Abstract
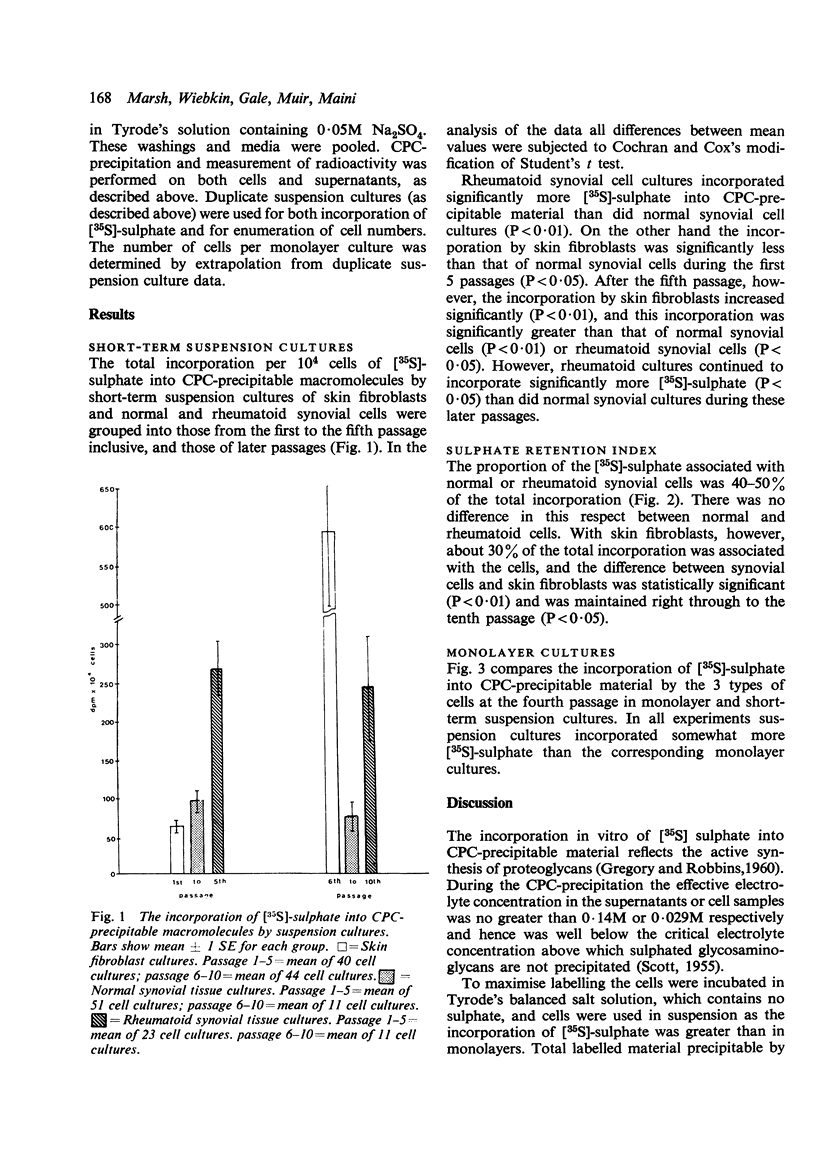
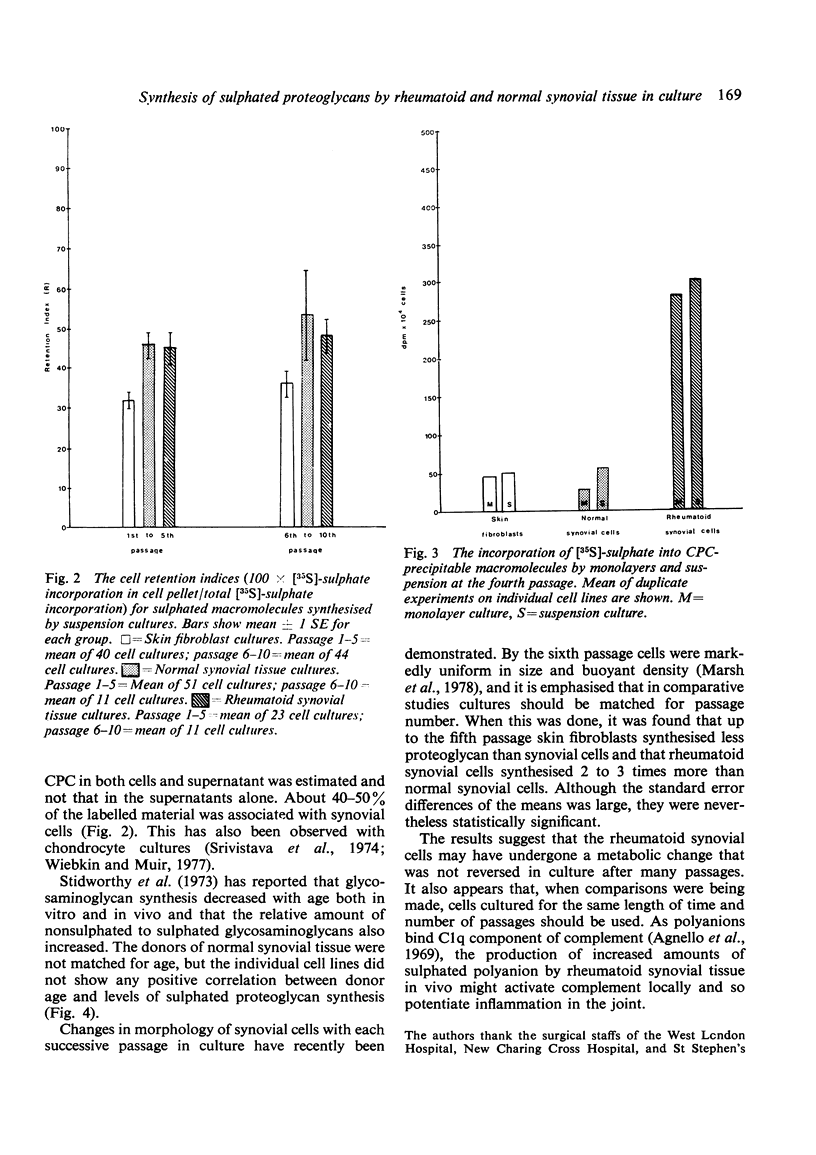
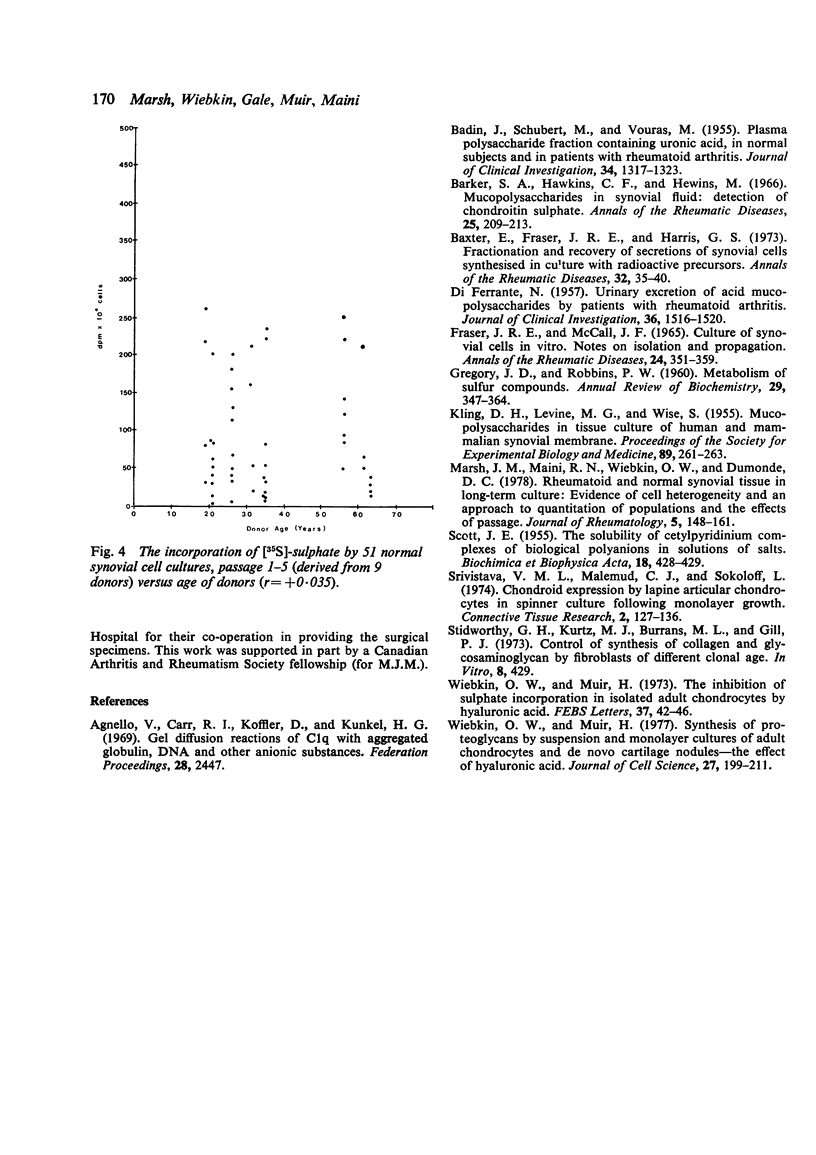
Synthesis of sulphated proteoglycans by cell lines derived from explants of 7 rheumatoid and 9 normal specimens of synovial tissue, as well as by 7 lines of skin fibroblasts from non-rheumatoid patients, was examined. Cells of all 3 types were cultured as monolayers. They were then disaggregated and their capacity to synthesise proteoglycan estimated in cell suspensions by the incorporation of [35S]-sulphate into CPC-precipitable material during 2 hours of incubation. Cell suspensions incorporated somewhat more [35S]-sulphate than corresponding duplicate monolayers. Synovial cells from rheumatoid patients incorporated 2 to 3 times as much [35S]-sulphate as synovial cells from normals. Skin fibroblasts, however, incorporated less [35S]-sulphate than rheumatoid or normal synovial cells up to the fifth passage. Thereafter their incorporation gradually increased to overtake that of synovial cells. About one-half to one-third of the total [35S]-sulphate labelled material was closely associated with cells from synovial tissues and fibroblasts respectively.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BADIN J., SCHUBERT M., VOURAS M. Plasma polysaccharide fraction containing uronic acid, in normal subjects and in patients with rheumatoid arthritis. J Clin Invest. 1955 Aug;34(8):1317–1323. doi: 10.1172/JCI103178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker S. A., Hawkins C. F., Hewins M. Mucopolysaccharides in synovial fluid detection of chondroitin sulphate. Ann Rheum Dis. 1966 May;25(3):209–213. doi: 10.1136/ard.25.3.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DI FERRANTE N. Urinary excretion of acid mucopolysaccharides by patients with rheumatoid arthritis. J Clin Invest. 1957 Oct;36(10):1516–1520. doi: 10.1172/JCI103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J. R., McCall J. F. Culture of synovial cells in vitro. Notes on isolation and propagation. Ann Rheum Dis. 1965 Jul;24(4):351–359. doi: 10.1136/ard.24.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREGORY J. D., ROBBINS P. W. Metabolism of sulfur compounds (sulfate metabolism). Annu Rev Biochem. 1960;29:347–364. doi: 10.1146/annurev.bi.29.070160.002023. [DOI] [PubMed] [Google Scholar]
- KLING D. H., LEVINE M. G., WISE S. Mucopolysaccharides in tissue cultures of human and mammalian synovial membrane. Proc Soc Exp Biol Med. 1955 Jun;89(2):261–263. doi: 10.3181/00379727-89-21777. [DOI] [PubMed] [Google Scholar]
- Marsh J. M., Maini R. N., Wiebkin O. W., Dumonde D. C. Rheumatoid and normal synovial tissue in longterm culture. Evidence of cell heterogeneity and an approach to quantitation of of populations and the effect of passage. J Rheumatol. 1978 Summer;5(2):148–161. [PubMed] [Google Scholar]
- SCOTT J. E. The solubility of cetylpyridinium complexes of biological polyanions in solution of salts. Biochim Biophys Acta. 1955 Nov;18(3):428–429. doi: 10.1016/0006-3002(55)90108-6. [DOI] [PubMed] [Google Scholar]
- Srivastava V. M., MaleMud C. J., Sokoloff L. Chondroid expression by lapine articular chondrocytes in spinner culture following monolayer growth. Connect Tissue Res. 1974;2(2):127–136. doi: 10.3109/03008207409152098. [DOI] [PubMed] [Google Scholar]
- Wiebkin O. W., Muir H. Synthesis of proteoglycans by suspension and monolayer cultures of adult chondrocytes and de novo cartilage nodules-the effect of hyaluronic acid. J Cell Sci. 1977;27:199–211. doi: 10.1242/jcs.27.1.199. [DOI] [PubMed] [Google Scholar]


