Abstract
Antibodies reactive with phosphorylcholine (PC) are ubiquitous in human sera, but the antigens stimulating their production and their function are not clear. Previous studies have shown that a significant proportion of dental plaque bacteria contain PC as determined by reactivity with PC-specific mouse myeloma proteins and monoclonal antibodies. Additionally, serum antibody concentrations of immunoglobulin (IgG) G anti-PC are higher in sera of individuals who have experienced periodontal attachment loss than those who are periodontally healthy. These data implicate the oral microflora as a source of antigen-stimulating anti-PC responses. Recent data also indicate that antibodies with specificity for PC are elevated in ApoE-deficient mice, a model for studies of athersclerosis, and that such antibodies bound oxidized low-density lipoproteins (LDL) (oxLDL) in atherosclerotic plaques. These data prompted the hypothesis that human anti-PC could bind to both oral bacteria and human oxLDL, and that these antigens are cross-reactive. We therefore examined the ability of human anti-PC to bind to PC-bearing strains of oral bacteria using enzyme-linked immunosorbent inhibition assays and by assessment of direct binding of affinity-purified human anti-PC to PC-bearing Actinobacillus actinomycetemcomitans. Our results indicated that PC-bearing strains of Streptococcus oralis, Streptococcus sanguis, Haemophilus aphrophilus, Actinomyces naeslundii, Fusobacterium nucleatum, and A. actinomycetemcomitans, as well as a strain of Streptococcus pneumoniae, absorbed up to 80% of anti-PC IgG antibody from human sera. Furthermore, purified anti-PC bound to a PC-bearing strain of A. actinomycetemcomitans but only poorly to a PC-negative strain. OxLDL also absorbed anti-PC from human sera, and oxLDL but not LDL reacted with up to 80% of the anti-PC in human sera. Furthermore, purified anti-PC bound directly to oxLDL but not to LDL. The data indicate that PC-containing antigens on a variety of common oral bacteria are cross-reactive with neoantigens expressed in oxLDL. We propose that PC-bearing dental plaque microorganisms may induce an antibody response to PC that could influence the inflammatory response associated with atherosclerosis.
Antibodies reactive with phosphorylcholine (PC) are found in all human sera, though the sources of antigen responsible for induction of such antibodies are not entirely known. Phosphorylcholine antigens are most notably present in the C polysaccharide of the cell wall of Streptococcus pneumoniae (32). Recent studies of the oral and respiratory tract flora have identified additional species which have structural molecules bearing choline (13–15, 23, 40); these molecules have invariably been shown to contain PC by specific reactivity with monoclonal antibodies or myeloma proteins which react only with PC. PC has additionally been detected on a number of pathogenic prokaryocytes including S. pneumonia, and other gram-positive bacteria such other streptococci, Bacillius spp., Clostridium spp., and bacilli, as well as the gram-negative species Haemophilus influenzae (17).
Recent studies performed in our laboratories (40) and an extensive survey of plaque bacteria by Gmur and coworkers (15) indicate that a significant proportion of supragingival and subgingival plaque bacteria react with TEPC-15, a mouse myeloma protein with specificity for PC, or with PC specific MAb. Furthermore, we demonstrated that individuals with all forms of periodontal attachment loss have elevated serum concentrations of antibody to PC compared with those with no past history of periodontal attachment loss (40). These studies implicate the oral flora, and particularly dental plaque, as a significant source of antigen participating in induction of anti-PC antibodies.
Recently, Shaw and coworkers (42) noted that during development of atherosclerosis, ApoE-deficient mice produce high titers of immunoglobulin M (IgM) autoantibodies to oxidation-specific neoepitopes of oxidized low-density lipoproteins (LDL) (oxLDL). They noted that hybridoma antibodies derived from such mice with specificity for oxLDL bind to phospholipids in Cu-oxidized LDL and further that the antibodies were structurally and functionally identical to T15 anti-PC antibodies. The data suggest that oxidized LDL, in addition to bacteria, could serve as sources of antigen for induction of anti-PC. Interestingly, these antibodies are deposited in atherosclerotic lesions and appear to function in this mouse model by blocking uptake of oxLDL by macrophages, suggesting that these naturally occurring IgM antibodies may inhibit foam cell formation. In contrast with this murine anti-PC response, human anti-PC is overwhelmingly IgG, with more than 80% of all immunoglobulin being IgG2 (8). Nevertheless, a large proportion of this human IgG2 does bear the T 15 idiotype (8).
Some epidemiological studies have implicated periodontal disease as a risk factor for cardiovascular diseases (3–6). Risk for both myocardial infarction (2) and stroke (50, 51) has been shown to be associated with severity of periodontitis. It has been hypothesized that systemic indicators of inflammation, such as acute-phase reactants, are characteristic of both diseases and provide a common link that may explain disease pathology (44, 51). The possible participation of oral bacteria in the pathology of these diseases has been explained by the capacity of oral pathogens both to invade endothelial cells (10, 35) and further to stimulate production of proinflammatory cytokines (30). It is noteworthy, however, that some investigators have failed to find such a relationship between periodontitis and cardiovascular diseases and attribute positive findings to inadequate management of confounding variables in management of epidemiological data (20).
The observation that plaque bacteria and mouse oxLDL both contain PC-bearing epitopes reactive with TEPC-15 myeloma protein prompted the hypothesis that human anti-PC could bind to both oral bacteria and human oxLDL and that these antigens are cross-reactive. We present data indicating that a number of plaque bacteria react with human anti-PC antibodies and that oxLDL likewise react with such antibodies. Thus, it appears that there is cross-reactivity between human antibodies to PC with respect to oxLDL and oral bacterial antigens.
MATERIALS AND METHODS
Bacteria.
Bacterial strains of Streptococcus oralis ATCC 35037, Streptococcus sanguis ATCC 49295 & 10556, Haemophilus aphrophilus ATCC 13252, Actinomyces naeslundii ATCC 49340, and Actinobacillus actinomycetemcomitans VPI D045D-40 & DB03A-42 were maintained in brain heart infusion medium (Difco) and incubated at 37°C in humidified 5% CO2. S. pneumoniae ATCC 39937 was maintained in brain heart infusion medium and incubated at 37°C under atmospheric conditions. Fusobacterium nucleatum VPI D052B-16 and D021B-13 were maintained in commercially prepared broth (BBL) supplemented with yeast extract, l-cysteine, vitamin K1, and hemin and were incubated at 37°C in an anaerobe chamber forced-air incubator. Bacteria were incubated for 16 h under prescribed conditions prior to usage.
Absorption of human serum with bacteria.
Sera were obtained from systemically healthy human volunteers after obtaining informed consent. The bacteria were washed three times in phosphate-buffered saline (PBS), pH 7.4, at 4°C. The optical density of each strain was measured at 650 nm, and the volume was adjusted so that the optical density reading was 2.5, which was nominally equivalent to a concentration of 1.25 × 109 bacteria/ml. For each strain, dilutions were prepared in PBS, pH 7.4, and 1 ml of each dilution was pipetted into microcentrifuge tubes and centrifuged at 6,000 rpm (Eppendorf 5403 centrifuge) at 4°C for 20 min. The supernatants were discarded. The pellets were resuspended in 500 μl of a 1/500 dilution of human serum. The tubes were incubated for 60 min in a 37°C shaker water bath and rocked overnight at 4°C. The tubes were centrifuged, and the supernatants were collected.
ELISA for anti-PC IgG.
Measurement of anti-PC concentrations in serum was carried out using a modification of the method we previously described (45). Immulon 4 HBX ELISA (Dynatech) plates were coated with 0.1 ml of PC-BSA diluted to 1.25 μg of PC/ml in 0.005 M phosphate buffer, pH 7.5, and incubated at room temperature, with shaking, for 15 min. Plates were washed two times, with shaking, in a solution containing 0.01 M phosphate buffer, 0.14 M NaCl, and 0.01% Tween 20 (Fisher) (PBS-Tween). Next, 0.1 ml of sample diluted in PBS-Tween was loaded into the coated wells and incubated at room temperature, with shaking, for 30 min. Following another two washes with PBS-Tween, 0.1 ml of peroxidase-conjugated, affinity-purified, rabbit anti-human IgG (Jackson ImmunoResearch) diluted 1/10,000 in PBS-Tween was added and incubated for 30 min, with shaking, at room temperature. The plates were then washed twice in PBS-Tween as above and then washed twice in dH2O (to remove residual Tween). Then, 0.1 ml of 3,3′,5,5′-tetramethyl-benzidine (100 μg/ml; CalBiochem)–0.006% H2O2 in 0.1 M acetate buffer, pH 6.0, was added and incubated at room temperature, with shaking, for 30 min, and 25 μl of 2.5 M H2SO4 was then added to stop the reaction and initiate the final color change. Plates were read at A450 on a Molecular Devices Vmax enzyme-linked immunosorbent assay (ELISA) reader. Assays were performed four to six times, and data in the figures are reported as means ± standard deviation for replicate determinations.
Preparation of human LDL and OxLDL.
Human LDL were isolated from serum by ultracentrifugation using a modification of the methods described in references 9 and 18. Briefly, 2 ml of a saline solution (density, 1.346) was added to 10 ml of serum to give a mixture solvent density of 1.019 to 1.063. The mixture was centrifuged at 105,000 × g for 22 h at 12°C, and the top layer containing the LDL was removed. The LDL protein content was estimated using the Lowry assay (27). The LDL preparation was oxidized by incubating 100 μg of LDL protein/ml in PBS, pH 7.4, with 5 μM CuSO4 (33) at 37°C for 16 h with shaking. The solution was then dialyzed against PBS, pH 7.4, containing 10 mM EDTA. The preparation was concentrated using a 2-ml 10,000-molecular-weight cutoff Centricon centrifugal filter device (Amicon Corporation), and the protein content was determined using the Lowry assay.
Absorption of human sera with LDL.
Dilutions of LDL or oxLDL, prepared in PBS containing 10 mM Na-EDTA, were added to human sera to achieve final concentrations of 0.05 to 250 μg/ml. The sera were incubated for 60 min ate 37°C with shaking, and then were incubated overnight at 4°C.
Purification of anti-PC.
Antibodies to PC were isolated from Cohn Fraction II (Sigma catalog no. G-4386). Cohn Fraction II was resuspended in 0.01 M EDTA Hanks balanced salt solution (HBSS) (GibcoBRL catalog no. 14185-052) at 100 mg/ml. Aliquots of the resuspended Cohn Fraction II were passed through an immobilized p-aminophenyl PC column (Pierce catalog no. 20307). After washing until the effluent gave a baseline absorbance of less than 0.01 at 280 nm UV, a step gradient of 0.01 M PC chloride (Sigma catalog no. P-0378) followed by 0.01 M p-aminophenyl-PC (Sigma catalog no. A-9278) was applied to elute the bound antibodies to PC. Pools of the two eluates were collected and concentrated on Centriprep YM-30 (Amicon 4306) filter devices. The pH of the concentrated samples was adjusted to 3.0 with 0.4 M HCl, and the IgG and PC-chloride or p-aminophenyl PC were separated on a Sephadex G-25 (Pharmacia) column equilibrated with 0.01 M EDTA HBSS. Pools, as determined by A280 and pH, were made and concentrated as above.
Binding of anti-PC to bacteria.
Binding of anti-PC was accomplished by diluting the bacteria to 108/ml in HBSS–0.1% low-endotoxin BSA (Sigma catalog no. A-2934), adding antibodies at 30 μg/ml, and then incubating, at room temperature, for 30 min. Bacteria were washed three times in HBSS-BSA, resuspended to their initial volume, and incubated with a biotinylated F(ab′)2 goat anti-human IgG (5 μg/ml) for 30 min. Bacteria were washed three times in HBSS-BSA, resuspended to their initial volume, and incubated with Alexa-488-streptavidin (Molecular Probes catalog no. S-11223) for 30 min at room temperature. Following three washes with HBSS-BSA the cells were examined on a FACScan device (Becton Dickinson).
RESULTS
Binding of human serum anti-PC to oral bacteria.
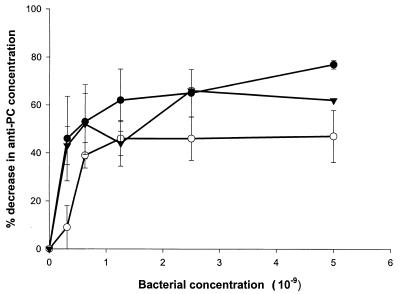
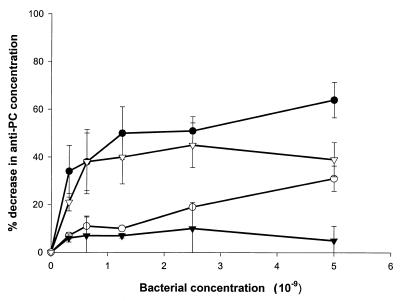
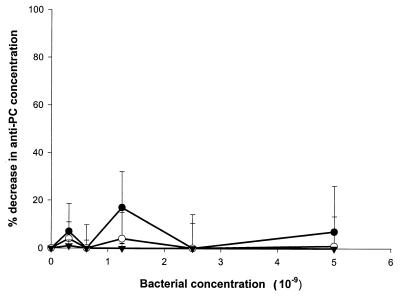
We first sought to demonstrate that human anti-PC binds to oral bacteria bearing PC-containing antigens. ELISA inhibition assays were carried out in which bacterial samples were incubated in serum prior to determination of residual anti-PC antibody. As shown in Fig. 1 and 2, a variety of strains of gram-positive and gram-negative plaque bacteria, including strains of S. oralis, S. sanguis, A. actinomycetemcomitans, F. nucleatum, and H. aphrophilus, as well as the nonoral pathogen S. pneumoniae, bound anti-PC. Other strains of oral isolates previously shown not to incorporate choline or react with TEPC-15, including strains of A. actinomycetemcomitans, S. sanguis, and A. naeslundii, failed to remove anti-PC from human serum (Fig. 3). These results demonstrate that human anti-PC binds to a variety of oral microorganisms, specifically to those bearing phosphorylcholine.
FIG. 1.
Decrease in anti-PC concentration following absorption of human serum with gram-positive bacteria. Human serum diluted 1:500 was absorbed with the indicated concentrations of S. pneumoniae 39937 (●), S. sanguis 49295 (○), and S. oralis 35037 (▾) as indicated in Materials and Methods. Subsequently, anti-PC antibody concentrations were determined by ELISA. Data are expressed as the decrease in anti-PC concentration following absorption ±1 standard deviation (error bars).
FIG. 2.
Decrease in anti-PC concentration following absorption of human serum with gram-negative bacteria. Human serum diluted 1:500 was absorbed with the indicated concentrations of A. actinomycetemcomitans D045D-40 (●), F. nucleatum D052B-16 (○), and H. aphrophilus 13252 (▾), or F. nucleatum D021B-13 (▿) as indicated in Materials and Methods. Subsequently, anti-PC antibody concentrations were determined by ELISA. Data are expressed as the decrease in anti-PC concentration following absorption ±1 standard deviation (error bars).
FIG. 3.
Decrease in anti-PC concentration following absorption of human serum with PC-negative bacteria. Human serum diluted 1:500 was absorbed with the indicated concentrations of A. actinomycetemcomitans DB03A-42 (●), S. sanguis 10556 (○), and A. naeslundii 49340 (▾) as indicated in Materials and Methods. Subsequently, anti-PC antibody concentrations were determined by ELISA. Data are expressed as the decrease in anti-PC concentration following absorption ±1 standard deviation (error bars).
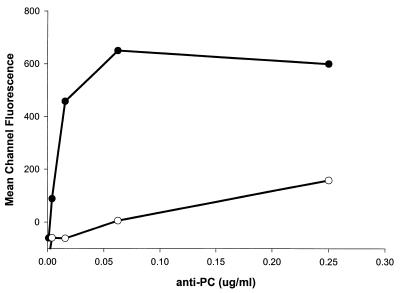
Since ELISA inhibition assays identified strains of A. actinomycetemcomitans that were either PC-positive (strain D045D-40) or PC-negative (strain DB03A-42), we chose to verify results of the ELISA inhibition assays by assessing direct binding of anti-PC to these bacterial strains. Purified anti-PC was incubated with either bacterial strain and then with FITC-labeled biotinylated F(ab′)2 goat anti-human IgG, and bound antibody was detected by fluorescence-activated cell sorter analysis. As shown in Fig. 4, purified anti-PC binds specifically to the strain of A. actinomycetemcomitans (D045D-40) that has been shown both to contain choline and to react with TEPC-15, while little binding could be demonstrated to strain DB03A-42.
FIG. 4.
Binding of purified anti-PC to A. actinomycetemcomitans strains. Affinity-purified human anti-PC antibody was incubated with either a PC-containing strain (D045D-40 [●]) or a PC-negative strain (DB03A-42 [○]) of A. actinomycetemcomitans.
Binding of human anti-PC to oxLDL.
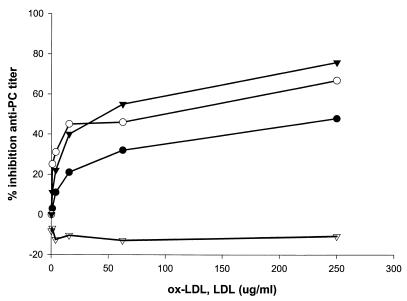
To demonstrate cross-reactivity of PC-bearing oral bacteria with oxLDL we performed ELISA inhibition assays with purified LDL and oxLDL to demonstrate decrease in anti-PC serum titers following absorption. As shown in Fig. 5, absorption of serum with three different preparations of oxLDL from three serum donors inhibited anti-PC titers by up to 80%. In contrast, absorption with unoxidized LDL consistently failed to deplete IgG anti-PC from human sera. This verifies for human IgG anti-PC the observation by Shaw in the mouse model that oxidation of LDL reveals neoepitopes containing PC that react with mouse anti-PC. Furthermore, this establishes the cross-reactivity of oxLDL with a series of bacteria commonly found in dental plaque due to their common reactivity with anti-PC.
FIG. 5.
Inhibition of anti-PC by oxLDL and LDL. Preparations of oxLDL from serum of three donors (●, ○, ▾) or unoxidized LDL (▿) were incubated in human serum as described in Materials and Methods. Following incubation, the level of residual anti-PC was determined by ELISA.
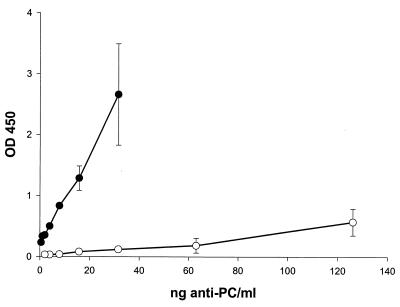
Attempts to directly demonstrate that oral bacteria absorb anti-oxLDL from human sera were attempted. However, the small proportion of PC-bearing antigens in the oxLDL preparations used as ELISA antigens made it impossible to convincingly demonstrate removal of anti-PC from the anti-oxLDL antibody pool. This was due to an 85 to 90% reduction in sensitivity of the ELISA to detect anti-PC when using oxLDL as the antigen (data not shown). As shown in Fig. 6, however, purified human anti-PC can be shown to react directly with oxLDL but not LDL in ELISA assays, further verifying its cross-reactivity with oral bacterial strains.
FIG. 6.
Binding of purified anti-PC to oxLDL. LDL, purified from human sera by ultracentrifugation, and oxLDL, generated from LDL using CuSO4, were used to coat ELISA plates. Affinity-purified human anti-PC was added to the plates, and binding was determined as described in the text. Symbols: ●, oxLDL; ○, LDL. Error bars represent ±1 standard deviation.
DISCUSSION
The data demonstrate that human anti-PC IgG antibody reacts both with a variety of plaque microorganisms and with oxidized, but not native, LDL. Our previous data indicate that patients with periodontal attachment loss have higher concentrations of anti-PC IgG than do individuals who demonstrate no attachment loss (40). The implication of this finding is that it is possible that inflamed periodontal tissues permit ingress of antigens from oral bacteria which leads to increased systemic production of anti-PC. These antibodies in turn can react with autoantigens bearing PC, one of which is oxLDL. Although it is also possible that members of the intestinal or respiratory flora that bear PC may also contribute to the production of these antibodies, the data linking periodontal diseases with levels of anti-PC strongly implicate the oral flora as a major source of such antigen. We have established that PC-bearing strains of A. actinomycetemcomitans can invade endothelial cells via the receptor for platelet-activating factor (39), implying that oral bacteria bearing PC may be able to readily access the systemic circulation due to their ability to mimic platelet-activating factor.
Evidence from both animal models and human disease populations characterized by elevated immune responses against oxidatively modified lipids suggests a role for the immune system in the chronic inflammatory response observed in atherosclerosis (11, 22, 29, 34, 37, 43, 47, 49). These studies suggest that antibody responses to oxidized phospholipids may be targeted at removal of damaged lipoproteins and apoptotic cells. In the process of immune clearance it is further suggested that some of these antibodies may also participate in enhancing the chronic inflammation characteristic of atherosclerotic lesions. Alternatively, it has also been demonstrated that immunization with oxLDL or atheromatous plaques generates a T-cell-dependent IgG response that is protective (52). Although the specific function of such antibodies in animal models is not clear, the presence of these antibodies may mark the appearance and progression of atherosclerotic lesions.
A characteristic of some diseases demonstrating accelerated atherosclerosis, such as systemic lupus erythematosus and antiphospholipid syndrome, is the appearance of antiphospholipid antibodies (12, 19, 29, 37, 47–49). Such antibodies are viewed by some investigators as an autoimmune response against oxidized phospholipids. It has been suggested that these autoantibodies could contribute to the accelerated pathogenicity of oxidized phospholipids in these syndromes by contributing to a chronic inflammatory reaction in the arterial wall.
The observation that commonly found oral bacterial species and up to 50% of the supragingival and subgingival flora of some individuals contain PC (40) may provide a link between oral colonization and infection and a variety of systemic conditions. It is possible that such bacteria are an antigenic source that stimulates production of anti-PC antibodies. Since PC is also a component of oxLDL, then the oral flora of periodontitis patients may be responsible for increased antibody titers against oxLDL. Elevated titers of antibody to oxLDL (which include anti-PC) have been shown to occur in angiographically verified coronary artery disease and coronary stenosis (24), in diabetes mellitus (7, 26, 31), in early hypertension (28), prior to myocardial infarction (36, 38), and in endometriosis and preeclampsia (41, 46). Many of these conditions have been demonstrated to be associated with periodontal disease, and periodontal disease is thought by some investigators to be a risk factor for these conditions. Furthermore, oral bacterial antigens and DNA have been observed within atheromatous plaques (16), indicating that oral bacteria access the circulation and become trapped within the plaques. The finding that PC is a common component of plaque bacteria and that patients with attachment loss have elevated antibody levels against PC therefore argue that the anti-PC response is a common tie between many systemic conditions and periodontal infections. It is also noteworthy that some investigators have reported that anti-oxLDL antibodies are present in newborns and children and increase during childhood (21). It has been speculated that such antibodies may reflect oxidative damage to LDLs; however, a portion of such antibody could reflect exposure to the oral flora during inflammatory episodes.
As previously noted, anti-oxLDL has been identified in atheromatous lesions and in circulating immune complexes in atherosclerosis and diabetes. Although the functional consequences of such antibodies are not completely clear, is has been suggested that such antibodies enhance LDL cholesterol turnover via opsonization, promote intracellular accumulation of cholesteryl esters in macrophages and fibroblasts (1), and promote cytokine (interleukin β and tumor necrosis factor alpha) production by macrophages (25). Thus, these antibodies could have either a protective or proinflammatory function. The outcome of binding of anti-PC to oxLDL is likely to depend on the balance between the amount of PC-bearing autoantigens in the circulation and in atheromatous plaques and the capacity of phagocytes relative to the antigenic load.
In conclusion, our observations that patients with periodontal attachment loss have elevated anti-PC antibody levels and that a large percentage of dental plaque bacteria contain PC suggest that elevations in serum anti-PC could be the result of exposure of the systemic immune system to oral bacterial antigens. PC and other oral bacterial antigens that are cross-reactive with LDL may therefore provide a biological link between periodontal diseases and cardiovascular diseases, diabetes complications, and adverse pregnancy outcomes, all of which are thought to be influenced by the presence of periodontitis.
ACKNOWLEDGMENTS
This work was supported in part by Public Health Service grant number U19 DE13102 from the National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Alving C R, Wassef N M. Naturally occurring antibodies to cholesterol: a new theory of LDL cholesterol metabolism. Immunol Today. 1999;20:362–366. doi: 10.1016/s0167-5699(99)01496-6. [DOI] [PubMed] [Google Scholar]
- 2.Arbes S J, Slade G D, Beck J D. Association between extent of periodontal attachment loss and self-reported history of heart attack: an analysis of NHANES III data. J Dent Res. 1999;78:1777–1782. doi: 10.1177/00220345990780120301. [DOI] [PubMed] [Google Scholar]
- 3.Beck J, Garcia R, Heiss G, Vokonas P S, Offenbacher S. Periodontal disease and cardiovascular disease. J Periodontol. 1996;67:1123–1137. doi: 10.1902/jop.1996.67.10s.1123. [DOI] [PubMed] [Google Scholar]
- 4.Beck J D, Offenbacher S, Williams R, Gibbs P, Garcia R. Periodontitis: a risk factor for coronary heart disease? Ann Periodontol. 1998;3:127–141. doi: 10.1902/annals.1998.3.1.127. [DOI] [PubMed] [Google Scholar]
- 5.Beck J D, Pankow J, Tyroler H A, Offenbacher S. Dental infections and atherosclerosis. Am Heart J. 1999;138:S528–S533. doi: 10.1016/s0002-8703(99)70293-0. [DOI] [PubMed] [Google Scholar]
- 6.Beck J D, Slade G D. Epidemiology of periodontal diseases. Curr Opin Periodontol. 1996;3:3–9. [PubMed] [Google Scholar]
- 7.Bellomo G, Maggi E, Poli M, Agosta F G, Bollati P, Finardi G. Autoantibodies against oxidatively modified low-density lipoproteins in NIDDM. Diabetes. 1995;44:60–66. doi: 10.2337/diab.44.1.60. [DOI] [PubMed] [Google Scholar]
- 8.Brown M, Schiffman G, Rittenberg M B. Subpopulations of antibodies to phosphocholine in human serum. J Immunol. 1984;132:1323–1328. [PubMed] [Google Scholar]
- 9.Craig W, Poulin S, Nelson C, Ritchie R. ELISA of IgG antibody to oxidized low-density lipoprotein: effects of blocking buffer and method of data expression. Clin Chem. 1994;40:882–888. [PubMed] [Google Scholar]
- 10.Dorn B R, Dunn W A, Jr, Progulske-Fox A. Invasion of human coronary artery cells by periodontal pathogens. Infect Immun. 1999;67:5792–5798. doi: 10.1128/iai.67.11.5792-5798.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.George J, Afek A, Gilburd B, Harats D, Shoenfeld Y. Autoimmunity in atherosclerosis: lessons from experimental models. Lupus. 2000;9:223–227. doi: 10.1191/096120300678828190. [DOI] [PubMed] [Google Scholar]
- 12.George J, Harats D, Shoenfeld Y. Autoimmunity in atherosclerosis. The role of autoantigens. Clin Rev Allergy Immunol. 2000;18:73–86. doi: 10.1385/CRIAI:18:1:73. [DOI] [PubMed] [Google Scholar]
- 13.Gillespie S H, Ainscough S, Dickens A, Lewin J. Phosphorylcholine-containing antigens in bacteria from the mouth and respiratory tract. J Med Microbiol. 1996;44:35–40. doi: 10.1099/00222615-44-1-35. [DOI] [PubMed] [Google Scholar]
- 14.Gillespie S H, McWhinney P H, Patel S, Raynes J G, McAdam K P, Whiley R A, Hardie J M. Species of alpha-hemolytic streptococci possessing a C-polysaccharide phosphorylcholine-containing antigen. Infect Immun. 1993;61:3076–3077. doi: 10.1128/iai.61.7.3076-3077.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gmur R, Thurnheer T, Guggenheim B. Dominant cross-reactive antibodies generated during the response to a variety of oral bacterial species detect phosphorylcholine. J Dent Res. 1999;78:77–85. doi: 10.1177/00220345990780011201. [DOI] [PubMed] [Google Scholar]
- 16.Haraszthy V I, Zambon J J, Trevisan M, Zeid M, Genco R J. Identification of periodontal pathogens in atheromatous plaques. J Periodontol. 2000;71:1554–1560. doi: 10.1902/jop.2000.71.10.1554. [DOI] [PubMed] [Google Scholar]
- 17.Harnett W, Harnett M M. Phosphorylcholine: friend or foe of the immune system? Immunol Today. 1999;20:125–129. doi: 10.1016/s0167-5699(98)01419-4. [DOI] [PubMed] [Google Scholar]
- 18.Havel R, Eder H, Bragdon J. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Investig. 1955;34:1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Horkko S, Binder C J, Shaw P X, Chang M K, Silverman G, Palinski W, Witztum J L. Immunological responses to oxidized LDL. Free Radic Biol Med. 2000;28:1771–1779. doi: 10.1016/s0891-5849(00)00333-6. [DOI] [PubMed] [Google Scholar]
- 20.Hujoel P P, Drangsholt M, Spiekerman C, DeRouen T A. Periodontal disease and coronary heart disease risk. JAMA. 2000;284:1406–1410. doi: 10.1001/jama.284.11.1406. [DOI] [PubMed] [Google Scholar]
- 21.Iughetti L, Volta C, Maggi E, Palladini G, Perugini C, Bellomo G, Bernasconi S. Circulating antibodies recognizing oxidatively modified low-density lipoprotein in children. Pediatr Res. 1999;45:94–99. doi: 10.1203/00006450-199901000-00016. [DOI] [PubMed] [Google Scholar]
- 22.Kearney J F. Immune recognition of OxLDL in atherosclerosis. J Clin Investig. 2000;105:1683–1685. doi: 10.1172/JCI10426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolberg J, Hoiby E A, Jantzen E. Detection of the phosphorylcholine epitope in streptococci, Haemophilus and pathogenic Neisseriae by immunoblotting. Microb Pathog. 1997;22:321–329. doi: 10.1006/mpat.1996.0114. [DOI] [PubMed] [Google Scholar]
- 24.Lehtimaki T, Lehtinen S, Solakivi T, Nikkila M, Jaakkola O, Jokela H, Yla-Herttuala S, Luoma J S, Koivula T, Nikkari T. Autoantibodies against oxidized low density lipoprotein in patients with angiographically verified coronary artery disease. Arterioscler Thromb Vasc Biol. 1999;19:23–27. doi: 10.1161/01.atv.19.1.23. [DOI] [PubMed] [Google Scholar]
- 25.Lopes-Virella M F, Virella G. Cytokines, modified lipoproteins, and arteriosclerosis in diabetes. Diabetes. 1996;45(Suppl. 3):S40–S44. doi: 10.2337/diab.45.3.s40. [DOI] [PubMed] [Google Scholar]
- 26.Lopes-Virella M F, Virella G, Orchard T J, Koskinen S, Evans R W, Becker D J, Forrest K Y. Antibodies to oxidized LDL and LDL-containing immune complexes as risk factors for coronary artery disease in diabetes mellitus. Clin Immunol. 1999;90:165–172. doi: 10.1006/clim.1998.4631. [DOI] [PubMed] [Google Scholar]
- 27.Lowry O, Rosebrough N, Farr A, Randall R. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 28.Maggi E, Marchesi E, Ravetta V, Martignoni A, Finardi G, Bellomo G. Presence of autoantibodies against oxidatively modified low-density lipoprotein in essential hypertension: a biochemical signature of an enhanced in vivo low-density lipoprotein oxidation. J Hypertens. 1995;13:129–138. [PubMed] [Google Scholar]
- 29.Matsuura E, Kobayashi K, Yasuda T, Koike T. Antiphospholipid antibodies and atherosclerosis. Lupus. 1998;7(Suppl. 2):S135–S139. doi: 10.1177/096120339800700230. [DOI] [PubMed] [Google Scholar]
- 30.Meyer D H, Fives-Taylor P M. Oral pathogens: from dental plaque to cardiac disease. Curr Opin Microbiol. 1998;1:88–95. doi: 10.1016/s1369-5274(98)80147-1. [DOI] [PubMed] [Google Scholar]
- 31.Mironova M A, Klein R L, Virella G T, Lopes-Virella M F. Anti-modified LDL antibodies, LDL-containing immune complexes, and susceptibility of LDL to in vitro oxidation in patients with type 2 diabetes. Diabetes. 2000;49:1033–1041. doi: 10.2337/diabetes.49.6.1033. [DOI] [PubMed] [Google Scholar]
- 32.Mosser J L, Tomasz A. Choline-containing teichoic acid as a structural component of pneumococcal cell wall and its role in sensitivity to lysis by an autolytic enzyme. J Biol Chem. 1970;245:287–298. [PubMed] [Google Scholar]
- 33.Palinski W, Horkko S, Miller E, Steinbrecher U P, Powell H C, Curtiss L K, Witztum J L. Cloning of monoclonal autoantibodies to epitopes of oxidized lipoproteins from apolipoprotein E-deficient mice. Demonstration of epitopes of oxidized low density lipoprotein in human plasma. J Clin Investig. 1996;98:800–814. doi: 10.1172/JCI118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Palinski W, Witztum J L. Immune responses to oxidative neoepitopes on LDL and phospholipids modulate the development of atherosclerosis. J Intern Med. 2000;247:371–380. doi: 10.1046/j.1365-2796.2000.00656.x. [DOI] [PubMed] [Google Scholar]
- 35.Progulske-Fox A, Kozarov E, Dorn B, Dunn W, Burks J, Wu Y. Porphyromonas gingivalis virulence factors and invasion of cells of the cardiovascular system. J Periodont Res. 1999;34:393–399. doi: 10.1111/j.1600-0765.1999.tb02272.x. [DOI] [PubMed] [Google Scholar]
- 36.Puurunen M, Manttari M, Manninen V, Tenkanen L, Alfthan G, Ehnholm C, Vaarala O, Aho K, Palosuo T. Antibody against oxidized low-density lipoprotein predicting myocardial infarction. Arch Intern Med. 1994;154:2605–2609. [PubMed] [Google Scholar]
- 37.Romero F I, Khamashta M A, Hughes G R. Lipoprotein(a) oxidation and autoantibodies: a new path in atherothrombosis. Lupus. 2000;9:206–209. doi: 10.1191/096120300678828253. [DOI] [PubMed] [Google Scholar]
- 38.Salonen J T, Yla-Herttuala S, Yamamoto R, Butler S, Korpela H, Salonen R, Nyyssonen K, Palinski W, Witztum J L. Autoantibody against oxidised LDL and progression of carotid atherosclerosis. Lancet. 1992;339:883–887. doi: 10.1016/0140-6736(92)90926-t. [DOI] [PubMed] [Google Scholar]
- 39.Schenkein H A, Barbour S E, Berry C R, Kipps B, Tew J G. Invasion of human vascular endothelial cells by Actinobacillus actinomycetemcomitans via the receptor for platelet-activating factor. Infect Immun. 2000;68:5416–5419. doi: 10.1128/iai.68.9.5416-5419.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenkein H A, Gunsolley J C, Best A M, Harrison M T, Hahn C L, Wu J, Tew J G. Antiphosphorylcholine antibody levels are elevated in humans with periodontal diseases. Infect Immun. 1999;67:4814–4818. doi: 10.1128/iai.67.9.4814-4818.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shanti A, Santanam N, Morales A J, Parthasarathy S, Murphy A A. Autoantibodies to markers of oxidative stress are elevated in women with endometriosis. Fertil Steril. 1999;71:1115–1118. doi: 10.1016/s0015-0282(99)00145-4. [DOI] [PubMed] [Google Scholar]
- 42.Shaw P X, Horkko S, Chang M K, Curtiss L K, Palinski W, Silverman G J, Witztum J L. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Investig. 2000;105:1731–1740. doi: 10.1172/JCI8472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silverman G J, Shaw P X, Luo L, Dwyer D, Chang M, Horkko S, Palinski W, Stall A, Witztum J L. Neo-self antigens and the expansion of B-1 cells: lessons from atherosclerosis-prone mice. Curr Top Microbiol Immunol. 2000;252:189–200. doi: 10.1007/978-3-642-57284-5_20. [DOI] [PubMed] [Google Scholar]
- 44.Slade G D, Offenbacher S, Beck J D, Heiss G, Pankow J S. Acute-phase inflammatory response to periodontal disease in the US population. J Dent Res. 2000;79:49–57. doi: 10.1177/00220345000790010701. [DOI] [PubMed] [Google Scholar]
- 45.Tangada S D, Califano J V, Nakashima K, Quinn S M, Zhang J B, Gunsolley J C, Schenkein H A, Tew J G. The effect of smoking on serum IgG2 reactive with Actinobacillus actinomycetemcomitans in early-onset periodontitis patients. J Periodontol. 1997;68:842–850. doi: 10.1902/jop.1997.68.9.842. [DOI] [PubMed] [Google Scholar]
- 46.Uotila J, Solakivi T, Jaakkola O, Tuimala R, Lehtimaki T. Antibodies against copper-oxidised and malondialdehyde-modified low density lipoproteins in pre-eclampsia pregnancies. Br J Obstet Gynaecol. 1998;105:1113–1117. doi: 10.1111/j.1471-0528.1998.tb09945.x. [DOI] [PubMed] [Google Scholar]
- 47.Vaarala O. Antibodies to oxidised LDL. Lupus. 2000;9:202–205. doi: 10.1191/096120300678828280. [DOI] [PubMed] [Google Scholar]
- 48.Vaarala O. Antiphospholipid antibodies and myocardial infarction. Lupus. 1998;7(Suppl. 2):S132–S134. doi: 10.1177/096120339800700229. [DOI] [PubMed] [Google Scholar]
- 49.Vaarala O. Autoantibodies to modified LDLs and other phospholipid-protein complexes as markers of cardiovascular diseases. J Intern Med. 2000;247:381–384. doi: 10.1046/j.1365-2796.2000.00657.x. [DOI] [PubMed] [Google Scholar]
- 50.Wu T, Trevisan M, Genco R J, Dorn J P, Falkner K L, Sempos C T. Periodontal disease and risk of cerebrovascular disease: the first national health and nutrition examination survey and its follow-up study. Arch Intern Med. 2000;160:2749–2755. doi: 10.1001/archinte.160.18.2749. [DOI] [PubMed] [Google Scholar]
- 51.Wu T, Trevisan M, Genco R J, Falkner K L, Dorn J P, Sempos C T. Examination of the relation between periodontal health status and cardiovascular risk factors: serum total and high density lipoprotein cholesterol, C-reactive protein, and plasma fibrinogen. Am J Epidemiol. 2000;151:273–282. doi: 10.1093/oxfordjournals.aje.a010203. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X, Caligiuri G, Hamsten A, Lefvert A K, Hansson G K. LDL immunization induces T-cell-dependent antibody formation and protection against atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:108–114. doi: 10.1161/01.atv.21.1.108. [DOI] [PubMed] [Google Scholar]