Abstract
Information regarding genetic alterations of driver cancer genes in circulating tumour cells (CTCs) and their surrounding immune microenvironment nowadays can be employed as a real-time monitoring platform for translational applications such as patient response to therapeutic targets, including immunotherapy. This study aimed to investigate the expression profiling of these genes along with immunotherapeutic target molecules in CTCs and peripheral blood mononuclear cells (PBMCs) in patients with colorectal carcinoma (CRC). Expression of p53, APC, KRAS, c-Myc, and immunotherapeutic target molecules PD-L1, CTLA-4, and CD47 in CTCs and PBMCs were analysed by qPCR. Their expression in high versus low CTC-positive patients with CRC was compared and clinicopathological correlations between these patient groups were analysed. CTCs were detected in 61% (38 of 62) of patients with CRC. The presence of higher numbers of CTCs was significantly correlated with advanced cancer stages (p = 0.045) and the subtypes of adenocarcinoma (conventional vs. mucinous, p = 0.019), while being weakly correlated with tumour size (p = 0.051). Patients with lower numbers of CTCs had higher expression of KRAS. Higher KRAS expression in CTCs was negatively correlated with tumour perforation (p = 0.029), lymph node status (p = 0.037), distant metastasis (p = 0.046) and overall staging (p = 0.004). CTLA-4 was highly expressed in both CTCs and PBMCs. In addition, CTLA-4 expression was positively correlated with KRAS (r = 0.6878, p = 0.002) in the enriched CTC fraction. Dysregulation of KRAS in CTCs might evade the immune system by altering the expression of CTLA-4, providing new insights into the selection of therapeutic targets at the onset of the disease. Monitoring CTCs counts, as well as gene expression profiling of PBMCs, can be helpful in predicting tumour progression, patient outcome and treatment.
Keywords: circulating tumour cells, KRAS, CTLA-4, immune checkpoint molecules, immune escape mechanism, molecular characterisation
1. Introduction
Circulating tumour cells (CTCs)—the subpopulations of primary tumour cells that are released into the bloodstream—are believed to be the key player in cancer metastases and recurrence [1]. Over the past decades, CTCs have been studied frequently for the clinical management of patients with localized, metastatic and recurrent disease, demonstrating the potential clinical significance of CTC counts in many cancers including colorectal carcinoma (CRC) [2,3]. Immune evasion by cancer cells is one of the major events in tumour progression. In an immunosuppressive setting in circulating blood, CTCs become susceptible to immune surveillance. It is reported that a high number of CTCs can hinder the antitumour immune responses via immune escape pathways, thereby promoting cancer progression [4]. Immune checkpoint inhibitors are important regulators that induce tumour cell immune escape mediated by CTCs [4,5,6].
In addition to CTC detection, molecular characterization of these cells is important to understand their biology, predict metastasis formation and select appropriate therapeutic interventions [7,8]. With the advent of immune checkpoint inhibitors, the cancer treatment paradigm has dramatically changed. On the other hand, accumulating evidence suggested that oncogene- and tumour suppressor gene-dependent signalling pathways might play an important role during the malignant transformation by altering the expression of immune checkpoint molecules [9,10,11,12,13,14,15,16]. For instance, Chen et al. (2017) demonstrated a correlation between high PD-L1 (programmed death-ligand 1) expression and KRAS (Kirsten rat sarcoma viral oncogene) mutation in non-small cell lung carcinoma, suggesting that blocking the programmed cell death protein 1(PD-1)/PD-L1 pathway could be a novel therapeutic option for lung cancer with genetic alteration in KRAS [17].
As CTCs have been studied a great deal as minimally invasive and reliable real-time liquid biomarkers in the clinical management of cancer patients, including CRC, tracking the number of CTCs before, during and after cancer treatment is important to better anticipate outcomes and provide insight into the efficacy of treatments such as molecular targeted therapy and immunotherapy in CRC. In addition, the surrounding haematopoetic cells may have impacts on CTCs. Thus, the gene expression profiling of their surrounding immune microenvironment can be used for translational applications, such as the selection of therapeutic targets, including immunotherapy, and monitoring patient response to treatment.
However, the clinical relevance of cancer cell-intrinsic genetic events that may cause immune failure in patients with CRC during immunotherapeutic application remains largely unknown. More attention to this gap in knowledge is required to evaluate, and thoroughly to discuss, the unique perspective of CTCs and their surrounding immune cells on the relationship between the expression of genes involved in carcinogenesis of CRC and the molecules that involved in immune escape pathways. Therefore, this study aims to explore the expression profiling of the tumour suppressor genes p53 and APC (adenomatous polyposis coli), the oncogenes KRAS and c-Myc and selected immune checkpoints (PD-L1, CTLA-4 (cytotoxic T-lymphocyte-associated protein 4, CD152) and CD47 in CTCs and peripheral blood mononuclear cells (PBMCs) isolated from patients with CRC. The implications of clinicopathological factors in the expressions of these genes were also studied. The clinicopathological correlation between high versus low CTC-positive patients with CRC was also compared.
2. Results
2.1. Enumeration of CTCs in Patients with CRC
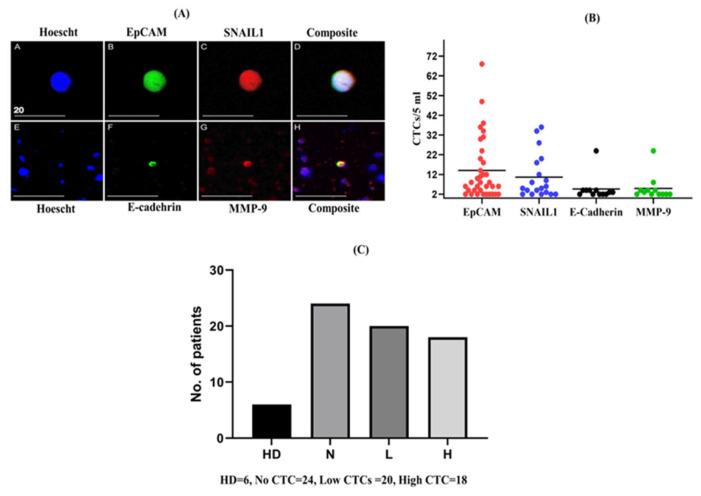
Preliminary experiments had been performed previously, in which different numbers of colon cancer cell lines were added to peripheral blood collected from healthy donors to evaluate the sensitivity and specificity of the CTC enrichment technique (negative selection method) by estimating the recovery rate of CTCs from a 5 mL blood sample [18]. Screening for different subpopulations of CTC was also validated using a panel of six antibodies (EpCAM, CK18, SNAIL1, MMP-9, E-cadherin, and BCL-2) described in detail previously [18]. In this present study, we selected four epithelial and EMT-related markers from the panel of antibodies (EpCAM, SNAIL1, MMP-9 and E-cadherin) to characterise different subpopulations of CTCs in different patients. The detection images of CTCs and marker expression for different subpopulations are presented in Figure 1. Thirty-eight (61%) of the 62 patients with CRC were positive for at least one marker. Among the 38 patients who tested positive, EpCAM was detected in 35 (92%; mean 14.02, range 2–68), SNAIL1 in 19 (50%; mean 10.6, range 2–36), E-cadherin in 11 (28.9%; mean 5.2, range 2–24), and MMP-9 in 11 (28.9%; mean 4.8, range 2–24) patients (Figure 1B). In 38 patients with CTCs, 7 (18.4%) patients were positive for all four markers, 2 (5.3%) for three markers, 13 (34.2%) for two markers, and 16 (42.1%) for one marker. The cells which were positive for at least one of the markers (EpCAM, SNAIL-1, E-cadherin, and MMP-9) and which had an enlarged nucleus and cell size > 8 μm were considered as CTC positive. Among the 62 CRC-positive patients, 24 patients did not express any of the markers (no CTC), while 20 had < 10 CTCs (low CTC group) and 18 showed ≥ 10 CTCs (high CTC group) in their blood samples (Figure 1C). For the downstream analysis of this study, the population was categorized into two groups: low CTC-positive and high CTC-positive. However, cells isolated from the peripheral blood of healthy donors (n = 6) were also screened for CTCs. We found only one healthy donor positive for EpCAM. Although there is no universal standard cut-off value for CTC positivity, to avoid false-positive counts, the cut-off of ≥2 CTCs/5 mL was chosen to define the presence of CTCs as positive, as described in the previous report [19,20]. Previously, Allard et al. observed that whereas eight of the 145 healthy volunteers recruited had one CTC (5.5%), they found that malignant patients had more than one CTC, suggesting that detection of more than two CTCs per 7.5 mL of blood might be unusual [20].
Figure 1.
Enumeration of circulating tumour cells (CTCs) and numbers of different subpopulation of CTCs in patients with colorectal carcinoma (CRC). The figure depicts: (A) representative images of CTCs detected from patients with CRC captured using an Olympus Fluoview FV1000 Confocal Microscope (scale bar: 20 µm); (B) a comparison of the number of different subpopulations of CTCs detected in patients with CRC; and (C) the number of populations recruited in different groups based on the range of CTC counts, along with healthy donors. (HD, healthy donor; N, No CTC; L, low CTC-positive group; H, high CTC-positive group).
While a significant number of leukocytes (up to 3 log depletion rate) were removed during the CTC enrichment step, a substantial number of leukocytes were still detected in the CTC-enriched fractions from patients with CRC. In a subset of 27 patients with CTC, we counted the total numbers of nucleated cells in patients with CRC (n = 27, mean 6101.67, range 2272–14096) and heathy donors (n = 6, mean 4550.8, range 3309–5913) using NIS-element AR imaging software (version 5.20) via Widefield Microscope, Nikon Ti-2. Figure S1 shows the number of contaminating leukocytes in enriched fractions isolated from both healthy donors and patients.
2.2. Correction for Gene Expression by RT-PCR Due to Leukocyte Contamination in CTC-Enriched Fractions
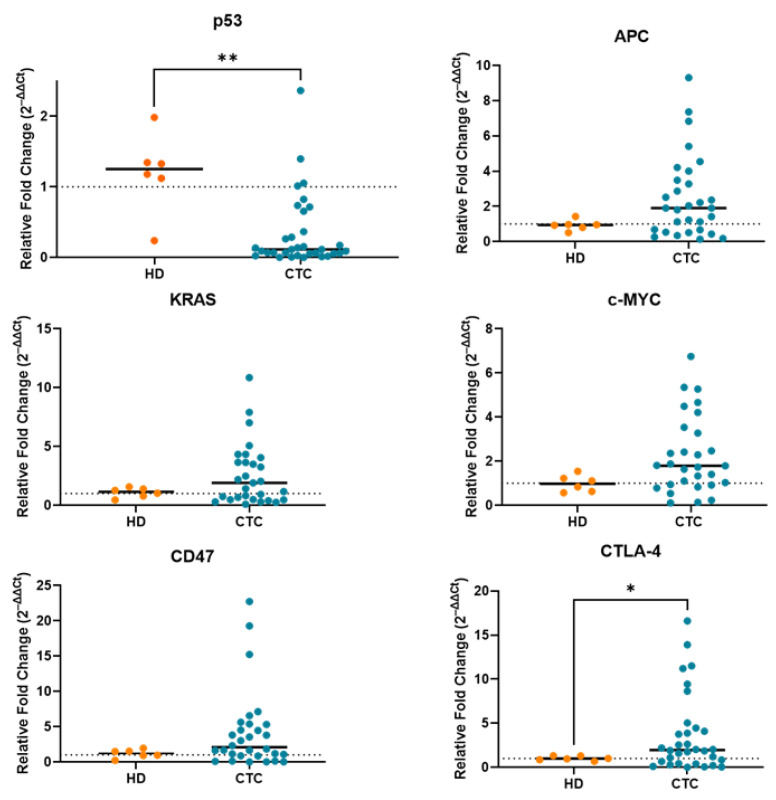
Due to leukocyte contamination, it is obvious that the target genes were still expressed to some extent in CTC-enriched fractions, which may have affected the gene expression level in a low number of CTCs against the thousands of leukocytes that remained after CTC enrichment. To eliminate the effects arising from contaminating cells, we processed 5 mL of blood from healthy donors (n = 6), performed in the same way as previously for peripheral blood samples from patients, and used this as the control. Next, we performed gene expression profiling in CTC-enriched fractions from the blood of patients with CRC and calibrated the results with those of samples prepared from healthy donors. The relative fold change (2–ΔΔCt) of p53, APC, KRAS, c-Myc and CD47, CTLA-4 in CTCs was calculated by subtracting the average delta Ct values derived from the HD group. If the fold change value was more than 1, the genes were considered as positively expressed. All the target genes except p53 were expressed at lower levels in the blood of healthy donors compared with that in CTC-enriched fractions from patients with CRC (Figure 2). We also evaluated the expression level of CD45 gene (PTPRC) in CTC-enriched fractions, which is a generic leukocyte marker, indicating the presence of contamination by leukocytes. Expression of CD45 was noted to be lower in the CTC-enriched fractions (Figure S2).
Figure 2.
Relative fold change values (2–ΔΔCt) of p53, APC, KRAS, c-Myc and CD47, CTLA-4 in CTC-enriched fraction from patients with CRC and from healthy donors (HDs) (n = 6). All the values are plotted as a scatter plot with the median. Line indicates the normal fold change value. ** p < 0.005, * p < 0.05.
2.3. Gene Expression Profile of CTC-Fractions and PBMCs
Finally, we performed mRNA expression analysis of target genes in CTC-enriched fractions and PBMCs. The Ct values of < 35 for all target genes, and < 30 for housekeeping genes, were included for the gene expression analysis. Of the 38 CTC-positive patients, 8 patients were excluded from further research analysis who had no expression or lower expression because of poor mRNA quality in CTC-enriched fractions.
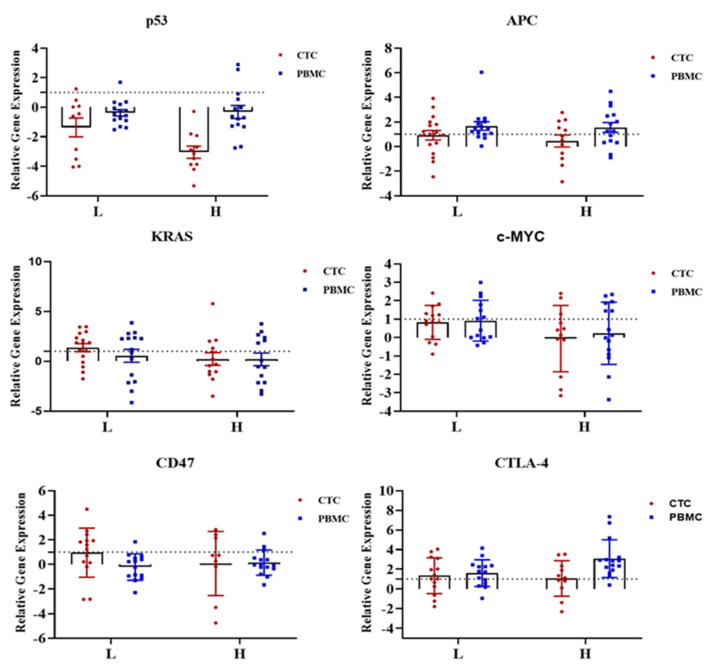
In this study, we found increasing KRAS and CTLA-4 expressions in the low CTC-positive group and decreasing p53, APC, c-Myc and CD47 mRNA expressions in both high and low CTC-positive groups when compared with that of healthy donors (Figure 3). However, we found very few CTC-positive patients expressing PD-L1, hence we excluded PD-L1 from further analysis. We also analysed gene expression in PBMC samples from patients with CRC using the same panel of genes that were used for CTCs. Decreased expression was noted for most of the genes in the matched PBMC samples compared with that in CTCs, while higher expression of APC and CTLA-4 in both groups of patients was noted (Figure 3).
Figure 3.
Comparison of the gene expression of oncogenes (KRAS, c-Myc), tumour suppressor genes (p53, APC) and immune checkpoint molecules (CTLA-4, CD47) between high versus low CTC-positive groups in CTCs and PBMCs from patients with colorectal carcinoma (CRC). Data are depicted as scatter plots interleaved with bar plots, indicating min. to max. value. All the values are plotted as mean ± SEM. The dashed line indicates the normal fold change value. The PCR data were shown on a log2 scale and analysed by unpaired two-way ANOVA (Bonferroni’s multiple comparison test). Comparisons were considered significant at p ≤ 0.05.
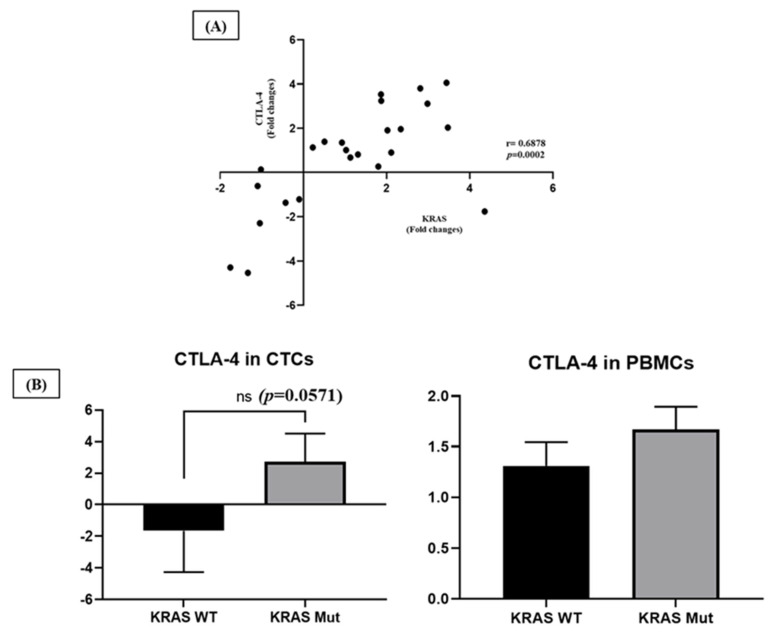
Next, we compared the expression levels of p53, APC, KRAS and c-Myc with CD47 and CTLA-4 between CTCs and PBMCs. Interestingly, the mRNA expression of CTLA-4 was positively correlated with KRAS (r = 0.6878, p = 0.0002) expression in CTCs (Figure 4A). In addition, CTLA-4 gene expression level tended to be higher in CTCs and PBMCs in patients with KRAS mutation than in patients with KRAS wild type (data obtained from Gold Coast University Hospital and detected by next-generation sequencing on cancer tissue) (Figure 4B).
Figure 4.
Correlation between KRAS gene and CTLA-4 expression in CTCs. (A) The mRNA expression level of CTLA-4 correlated positively with KRAS (r = 0.6878, p = 0.0002). r; coefficient correlation value (Spearman’s rank test). (B) The mRNA expression levels of CTLA-4 in CTCs and PBMCs from patients with CRC according to the KRAS mutation status of the primary tumour.
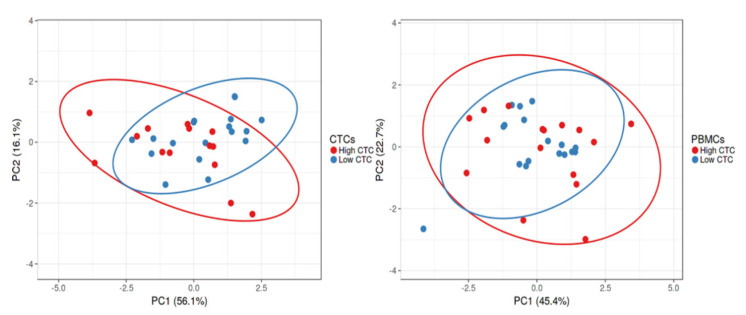
A PCA plot was derived from normalised gene expression data to show variation between high and low CTC groups and PBMCs (Figure 5). However, we found no significant variation in mRNA expression level between these two groups.
Figure 5.
Relationship of the gene expressions (P53, APC, KRAS, c-Myc, CD47 and CTLA-4) between high and low CTC groups in CTCs and PBMCs, in patients with CRC.
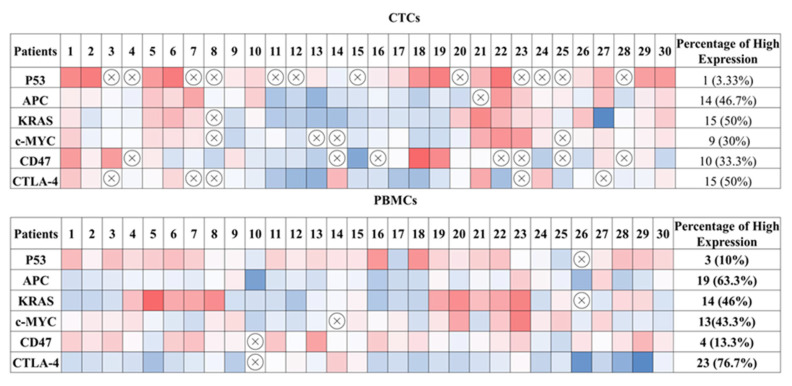
In addition, heat map imaging of the gene expression data was generated to demonstrate the heterogeneity of gene expression in CTC fractions and PBMCs, showing variable expression pattern and the percentage of positive expression among individual patients with CRC (Figure 6).
Figure 6.
Heat map depicting the mRNA expression level and percentage of positive expression of the tumour suppressor genes p53 and APC; the oncogenes, KRAS and c-Myc; and the immune-regulatory molecules CD47 and CTLA-4 in CTCs and PBMCs among individual patients. The values were calculated from the log2 value of the relative quantification of each gene. The colour indicates the expression level for each gene. Red fields represent downregulated genes; blue fields represent upregulated genes; crossed-out fields represent no expression.
2.4. Clinical and Pathological Correlations
The correlations between patients’ clinical characteristics and different groups of CTCs based on CTC counts are presented in Table 1. Approximately 64% (7/11) of patients with mucinous adenocarcinoma showed high levels of CTCs, while patients with conventional adenocarcinomas were often presented with lower levels of CTCs (41%, 21/51) (p = 0.019). Additionally, patients with advanced pathological stages (stages III or IV) reported high levels of CTC count when compared with those with early-stage (stage I or II) CRC (44% vs. 21%). Conversely, the high prevalence of zero or low CTC counts was noted among patients with early-stage CRC (44% vs. 30% and 36% vs 27%, respectively) (p = 0.045). In addition, half of the patients with higher CTC counts (9/18) had larger tumour sizes (50 mm or above), while approximately 80% of patients (35/44) with no or low CTC counts had smaller tumour sizes (below 50 mm) (p = 0.051). On the other hand, the number of CTCs had no association with the age or gender of the patients, or with the grade or microsatellite instability (MSI) status of the tumour.
Table 1.
The correlations of high vs. low CTC-positive groups with clinicopathological features in patients with CRC.
| Characteristics | Total (n = 62) | CTC = 0 (n = 24) | CTC < 10 (n = 20) | CTC ≥ 10 (n = 18) | p-Value |
|---|---|---|---|---|---|
| Gender | |||||
| Female | 27 (43.55%) | 12 (44.44%) | 10 (37.04%) | 5 (18.52%) | 0.266 |
| Male | 35 (56.45%) | 12 (34.29%) | 10 (28.57%) | 13 (37.14%) | |
| Age | |||||
| ≤60 years | 13 (20.97%) | 4 (30.77%) | 6 (46.15%) | 3 (23.08%) | 0.483 |
| >60 years | 49 (79.03%) | 20 (40.82%) | 14 (28.57%) | 15 (30.61%) | |
| Size | |||||
| ≤50 mm | 44 (70.98%) | 19 (43.18%) | 16 (36.36%) | 9 (20.45%) | 0.051 |
| >50 mm | 18 (29.03%) | 5 (27.78%) | 4 (22.22%) | 9 (50%) | |
| Tumour perforation | |||||
| No | 58 (93.5%) | 23 (39.7%) | 20 (34.5) | 15 (25.9%) | 0.077 |
| With perforation | 4 (6.5%) | 1 (25%) | 0 (0.00%) | 3 (75%) | |
| Site | |||||
| Colon | 44 (70.97%) | 18 (40.91%) | 12 (27.27%) | 14 (31.82%) | 0.414 |
| Rectum | 18 (29.03%) | 6 (33.33%) | 8 (44.44%) | 4 (22.22%) | |
| Subtype | |||||
| Conventional | 51 (82.25%) | 21 (41.18%) | 19 (37.25%) | 11 (21.57%) | 0.019 |
| Mucinous | 11 (17.74%) | 3 (27.27%) | 1 (9.09%) | 7 (63.64%) | |
| Grade | |||||
| Well | 11 (17.74%) | 5 (45.45%) | 4 (36.36%) | 2 (18.18%) | 0.810 |
| Moderate | 46 (74.19%) | 18 (39.13%) | 14 (30.43%) | 14 (30.43%) | |
| Poor | 5 (8.06%) | 1 (20%) | 2 (40%) | 2 (40%) | |
| T stage | |||||
| I or II | 20 (32.26%) | 9 (45%) | 8 (40%) | 3 (15%) | 0.128 |
| III or IV | 42 (67.74%) | 15 (35.71%) | 12 (28.57%) | 15 (35.71%) | |
| Overall pathological stage | |||||
| I or II | 39 (62.9%) | 17 (43.59%) | 14 (35.90%) | 8 (20.51%) | 0.045 |
| III or IV | 23 (37.10%) | 7 (30.43%) | 6 (26.09%) | 10 (43.48%) | |
| Lymph node status | |||||
| Positive | 20 (32.26%) | 6 (30%) | 5 (25%) | 9 (45%) | 0.104 |
| Negative | 42 (67.74%) | 18 (42.86%) | 15 (35.71%) | 9 (21.43%) | |
| Distant metastasis | |||||
| Positive | 9 (14.52%) | 2 (22.22%) | 2 (22.22%) | 5 (55.56%) | 0.090 |
| Negative | 53 (85.48%) | 22 (30.2%) | 18 (69.8%) | 13 (24.53%) | |
| MSI status | |||||
| Stable | 52 (83.87%) | 20 (38.46%) | 16 (30.77%) | 16 (30.77%) | 0.643 |
| High | 10 (16.13%) | 4 (40%) | 4 (40%) | 2 (20%) |
Clinical correlations were also evaluated with the mRNA expression level of tested genes. Samples that had no expression were excluded from the clinical analysis. Among those exhibiting mRNA expression, we found significant pathophysiological correlations between KRAS expression in CTCs. Table 2 shows the correlation of the mRNA expression of KRAS in CTCs with the clinical and pathological factors in patients with CRC. High expression of KRAS mRNA was predominantly seen among patients with early-stage compared with those with advanced-stage CRC (75% versus 25%, p = 0.004). Approximately 69% of patients having low CTCs had higher expression of the KRAS gene (approx. 69%, versus 31%, p = 0.039). KRAS gene expression was negatively correlated with lymph node metastasis and distant metastasis (approx. 67% versus 27%, p = 0.037, 61% versus 17%, p = 0.046). Around 58% (15/26) of patients without perforated CRC adenocarcinoma had high expression of the KRAS gene compared with those with cancer that showed perforation (p = 0.029).
Table 2.
The correlations of KRAS gene expression levels in CTCs with clinicopathological features in patients with CRC.
| Characteristics | Total (29) | Low | High | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 11 (37.9%) | 4 (36.4%) | 7 (63.6%) | 0.313 |
| Male | 18 (62.1%) | 10 (55.6%) | 8 (44.4%) | |
| Age | ||||
| ≤60 years | 9 (31%) | 3 (33.3%) | 6 (66.7%) | 0.276 |
| >60 years | 20(69%) | 11 (55%) | 9 (45%) | |
| Size | ||||
| ≤50 mm | 21 (72.4%) | 8 (38.1%) | 13 (61.9%) | 0.071 |
| >50 mm | 8 (27.6%) | 6 (75.0%) | 2 (25.0%) | |
| Tumour perforation | ||||
| No | 26 (89.7%) | 11 (42.3%) | 15 (57.75%) | 0.029 |
| With perforation | 3 (10.3%) | 3 (100%) | 0 (0.0%) | |
| Site | ||||
| Colon | 16 (55.2%) | 9 (56.3%) | 7 (43.8%) | 0.339 |
| Rectum | 13 (44.8%) | 5 (38.5%) | 8 (61.5%) | |
| Grade | ||||
| Well | 6 (20.7%) | 2 (33.3%) | 4 (66.7%) | 0.082 |
| Moderate | 20 (69.0%) | 9(45.0%) | 11 (55.0%) | |
| Poor | 3 (10.3%) | 3 (100%) | 0 (0.0%) | |
| T stage | ||||
| I or II | 9 (31.0%) | 5 (55.6%) | 4 (44.4%) | 0.599 |
| III or IV | 20 (69.0%) | 9 (45.0%) | 11 (55.0%) | |
| Lymph node status | ||||
| Negative | 18 (62.1%) | 6 (33.3%) | 12 (66.7%) | 0.037 |
| Positive | 11 (37.9%) | 8 (72.7%) | 3 (27.3%) | |
| Distant metastasis | ||||
| Negative | 23 (79.3%) | 9 (39.1%) | 14(60.9%) | 0.046 |
| Positive | 6 (20.7%) | 5 (83.3%) | 1 (16.7%) | |
| Overall stage | ||||
| I or II | 16 (55.2%) | 4 (25.0%) | 12 (75.0%) | 0.004 |
| III or IV | 13 (44.8%) | 10 (76.9%) | 3 (23.1%) | |
| MSI status | ||||
| Stable | 26 (89.7%) | 12 (46.2%) | 14 (53.8%) | 0.498 |
| High | 3 (10.3%) | 2 (66.7%) | 1 (33.3%) | |
| CTC group | ||||
| Low | 16 (55.2%) | 5 (31.3%) | 11 (68.8%) | 0.039 |
| High | 13 (44.8%) | 9 (69.2%) | 4 (30.8%) |
Conversely, in PBMCs, patients with high KRAS expression were less likely to have a small tumour size. However, we did not find any significant clinical associations. In addition, we found that higher CTLA-4 expression in CTCs was weakly correlated with those cancers with the KRAS mutant (p = 0.06) (Figure 4B), while in PBMCs, CTLA-4 was more likely have higher expression in patients detected with high CTC counts (64% vs. 93%, p = 0.046) and with lymph node metastasis (65% vs. 100%, p = 0.006) (Table 3). CTLA-4 expression was also correlated with pathological stages (63% vs 100%, p = 0.004). The expression levels of other genes did not significantly correlate with clinical or pathological features.
Table 3.
The correlations of CTLA-4 gene expression levels in PBMCs with clinicopathological features in patients with colorectal carcinoma (CRC).
| Characteristics | Total (n = 29) | Low | High | p-Value |
|---|---|---|---|---|
| Gender | ||||
| Female | 11 (37.9%) | 4 (36.4%) | 7 (63.6%) | 0.104 |
| Male | 18 (62.1%) | 2 (11.1%) | 16 (88.9%) | |
| Age | ||||
| ≤60 years | 8 (27.6%) | 3 (37.5%) | 5 (62.5%) | 0.185 |
| >60 years | 21(72.4%) | 3 (14.3%) | 18 (85.7%) | |
| Size | ||||
| ≤50 mm | 20 (69.0%) | 4 (20.0%) | 16 (80.0%) | 0.892 |
| >50 mm | 9 (31.0%) | 2 (22.2%) | 7 (77.8%) | |
| Tumour perforation | ||||
| No | 26 (89.7%) | 6 (23.1%) | 20 (76.9%) | 0.224 |
| With perforation | 3 (10.3%) | 0 (0.0%) | 3 (100.0%) | |
| Site | ||||
| Colon | 18 (62.1%) | 4 (22.2%) | 14 (77.8%) | 0.793 |
| Rectum | 11 (37.9%) | 2 (18.2%) | 9 (81.8%) | |
| Grade | ||||
| Well | 5 (17.2%) | 1 (20.0%) | 4 (80.0%) | 0.355 |
| Moderate | 20 (69.0%) | 5 (25.0%) | 15 (75.0%) | |
| Poor | 4 (13.8%) | 0 (0.0%) | 4 (100.0%) | |
| T stage | ||||
| I or II | 12 (41.40%) | 4 (33.3%) | 8 (66.7%) | 0.160 |
| III or IV | 17 (58.6%) | 2 (11.8%) | 15 (88.2%) | |
| Lymph node status | ||||
| Negative | 17 (58.6%) | 6 (35.3%) | 11 (64.7%) | 0.006 |
| Positive | 12 (41.4%) | 0 (0.0%) | 12 (100.0%) | |
| Distant metastasis | ||||
| Negative | 23 (79.3%) | 6 (26.1%) | 17 (73.9%) | 0.213 |
| Positive | 6 (20.7%) | 0 (0.0%) | 6 (100.0%) | |
| Overall stage | ||||
| I or II | 16 (55.2%) | 6 (37.5%) | 10 (62.5%) | 0.004 |
| III or IV | 13 (44.8%) | 0 (0.0%) | 13 (100.0%) | |
| MSI status | ||||
| Stable | 25 (86.2%) | 4 (16.0%) | 21 (84.0%) | 0.180 |
| High | 4 (13.8%) | 2 (50.0%) | 2 (50.0%) | |
| CTC group | ||||
| Low | 14 (48.3%) | 5 (35.7%) | 9 (64.3%) | 0.046 |
| High | 15 (51.7%) | 1 (6.7%) | 14 (93.3%) |
3. Discussion
The identification of CTCs and their specific gene profiles could offer new perspectives that may improve the prediction of metastasis formation, as well as representing a promising approach for determining better therapeutic targets. Previous studies have reported a significant correlation between CTC counts and pathological stages of various cancers, including CRC [2,18,19,21,22,23,24,25]. In this study, we noted that late-stage cancers more often had higher CTC counts when compared with early-stage cancers (p = 0.045). Patients with mucinous adenocarcinoma, a specific subtype of CRC characterized by over 50% tumour volume composed of extracellular mucin [26], were likely to have high levels of CTCs. In addition, CTC levels were higher in patients with larger tumours. It has been previously reported that patients with colorectal mucinous adenocarcinoma often present with advanced pathological stages and a larger tumour size compared with conventional CRC [27]. Taken together, these clinical correlations imply that a relatively high number of CTCs associated with pathological features of cancer patients can predict tumour aggressiveness and may become more pronounced over time. However, patients with distant metastases were not associated with high CTC counts, implying that the low number of metastatic patients in the study may be the contributing factor for this discrepancy. We did not perform survival analysis in this study due to the limited follow up time.
The immune-suppressive microenvironment is significantly involved in CRC carcinogenesis [17]. Immune checkpoint molecules, such as PD-L1, CTLA-4 and CD47, are important regulators that induce tumour cell immune escape [5,6,7]. Immunotherapy using immune checkpoint inhibitors (ICIs) has revolutionised the treatment of many cancers [28]. However, cancer-causing genetic abnormalities determine the tumour immunological context and significantly contribute to therapeutic resistance, including immunotherapy [29]. In this study, we noted that the upregulation of the proto-oncogene KRAS was more prevalent in patients with low CTC counts. Significant correlation with clinical parameters (pathological stage, distant metastasis, lymph node status, perforation) was also noted, which is in line with a previous study suggesting that activation of the KRAS gene may be more prevalent and could be a significant prognostic factor in patients with early-stage cancer [30]. It is worth noting that decreased expression of p53 and APC expression lowers the tumour-suppressive capability of a cell and leads to cell cycle dysregulation and uncontrolled cell growth [31,32]. Further, reduced expression of c-Myc was noted and is well in line with other reports [33,34]. Steinert et al. noted that downregulation of c-Myc may indicate the state of dormancy of CTCs (if Ki-67 expression is also low) [34]. Though no significant variations were found between high vs. low CTC-positive groups, possibly due to the extensive heterogenous nature of CTCs [35], we found comparatively higher expression of these genes in patients detected with low CTC counts. Taken together, our findings suggest that changes in the mRNA expression level of tumour suppressor genes and oncogenes, especially KRAS in CTCs, may become more aggressive at the early stages; thus, CTCs could play a significant role in predicting targeted therapy at the onset of the disease.
As the majority of patients with CRC are MSI-stable, discovering novel immunotherapeutic targets are vital in improving the efficacy of immunotherapy [9]. For the first time, we investigated CTLA-4 gene expression in CTCs in CRC, which is usually expressed in immune cells [36]. In a recent study, CTLA-4 expression was evaluated in CRC tissues and different cancer cell lines (HT-29, HCT-166, and SW480) [37]. Only one report found CTLA-4 expression in CTCs in metastatic prostate cancer (mPC), which was rare [6]. Interestingly, we found overexpression of CTLA-4 in patients detected with lower CTC count. In addition, a significant upregulation of CD47 in CTCs plays a potential role in immune escape and thus may also promote the spread of CRC and enhances the stemness of cancer cells [34,38]. However, our data showed decreasing expression level of CD47 in patients with CRC compared with healthy donors, though positive expression were seen in a number of individual patients. This may have happened because of the heterogeneous characteristics of CTCs [35]. The above findings may suggest that CTLA-4 is also expressed in CTCs along with other immune checkpoint molecules, so blocking these inhibitory molecules could improve their therapeutic efficacy. However, additional studies would help to confirm these findings.
Current research evidence suggests a significant influence of genetic alterations of tumour suppressor genes and oncogenes in controlling tumour–immune system crosstalk in a variety of malignancies by modulation of the expression of immune checkpoint molecules [10,11,12,13,14,16,17,39]. It is suggested that these driver cancer genes may directly bind to the promoters of immune checkpoint molecules, thereby altering their expression [11]. The positive correlation between CTLA-4 and KRAS expression, and the higher CTLA-4 gene expression in CTC-positive patients having KRAS mutation, therefore, suggest that activation of KRAS may aid CTCs in evading immune surveillance by modifying the expression of CTLA-4.
Due to the challenges in isolating and identifying rare CTCs from excessive background cells in peripheral blood, the gene expression profiling of PBMCs could be another hallmark in the clinical management of cancer patients [33]. Interestingly, our data showed a differential expression pattern for the tested genes in PBMCs compared with CTCs. Positive expression of CD47 was noted in a few patients, while APC and CTLA-4 positive expression levels were higher in PBMCs than those in CTCs. Thus, the above findings and the pathophysiological correlations between CTLA-4 gene expression levels suggest that haematopoietic cells may regulate the expression of these genes, providing important information in the clinical management of patients with CRC.
It is already known that immune checkpoint molecules are expressed not only in tumour cells but also in a wide variety of haematopoietic cells [36,40], and we acknowledge that there was still a considerable number of leukocytes in the CTC enrichment fraction obtained from peripheral blood of patients with CRC, which might have affected the gene expression profiling of CTCs. Hence, gene expression profiling of single CTCs would be more beneficial in providing more accurate information. Nowadays, the molecular profiling of single CTCs in different cancers, including colorectal carcinomas, is receiving more attention [34,35,41,42]. As this study is an ongoing project, we have obtained some preliminary results with single CTCs isolated from individual patients, which further confirm our findings. Detailed studies are needed to validate and confirm these findings at single-cell level.
Nevertheless, this study provides a preliminary concept for understanding that alteration in oncogene KRAS expression may regulate the expression of immune checkpoint molecules, which has a direct role in the initiation and maintenance of cancer gene-driven tumorigenesis. KRAS overexpression may be one general mechanism by which tumour cells upregulate the expression of the immune checkpoint regulator CTLA-4, thereby evading immune surveillance. Additional molecular biology investigations in a large cohort may be necessary to confirm and to elucidate the mechanism underlying this hypothesis. This study also revealed that CTC detection and gene expression profiling of PBMCs, especially for immune regulatory genes, can be another platform to study the cellular heterogeneities, resistance mechanisms and therapeutic targets in cancer.
4. Materials and Methods
4.1. Patients
A total of sixty-two patients (35 males, 27 females) with pathologically confirmed CRC and six healthy individuals were prospectively recruited from Gold Coast University Hospital during the period of May 2017 to November 2021 for this study. Ethical approval was obtained from the Griffith University Human Research Ethics Committee (GU Ref No: MSC/17/10/HREC). These patients signed a written informed consent form before participating in the study. Clinical and pathological parameters, including age and gender of patient, as well as the size, location, histological subtype, and microsatellite instability (MSI) status of the patient’s tumour, as detected by immunohistochemistry and pathological staging, were recorded as previously reported [43]. Blood samples were collected from the patient on the day of resection and were processed within one hour of collection. From each of these patients, 15 mL of peripheral blood was collected in heparin-containing BD (Becton Dickinson, Franklin Lakes, NJ, USA) vacutainer tubes at the time of surgery for CRC.
4.2. Enrichment and Isolation of CTCs
In this study, 5 mL of freshly collected blood from each patient was enriched for CTC isolation using a negative selection method (EasySepTM Direct Human CTC Enrichment Kit, STEMCELL Technologies., Vancouver, BC, Canada) according to the manufacturer’s protocol. Briefly, blood was incubated for 10 min twice with a cocktail of different antibody-labelled magnetic beads targeting CD2, CD14, CD16, CD19, CD45, CD61, CD66b, and glycophorin A surface markers at room temperature, allowing the enriched cell suspension collection in a new tube to obtain a pure suspension of CTCs. The enriched CTCs were centrifuged at 450 rcf (relative centrifugal force) for 7 min and resuspended in a CTC growth medium. Then, the enriched CTCs were seeded in a 96-well plate (50 µL per well) for immunofluorescence and in a 6-well plate for downstream analysis, followed by overnight incubation. The composition of CTC growth medium was described in a previously published article [18]. Peripheral blood samples from 6 healthy donors were processed as negative controls in the same way as previously performed for patients with CRC.
4.3. Immunofluorescence Staining
Immunofluorescence staining was performed for the identification of CTCs using a cocktail of four primary antibodies for EpCAM (Thermo Fisher Scientific, Waltham, MA, USA), SNAIL1, E-cadherin and MMP-9 (Santa Cruz Biotechnology, CA, USA) as described previously [18]. In brief, the enriched cells were fixed with 100% methanol for 10 min at −20 °C and were permeabilised with 0.2% Triton X-100 for 10 min. Cells were then stained with primary antibodies followed by secondary antibodies: rabbit-anti-mouse IgG fluorescein isothiocyanate (FITC) and rabbit anti-goat IgG (H + L) Texas Red (Sigma Aldrich, St. Louis, MO, USA), and labelled with Hoechst 33,342 (ThermoFisher Scientific, Waltham, MA, USA) to stain the nucleus. The cells were counted using a Nikon Ti2 widefield microscope (Nikon Corporation, Tokyo, Japan). The size and fluorescent intensity of CTCs were measured using Nikon NIS-element AR imaging software, version 5.20 (Nikon Corporation, Tokyo, Japan). The number of total nucleated events was also counted using similar software to visualise, annotate and quantify the contaminating cells. High-resolution images were captured using an Olympus Fluoview FV1000 Confocal Microscope (Shinjuku, Tokyo, Japan) at 40× magnification. To avoid the overlapping results between the primary antibodies, we checked for possible cross-species binding of selected secondary antibodies (Figure S3).
4.4. Isolation of Peripheral Blood Mononuclear Cells (PBMCs)
PBMCs were isolated from 5 mL peripheral blood using Histopaque®-1077 (Sigma Aldrich, St. Louis, MO, USA) gradient centrifugation, following the manufacturer’s guidelines. Briefly, 7 mL of Histopaque was pipetted into a 15 mL falcon tube. The blood sample was carefully layered over the Histopaque gradient and then centrifuged at 400 rcf for 30 min at room temperature. The PBMC layer was collected, and the cell pellet was washed twice in PBS-EDTA (ethylenediaminetetraacetic acid) (10 min/250 rcf at room temperature). The cell pellet was resuspended in RPMI stocking media and stored at −80 °C for further analysis.
4.5. RNA Extraction and cDNA Conversion
The total RNA from CTC fractions and PBMCs was extracted using the RNeasy mini kit (Qiagen, Hilden, North Rhine-Westphalia, Germany) according to the manufacturer’s instructions. DNase was used to remove contaminating genomic DNA from the RNA sample.
cDNA synthesis was performed using the SensiFAST cDNA synthesis kit (Meridian Bioscience, Cincinnati, OH, USA) following the manufacturer’s guidelines. The resulting cDNA was diluted in nuclease-free water to a final concentration of 100 ng/μL and stored at –20 °C. The values for cDNA and RNA purity (260/280 ratio) and concentration (ng/μL) were measured using a nanoDrop (BioLab, Milford, MA, USA) spectrophotometer.
4.6. Quantitative Real-Time Polymerase Chain Reaction
Before performing gene expression analysis, we validated the technical feasibility of qRT-PCR (Figure S4). The pre-amplified products were then analysed for target gene expression using real-time quantitative PCR (qPCR) (QuantStudio, Thermo Fisher Scientific, MA, USA). qPCR was performed using the SensiFAST SYBR No-ROX kit (Meridian Bioscience) according to the manufacturer’s protocol. A total of nine primers for the 7 targets, PTPRC (CD45) and ACTB (β-actin) as endogenous control were purchased from Sigma Aldrich. The list and sequence of chosen primer sets are summarized in Table S1. The relative gene expression levels of target genes were estimated as log2 value of the fold change by the relative quantification 2−ΔΔCt method. Fold changes were calculated as previously reported [44,45].
4.7. Statistical Analysis
The statistical analyses of gene expression levels were performed using GraphPad Prism Software 5.03 (GraphPad Software Inc., San Diego, CA, USA). The Kolmogorov–Smirnov non-parametric test was used to compare fold changes between the HD and CTC groups. A two-way ANOVA test (Bonferroni’s multiple comparisons test) was used to compare the various groups of CTCs and PBMCs based on the quantification of CTCs. The values were estimated from the log2 value of the relative quantification of each gene. Spearman’s rank test was performed to check the correlations of p53, APC, KRAS and c-Myc gene expression levels with CD47 and CTLA-4 in CTCs and PBMCs. Principal component analysis (PCA) plots of gene expression data in different groups were generated with log2 transformation of the data with a 95% confidence interval using the ClustVis web tool (https://biit.cs.ut.ee/clustvis/, accessed on 29 August 2022). Association of patient groups based on CTC numbers and gene expression level against clinicopathological parameters of each patient’s cohort were performed using IBM SPSS (Statistical Package for the Social Sciences) statistics, version 29 (International Business Machines, Armonk, NY, USA). The Chi-square test or likelihood ratio was used for categorical variables. A p-value of < 0.05 was considered statistically significant.
Acknowledgments
The authors would like to acknowledge the School of Medicine and Dentistry, Griffith University for the HDR scholarship. The authors also wish to thank other members from our group for their valuable suggestions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/1422-0067/24/5/5051/s1.
Author Contributions
Conceptualization, methodology, investigation, data interpretation, and writing—original draft preparation: S.A.; sample collection, methodology, and validation: F.B.H.; data acquisition: S.M.K.G., T.C., N.P. and C.T.L.; supervision, conceptualization, and writing—review and editing: F.I., V.G. and A.K.-y.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data presented in this study are available in Gene expression analysis of immune regulatory genes in circulating tumour cells and peripheral blood mononuclear cells in patients with colorectal carcinoma and its Supplementary Material within.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Pantel K., Alix-Panabières C., Riethdorf S. Cancer micrometastases. Nat. Rev. Clin. Oncol. 2009;6:339–351. doi: 10.1038/nrclinonc.2009.44. [DOI] [PubMed] [Google Scholar]
- 2.Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Matera J., Miller M.C., Reuben J.M., Doyle G.V., Allard W.J., Terstappen L.W. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 2004;351:781–791. doi: 10.1056/NEJMoa040766. [DOI] [PubMed] [Google Scholar]
- 3.Allen J.E., El-Deiry W.S. Circulating tumor cells and colorectal cancer. Curr. Colorectal Cancer Rep. 2010;6:212–220. doi: 10.1007/s11888-010-0069-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kallergi G., Vetsika E.-K., Aggouraki D., Lagoudaki E., Koutsopoulos A., Koinis F., Katsarlinos P., Trypaki M., Messaritakis I., Stournaras C., et al. Evaluation of PD-L1/PD-1 on circulating tumor cells in patients with advanced non-small cell lung cancer. Ther. Adv. Med. Oncol. 2018;10:1758834017750121. doi: 10.1177/1758834017750121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mohme M., Riethdorf S., Pantel K. Circulating and disseminated tumour cells—Mechanisms of immune surveillance and escape. Nat. Rev. Clin. Oncol. 2016;14:155. doi: 10.1038/nrclinonc.2016.144. [DOI] [PubMed] [Google Scholar]
- 6.Zhang T., Agarwal A., Almquist R.G., Runyambo D., Park S., Bronson E., Boominathan R., Rao C., Anand M., Oyekunle T., et al. Expression of immune checkpoints on circulating tumor cells in men with metastatic prostate cancer. Biomark. Res. 2021;9:14. doi: 10.1186/s40364-021-00267-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mostert B., Sieuwerts A.M., Bolt-de Vries J., Kraan J., Lalmahomed Z., van Galen A., van der Spoel P., de Weerd V., Ramírez-Moreno R., Smid M., et al. mRNA expression profiles in circulating tumor cells of metastatic colorectal cancer patients. Mol. Oncol. 2015;9:920–932. doi: 10.1016/j.molonc.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kong S.L., Liu X., Suhaimi N.M., Koh K.J.H., Hu M., Lee D.Y.S., Cima I., Phyo W.M., Lee E.X.W., Tai J.A., et al. Molecular characterization of circulating colorectal tumor cells defines genetic signatures for individualized cancer care. Oncotarget. 2017;8:68026–68037. doi: 10.18632/oncotarget.19138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu J., Huang X., Liu H., Wei C., Ru H., Qin H., Lai H., Meng Y., Wu G., Xie W., et al. Immune landscape and prognostic immune-related genes in KRAS-mutant colorectal cancer patients. J. Transl. Med. 2021;19:27. doi: 10.1186/s12967-020-02638-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim E.Y., Kim A., Kim S.K., Chang Y.S. MYC expression correlates with PD-L1 expression in non-small cell lung cancer. Lung Cancer. 2017;110:63–67. doi: 10.1016/j.lungcan.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Casey S.C., Tong L., Li Y., Do R., Walz S., Fitzgerald K.N., Gouw A.M., Baylot V., Gütgemann I., Eilers M., et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science. 2016;352:227–231. doi: 10.1126/science.aac9935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thiem A., Hesbacher S., Kneitz H., di Primio T., Heppt M.V., Hermanns H.M., Goebeler M., Meierjohann S., Houben R., Schrama D. IFN-gamma-induced PD-L1 expression in melanoma depends on p53 expression. J. Exp. Clin. Cancer Res. 2019;38:397. doi: 10.1186/s13046-019-1403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Glorieux C., Xia X., He Y.Q., Hu Y., Cremer K., Robert A., Liu J., Wang F., Ling J., Chiao P.J., et al. Regulation of PD-L1 expression in K-ras-driven cancers through ROS-mediated FGFR1 signaling. Redox. Biol. 2021;38:101780. doi: 10.1016/j.redox.2020.101780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu X., Wang X., Duanmu J., Li T., Jiang Q. KRAS mutations are negatively correlated with immunity in colon cancer. Aging. 2020;13:750–768. doi: 10.18632/aging.202182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ischenko I., D’Amico S., Rao M., Li J., Hayman M.J., Powers S., Petrenko O., Reich N.C. KRAS drives immune evasion in a genetic model of pancreatic cancer. Nat. Commun. 2021;12:1482. doi: 10.1038/s41467-021-21736-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zou J., Zhuang M., Yu X., Li N., Mao R., Wang Z., Wang J., Wang X., Zhou H., Zhang L., et al. MYC inhibition increases PD-L1 expression induced by IFN-γ in hepatocellular carcinoma cells. Mol. Immunol. 2018;101:203–209. doi: 10.1016/j.molimm.2018.07.006. [DOI] [PubMed] [Google Scholar]
- 17.Chen N., Fang W., Lin Z., Peng P., Wang J., Zhan J., Hong S., Huang J., Liu L., Sheng J. KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol. Immunother. 2017;66:1175–1187. doi: 10.1007/s00262-017-2005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamid F.B., Lu C.T., Matos M., Cheng T., Gopalan V., Lam A.K. Enumeration, characterisation and clinicopathological significance of circulating tumour cells in patients with colorectal carcinoma. Cancer Genet. 2021;254–255:48–57. doi: 10.1016/j.cancergen.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 19.Sastre J., Maestro M.L., Puente J., Veganzones S., Alfonso R., Rafael S., García-Saenz J.A., Vidaurreta M., Martín M., Arroyo M., et al. Circulating tumor cells in colorectal cancer: Correlation with clinical and pathological variables. Ann. Oncol. 2008;19:935–938. doi: 10.1093/annonc/mdm583. [DOI] [PubMed] [Google Scholar]
- 20.Allard W.J., Matera J., Miller M.C., Repollet M., Connelly M.C., Rao C., Tibbe A.G.J., Uhr J.W., Terstappen L.W.M.M. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 2004;10:6897–6904. doi: 10.1158/1078-0432.CCR-04-0378. [DOI] [PubMed] [Google Scholar]
- 21.Zhao X.H., Wang Z.R., Chen C.L., Di L., Bi Z.F., Li Z.H., Liu Y.M. Molecular detection of epithelial-mesenchymal transition markers in circulating tumor cells from pancreatic cancer patients: Potential role in clinical practice. World J. Gastroenterol. 2019;25:138–150. doi: 10.3748/wjg.v25.i1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang H.-M., Wu M.-H., Chang P.-H., Lin H.-C., Liao C.-D., Wu S.-M., Hung T.-M., Lin C.-Y., Chang T.-C., Tzu-Tsen Y., et al. The change in circulating tumor cells before and during concurrent chemoradiotherapy is associated with survival in patients with locally advanced head and neck cancer. Head Neck. 2019;41:2676–2687. doi: 10.1002/hed.25744. [DOI] [PubMed] [Google Scholar]
- 23.Cohen S.J., Punt C., Iannotti N., Saidman B.H., Sabbath K.D., Gabrail N.Y., Picus J., Morse M., Mitchell E., Miller M.C. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 2008;26:3213–3221. doi: 10.1200/JCO.2007.15.8923. [DOI] [PubMed] [Google Scholar]
- 24.De Bono J.S., Scher H.I., Montgomery R.B., Parker C., Miller M.C., Tissing H., Doyle G.V., Terstappen L.W., Pienta K.J., Raghavan D. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 2008;14:6302–6309. doi: 10.1158/1078-0432.CCR-08-0872. [DOI] [PubMed] [Google Scholar]
- 25.Hayes D.F., Cristofanilli M., Budd G.T., Ellis M.J., Stopeck A., Miller M.C., Matera J., Allard W.J., Doyle G.V., Terstappen L.W.W.M. Circulating Tumor Cells at Each Follow-up Time Point during Therapy of Metastatic Breast Cancer Patients Predict Progression-Free and Overall Survival. Clin. Cancer Res. 2006;12:4218–4224. doi: 10.1158/1078-0432.CCR-05-2821. [DOI] [PubMed] [Google Scholar]
- 26.Luo C., Cen S., Ding G., Wu W. Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun. 2019;39:1–13. doi: 10.1186/s40880-019-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lam A.K., Ong K., Ho Y.H. Colorectal mucinous adenocarcinoma: The clinicopathologic features and significance of p16 and p53 expression. Dis. Colon Rectum. 2006;49:1275–1283. doi: 10.1007/s10350-006-0650-y. [DOI] [PubMed] [Google Scholar]
- 28.Ganesh K., Stadler Z.K., Cercek A., Mendelsohn R.B., Shia J., Segal N.H., Diaz L.A. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019;16:361–375. doi: 10.1038/s41575-019-0126-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cree I.A., Charlton P. Molecular chess? Hallmarks of anti-cancer drug resistance. BMC Cancer. 2017;17:1–8. doi: 10.1186/s12885-016-2999-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kadota K., Sima C.S., Arcila M.E., Hedvat C., Kris M.G., Jones D.R., Adusumilli P.S., Travis W.D. KRAS Mutation Is a Significant Prognostic Factor in Early-stage Lung Adenocarcinoma. Am. J. Surg. Pathol. 2016;40:1579–1590. doi: 10.1097/PAS.0000000000000744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Al-Sohaily S., Biankin A., Leong R., Kohonen-Corish M., Warusavitarne J. Molecular pathways in colorectal cancer. J. Gastroenterol. Hepatol. 2012;27:1423–1431. doi: 10.1111/j.1440-1746.2012.07200.x. [DOI] [PubMed] [Google Scholar]
- 32.Lam A.K., Ong K., Ho Y.H. hTERT expression in colorectal adenocarcinoma: Correlations with p21, p53 expressions and clinicopathological features. Int. J. Colorectal Dis. 2008;23:587–594. doi: 10.1007/s00384-008-0455-7. [DOI] [PubMed] [Google Scholar]
- 33.Hensler M., Vančurová I., Becht E., Palata O., Strnad P., Tesařová P., Čabiňaková M., Švec D., Kubista M., Bartůňková J., et al. Gene expression profiling of circulating tumor cells and peripheral blood mononuclear cells from breast cancer patients. Oncoimmunology. 2015;5:e1102827. doi: 10.1080/2162402X.2015.1102827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinert G., Schölch S., Niemietz T., Iwata N., García S.A., Behrens B., Voigt A., Kloor M., Benner A., Bork U., et al. Immune Escape and Survival Mechanisms in Circulating Tumor Cells of Colorectal Cancer. Cancer Res. 2014;74:1694. doi: 10.1158/0008-5472.CAN-13-1885. [DOI] [PubMed] [Google Scholar]
- 35.Hamid F.B., Gopalan V., Matos M., Lu C.-T., Lam A.K.-y. Genetic Heterogeneity of Single Circulating Tumour Cells in Colorectal Carcinoma. Int. J. Mol. Sci. 2020;21:7766. doi: 10.3390/ijms21207766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jacobs J., Smits E., Lardon F., Pauwels P., Deschoolmeester V. Immune Checkpoint Modulation in Colorectal Cancer: What’s New and What to Expect. J. Immunol. Res. 2015;2015:158038. doi: 10.1155/2015/158038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Derakhshani A., Hashemzadeh S., Asadzadeh Z., Shadbad M.A., Rasibonab F., Safarpour H., Jafarlou V., Solimando A.G., Racanelli V., Singh P.K., et al. Cytotoxic T-Lymphocyte Antigen-4 in Colorectal Cancer: Another Therapeutic Side of Capecitabine. Cancers. 2021;13:2414. doi: 10.3390/cancers13102414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujiwara-Tani R., Sasaki T., Ohmori H., Luo Y., Goto K., Nishiguchi Y., Mori S., Nakashima C., Mori T., Miyagawa Y., et al. Concurrent Expression of CD47 and CD44 in Colorectal Cancer Promotes Malignancy. Pathobiology. 2019;86:182–189. doi: 10.1159/000496027. [DOI] [PubMed] [Google Scholar]
- 39.Wellenstein M.D., de Visser K.E. Cancer-Cell-Intrinsic Mechanisms Shaping the Tumor Immune Landscape. Immunity. 2018;48:399–416. doi: 10.1016/j.immuni.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Chikamatsu K., Tada H., Takahashi H., Kuwabara-Yokobori Y., Ishii H., Ida S., Shino M. Expression of immune-regulatory molecules in circulating tumor cells derived from patients with head and neck squamous cell carcinoma. Oral Oncol. 2019;89:34–39. doi: 10.1016/j.oraloncology.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 41.Kondo Y., Hayashi K., Kawakami K., Miwa Y., Hayashi H., Yamamoto M. KRAS mutation analysis of single circulating tumor cells from patients with metastatic colorectal cancer. BMC Cancer. 2017;17:1–10. doi: 10.1186/s12885-017-3305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miyamoto D.T., Zheng Y., Wittner B.S., Lee R.J., Zhu H., Broderick K.T., Desai R., Fox D.B., Brannigan B.W., Trautwein J. RNA-Seq of single prostate CTCs implicates noncanonical Wnt signaling in antiandrogen resistance. Science. 2015;349:1351–1356. doi: 10.1126/science.aab0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gopalan V., Ebrahimi F., Islam F., Vider J., Qallandar O.B., Pillai S., Lu C.-T., Lam A.K.-y. Tumour suppressor properties of miR-15a and its regulatory effects on BCL2 and SOX2 proteins in colorectal carcinomas. Exp. Cell Res. 2018;370:245–253. doi: 10.1016/j.yexcr.2018.06.025. [DOI] [PubMed] [Google Scholar]
- 44.Islam F., Gopalan V., Wahab R., Smith R.A., Qiao B., Lam A.K.-Y. Stage dependent expression and tumor suppressive function of FAM134B (JK1) in colon cancer. Mol. Carcinog. 2017;56:238–249. doi: 10.1002/mc.22488. [DOI] [PubMed] [Google Scholar]
- 45.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in Gene expression analysis of immune regulatory genes in circulating tumour cells and peripheral blood mononuclear cells in patients with colorectal carcinoma and its Supplementary Material within.