Abstract
A clinical trial is the most foolproof method to evaluate the efficacy of a new intervention. Successful completion of clinical trials depends on the retention of the participants enrolled. Poor participant retention can lead to significant time and cost burden and have potentially adverse biases on the results. A high retention rate of participants is an important criterion for the validity and credibility of randomized controlled clinical trials. Many long-term trials fail due to low retention of study participants. Efforts at participant retention should start even before the first participant is recruited into the study. Retention is not only the responsibility of the investigators but also all other stakeholders in a clinical trial. In recent years, retention materials, participant camps, and introduction of national study coordinators have helped in improving retention. Quality of the relationship developed between the research staff and the study participant is a key factor for success of any trial. In our experience, in the context of resource-challenged low- and middle-income countries, we have found that it is possible to achieve high retention rates, 95%–100%. The rapport built between the investigating team and the participant plays a vital role in retention. In addition, personalized care, including listening to the participant's problems and enabling to contact investigators or study team at any time of the day, has shown benefits in retention.
Keywords: Clinical trials, retention, stakeholders
INTRODUCTION
The randomized clinical trial is the most scientific method to access the effectiveness of treatment.[1] The success of a clinical trial depends on the ability to recruit participants in a systematic manner, to ensure adherence to and integrity of intervention, and to retain the participants through the entire study duration and follow-up. Power of the study will be affected if the retention rate is poor, and selection bias is introduced.[2] The reliability of results and validity of the study are affected by poor retention.[3] Successful recruitment and retention of participants is the most challenging aspect in the conduct of clinical trials.[4] Participant selection is crucial for retention. Retention is a continuous process, and plans for retention strategies should start during protocol development and from the onset of recruitment. Therefore, it is essential to understand effective retention strategies, as participant retention is a major practical challenge – often the “Achilles Heel” of trials – especially in resource-constrained situations, as in low- and middle-income countries.
There are many challenges in achieving high retention, which include lack of trust, socioeconomic barriers, inconveniences, real or perceived adverse events from intervention, migration of the subject from the study site, personal reasons, and influence of media.[5] Complexity of the study, nature of disease studied, need to take time off from work, education and occupation level, and interference from physicians not involved in the study and family members are other factors to be considered.[6] At times, retention remains a challenge even if recruitment is successful. Recent data between the years 2000 and 2006 examining trends in recruitment and retention showed that recruitment dropped by 16% whereas retention dropped by 21%.[7] Studies have shown an 88% dropout due to the following reasons – lost to follow-up, nonadherence to protocol, and withdrawal of consent.[8]
On the other hand, multiple strategies which include the presence of a study team that is respectful and supportive of participants during the entire study period, developing a good relationship with the participant, access to the investigators at any time of the day, and meticulous monitoring and corrective action on an ongoing basis, when all these are well employed, can help enhance retention.[9] In some cases, provision of additional investigations related to clinical needs other than the trial investigations may also help in retention.[10]
CHALLENGES IN RETENTION
Retention and recruitment are interlinked. When the duration of the study becomes long and the number of study visits increase, it becomes an added burden to the participants and to the study team.[11] This factor needs to be considered during the design of the study, and strategies put in place to ensure unbiased recruitment and high retention.
On average, 25%–26% of the participants drop out after expressing their consent.[12,13] More than 90% of the studies are delayed due to failed enrollment or challenges to participant retention, which includes loss of participants to follow-up.[14] In a survey assessing the challenges in participant retention, it was found that 44% of the participants feared side effects. Fear of study procedures and change of residence were reported by 47% as the reasons for dropping. Lack of support from the family physician and family members was reported by 27%, while 55% of the participants stated that the lack of dedicated approach by an investigator's team and 9% each reported negative media publicity and perceived lack of efficacy of the treatment as reasons for dropping out.[4] Other retention challenges include inconvenient location, schedule conflicts, perception that the disease condition is not improving, lack of appreciation for their participation, and unrealistic expectations from the treatment.
Retaining recruited participants is also a major challenge. Regardless of the objective of research projects, the required data collection is only possible if adequate and enough of the selected subjects continue to participate throughout the trial.[15] There are, however, several reasons beyond design, experimental drug, or type of study that prevent high retention of the study population. In recent years, the rate of retention in major global trials has increased, on average, significantly, as shown in Table 1, and this is largely due to the involvement of national study coordinators in long-term global clinical trials.[4]
Table 1.
Participant completion figures in clinical trials
HOW TO OVERCOME RETENTION CHALLENGES
It is important to recognize the symptoms and signs of nonadherence of participants during the study, which include missed study visits, difficulty in reaching by phone or failure to return calls, and impatience during clinic visits. To address these challenges, there are retention strategies and the role of respective stakeholders plays a major role, as shown in Figure 1.
Figure 1.
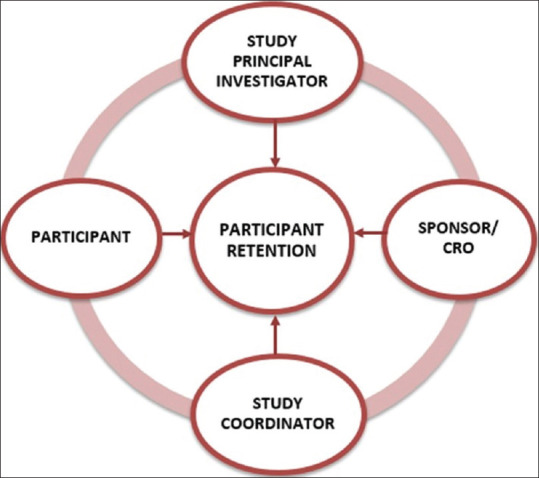
Stakeholders needed to ensure success in retention
ROLE OF VARIOUS STAKEHOLDERS
Drug discovery and development are possible only with the participation of key stakeholders within the industry. These stakeholders include the participants, research team, sponsors, regulators, monitors, government agencies, and contract research organizations Contract Research Organization (CROs).
The primary stakeholders who discover the drug and initiate the research process are the sponsors. In recent years, sponsors increasingly utilize the assistance of clinical research organizations (CROs) for conducting a clinical trial. The CROs conduct the trial as directed by the sponsor and they act as one of the stakeholders.
Researchers or the principal investigators (PIs) are the stakeholders, hired by the sponsor to conduct the clinical trial. In a study team led by the PIs, the study coordinators play a major role in ensuring high retention. However, the most important stakeholders are the participants who contribute by providing clinical data to evaluate the efficacy of an investigational product.
ROLE OF THE PRINCIPAL INVESTIGATOR
The PI is a key responsible person and accountable for conducting the clinical trial. The PI ensures that the study is ethically conducted in compliance with the protocol and ensuring access to medical care of the study subjects. Recruitment, retention, training, appraisal, and supervision of team members is also the responsibility of the PI.
ROLE OF THE STUDY COORDINATOR
In most of the instances, the participant dropout is well managed by the study team except for those due to adverse events which makes it difficult to achieve a 100% retention rate, even as a best-case scenario.[12,13,14] The key person for the successful retention in the study team is the study coordinator. The Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications and Outcome Reduction with Initial Glargine Intervention studies have served as a model for the role of the study coordinator in a clinical trial.[22,23] In addition, study coordinators have made remarkable contributions to the overall research endeavor for high participant retention and data completion rates.[24] In recent years, some sponsors have introduced the concept of a national study coordinator, who guides all the study coordinators at their respective sites. The national coordinators work closely with all the sites with the help of the sponsors, a development that has led to very high retention rates.[25]
STUDY PARTICIPANTS
Participants are volunteers who have the liberty to withdraw from the study at any point of time. It is very important to ensure that the participants are taken care of in terms of the following –answering their concerns and attending to their medical management and investigations. Challenges for the study participants to continue in the study include adverse events, migration, and physician and family interference.
Providing a comfortable waiting room and also spending more time with the participants will ensure a lot of trust and confidence. The goal of the study coordinator is to build such a rapport with the participants and also to inform the participants of their value in the study.[26]
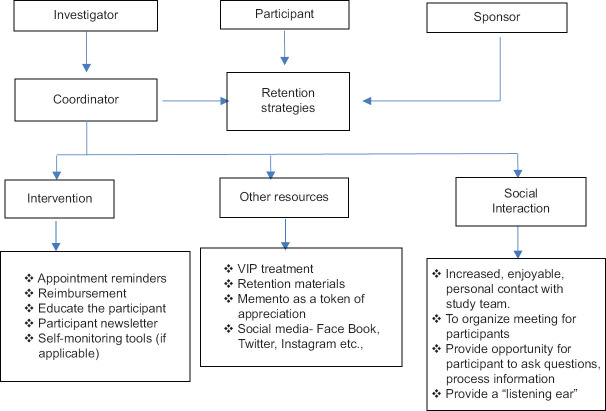
Table 2 shows the various retention methods which have been used for many long-term studies. Listening to the participant problems and spending more time with the person is essential and helps in achieving high retention.
Table 2.
Participant retention methods and tools
| Intervention | Social interaction |
|---|---|
| Appointment reminders | Increased, enjoyable contact with the study team |
| Reimbursement | Provide opportunity for participants to ask questions |
| Participant newsletter Self-monitoring tools[27] | Provide a “listening ear” |
Methods used to improve retention
Appointment reminders
This is very important, as one of the most common mistakes many of research sites make is to rely on participants to remember their next appointment. Although some participants will remember when they are supposed to come back, it is still the task of the team to remind them. Phone calls, emails, and reminder cards will jog their memory and remind them to come back for their scheduled appointment.
Reimbursement/incentives
Travel reimbursement to the participants at each visit and meal vouchers help in retention. Participants in trials are offered rewards such as monetary payments, free medical care, and food. Such rewards are not considered benefits of study participation but rather incentives and motivation for participation. Receiving a monetary payment or receiving something free that would normally have to be paid, is considered as an inducement. Any inducement may be coercive or an undue influence on a potential study participant, and therefore, incentives are to be planned accordingly with approval of the Ethics Committee. As incentives for participation are potentially coercive, the amount and conditions of such incentives must be reviewed and approved by the Institutional Ethics Committee.
Participant newsletter
Newsletters that highlight the importance of research or offer tips on daily living with their condition, would really help them. Participants can be engaged with E-mail newsletters to keep them connected throughout the trial. Surveys showed that participants are eager to receive ongoing education about their disease state, which could be established through the distribution of participant newsletters, brochures, etc.
Self-monitoring tools
In many diabetes trials, capillary glucose meters are provided to measure the blood glucose, and in some cardiology studies, blood pressure monitors are provided to the participants.
Making clinic visit pleasant
Minimize waiting time
Participants in trials always need special attention. It is important that the study procedures are done quickly in order to reduce the waiting time.
Waiting room facilities
Separate room for the study participants and availability of newspapers, magazines to read, and television to watch at the waiting room would make them more comfortable. Availability of free WiFi may be an additional benefit.
Spending more time
Talking to the participants and spending some time by listening to their problems gives a lot of comfort to the participants.
SOCIAL INTERACTION
Increased, enjoyable contact with the study team
Provide opportunity for participants to ask questions
Provide a “listening ear.”
INFORMED CONSENT FORMS
Strategies for retention start from day 1 when the informed consent form has been signed. As it is important to provide participants with all the details regarding the study at the beginning itself, the handouts, especially the informed consent forms, should provide a good sense of what to expect and what the trial would involve. It is also important to make sure that the informed consent is easy to understand, so that participants know exactly what to expect from the trial.
INVOLVING FAMILIES
A common reason for participants to withdraw from clinical trials prematurely is due to the influence of friends or family who are uncomfortable or unfamiliar with clinical trials. Participants may be enthusiastic at the start of the trials, but later, the enthusiasm declines. This change can be prevented by providing participants with educational materials and involving family members.
The most common retention techniques involve phone calls, visit reminders, birthday or anniversary cards, reimbursement of study participants for their time, and expenses incurred because of trial participation.
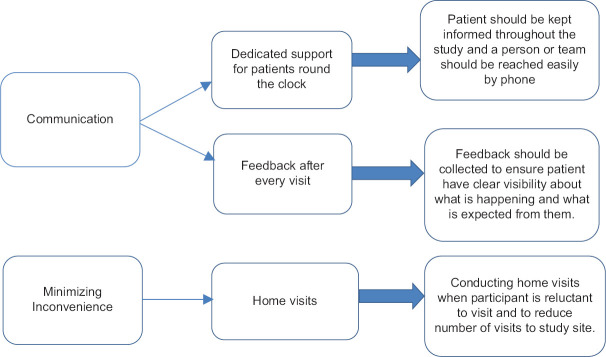
Figure 2 shows other retention strategies followed at our centre.
Figure 2.
Other retention strategies
Table 3 shows the various retention challenges and the solutions adapted at the Madras Diabetes Research Foundation (MDRF). MDRF is a 100% nonprofit organization, located in Chennai, India, which conducts full-fledged research on diabetes and related complications. MDRF is designated as the Indian Council of Medical Research Centre for Advanced Research on Diabetes and the International Diabetes Federation Centre of Excellence in Diabetes Care. It has an established track record of conducting high-quality surveillance and clinical trials.
Table 3.
Retention challenges and solutions - the Madras Diabetes Research Foundation experience
| Challenges | Solutions |
|---|---|
| Migration | Arrangements can be made to follow up in other cities or countries |
| Obtaining 3-4 contact details from the participants | |
| Lack of cooperation from family | Educating family members can be a good support for the follow-up Creating materials especially for family members to support their loved ones Retention camp for the participants (participant education camp, cookery demonstration, and simple exercise program) Family members of the participant are encouraged to attend |
| Interference of the family physicians | Providing informed consent, which gives a good sense of what to expect from the study |
| Influence of media | A copy of the informed consent is given a few days or weeks, before the participant shows interest to participate |
| Fear of side effects | Negative influence of the media can be avoided if the participant is in regular contact with the sites and doubts regarding the study drug are addressed periodically Providing an opportunity to ask questions, updating the participants about the progress of the study and also make them understand the side effects of the drug |
| Having to take time off from work, impatience during study visit | Spending more time with participants and providing a listening ear Providing comfortable waiting room and minimized waiting time Providing opportunity to ask questions |
| Lack of motivation and appreciation | Giving memento as a token of appreciation Patient's camps to be conducted once or twice a year |
| Fear of placebo by the participants | Face-to-face communication Ensuring more learning |
| Forgetting their appointments | Reminder letters and reminder calls for the next visit are given |
| Financial barriers | Monitoring tools such as glucose meter and BP monitor to be provided to the participants Travel incentives, free medical investigations, and concomitant medications are provided |
BP=Blood pressure
RETENTION TOOLS
In recent years, many retention materials have been developed, which have helped to retain the participants in long-term studies. It is the duty of the sponsor as well as the investigator to ensure that these materials are culturally appropriate.
Participant education's camps have taken off in a big way retain participants for long-term studies. These camps are mostly on participant education, cookery demonstrations, and simple exercise programs, which are conducted at the study site. During these camps, the participant's family members are invited and this in turn gives confidence and faith to the participant's family. Honoring the research participants and arranging annual luncheons is one of the most effective retention strategies. All these activities require approval from the Ethics Committee.
Figure 3 shows the overall retention strategies that can be used for a long- term follow-up.
Figure 3.
Conceptual diagram - Participant retention in long-term trials
CONCLUSION AND RECOMMENDATIONS
Retention of patients in clinical trials is a continuous process and remains a key factor for the success of the clinical trial. Participant retention remains a significant problem in many trials. Adopting a more participant-centric approach by listening to participants and by implementing their feedback is essential. Motivation for participation in clinical trials is multifactorial but can effectively attack the problem of participant dropouts. Respect for participants, helps in establishing trust and rapport, which in turn leads to better retention. Effective informed consent, showing appreciation to make their participation personal, and meaningful and building good relationship with participants by sending greeting cards for their birthday and for festivals between visits encourage retention. Creating a social community for the participants gives them an opportunity to feel like they are an integral part of the study, which would help them to share their experience. Maintaining a health record for each participant enhances retention.
Apart from the different retention strategies discussed for both clinical trials as well as for mental health studies, quality of the relationship developed between the research staff and the study participant is a key factor to overcome the challenges faced in retention.
It is important to put the patient first at every stage of the study journey. Hence, there is a need to value and support the participants throughout the study, which has shown evidence for less dropouts from the trial. Therefore, our emphasis is to develop patient-focused protocols by using the patient feedback. This would help us resolve the most common issues around patient retention, which in turn influence the success of a clinical trial.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
The authors would like to acknowledge and thank Ms. Mokkapati Lalasa, of Madras Diabetes Research Foundation for her assistance in the research paper.
REFERENCES
- 1.Sibbald B. Understanding controlled trails: Why are randomised controlled trials important. Br Med J. 1998;316:201.. doi: 10.1136/bmj.316.7126.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cramer JA. Patient recruitment and compliance issues in clinical trials. Epilepsy Res Suppl. 1993;10:211–22. [PubMed] [Google Scholar]
- 3.Schulz K, Grimes D. Sample size slippages in randomised trials: Exclusions and the lost and wayward. Lancet. 2002;359:781–5. doi: 10.1016/S0140-6736(02)07882-0. [DOI] [PubMed] [Google Scholar]
- 4.Kadam RA, Borde SU, Madas SA, Salvi SS, Limaye SS. Challenges in recruitment and retention of clinical trial subjects. Perspect Clin Res. 2016;7:137–43. doi: 10.4103/2229-3485.184820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaudhari N, Ravi R, Gogtay NJ, Thatte UM. Recruitment and retention of the participants in clinical trials: Challenges and solutions. Perspect Clin Res. 2020;11:64–9. doi: 10.4103/picr.PICR_206_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Robinson KA, Dennison CR, Wayman DM, Pronovost PJ, Needham DM. Systematic review identifies number of strategies important for retaining study participants. J Clin Epidemiol. 2007;60:757–65. doi: 10.1016/j.jclinepi.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COUCH Health. Clinical Trial Recruitment and Retention Challenges. 2019. Apr 14, Available at Clinical Trial Recruitment and Retention Challenges. Available from: http://couchhealth.co . [Last accessed on 2021 Apr 06]
- 8.Desai M. Recruitment and retention of participants in clinical studies: Critical issues and challenges. Perspect Clin Res. 2020;11:51–3. doi: 10.4103/picr.PICR_6_20. Available from: https://www.picronline.org/text.asp?2020/11/2/51/283844 . [Last accessed on 2021 Sep 09]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassidy EL, Baird E, Sheikh JI. Recruitment and retention of elderly patients in clinical trials: Issues and strategies. Am J Geriatr Psychiatry. 2001;9:136–40. [PubMed] [Google Scholar]
- 10.Ellis PM. Attitudes towards and participation in randomised clinical trials in oncology: A review of the literature. Ann Oncol. 2000;11:939–45. doi: 10.1023/a:1008342222205. [DOI] [PubMed] [Google Scholar]
- 11.Zweben A, Fucito LM, O'Malley SS. Effective Strategies for Maintaining Research Participation in Clinical Trials. Drug Inf J. 2009;43 doi: 10.1177/009286150904300411. doi: 10.1177/009286150904300411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Overcoming Clinical Trial Marketing Challenges: Patient Recruitment and Retention. Healthcare Success. 2014. Available from: https://healthcaresuccess.com/blog/healthcare-marketing/overcoming-clinical-trial-marketing-challenges-patient-recruitment-retention.html . [Last accessed on 2021 Jun 15].
- 13.David A. Marketing Strategies for Clinical Trial Recruitment and Patient Retention; 2006. Available from: https://cdn2.hubspot.net/hub/30312/file-452156912-pdf/news-page-pdfs/avitable 1206.pdf?t=1509126515748 . [Last accessed on 2021 Jun 15].
- 14.Betz D. Addressing Clinical Trial Recruitment and Participant Retention Issues Imposed by HIPAA Step by Step (third of a series); 30 September, 2008. Available from: http://lost-to-followup.com/participant-retention . [Last retrieved on 2010 Apr 14].
- 15.Moyses S, Javier N. Epidemiology Beyond the Basics; 2004. Available from: https://shmu.ac.ir/file/download/news/1564213580-epidemiology-beyod.pdf . [Last accessed on 2021 Jun 15].
- 16.Marso S, McGuire D, Zinman B, Poulter N, Emerson S, Pieber T, et al. Efficacy and safety of degludec versus glargine in type 2 diabetes. N Engl J Med. 2017;377:723–32. doi: 10.1056/NEJMoa1615692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanjay K. Key CVOTs with Semaglutide: SUSTAIN 6 and PIONEER 6; 2020. Available from: https://www.acc.org/latest-in-cardiology/articles/2020/01/02/15/05/key-cvots-with-semaglutide . [Last accessed on 2021 Jun 15].
- 18.Zinman B, Aroda VR, Buse JB, Cariou B, Harris SB, Hoff ST, et al. Efficacy, safety, and tolerability of oral semaglutide versus placebo added to insulin with or without metformin in patients with type 2 diabetes: The PIONEER 8 Trial. Diabetes Care. 2019;42:2262–71. doi: 10.2337/dc19-0898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–44. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 20.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311–22. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kowalski AJ, Poongothai S, Chwastiak L, Hutcheson M, Tandon N, Khadgawat R, et al. The INtegrating DEPrEssioN and Diabetes treatmENT (INDEPENDENT) study: Design and methods to address mental healthcare gaps in India. Contemp Clin Trials. 2017;60:113–24. doi: 10.1016/j.cct.2017.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crofford OB, Carolyn S, Patricia AC. Diabetes Control and Complications Trial (DCCT); 2010. Clinical.Trials.Gov. Available from: https://clinicaltrials.gov/ct2/show/NCT00360815 . [Last accessed on 2021 Jun 15].
- 23.ORIGIN Trial Investigators. Gerstein HC, Bosch J, Dagenais GR, Díaz R, Jung H, et al. Basal insulin and cardiovascular and other outcomes in dysglycemia. N Engl J Med. 2012;367:319–28. doi: 10.1056/NEJMoa1203858. [DOI] [PubMed] [Google Scholar]
- 24.Larkin ME, Lorenzi GM, Bayless M, Cleary PA, Barnie A, Golden E, et al. Evolution of the study coordinator role: The 28-year experience in Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Clin Trials. 2012;9:418–25. doi: 10.1177/1740774512449532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Buchanan DA, Goldstein J, Pfalzer AC, Lin YC, Kang H, Claassen DO. Empowering the clinical research coordinator in academic medical centers. Mayo Clin Proc Innov Qual Outcomes. 2021;5:265–73. doi: 10.1016/j.mayocpiqo.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cyenthia W, Karen B, Tawni KH, Conor M, Aliya A, Danielle B, et al. The Anatomy of a Great Clinical Research Coordinator. Clinical Researcher; 2018. Available from: https://acrpnet.org/2018/08/14/the-anatomy-of-a-great-clinical-research-coordinator/ . [Last accessed on 2021 Jun 15].
- 27.Abshire M, Dinglas VD, Cajita MI, Eakin MN, Needham DM, Himmelfarb CD. Participant retention practices in longitudinal clinical research studies with high retention rates. BMC Med Res Methodol. 2017;17:30.. doi: 10.1186/s12874-017-0310-z. [DOI] [PMC free article] [PubMed] [Google Scholar]