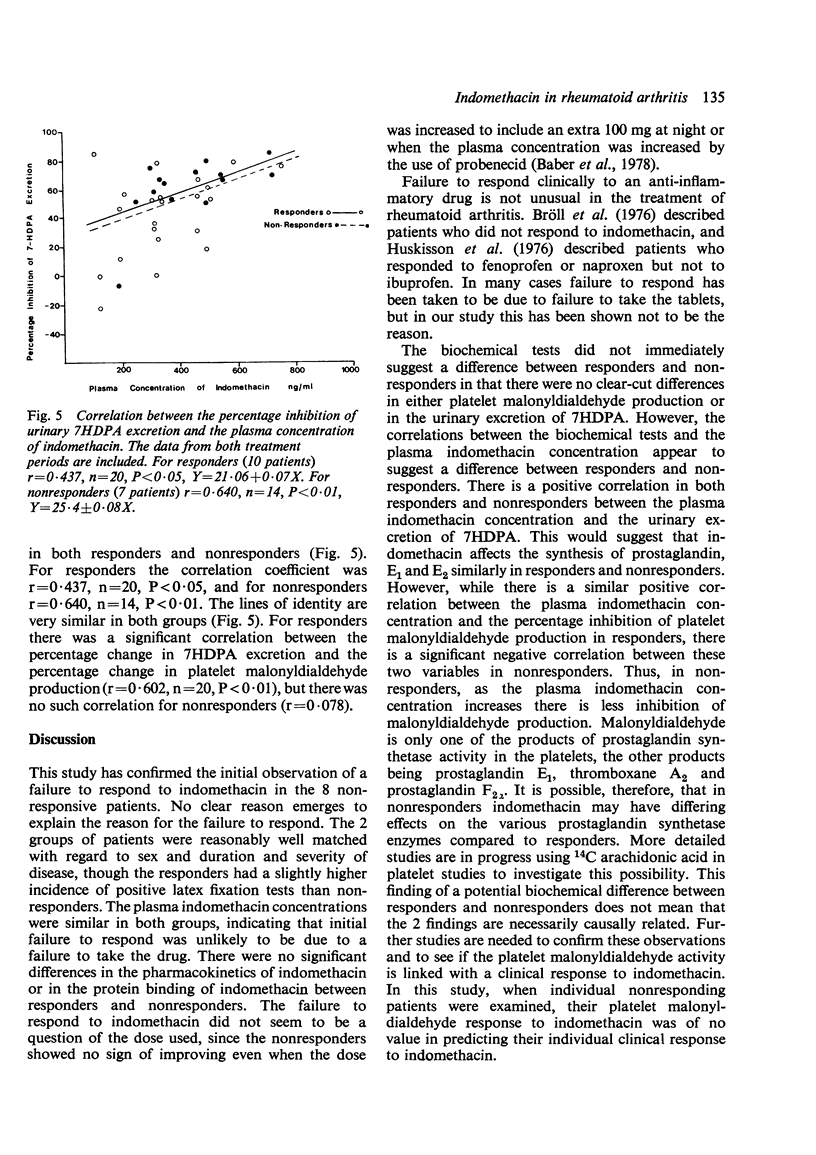
Abstract
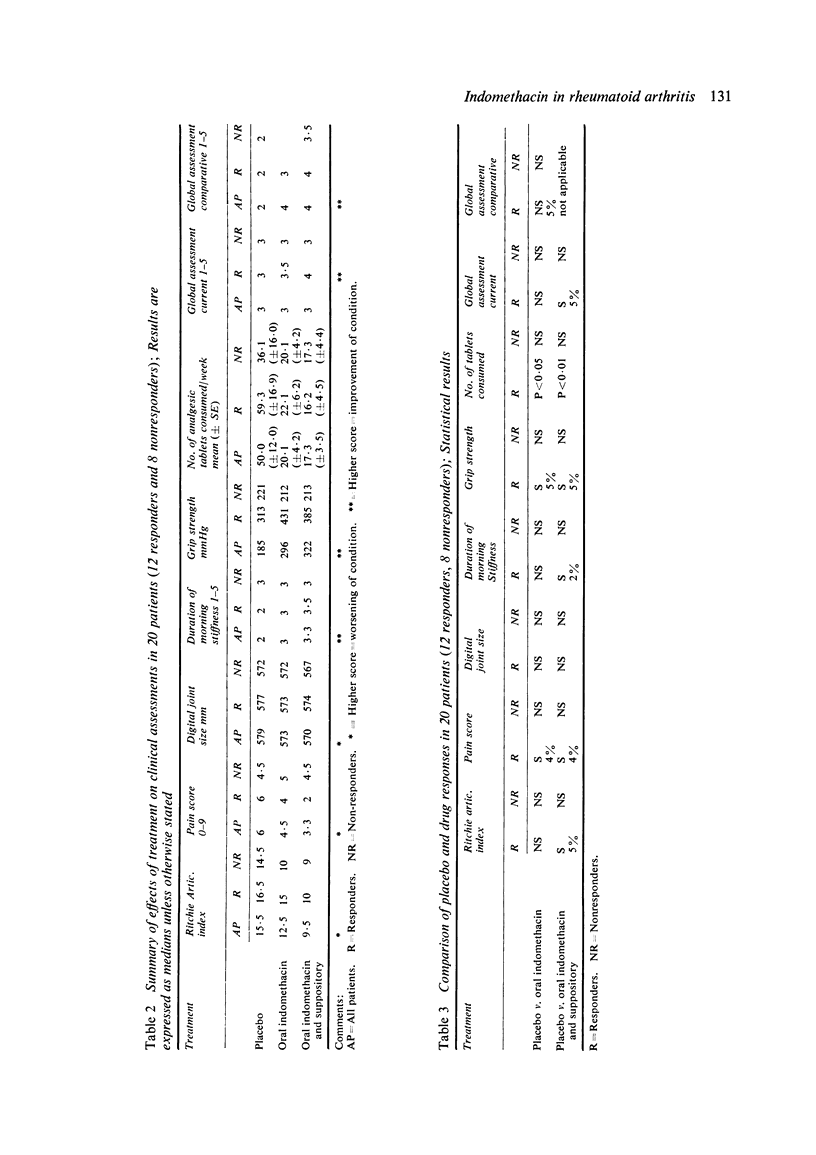
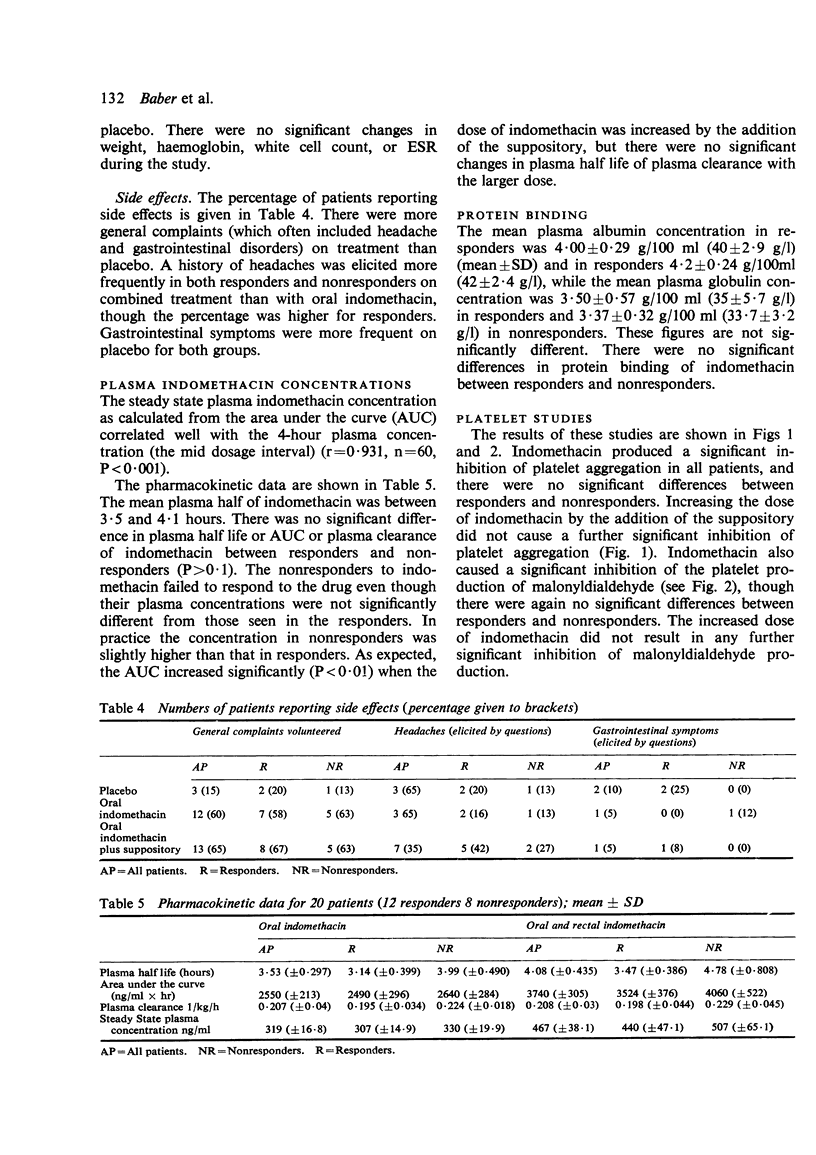
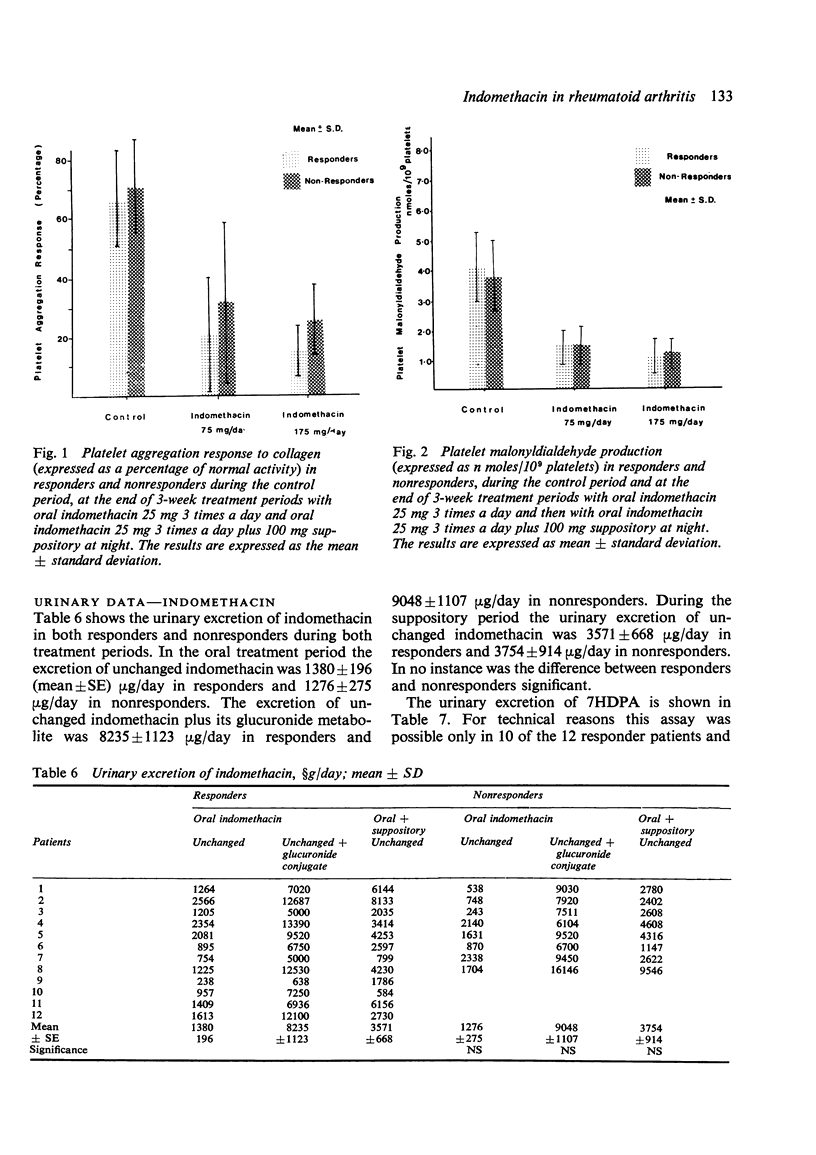
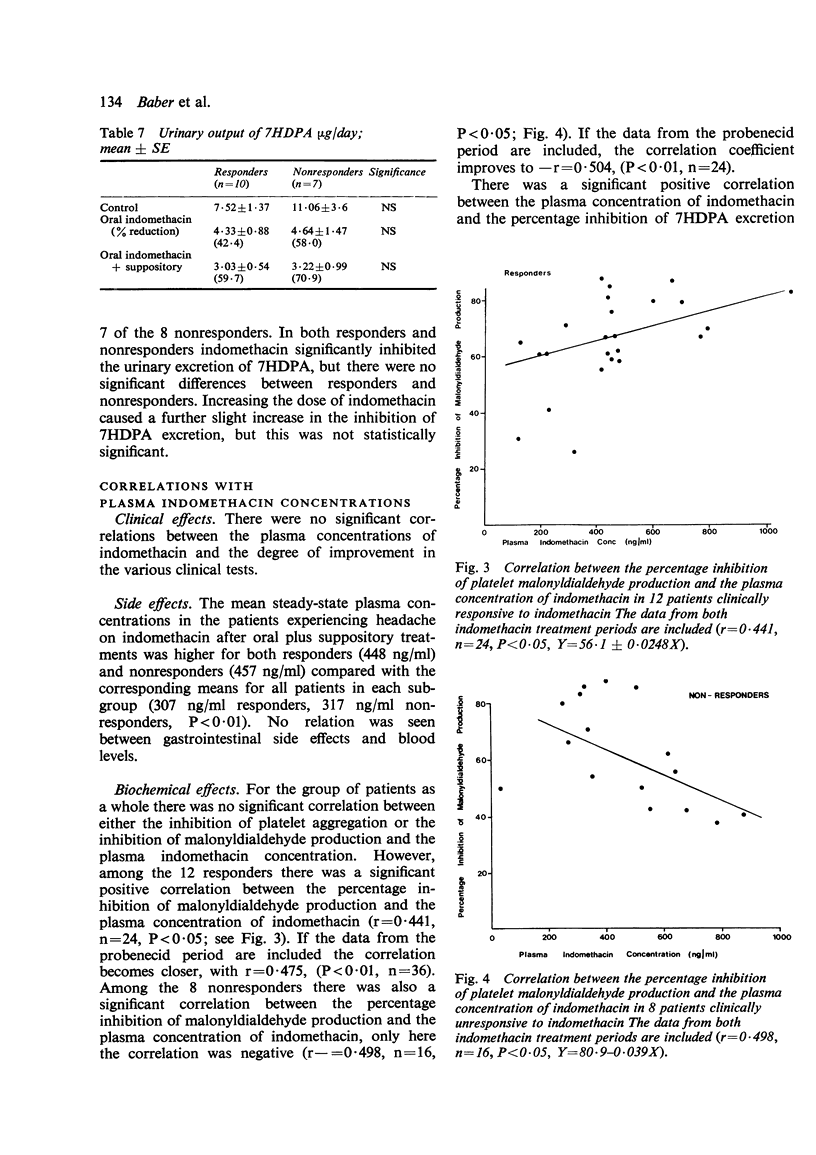
Twenty patients with definite or classical rheumatoid arthritis entered and completed a sequential study of placebo for 1 week, oral indomethacin 25 mg 3 times a day for 3 weeks, and oral indomethacin 25 mg 3 times a day plus 100 mg indomethacin suppository at night for 3 weeks. Twelve of the patients had previously been classified as responders and eight as nonresponders to indomethacin by an independent assessor. At the end of each period patients were assessed by a blind observer for duration of morning stiffness, pain score, digital joint size, grip strength, articular index, analgesic tablet usage, and the patient's own overall global assessment and comparative global assessment. In 8 of the 9 tests used responders improved on indomethacin in comparison with placebo, while nonresponders did not improve. There were no significant differences between responders and nonresponders in the plasma half-life, plasma clearance of indomethacin, protein binding of indomethacin, or urinary excretion of free or conjugated indomethacin. There were no significant differences between responders and nonresponders in the urinary excretion of 7HDPA or in the platelet aggregation or platelet malonyldialdehyde production tests. In responders there was a significant positive correlation between the plasma indomethacin concentration (r=0·44, P<0·05) and the percentage inhibition of malonyldialdehyde production by the platelets. However, in nonresponders this correlation, while significant (P<0·05), was negative (r=−0·498). Both for responders and nonresponders there was a significant correlation between plasma indomethacin concentration and the percentage reduction in 7HDPA. There was no correlation between the clinical response and the plasma concentration of indomethacin. There appears to be a biochemical difference between responders and nonresponders, which, while not necessarily causally linked with the clinical response to indomethacin, is worthy of further study.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alván G., Orme M., Bertilsson L., Ekstrand R., Palmér L. Pharmacokinetics of indomethacin. Clin Pharmacol Ther. 1975 Sep;18(3):364–373. doi: 10.1002/cpt1975183364. [DOI] [PubMed] [Google Scholar]
- Baber N., Halliday L., Sibeon R., Littler T., Orme M. L. The interaction between indomethacin and probenecid. A clinical and pharmacokinetic study. Clin Pharmacol Ther. 1978 Sep;24(3):298–307. doi: 10.1002/cpt1978243298. [DOI] [PubMed] [Google Scholar]
- Boardman P. L., Hart F. D. Clinical measurement of the anti-inflammatory effects of salicylates in rheumatoid arthritis. Br Med J. 1967 Nov 4;4(5574):264–268. doi: 10.1136/bmj.4.5574.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DIAGNOSTIC criteria for rheumatoid arthritis: 1958 revision by a committee of the American Rheumatism Association. Ann Rheum Dis. 1959 Mar;18(1):49–53. [PMC free article] [PubMed] [Google Scholar]
- Huskisson E. C., Woolf D. L., Balme H. W., Scott J., Franklin S. Four new anti-inflammatory drugs: responses and variations. Br Med J. 1976 May 1;1(6017):1048–1049. doi: 10.1136/bmj.1.6017.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keenan J. P., Wharton J., Shepherd A. J., Bellingham A. J. Defective platelet lipid peroxidation in myeloproliferative disorders: a possible defect of prostaglandin synthesis. Br J Haematol. 1977 Feb;35(2):275–283. doi: 10.1111/j.1365-2141.1977.tb00584.x. [DOI] [PubMed] [Google Scholar]
- Ritchie D. M., Boyle J. A., McInnes J. M., Jasani M. K., Dalakos T. G., Grieveson P., Buchanan W. W. Clinical studies with an articular index for the assessment of joint tenderness in patients with rheumatoid arthritis. Q J Med. 1968 Jul;37(147):393–406. [PubMed] [Google Scholar]
- Sibeon R. G., Baty J. D., Baber N., Chan K., Orme M. L. Quantitative gas-liquid chromatographic method for the determination of indomethacin in biological fluids. J Chromatogr. 1978 Jun 1;153(1):189–194. doi: 10.1016/s0021-9673(00)89871-4. [DOI] [PubMed] [Google Scholar]
- TRINDER P. Rapid determination of salicylate in biological fluids. Biochem J. 1954 Jun;57(2):301–303. doi: 10.1042/bj0570301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weder J. H., Bickel M. H. Interactions of drugs with proteins. II. Experimental methods, treatment of experimental data, and thermodynamics of binding reactions of thymoleptic drugs and model dyes. J Pharm Sci. 1970 Nov;59(11):1563–1569. doi: 10.1002/jps.2600591104. [DOI] [PubMed] [Google Scholar]


