Abstract
Background: The identification of asymptomatic structural and functional cardiac abnormalities can help us to recognize early and intervene in patients at pre-heart failure (HF). However, few studies have adequately evaluated the associations of renal function and left ventricular (LV) structure and function in patients at high risk of cardiovascular diseases (CVD). Methods: Patients undergoing coronary angiography and/or percutaneous coronary interventions were enrolled from the Cardiorenal ImprovemeNt II (CIN-II) cohort study, and their echocardiography and renal function were assessed at admission. Patients were divided into five groups according to their estimated glomerular filtration rate (eGFR). Our outcomes were LV hypertrophy and LV systolic and diastolic dysfunction. Multivariable logistic regression analyses were conducted to investigate the associations of eGFR with LV hypertrophy and LV systolic and diastolic dysfunction. Results: A total of 5610 patients (mean age: 61.6 ± 10.6 years; 27.3% female) were included in the final analysis. The prevalence of LV hypertrophy assessed by echocardiography was 29.0%, 34.8%, 51.9%, 66.7%, and 74.3% for the eGFR categories >90, 61–90, 31–60, 16–30, and ≤15 mL/min per 1.73 m2 or for patients needing dialysis, respectively. Multivariate logistic regression analysis showed that subjects with eGFR levels of ≤15 mL/min per 1.73 m2 or needing dialysis (OR: 4.66, 95% CI: 2.96–7.54), as well as those with eGFR levels of 16–30 (OR: 3.87, 95% CI: 2.43–6.24), 31–60 (OR: 2.00, 95% CI: 1.64–2.45), and 61–90 (OR: 1.23, 95% CI: 1.07–1.42), were significantly associated with LV hypertrophy. This reduction in renal function was also significantly associated with LV systolic and diastolic dysfunction (all P for trend <0.001). In addition, a per one unit decrease in eGFR was associated with a 2% heightened combined risk of LV hypertrophy and systolic and diastolic dysfunction. Conclusions: Among patients at high risk of CVD, poor renal function was strongly associated with cardiac structural and functional abnormalities. In addition, the presence or absence of CAD did not change the associations. The results may have implications for the pathophysiology behind cardiorenal syndrome.
Keywords: renal function, echocardiography, left ventricular function, left ventricular hypertrophy
1. Introduction
Heart failure (HF) is globally recognized as a major public health problem, with increasing prevalence and mortality in developing countries [1]. In the latest guideline for the management of HF, pre-HF is defined as a phase of asymptomatic structural and functional cardiac abnormalities [2]. Early recognition and intervention can delay the development of HF and improve the prognosis of patients with HF. Therefore, the early detection of structural and functional changes may help clinicians to recognize patients at pre-HF earlier.
Prior studies and guidelines suggest that renal function insufficiency is one of the most important risk factors for the progression and poorer prognosis of HF [2,3,4]. The changes in heart structure and function are the key pathophysiological elements of heart failure, which may meanwhile underlie the renal pathology, based on interactions through the sympathetic signaling changing of the renin–angiotensin–aldosterone system (RAAS) [5,6,7]. Previous studies indicated that in the general population or patients with CKD, poor renal function was associated with abnormal LV structure and dysfunction [8,9,10]. However, some studies demonstrated inconsistently negative associations between renal function and LV structure and function among the general population or CKD patients [10,11,12]. Few studies have investigated the associations between the cardiac profile and renal function in patients at high risk of HF or cardiovascular diseases (CVD). Therefore, we design this study to systematically examine the associations of renal function with LV structure and systolic diastolic function in high-risk CVD patients.
2. Methods
2.1. Study Population
This multicenter study cohort was recruited from the Cardiorenal ImprovemeNt II (CIN-II, NCT05050877) study, conducted among five regional central tertiary teaching hospitals in China. Patients who underwent coronary angiography (CAG) and/or percutaneous coronary intervention (PCI) were included. The indications of CAG or PCI were signs or symptoms of ischemia, elevated diagnostic enzymes, or abnormal electrocardiogram findings. All treatments were performed based on the standard clinical practice guidelines [13,14].
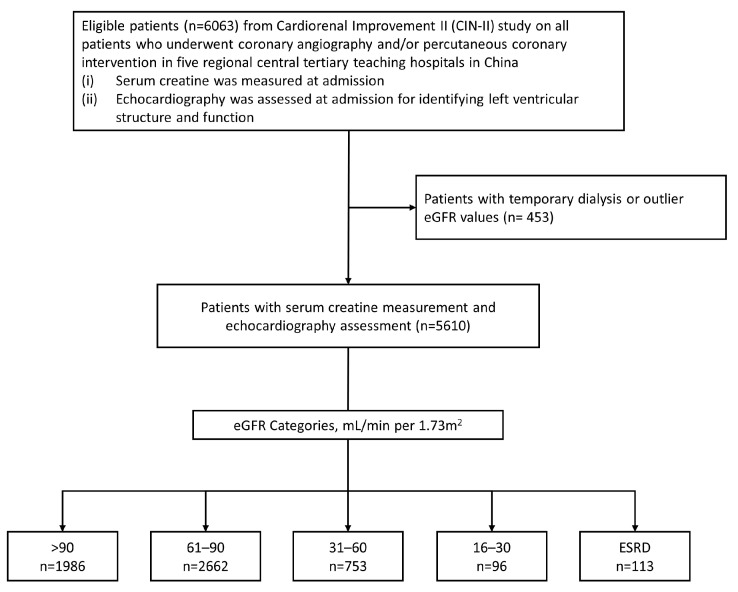
We enrolled patients (≥18 years) who underwent echocardiographic assessment (structure, systolic, and diastolic function) and had measurements of serum creatinine, height, and weight on admission. Patients with temporary dialysis or outlier eGFR values (>120 mL/min per 1.73 m2) were excluded. Finally, 5610 patients were included in this study (Figure 1). The study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No.GDREC2019555H[R1]) and conducted in accordance with the principles of the Declaration of Helsinki. All participating sites received institutional review board approval from their own ethics committees.
Figure 1.
Patient flow diagram.
2.2. Data Collection and Definitions
All clinical data of the enrolled patients were collected from the electronic medical record system for all the participant hospitals, including demographic characteristics, medical history, procedures, laboratory examinations, echocardiographic data, and discharge medications. The eGFR was calculated using the Chronic Kidney Diseases Epidemiology Collaboration equation [15]. CKD was defined as eGFR < 60 mL/min per 1.73 m2 and end-stage renal disease (ESRD) was defined as eGFR < 15 mL/min per 1.73 m2 or the maintenance of dialysis [16,17]. Congestive heart failure (CHF) was defined as New York Heart Association (NYHA) functional class > 2 or Killip class > 1 [18].
2.3. Echocardiography Assessment
Echocardiography was performed by the same team of trained cardiac ultrasound doctors at Guangdong Provincial People’s Hospital for all the patients at the time of admission using Philips EPIQ5. The structural indices assessed on echocardiography included the left ventricular (LV) thickness (interventricular septal wall thickness (IVS), posterior wall thickness (PWT), and relative wall thickness (RWT)), LV size (LV end-systolic diameter (LVESD) and LV end-diastolic diameter (LVEDD)), LV systolic function (LV ejection fraction (LVEF)), and LV diastolic function (early mitral inflow peak velocity (E), early mitral annulus TDI velocity (e’), peak velocity flow in the early to late diastole (E/A)). The LV mass was calculated using the linear method and indexed to the body surface area as the LV mass index (LVMI). The RWT was calculated using the formula (2 × diastolic PWT)/LVEDD and was considered to be increased if the result was >0.42. LV hypertrophy was defined as LVMI > 115 g/m2 in men and LVMI > 95 g/m2 in women. The LV geometry was classified using the LVMI and RWT as normal (no LV hypertrophy and normal RWT), concentric remodeling (no LV hypertrophy and increased RWT), concentric hypertrophy (LV hypertrophy and increased RWT), and eccentric hypertrophy (LV hypertrophy and normal RWT) [19]. LV systolic dysfunction was defined as LVEF < 55%, and LV diastolic dysfunction was defined as E/e’ > 14 [20].
2.4. Statistical Analysis
The patients were divided into five groups according to their eGFR levels (>90, 61–90, 31–60, 16–30, and ≤15 mL/min per 1.73 m2 or the need for dialysis). Data were presented as the mean with standard deviation (SD) or median with interquartile range (IQR) for continuous variables and as the quantity and frequency (%) for categorical variables. The categorical variables were compared using Pearson’s chi-squared test, and the continuous variables were compared using t-test. Univariable and multivariable logistic regression was used to test the associations between the eGFR categories and LV hypertrophy, as well as LV systolic and diastolic dysfunction. A linear trend test was applied using 5 groups as a continuous variable by assigning the median value of each group to the variable. Restricted cubic splines were plotted to reveal the potential linear associations between the eGFR as a continuous variable and the odds ratio (OR) of LV hypertrophy and LV systolic and diastolic dysfunction. Model 1 was unadjusted, Model 2 was adjusted for age, gender, and body mass index, and Model 3 was adjusted according to Model 2, adding diabetes mellitus (DM), hypertension (HT), CHF, high-density lipoprotein cholesterol, β-blocker, and angiotensin-converting enzyme inhibitor (ACEI) or angiotensin receptor blocker (ARB). All analyses were performed using R software (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria). A two-sided p-value < 0.05 indicated significance for all the analyses.
3. Results
3.1. Baseline Characteristics
The baseline characteristics of the study cohort are presented in Table 1. The average age of the 5610 patients was 61.6 ± 10.6 years, and 1535 (27.3%) were female. Of those patients, 17.1% (n = 962) had CKD and 2% (n = 113) had ESRD. In comparison with those who had a higher eGFR, patients with a lower eGFR tended to have a worse cardiovascular risk profile (older, with a higher prevalence of hypertension, diabetes mellitus, CHF, CAD, and stroke). With a lower eGFR, there were progressive decreases in the mean LVEF and increases in the mean echo parameters of the LV thickness (IVS and PWT), LV size (LVEDD and LVESD), and LV diastolic dysfunction (E/e’). There was also a correlation between a lower eGFR and a higher proportion of concentric hypertrophy.
Table 1.
Baseline characteristics of the study population.
| eGFR Categories, mL/min per 1.73 m2 | |||||||
|---|---|---|---|---|---|---|---|
| Characteristics | Overall (N = 5610) |
>90 (N = 1986) |
61–90 (N = 2662) |
31–60 (N = 753) |
16–30 (N = 96) |
≤15 or Dialysis (N = 113) |
p-Value |
| Demographic | |||||||
| Age, years | 61.6 (10.6) | 56.2 (9.4) | 63.6 (10.0) | 67.4 (9.6) | 68.6 (9.6) | 63.7 (9.6) | <0.001 |
| Female, n (%) | 1534 (27.3) | 546 (27.5) | 699 (26.3) | 230 (30.5) | 33 (34.4) | 26 (23.0) | 0.063 |
| Height, cm | 162.9 (15.5) | 163.4 (16.4) | 163.0 (13.9) | 161.3 (16.9) | 160.7 (18.5) | 160.7 (22.8) | 0.007 |
| Weight, kg | 65.7 (11.8) | 66.9 (12.0) | 65.4 (11.1) | 64.1 (12.4) | 62.6 (14.5) | 64.6 (13.1) | <0.001 |
| Body mass index, kg/m2 | 24.32 (3.37) | 24.56 (3.39) | 24.26 (3.24) | 24.00 (3.29) | 23.75 (5.43) | 24.04 (3.80) | <0.001 |
| Medical history | |||||||
| CAD, n (%) | 4254 (75.90) | 1433 (72.23) | 2029 (76.28) | 611 (81.25) | 81 (84.38) | 100 (88.50) | <0.001 |
| Hypertension, n (%) | 3171 (56.6) | 938 (47.3) | 1508 (56.7) | 547 (72.7) | 78 (81.3) | 100 (88.5) | <0.001 |
| Diabetes mellitus, n (%) | 1977 (35.2) | 600 (30.2) | 890 (33.4) | 367 (48.7) | 49 (51.0) | 71 (62.8) | <0.001 |
| Atrial Fibrillation, n (%) | 447 (8.0) | 98 (4.9) | 229 (8.6) | 99 (13.2) | 14 (14.6) | 7 (6.2) | <0.001 |
| CHF, n (%) | 767 (13.7) | 170 (8.6) | 321 (12.1) | 202 (26.9) | 25 (26.0) | 49 (43.4) | <0.001 |
| Stroke, n (%) | 388 (6.9) | 91 (4.6) | 195 (7.3) | 78 (10.4) | 11 (11.5) | 13 (11.5) | <0.001 |
| Procedure | |||||||
| PCI, n (%) | 3227 (57.5) | 1069 (53.8) | 1535 (57.7) | 475 (63.1) | 64 (66.7) | 84 (74.3) | <0.001 |
| Laboratory test | |||||||
| HDL-C, mmol/L | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 1.0 (0.3) | 0.9 (0.3) | <0.001 |
| LDL-C, mmol/L | 2.9 (0.9) | 2.9 (0.9) | 2.9 (0.9) | 2.9 (0.9) | 3.0 (1.2) | 2.6 (0.8) | <0.001 |
| Echo parameters | |||||||
| LVEF, % | 58.1 (12.8) | 60.1 (11.4) | 58.1 (13.0) | 54.4 (14.4) | 52.6 (12.5) | 52.6 (13.1) | <0.001 |
| LVEDD, mm | 48.7 (8.0) | 47.9 (7.1) | 48.7 (8.1) | 50.2 (8.9) | 50.7 (8.6) | 52.7 (7.4) | <0.001 |
| LVESD, mm | 32.9 (9.6) | 31.6 (8.5) | 32.9 (9.9) | 35.2 (10.8) | 35.9 (9.7) | 37.6 (8.9) | <0.001 |
| LVRWT, mm | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) | 0.484 |
| LVPWT, mm | 9.9 (2.1) | 9.8 (1.6) | 9.9 (2.4) | 10.1 (1.7) | 10.4 (1.8) | 11.2 (2.0) | <0.001 |
| IVS, mm | 10.4 (1.9) | 10.2 (1.9) | 10.3 (1.9) | 10.7 (2.0) | 11.1 (1.9) | 11.8 (1.8) | <0.001 |
| E/A | 1.0 (0.5) | 1.0 (0.5) | 1.0 (0.5) | 0.9 (0.6) | 1.0 (0.7) | 1.0 (0.6) | <0.001 |
| E/e’ | 14.0 (7.2) | 12.6 (5.9) | 13.9 (6.5) | 16.8 (9.5) | 17.2 (7.8) | 21.2 (11.7) | <0.001 |
| Left ventricular geometry | <0.001 | ||||||
| Normal, n (%) | 3153 (56.2) | 1265 (63.7) | 1526 (57.3) | 311 (41.3) | 25 (26.0) | 26 (23.0) | |
| Concentric remodeling, n (%) | 413 (7.4) | 143 (7.2) | 209 (7.9) | 51 (6.8) | 7 (7.3) | 3 (2.7) | |
| Concentric hypertrophy, n (%) | 1728 (30.8) | 474 (23.9) | 796 (29.9) | 327 (43.4) | 55 (57.3) | 76 (67.3) | |
| Eccentric hypertrophy, n (%) | 316 (5.6) | 104 (5.2) | 131 (4.9) | 64 (8.5) | 9 (9.4) | 8 (7.1) | |
| Medication | |||||||
| β-blockers, n (%) | 4275 (77.4) | 1457 (75.2) | 2064 (78.4) | 598 (80.0) | 66 (70.2) | 90 (80.4) | 0.013 |
| ACEI/ARB, n (%) | 3239 (58.62) | 1086 (56.07) | 1591 (60.40) | 486 (64.97) | 34 (36.17) | 42 (37.50) | <0.001 |
| CCB, n (%) | 1221 (22.1) | 377 (19.5) | 569 (21.6) | 188 (25.1) | 39 (41.5) | 48 (42.9) | <0.001 |
| Statins, n (%) | 4194 (75.9) | 1433 (74.0) | 2015 (76.5) | 589 (78.7) | 68 (72.3) | 89 (79.5) | 0.057 |
Abbreviation: ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; ACS, acute coronary syndrome; AMI, acute myocardial infarction; CAD, coronary artery diseases; CCB, calcium channel blockers; CHF, congestive heart failure; E, early mitral inflow peak velocity; eʹ, early mitral annulus Doppler tissue imaging velocity; E/A, peak velocity flow in early to late diastole; eGFR, estimated glomerular filtration rate; HDL-C, high-density lipoprotein cholesterol; IVS, interventricular septal wall thickness; LDL-C, low-density lipoprotein cholesterol; LVEDD, left ventricular end-diastolic dimension; LVEF, left ventricular ejection fraction; LVESD, ventricular end-systolic diameter; LVPWT, left ventricular posterior wall thickness; LVRWT, left ventricular relative wall thickness; PCI, percutaneous coronary intervention. Association between eGFR and echocardiographic parameters.
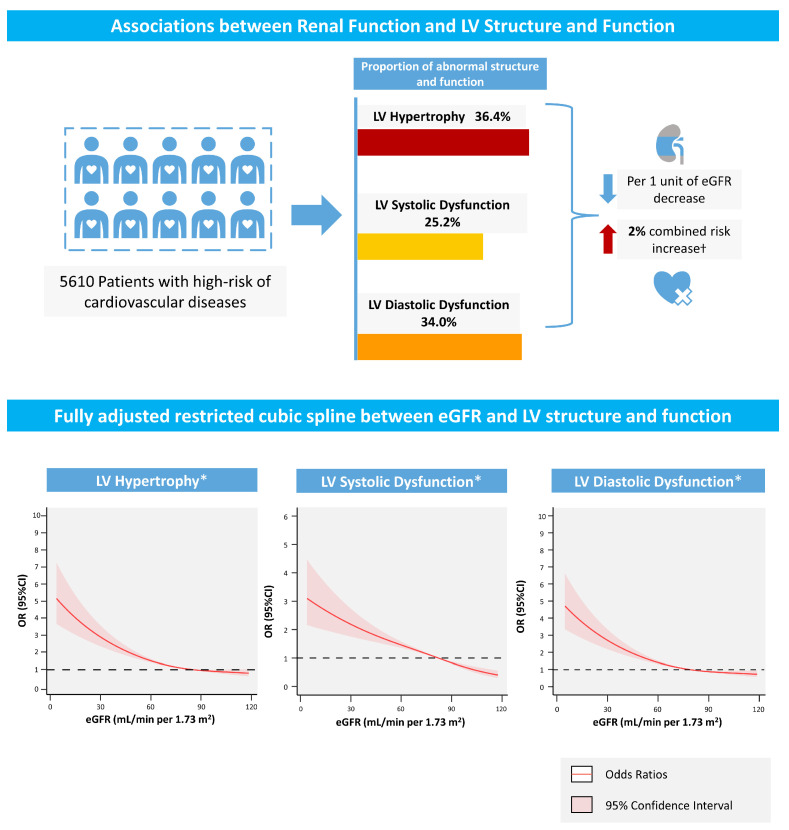
In total, 36.4%, 25.4%, and 34.0% of the patients had combined LV hypertrophy and LV systolic and diastolic dysfunction, respectively. The associations of the ORs for LV hypertrophy and LV systolic and diastolic dysfunction with eGFR in the fully adjusted restricted cubic spline plots are presented in Figure 2. When fully adjusted for confounders, the associations between eGFR and LV hypertrophy, as well as LV systolic and diastolic dysfunction, remained significant. As depicted in Figure 2, the combined risk of LV hypertrophy and LV systolic and diastolic dysfunction rapidly increases in patients with a lower eGFR.
Figure 2.
Central Illustration. † All p < 0.05. * Adjusted for age, gender, body mass index, diabetes mellitus, hypertension, congestive heart failure, high-density lipoprotein cholesterol, β-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker.
Subsequently, we ran logistic regression models to evaluate the associations between the eGFR categories and LV hypertrophy, as well as LV systolic and diastolic dysfunction. In the multivariate logistic regression analysis, patients with a lower eGFR were still significantly associated with a higher risk of LV hypertrophy and LV systolic and diastolic dysfunction (Table 2), and a per one unit decrease in eGFR was associated with a 2% heightened risk of combining with LV hypertrophy and systolic and diastolic dysfunction. Compared with the reference of eGFR > 90 mL/min per 1.73 m2, patients in the four groups based on the eGFR categories of 61–90, 31–60, 16–30, and ESRD had a higher risk of LV hypertrophy (OR: 1.23; 95% CI: 1.07–1.42, OR: 2.00; 95% CI: 1.64–2.45, OR: 3.87; 95% CI: 2.43–6.24, and OR: 4.66; 95% CI: 2.96–7.54, respectively, P for trend <0.001). Meanwhile, the same increased risk of LV systolic and diastolic dysfunction was observed in the unadjusted and multi-variables adjusted models (all P for trend <0.001).
Table 2.
Associations between the categories of eGFR and LV structure and function in the logistic analysis.
| eGFR (mL/min/1.73 m2) | Model 1 | Model 2 | Model 3 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| LV Hypertrophy | ||||||
| Per 1 unit decrease | 1.02 (1.02–1.02) | <0.001 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| >90 | Ref | - | Ref | - | Ref | - |
| 61–90 | 1.30 (1.15–1.48) | <0.001 | 1.35 (1.18–1.54) | <0.001 | 1.23(1.07–1.42) | 0.005 |
| 31–60 | 2.63 (2.21–3.13) | <0.001 | 2.71 (2.25–3.26) | <0.001 | 2.00 (1.64–2.45) | <0.001 |
| 16–30 | 4.87 (3.18–7.61) | <0.001 | 4.98 (3.21–7.85) | <0.001 | 3.87 (2.43–6.24) | <0.001 |
| ≤15 or dialysis | 7.06 (4.63–11.05) | <0.001 | 7.68 (5.01–12.08) | <0.001 | 4.66 (2.96–7.54) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| LV Systolic Dysfunction | ||||||
| Per 1 unit decrease | 1.02 (1.02–1.02) | <0.001 | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| >90 | Ref | - | Ref | - | Ref | - |
| 61–90 | 1.39 (1.21–1.61) | <0.001 | 1.63 (1.40–1.90) | <0.001 | 1.53 (1.29–1.81) | <0.001 |
| 31–60 | 2.71 (2.25–3.26) | <0.001 | 3.58 (2.92–4.39) | <0.001 | 2.67 (2.12–3.37) | <0.001 |
| 16–30 | 3.34 (2.19–5.07) | <0.001 | 4.62 (2.97–7.41) | <0.001 | 3.50 (2.11–5.75) | <0.001 |
| ≤15 or dialysis | 4.22 (2.87–6.21) | <0.001 | 4.98 (3.35–7.41) | <0.001 | 2.83 (1.76–4.52) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
| LV Diastolic Dysfunction | ||||||
| Per 1 unit decrease | 1.02 (1.02–1.03) | <0.001 | 1.02 (1.02–1.02) | <0.001 | 1.02 (1.01–1.02) | <0.001 |
| >90 | Ref | - | Ref | - | Ref | - |
| 61–90 | 1.49 (1.31–1.70) | <0.001 | 1.37 (1.19–1.58) | <0.001 | 1.26 (1.09–1.47) | 0.002 |
| 31–60 | 3.25 (2.72–3.87) | <0.001 | 2.81 (2.32–3.40) | <0.001 | 2.08 (1.70–2.56) | <0.001 |
| 16–30 | 3.42 (2.26–5.19) | <0.001 | 2.85 (1.86–4.38) | <0.001 | 2.30 (1.46–3.65) | <0.001 |
| ≤15 or dialysis | 11.20 (7.17–18.15) | <0.001 | 11.01 (7.01–17.94) | <0.001 | 6.74 (4.16–11.29) | <0.001 |
| P for trend | <0.001 | <0.001 | <0.001 | |||
Abbreviation: eGFR, estimated glomerular filtration rate; LV, left ventricular; OR, odds ratio; CI, confidence interval. Model 1: unadjusted. Model 2: adjusted for age, gender, and body mass index. Model 3: adjusted for multiple variables (age, gender, body mass index, diabetes mellitus, hypertension, congestive heart failure, high-density lipoprotein cholesterol, β-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker).
3.2. Subgroup Analysis
The subgroup analyses were consistent with the primary results (Table 3). When the analysis was stratified according to the coronary artery disease (CAD) status, the associations of the eGFR with LV hypertrophy and LV systolic and diastolic dysfunction did not differ significantly among individuals stratified by CAD without interaction (P for interaction: 0.519, 0.348, and 0.779, respectively).
Table 3.
Subgroup analysis stratified by CAD of the associations between eGFR and LV structure and function.
| eGFR (mL/min/1.73 m2) |
All Patients | CAD | Non-CAD | P for Interaction |
|||
|---|---|---|---|---|---|---|---|
| aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | aOR (95% CI) | p-Value | ||
| LV Hypertrophy | |||||||
| >90 | Ref | - | Ref | - | Ref | - | 0.519 |
| 61–90 | 1.23(1.07–1.42) | 0.005 | 1.13 (0.95–1.33) | 0.167 | 1.38 (1.04–1.83) | 0.027 | |
| 31–60 | 2.00 (1.64–2.45) | <0.001 | 1.85 (1.47–2.32) | <0.001 | 2.45 (1.57–3.87) | <0.001 | |
| 16–30 | 3.87 (2.43–6.24) | <0.001 | 3.48 (2.11–5.84) | <0.001 | 6.85 (1.92–32.47) | 0.006 | |
| ≤15 or dialysis | 4.66 (2.96–7.54) | <0.001 | 4.83 (2.98–8.07) | <0.001 | 3.98 (1.09–18.97) | 0.06 | |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| LV Systolic Dysfunction | |||||||
| >90 | Ref | - | Ref | - | Ref | - | 0.348 |
| 61–90 | 1.53 (1.29–1.81) | <0.001 | 1.40 (1.16–1.70) | 0.001 | 2.02 (1.37–3.01) | <0.001 | |
| 31–60 | 2.67 (2.12–3.37) | <0.001 | 2.30 (1.77–2.97) | <0.001 | 4.96 (2.82–8.74) | <0.001 | |
| 16–30 | 3.50 (2.11--5.75) | <0.001 | 2.90 (1.67–4.98) | <0.001 | 5.84 (1.46–21.38) | 0.009 | |
| ≤15 or dialysis | 2.83 (1.76–4.52) | <0.001 | 2.62 (1.59–4.29) | <0.001 | 3.14 (0.62–13.01) | 0.133 | |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
| LV Diastolic Dysfunction | |||||||
| >90 | Ref | - | Ref | - | Ref | - | 0.779 |
| 61–90 | 1.26 (1.09–1.47) | 0.002 | 1.14 (0.96–1.36) | 0.132 | 1.54 (1.15–2.06) | 0.004 | |
| 31–60 | 2.08 (1.70–2.56) | <0.001 | 1.76 (1.39–2.22) | <0.001 | 3.12 (2.00–4.91) | <0.001 | |
| 16–30 | 2.30 (1.46–3.65) | <0.001 | 1.95 (1.18–3.24) | 0.010 | 4.29 (1.37–14.20) | 0.013 | |
| ≤15 or dialysis | 6.74 (4.16–11.29) | <0.001 | 6.21 (3.49–10.85) | <0.001 | 7.72 (2.11–36.89) | 0.004 | |
| P for trend | <0.001 | <0.001 | <0.001 | ||||
Abbreviation: aOR, adjusted odds ratio; CAD, coronary artery diseases; CI, confidence interval; eGFR, estimated glomerular filtration rate; LV, left ventricular. Multivariable logistic regression was adjusted for multiple variables (age, gender, body mass index, diabetes mellitus, hypertension, congestive heart failure, high-density lipoprotein cholesterol, β-blockers, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker).
4. Discussion
To our knowledge, this is the first study to systematically evaluate the association between renal function and LV structure and systolic and diastolic function among patients at high risk of CVD. Among these patients, more than 1/3 had combined LV hypertrophy, of which concentric hypertrophy formed the highest proportion among the abnormal LV geometries. Almost 1/4 and 1/3 of the patients combined systolic and diastolic dysfunction, respectively. The proportion of LV hypertrophy and systolic and diastolic dysfunction increases directly to the renal function, and a per one unit decrease in eGFR was associated with a 2% heightened combined risk of LV hypertrophy and systolic and diastolic dysfunction.
LV hypertrophy is considered a key pathophysiological feature of patients at pre-HF and a strong predictor of poor prognosis among the general population [2,21]. In our study, 36.4% of patients at high risk of CVD had LV hypertrophy, and 30.8% had concentric hypertrophy. Among patients with CKD stages 3–5 of the Chronic Renal Insufficiency Cohort, Park M. showed that almost half of 3487 patients were classified as having LV hypertrophy, and concentric hypertrophy formed the highest proportion of cases of abnormal LV geometry [10], which is comparable to our study. Matsushita K. reported that the prevalence of LV hypertrophy was 10.6% among 4175 patients in a cohort from Atherosclerosis Risk in Communities population [8]. In addition, the above and other studies of the CKD cohort showed that renal insufficiency was independently associated with LV hypertrophy, especially in advanced CKD. However, several studies demonstrated no independent relations between lower renal function and abnormal LV structure [11,22,23]. The negative results of these studies should be interpreted as aimed at patients with a low risk of a poor cardiovascular profile due to the restricted enrollment of patients with cardiac conditions. In our study, concerning the patients at high risk of CVD, poor renal function was independently associated with LV hypertrophy after adjusting the confounders. Although no strong associations were observed in the relation of renal function and LV hypertrophy in the patients stratified by CAD, it should be noted that a lower eGFR is an independent risk factor for abnormal LV structure, which may require attention in clinical practice.
Systolic and diastolic dysfunction denotes the subsequent changes in LV remodeling and are important predictors for HF and poor prognosis. Previous studies have been inconsistent in determining the association between eGFR and LV systolic and diastolic function, regardless of whether they examined general patients, patients with CKD, or patients with DM [10,12,24]. In addition, strong associations related to LV dysfunction were observed more frequently in patients with advanced CKD. All these studies enrolled patients at a lower risk of CVD than those in our study. Previous studies showed the high prevalence of abnormal LV structure and function in patients with CVD and demonstrated that left ventricular hypertrophy was reversible, whereas diastolic dysfunction was difficult to be improved in patients at high risk of CVD [25,26]. Our study highlighted the high prevalence of LV systolic and diastolic dysfunction in patients at high risk of CVD and showed that even a mild eGFR reduction was consistently associated with a higher proportion of LV systolic and diastolic dysfunction.
Renal insufficiency is associated with structural and functional abnormalities based on multiple mechanisms, representing one of the essential risk factors for patients with pre-HF, and is associated with the development and poor prognosis of HF. There are several possible explanations for these cardiac changes with poor renal function. Firstly, poor renal function increases the burden of salt retention and volume overload, which leads to cardiovascular compensatory changes such as LV remodeling, hypertrophy, and even decompensatory alternations in function [5]. Secondly, patients with CKD are more likely to have diabetes or insulin resistance which may aggravate LV hypertrophy and, subsequently, diastolic and systolic dysfunction through the phosphoinositide-3 kinase–AKT pathway [17,27]. Thirdly, fibroblast growth factor-23 (FGF-23) was found to be elevated in the cases of poor renal function, as it can directly upregulate RAAS by inhibiting angiotensin 2 [28]. Additionally, elevations in FGF-23 can lead to 1,25(OH)2D deficiency through 1-α-hydroxylase suppression [29]. All of these factors may contribute to accelerated hypertrophy and dysfunction.
This study has clinical significance and several research implications. Our results demonstrated that poor renal function is an independent predictor of LV hypertrophy and dysfunction among patients at high risk of CVD. Poor renal function is considered a major risk factor for HF, and in recent years, the evaluation of renal function has received more attention from clinicians. However, the assessment of the phenotype of pre-HF and the impact of renal function on the heart are undervalued in clinical practice. This highlights the need for physicians to integrate the early recognition of changes in LV structure and function in clinical routines, especially for patients with mild renal insufficiency. Albuminuria is a well-established risk factor of CVD, and ACEI/ARB use may halt or reverse the progression of albuminuria [30]. In addition, the progression of renal function is significantly associated with increased cardiovascular risk, and multifactorial interventions for risk factors could improve cardiovascular renal outcomes and prognosis. Therefore, early intervention aiming to delay renal function progression or improve the LV structure and function is required in clinical practices [31,32,33,34]. Therefore, future study is needed to verify the efficacy of interventions in protecting renal function upon changes in LV structure and function and their prognostic value for patients with high-risk CVD.
5. Limitations
There are several limitations to our study that should be taken into consideration. Firstly, this study was a retrospectively multicenter study, and, thus, our findings reflect statistical associations and do not imply cause–effect relationships. Secondly, our study only used the LVEF and E/e’ for the evaluation of LV systolic and diastolic function, which is one of several parameters used to assess systolic and diastolic function. However, these are easily accessible parameters in clinical practice and can represent the cardiac function of patients. Thirdly, other validated renal function measurements such as the albuminuria level were not incorporated into our study, which could enhance better determination of patients. Fourthly, echocardiography was performed by a team of cardiac ultrasound doctors which may elevate variability and bring an erroneous stratification. Fifthly, the study was aimed at patients with high-risk CVD, who were mainly elderly patients. Although we adjusted for age, the findings should be cautious to extrapolate for other populations. Therefore, more prospective studies are required to evaluate the influence and mechanisms of the relationship between renal function and LV structure and function.
6. Conclusions
In summary, a reduction in eGFR was associated with increased LV hypertrophy and reduced systolic and diastolic function among patients at high risk of CVD. In addition, the presence or absence of CAD did not change the outcome. Further studies should be encouraged to explore the underlying risk factors and pathophysiological processes behind cardiorenal syndrome.
Acknowledgments
Amanda Y. Wang is supported by National Heart Foundation Vanguard grant, Australia.
Author Contributions
Research concept and study design: Y.L., A.Y.W., J.L. (Jin Liu) and X.L.; data acquisition: Q.L., X.L., J.D., Y.K., L.D., G.L., L.G., H.R., Z.P., J.L. (Jiaxi Li) and J.L. (Jin Liu); data analysis/interpretation: J.D., X.L., Q.L. and J.L. (Jin Liu); statistical analysis: X.L., G.L. and Q.L.; supervision and mentorship: Y.L., J.C., N.T. and A.Y.W. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any element of the work are appropriately investigated and resolved. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was approved by the Ethics Committee of Guangdong Provincial People’s Hospital (No.GDREC2019555H[R1]) and conducted in accordance with the principles of the Declaration of Helsinki. All participating sites received institutional review board approval from their own ethics committees.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to the institution’s policy but are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors have no conflict of interest to declare that are relevant to the content of this article.
Funding Statement
This research was funded and supported by the Guangdong Provincial Science and Technology Project (2020B1111170011), the Guangdong Provincial Science and Technology Project (KJ022021049) and Guangdong Provincial Key Laboratory of Coronary Heart Disease Prevention (No.Y0120220151). The funders had no role in the study design; data collection and analysis; the decision to publish the results; or the preparation of the manuscript. The work was not funded by any industry sponsors.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bragazzi N.L., Zhong W., Shu J., Abu Much A., Lotan D., Grupper A., Younis A., Dai H. Burden of heart failure and underlying causes in 195 countries and territories from 1990 to 2017. Eur. J. Prev. Cardiol. 2021;28:1682–1690. doi: 10.1093/eurjpc/zwaa147. [DOI] [PubMed] [Google Scholar]
- 2.Heidenreich P.A., Bozkurt B., Aguilar D., Allen L.A., Byun J.J., Colvin M.M., Deswal A., Drazner M.H., Dunlay S.M., Evers L.R., et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022;79:e263–e421. doi: 10.1016/j.jacc.2021.12.012. [DOI] [PubMed] [Google Scholar]
- 3.Janus S.E., Hajjari J., Chami T., Mously H., Badhwar A.K., Karnib M., Carneiro H., Rahman M., Al-Kindi S.G. Multi-variable biomarker approach in identifying incident heart failure in chronic kidney disease: Results from the Chronic Renal Insufficiency Cohort study. Eur. J. Heart Fail. 2022;24:988–995. doi: 10.1002/ejhf.2543. [DOI] [PubMed] [Google Scholar]
- 4.Kottgen A., Russell S.D., Loehr L.R., Crainiceanu C.M., Rosamond W.D., Chang P.P., Chambless L.E., Coresh J. Reduced kidney function as a risk factor for incident heart failure: the atherosclerosis risk in communities (ARIC) study. J. Am. Soc. Nephrol. 2007;18:1307–1315. doi: 10.1681/ASN.2006101159. [DOI] [PubMed] [Google Scholar]
- 5.Kaesler N., Babler A., Floege J., Kramann R. Cardiac Remodeling in Chronic Kidney Disease. Toxins. 2020;12:161. doi: 10.3390/toxins12030161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X., Shapiro J.I. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nat. Rev. Nephrol. 2019;15:159–175. doi: 10.1038/s41581-018-0101-8. [DOI] [PubMed] [Google Scholar]
- 7.Jankowski J., Floege J., Fliser D., Böhm M., Marx N. Cardiovascular Disease in Chronic Kidney Disease: Pathophysiologi-cal Insights and Therapeutic Options. Circulation. 2021;143:1157–1172. doi: 10.1161/CIRCULATIONAHA.120.050686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matsushita K., Kwak L., Sang Y., Ballew S.H., Skali H., Shah A.M., Coresh J., Solomon S. Kidney Disease Measures and Left Ventricular Structure and Function: The Atherosclerosis Risk in Communities Study. J. Am. Heart Assoc. 2017;6:e006259. doi: 10.1161/JAHA.117.006259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsumoto M., Io H., Furukawa M., Okumura K., Masuda A., Seto T., Takagi M., Sato M., Nagahama L., Omote K., et al. Risk factors associated with increased left ventricular mass index in chronic kidney disease patients evaluated using echocardiography. J. Nephrol. 2012;25:794–801. doi: 10.5301/jn.5000066. [DOI] [PubMed] [Google Scholar]
- 10.Park M., Hsu C.-Y., Li Y., Mishra R.K., Keane M., Rosas S.E., Dries D., Xie D., Chen J., He J., et al. Associations between Kidney Function and Subclinical Cardiac Abnormalities in CKD. J. Am. Soc. Nephrol. 2012;23:1725–1734. doi: 10.1681/ASN.2012020145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah A.M., Lam C.S., Cheng S., Verma A., Desai A.S., Rocha R.A., Hilkert R., Izzo J., Oparil S., Pitt B., et al. The relationship between renal impairment and left ventricular structure, function, and ventricular–arterial interaction in hypertension. J. Hypertens. 2011;29:1829–1836. doi: 10.1097/HJH.0b013e32834a4d38. [DOI] [PubMed] [Google Scholar]
- 12.Zhou J., Cui X., Jin X., Zhou J., Zhang H., Tang B., Fu M., Herlitz H., Cui J., Zhu H., et al. Association of Renal Biochemical Parameters with Left Ventricular Diastolic Dysfunction in a Community-Based Elderly Population in China: A Cross-Sectional Study. PLoS ONE. 2014;9:e88638. doi: 10.1371/journal.pone.0088638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jneid H., Anderson J.L., Wright R.S., Adams C.D., Bridges C.R., Casey D.E., Jr., Ettinger S.M., Fesmire F.M., Ganiats T.G., Lincoff A.M., et al. 2012 ACCF/AHA focused update of the guideline for the management of patients with unstable angina/non-ST-elevation myocardial infarction (updating the 2007 guideline and replacing the 2011 focused update): A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2012;60:645–681. doi: 10.1016/j.jacc.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Levine G.N., Bates E.R., Blankenship J.C., Bailey S.R., Bittl J.A., Cercek B., Chambers C.E., Ellis S.G., Guyton R.A., Hollenberg S.M., et al. 2015 ACC/AHA/SCAI Focused Update on Primary Percutaneous Coronary Intervention for Patients With ST-Elevation Myocardial Infarction: N Update of the 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention and the 2013 ACCF/AHA Guideline for the Management of ST-Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2016;67:1235–1250. doi: 10.1016/j.jacc.2015.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Levey A.S., Stevens L.A., Schmid C.H., Zhang Y.L., Castro A.F., 3rd, Feldman H.I., Kusek J.W., Eggers P., Van Lente F., Greene T., et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aguiar-Souto P., Ferrante G., Del Furia F., Barlis P., Khurana R., Di Mario C. Frequency and predictors of contrast-induced nephropathy after angioplasty for chronic total occlusions. Int. J. Cardiol. 2010;139:68–74. doi: 10.1016/j.ijcard.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Stevens P.E., Levin A. Kidney Disease: Improving Global Outcomes Chronic Kidney Disease Guideline Development Work Group Members. Evaluation and Management of Chronic Kidney Disease: Synopsis of the Kidney Disease: Improving Global Outcomes 2012 Clinical Practice Guideline. Ann. Intern. Med. 2013;158:825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 18.Qian G., Fu Z., Guo J., Cao F., Chen Y. Prevention of Contrast-Induced Nephropathy by Central Venous Pressure–Guided Fluid Administration in Chronic Kidney Disease and Congestive Heart Failure Patients. JACC Cardiovasc. Interv. 2016;9:89–96. doi: 10.1016/j.jcin.2015.09.026. [DOI] [PubMed] [Google Scholar]
- 19.Verma A., Meris A., Skali H., Ghali J.K., Arnold J.M.O., Bourgoun M., Velazquez E.J., McMurray J.J., Kober L., Pfeffer M.A., et al. Prognostic Implications of Left Ventricular Mass and Geometry Following Myocardial Infarction: The VALIANT (VALsartan in Acute myocardial iNfarcTion) Echocardiographic Study. JACC Cardiovasc. Imaging. 2008;1:582–591. doi: 10.1016/j.jcmg.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 20.Nagueh S.F., Smiseth O.A., Appleton C.P., Byrd B.F., 3rd, Dokainish H., Edvardsen T., Flachskampf F.A., Gillebert T.C., Klein A.L., Lancellotti P., et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016;29:277–314. doi: 10.1016/j.echo.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Kannel W.B., Doyle J.T., McNamara P.M., Quickenton P., Gordon T. Precursors of sudden coronary death. Factors related to the incidence of sudden death. Circulation. 1975;51:606–613. doi: 10.1161/01.CIR.51.4.606. [DOI] [PubMed] [Google Scholar]
- 22.Lieb W., Mayer B., Stritzke J., Doering A., Hense H.-W., Loewel H., Erdmann J., Schunkert H. Association of low-grade urinary albumin excretion with left ventricular hypertrophy in the general population: The MONICA/KORA Augsburg Echocardiographic Substudy. Nephrol. Dial. Transplant. 2006;21:2780–2787. doi: 10.1093/ndt/gfl364. [DOI] [PubMed] [Google Scholar]
- 23.Perrone R.D., Abebe K.Z., Schrier R.W., Chapman A.B., Torres V.E., Bost J., Kaya D., Miskulin D.C., Steinman T.I., Braun W., et al. Cardiac Magnetic Resonance Assessment of Left Ventricular Mass in Autosomal Dominant Polycystic Kidney Disease. Clin. J. Am. Soc. Nephrol. 2011;6:2508–2515. doi: 10.2215/CJN.04610511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nguyen M., Cosson E., Valensi P., Poignard P., Nitenberg A., Pham I. Transthoracic echocardiographic abnormalities in asymptomatic diabetic patients: Association with microalbuminuria and silent coronary artery disease. Diabetes Metab. 2011;37:343–350. doi: 10.1016/j.diabet.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 25.Rzeznik D., Przewlocki T., Kablak-Ziembicka A., Kozanecki A., Roslawiecka A., Lach J., Tracz W., Podolec P. Effect of renal artery revascularization on left ventricular hypertrophy, diastolic function, blood pressure, and the one-year outcome. J. Vasc. Surg. 2011;53:692–697. doi: 10.1016/j.jvs.2010.09.054. [DOI] [PubMed] [Google Scholar]
- 26.Abdi-Ali A., Miller R., Southern D., Zhang M., Mikami Y., Knudtson M., Heydari B., Howarth A.G., Lydell C.P., James M.T., et al. LV Mass Independently Predicts Mortality and Need for Future Revascularization in Patients Undergoing Diagnostic Coronary Angiography. JACC Cardiovasc. Imaging. 2018;11:423–433. doi: 10.1016/j.jcmg.2017.04.012. [DOI] [PubMed] [Google Scholar]
- 27.Semple D., Smith K., Bhandari S., Seymour A.-M.L. Uremic Cardiomyopathy and Insulin Resistance: A critical role for akt? J. Am. Soc. Nephrol. 2011;22:207–215. doi: 10.1681/ASN.2009090900. [DOI] [PubMed] [Google Scholar]
- 28.Jovanovich A., Ix J.H., Gottdiener J., McFann K., Katz R., Kestenbaum B., de Boer I.H., Sarnak M., Shlipak M.G., Mukamal K.J., et al. Fibroblast growth factor 23, left ventricular mass, and left ventricular hypertrophy in community-dwelling older adults. Atherosclerosis. 2013;231:114–119. doi: 10.1016/j.atherosclerosis.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai B., David V., Martin A., Huang J., Li H., Jiao Y., Gu W., Quarles L.D. A Comparative Transcriptome Analysis Identifying FGF23 Regulated Genes in the Kidney of a Mouse CKD Model. PLoS ONE. 2012;7:e44161. doi: 10.1371/journal.pone.0044161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sasso F.C., Carbonara O., Persico M., Iafusco D., Salvatore T., D’Ambrosio R., Torella R., Cozzolino D. Irbesartan Reduces the Albumin Excretion Rate in Microalbuminuric Type 2 Diabetic Patients Independently of Hypertension: A randomized double-blind placebo-controlled crossover study. Diabetes Care. 2002;25:1909–1913. doi: 10.2337/diacare.25.11.1909. [DOI] [PubMed] [Google Scholar]
- 31.Pontremoli R., Borghi C., Filardi P.P. Renal protection in chronic heart failure: Focus on sacubitril/valsartan. Eur. Heart J.-Cardiovasc. Pharmacother. 2021;7:445–452. doi: 10.1093/ehjcvp/pvab030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zannad F., Ferreira J.P., Pocock S.J., Anker S.D., Butler J., Filippatos G., Brueckmann M., Ofstad A.P., Pfarr E., Jamal W., et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: A meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9. [DOI] [PubMed] [Google Scholar]
- 33.Sasso F.C., Pafundi P.C., Simeon V., De Nicola L., Chiodini P., Galiero R., Rinaldi L., Nevola R., Salvatore T., Sardu C., et al. Efficacy and durability of multifactorial intervention on mortality and MACEs: A randomized clinical trial in type-2 diabetic kidney disease. Cardiovasc. Diabetol. 2021;20:145. doi: 10.1186/s12933-021-01343-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sasso F.C., Simeon V., Galiero R., Caturano A., De Nicola L., Chiodini P., Rinaldi L., Salvatore T., Lettieri M., Nevola R., et al. The number of risk factors not at target is associated with cardiovascular risk in a type 2 diabetic population with albuminuria in primary cardiovascular prevention. Post-hoc analysis of the NID-2 trial. Cardiovasc. Diabetol. 2022;21:235. doi: 10.1186/s12933-022-01674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and analyzed during the current study are not publicly available due to the institution’s policy but are available from the corresponding author upon reasonable request.