Abstract
Aim:
The aim of this study was to compare the cytotoxic effect of calcium silicate-based Bio-C, CeraSeal, and mineral trioxide aggregate (MTA)-based (MTA-Fillapex) sealers to a widely used resin-based sealer (AH Plus) using 3-(4, 5 dimethythiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay and microscopic examination.
Materials and Methods:
A total of (n = 36) samples divided into four groups with three sealer samples per time period of 0 h, 24 h, and 7 days after mixing were extracted in cell culture medium using Dulbecco's Modified Eagle's Medium. The cytotoxicity of these sealers was evaluated using an MTT assay on L929 mouse fibroblasts, and changes in the cell morphology were observed under an inverted phase-contrast microscope (×20). The values obtained were analyzed statistically using one-way ANOVA, Tukey's post hoc, and Bonferroni's test.
Results:
Bio-C and CeraSeal showed a reduction in cytotoxicity from severe at 0 h to no cytotoxicity at 24 h and 7 day time period. AH Plus showed severe cytotoxicity at all time periods. MTA-Fillapex showed severe cytotoxicity at 0 h which decreased to moderate at 24 h and 7 days. The differences between groups were statistically significant (P < 0.05).
Conclusions:
The sealers with resin constituents (AH Plus and MTA-Fillapex) showed severe-to-moderate cytotoxicity at different time periods, whereas calcium silicate-based sealers (Bio-C and CeraSeal) were relatively biocompatible as their cytotoxicity decreased significantly from severe initially to noncytotoxic with time.
Keywords: 3-(4, 5 dimethythiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay; bioceramic sealers; cell culture; cytotoxicity
INTRODUCTION
The leaching of extracts or elutes of sealers from the root canal because of contact with extracellular fluids[1] or inadvertent extrusion of root canal sealers periradicularly during obturation may result in persistent inflammation manifesting as pain, tenderness, swelling of the affected area, and delayed healing.[2] Hence, root canal sealer should be biocompatible and well tolerated by the periradicular tissues.[3]
AH Plus (Dentsply, Germany) is a two-paste conventional epoxy resin-based sealer which is widely used, well-investigated, and employed as the “gold standard” in scientific studies due to its excellent physicochemical properties.[4] However, its poor cytocompatibility is its biggest limitation.[5,6] Hence, sealers with improved biocompatibility are always sought.
MTA-Fillapex (Angelus Brazil) is a salicylate resin-based sealer containing MTA particles, bismuth trioxide, pigments, and silica particles. It has suitable physicochemical properties, such as good radiopacity, flow, and alkaline pH,[7,8] but there are conflicting results in different studies regarding its biocompatibility.[9]
Bio-C Sealer (Angelus, Brazil) and CeraSeal (Meta Biomed, Korea) are premixed ready-to-use bioceramic sealers based on calcium silicate and its compounds and various metal oxides. Both these sealers have good physiochemical properties.[10] Due to limited comparative data about their biological properties, their cytotoxicity needs to be evaluated in a quest to find more biocompatible sealers.
Therefore, this study was performed to compare the cytotoxicity and microscopic cell morphological changes due to calcium silicate-based (Bio-C and Ceraseal) and MTA-based (MTA-Fillapex) root canal sealers to a widely used resin-based sealer (AH Plus) on L929 mouse fibroblast using 3-(4, 5 dimethythiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. The hypothesis tested was that all sealers have the same cytotoxicity effect on L929 mouse fibroblast.
MATERIALS AND METHODS
Growing cell cultures
L929 mouse fibroblasts (ATCC, USA), were placed in 25-cm2 culture flasks, and cultured in Dulbecco's Modified Eagle's Medium (HiMedia Laboratory, India) supplemented with 10% fetal bovine serum (Sigma-Aldrich), containing 4 mmol/L glutamine (Sigma-Aldrich), 100 IU/ml penicillin, and 100 μg/ml streptomycin. After rinsing and trypsinization, cells were seeded (5 × 103 cells per well) in 96 well-culture plates (Nunc, Denmark) and incubated for 24 h in 5% CO2 and 95% air at 37°C, to get a subconfluent monolayer of cells.
Sample preparation and elution
All sealers were mixed according to the manufacturer's directions and placed into sterile Teflon molds (4 mm × 2 mm). Samples are eluted as per ISO-10993-12, 2021[11] by adding 1 ml of media containing serum in each vial at an extraction ratio of 0.1 g/ml.
Addition of elute or extract to the cells
In vitro cytotoxicity test using extract on cells was done based on the ISO 10993-5, 2009,[12] guidelines. Three discs per material per time period were made, i.e., 0 h freshly mixed sealer, 24 h, and 7 days. Hence, 9 samples per material and a total of 36 (9 × 4) samples are taken for study. At the end of elution, 100 ml of test elute is added to 6 wells per material per time period of already cultured L929 mouse fibroblasts in 96 well-culture plates. The same number of cell wells per material with 100 ml of 130 mg% phenol act as a positive control. An equal number of cell wells without any addition serve as a negative control. Then, these cells are incubated for 48 h in 5% CO2 at 37°C in an incubator (Nuaire, USA).
3-(4, 5 dimethythiazol-2yl)-2, 5-diphenyltetrazolium bromide (MTT) assay
MTT assay (Sigma-Aldrich Co.) solution was prepared as 1 mg/mL in phosphate buffer saline just before use. 100-μL MTT dye was added to each well-containing cell treated with various extracts of sealers. Plates were incubated in a CO2 incubator for 3 h. Optical density was determined by eluting the dye, and the spectrophotometric absorbance was measured at 550 nm using a Bio-Rad ELISA plate reader (Alfred Nobel Drive, Hercules, CA, USA). The percentage of cell viability was calculated from the formula:
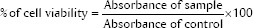
Cytotoxicity was rated based on % age of cell viability relative to the control group,[13] severe (<30%), moderate (30%–60%), slight (61%–90%), or noncytotoxic (>90%).
Microscopic examination
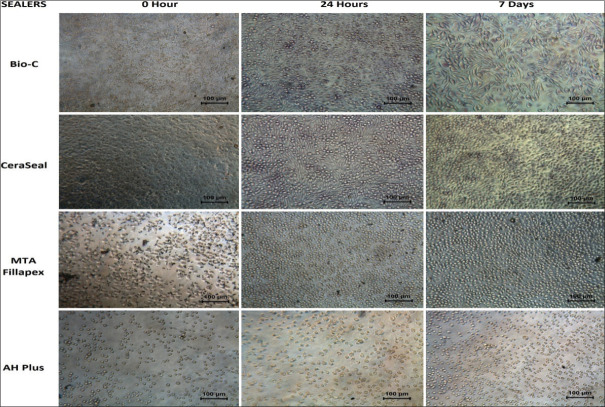
After 48 h of incubation with test elutes of various sealers, and before doing the MTT assay, the cells were observed under a phase-contrast inverted microscope (Leica, DM IL, Germany) to see cell morphology, and then these cells were photographed at ×20 magnification with a camera inbuilt in the microscope (Leica DFC 180, Germany) looking at vacuolization, cell lysis, membrane integrity, confluency, and a number of empty spaces between cells. The various cell culture wells were graded according to the criteria of Reactivity Grades for Elution Test given by United States Pharmacopeia.[14]
Statistical analysis
Statistical analysis was performed using Statistical package for social sciences [SPSS] for windows Version 22.0 Released 2013 NY: IBM Corp. Descriptive analysis includes expression of the percentage of cell viability in terms of mean and standard deviation. One-way ANOVA followed by Tukey's post hoc test was used to compare the mean percentage of cell viability between four groups at different time intervals [Table 1]. Repeated measures of ANOVA followed by Bonferroni's post hoc test were used to compare the mean percentage of cell viability between different time intervals in each group [Table 2]. The level of significance was set at P < 0.05.
Table 1.
Multiple comparison of mean difference in the percentage of cell viability between groups at different time intervals using Tukey’s post hoc test
| Time | Groups (I) | Groups (J) | Mean difference (I-J) | 95% CI for the difference | P | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
| 0 h | Bio-C | CeraSeal | −0.147 | −3.288 | 2.994 | 0.99 |
| MTA-Fillapex | 14.862 | 11.721 | 18.003 | <0.001* | ||
| AH Plus | 17.305 | 14.164 | 20.446 | <0.001* | ||
| CeraSeal | MTA-Fillapex | 15.008 | 11.868 | 18.149 | <0.001* | |
| AH Plus | 17.452 | 14.311 | 20.593 | <0.001* | ||
| MTA-Fillapex | AH Plus | 2.443 | −0.698 | 5.584 | 0.16 | |
| 24 h | Bio-C | CeraSeal | −4.957 | −8.595 | −1.318 | 0.006* |
| MTA-Fillapex | 39.967 | 36.328 | 43.605 | <0.001* | ||
| AH Plus | 68.703 | 65.065 | 72.342 | <0.001* | ||
| CeraSeal | MTA-Fillapex | 44.923 | 41.285 | 48.562 | <0.001* | |
| AH Plus | 73.660 | 70.021 | 77.299 | <0.001* | ||
| MTA-Fillapex | AH Plus | 28.737 | 25.098 | 32.375 | <0.001* | |
| 7 days | Bio-C | CeraSeal | −3.618 | −7.029 | −0.208 | 0.04* |
| MTA-Fillapex | 33.925 | 30.514 | 37.336 | <0.001* | ||
| AH Plus | 64.687 | 61.276 | 68.097 | <0.001* | ||
| CeraSeal | MTA-Fillapex | 37.543 | 34.133 | 40.954 | <0.001* | |
| AH Plus | 68.305 | 64.894 | 71.716 | <0.001* | ||
| MTA-Fillapex | AH Plus | 30.762 | 27.351 | 34.172 | <0.001* | |
CI: Confidence interval. *P<0.05 (Significant)
Table 2.
Comparison of mean percentage of cell viability between different time intervals in each group using repeated measures of ANOVA test followed by Bonferroni’s post hoc test
| Groups | Time | n | Mean | SD | Minimum | Maximum | P a | Significant diff | P b |
|---|---|---|---|---|---|---|---|---|---|
| Bio-C | 0 h | 6 | 29.797 | 2.343 | 25.76 | 32.76 | <0.001* | T0 versus T1 | <0.001* |
| 24 h | 6 | 91.433 | 1.339 | 89.88 | 93.65 | T0 versus T2 | <0.001* | ||
| 7 days | 6 | 93.575 | 2.602 | 89.98 | 96.87 | T1 versus T2 | 0.55 | ||
| CeraSeal | 0 h | 6 | 29.943 | 0.899 | 28.79 | 31.34 | <0.001* | T0 versus T1 | <0.001* |
| 24 h | 6 | 96.390 | 1.447 | 93.76 | 97.65 | T0 versus T2 | <0.001* | ||
| 7 days | 6 | 97.193 | 1.506 | 94.65 | 98.88 | T1 versus T2 | 0.02* | ||
| MTA-Fillapex | 0 h | 6 | 14.935 | 1.487 | 12.78 | 16.54 | <0.001* | T0 versus T1 | <0.001* |
| 24 h | 6 | 51.467 | 2.993 | 46.98 | 54.76 | T0 versus T2 | <0.001* | ||
| 7 days | 6 | 59.650 | 2.188 | 56.43 | 61.65 | T1 versus T2 | 0.02* | ||
| AH Plus | 0 h | 6 | 12.492 | 2.569 | 9.93 | 16.61 | <0.001* | T0 versus T1 | 0.01* |
| 24 h | 6 | 22.730 | 2.727 | 19.09 | 26.98 | T0 versus T2 | <0.001* | ||
| 7 days | 6 | 28.888 | 1.998 | 25.98 | 30.87 | T1 versus T2 | 0.001* |
SD: Standard deviation. *P<0.05 (Significant)
RESULTS
Bio-C and CeraSeal group showed higher mean cell viability as compared to AH Plus and MTA-Fillapex at all time intervals (P < 0.001). Comparing the results, all sealers showed severe cytotoxicity at 0 h as <30% of cells are viable [Figure 1]. However, the cytotoxicity was greatly reduced at 24 h and 7 days in the case of Bio-C and CeraSeal sealers, becoming noncytotoxic with a percentage of viable cells of more than 90%, but in the case of MTA-Fillapex, it was moderate at 24 h and 7 day interval with <60% cells viable. Although there is a significant reduction in cytotoxicity in the AH Plus group, it continued to remain severe cytotoxic even after 7 days.
Figure 1.
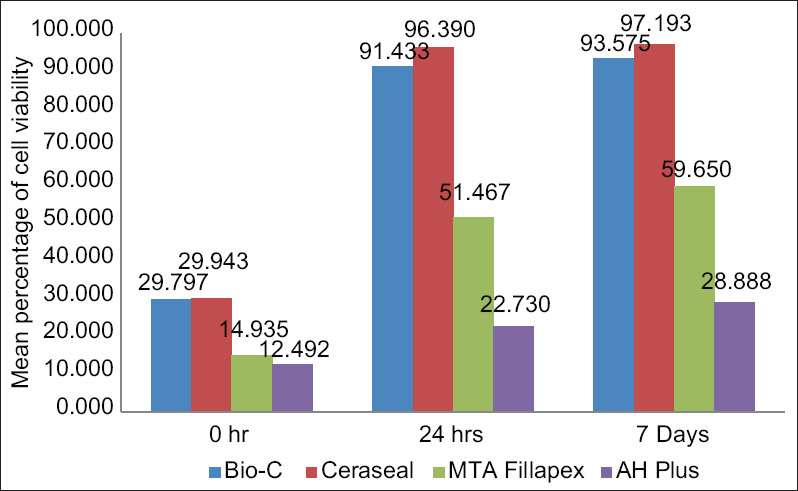
Mean percentage of cell viability between four groups at different time intervals
From the phase-contrast microscopic observations at ×20 [Figure 2], all the groups at 0 h, show empty spaces between cells, filled with abundant debris and particulate matter, very few normal cells are seen, and the majority of the cells are small, rounded, granulated, and vacuolated. These changes were suggestive of severe cytotoxicity at 0 h. For Bio-C and CeraSeal groups, at 24 h and 7 days, the cells appear normal in morphology and were in subconfluent monolayers. No cell lysis was seen as indicative of noncytoxicity at these time periods. The cells in contact with MTA-Fillapex at 24-h evaluation appear smaller, more in number than 0 h, and are mostly round with few spindle-shaped cells and normal morphology indicative of moderate toxicity which although have increased in number and size at 7th day, yet showed vacuolization and disruption of cell membranes in few cells but overall <60% of cells are lysed indicative of moderate toxicity. AH Plus group continued to show cell morphologic changes suggestive of severe cytoxicity reaction both at 24 h and 7 days.
Figure 2.
Microscopic examination at ×20 magnifications showing cells at different time periods
DISCUSSION
The null hypothesis was rejected, as Bio-C, CeraSeal, and MTA-Fillapex were significantly less toxic than AH Plus, whereas Bio-C and CeraSeal sealers showed comparative cytotoxicity which was severe at 0 h and decreased to noncytotoxic level at 24 h and 7 day period. This study was done to check the cytotoxic effect of sealers in both unset and set conditions at 0 h, 24 h, and 7 days after mixing. As stated by Huang et al.,[15] the difference in toxicity patterns for different sealers at various elution times may be related to the degree of their setting. Using established cell lines like L929 fibroblasts for cytotoxicity evaluation by MTT assay is a common method of in vitro testing of sealers, which allows for quantifiable and reproducible results in a controlled laboratory setting even for multiple materials simultaneously using the same cells under the same conditions.[16] This assay allows us to differentiate between viable and nonviable cells which microscopic assay alone cannot differentiate.
The differences in cytotoxicity between the sealers analyzed in this study may be due to their specific chemical composition, solubility, flow, and pH immediately after mixing and post setting.[17] In this study, Bio-C and CeraSeal showed statistically significant lesser cytotoxicity at all time periods compared to MTA-Fillapex and AH plus.
Both Bio-C and CeraSeal bioceramic sealers are based on calcium silicates which hydrate to calcium silicate gel and form calcium hydroxide and ultimately release various ions including Si4+, Ca++, and OH ions during setting.[18] The freshly mixed elutes, release very high amounts of Ca++ and OH-that affect calcium homeostasis around cells due to raised pH and high concentration of Ca++ ions, negatively influencing cell's metabolism and resulting in cell apoptosis and cell death decreasing the cell viability rate.[19,20] This will explain the severe toxicity of Bio-C and CeraSeal sealers at 0 h. Bio-C Sealer as compared to CeraSeal has traces of aluminum tungsten and iron oxide which are known to have slight cytotoxic effects[21] and this difference accounts for slight less cell viability in Bio-C than CeraSeal. Viability of cells in CeraSeal group significantly increased at 24 h compared to that of other groups. The results of this study correspond with a study done by López-García et al.[22] which reported that CeraSeal displayed higher cell viability, cell attachment, cell migration rates, and ion release rates. In other studies done by Oh et al.[23] evaluating the level of TGF-β an anti-inflammatory cytokine, CeraSeal showed higher levels of TGF-β than AH Plus, thus corroborating the excellent cell viability and biocompatibility of CeraSeal. The results of this study are in accordance with another study done by López-García et al.[24] which evaluated the physicochemical properties of the Bio-C Sealer in comparison with the total fill-Bio-C Sealer and the AH Plus sealer and concluded that the Bio-C Sealer has a short setting time, alkalinization ability, and superior cytocompatibility in comparison with AH Plus.
MTA-Fillapex is a salicylate resin-based sealer. It should not be regarded as a tricalcium silicate sealer as it has no intrinsic bioactive potential and its composition is primarily resin with 15% MTA powder as a minor additive.[25,26] The toxicity of MTA-Fillapex may be attributed mainly to the presence of salicylate resin matrix and bismuth oxide as radiopacifying agents, both of these are known to induce apoptosis and have cell toxicity effects.[27] This sealer absorbs less water, releases significantly less Ca2+, it shows less alkalinizing and apatite deposition activity as compared to calcium silicate sealers, the resin matrix renders the MTA component relatively inert in terms of bioactivity.[28] MTA-Fillapex shows high flowability and setting time because of the unbalanced ratio between resin and MTA.[29] This may contribute to the prolonged dissolution of toxic compounds and increased cytotoxicity as compared with CeraSeal and Bio-C at all time periods.
In the present study, fresh extracts of AH Plus exerted a severe cytotoxic effect which lasted for all time periods. AH Plus contains formaldehyde and Bisphenol A, along with epoxy resins, all have cytotoxic profiles.[30] The cytotoxicity of resin-based materials is commonly attributed to leaching out of unpolymerized monomers due to incomplete polymerization.[31,32] The epoxy resin present in AH Plus is mutagenic and may cause breaks in the chain of cellular DNA resulting in cellular deaths.[5] This is in agreement with previous studies that have documented the cytotoxic effect of AH Plus.[4,5] Although fresh AH Plus was strongly cytotoxic and gradually became lesser cytotoxic over increasing time intervals, it continued to be severely detrimental to cell viability even after the sealer is fully set.
The findings of microscopic examination observations of this study gave us a qualitative assessment of cells in contact with elutes of all the sealers tested and these findings corroborate with the MTT assay findings of the study.
The findings of the present study are relevant for clinicians looking to explore the use of the Bio-C and CeraSeal sealers in clinical practice to achieve better outcomes in their patients. However, since this study has limitations as an in vitro study, it did not take into account that the products leached out in the periapical area are constantly washed out by circulating blood. Future in vivo studies such as the subcutaneous tissue implantation model or endodontic usage test models in higher animal studies would be necessary to strengthen the rationale for the clinical use of these sealers.
CONCLUSIONS
According to this study, it was seen that calcium silicate-based sealers are less cytotoxic and more biocompatible than MTA-Fillapex and AH Plus sealers.
The sealers with resin constituents (AH-Plus and MTA-Fillapex) showed severe-to-moderate cytotoxicity at different time periods, whereas calcium silicate-based sealers (Bio-C sealer and CeraSeal) were relatively biocompatible as their cytotoxicity decreased significantly from severe initially to noncytotoxic with time.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Costa F, Sousa Gomes P, Fernandes MH. Osteogenic and angiogenic response to calcium silicate-based endodontic sealers. J Endod. 2016;42:113–9. doi: 10.1016/j.joen.2015.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Shashirekha G, Jena A, Pattanaik S, Rath J. Assessment of pain and dissolution of apically extruded sealers and their effect on the periradicular tissues. J Conserv Dent. 2018;21:546–50. doi: 10.4103/JCD.JCD_224_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spångberg L. Biological effects of root canal filling materials. 7. Reaction of bony tissue to implanted root canal filling material in guineapigs. Odontol Tidskr. 1969;77:133–59. [PubMed] [Google Scholar]
- 4.Schäfer E, Bering N, Bürklein S. Selected physicochemical properties of AH Plus, EndoREZ and RealSeal SE root canal sealers. Odontology. 2015;103:61–5. doi: 10.1007/s10266-013-0137-y. [DOI] [PubMed] [Google Scholar]
- 5.Schweikl H, Schmalz G, Federlin M. Mutagenicity of the root canal sealer AHPlus in the Ames test. Clin Oral Investig. 1998;2:125–9. doi: 10.1007/s007840050057. [DOI] [PubMed] [Google Scholar]
- 6.Eldeniz AU, Mustafa K, Ørstavik D, Dahl JE. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int Endod J. 2007;40:329–37. doi: 10.1111/j.1365-2591.2007.01211.x. [DOI] [PubMed] [Google Scholar]
- 7.Silva EJ, Rosa TP, Herrera DR, Jacinto RC, Gomes BP, Zaia AA. Evaluation of cytotoxicity and physicochemical properties of calcium silicate-based endodontic sealer MTA Fillapex. J Endod. 2013;39:274–7. doi: 10.1016/j.joen.2012.06.030. [DOI] [PubMed] [Google Scholar]
- 8.Mangala MG, Chandra SM, Bhavle RM. To evaluate the biocompatibility of the Indian Portland cement with potential for use in dentistry: An animal study. J Conserv Dent. 2015;18:440–4. doi: 10.4103/0972-0707.168800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bin CV, Valera MC, Camargo SE, Rabelo SB, Silva GO, Balducci I, et al. Cytotoxicity and genotoxicity of root canal sealers based on mineral trioxide aggregate. J Endod. 2012;38:495–500. doi: 10.1016/j.joen.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Zordan-Bronzel CL, Esteves Torres FF, Tanomaru-Filho M, Chávez-Andrade GM, Bosso-Martelo R, Guerreiro-Tanomaru JM. Evaluation of physicochemical properties of a new calcium silicate-based sealer, bio-c sealer. J Endod. 2019;45:1248–52. doi: 10.1016/j.joen.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 11.Biological evaluation of medical devices - Part 12: Sample preparation and reference materials (ISO 10993-12:2021) https://www.iso.org/standard/75769.html. [Google Scholar]
- 12.Biological evaluation of medical devices — Part 5: Tests for in vitro cytotoxicity ISO 10993-5:2009. https://www.iso.org/standard/36406.html. [Google Scholar]
- 13.Dahl JE, Frangou-Polyzois MJ, Polyzois GL. In vitro biocompatibility of denture relining materials. Gerodontology. 2006;23:17–22. doi: 10.1111/j.1741-2358.2006.00103.x. [DOI] [PubMed] [Google Scholar]
- 14.United States Pharmacopeia and National Formulary (USP 41). Ch. 87. Biological Reactivity Tests, in vitro – Elution Test. 2018 https://www.uspnf.com/sites/default/files/usp_pdf/EN/USPNF/iras/gc-87-biolgoicalreactivity.pdf. [Google Scholar]
- 15.Huang FM, Tai KW, Chou MY, Chang YC. Cytotoxicity of resin-, zinc oxide-eugenol-, and calcium hydroxide-based root canal sealers on human periodontal ligament cells and permanent V79 cells. Int Endod J. 2002;35:153–8. doi: 10.1046/j.1365-2591.2002.00459.x. [DOI] [PubMed] [Google Scholar]
- 16.Key JE, Rahemtulla FG, Eleazer PD. Cytotoxicity of a new root canal filling material on human gingival fibroblasts. J Endod. 2006;32:756–8. doi: 10.1016/j.joen.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 17.Collado-González M, Tomás-Catalá CJ, Oñate-Sánchez RE, Moraleda JM, Rodríguez-Lozano FJ. Cytotoxicity of guttaflow bioseal, GuttaFlow2, MTA Fillapex, and AH Plus on human periodontal ligament stem cells. J Endod. 2017;43:816–22. doi: 10.1016/j.joen.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 18.Torres FFE, Zordan-Bronzel CL, Guerreiro-Tanomaru JM, Chávez-Andrade GM, Pinto JC, Tanomaru-Filho M. Effect of immersion in distilled water or phosphate-buffered saline on the solubility, volumetric change and presence of voids within new calcium silicate-based root canal sealers. Int Endod J. 2020;53:385–91. doi: 10.1111/iej.13225. [DOI] [PubMed] [Google Scholar]
- 19.Mohammadi Z, Dummer PM. Properties and applications of calcium hydroxide in endodontics and dental traumatology. Int Endod J. 2011;44:697–730. doi: 10.1111/j.1365-2591.2011.01886.x. [DOI] [PubMed] [Google Scholar]
- 20.Anjaneyulu K, Nivedhitha MS. Influence of calcium hydroxide on the post-treatment pain in Endodontics: A systematic review. J Conserv Dent. 2014;17:200–7. doi: 10.4103/0972-0707.131775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.López-García S, Lozano A, García-Bernal D, Forner L, Llena C, Guerrero-Gironés J, et al. Biological effects of new hydraulic materials on human periodontal ligament stem cells. J Clin Med. 2019;8:E1216. doi: 10.3390/jcm8081216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.López-García S, Myong-Hyun B, Lozano A, García-Bernal D, Forner L, Llena C, et al. Cytocompatibility, bioactivity potential, and ion release of three premixed calcium silicate-based sealers. Clin Oral Investig. 2020;24:1749–59. doi: 10.1007/s00784-019-03036-2. [DOI] [PubMed] [Google Scholar]
- 23.Oh H, Kim E, Lee S, Park S, Chen D, Shin SJ, et al. Comparison of biocompatibility of calcium silicate-based sealers and epoxy resin-based sealer on human periodontal ligament stem cells. Materials (Basel) 2020;13:E5242. doi: 10.3390/ma13225242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.López-García S, Pecci-Lloret MR, Guerrero-Gironés J, Pecci-Lloret MP, Lozano A, Llena C, et al. Comparative cytocompatibility and mineralization potential of Bio-C Sealer and TotalFill BC Sealer. Materials (Basel) 2019;12:E3087. doi: 10.3390/ma12193087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalil I, Naaman A, Camilleri J. Properties of Tricalcium Silicate Sealers. J Endod. 2016;42:1529–35. doi: 10.1016/j.joen.2016.06.002. [DOI] [PubMed] [Google Scholar]
- 26.Vitti RP, Prati C, Sinhoreti MA, Zanchi CH, Souza E Silva MG, Ogliari FA, et al. Chemical-physical properties of experimental root canal sealers based on butyl ethylene glycol disalicylate and MTA. Dent Mater. 2013;29:1287–94. doi: 10.1016/j.dental.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Antonijević D, Despotović A, Biočanin V, Milošević M, Trišić D, Lazović V, Zogović N, Milašin J, Ilić D. Influence of the addition of different radiopacifiers and bioactive nano-hydroxyapatite on physicochemical and biological properties of calcium silicate based endodontic ceramic. Cerami CeraSeal Int. 2021;47:28913–23. [Google Scholar]
- 28.Portella FF, Collares FM, Dos Santos LA, dos Santos BP, Camassola M, Leitune VC, et al. Glycerol salicylate-based containing α-tricalcium phosphate as a bioactive root canal sealer. J Biomed Mater Res B Appl Biomater. 2015;103:1663–9. doi: 10.1002/jbm.b.33326. [DOI] [PubMed] [Google Scholar]
- 29.Silva EJ, Accorsi-Mendonça T, Pedrosa AC, Granjeiro JM, Zaia AA. Long-Term cytotoxicity, pH and dissolution rate of AH Plus and MTA Fillapex. Braz Dent J. 2016;27:419–23. doi: 10.1590/0103-6440201600735. [DOI] [PubMed] [Google Scholar]
- 30.Schweikl H, Schmalz G, Spruss T. The induction of micronuclei in vitro by unpolymerized resin monomers. J Dent Res. 2001;80:1615–20. doi: 10.1177/00220345010800070401. [DOI] [PubMed] [Google Scholar]
- 31.Caughman WF, Caughman GB, Shiflett RA, Rueggeberg F, Schuster GS. Correlation of cytotoxicity, filler loading and curing time of dental composites. Biomaterials. 1991;12:737–40. doi: 10.1016/0142-9612(91)90022-3. [DOI] [PubMed] [Google Scholar]
- 32.Stanislawski L, Daniau X, Lauti A, Goldberg M. Factors responsible for pulp cell cytotoxicity induced by resin-modified glass ionomer cements. J Biomed Mater Res. 1999;48:277–88. doi: 10.1002/(sici)1097-4636(1999)48:3<277::aid-jbm11>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]