Abstract
Drought has severe effects on plant growth, forest productivity, and survival throughout the world. Understanding the molecular regulation of drought resistance in forest trees can enable effective strategic engineering of novel drought-resistant genotypes of tree species. In this study, we identified a gene, PtrVCS2, encoding a zinc finger (ZF) protein of the ZF-homeodomain transcription factor in Populus trichocarpa (Black Cottonwood) Torr. & A. Gray. ex Hook. Overexpression of PtrVCS2 (OE-PtrVCS2) in P. trichocarpa resulted in reduced growth, a higher proportion of smaller stem vessels, and strong drought-resistance phenotypes. Stomatal movement experiments revealed that the OE-PtrVCS2 transgenics showed lower stomata apertures than wild-type plants under drought conditions. RNA-seq analysis of the OE-PtrVCS2 transgenics showed that PtrVCS2 regulates the expression of multiple genes involved in regulation of stomatal opening and closing, particularly the PtrSULTR3;1-1 gene, and several genes related to cell wall biosynthesis, such as PtrFLA11-12 and PtrPR3-3. Moreover, we found that the water use efficiency of the OE-PtrVCS2 transgenic plants was consistently higher than that of wild type plants when subjected to chronic drought stress. Taken together, our results suggest that PtrVCS2 plays a positive role in improving drought adaptability and resistance in P. trichocarpa.
Keywords: Populus trichocarpa (Black Cottonwood), stem developing xylem, drought stress, stomata aperture, transcriptomic sequencing, chronic stress
1. Introduction
In addition to being a source of food, fuel, and materials, forests can be used to sequester carbon dioxide through photosynthesis, affecting soils and weather around the world [1]. The vascular cambium of trees differentiates into xylem and phloem, in which xylem mainly transports water and mineral nutrients [2]. However, drought, causing loss in xylem hydraulic conductivity, critically impairs metabolic and hydraulic function [3]. When the water potential of root cells is lower than that of soil, water enters the cells in the root cortex through symplastic movement and moves into the vascular system [4]. However, under drought stress, insufficient water enters the vascular system, and gas enters a single vessel cell, forming an embolism, which travels from one vessel to another. Embolisms can cause serious damage to water transport [5]. It is usually measured by the percentage loss of conductance (PLC) [6]. The broken water column causes the water to not be transported to shoot, which can result in desiccation and mortality [7]. To cope with drought stress, trees have developed many strategies, such as reducing water loss and protecting the water transport capacity of the plant [8].
In response to the biotic stress of bacteria, fungi, viruses, and insects, plants often protect themselves by regulating some ion signals and hormone signals [9,10]. Similarly, signaling of hormones, such as abscisic acid (ABA), jasmonic acid (JA), brassinosteroid (BR), etc., also plays various roles in plant response to abiotic stress [11]. The main way water is lost in plants is by gas exchange in leaves; closing stomata is one of the most effective way to reduce water loss [12]. The most common light-induced stomatal closure is mediated by ABA [13]. ABA induces the increase of cytoplasmic calcium (Ca2+) in guard cells. Activation of anion channels leads to membrane depolarization and potassium (K+) outflow, which both promote water outflow, resulting in filling loss and thus stomatal closure [14]. Water use efficiency (WUE) is the ratio of carbon taken by plants with respect to water use. The effects of photosynthesis and stomata on WUE determine the accumulation of plant biomass [15]. Various metabolites from photosynthesis increase WUE by controlling sucrose metabolism in guard cells [16].
In addition to reducing water loss, it is important to ensure water transport in plants. The key factor is prevention of embolism. For tree species, wider vessels may lead to more embolism [17]. Polar auxin transport in the xylem to form small diameter highly aggregated vessels can alter the hydraulic properties of the stem [18]. Small-diameter vessels do not develop severe embolism or horizontal deficiency [19]. Additionally, through a complex regulatory network, cellulose, hemicellulose, and lignin are biosynthesized and deposited to thicken secondary cell walls and form wood [20]. For the long distance transport of water, lignin is deposited in the cell wall, a process that can be triggered by abiotic stresses [21]. Xyloglucan is the main hemicellulose of the cell wall, and xyloglucan endotransglycosylase/hydrolase activity is involved in xylem cell wall thickening and regulation of xylem water transport [22].
Stress signals are transmitted through hormone signals to functional genes and transcription factors involved in cell protection, including v-myb avian myeloblastosis viral oncogene homolog (MYB), basic leucine zipper (bZIP), zinc finger homeodomain (ZF-HD), etc., which have been proven to be involved in responding to abiotic stresses such as salt tolerance, cold tolerance, and drought tolerance [23,24]. Plant-specific ZF-HD transcription factors are induced by polyethylene glycol, sodium chloride, and cold and hot stress in wheat [25]. In Chenopodium quinoa (Willd.), CqZF-HD14 regulates CqNAC79 and CqHIPP34 promotes photosynthetic pigment accumulation, maintains antioxidant capacity, and enhances drought resistance [26]. DcHB30-encoding ZF-HD protein and DcWRKY75 are antagonistic to the regulation of petal senescence in carnation (Dianthus caryophyllus L.) [27]. The PpOFP1 gene of peach (Prunus persica Batsch) has been allogeneously transformed in yeast and tomato to exhibit salt tolerance in interaction with PpZFHD1 [28]. The members of the ZF-HD family in Arabidopsis thaliana (Heynh.) are divided into seven groups except for MINI ZINC FINGER proteins (MIFs), which are only found in seed plants [29]. AtMIF1 is involved in the regulation of various hormones in Arabidopsis thaliana development, including gibberellins, ABA, auxin, ethylene, etc. [30]. In tomato (Solanum lycopersicum L.), SIIMA recruits SIKNU to form a complex with TOPLESS and HDA19 that binds and inhibits SIWUS transcription to regulate carpels and fruit size [31]. Although some ZF-HD family genes respond to drought stress [32], the function of MIFs in response to abiotic stresses has not been previously reported. Moreover, little is known about the ZF-HD subfamily in woody plants except that it contains 21 members in Populus trichocarpa [33].
Recently, we have identified a ZF-HD TF family gene, PtrVCS2, in P. trichocarpa. PtrVCS2 is involved in regulating cambium proliferation by directly binding to PtrWOX4a gene promoter through interaction with PtrWOX13 in P. trichocarpa [34]. The activity of vascular cambium also affects xylem development [35]. In this study, we revealed the role of PtrVCS2 in regulating xylem development and stomatal closure of P. trichocarpa and found that overexpression of PtrVCS2 decreases the percentage of embolism in xylem and improves drought resistance of P. trichocarpa.
2. Results
2.1. Overexpressing PtrVCS2 Gene Results in More and Smaller Vessels in Xylem Tissue
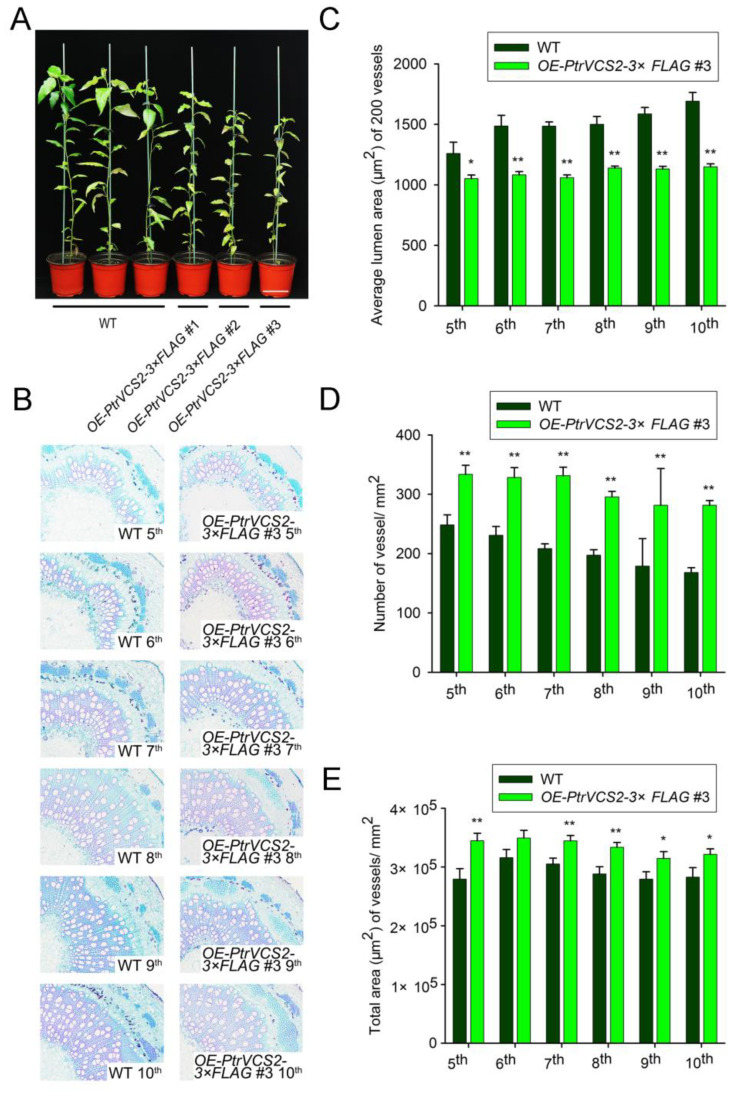
We previously generated transgenic P. trichocarpa plants overexpressing PtrVCS2-3×FLAG (OE-PtrVCS2-3×FLAG) under the control of a CaMV 35S promoter (Figure 1A). The transgenic line #3 (OE-PtrVCS2-3×FLAG #3) with the highest levels of transgene overexpression was selected and propagated with the wild type (WT) and maintained in a greenhouse. Stem cross-sections analysis revealed that the xylem vessels in OE-PtrVCS2-3×FLAG #3 plants were smaller than those in wild-type plants from the 5th stem internode (Figure 1B, C) where stem secondary growth begins. The vessel number per unit area in OE-PtrVCS2-3×FLAG #3 plants was greater than that in wild-type plants (Figure 1D), which resulted in an increase in the total vessel lumen area (void area) in OE-PtrVCS2-3×FLAG #3 plants (Figure 1E).
Figure 1.
Overexpressing PtrVCS2 gene affects the size and number of vessels in xylem tissue of P. trichocarpa. (A) The growth phenotypes of 4-month-old OE-PtrVCS2-3×FLAG #1, #2, #3 and wild-type (WT) plants. Bars = 10 cm. (B) Stem cross-sections of wild-type (WT) and OE-PtrVCS2-3×FLAG #3 transgenic plants. Bars = 200 µm. (C–E) Average lumen area (μm2) of 200 vessels cells (C), total number of vessel/ mm2 (D), and total area (μm2) of vessels/ mm2 (E) using vessel cells from (B). Error bars represent standard errors for three independent replicates with at least 200 OE-PtrVCS2 transgenic vessel cells and 200 WT plant vessel cells for each genotype in each replicate, and asterisks indicate significant differences between the OE-PtrVCS2 transgenics and WT plants. * p < 0.05, ** p < 0.01 (Student’s t-test).
2.2. Overexpressing PtrVCS2 Gene Improves Drought Resistance
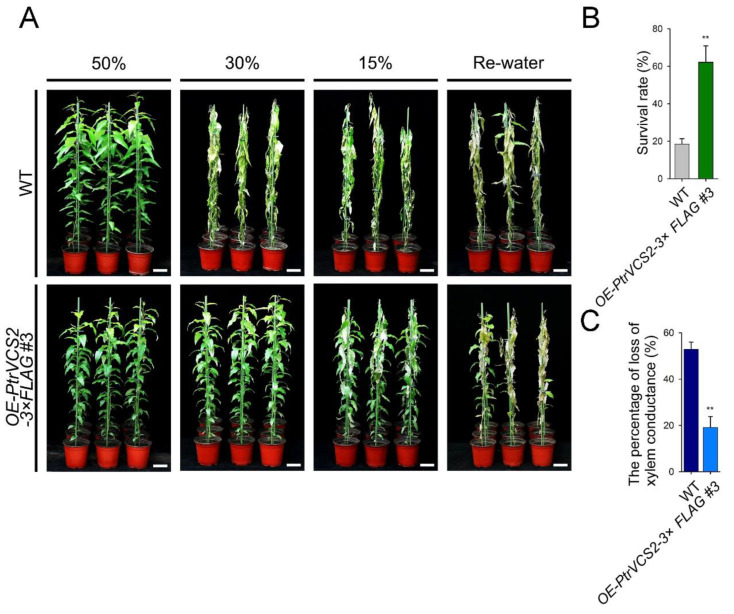
Next, we tested the drought response of OE-PtrVCS2-3×FLAG #3 and wild-type plants. Four-month-old OE-PtrVCS2 and wild-type plants were subjected to drought treatments by withholding water. When plants were adequately watered, the soil was saturated, having 50% volumetric soil water content (VWC) (Figure 2A). In the absence of water replenishment, the VWC decreased to 30%, and the leaves of wild-type plants wilted, while most of the OE-PtrVCS2 transgenics remained turgid (Figure 2A). Under the severe drought condition with 15% VWC, the wild-type plants showed severe wilting symptoms, compared with the OE-PtrVCS2 transgenics (Figure 2A). After rehydration in 3 days, the survival rate for the stressed plants was estimated (Figure 2A). Most of the wild-type plants did not recover, with an 18.4% survival rate (Figure 2B). By contrast, the OE-PtrVCS2 transgenics recovered rapidly, with a survival rate of 62.2% (Figure 2B).
Figure 2.
Overexpressing PtrVCS2 gene improves drought resistance of P. trichocarpa. (A) Drought resistance phenotypes of wild-type and OE-PtrVCS2 transgenic plants. Plants (before drought, on the left) were dehydrated by reducing soil water content to 30%, 15% and then rehydrated for 3 days (Rehydrated for 3 days, on the right). Bars = 10 cm. (B) Statistical analysis of survival rates after drought treatment and recovery. The average percentage of survival and standard errors were calculated from three independent experiments with at least 12 plants of each genotype in each replicate. (C) Percentage loss of xylem conductance (PLC) of wild-type and OE-PtrVCS2 transgenic plants. In the statistical analysis, the average percentage of PLC was calculated from three independent experiments and standard errors were calculated from three independent experiments. Asterisks indicate significant differences between the OE-PtrVCS2 transgenics and WT plants. ** p < 0.01 (Student’s t-test).
Although the leaves of the OE-PtrVCS2 plants were smaller than those of the wild-type plants, the number of functional leaves of the transgenics were much greater than those of the wild-type plants under drought stress conditions (Supplemental Figure S1). We measured PLC in the stems of the OE-PtrVCS2 and wild-type plants. Under the drought condition with 15% VWC, the OE-PtrVCS2 transgenics have lower PLC (19.1%, Figure 2C) compared with wild-type plants (52.9%, Figure 2C).
2.3. PtrVCS2 Regulates Stomatal Closure-Related Genes
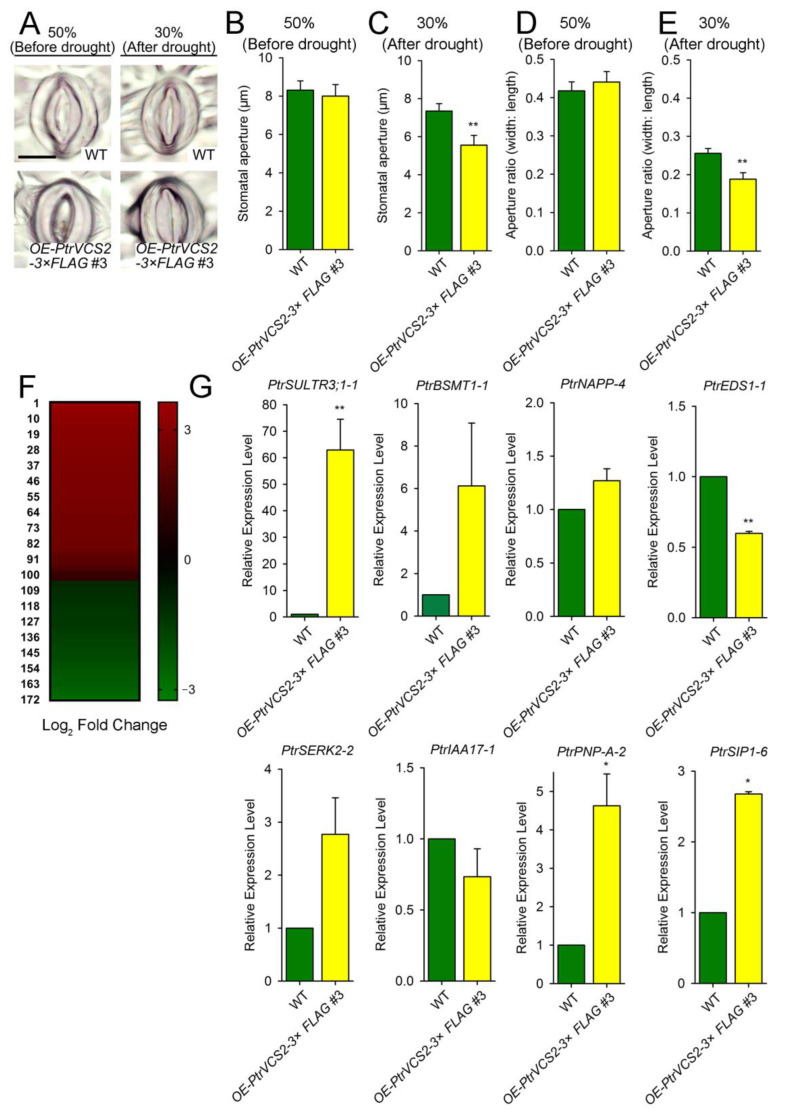
Stomatal movement experiments revealed that the OE-PtrVCS2 transgenics showed lower stomata aperture than wild-type plants under the drought condition with 30% VWC (Figure 3A–E). These results suggested that overexpression of PtrVCS2 may improve drought resistance by regulating stomatal aperture.
Figure 3.
The stomata aperture of the wild-type and OE-PtrVCS2 plants in response to drought stress. (A) Photographs of stomata on the leaves of the wild-type (WT) and OE-PtrVCS2 plants before or after drought stress treatments. Bars = 20 μm. (B) The stomatal aperture under the condition with 50% VWC (before drought stress treatments). (C) The stomatal aperture under the condition with 30% VWC (after drought stress treatments). (D) The aperture ratio under the condition with 50% VWC (before drought stress treatments). (E) The aperture ratio under the condition with 30% VWC (after drought stress treatments). (F) Identification of differentially expressed genes in the OE-PtrVCS2 transgenics under the drought condition. (G) The relative expression levels of the genes related to stomata closure in the leaves of the WT and OE-PtrVCS2 plants under the drought condition. The error bars represent one SE of the three biological replicates. “*”,” **” denote significant differences: * p < 0.05, ** p < 0.01 (Student’s t-test).
To explore the molecular regulation of PtrVCS2 in drought resistance of P. trichocarpa plants, we performed RNA-seq analysis of WT and OE-PtrVCS2 plants with stem-developing xylem (SDX) tissues under the drought condition with 30% VWC (Figure 2). We identified 172 differentially expressed genes (DEGs) in the OE-PtrVCS2 transgenics with 103 up-regulated and 69 down-regulated genes (Figure 3F) (Supplemental Dataset S1). Of these DEGs, the expression levels of multiple genes involved in regulation of stomatal opening and closing were obviously changed (Supplemental Table S1). The expression levels of these stomatal closure-related genes were analyzed in leaves of WT and OE-PtrVCS2 plants with drought treatments. We found that the expression levels of five genes, whose homologous genes such as PtrSULTR3;1-1, PtrNAPP-4, PtrEDS1-1, and PtrSERK2-2 which have functions in promoting stomatal closure, were highly induced by drought stress (Figure 3G). Considering that induction by drought stress is particularly apparent in PtrSULTR3;1-1, we speculated that PtrVCS2 may control stomatal closure by regulating the expression of the PtrSULTR3;1-1 gene in P. trichocarpa.
2.4. PtrVCS2 Regulates the Genes Related to Cell Wall Biosynthesis
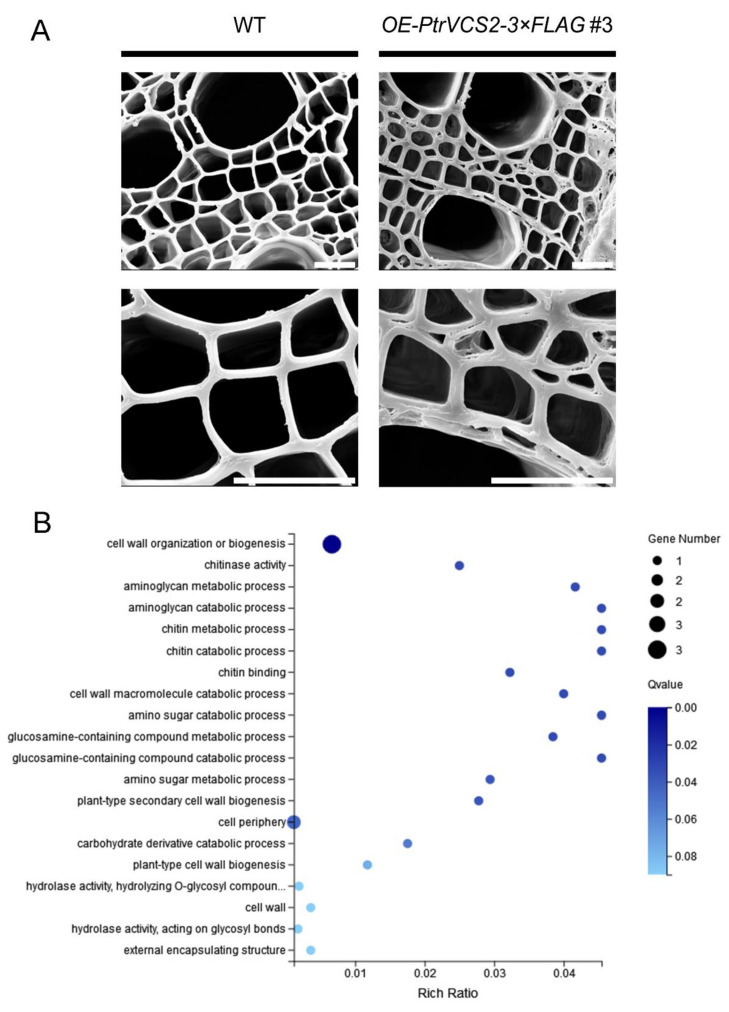
Scanning electron micrographs show that elevated PtrVCS2 expression resulted in thicker fiber and vessel cell walls in the xylem of the OE-PtrVCS2 transgenics compared with the WT plants (Figure 4A). Next, we conducted gene ontology (GO) analysis (Figure 4B), with 172 DEGs identified from the RNA-seq of WT and OE-PtrVCS2 plants under drought conditions, and found three genes: PtrPR3-3, PtrFLA11-12, and a novel one, related to cell wall organization or biogenesis (Supplementary Table S2). Moreover, we found that PtrVCS2 regulates three genes, PtrGH9B1-1, PtrLAC44, and PtrLAC45 under drought conditions, and their homologous genes function in regulating cellulose and lignin biosynthesis.
Figure 4.
PtrVCS2 regulates the cell wall biosynthesis-related genes. (A) Wild-type (WT) and OE-PtrVCS2 transgenic plants were imaged by SEM with the 12th internode imaged at ×1000 (upper panels) and ×3000 (lower panels) magnification. Bars = 20 μm. (B) Gene ontology categories related to cell wall biosynthesis.
2.5. Overexpressing PtrVCS2 Gene Improves the Adaptability of P. trichocarpa to Chronic Drought Stress
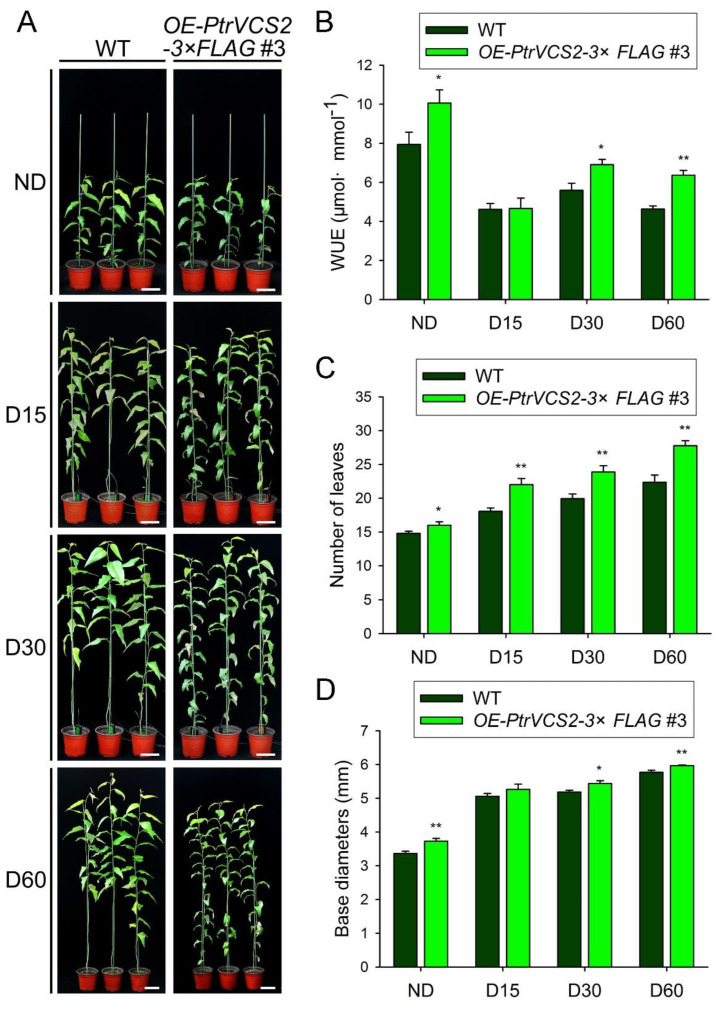
Chronic drought, a continuous stress, inhibits the biomass accumulation of plants. To further elucidate the role of PtrVCS2 in response to drought stress, we employed a chronic water deficit assay (Figure 5A).
Figure 5.
Overexpressing PtrVCS2 gene improves the adaptability of P. trichocarpa to chronic drought stress. (A) Phenotypes of the OE-PtrVCS2 transgenic and wild-type plants with chronic stress treatments. Plants (before drought, on the left) were dehydrated by reducing soil water content to 40% and maintaining it for 60 days. ND (normal condition, 50% VWC), D15 (15-day, 40% VWC), D30 (30-day, 40% VWC), and D60 (60-day, 40% VWC). Bars = 10 cm. (B) Water use efficiency of the OE-PtrVCS2 transgenic and WT plants. (C) Number of leaves of the OE-PtrVCS2 transgenic and WT plants. (D) Base diameters of the OE-PtrVCS2 transgenic and WT plants. The error bars indicate one standard error of three biological replicates from independent pools of P. trichocarpa plants. “*”, “**” indicate significant differences between the OE-PtrVCS2 transgenics and WT plants. * p < 0.05, ** p < 0.01 (Student’s t-test).
Under normal conditions (ND (normal condition, 50% VWC)), the transgenics had higher WUE (10.1 μmol·mmol−1) than the WT. After 15-day drought treatments, there was no significant difference in WUE between the OE-PtrVCS2 transgenics and the WT plants. After 30-day drought treatments, the WUE of the OE-PtrVCS2 transgenics (6.9 μmol·mmol−1) was higher than that of the WT plants (5.6 μmol·mmol−1). After 60-day drought treatments, the WUE of the OE-PtrVCS2 transgenics (6.4 μmol·mmol−1) was significantly higher than that of the WT plants (4.6 μmol·mmol−1) (Figure 5B). Moreover, the OE-PtrVCS2 transgenic plants had more leaves (Figure 5C) and larger base diameters (Figure 5D) than the WT plants. These results indicated that overexpressing PtrVCS2 could improve the adaptability of P. trichocarpa to chronic drought stress.
3. Discussion
Changes in the duration and frequency of drought led to a gradual increase in tree mortality [36]. Land drought has become a key global problem [37]. Transpiration forces transport water and solutes absorbed from the roots through the vessel cells to the leaves, and the water deficit can cause the water column in vessels to break, thus forming an embolism that blocks water transport in the stem [7,38]. In this study, we identified a gene in P. trichocarpa, PtrVCS2, which plays a vital role in plant response to drought. Overexpressing PtrVCS2 in P. trichocarpa resulted in reduced growth and enhanced development of more and smaller vessel cells (Figure 1), which promoted the ability of plants to resist embolism (Figure 2C) [19]. The OE-PtrVCS2 transgenic plants had higher survival rate (Figure 2B) and lower water conductivity loss (Figure 2C) compared with wild-type plants under drought conditions, indicating that overexpression of PtrVCS2 is conducive to drought adaptation [39,40]. The overexpression plants had a large number of functional leaves after drought stress, suggesting that these transgenics do not require less water than the wild-type plants under drought stress conditions. Moreover, drought-induced stomatal closure of leaves reduced gas exchange and water loss [12], which protected the OE-PtrVCS2 transgenics from stress.
There is a trade-off between growth and stress responses such that loss of growth and development tends to enhance the stress resistance of plants [41]. Overexpression of PtrVCS2 gene led to reduced growth phenotype of the transgenic plants (Figure 1A). RNA-seq analysis of the transgenics revealed that two auxin related genes, PtrIAA17-1 (Potri.001G177400) and PtrIAA4-4 (Potri.008G161100), were inhibited by PtrVCS2 (Supplemental Dataset S1). There is one possibility that the inhibition of PtrVCS2 on auxin response genes may be the reason for reduced growth of the transgenic plants. PtrVCS2 encodes a ZF-HD transcription factor, and the homologs of PtrVCS2 in other species were also found to be involved in regulation of plant development. In soybean, GhMIF regulates petal elongation by activating the expression of GEG gene of GASA family members [42]. MIF1 is related to root growth reduction and dwarfism in Amaranthus hypochondriacus L. [43]. Recently, we demonstrated that PtrVCS2 regulates cambium development by directly binding to PtrWOX4a gene promoter through interaction with PtrWOX13 in P. trichocarpa [34]. In this study, we found that PtrVCS2 not only reduces the number of cambium layers but also inhibits the enlargement of xylem vessel cells. The individual vessel cell area was smaller in the OE-PtrVCS2 transgenics than in wild-type plants (Figure 1C). Small vessels are less prone to embolism [17]; therefore, the loss of xylem conductance of OE-PtrVCS2 transgenics was lower than that of the WT (Figure 2C). Reduction in the size of the vessel is one approach to improve WUE. The vessel diameter correlated negatively with intrinsic WUE, and the correlation was stronger under water deficit conditions [44]. We speculate that WUE of OE-PtrVCS2 transgenics was also higher than that of WT under drought stress. Although smaller vessels have little embolism, they also transport less water, which is not sufficient for the growth of the plants [4]. We found that the vessel cell number (Figure 1D) and the area of vessels in the transverse section of the woody stem (Figure 1E) were significantly increased in the OE-PtrVCS2 transgenics compared with wild-type plants. The increase in the number of vessels solves the problem of water loss caused by the smaller area of the individual cells in OE-PtrVCS2 transgenic plants. Moreover, we demonstrated that PtrVCS2 plays a positive role in improving drought adaptability and resistance in P. trichocarpa (Figure 2 and Figure 5). Previous studies and our findings suggest that PtrVCS2 and its homologs in different species have diverse functions not only in the regulation of plant development but also in plant response to abiotic stresses.
Plant growth adapts to the changing environments. Under drought conditions, plants alter their physiology to reduce growth and enhance drought resistance for adaptation [39,45,46,47,48]. Drought adaptation also includes development of cell wall structures that are conducive to water transport, i.e., structures with smaller stem xylem vessels to minimize xylem cavitation (failure of upward water transport) [49,50]. Cell walls limit cells to different shapes and protect the cells in response to abiotic stresses [51,52]. The secondary cell wall acts as a conduit for water and resistance to the tension generated by the transpiration pull [53]. We observed the cross section of the stem by scanning electron microscope and found that the xylem cell wall of the OE-PtrVCS2 plants was thicker than that of the wild-type plants (Figure 4A). Therefore, it is possible that PtrVCS2 may restrict xylem cell morphological changes by regulating cell wall thickening. Moreover, PtrVCS2 regulated the expression of the cell wall synthesis genes, such as PtrFLA11-12 and PtrPR3-3 (Figure 4B). The homologous gene of PtrFLA11-12 is associated with cell wall expansion [54], and the homologous gene of PtrPR3-3, as a marker gene of Botrytis cinerea, has been shown to promote disease resistance in plants [55]. In Arabidopsis thaliana, AtGH9B1-1 is involved in cellulose biosynthesis [56]. In Cleome hassleriana L., LACs promote the biosynthesis of lignin components [57]. Together, these results suggest that PtrVCS2 may control xylem cell morphological changes by regulating the expression of these cell wall biosynthesis-related genes in P. trichocarpa. The increase of cell wall thickness increases the density of wood, thus changing the wood properties and increasing the biomass of plants [53,58]. The key traits in the OE-PtrVCS2 transgenics, i.e., reduced plant growth and a larger number of small xylem vessels that are consistent with overexpression of PtrVCS2, are all conducive to drought adaptation. The recovery of plant stems with small and abundant cells and thick cell walls may depend on the recovery of xylem function and the regulation of carbohydrate metabolism in xylem after rehydration after drought stress [59]. These apparent adaptative traits make the OE-PtrVCS2 lines unique as potential sources for the attainment of new knowledge of the complex regulations involved in cell-type biosynthesis in wood formation and growth under stresses.
Hydraulic conductivity is positively correlated with stomatal conductance, and a decrease in water transport leads to stomatal closure [60,61]. In response to drought stress, plants use hormones to trigger stomatal closure, reducing water loss [62]. Using transgenesis (Figure 2) and RNA-seq (Figure 3) we found that PtrVCS2 controls stomatal closure by regulating the expression of stomatal closure-related genes in plant response to drought (Supplemental Table S1) under drought conditions [63,64,65,66,67,68,69,70]. Of these genes, the expression levels of PtrSULTR3; 1-1 were significantly increased in xylem and leaves of the OE-PtrVCS2 transgenic plants compared with wild-type plants under drought conditions (Figure 3G). The homolog of PtrSULTR3;1-1 in Arabidopsis, AtSULTR3, moves into chloroplasts via sulfate transport to promote cysteine synthesis, trigger ABA biosynthesis, and regulate stomatal closure under stress conditions [63]. The most stomata of the OE-PtrVCS2 transgenic plants were closed under drought stress (Figure 3A,E), suggesting that PtrVCS2 may regulate the expression of these stomatal closure-related genes and thus control water losses through transpiration of the leaves by an ABA-dependent pathway. Additionally, the expression levels of PtrPNP-A-2 were also induced by PtrVCS2, but the relative expression abundance was lower than that of PtrSULTR3; 1-1 (Figure 3G). The homologue of PtrPNP-A-2 in Arabidopsis, AtPNP-A, as an antagonist of ABA, limits gas exchange in stomata [66]. There is one possibility that PtrSULTR3; 1-1 and PtrPNP-A-2 may play opposite roles in ABA-induced stomatal closure, but we do not know when PtrPNP-A-2 is dominant. Usually, ABA signaling receptors inhibit PP2C phosphatase activity and promote the activation of SnRK2s protease phosphorylation to trigger stomatal closure [71]. Whether PtrVCS2 is involved in this process, what role it plays, and the target of phosphorylation still needs to be further investigated.
Moreover, gene ontology (GO) analysis showed that PtrVCS2 regulates the expression of genes related to the ABA signaling pathway in xylem (Supplemental Figure S2). The ZF-HD transcription factor also responded to abiotic stress and the ABA hormone signal in tomato [72]. Thus, PtrVCS2 may control stomatal closure to improve drought resistance by integrating ABA signals in P. trichocarpa. Further exploration of the connections between the PtrVCS2-mediated control of stomatal closure and ABA signaling should yield new insights into the regulation of drought resistance in P. trichocarpa. In addition to the ABA signaling pathway, PtrVCS2 also regulates the expression of the BR response gene PtrCYP67A2-13 (Potri.009G064800) and the ethylene response gene PtrPR4-3 (Potri.013g041600) (Supplemental Dataset S1). Considering the complexity of the regulatory network of plant stress response [73,74], it is possible that multiple hormone signaling pathways are involved in improving drought resistance of OE-PtrVCS2 plants.
Water deficit often results in the decrease of aboveground biomass and retardation of plant growth [75]. Under chronic drought stress, the leaves of wild plants fall off (Figure 5A), but there was no obvious leaf loss in OE-PtrVCS2 plants. The number of leaves of OE-PtrVCS2 transgenics was significantly more than that of wild type plants (Figure 5C). WUE is a key ecosystem attribute of plant water cycles [76]. The OE-PtrVCS2 transgenic plants had strong photosynthesis and high water use efficiency under drought conditions (Supplemental Figure S3), indicating that OE-PtrVCS2 plants produced more biomass for the same amount of water consumption [77]. This is consistent with the increase in stem size and wood yield of OE-PtrVCS2 transgenics (Figure 5D). We demonstrated that the OE-PtrVCS2 transgenics could maintain high WUE (Figure 5B) and thus accumulate a large amount of biomass under chronic drought stress condition.
In conclusion, we present a novel gene, PtrVCS2, in regulation of secondary xylem development and drought resistance. Knowledge of PtrVCS2 regulation may help design genetic controls that could maximize beneficial wood traits while minimizing negative effects on growth under drought conditions.
4. Materials and Methods
Nisqually-1, a genotype of P. trichocarpa Torr. & A. Gray ex. Hook., was used for all experiments with P. trichocarpa genotype. Wild-type and transgenic plants were grown in a greenhouse as previously described [39]. PtrVCS2 (Potri.004G126600) was identified as a high-expression gene in the stem from RNA-seq data [39]. Amino acid sequences of PtrVCS2 and other homologous genes mentioned in the article were obtained from the Phytozome, a comparative platform for green plant genomics (https://phytozome-next.jgi.doe.gov/, accessed on 25 August 2021), and conserved motifs were identified in NCBI (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi, accessed on 8 October 2021). The full CDS of the PtrVCS2 gene was cloned into pENTR/D-TOPO vector (Invitrogen), and then a pBI121-35Spro-PtrVCS2-3×FLAG plant expression vector was generated. Transgenic plants were obtained by using the P. trichocarpa transformation system [78]. Three independent transgenic lines, OE-PtrVCS2-3×FLAG #1, OE-PtrVCS2-3×FLAG #2, OE-PtrVCS2-3×FLAG #3 were generated as previously described [34].
4.1. RNA-Seq Assay and Data Analyses
In RNA-seq experiments, 4-month-old wild-type P. trichocarpa plants and OE-PtrVCS2 transgenic plants were placed in a separate 15-cm pot, which were used for drought treatments (30% VWC). The plants were divided into two groups: (1) control (WT), and (2) OE-PtrVCS2 transgenic plants (OE). Before drought treatment, all plants were thoroughly watered until the soil was saturated. Short-term drought treatments were conducted according to established procedures [39] with a few modifications. Plants in saturated soil were placed in a greenhouse to stand, without replenishing water, and soil water content was reduced to 30%. All the plants were harvested at 30% VWC. Stem-developing xylem tissues for RNA-seq were packed in aluminum foil and collected in liquid nitrogen [79,80]. Total RNA was extracted using the Qiagen RNeasy Mini Kit (Qiagen, Hilden, NRW, Germany) as previously described [81]. RNase-Free DNase Set (Qiagen) was used to remove DNA impurities. Purified RNA was used for RNA-seq. RNA-seq library construction of each sample was performed using the Illumina TruSeq RNA sample preparation kit. The quality of libraries was examined using a Bioanalyzer 2100 (Agilent, Santa Clara, CA, USA). Six libraries were sequenced using the Illumina Genome Analyzer, with an average reading length of 100-bp. After the library index sequences from each read were removed by HISAT [82] with parameters (--dta --phred64 unstranded --new-summary -x index -1 read_r1 -2 read_r2 (PE)), the remaining RNA-seq reads were mapped to the P. trichocarpa genome v.4.0. The frequency of raw counts was determined by DIAMOND tools [83,84,85] for all annotated genes. DEGs between the WT and OE samples were identified using DEseq2 [86,87] based on raw counts of mapped RNA-seq reads to annotated genes. Gene ontology (GO) was analyzed using R (3.1.2) statistical software with the Phyper function by Fisher’s exact test with FDR multiple test correction (FDR ≤ 0.05).
4.2. RT-qPCR Analyses
Mature leaves of wild-type and OE-PtrVCS2 transgenics after drought stress treatments were harvested for RNA extraction. RNase-Free DNase Set (Qiagen) was used to remove DNA. Purified RNA was stored in the refrigerator at −80 °C. cDNAs were synthesized by reverse transcription using SrimeScript™ RT reagent Kit (TAKARA) according to the manufacturer’s protocol. FastStart Universal SYBR Green Master (Roche) was used to formulate the reaction system and an Agilent Mx3000P Real-time PCR System was used for the RT-qPCR experiment. Primers for RT-qPCR are listed in Supplemental Table S3.
4.3. Histochemical and Histological Analyses
The 5th–10th internodes of OE-PtrVCS2 transgenics and wild-type plants were harvested and cut into 2–3 mm fragments, and then immersed in 4% formaldehyde following established procedures [39]. The cut wax tape was fixed on the slide and immersed in 2.5% safranin O and 1.25% fast green. The size and area of vessels were measured by using PreciPoint Streaming Software of Precipoint Digital Scanning microscope imaging System (M8). The observation and statistical methods of stomata were conducted as previously described [88]. The images were scanned with ViewPoint Light. IBM SPSS Statistics 19 was used for statistical analyses.
4.4. Scanning Electron Micrograph (SEM) Analyses
Fresh stem segments from the 12th internode of OE-PtrVCS2 transgenic and WT plants were plated with gold (Au) for 60 s at 10 mA. Fresh leaves of OE-PtrVCS2 transgenic and WT plants were harvested, and the lower epidermis was coated by nail polish. The leaves were then placed on ice to keep fresh. The leaves were cut into small squares, about 1 cm2 in area, for observation of the stomatal aperture. The samples were observed under high vacuum at 15 kV using a Nanotech JCM-5000 [89].
4.5. Physiological Index Measurement
Four time points (ND (normal condition, 50% VWC), D15 (15-day, 40% VWC), D30 (30-day, 40% VWC), and D60 (60-day, 40% VWC)) were selected to test key photosynthesis indicators and calculate WUE of the WT and OE-PtrVCS2 transgenics. At least 12 transgenics and 12 wild-type plants were used for each physiological index measurement. Three independent experiments were carried out. Net photosynthetic rate (Pn) and transpiration rate (Tr) were measured by Yaxin-1102G Portable photosynthesis system. Water use efficiency (WUE) = Pn/Tr × 100%. The base internode was used for measuring the percentage of loss of xylem conductance by XYL’EM- Plus (Bronkhorst). Volumetric soil water content (VWC, %) was measured by TDR 350 Soil Moisture Meter (Spectrum).
4.6. Drought Treatments
For short-term drought treatments, 4-month-old plants in saturated soil (50% VWC) were placed in a greenhouse without replenishing water, and soil water content was reduced from 50% to 30% to 15% and then finally rehydrated for 3 days. At least 12 transgenic plants and 12 wild-type plants were used for each drought treatment. Three independent experiments were performed. The screening experiments revealed that 15% VWC caused shoot dieback to 4-month-old wild-type P. trichocarpa plants; this was used for the estimation of the survival rate. After the soil water content was reduced to 15% VWC, all plants were rehydrated for 3 days to estimate their survival rates. For chronic drought treatments, 3-month-old OE-PtrVCS2 and WT plants were subjected to drought treatments by withholding water. After 12-day drought treatments, the VWC was reduced to 40% and maintained for 60 days [90].
Acknowledgments
We acknowledge the financial support from the Heilongjiang Touyan Innovation Team Program (Tree Genetics and Breeding Innovation Team).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/ijms24054458/s1.
Author Contributions
W.L., V.L.C. and M.L. conceived the research and designed the experiments. M.L., H.D., J.L. (Jiyuan Li), X.D. and J.L. (Jiaojiao Lin) conducted the experiments. M.L., S.L., C.Z., V.L.C. and W.L. analyzed the data. W.L., V.L.C. and M.L. wrote the manuscript with input from all co-authors. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplemental Information files.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the National Key Research and Development Program of China (grant no. 2021YFD2200700), the Fundamental Research Funds for the Central Universities of China (grant no. 2572022DQ01), and the National Natural Science Foundation of China (grant nos. 32001332 and 32001331).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.At the human-forest interface. Nat. Commun. 2018;9:1153. doi: 10.1038/s41467-018-03586-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agustí J., Blázquez M.A. Plant vascular development: Mechanisms and environmental regulation. Cell Mol. Life Sci. 2020;77:3711–3728. doi: 10.1007/s00018-020-03496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rehschuh R., Cecilia A., Zuber M., Faragó T., Baumbach T., Hartmann H., Jansen S., Mayr S., Ruehr N. Drought-Induced Xylem Embolism Limits the Recovery of Leaf Gas Exchange in Scots Pine. Plant Physiol. 2020;184:852–864. doi: 10.1104/pp.20.00407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rodriguez-Zaccaro F.D., Groover A. Wood and water: How trees modify wood development to cope with drought. Plant People Planet. 2019;1:346–355. doi: 10.1002/ppp3.29. [DOI] [Google Scholar]
- 5.Schenk H.J., Steppe K., Jansen S. Nanobubbles: A new paradigm for air-seeding in xylem. Trends Plant Sci. 2015;20:199–205. doi: 10.1016/j.tplants.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 6.Salleo S., Gullo M.A.L., De Paoli D., Zippo M. Xylem recovery from cavitation-induced embolism in young plants of Laurus nobilis: A possible mechanism. New Phytol. 1996;132:47–56. doi: 10.1111/j.1469-8137.1996.tb04507.x. [DOI] [PubMed] [Google Scholar]
- 7.Choat B., Jansen S., Brodribb T.J., Cochard H., Delzon S., Bhaskar R., Bucci S.J., Feild T.S., Gleason S.M., Hacke U.G., et al. Global convergence in the vulnerability of forests to drought. Nature. 2012;491:752–755. doi: 10.1038/nature11688. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z., Li S., Wan X., Liu S. Strategies of tree species to adapt to drought from leaf stomatal regulation and stem embolism resistance to root properties. Front. Plant Sci. 2022;13:926535. doi: 10.3389/fpls.2022.926535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aldon D., Mbengue M., Mazars C., Galaud J.-P. Calcium Signalling in Plant Biotic Interactions. Int. J. Mol. Sci. 2018;19:665. doi: 10.3390/ijms19030665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chrigui N., Sari D., Sari H., Eker T., Cengiz M.F., Ikten C., Toker C. Introgression of Resistance to Leafminer (Liriomyza cicerina Rondani) from Cicer reticulatum Ladiz. to C. arietinum L. and Relationships between Potential Biochemical Selection Criteria. Agronomy. 2021;11:57. doi: 10.3390/agronomy11010057. [DOI] [Google Scholar]
- 11.Chen Y., Chen Y., Shi Z., Jin Y., Sun H., Xie F., Zhang L. Biosynthesis and Signal Transduction of ABA, JA, and BRs in Response to Drought Stress of Kentucky Bluegrass. Int. J. Mol. Sci. 2019;20:1289. doi: 10.3390/ijms20061289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hetherington A.M., Woodward F.I. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Bai L., Wang W., Shi H., Ramón Botella J., Zhan Q., Liu K., Yang H.-Q., Song C.-P. COP1 promotes ABA-induced stomatal closure by modulating the abundance of ABI/HAB and AHG3 phosphatases. New Phytol. 2021;229:2035–2049. doi: 10.1111/nph.17001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sirichandra C., Wasilewska A., Vlad F., Valon C., Leung J. The guard cell as a single-cell model towards understanding drought tolerance and abscisic acid action. J. Exp. Bot. 2009;60:1439–1463. doi: 10.1093/jxb/ern340. [DOI] [PubMed] [Google Scholar]
- 15.Leakey A.D.B., Ferguson J.N., Pignon C.P., Wu A., Jin Z., Hammer G.L., Lobell D.B. Water Use Efficiency as a Constraint and Target for Improving the Resilience and Productivity of C3 and C4 Crops. Annu. Rev. Plant Biol. 2019;70:781–808. doi: 10.1146/annurev-arplant-042817-040305. [DOI] [PubMed] [Google Scholar]
- 16.Lawson T., Simkin A.J., Kelly G., Granot D. Mesophyll photosynthesis and guard cell metabolism impacts on stomatal behaviour. New Phytol. 2014;203:1064–1081. doi: 10.1111/nph.12945. [DOI] [PubMed] [Google Scholar]
- 17.Christman M.A., Sperry J.S., Smith D.D. Rare pits, large vessels and extreme vulnerability to cavitation in a ring-porous tree species. New Phytol. 2012;193:713–720. doi: 10.1111/j.1469-8137.2011.03984.x. [DOI] [PubMed] [Google Scholar]
- 18.Johnson D., Eckart P., Alsamadisi N., Noble H., Martin C., Spicer R. Polar auxin transport is implicated in vessel differentiation and spatial patterning during secondary growth in Populus. Am. J. Bot. 2018;105:186–196. doi: 10.1002/ajb2.1035. [DOI] [PubMed] [Google Scholar]
- 19.Taneda H., Tateno M. Hydraulic conductivity, photosynthesis and leaf water balance in six evergreen woody species from fall to winter. Tree Physiol. 2005;25:299–306. doi: 10.1093/treephys/25.3.299. [DOI] [PubMed] [Google Scholar]
- 20.Chen H., Wang J.P., Liu H., Li H., Lin Y.-C.J., Shi R., Yang C., Gao J., Zhou C., Li Q., et al. Hierarchical Transcription Factor and Chromatin Binding Network for Wood Formation in Black Cottonwood (Populus trichocarpa) Plant Cell. 2019;31:602–626. doi: 10.1105/tpc.18.00620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barros J., Serk H., Granlund I., Pesquet E. The cell biology of lignification in higher plants. Ann. Bot. 2015;115:1053–1074. doi: 10.1093/aob/mcv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu X., Cui Y., Lu X., Song W., Lei L., Zhu J., Lai J., E L., Zhao H. Maize WI5 encodes an endo-1,4-β-xylanase required for secondary cell wall synthesis and water transport in xylem. J. Integr. Plant Biol. 2020;62:1607–1624. doi: 10.1111/jipb.12923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan S.-A., Li M.-Z., Wang S.-M., Yin H.-J. Revisiting the Role of Plant Transcription Factors in the Battle against Abiotic Stress. Int. J. Mol. Sci. 2018;19:1634. doi: 10.3390/ijms19061634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang W., Wu P., Li Y., Hou X. Genome-wide analysis and expression patterns of ZF-HD transcription factors under different developmental tissues and abiotic stresses in Chinese cabbage. Mol. Genet Genom. 2016;291:1451–1464. doi: 10.1007/s00438-015-1136-1. [DOI] [PubMed] [Google Scholar]
- 25.Liu H., Yang Y., Zhang L. Zinc Finger-Homeodomain Transcriptional Factors (ZF-HDs) in Wheat ( Triticum aestivum L.): Identification, Evolution, Expression Analysis and Response to Abiotic Stresses. Plants. 2021;10:593. doi: 10.3390/plants10030593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun W., Wei J., Wu G., Xu H., Chen Y., Yao M., Zhan J., Yan J., Wu N., Chen H., et al. CqZF-HD14 enhances drought tolerance in quinoa seedlings through interaction with CqHIPP34 and CqNAC79. Plant Sci. 2022;323:111406. doi: 10.1016/j.plantsci.2022.111406. [DOI] [PubMed] [Google Scholar]
- 27.Xu H., Wang S., Larkin R.M., Zhang F. The transcription factors DcHB30 and DcWRKY75 antagonistically regulate ethylene-induced petal senescence in carnation (Dianthus caryophyllus) J. Exp. Bot. 2022;73:7326–7343. doi: 10.1093/jxb/erac357. [DOI] [PubMed] [Google Scholar]
- 28.Tan Q., Jiang S., Wang N., Liu X., Zhang X., Wen B., Fang Y., He H., Chen X., Fu X., et al. OVATE Family Protein PpOFP1 Physically Interacts With PpZFHD1 and Confers Salt Tolerance to Tomato and Yeast. Front. Plant Sci. 2021;12:759955. doi: 10.3389/fpls.2021.759955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu W., de Pamphilis C.W., Ma H. Phylogenetic analysis of the plant-specific zinc finger-homeobox and mini zinc finger gene families. J. Integr. Plant Biol. 2008;50:1031–1045. doi: 10.1111/j.1744-7909.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 30.Hu W., Ma H. Characterization of a novel putative zinc finger gene MIF1: Involvement in multiple hormonal regulation of Arabidopsis development. Plant J. 2006;45:399–422. doi: 10.1111/j.1365-313X.2005.02626.x. [DOI] [PubMed] [Google Scholar]
- 31.Bollier N., Sicard A., Leblond J., Latrasse D., Gonzalez N., Gévaudant F., Benhamed M., Raynaud C., Lenhard M., Chevalier C., et al. At-MINI ZINC FINGER2 and Sl-INHIBITOR OF MERISTEM ACTIVITY, a Conserved Missing Link in the Regulation of Floral Meristem Termination in Arabidopsis and Tomato. Plant Cell. 2018:30. doi: 10.1105/tpc.17.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bollier N., Gonzalez N., Chevalier C., Hernould M. Zinc Finger-Homeodomain and Mini Zinc Finger proteins are key players in plant growth and responses to environmental stresses. J. Exp. Bot. 2022;73:4662–4673. doi: 10.1093/jxb/erac194. [DOI] [PubMed] [Google Scholar]
- 33.Hou J., Sun Y., Wang L., Jiang Y., Chen N., Tong S. Genome-Wide Analysis of the Homeobox Gene Family and Identification of Drought-Responsive Members in Populus trichocarpa. Plants. 2021;10:2284. doi: 10.3390/plants10112284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dai X., Zhai R., Lin J., Wang Z., Meng D., Li M., Mao Y., Gao B., Ma H., Zhang B., et al. Cell-type-specific PtrWOX4a and PtrVCS2 form a regulatory nexus with a histone modification system for stem cambium development in Populus trichocarpa. Nat. Plants. :2023. doi: 10.1038/s41477-022-01315-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye Z.-H. Vascular tissue differentiation and pattern formation in plants. Annu. Rev. Plant Biol. 2002;53:183–202. doi: 10.1146/annurev.arplant.53.100301.135245. [DOI] [PubMed] [Google Scholar]
- 36.Allen C.D., Macalady A.K., Chenchouni H., Bachelet D., McDowell N., Vennetier M., Kitzberger T., Rigling A., Breshears D.D., Hogg E.H., et al. A global overview of drought and heat-induced tree mortality reveals emerging climate change risks for forests. For. Ecol. Manag. 2010;259:660–684. doi: 10.1016/j.foreco.2009.09.001. [DOI] [Google Scholar]
- 37.Schwalm C.R., Anderegg W.R.L., Michalak A.M., Fisher J.B., Biondi F., Koch G., Litvak M., Ogle K., Shaw J.D., Wolf A., et al. Global patterns of drought recovery. Nature. 2017;548:202–205. doi: 10.1038/nature23021. [DOI] [PubMed] [Google Scholar]
- 38.Chen G., Li Y., Liu S., Junaid M., Wang J. Effects of micro(nano)plastics on higher plants and the rhizosphere environment. Pt 1Sci. Total Environ. 2022;807:150841. doi: 10.1016/j.scitotenv.2021.150841. [DOI] [PubMed] [Google Scholar]
- 39.Li S., Lin Y.-C.J., Wang P., Zhang B., Li M., Chen S., Shi R., Tunlaya-Anukit S., Liu X., Wang Z., et al. The AREB1 Transcription Factor Influences Histone Acetylation to Regulate Drought Responses and Tolerance in Populus trichocarpa. Plant Cell. 2018;31:663–686. doi: 10.1105/tpc.18.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu H., Gao J., Sun J., Li S., Zhang B., Wang Z., Zhou C., Sulis D.B., Wang J.P., Chiang V.L., et al. Dimerization of PtrMYB074 and PtrWRKY19 mediates transcriptional activation of PtrbHLH186 for secondary xylem development in Populus trichocarpa. New Phytol. 2022;234:918–933. doi: 10.1111/nph.18028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen J., Nolan T.M., Ye H., Zhang M., Tong H., Xin P., Chu J., Chu C., Li Z., Yin Y. Arabidopsis WRKY46, WRKY54, and WRKY70 Transcription Factors Are Involved in Brassinosteroid-Regulated Plant Growth and Drought Responses. Plant Cell. 2017;29:1425–1439. doi: 10.1105/tpc.17.00364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Han M., Jin X., Yao W., Kong L., Huang G., Tao Y., Li L., Wang X., Wang Y. A Mini Zinc-Finger Protein (MIF) from Activates the GASA Protein Family Gene, GEG, to Inhibit Ray Petal Elongation. Front. Plant Sci. 2017;8:1649. doi: 10.3389/fpls.2017.01649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huerta-Ocampo J.A., León-Galván M.F., Ortega-Cruz L.B., Barrera-Pacheco A., De León-Rodríguez A., Mendoza-Hernández G., de la Rosa A.P.B. Water stress induces up-regulation of DOF1 and MIF1 transcription factors and down-regulation of proteins involved in secondary metabolism in amaranth roots (Amaranthus hypochondriacus L.) Plant Biol. 2011;13:472–482. doi: 10.1111/j.1438-8677.2010.00391.x. [DOI] [PubMed] [Google Scholar]
- 44.Fichot R., Laurans F., Monclus R., Moreau A., Pilate G., Brignolas F. Xylem anatomy correlates with gas exchange, water-use efficiency and growth performance under contrasting water regimes: Evidence from Populus deltoides x Populus nigra hybrids. Tree Physiol. 2009;29:1537–1549. doi: 10.1093/treephys/tpp087. [DOI] [PubMed] [Google Scholar]
- 45.Halevy A.H., Kessler B. Increased Tolerance of Bean Plants to Soil Drought by means of Growth-retarding Substances. Nature. 1963;197:310–311. doi: 10.1038/197310a0. [DOI] [Google Scholar]
- 46.Kasuga M., Liu Q., Miura S., Yamaguchi-Shinozaki K., Shinozaki K. Improving plant drought, salt, and freezing tolerance by gene transfer of a single stress-inducible transcription factor. Nat. Biotechnol. 1999;17:287–291. doi: 10.1038/7036. [DOI] [PubMed] [Google Scholar]
- 47.Skirycz A., Inzé D. More from less: Plant growth under limited water. Curr. Opin. Biotechnol. 2010;21:197–203. doi: 10.1016/j.copbio.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida T., Mogami J., Yamaguchi-Shinozaki K. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 2014;21:133–139. doi: 10.1016/j.pbi.2014.07.009. [DOI] [PubMed] [Google Scholar]
- 49.Tyree M.T., Sperry J.S. Vulnerability of Xylem to Cavitation and Embolism. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:19–36. doi: 10.1146/annurev.pp.40.060189.000315. [DOI] [Google Scholar]
- 50.Fisher J.B., Goldstein G., Jones T.J., Cordell S. Wood vessel diameter is related to elevation and genotype in the Hawaiian tree Metrosideros polymorpha (Myrtaceae) Am. J. Bot. 2007;94:709–715. doi: 10.3732/ajb.94.5.709. [DOI] [PubMed] [Google Scholar]
- 51.Hoffmann N., King S., Samuels A.L., McFarlane H.E. Subcellular coordination of plant cell wall synthesis. Dev. Cell. 2021;56:933–948. doi: 10.1016/j.devcel.2021.03.004. [DOI] [PubMed] [Google Scholar]
- 52.Ezquer I., Salameh I., Colombo L., Kalaitzis P. Plant Cell Walls Tackling Climate Change: Insights into Plant Cell Wall Remodeling, Its Regulation, and Biotechnological Strategies to Improve Crop Adaptations and Photosynthesis in Response to Global Warming. Plants. 2020;9:212. doi: 10.3390/plants9020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ko J.H., Jeon H.W., Kim W.C., Kim J.Y., Han K.H. The MYB46/MYB83-mediated transcriptional regulatory programme is a gatekeeper of secondary wall biosynthesis. Ann. Bot. 2014;114:1099–1107. doi: 10.1093/aob/mcu126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wolny E., Skalska A., Braszewska A., Mur L.A.J., Hasterok R. Defining the Cell Wall, Cell Cycle and Chromatin Landmarks in the Responses of to Salinity. Int. J. Mol. Sci. 2021;22:949. doi: 10.3390/ijms22020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lu X., Zhang L., Zhang F., Jiang W., Shen Q., Zhang L., Lv Z., Wang G., Tang K. AaORA, a trichome-specific AP2/ERF transcription factor of Artemisia annua, is a positive regulator in the artemisinin biosynthetic pathway and in disease resistance to Botrytis cinerea. New Phytol. 2013;198:1191–1202. doi: 10.1111/nph.12207. [DOI] [PubMed] [Google Scholar]
- 56.Shani Z., Dekel M., Roiz L., Horowitz M., Kolosovski N., Lapidot S., Alkan S., Koltai H., Tsabary G., Goren R., et al. Expression of endo-1,4-beta-glucanase (cel1) in Arabidopsis thaliana is associated with plant growth, xylem development and cell wall thickening. Plant Cell Rep. 2006;25:1067–1074. doi: 10.1007/s00299-006-0167-9. [DOI] [PubMed] [Google Scholar]
- 57.Zhuo C., Wang X., Docampo-Palacios M., Sanders B.C., Engle N.L., Tschaplinski T.J., Hendry J.I., Maranas C.D., Chen F., Dixon R.A. Developmental changes in lignin composition are driven by both monolignol supply and laccase specificity. Sci. Adv. 2022;8:eabm8145. doi: 10.1126/sciadv.abm8145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jyske T., Hölttä T., Mäkinen H., Nöjd P., Lumme I., Spiecker H. The effect of artificially induced drought on radial increment and wood properties of Norway spruce. Tree Physiol. 2010;30:103–115. doi: 10.1093/treephys/tpp099. [DOI] [PubMed] [Google Scholar]
- 59.Liu M., Zhao Y., Wang Y., Korpelainen H., Li C. Stem xylem traits and wood formation affect sex-specific responses to drought and rewatering in Populus cathayana. Tree Physiol. 2022;42:1350–1363. doi: 10.1093/treephys/tpac011. [DOI] [PubMed] [Google Scholar]
- 60.Fan D.-Y., Dang Q.-L., Xu C.-Y., Jiang C.-D., Zhang W.-F., Xu X.-W., Yang X.-F., Zhang S.-R. Stomatal Sensitivity to Vapor Pressure Deficit and the Loss of Hydraulic Conductivity Are Coordinated in Populus euphratica, a Desert Phreatophyte Species. Front. Plant Sci. 2020;11:1248. doi: 10.3389/fpls.2020.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodriguez-Dominguez C.M., Brodribb T.J. Declining root water transport drives stomatal closure in olive under moderate water stress. New Phytol. 2020;225:126–134. doi: 10.1111/nph.16177. [DOI] [PubMed] [Google Scholar]
- 62.Schroeder J.I., Kwak J.M., Allen G.J. Guard cell abscisic acid signalling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- 63.Chen Z., Zhao P.-X., Miao Z.-Q., Qi G.-F., Wang Z., Yuan Y., Ahmad N., Cao M.-J., Hell R., Wirtz M., et al. SULTR3s Function in Chloroplast Sulfate Uptake and Affect ABA Biosynthesis and the Stress Response. Plant Physiol. 2019;180:593–604. doi: 10.1104/pp.18.01439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zheng X.-Y., Spivey N.W., Zeng W., Liu P.-P., Fu Z.Q., Klessig D.F., He S.Y., Dong X. Coronatine promotes Pseudomonas syringae virulence in plants by activating a signaling cascade that inhibits salicylic acid accumulation. Cell Host Microbe. 2012;11:587–596. doi: 10.1016/j.chom.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huang L., Chen L., Wang L., Yang Y., Rao Y., Ren D., Dai L., Gao Y., Zou W., Lu X., et al. A Nck-associated protein 1-like protein affects drought sensitivity by its involvement in leaf epidermal development and stomatal closure in rice. Plant J. 2019;98:884–897. doi: 10.1111/tpj.14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang Y.H., Gehring C., Irving H.R. Plant natriuretic peptides are apoplastic and paracrine stress response molecules. Plant Cell Physiol. 2011;52:837–850. doi: 10.1093/pcp/pcr036. [DOI] [PubMed] [Google Scholar]
- 67.Zheng X.-Y., Zhou M., Yoo H., Pruneda-Paz J.L., Spivey N.W., Kay S.A., Dong X. Spatial and temporal regulation of biosynthesis of the plant immune signal salicylic acid. Proc. Natl. Acad. Sci. USA. 2015;112:9166–9173. doi: 10.1073/pnas.1511182112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Meng X., Chen X., Mang H., Liu C., Yu X., Gao X., Torii K.U., He P., Shan L. Differential Function of Arabidopsis SERK Family Receptor-like Kinases in Stomatal Patterning. Curr. Biol. 2015;25:2361–2372. doi: 10.1016/j.cub.2015.07.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Balcerowicz M., Ranjan A., Rupprecht L., Fiene G., Hoecker U. Auxin represses stomatal development in dark-grown seedlings via Aux/IAA proteins. Development. 2014;141:3165–3176. doi: 10.1242/dev.109181. [DOI] [PubMed] [Google Scholar]
- 70.Xu H., Shi X., He L., Guo Y., Zang D., Li H., Zhang W., Wang Y. Arabidopsis thaliana Trihelix Transcription Factor AST1 Mediates Salt and Osmotic Stress Tolerance by Binding to a Novel AGAG-Box and Some GT Motifs. Plant Cell Physiol. 2018;59:946–965. doi: 10.1093/pcp/pcy032. [DOI] [PubMed] [Google Scholar]
- 71.Hsu P.-K., Dubeaux G., Takahashi Y., Schroeder J.I. Signaling mechanisms in abscisic acid-mediated stomatal closure. Plant J. 2021;105:307–321. doi: 10.1111/tpj.15067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Khatun K., Nath U.K., Robin A.H.K., Park J.-I., Lee D.-J., Kim M.-B., Kim C.K., Lim K.-B., Nou I.S., Chung M.-Y. Genome-wide analysis and expression profiling of zinc finger homeodomain (ZHD) family genes reveal likely roles in organ development and stress responses in tomato. BMC Genom. 2017;18:695. doi: 10.1186/s12864-017-4082-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang H., Tang J., Liu J., Hu J., Liu J., Chen Y., Cai Z., Wang X. Abscisic Acid Signaling Inhibits Brassinosteroid Signaling through Dampening the Dephosphorylation of BIN2 by ABI1 and ABI2. Mol. Plant. 2018;11:315–325. doi: 10.1016/j.molp.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 74.Verma V., Ravindran P., Kumar P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016;16:86. doi: 10.1186/s12870-016-0771-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Eziz A., Yan Z., Tian D., Han W., Tang Z., Fang J. Drought effect on plant biomass allocation: A meta-analysis. Ecol. Evol. 2017;7:11002–11010. doi: 10.1002/ece3.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Smith W.K. Importance of Aerodynamic Resistance to Water Use Efficiency in Three Conifers under Field Conditions. Plant Physiol. 1980;65:132–135. doi: 10.1104/pp.65.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bhaskara G.B., Lasky J.R., Razzaque S., Zhang L., Haque T., Bonnette J.E., Civelek G.Z., Verslues P.E., Juenger T.E. Natural variation identifies new effectors of water-use efficiency in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2022;119:e2205305119. doi: 10.1073/pnas.2205305119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li S., Zhen C., Xu W., Wang C., Cheng Y. Simple, rapid and efficient transformation of genotype Nisqually-1: A basic tool for the first sequenced model tree. Sci. Rep. 2017;7:2638. doi: 10.1038/s41598-017-02651-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li Q., Lin Y.-C., Sun Y.-H., Song J., Chen H., Zhang X.-H., Sederoff R.R., Chiang V.L. Splice variant of the SND1 transcription factor is a dominant negative of SND1 members and their regulation in Populus trichocarpa. Proc. Natl. Acad. Sci. USA. 2012;109:14699–14704. doi: 10.1073/pnas.1212977109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lin Y.-C., Li W., Sun Y.-H., Kumari S., Wei H., Li Q., Tunlaya-Anukit S., Sederoff R.R., Chiang V.L. SND1 transcription factor-directed quantitative functional hierarchical genetic regulatory network in wood formation in Populus trichocarpa. Plant Cell. 2013;25:4324–4341. doi: 10.1105/tpc.113.117697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lin Y.-C., Li W., Chen H., Li Q., Sun Y.-H., Shi R., Lin C.-Y., Wang J.P., Chen H.-C., Chuang L., et al. A simple improved-throughput xylem protoplast system for studying wood formation. Nat. Protoc. 2014;9:2194–2205. doi: 10.1038/nprot.2014.147. [DOI] [PubMed] [Google Scholar]
- 82.Kim D., Langmead B., Salzberg S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods. 2015;12:357–360. doi: 10.1038/nmeth.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Buchfink B., Xie C., Huson D.H. Fast and sensitive protein alignment using DIAMOND. Nat. Methods. 2015;12:59–60. doi: 10.1038/nmeth.3176. [DOI] [PubMed] [Google Scholar]
- 84.Sanseverino W., Roma G., De Simone M., Faino L., Melito S., Stupka E., Frusciante L., Ercolano M.R. PRGdb: A bioinformatics platform for plant resistance gene analysis. Nucleic Acids Res. 2010;38:D814–D821. doi: 10.1093/nar/gkp978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhou X., Bao S., Liu J., Yong Z. De Novo Sequencing and Analysis of the Transcriptome of the Wild Eggplant Species Solanum Aculeatissimum in Response to Verticillium dahliae. Plant Mol. Biol. Report. 2016;34:1193–1203. doi: 10.1007/s11105-016-0998-7. [DOI] [Google Scholar]
- 86.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Anders S., Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Miao J., Li X., Li X., Tan W., You A., Wu S., Tao Y., Chen C., Wang J., Zhang D., et al. OsPP2C09, a negative regulatory factor in abscisic acid signalling, plays an essential role in balancing plant growth and drought tolerance in rice. New Phytol. 2020;227:1417–1433. doi: 10.1111/nph.16670. [DOI] [PubMed] [Google Scholar]
- 89.Wang Z., Mao Y., Guo Y., Gao J., Liu X., Li S., Lin Y.-C.J., Chen H., Wang J.P., Chiang V.L., et al. MYB Transcription Factor161 Mediates Feedback Regulation of Family Genes for Wood Formation. Plant Physiol. 2020;184:1389–1406. doi: 10.1104/pp.20.01033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Georgii E., Kugler A.K., Pfeifer B.M., Vanzo B.E., Block C.K. The Systems Architecture of Molecular Memory in Poplar after Abiotic Stress. Plant Cell. 2019;31:tpc0043102018. doi: 10.1105/tpc.18.00431. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplemental Information files.