulty in handling and prolonged setting time.[4] To overcome these disadvantages, Biodentine has been introduced and to the best of our knowledge, there is no published literature on its retrieval with organic solvents so far. Thus, in this study, it was evaluated whether organic solvents reduce the microhardness of Biodentine which would help in its maximum removal when used as an adjunct to rotary and ultrasonic instruments.
As per previous literature, organic acids such as citric acid and tartaric acid have calcium depleting tendency and their carboxyl groups possess the ability to reduce dentin microhardness.[7] The acids on contacting with the set cement matrix of Biodentine produce mainly calcium and aluminum salts, which increases the solubility of Biodentine. Consequently, porosity increases and the mechanical strength decreases, thus enhancing its retrievability with rotary instruments. However, organic solvents may also have adverse effects such as softening potential of dentinal walls while retrieving Biodentine. Changes in mineral content ratio alter the original proportion of organic and inorganic components of dentin, increasing the permeability and solubility of radicular dentin, hence permitting leakage. Thus, it poses a question of interest to investigate as to what extent the radicular dentin is affected by the use of various organic solvents in this study.[7]
Hence, the purpose of this study was to analyze the influence of various organic solvents on the microhardness of Biodentine and radicular dentin. The null hypothesis tested was that organic solvents did not have any effect on the microhardness of Biodentine and radicular dentin.
MATERIALS AND METHODS
This study obtained ethical clearance from the institutional ethics committee (Pr. 2114/IEC/SIBAR (UG)/2021). The inclusion criteria included healthy single-rooted maxillary and mandibular teeth, teeth free of caries, cracks, calcification, resorption, and teeth extracted for periodontal or orthodontic reasons. Discolored teeth, teeth with developmental anomalies, deciduous teeth, and endodontically treated teeth were excluded from the study.
Specimen preparation
Forty freshly extracted teeth according to selection criteria were chosen. The collected samples were thoroughly cleaned free of debris or calculus by ultrasonic scaler (EMS, Switzerland) and kept in 0.1% thymol solution (Sigma-Aldrich chemicals Pvt. Ltd., USA) at room temperature for disinfection and later stored in normal saline (Otsuka Pharmaceutical India Pvt. Ltd., Ahmedabad, India) until use. Each tooth was decoronated horizontally at cementoenamel junction labiolingually using a flat diamond abrasive disc (Sunny Superhard Tools Co Ltd, India) mounted on a slow-speed micromotor straight handpiece (NSK, Japan) under water coolant to obtain 12 mm standardized length of root. The coronal part of each canal for all decoronated teeth was preflared with round diamond abrasive (BR-46; Mani Inc., Japan) mounted on airotor handpiece (W&H, Austria) under water coolant.
The working length was determined just 0.5 mm short of apical foramen using size #15 K-file (Mani Inc., Japan) for each root canal. Biomechanical preparation of root canals was done with K-files by step back technique, initially with size #15 up to master apical size #40, along with copious irrigation using saline in a syringe with 30-gauge side-vented needle throughout the instrumentation to prevent clogging of debris in root canals. For achieving a uniform internal diameter of root canals, the canals were prepared with peeso reamers mounted on a slow-speed contrangled micromotor (NSK Japan), from size #1 to size #4 (Mani Inc., Japan). Two horizontal slices of thickness 2 mm were sectioned from the middle third of the root with a flat diamond abrasive disc mounted on a slow-speed straight micromotor handpiece under water coolant. The thickness was reconfirmed using a digital vernier caliper (Panama Orthodontics Inc., USA) with an accuracy of 0.001 mm to maintain the standardization of slices.
All the sectioned samples were cleaned by flushing them in individual containers, measuring 20 ml each, with 5.25% sodium hypochlorite (Coltene Whaledent Pvt. Ltd., Maharashtra, India) for 1 min, followed by distilled water for 1 min and finally with 17% EDTA (Prime dental Pvt. Ltd., Thane) for 1 min to remove the smear layer. This procedure for removing the smear layer was repeated for two cycles for thorough removal of both organic and inorganic debris and at the end, the samples were cleaned with distilled water for 1 min. After smear layer removal, the samples were dried with 80-size absorbent paper points (Medicept Dental Pvt. Ltd., India) and arranged on a clean, dry glass slab.
Biodentine (Septodont, St. Maur-des-Fosses, France) was manipulated as per manufacturer's instructions. The mixed Biodentine, which had putty-like consistency, was collected and packed into prepared root canals, using an MTA carrier (Waldent Innovations Pvt. Ltd., India), followed by condensation with hand plugger (GDC, India) and was left undisturbed for 12 min to allow initial setting of the material. The samples were later stored at 37°C and 100% humidity (Remi Instruments Ltd., India) for 1 week to enable a complete set of Biodentine.
After the complete set, all the samples were embedded horizontally into self-cure acrylic resin (DPI, India) using plastic molds to obtain the required 80 specimens [Figure 1]. The prepared specimens were polished using 600 grit silicon carbide paper to get a smooth surface and subjected to Vicker's microhardness test (Shimadzu HMV-G31DT ENG 230V Micro Vickers Hardness Tester, Japan) [Figure 2]. For the measurement of microhardness on radicular dentin, an indentation was placed 0.5 mm away from the root canal wall. Each determined location on Biodentine and radicular dentin was loaded with a pyramid diamond indenter point with weight of 100 g and dwell time of 5s. The Vickers hardness number (VHN) values were displayed digitally and noted.
Figure 1.
Specimen before subjecting to Vicker's microhardness test
Figure 2.

Specimen being subjected to Vicker's microhardness test
The specimens were randomly divided into four groups (n = 20) based on the organic solvents used,
Group 1: Specimens treated with distilled water (control)
Group 2: Specimens treated with 10% citric acid
Group 3: Specimens treated with 2% acetic acid
Group 4: Specimens treated with 20% tartaric acid.
In all groups, the specimens were immersed in a beaker containing the respective organic solvents for a period of 10 min and a magnetic stirrer was placed to ensure complete wetting of all specimens. After removal from respective solutions, specimens were dried with absorbent paper points. The Biodentine and radicular dentin of each specimen was again subjected to Vicker's microhardness test. The mean microhardness values before and after exposure to respective solutions for all groups were calculated and subjected to statistical analysis.
Statistical analysis
The data obtained were subjected to Kruskal–Wallis ANOVA test, followed by Wilcoxon signed-rank test with a level of significance set at P ≤ 0.05 using version 28.0 SPSS (Statistical Package for Social Sciences, IBM Corporation, USA).
RESULTS
The results of microhardness testing for Biodentine before and after exposure to organic solvents are summarized in Table 1 and graphically represented in Graph 1. The differences in Vicker's microhardness values after exposure to solvents in Groups 2, 3, and 4 were found to be statistically significant. Exposure of samples to 10% citric acid, 2% acetic acid, and 20% tartaric acid for 10 min substantially decreased the microhardness of Biodentine to 39.61 VHN (approximately by 33.89%), 48.455 VHN (approximately by 19.64%), and 50.7 VHN (approximately 16.03%), respectively. 10% citric acid showed the greatest reduction in microhardness of Biodentine as compared to other experimental organic solvents used in the study.
Table 1.
Comparison of mean microhardness values for Biodentine before and after immersion into respective solutions
| Groups | Before and after immersion | n | Mean±SD | P |
|---|---|---|---|---|
| Group 1 (distilled water) | Before | 20 | 61.180±4.5095 | 0.502 |
| After | 20 | 61.720±5.0298 | ||
| Group 2 (10% citric acid) | Before | 20 | 59.910±5.3102 | 0.000* |
| After | 20 | 39.610±3.1878 | ||
| Group 3 (2% acetic acid) | Before | 20 | 60.290±4.9687 | 0.000* |
| After | 20 | 48.455±2.3734 | ||
| Group 4 (20% tartaric acid) | Before | 20 | 60.375±5.3975 | 0.000* |
| After | 20 | 50.7±3.2195 |
*P≤0.05 considered statistically significant. Test: Wilcoxon signed-rank test. Values are mentioned in VHN. n: Number of samples, VHN: Vickers hardness number, SD: Standard deviation
Graph 1.
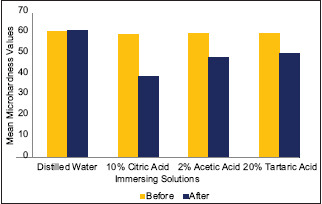
Comparison of mean microhardness values for Biodentine before and after immersion into respective solvents
The results of microhardness testing of radicular dentin before and after exposure to organic solvents are summarized in Table 2 and graphically represented in Graph 2. The differences in Vicker's microhardness values after exposure to solvents in Groups 2 and 3 were found to be statistically significant. Exposure of samples to 10% citric acid and 2% acetic acid for 10 min decreased the microhardness of radicular dentin to 41.64 VHN (approximately by 39.36%) and 58.585 VHN (approximately by 12.19%), respectively. Samples when exposed to 20% tartaric acid showed minimal reduction effect on the microhardness of radicular dentin to 65.775 VHN (approximately 0.77%). Therefore, 10% citric acid showed the greatest reduction in the microhardness of radicular dentin as compared to other experimental organic solvents used in the study.
Table 2.
Comparison of mean microhardness values for radicular dentin before and after immersion into respective solutions
| Groups | Before and after immersion | n | Mean±SD | P |
|---|---|---|---|---|
| Group 1 (distilled water) | Before | 20 | 65.840±6.4994 | 0.478 |
| After | 20 | 63.960±6.7183 | ||
| Group 2 (10% citric acid) | Before | 20 | 68.665±4.3518 | 0.001* |
| After | 20 | 41.640±2.6321 | ||
| Group 3 (2% acetic acid) | Before | 20 | 66.710±5.8484 | 0.001* |
| After | 20 | 58.585±5.2636 | ||
| Group 4 (20% tartaric acid) | Before | 20 | 66.285±5.5026 | 0.681 |
| After | 20 | 65.775±4.8010 |
*P≤0.05 considered statistically significant. Test: Wilcoxon signed-rank test. Values are mentioned in VHN. n: Number of samples, VHN: Vickers hardness number, SD: Standard deviation
Graph 2.
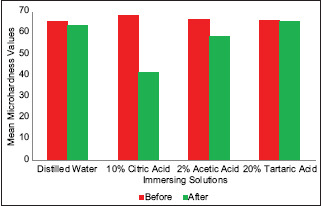
Comparison of mean microhardness values for radicular dentin before and after immersion into respective solvents
DISCUSSION
Based on the results, the null hypothesis was rejected as the organic solvents showed a reduction in the microhardness of Biodentine and radicular dentin. Vicker's microhardness test is used for assessment of mechanical changes of deeper dental hard tissue after exposure to various chemicals and has an inverse relationship with porosity based on crystal structure stability.[8] A positive correlation exists between microhardness values and loss or gain of the mineral content of teeth.[7]
10% citric acid exhibited a maximum reduction in microhardness of Biodentine and radicular dentin in the present study. According to the study carried out by Katarzyna Dąbrowska et al., irrigation of Biodentine samples with citric acid produced pronounced dissolution of calcium ions, especially after ultrasonic activation. The acid was able to dissolve the set cement matrix and extract the crystalline forms leaving irregularities on the surface of Biodentine as observed under scanning electron microscopic images. This is attributed to the fact that citric acid on reaction with calcium silicate leads to the formation of hydrosoluble chelate which is easier to remove during ultrasonic activation.[9] This material disintegration phenomenon explains the reduced microhardness values in the present study when Biodentine is treated with 10% citric acid. Furthermore, study by Kastiukas et al. showed a reduction in the strengths of portland cement with different concentrations of citric acid which can be correlated to the present study as both portland cement and Biodentine have similar compositions.[10]
The similar reduction in Biodentine and dentin microhardness by citric acid can be accredited to the factor that Biodentine is claimed to be similar to dentin in physical and mechanical properties.[11] Citric acid, due to chelating properties, has a demineralizing effect on calcified dentin components leading to the removal of calcium ions, thus producing a greater reduction of dentin microhardness.[12] In a study carried out by Muana et al., 10% citric acid reduced dentin microhardness which is similar to the results of the present study although the specimens were treated for 1 min in the former study in contrast to 10 min in the current study.[13] The microstructure of the product is present as needle- and cubic-shaped crystals, although further investigation is necessary.[14,15]
After reacting with tartaric acid, the microhardness of Biodentine was reduced significantly whereas that of radicular dentin was not significant in this study. Rai et al. reported the effect of 20% tartaric acid in the hydration process of portland cement. The organic acid interacts chemically with cement mineral phases leading to the formation of calcium tartrate hydrate which showed decreased amounts of C3S, C2S, and C3A phases explaining the fact that tartaric acid can help dissolve calcium silicate-based material.[16] Since Biodentine and portland cement have similar composition, this material phenomenon can help explain the reduced microhardness of Biodentine in the present study although more detailed research is needed regarding the microhardness and material strength of Biodentine and radicular dentin after using tartaric acid. Fu et al. investigated the interfacial interaction of tartaric acid with hydroxyapatite and concluded that enamel etched with tartaric acid revealed decalcification of the periphery of the enamel rods.[17] The above findings indicate tartaric acid is a weak acid that explains the least reduction of dentin microhardness with tartaric acid in this study.
Acetic acid has shown a significant reduction of the microhardness of Biodentine and radicular dentin. This is due to higher pH compared to citric acid but lower than that of tartaric acid. According to a study by Sathish Abraham et al., the results suggested that 2% acetic acid for 10 min significantly decreased the microhardness of root canal dentin which is similar to the present study. This change was attributed to the fact that acetic acid has calcium-depleting action and their carboxyl groups possess efficiency in reducing the microhardness of dentin.[12] These mild agents can be considered for use in clinical situations to bring surface changes of Biodentine with a minimal decrease of surface microhardness of dentin, thus preventing unnecessary damage to the latter. Further research is needed as there is no published literature that reports the action of 2% acetic acid on Biodentine.
As per the evidence in a study carried out by Ashofteh Yazdi et al., it was reported that exposure to acidic pH decreased calcium hydroxide crystalline formation in Biodentine.[18] This explains the decreased microhardness values in the present study, after exposure to organic solvents. Elnaghy AM and Koral Bayratkar et al. reported microhardness variations of Biodentine at different pH levels.[19,20] The present study used three different organic solvents with different pH levels. Citric acid impaired the surface microhardness of Biodentine more in comparison to acetic and tartaric acid. This variation is attributed to the fact that mechanical properties get deteriorated in the presence of an acidic environment, their chemical structure, and pH levels.[21] Ballal et al. concluded in their study that the action of chelating agents on the microhardness of Biodentine is associated not only with pH but also the type of acid used.[4]
Remnants of previous restorative material can affect the root canal sealer penetration into the dentinal tubules by acting as a physical barrier and its consequent adhesion. Hand or mechanical instrumentation and irrigation techniques along with exposure to organic acids might influence in quick as well as maximum removal of Biodentine or other root canal restorative materials. Factors such as concentration of organic acids, exposure time, type of acid used, chemical reaction of the acids with Biodentine and radicular dentin, and instrumentation and irrigation techniques used might influence the retrieval of Biodentine.
The present study had some limitations. First, an attempt was made to simulate the clinical situation, however, there was a difference in how the specimens were subjected to organic solvents as clinically they would be irrigated with a small amount of organic solvents, whereas in the present study, the specimens were completely immersed in organic solvents. Second, morphological alterations caused by organic solvents on Biodentine were not assessed.
CONCLUSION
Within the limitations of this in vitro study, the microhardness of Biodentine and radicular dentin was highly altered when subjected to citric acid followed by acetic acid and tartaric acid. 10% citric acid can be used judiciously for retrieval of Biodentine from root canals in case of retreatment but for a limited period of time to prevent significant alterations in the mechanical and physical properties of radicular dentin. As an alternative, clinicians can opt for milder organic solvents such as 20% tartaric acid because of its minimal effect on radicular dentin. A combination of mild organic solvents with rotary/ultrasonic instrumentation in the removal of Biodentine in vivo may be effective and has to be evaluated further.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors of this article would like to acknowledge Mr. P. Prachet for helping in storing samples at 37°C and 100% humidity for a complete set of Biodentine, Dr. Jayalakshmi for help in obtaining microhardness values, and Dr. Gnana Saritha for help in statistical analysis of data.
REFERENCES
- 1.Ballal NV, Mishra P, Rao S, Upadhyay ST. Effect of chelating agents on the microhardness of Biodentine. Saudi Endod J. 2019;9:109–12. [Google Scholar]
- 2.Kaur M, Singh H, Dhillon JS, Batra M, Saini M. MTA versus Biodentine: Review of literature with a comparative analysis. J Clin Diagn Res. 2017;11:ZG01–5. doi: 10.7860/JCDR/2017/25840.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Priyalakshmi S, Ranjan M. Review on Biodentine – A bioactive dentin substitute. IOSR J Dent Med Sci. 2014;13:13–7. [Google Scholar]
- 4.Ballal V, Marques JN, Campos CN, Lima CO, Simão RA, Prado M. Effects of chelating agent and acids on Biodentine. Aust Dent J. 2018;63:170–6. doi: 10.1111/adj.12609. [DOI] [PubMed] [Google Scholar]
- 5.Kwak Y, Choi J, Kim K, Shin SJ, Kim S, Kim E. The 5-year survival rate of nonsurgical endodontic treatment: A population-based cohort study in Korea. J Endod. 2019;45:1192–9. doi: 10.1016/j.joen.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 6.Shojaee NS, Adl A, Sobhnamayan F, Khademi A, Hamedi M. In vitro evaluation of different solvents for retrieval of mineral trioxide aggregate and calcium-enriched mixture. Iran Endod J. 2016;11:223–7. doi: 10.7508/iej.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt N, Talwar S. In-vitro evaluation of various solvents for retrieval of mineral trioxide aggregate and their effect on microhardness of dentin. J Conserv Dent. 2013;16:199–202. doi: 10.4103/0972-0707.111313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neekofar MH, Oloomi K, Sheykhrezae MS, Salariyeh S, Hayes SJ, Bryant ST, et al. An evaluation of the effect blood and human serum on the surface microhardness and surface microstructure of mineral trioxide aggregate. Int Endod J. 2010;43:849–58. doi: 10.1111/j.1365-2591.2010.01750.x. [DOI] [PubMed] [Google Scholar]
- 9.Dąbrowska K, Palatyńska-Ulatowska A, Klimek L. The effect of irrigation with citric acid on Biodentine tricalcium silicate-based cement: SEM-EDS in vitro study. Materials (Basel) 2022;15:3467. doi: 10.3390/ma15103467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kastiukas G, Zhou X, Castro-Gomes J, Huang S, Saafi M. Effects of lactic and citric acid on early-age engineering properties of Portland/calcium aluminate blended cements. Constr Build Mater. 2015;101:389–95. [Google Scholar]
- 11.Eldeniz AU, Erdemir A, Belli S. Effect of EDTA and citric acid solutions on the microhardness and the roughness of human root canal dentin. J Endod. 2005;31:107–10. doi: 10.1097/01.don.0000136212.53475.ad. [DOI] [PubMed] [Google Scholar]
- 12.Abraham S, Kamble AB, Gupta P, Satpute A, Chaudhari S, Ladhe P. In vitro evaluation of the efficacy of 2% carbonic acid and 2% acetic acid on retrieval of mineral trioxide aggregate and their effect on microhardness of dentin. J Contemp Dent Pract. 2016;17:568–73. [PubMed] [Google Scholar]
- 13.Muana HL, Nassar M, Dargham A, Hiraishi N, Tagami J. Effect of smear layer removal agents on the microhardness and roughness of radicular dentin. Saudi Dent J. 2021;33:661–5. doi: 10.1016/j.sdentj.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alzraikat H, Taha NA, Salameh A. A comparison of physical and mechanical properties of Biodentine and mineral trioxide aggregate. J Res Med Dent Sci. 2016;4:121–6. [Google Scholar]
- 15.Deepthi V, Mallikarjun E, Nagesh B, Mandava P. Effect of acidic pH on microhardness and microstructure of theraCal LC, endosequence, mineral trioxide aggregate, and biodentine when used as root repair material. J Conserv Dent. 2018;21:408–12. doi: 10.4103/JCD.JCD_308_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rai S, Singh NB, Singh NP. Interaction of tartaric acid during hydration of Portland cement. Indian J Chem Technol. 2006;13:255–61. [Google Scholar]
- 17.Fu B, Shen Q, Qian W, Zeng Y, Sun X, Hannig M. Interfacial interaction of tartaric acid with hydroxyapatite and enamel. J Mater Sci Mater Med. 2005;16:827–31. doi: 10.1007/s10856-005-3581-6. [DOI] [PubMed] [Google Scholar]
- 18.Ashofteh Yazdi K, Ghabraei S, Bolhari B, Kafili M, Meraji N, Nekoofar MH, et al. Microstructure and chemical analysis of four calcium silicate-based cements in different environmental conditions. Clin Oral Investig. 2019;23:43–52. doi: 10.1007/s00784-018-2394-1. [DOI] [PubMed] [Google Scholar]
- 19.Elnaghy AM. Influence of acidic environment on properties of biodentine and white mineral trioxide aggregate: A comparative study. J Endod. 2014;40:953–7. doi: 10.1016/j.joen.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 20.Bayraktar K, Basturk FB, Turkaydin D, Gunday M. Long-term effect of acidic pH on the surface microhardness of ProRoot mineral trioxide aggregate, Biodentine, and total fill root repair material putty. Dent Res J (Isfahan) 2021;18:2. [PMC free article] [PubMed] [Google Scholar]
- 21.Mohebbi P, Asgary S. Effect of pH on physical properties of two endodontic biomaterials. J Conserv Dent. 2016;19:212–9. doi: 10.4103/0972-0707.181935. [DOI] [PMC free article] [PubMed] [Google Scholar]


