Abstract
Background. The “weekend effect” has been associated with worse clinical outcomes. Our aim was to compare off-hours vs. regular-hours peripheral venoarterial extracorporeal membrane oxygenation (VA-ECMO) in cardiogenic shock patients. Methods. We analyzed in-hospital and 90-day mortality among 147 consecutive patients treated with percutaneous VA-ECMO for medical reasons between July 1, 2013, and September 30, 2022, during regular-hours (weekdays 8:00 a.m.–10:00 p.m.) and off-hours (weekdays 10:01 p.m.–7:59 a.m., weekends, and holidays). Results. The median patient age was 56 years (interquartile range [IQR] 49–64 years) and 112 (72.6%) were men. The median lactate level was 9.6 mmol/L (IQR 6.2–14.8 mmol/L) and 136 patients (92.5%) had a Society for Cardiovascular Angiography and Interventions (SCAI) stage D or E. Cannulation was performed off-hours in 67 patients (45.6%). In-hospital mortality was similar in off-hours and regular hours (55.2% vs. 56.3%, p = 0.901), as was the 90-day mortality (58.2% vs. 57.5%, p = 0.963), length of hospital stay (31 days [IQR 16–65.8 days] vs. 32 days [IQR 18–63 days], p = 0.979), and VA-ECMO related complications (77.6% vs. 70.0%, p = 0.305). Conclusions. Off-hours and regular-hours percutaneous VA-ECMO implantation in cardiogenic shock of medical cause have similar results. Our results support well-designed 24/7 VA-ECMO implantation programs for cardiogenic shock patients.
Keywords: extracorporeal membrane oxygenation, off-hours, regular-hours, cardiogenic shock, percutaneous cannulation, prognosis
1. Introduction
Venoarterial extracorporeal membrane oxygenation support (VA-ECMO) use has risen steeply over the last years for several cardiac conditions refractory to conventional measures (heart failure, pulmonary hypertension, myocarditis, post-cardiotomy shock, and intractable arrhythmias) [1], as a bridge to recovery or to cardiac transplantation [2]. However, the best timing to start VA-ECMO and the risk/benefit of this therapy remains unclear, as complications such as vascular damage, lower limb ischemia, and bleeding are common [3].
The “weekend effect” has been associated with an increased risk of in-hospital mortality in patients admitted during the night, weekends, and holidays [4]. This effect has been attributed to a reduction in the number of health-care professionals [5], greater severity of clinical presentation [6], and tiredness associated with long working hours [7]. In fact, admission to intensive care units during the weekend seems to be associated with in-hospital mortality [8]. In patients supported with VA-ECMO, previous studies have suggested that the prognosis may also worsen when this therapy is started during the weekend [9,10,11].
The aim of this study is to evaluate whether the results of off-hours and regular-hours peripheral VA-ECMO are comparable in an unselected population of patients with cardiogenic shock in a center with a “Shock Team” [12] and specific training of off-hours health-care professionals.
2. Materials and Methods
2.1. Study Population and Procedure
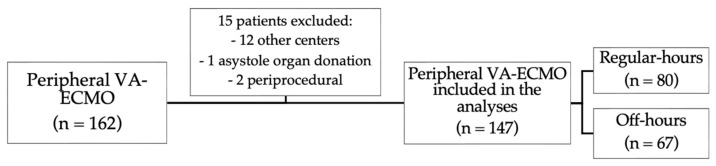
This observational, retrospective, single-center study included all patients who underwent peripheral VA-ECMO implantation for medical reasons at a high-volume tertiary hospital between 1 July 2013, and 30 September 2022, during regular-hours (weekdays 8:00 a.m. to 10:00 p.m.) and off-hours (weekdays 10:01 p.m. to 7:59 a.m., weekends, and holidays). The exclusion criteria were implantation in another center and periprocedural support (high-risk percutaneous coronary intervention or solid organ donation) (Figure 1). We registered the demographic characteristics, comorbidities, VA-ECMO indication, lactate level [13], and Society for Cardiovascular Angiography and Interventions (SCAI) stage [14] at the time of implantation, as well as complications and mortality (in-hospital and 90-day). All cannulations were performed percutaneously by an interventional cardiology team under ultrasound and/or fluoroscopy guidance. The cannulation team included one field nurse and one circulating nurse, and during regular hours two interventional cardiologists and during off hours only one interventional cardiologist. Initially, the membrane was primed immediately before implant; however, since January 2021, pre-primed membrane was available at the catheterization lab and was periodically recirculated. Implantation of an intra-aortic balloon pump or an Impella CP (Abiomed, Denver, CO, USA) for left ventricle venting was recommended in all cases after VA-ECMO implant, but the final decision was made by the on-call team on a case-by-case basis. Our protocol includes periodic training of the off-hours health professionals with bimonthly theoretical–practical sessions.
Figure 1.
Flow chart. VA-ECMO: venoarterial extracorporeal membrane oxygenation.
2.2. Definition and Outcomes
VA-ECMO indications due to medical reasons included refractory cardiogenic shock SCAI D-E (systolic blood pressure <90 mmHg, heart rate >120 beats per minute, lactate level >4 mmol/L, cardiac index <1.5 L/min/m2 despite treatment with norepinephrine >0.5 mcg/kg/min plus dobutamine or epinephrine), ventricular arrhythmia and arrhythmic storm, cardiopulmonary resuscitation, and acute pulmonary embolism. Complications included: acute kidney injury (increase in plasma creatinine value > 100% of baseline or the need of renal replacement therapy); major bleeding events (intracranial hemorrhage, bleeding requiring intervention to control, cardiac tamponade, or bleeding requiring transfusion >2 packed red blood cells in 8 h or >4 per day), hemolysis (plasma free hemoglobin levels >50 mg/dL), distal ischemia (requiring intervention, urgent decannulation, fasciotomy or amputation), ischemic or hemorrhagic stroke, and vascular lesion (hematoma with hemoglobin drop ≥1 g/dL, pseudoaneurysm, arterio-venous fistula, arterial thrombosis or deep venous thrombosis); and infection (all acquired during VA-ECMO support: ventilator-associated pneumonia, catheter-associated bacteriemia, or urinary tract infection; we excluded those acquired previous to VA-ECMO implant, e.g., aspiration pneumonia).
2.3. Statistical Analysis
Continuous variables were presented as median and interquartile range (IQR) or mean ± standard deviation when the normal distribution was observed, and were compared using the Kruskal–Wallis, Mann–Whitney U, and Student’s t-tests. Categorical variables were presented as number of patients and percentages, and were tested with the Pearson’s χ² test. The 90-day mortality was assessed using survival tables, Kaplan–Meier curves, and the log-rank test. All the tests were two-tailed. Statistical analyses were performed using SPSS Statistics 25.0 software (SPSS Inc., IBM, Chicago, IL, USA).
3. Results
3.1. Patients Characteristics
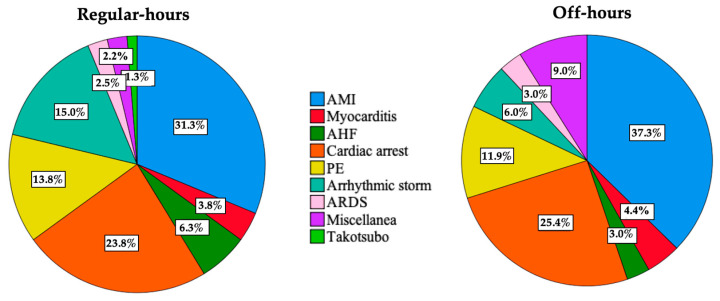
VA-ECMO was implanted in 147 patients with a median age of 56 years (IQR 49–64 years), and in 67 (45.6%) during off-hours. Figure S1 shows the number of VA-ECMO implants made each year. Baseline characteristics are shown in Table 1 and were similar between regular-hours and off-hours groups. Patients were frequently male (76.2%) and had severe cardiogenic shock (median baseline lactate of 9.6 mmol/L [IQR 6.2–14.8 mmol/L] and >90% SCAI D or E stages). The most frequent indications were cardiogenic shock due to acute myocardial infarction (34%) and cardiopulmonary resuscitation (25%) (Figure 2).
Table 1.
Baseline characteristics according to the moment of venoarterial extracorporeal membrane oxygenation implantation.
| Total (n = 147) |
Regular-Hours (n = 80) |
Off-Hours (n = 67) |
p | |
|---|---|---|---|---|
| Age, years | 56 (49–64) | 55 (49–63) | 58 (49–65) | 0.328 |
| Male sex, n (%) | 112 (76.2) | 62 (77.5) | 50 (74.6) | 0.684 |
| BMI 1, kg/m2 | 27.7 (25–31.1) | 27.7 (25.2–31.4) | 27.7 (24.8–29.9) | 0.562 |
| Hypertension, n (%) | 62 (42.2) | 35 (43.8) | 27 (40.3) | 0.673 |
| Diabetes mellitus, n (%) | 33 (22.4) | 20 (25) | 13 (19.4) | 0.418 |
| Dyslipidemia, n (%) | 64 (43.5) | 32 (40.0) | 32 (47.8) | 0.345 |
| Active smoker, n (%) | 49 (33.3) | 27 (33.8) | 22 (32.8) | 0.988 |
| Ischemic heart disease, n (%) | 26 (17.7) | 11 (13.8) | 15 (22.4) | 0.172 |
| Atrial fibrillation, n (%) | 16 (10.9) | 10 (12.5) | 6 (9.0) | 0.492 |
| Peripheral artery disease, n (%) | 15 (10.2) | 10 (12.5) | 5 (7.5) | 0.315 |
| Creatinine, mg/dL | 1.3 (1.1–1.8) | 1.3 (1.1–1.7) | 1.3 (1.1–1.8) | 0.949 |
| Lactate, mmol/L | 9.6 (6.2–14.8) | 8.9 (4.1–14) | 10.4 (7.5–14.9) | 0.122 |
| LVEF 2, % | 20 (10–40) | 23 (10–45) | 20 (10–30) | 0.521 |
| SCAI 3 D or E, n (%) | 136 (92.5) | 75 (93.8) | 61 (91.0) | 0.272 |
1 BMI: body mass index. 2 LVEF: left ventricular ejection fraction. 3 Society for Cardiovascular Angiography and Interventions shock stage classification.
Figure 2.
Reasons for venoarterial extracorporeal membrane oxygenation indication. No significant differences were found between regular-hours and off-hours groups (p = 0.467). AMI: acute myocardial infarction. AHF: acute heart failure. PE: pulmonary embolism. ARDS: acute respiratory distress syndrome.
In all but one case, femoro-femoral cannulation was performed. In most cases, a 23 French (F) extraction cannula (76%), 15 F return cannula (71%), and 6 F peripheral perfusion cannula (86%) were used. A left ventricular unloading device was implanted in 106 (72.1%) patients (intra-aortic balloon pump 96–65.3%, and Impella CP 20–13.6%). Compared with the regular-hours group, the off-hours group was more frequently treated with Impella CP (25.4% vs. 3.8%, p < 0.001) and a pre-primed membrane (27.9% vs. 13.2%, p = 0.039).
3.2. Clinical Outcomes
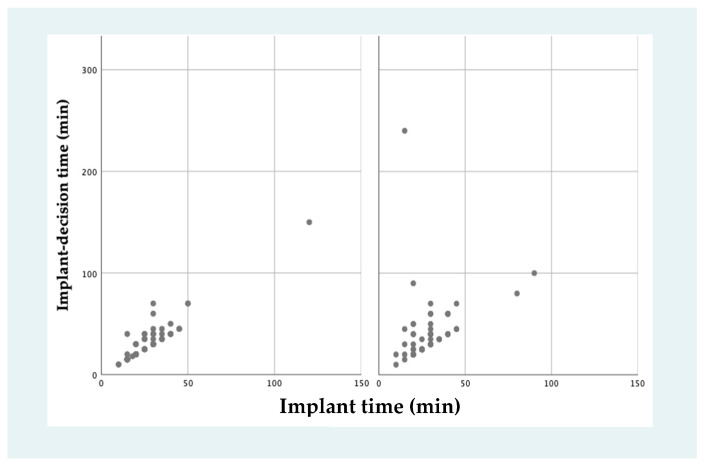
The mean time between the decision of VA-ECMO implant and the start of extracorporeal circulation (decision–implant time) was longer in the off-hours group (40.0 ± 30.7 min vs. 28.4 ± 13.1 min, p = 0.041). The mean duration of the implant procedure, from the first skin puncture during cannulation to the start of the extracorporeal circulation (implant time) was similar in both groups (28.4 ± 13.1 min vs. 26.9 ± 13.9 min, p = 0.518) (Figure 3). Figure S2 shows implant time and decision–implant time according to year of implant.
Figure 3.
Venoarterial extracorporeal membrane oxygenation implant times. Time between venoarterial extracorporeal membrane oxygenation implant decision and the end of the implant procedure was slightly higher in off-hours group (40.0 ± 30.7 min vs. 28.4 ± 13.1 min, p = 0.041), without differences in implant procedure times.
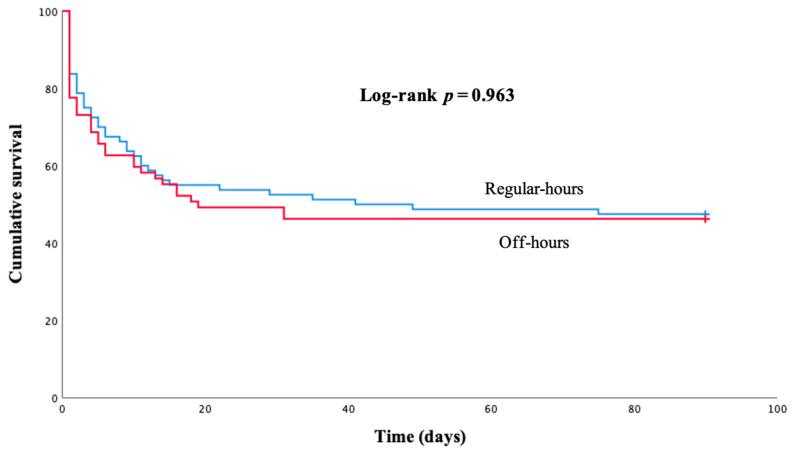
The median duration of VA-ECMO support was 4 days (IQR 2–6 days) and the length of hospital stay was 32 days (IQR 17–64 days), with no relevant differences between regular-hours and off-hours groups. Decannulation was achieved in 50 regular-hours patients (62.5%) and 40 off-hours patients (59.7%), p = 0.729. In-hospital mortality was similar in off-hours (37–55.2%) and regular hours (45–56.3%), p = 0.901 (Table 2). In addition, no relevant differences were found in mortality after the 90-day follow-up (log-rank p = 0.963) (Figure 4). The results were similar irrespective of VA-ECMO indications (Table S1) and type of left ventricular unloading device (Table S2). Most frequent causes of death were multiorgan failure (30–34.1%), severe brain injury (21–23.9%), uncontrollable bleeding (9–10.2%), intracranial hemorrhage (6–6.8%), sepsis (5–5.7%), arrhythmic storm (4–4.5%), and end-stage heart failure (4–4.5%); without differences between regular-hours and off-hours groups (p = 0.76).
Table 2.
Clinical outcomes according to the moment of venoarterial extracorporeal membrane oxygenation implantation.
| Total (n = 147) |
Regular-Hours (n = 80) |
Off-Hours (n = 67) |
p | |
|---|---|---|---|---|
| In-hospital mortality, n (%) | 82 (55.8) | 45 (56.3) | 37 (55.2) | 0.901 |
| Length of VA-ECMO support, days | 4 (2–6) | 4 (2–6) | 4 (2–5) | 0.46 |
| Successful decannulation, n (%) | 90 (60.4) | 50 (62.5) | 40 (59.7) | 0.729 |
| Length of hospital stay, days | 32 (16.5–64) | 32 (18–63) | 31 (16–65.8) | 0.979 |
| Complications | ||||
| Acute kidney injury, n (%) | 55 (37.4) | 29 (36.3) | 26 (38.8) | 0.696 |
| RRT 1 need, n (%) | 15 (10.2) | 8 (10) | 7 (10.4) | 0.929 |
| Major bleeding, n (%) | 51 (34.7) | 25 (31.3) | 26 (38.8) | 0.338 |
| Distal ischemia, n (%) | 28 (19) | 15 (18.8) | 13 (19.4) | 0.92 |
| Vascular injury, n (%) | 25 (17) | 12 (15) | 13 (19.4) | 0.479 |
| Stroke, n (%) | 9 (6.1) | 2 (2.5) | 7 (10.4) | 0.045 |
| Infection, n (%) | 23 (15.6) | 14 (17.5) | 9 (13.4) | 0.499 |
| LV 2 overdistention, n (%) | 17 (11.6) | 10 (12.5) | 7 (10.5) | 0.698 |
1 RRT: renal replacement therapy. 2 LV: left ventricle.
Figure 4.
Kaplan–Meier survival curve for 90-day follow-up according to the moment of venoarterial extracorporeal membrane oxygenation implantation. Log-rank p = 0.963.
The most common complications were acute kidney injury (55–37.4%, with need of renal replacement therapy in 15 cases), major bleeding event (51–34.7%), severe lower limb ischemia (28–19.0%), vascular injuries (25–17.9%), infections (23–15.6%), and stroke (9–6.1%). Despite frequent use of a left ventricle unloading device, left ventricle overdistention occurred in 17 patients (11.6%). Complications were similar in both groups, except of a higher incidence of stroke off-hours (10.4% vs. 2.5%, p = 0.045) (Table 2).
4. Discussion
In our study, performed in a center with a “Shock Team” and specific training of off-hours health-care professionals, regular-hours and off-hours VA-ECMO implantation had similar results in terms of in-hospital and 90-day mortality, weaning success, and length of hospital stay. Complications related to VA-ECMO therapy were also comparable.
The “weekend effect” has been described in different conditions [4] and even meta-analyses have reported an association between off-hours admission and worse outcomes in heart failure, cardiorespiratory arrest, and acute coronary syndromes [15,16]. A few previous studies have suggested unfavorable VA-ECMO results in cardiopulmonary resuscitation patients cannulated off-hours [11,17,18]. This disadvantage might be due to a reduction in the number of health-care professionals [5], greater severity of clinical presentation [6], and tiredness associated with long working hours [7]. However, longer delays might also play a role. In our study, although the cannulation time was comparable in the two groups, the decision–implantation time was higher off-hours than regular-hours. Nevertheless, it is unclear if a 30-min delay to start VA-ECMO support has a prognostic impact [19].
In a single-center study that included 200 VA-ECMO implants in the setting of cardiorespiratory arrest, survival results were worse during the weekend [11]. As in our case, the time from the cardiac arrest to the start of VA-ECMO support was longer off-hours (47 min vs. 31 min) [11]. The relevance of this delay is probably much more important in cardiopulmonary resuscitation than in cardiogenic shock. In fact, a study that included 250 patients with VA-ECMO implantation in cardiogenic shock, found similar results to our series, as cannulation outside working hours was not associated with increased mortality, duration of ECMO support, or longer intensive care unit stay [10]. The only negative association with off-hours in this study were vascular complications [10], something we did not observe, probably due to the generalization in the implantation of peripheral perfusion cannula. In addition, in a pediatric population, off-hours VA-ECMO implantation is also not associated with a higher rate of complications or mortality [20,21], even in the setting of cardiopulmonary resuscitation [17].
Compared to patients on regular hours, off-hours patients had a higher incidence of stroke; the explanation of this possible association remains unclear. VA-ECMO support may increase the risk of stroke due to several factors such as need for systemic anticoagulation, possibility of aortic root or left ventricle thrombosis, systemic inflammation with capillary fragility, or hemolysis [22]. In addition, the concomitant use of Impella, more frequent in off-hours group, may increase the risk of thrombotic and hemorrhagic complications [23], although most cerebrovascular events occurred in patients not supported with Impella. A previous VA-ECMO series also reported a high incidence of stroke (5.8%), with hyperlactatemia being the only independent predictor [24].
The main interest of our work lies in the fact that we included patients with cardiogenic shock due to multiple medical (non-surgical) reasons, beyond VA-ECMO implant in the context of cardiopulmonary resuscitation, in which the cannulation was performed in all cases by an interventional cardiologist team. In our series, off-hours versus regular-hours comparable outcomes are probably thanks to off-hours staff training and the on-call Shock Team/interventional cardiologist availability to ensure a correct patient selection and VA-ECMO cannulation procedure.
Our study also has some limitations. As it is a retrospective observational revision, we could have underestimated differences in the severity of the clinical condition between groups, although the absence of relevant differences at baseline, including lactate values and SCAI stage, suggests that our two groups of patients are comparable. In addition, this is a single-center study, in a high-volume academic hospital, so our data results may not be generalizable to other centers with less experience in VA-ECMO implantation. Over 90% of VA-ECMO implants in our center are guided by ultrasound plus fluoroscopy and less than 10% only by fluoroscopy; unfortunately, we have not collected data about guidance of the implant with fluoroscopy or ultrasound in each patient. Finally, the sample size is smaller than some of the previously published series, but we have not included VA-ECMO implantation in cardiac surgery-related cardiogenic shock, a common cause in previous cohorts.
5. Conclusions
Off-hours and regular-hours percutaneous VA-ECMO implantation in cardiogenic shock of medical cause have similar results. Our results support well-designed 24/7 VA-ECMO implantation programs for cardiogenic shock patients that should include specific training of all health-care professionals that might be involved in off-hours VA-ECMO implantation and management.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12051875/s1, Table S1: In-hospital mortality rates according to VA-ECMO indications. Table S2: In-hospital mortality rates according to the use and type of left ventricle unloading device. Figure S1: Number of venoarterial extracorporeal membrane oxygenation implants made each year. Figure S2: Implant time and decision-implant time according to year of implant.
Author Contributions
Conceptualization, J.G.-C., I.S.-C. and M.M.-S.; investigation, R.G.-S., J.G.-C., J.M.-S., I.S.-C., M.J.-F., C.D.-C., R.S.-R. and E.G.-I.; data curation, J.G.-C., J.M.-S., I.S.-C. and R.G.-S.; writing—original draft preparation, R.G.-S., J.G.-C., J.M.-S., I.S.-C. and M.M.-S.; writing—review and editing, R.G.-S.; supervision, J.G.-C., I.S.-C., J.E., F.F.-A. and M.M.-S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Comunidad de Madrid (protocol code18/07, approval date 11 January 2008).
Informed Consent Statement
Patient consent was waived due to the retrospective nature of the study and the approval of the ethics committee.
Data Availability Statement
The datasets used and analyzed in the current study are made available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Guglin M., Zucker M.J., Bazan V.M., Bozkurt B., El Banayosy A., Estep J.D., Gurley J., Nelson K., Malyala R., Panjrath G.S., et al. Venoarterial ECMO for Adults: JACC Scientific Expert Panel. J. Am. Coll. Cardiol. 2019;73:698–716. doi: 10.1016/j.jacc.2018.11.038. [DOI] [PubMed] [Google Scholar]
- 2.Barge-Caballero E., González-Vílchez F., Almenar-Bonet L., Carmena M.D.G.-C., González-Costello J., Gómez-Bueno M., Castel-Lavilla M., Lambert-Rodríguez J.L., Martínez-Sellés M., Mirabet-Pérez S., et al. Temporal trends in the use and outcomes of temporary mechanical circulatory support as a bridge to cardiac transplantation in Spain. Final report of the ASIS-TC study. J. Heart Lung Transplant. 2022. in press . [DOI] [PubMed]
- 3.Cheng R., Hachamovitch R., Kittleson M., Patel J., Arabia F., Moriguchi J., Esmailian F., Azarbal B. Complications of extracorporeal membrane oxygenation for treatment of cardiogenic shock and cardiac arrest: A meta-analysis of 1866 adult patients. Ann. Thorac. Surg. 2014;97:610–616. doi: 10.1016/j.athoracsur.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Honeyford K., Cecil E., Lo M., Bottle A., Aylin P. The weekend effect: Does hospital mortality differ by day of the week? A systematic review and meta-analysis. BMC Health Serv. Res. 2018;18:870. doi: 10.1186/s12913-018-3688-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cram P., Hillis S.L., Barnett M., Rosenthal G.E. Effects of weekend admission and hospital teaching status on in-hospital mortality. Am. J. Med. 2004;117:151–157. doi: 10.1016/j.amjmed.2004.02.035. [DOI] [PubMed] [Google Scholar]
- 6.Meynaar I.A., Van Der Spoel J.I., Rommes J.H., Van Spreuwel-Verheijen M., Bosman R.J., Spronk P.E. Off hour admission to an intensivist-led ICU is not associated with increased mortality. Crit. Care. 2009;13:R84. doi: 10.1186/cc7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leso V., Fontana L., Caturano A., Vetrani I., Fedele M., Iavicoli I. Impact of shift work and long working hours on worker cognitive functions: Current evidence and future research needs. Int. J. Environ. Res. Public Health. 2021;18:6540. doi: 10.3390/ijerph18126540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Galloway M., Hegarty A., McGill S., Arulkumaran N., Brett S.J., Harrison D. The effect of ICU out-of-hours admission on mortality: A systematic review and meta-analysis. Crit. Care Med. 2018;46:290–299. doi: 10.1097/CCM.0000000000002837. [DOI] [PubMed] [Google Scholar]
- 9.Cho H.-W., Song I.-A., Oh T.K. Weekend effect in extracorporeal membrane oxygenation therapy initiation: A nationwide cohort study in South Korea. Ann. Transl. Med. 2021;9:742. doi: 10.21037/atm-21-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van der Wal P., Kraaijeveld A., van der Heijden J., van Laake L., Platenkamp M., de Heer L., Braithwaite S., van Eijk M., Hermens J., Cremer O., et al. Initiation of veno-arterial extracorporeal membrane oxygenation (VA-ECMO) for cardiogenic shock during out of hours versus working hours is not associated with increased mortality. Int. J. Artif. Organs. 2022;45:301–308. doi: 10.1177/03913988211073344. [DOI] [PubMed] [Google Scholar]
- 11.Lee D.-S., Chung C.R., Jeon K., Park C.-M., Suh G.Y., Bin Song Y., Hahn J.-Y., Choi S.-H., Choi J.-H., Gwon H.-C., et al. Survival after extracorporeal cardiopulmonary resuscitation on weekends in comparison with weekdays. Ann. Thorac. Surg. 2016;101:133–140. doi: 10.1016/j.athoracsur.2015.06.077. [DOI] [PubMed] [Google Scholar]
- 12.Martínez-Sellés M., Hernández-Pérez F.J., Uribarri A., Villén L.M., Zapata L., Alonso J.J., Amat-Santos I.J., Ariza-Solé A., Barrabés J.A., Barrio J.M., et al. Código shock cardiogénico 2023. Documento de expertos para una organización multidisciplinaria que permita una atención de calidad. Rev. Española De Cardiol. 2022 doi: 10.1016/j.recesp.2022.10.010. in press . [DOI] [Google Scholar]
- 13.Martínez-Solano J., Sousa-Casasnovas I., Bellón-Cano J.M., García-Carreño J., Juárez-Fernández M., Díez-Delhoyo F., Sanz-Ruiz R., Devesa-Cordero C., Elízaga-Corrales J., Fernández-Avilés F., et al. Lactate levels as a prognostic predict in cardiogenic shock under venoarterial extracorporeal membrane oxygenation support. Rev. Española De Cardiol. 2022;75:595–603. doi: 10.1016/j.recesp.2021.08.007. [DOI] [PubMed] [Google Scholar]
- 14.Baran D.A., Grines C.L., Bailey S., Burkhoff D., Hall S.A., Henry T.D., Hollenberg S.M., Kapur N.K., O’Neill W., Ornato J.P., et al. SCAI clinical expert consensus statement on the classification of cardiogenic shock: This document was endorsed by the American College of Cardiology (ACC), the American Heart Association (AHA), the Society of Critical Care Medicine (SCCM), and the Society of Thoracic Surgeons (STS) in April 2019. Catheter. Cardiovasc. Interv. 2019;94:29–37. doi: 10.1002/ccd.28329. [DOI] [PubMed] [Google Scholar]
- 15.Zhou Y., Li W., Herath C., Xia J., Hu B., Song F., Cao S., Lu Z. Off-Hour admission and mortality risk for 28 specific diseases: A systematic review and meta-analysis of 251 cohorts. J. Am. Heart Assoc. 2016;5:e003102. doi: 10.1161/JAHA.115.003102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwok C.S., Al-Dokheal M., Aldaham S., Rushton C., Butler R., Kinnaird T., Zaman A., Zaman M.J., Timmis A., Mamas M. Weekend effect in acute coronary syndrome: A meta-analysis of observational studies. Eur. Heart J. Acute Cardiovasc. Care. 2019;8:432–442. doi: 10.1177/2048872618762634. [DOI] [PubMed] [Google Scholar]
- 17.Burke C.R., Chan T., Brogan T.V., McMullan D.M. Pediatric extracorporeal cardiopulmonary resuscitation during nights and weekends. Resuscitation. 2017;114:47–52. doi: 10.1016/j.resuscitation.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 18.Achuff B.-J., Elias M.D., Ittenbach R.F., Ravishankar C., Nicolson S.C., Spray T.L., Fuller S., Gaynor J.W., O’Connor M.J. Risk factors for mortality in paediatric cardiac ICU patients managed with extracorporeal membrane oxygenation. Cardiol. Young. 2019;29:40–47. doi: 10.1017/S1047951118001774. [DOI] [PubMed] [Google Scholar]
- 19.Ostadal P., Rokyta R., Karasek J., Kruger A., Vondrakova D., Janotka M., Naar J., Smalcova J., Hubatova M., Hromadka M., et al. Extracorporeal membrane oxygenation in the therapy of cardiogenic shock: Results of the ECMO-CS randomized clinical trial. Circulation. 2023;147:454–464. doi: 10.1161/CIRCULATIONAHA.122.062949. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez K.W., Dalton B.G.A., Weaver K.L., Sherman A.K., Peter S.D.S., Snyder C.L. Effect of timing of cannulation on outcome for pediatric extracorporeal life support. Pediatr. Surg. Int. 2016;32:665–669. doi: 10.1007/s00383-016-3901-6. [DOI] [PubMed] [Google Scholar]
- 21.Steurer M.A.M., Tonna J.E.M., Coyan G.N.M., Burki S., Sciortino C.M.M., Oishi P.E. On-Hours compared to off-hours pediatric extracorporeal life support initiation in the United States between 2009 and 2018—An analysis of the extracorporeal life support organization registry. Crit. Care Explor. 2022;4:e0698. doi: 10.1097/CCE.0000000000000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pahuja M., Chehab O., Ranka S., Mishra T., Ando T., Yassin A.S., Mph K.L.T., Shah P., Kimmelstiel C.D., Salehi P., et al. Incidence and clinical outcomes of stroke in ST-elevation myocardial infarction and cardiogenic shock. Catheter. Cardiovasc. Interv. 2021;97:217–225. doi: 10.1002/ccd.28919. [DOI] [PubMed] [Google Scholar]
- 23.Hassett C.E., Cho S.-M., Hasan S., Rice C.J., Migdady I., Starling R.C., Soltesz E., Uchino K. Ischemic stroke and intracranial hemorrhages during impella cardiac support. ASAIO J. 2020;66:e105–e109. doi: 10.1097/MAT.0000000000001132. [DOI] [PubMed] [Google Scholar]
- 24.Omar H.R., Mirsaeidi M., Shumac J., Enten G., Mangar D., Camporesi E.M. Incidence and predictors of ischemic cerebrovascular stroke among patients on extracorporeal membrane oxygenation support. J. Crit. Care. 2016;32:48–51. doi: 10.1016/j.jcrc.2015.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and analyzed in the current study are made available from the corresponding author on reasonable request.