Abstract
SNARE protein is an essential factor driving vesicle fusion in eukaryotes. Several SNAREs have been shown to play a crucial role in protecting against powdery mildew and other pathogens. In our previous study, we identified SNARE family members and analyzed their expression pattern in response to powdery mildew infection. Based on quantitative expression and RNA-seq results, we focused on TaSYP137/TaVAMP723 and hypothesized that they play an important role in the interaction between wheat and Blumeria graminis f. sp. Tritici (Bgt). In this study, we measured the expression patterns of TaSYP132/TaVAMP723 genes in wheat post-infection with Bgt and found that the expression pattern of TaSYP137/TaVAMP723 was opposite in resistant and susceptible wheat samples infected by Bgt. The overexpression of TaSYP137/TaVAMP723 disrupted wheat’s defense against Bgt infection, while silencing these genes enhanced its resistance to Bgt. Subcellular localization studies revealed that TaSYP137/TaVAMP723 are present in both the plasma membrane and nucleus. The interaction between TaSYP137 and TaVAMP723 was confirmed using the yeast two-hybrid (Y2H) system. This study offers novel insights into the involvement of SNARE proteins in the resistance of wheat against Bgt, thereby enhancing our comprehension of the role of the SNARE family in the pathways related to plant disease resistance.
Keywords: wheat, powdery mildew resistance, TaVAMP723, TaSYP137, gene function
1. Introduction
SNARE proteins are core factors that drive vesicle fusion in eukaryotes [1,2,3]. The SNARE family of genes can be divided into target membrane Q-SNARE and vesicular R-SNARE by their core functional domains and subcellular localization differences [1,2,3,4]. Q-SNARE is subdivided into four categories by different core functional domains: Qa-SNARE, Qb-SNARE, Qc-SNARE, and Qbc SNARE [1,2,3,5]. When the SNARE protein performs its biological function, it forms different SNARE protein complexes for specific functions [1,2,3,6]. For example, the ternary complex, composed of three different SNARE proteins, plays a role in exocrine secretion [6,7,8,9]. In contrast, the quaternary complex, composed of four SNARE proteins, acts on the fusion of intracellular vesicles [9,10].
Penetration resistance is a critical component of the host’s immune response against fungal pathogens. The process mentioned above is considered an efficient and rapid signal transduction mechanism in plants, enabling them to defend against fungal pathogen invasions. The host’s immune response to fungal pathogens involves the rapid reaction and activation of papillae to prevents fungal penetration into plant cells [11,12]. A dome-shaped cell wall is deposited between the cell wall and the plasma membrane of the penetrated part of the epidermal cells [13]. In the non-host interaction of Arabidopsis thaliana, non-host resistance has been proven to be mediated by the protein complex composed of PEN1, SNAP33, and VAMP721 [14,15,16,17]. In addition, SYP121 can resist powdery mildew invasion in tomato [18]. Similarly, the wheat SNARE protein NPSN11 mediates wheat resistance to stripe rust [19]. In conclusion, SNARE proteins play a critical role as host defense signals against fungal pathogens.
Previous studies have shown that SNARE family members are involved in the plant’s response to biotic and abiotic stresses [18,20,21,22,23,24,25]. VAMP721/722 is resistant to oomycete infection in plants and plays a role in plant growth, cell division, and abiotic stress response [26,27,28,29,30]. SYP132 can drive hormone-regulated internal digestive transport and inhibit the density and function of the plasma membrane (PM) H+-ATPase [31,32]. In response to bacterial pathogens, it can promote the secretion and transportation of resistance bacteria (PR)-related proteins. These processes seem to have opposing functions, but they trigger a mechanical connection between two possible independent membrane transport pathways [32]. In addition, the overexpression of SYP132 can inhibit bacterial invasion through the stomatal pathway [32,33]. However, when bacteria successfully bypass the stomatal defense, the overexpression of SYP132 enhances bacterial infection [32].
While a total of 64 wheat powdery mildew resistance genes have been named and published [34], only genes at 13 loci have been successfully cloned. These genes have been identified through two main approaches: forward and reverse genetics. Mutant sequencing led to the cloning of Pm1a, Pm2, and Pm4 [35,36,37], while map cloning was used to find Pm3b, Pm5e, Pm24, Pm38, Pm41, Pm46, and Pm60 [35,37,38,39]. Homologous cloning was employed to identify Pm8, Pm17, and Pm21 [40,41].
In terms of defense response to Bgt infection, H2O2 accumulates at the Bgt penetration site in RLK overexpressing wheat, with TaRLK1/TaRLK2 potentially involved through SA and ROS [42]. Reducing the expression of TaBON1 or TaBON3 through virus-induced gene silencing (VIGS) can enhance wheat resistance to Bgt [43]. Similarly, MLA genes have been found to positively enhance disease resistance in both barley and wheat [44], while TaJAZ1 [45], TaNAC6s [46], TaRLK-V [47], TaEDS1 [48], and TaRPP13L1-3D [49] have also been shown to positively regulate disease resistance. On the other hand, TaMED25 [50], TaATG6 [51], and TuMYB46L [52] are among the negatively regulated disease resistance genes.
In this paper, the expression patterns of TaSYP132/TaVAMP723 genes in wheat were measured post-infection with Blumeria graminis f. sp. Tritici, known as powdery mildew, to elucidate wheat–Bgt interactions. Concurrent overexpression and virus-mediated gene silencing were used to provide evidence that the genes could inhibit wheat resistance to powdery mildew invasion while a yeast two-hybrid model provided evidence of their interaction.
2. Results
2.1. Isolation of Wheat TaSYP137 and TaVAMP723 and Characterization of the Encoding Proteins
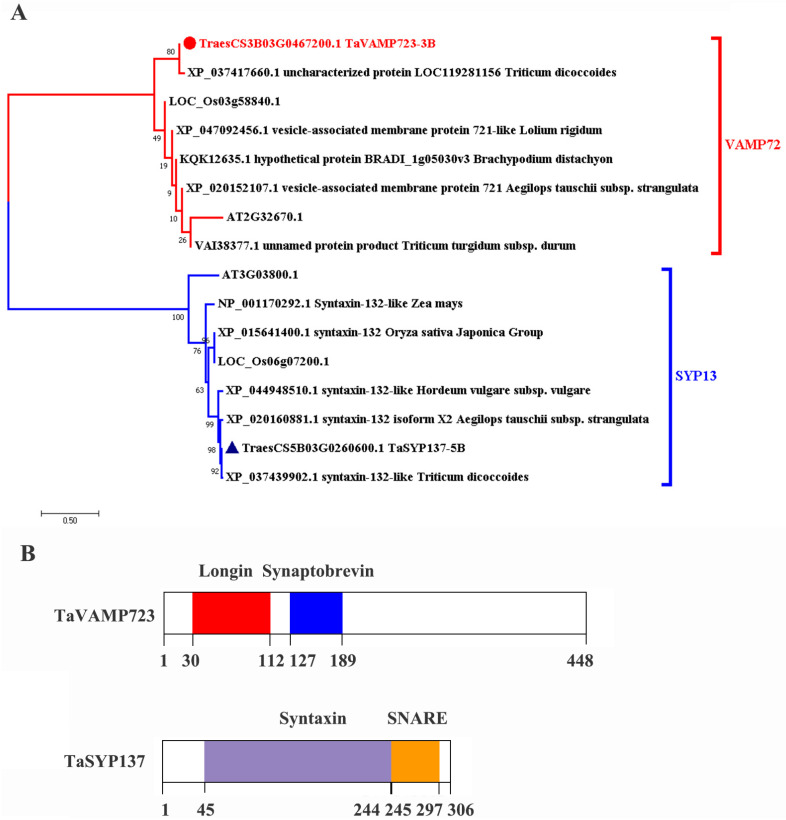
TraesCS5B03G0260600 (XP_044387391.1) and TraesCS3B03G0467200 (XP_044348247.1) were cloned from the near-isogenic Line N9134R. The TraesCS5B03G0260600 protein contains both syntaxin domains (45 aa-244 aa) and SNARE domains (245 aa-297 aa), while the TraesCS3B03G0467200 protein consists of longin domains (30 aa-112 aa) and synaptobrevin domains (127 aa-189 aa), as shown in Figure 1B. According to the domain information, they can be identified as members of the SNARE protein family SYP13 and VAMP72, named TaSYP137-5B and TaVAMP723-3B, respectively. The full-length coding sequences (CDSs) of TaSYP137-5B and TaVAMP723-3B contain a complete open reading frame (ORF) region consisting of 307 and 449 amino acid residues (Figure 1B), respectively, with molecular weights of 35 kDa and 49.8 kDa and isoelectric points of 5.9 and 6.8, respectively (Table S1). We then extracted similar protein sequences from NCBI and other species. A phylogenetic analysis was conducted on 16 proteins belonging to the VAMP72 and SYP13 protein classes, with 8 proteins in each class, using the maximum likelihood method (Figure 1A). It was observed that the proteins shared similarities with Triticum dicoccoides in XP_037417660.1 and that the XP_037439902.1 protein had the closest genetic relationship (Figure 1A).
Figure 1.
Analysis of TaVAMP723-3B and TaSYP137-5B. (A): Phylogenetic analysis of TaVAMP723 and TaSYP137 was conducted by constructing a phylogenetic tree using the maximum likelihood method with MEGA X software. Different SNARE proteins from Triticum aestivum (TaVAMP723-3B, TaSYP137-5B), Arabidopsis thaliana (AT2G32670.1, AT3G03800.1), Oryza sativa (LOC_Os03g58840.1, LOC_Os06g07200.1, XP_015641400.1), Triticum turgidum (VAI38377.1), Aegilops tauschii (XP_020152107.1, XP_020160881.1), Brachypodium distachyon (KQK12635.1), Lolium rigidum (XP_047092456.1), Triticum dicoccoides (XP_037417660.1, XP_037439902.1), Hordeum vulgare (XP_044948510.1), and Zea mays (NP_001170292.1). Red branches represent VAMP72-like proteins; Blue branches represent SYP13-like proteinsThe two wheat proteins TaSYP137 and TaVAMP723 are marked with blue triangle and red circles, respectively. The number on the evolutionary branches indicates the BOOTSTRAP value, and a value of 100 indicates that the probability of this branch is 100%. (B): The functional domains predicted by Pfam. TaVAMP723-3B has the longin domains (30 aa-112 aa) and synaptobrevin domains (127 aa-189 aa), while TaSYP137-5B contains the syntaxin domains (45 aa-244 aa) and SNARE domains (245 aa-297 aa).
2.2. Expression of TaSYP137 and TaVAMP723 in Wheat-Bgt and Their Subcellular Localization
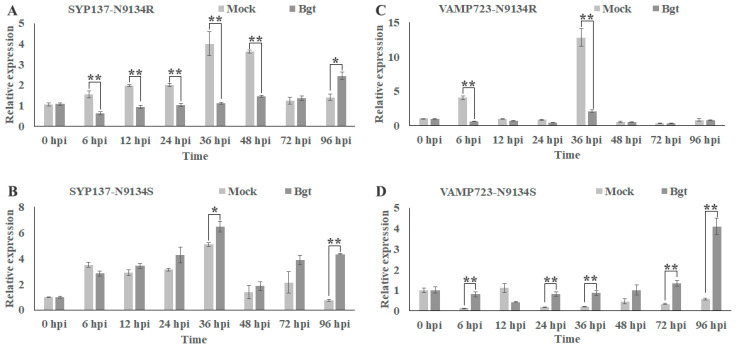
Analyzing their expression patterns in response to Bgt infection (Figure 2), we found that in N9134R, the expression of TaSYP137 was significantly reduced between 6 and 48 h post-infection (hpi) with a slight recovery at 96 hpi (Figure 2A). The expression of TaVAMP723 was also significantly reduced between 6 and 48 hpi in N9134R (Figure 2C). In N9134S, a significant increase in TaSYP137 was seen between 24 and 96 hpi (Figure 2B), and the expression of TaVAMP723 showed a significant reduction at 12 hpi but a significant increase at other times (Figure 2D).
Figure 2.
The expression patterns of TaSYP137 and TaVAMP723 genes were investigated in N9134R/N9134S at 0–96 h with Bgt/mock inoculation. TaSYP137 and TaVAMP723 expression levels were assessed in Bgt/mock inoculation-infected N9134R (resistant) and N9134S (susceptible) lines by qRT-PCR at 6, 12, 24, 36, 48, 72, and 96 hpi. Data were normalized to the β-Actin (GenBank: aK458277.1) expression level. (A): The expression level of TaSYP137 gene with Bgt/mock inoculation in N9134R; (B): The expression level of TaSYP137 gene with Bgt/mock inoculation in N9134S; (C): The expression level of TaVAMP723 gene with Bgt/mock inoculation in N9134R; (D): The expression level of TaVAMP723 gene with Bgt/mock inoculation in N9134S. Data were the mean of three biological replicates ± S.E. Asterisks denote significant differences by Student’s t-test analysis (t-tests, * p < 0.05, ** p < 0.01).
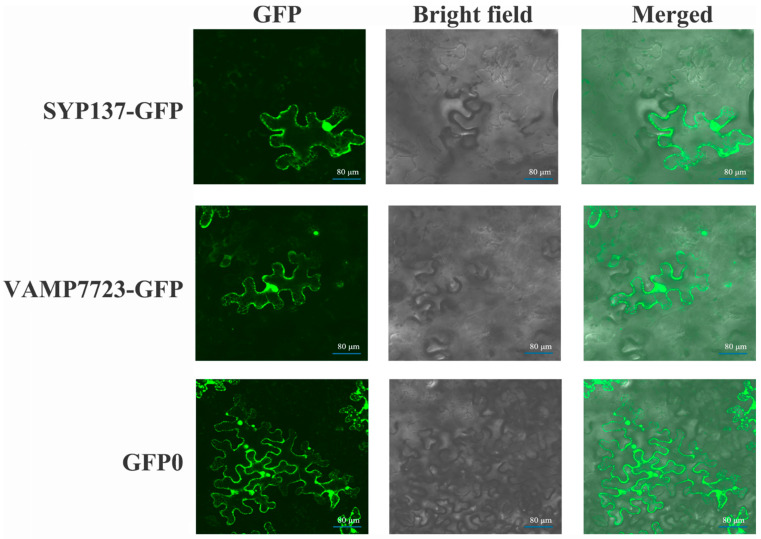
To investigate the subcellular localization of TaVAMP723 and TaSYP137 proteins, we constructed a GFP fusion vector and transformed it into Agrobacterium tumefaciens (Figure 3). Then, the Agrobacterium tumefaciens liquid containing the recombinant vector/blank vector was injected to infest the young leaves of Nicotiana benthamiana. Finally, GFP fluorescence detection was performed on the epidermal cells from the abaxial side of Nicotiana benthamiana. The results indicated that TaVAMP723 and TaSYP137 exhibited green fluorescent signals in both the cell membrane and the nucleus. In contrast, the control GFP0 showed a wide distribution of fluorescent signals in the cytoplasm, cell membrane, and nucleus.
Figure 3.
Transient expression and localization of TaSYP137 and TaVAMP723 fusion proteins in Nicotiana benthamiana non-plasmolyzed epidermal cells. The fusion vector pYJ:TaSYP137:GFP, pYJ:TaVAMP723:GFP and the pYJ::GFP control (Ck, marker with GFP0) vectors were transformed into tobacco epidermal cells by Agrobacterium. The subcellular distribution of GFP in the epidermal cells was revealed by fluorescence scanning microscopy.
2.3. Overexpression and Silencing of TaSYP137 and TaVAMP723 Substantiated Their Negative Roles in the Response of Wheat to Bgt
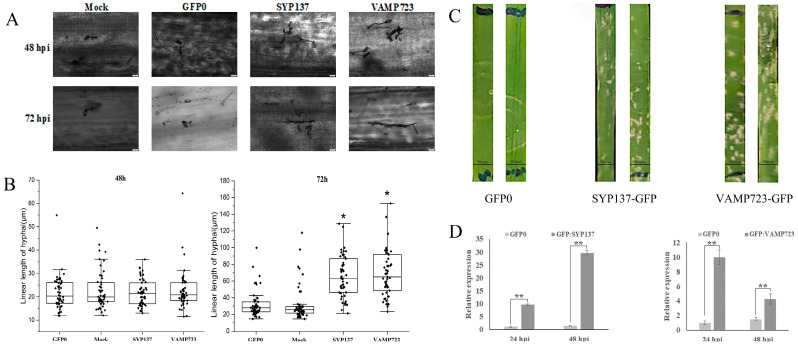
To explore the function of TaSYP137 and TaVAMP723 in the wheat–Bgt interaction, the genes were overexpressed in the leaves of N9134R prior to inoculation. The expression of the TaSYP137 gene was found to be upregulated by 10-fold and 30-fold at 24 hpi and 48 hpi, respectively (Figure 4D). The expression of the TaVAMP723 gene was found to be upregulated by 10-fold and 4-fold at 24 hpi and 48 hpi, respectively (Figure 4D). At 48 hpi, there was no significant difference in the mycelial length and cell size between the TaSYP137/TaVAMP723 overexpression and control groups (Figure 4A,B). At 72 hpi, the hyphal length of TaSYP137/TaVAMP723 overexpression was significantly longer than that of GFP0 and the control (Figure 4A,B). At 7 days post-infection (dpi), wheat leaves overexpressing TaSYP137/TaVAMP723 showed significant spore accumulation over the control (Figure 4C). To sum up, our results indicate that TaVAMP723 and TaSYP137 genes were expressed at lower levels in resistant wheat and higher levels in susceptible wheat, relative to the control, when infested with the pathogen. These data provide evidence that the overexpression of TaSYP137/TaVAMP723 inhibits wheat resistance to powdery mildew infection.
Figure 4.
Effect of TaSYP137 and TaVAMP723 overexpression on the response of N9134R leaves to Bgt stress. (A): The hyphal histology pictures in the TaSYP137 and TaVAMP723 overexpressed N9134R leaves. (B): Bgt hyphal length after infection. (C): Images of infection symptoms 10 days after inoculation with Bgt. The reconstructed vectors, pYJ:TaSYP137:GFP and pYJ:TaVAMP723:GFP, and the pYJ::GFP control were applied to the leaves before inoculation with Bgt pathogen. The mock group was treated with buffer in the same way. (D): Relative expression levels of TaSYP137 and TaVAMP723. Data were the mean of three biological replicates ± S.E. Asterisks denote significant differences by Student’s t-test analysis (t-tests, * p < 0.05, ** p < 0.01).
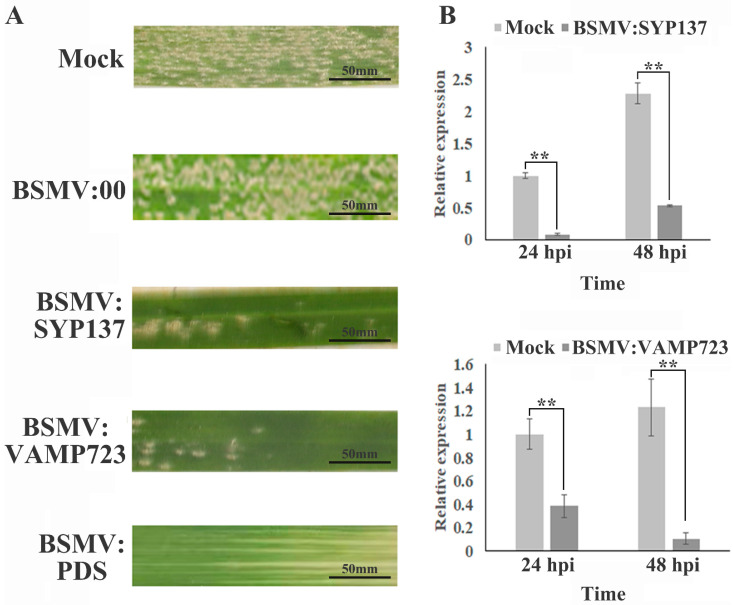
To further determine the function of TaSYP137/TaVAMP723 in wheat’s defense against powdery mildew infection, the barley stripe mosaic virus RNA-induced gene silencing system (BSMV-VIGS) was used to silence TaSYP137/TaVAMP723. Phytoene desaturase (PDS) silencing was used as a proof of concept, producing albino plants (Figure 5A). Silenced TaSYP137/TaVAMP723 plants and control plants were infected with Bgt, and the phenotypes were observed after 7 dpi. The results showed a significant decrease in the number and density of Bgt conidia in the silenced TaSYP137/TaVAMP723 plants (Figure 5A). The qPCR assays demonstrated a silencing efficiency of 50–70% (Figure 5B). These results suggest that the suppression of TaSYP137/TaVAMP723 significantly enhances wheat resistance to Bgt.
Figure 5.
Effect of TaSYP137 and TaVAMP723 silencing on the response of N9134S leaves to Bgt stress. (A): Images showing the appearance of the silenced phenotype 7 days after inoculation with BSMV-PDS; the effect of the reconstructed vectors BSMV-TaSYP137 and BSMV-TaVAMP723 on inoculated leaves of N9134S leaves after Bgt infection; and the mock and BSMV-Blank groups treated with buffer in the same way. (B): Relative expression level of TaSYP137 and TaVAMP723. Data were the mean of three biological replicates ± S.E. Asterisks denote significant differences by Student’s t-test analysis (t-tests, ** p < 0.01).
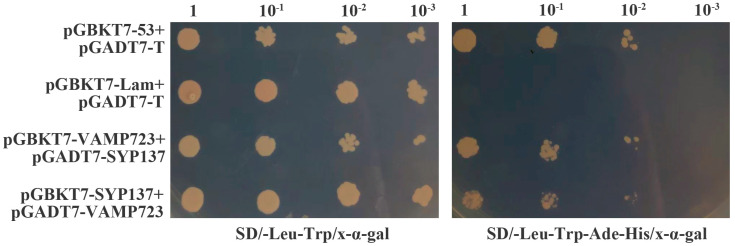
2.4. TaSYP137 Interacted with the TaVAMP723 Protein
According to previous reports, SNARE proteins form complexes to perform specific functions. In addition, SYP137 and VAMP723 can interact with each other in response to pathogenic bacteria in many other species. In this study, evidence of the interaction between TaSYP137 and TaVAMP723 was provided using the yeast two-hybrid system. We cloned TaVAMP723 and TaSYP137 genes into pGBKT7 and pGADT7 yeast vectors, respectively, with pGBKT7 containing the DNA-binding domain (BD) and pGADT7 containing the activation domain (AD). Positive control plasmids, pGBKT7-53 and pGADT7-T, and negative control plasmids, pGBKT7-lam and pGADT7-T, were used. For the experimental groups, pGBKT7-VAMP723 and pGADT7-SYP137 were designated as group 1, while pGBKT7-SYP137 and pGADT7-VAMP723 were designated as group 2. The yeast receptor state was transformed and then spotted on SD/-Leu-Trp to screen the transformants (Figure 6 left). The yeast solution containing transformants was then spotted on four-deficient medium SD/-Leu-Trp-Ade-His for further confirmation (Figure 6 right). The results of the study indicated that the yeast strains carrying AD-SYP137/BD-VAMP723 and BD-SYP137/AD-VAMP723 were able to grow normally on the four deficient media, indicating that the two proteins were capable of interacting in vitro (Figure 6).
Figure 6.
The interaction between TaSYP13 and TaVAMP723 proteins and their potential interactors in a yeast two-hybrid system. The interaction between SV40 large T-antigen (T) and murine p53 (53), T−AD + 53−BD, was used as the positive control, while the interaction between T-antigen and human lamin C (Lam), T−AD + Lam−BD, was used as the negative control.
3. Discussion
In this study, we isolated and characterized TaSYP137, a Q-SNARE subfamily gene, and TaVAMP723, an R-SNARE subfamily gene, from wheat. These genes possess distinct functional domains characteristic of their respective SNARE subfamilies. TaSYP137 has syntaxin and SNARE domains on the C-terminal and N-terminal, respectively. TaVAMP723 has longin and synaptobrevin domains. An evolutionary analysis of the genes TaSYP137 and TaVAMP723 shows that they are closely genetically related to XP_037417660.1 and XP_037439902.1, with high degrees of similarity to homologous proteins in other species. This suggests that TaSYP137/TaVAMP723 protein may perform similar biological functions in plant development and response to biotic and abiotic stresses, similar to the corresponding proteins in other species. It is possible to speculate on the functions of these two genes based on previous studies.
SNARE proteins play an essential role in the growth and development of all organisms [53]. The SNARE-mediated secretory pathway transfers antibacterial factors related to cell defense to the infection site during the plant’s exocytosis-related immune response [15]. VAMP721/722 is the main exocytosis-related R-SNARE of Arabidopsis thaliana [54]. It is involved in many physiological processes, such as cell division, growth, biotic and abiotic stress responses, and symbiosis between plants and bacteria [15,28,29,30,55,56]. Compared with VAMP721/722, plant PM synthesis proteins participate in specific biological processes. Although it is unknown how VAMP721/722 participates in these biological processes, they can interact with different PM synthetic proteins, such as SYP111, SYP121, SYP122, SYP123, and SYP132, which shows that VAMP721/722 can play corresponding biological functions by interacting with the corresponding PM synthetic elements [15,28,55,56]. In previous studies, it was found that VAMP721/722 played an essential role in the immune response of plants to the Pseudomonas syringae DC3000 bacteria [54]. The difference in the location of DC3000 bacteria, whether they are epiphytic or extracellular, could influence VAMP721/722′s role in bacterial immune response [54]. VAMP721/722 is important for plant response to epiphytic bacteria, but ineffective for the immune response of bacteria that proliferate outside the cells [27,54]. Although VAMP721 is very important for the plant immune response, there are just two associated immune factors: RPW8.2 and phospholipid alcohol D (PLD); other directly responding immune factors are not known to be transported by vesicles of VAMP721/722 [57,58].
Arabidopsis is a plant species that is resistant to Blumeria graminis f. sp. hordei (Bgh), a powdery mildew fungus, and it can resist the invasion of Bgh through two mechanisms: intracellular and extracellular immunity. PEN1-SNAP33-VAMP721/722 can form protein complexes to induce extracellular immunity [15]. However, TaSYP137 and TaVAMP723 are paralogous to AtPEN1 and AtVAMP721/722, respectively. They are divided into large groups in the evolutionary tree, so TaSYP137 and TaVAMP723 may also form complexes to jointly perform functions in wheat. As shown in previous reports, AtPEN1 [15], OsPEN1 [59], VvPEN1 [60], and SlPEN1 [61], which belong to SYP1, all play a positive role in the resistance to powdery mildew, while in this study, TaSYP137 plays a negative role, indicating that the function of genes in different species changes during differentiation.
In our earlier research, we focused on identifying the members of the SNARE family and examining their expression levels in response to powdery mildew infection. According to the quantitative expression and RNA-seq results, we focused on SYP137/VAMP723 and speculated that they might play an essential role in the interaction between wheat and Bgt [24]. In this study, we conducted a more detailed analysis of the functions of these two genes. First, the near-isogenic Line N9134R/N9134S was used as a template for quantitative expression analysis. The expression trend of TaSYP137/TaVAMP723 was similar. That is, the expression was downregulated in resistant and upregulated in susceptible plants at the early stage of Bgt induction. Further transient overexpression and silencing experiments confirmed that these genes negatively regulated wheat resistance to Bgt infection. Previous studies have shown that SNARE families often form complexes synergistically and that SYP13 and VAMP72 can interact in many other species. Therefore, yeast two-hybrid experiments were conducted in this paper to confirm that this interaction in vitro, providing evidence that TaSYP137/TaVAMP723 may form a complex to negatively regulate wheat’s response to Bgt infection.
In summary, this research highlights that TaSYP137/TaVAMP723 is present in both the nucleus and cell membrane and its expression pattern varies between resistant and susceptible wheat when infected by Bgt. Additionally, it was found that these two proteins can interact, which negatively regulates wheat’s resistance to Bgt invasion. The study of the role of SNARE in wheat resistance to Bgt offers new insights and expands our understanding of the role of the SNARE family in plant defense mechanisms against disease. The findings of this research demonstrate the importance of SNARE proteins in regulating the resistance of plants to fungal pathogens and contributes to a deeper understanding of the complex molecular pathways involved in plant–pathogen interactions. However, to verify the protein interactions between TaSYP137/TaVAMP723 and their function in response to powdery mildew invasion in wheat, further luciferase and wheat transgenic experiments are required.
4. Materials and Methods
4.1. Plant Materials and Pathogen Stress Treatment
The progeny of a pair of near isogenic lines, whose parents are Shaanyou225 and N9134 (containing the disease resistance gene PmAS846) [62], are named N9134R (resistance)/N9134S (susceptible), respectively. The near isogenic lines and their parents used in this paper are from our laboratory. The powdery mildew used was E09 (from our lab), which was stored and propagated in susceptible wheat Shaanyou225 [24]. N9134R/N9134S was incubated in a light incubator in an 16 h light/8 h dark cycle at 18 °C. Wheat plants were inoculated with powdery mildew conidia at the trilobal stage. Wheat leaves were collected at 0, 6, 12, 24, 48, 72, and 96 h after inoculation/simulated inoculation, quickly frozen in liquid nitrogen, and, finally, stored in an ultralow-temperature refrigerator at −80 °C.
4.2. TaSYP137 and TaVAMP723 Cloning and Sequence Analysis
PCR amplified TaSYP137/TaVAMP723 with specific primers (Table S2) covering the whole open reading frame and using cDNA from leaves of N9134R at two days post-inoculation with Bgt as the template. The PCR products were purified from agarose gel and cloned into the pGEM-T Easy Vector (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The nucleotide sequences of the positive clones were determined by AuGCT DNA-SYN Biotechnology Co., Ltd. (http://www.augct.com/ 28 September 2022) (Xianyang, China). The protein sequence encoded by TaSYP137 or TaVAMP723 was predicted using Pfam (http://pfam.xfam.org/ 28 September 2022). Sequence alignment was performed using the MEGA X software MUSCLE program, and the phylogenetic tree was constructed using the maximum likelihood method with MEGA X software (bootstrap test 1000 replicates, JTT matrix-based method).
4.3. Real-Time Quantitative PCR Analysis
TaSYP137/TaVAMP723 expression profiles in infected wheat leaves of NILs were determined by real-time quantitative PCR (qPCR) analysis of cDNA samples using SYBR Green. qPCR was performed on the QuantStudio 7 Flex Real-Time PCR System (Life Technologies Corporation, Carlsbad, USA). Sequence-specific primers were designed by NCBI primer blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/ 28 September 2022) (Table S2). The amplification was conducted in a 20 μL volume according to the SYBR Premix Ex Taq manual (Takara, Dalian, China) with the following conditions: 95 °C for 30 s followed by 40 cycles of 95 °C for 5 s and 63 °C for 34 s. For each sample, reactions were carried out in triplicate, and three non-template negative controls were included. Products were analyzed by melting curves obtained at the end of the process to confirm the amplification of a single product. The standard 2−ΔΔCT method was employed to quantify the relative gene expression. Mean values and standard errors were calculated with Microsoft Excel software. Student’s t-tests were used to analyze data with the Origin Pro program (Origin 2021b) to assess the significance of any differences between the control and treated samples or between time points, and the threshold for statistical significance was set at p < 0.05.
4.4. Vector Construction, Subcellular Localization, and Overexpression Assay
The overexpression plasmid was based on the pYJ::GFP vector driven by the Cauliflower mosaic virus (CaMV) 35S promoter (35Spro). To construct pYJ:GENE:GFP, the open reading frame sequences were ligated into the SpeI-cut plasmid using the ClonExpressII One Step Cloning Kit (Vazyme Biotech, Nanjing, China). The recombinant plasmid was transformed into A. tumefaciens using the freeze–thaw method. A single colony was inoculated into 50 mL liquid LB medium (50 mg/L rifampicin and 50 mg/L kanamycin) and cultured at 28 °C. The bacterial culture was then centrifuged for 10 min. For overexpression, the pellet was resuspended in suspension buffer (10 mM MgCl2, 10 mM MES, and 150 μm acetosyringone) to obtain an OD600 value of 0.8–1.0 according to a previously described protocol [63]. Bacteria suspended in infiltration media were injected into wheat leaves at the two-leaf stage with a syringe; leaves were injected with A. tumefaciens carrying the pYJ::GFP empty vector as a control. After 36 h, the wheat leaves were inoculated with Bgt. In our experiment, we evaluated the effectiveness of overexpression by measuring transcript levels in leaves collected at 12 and 48 h post-Bgt infection. The leaves that were treated with the powdery mildew fungus were also collected at 24, 48, and 72 h, and the development of spores was observed under a microscope after Coomassie brilliant blue R-250 staining. The mycelium length was determined using ImageJ software (https://cnij.imjoy.io/ 28 September 2022) [64]. A. tumefaciens grown in infiltration media were injected into 4-week-old Nicotiana benthamiana leaves, which were then grown for approximately 48 h in a growth chamber under normal conditions. The subcellular localization of 4-week-old Nicotiana benthamiana leaves cells was examined using a fluorescence confocal microscope with a detection wavelength of 488 nm (Nikon ECLIPSE Ti2, Tokyo, Japan).
4.5. Gene Silencing Induced by Tobacco Transcribed BSMV RNA in Wheat
The BSMV-VIGS was utilized to silence TaSYP137 or TaVAMP723. Briefly, a fragment of the TaSYP137 or TaVAMP723 homolog gene isolated by qPCR was inserted into SapI-digested pCB301-BSMV-γ by homologous recombination (Clontech Inc. Palo Alto, USA). The resulting plasmid, designated γSYP137/γVAMP723, was sequenced by AuGCT DNA-SYN Biotechnology Co., Ltd. After the pCB301-BSMV-α, -β, -γVAMP723, and -γSYP137 constructs were transformed into A. tumefaciens, the purified Agrobacterium cultures α, β, and γSYP137/γVAMP723 were mixed and transformed into Nicotiana benthamiana to transcribe RNA in vivo. The empty γ0 vector was used as a negative control, while a construct encoding a 214 bp fragment of the wheat phytoene desaturase (PDS) gene [49], designated γPDS, was also generated. Five days after being inoculated with phosphate-diluted tissue abrasive fluids such as BSMV-SYP137, BSMV-VAMP723, BSMV blank vectors, tap water (Called the mock-BSMV inoculation), and BSMV-PDS, the second leaves of N9134R/S seedlings at the 3-leaf stage were incubated for 12 h in the dark at 22 °C with 80% relative humidity, followed by 7 days at 18–22 °C in a growth cabinet (RLD-1000D-4DW, Ningbo, China). The infected seedlings and corresponding control plants (Shaanyou225) were then challenged with Bgt and kept at 18–22 °C until the susceptible variety Shaanyou225 showed signs of powdery mildew. The experiment was repeated three times. To assess the efficiency of TaSYP137 and TaVAMP723 silencing, the transcript levels in leaves collected at 0, 12, and 48 hpi with Bgt were quantified.
4.6. Yeast Two-Hybrid Assays
Protein interactions among TaSYP137 and TaVAMP723 were evaluated using the MatchMaker Y2H system (Clontech Inc. Palo Alto, USA), as previously described [63]. The full-length TaSYP137 and TaVAMP723 ORF was amplified by PCR with specific primers with a homologous arm designed using an NCBI primer blast and ligated into the PGBKT7 plasmid (Table S2). The transcriptional activity of the transformants was evaluated by preparing ten-fold serial dilutions and using 3 μL aliquots to inoculate SD/-Leu-Trp-Ade-His medium and SD/-Leu-Trp medium containing X-α-Gal (Clontech Inc. Palo Alto, USA). The inoculated media were incubated at 30 °C for 4 days. The coding sequences were then inserted into the BD and AD vectors.
4.7. Statistical Analysis
All experiments conducted in this study consisted of three or more biological replicates, with each biological replicate comprising at least three technical replicates. Statistical analysis, statistical comparisons, and plotting were carried out using ImageJ and Origin Pro program. Significance analysis of the data was conducted using Student’s t-test, and the threshold for statistical significance was set at p < 0.05.
5. Conclusions
In this paper, we identified two interacting genes, TaVAMP723 and TaSYP137, that exert negative regulation on wheat resistance to powdery mildew invasion. The subcellular localization of TaVAMP723 and TaSYP137 is in the cell membrane, and these genes exhibit similar expression patterns in near-isogenic lines of wheat with the same powdery mildew resistance, but opposite expression patterns in near-isogenic lines of wheat with different powdery mildew resistance.
Acknowledgments
The pYJ::GFP vector was kindly gifted by Cong Jiang.
Abbreviations
| Bgt | Blumeria graminis f. sp. tritici |
| Bgh | Blumeria graminis f. sp. hordei |
| BSMV | Barley stripe mosaic virus |
| VIGS | Virus-induced gene silencing |
| Hpi | Hours post-inoculation |
| PM | Plasma membrane |
| Y2H | Yeast two-hybrid |
| dpi | Days post-infection |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24054830/s1.
Author Contributions
Conceptualization, H.Z. and W.J.; methodology, G.W.; software, G.W.; validation, G.W., W.J. and C.C.; formal analysis, G.W.; investigation, G.W.; resources, C.Z.; data curation, X.Z.; writing—original draft preparation, G.W.; writing—review and editing, G.W.; visualization, H.G.; supervision, H.G.; project administration, C.C.; funding acquisition, W.J. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the Shaanxi Research Station of Crop Gene Resources and Germplasm Enhancement Program of China (No. Z100021811), the National Natural Science Foundation of China (31971741), and the Shaanxi Innovation Team Project (2018TD-004).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Jahn R., Scheller R.H. SNAREs—Engines for membrane fusion. Nat. Rev. Mol. Cell Biol. 2006;7:631–643. doi: 10.1038/nrm2002. [DOI] [PubMed] [Google Scholar]
- 2.Lipka V., Kwon C., Panstruga R. SNARE-Ware: The Role of SNARE-Domain Proteins in Plant Biology. Annu. Rev. Cell Dev. Biol. 2007;23:147–174. doi: 10.1146/annurev.cellbio.23.090506.123529. [DOI] [PubMed] [Google Scholar]
- 3.Yun H.S., Kwon C. Vesicle trafficking in plant immunity. Curr. Opin. Plant Biol. 2017;40:34–42. doi: 10.1016/j.pbi.2017.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Söllner T., Whiteheart S.W., Brunner M., Erdjument-Bromage H., Geromanos S., Tempst P., Rothman J.E. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- 5.Bock J.B., Matern H.T., Peden A.A., Scheller R.H. A genomic perspective on membrane compartment organization. Nature. 2001;409:839–841. doi: 10.1038/35057024. [DOI] [PubMed] [Google Scholar]
- 6.Kwon C., Lee J.-H., Yun H.S. SNAREs in Plant Biotic and Abiotic Stress Responses. Mol. Cells. 2020;43:501–508. doi: 10.14348/molcells.2020.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yang J., Jin H., Liu Y., Guo Y., Zhang Y. A dynamic template complex mediates Munc18-chaperoned SNARE assembly. Proc. Natl. Acad. Sci. USA. 2022;119:e2215124119. doi: 10.1073/pnas.2215124119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shaaban A., Dhara M., Frisch W., Harb A., Shaib A.H., Becherer U., Bruns D., Mohrmann R. The SNAP-25 linker supports fusion intermediates by local lipid interactions. eLife. 2019;8:e41720. doi: 10.7554/eLife.41720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han J., Pluhackova K., Böckmann R.A. The Multifaceted Role of SNARE Proteins in Membrane Fusion. Front. Physiol. 2017;8:5. doi: 10.3389/fphys.2017.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu M., Xu H., Jiang Y., Yu H., Liu Y. Epigallocatechin gallate inhibits SNARE-dependent membrane fusion by blocking trans-SNARE assembly. FEBS Open Bio. 2022;12:2111–2121. doi: 10.1002/2211-5463.13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rubiato H.M., Liu M., O’Connell R.J., Nielsen M.E. Plant SYP12 syntaxins mediate an evolutionarily conserved general immunity to filamentous pathogens. eLife. 2022;11:e73487. doi: 10.7554/eLife.73487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hückelhoven R., Panstruga R. Cell biology of the plant–powdery mildew interaction. Curr. Opin. Plant Biol. 2011;14:738–746. doi: 10.1016/j.pbi.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 13.Assaad F.F., Qiu J.-L., Youngs H., Ehrhardt D., Zimmerli L., Kalde M., Wanner G., Peck S.C., Edwards H., Ramonell K., et al. The PEN1 Syntaxin Defines a Novel Cellular Compartment upon Fungal Attack and Is Required for the Timely Assembly of Papillae. Mol. Biol. Cell. 2004;15:5118–5129. doi: 10.1091/mbc.e04-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Douchkov D., Nowara D., Zierold U., Schweizer P. A High-Throughput Gene-Silencing System for the Functional Assessment of Defense-Related Genes in Barley Epidermal Cells. Mol. Plant-Microbe Interact. 2005;18:755–761. doi: 10.1094/MPMI-18-0755. [DOI] [PubMed] [Google Scholar]
- 15.Kwon C., Neu C., Pajonk S., Yun H.S., Lipka U., Humphry M., Bau S., Straus M., Kwaaitaal M., Rampelt H., et al. Co-option of a default secretory pathway for plant immune responses. Nature. 2008;451:835–840. doi: 10.1038/nature06545. [DOI] [PubMed] [Google Scholar]
- 16.Lipka U., Fuchs R., Lipka V. Arabidopsis non-host resistance to powdery mildews. Curr. Opin. Plant Biol. 2008;11:404–411. doi: 10.1016/j.pbi.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 17.Wick P., Gansel X., Oulevey C., Page V., Studer I., Dürst M., Sticher L. The Expression of the t-SNARE AtSNAP33 Is Induced by Pathogens and Mechanical Stimulation. Plant Physiol. 2003;132:343–351. doi: 10.1104/pp.102.012633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He X., Huo Y., Liu X., Zhou Q., Feng S., Shen X., Li B., Wu S., Chen X. Activation of disease resistance against Botryosphaeria dothidea by downregulating the expression of MdSYP121 in apple. Hortic. Res. 2018;5:24. doi: 10.1038/s41438-018-0030-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang X., Wang X., Deng L., Chang H., Dubcovsky J., Feng H., Han Q., Huang L., Kang Z. Wheat TaNPSN SNARE homologues are involved in vesicle-mediated resistance to stripe rust (Puccinia striiformis f. sp. tritici) J. Exp. Bot. 2014;65:4807–4820. doi: 10.1093/jxb/eru241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eschen-Lippold L., Landgraf R., Smolka U., Schulze S., Heilmann M., Heilmann I., Hause G., Rosahl S. Activation of defense against Phytophthora infestans in potato by down-regulation of syntaxin gene expression. New Phytol. 2012;193:985–996. doi: 10.1111/j.1469-8137.2011.04024.x. [DOI] [PubMed] [Google Scholar]
- 21.Sugano S., Hayashi N., Kawagoe Y., Mochizuki S., Inoue H., Mori M., Nishizawa Y., Jiang C.-J., Matsui M., Takatsuji H. Rice OsVAMP714, a membrane-trafficking protein localized to the chloroplast and vacuolar membrane, is involved in resistance to rice blast disease. Plant Mol. Biol. 2016;91:81–95. doi: 10.1007/s11103-016-0444-0. [DOI] [PubMed] [Google Scholar]
- 22.Chung K.P., Zeng Y., Li Y., Ji C., Xia Y., Jiang L. Signal motifs-dependent ER export of Qc-SNARE BET12 interacts with MEMB12 and affects PR1 trafficking in Arabidopsis. J. Cell Sci. 2018;131:jcs202838. doi: 10.1242/jcs.202838. [DOI] [PubMed] [Google Scholar]
- 23.Ma J., Chen J., Wang M., Ren Y., Wang S., Lei C., Cheng Z. Sodmergen, Disruption of OsSEC3A increases the content of salicylic acid and induces plant defense responses in rice. J. Exp. Bot. 2018;69:1051–1064. doi: 10.1093/jxb/erx458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang G., Long D., Yu F., Zhang H., Chen C., Wang Y., Ji W. Genome-wide identification, evolution, and expression of the SNARE gene family in wheat resistance to powdery mildew. PeerJ. 2021;9:e10788. doi: 10.7717/peerj.10788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xue Y., Yang Y., Yang Z., Wang X., Guo Y. VAMP711 Is Required for Abscisic Acid-Mediated Inhibition of Plasma Membrane H+-ATPase Activity. Plant Physiol. 2018;178:1332–1343. doi: 10.1104/pp.18.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yun H.S., Kwaaitaal M., Kato N., Yi C., Park S., Sato M.H., Schulze-Lefert P., Kwon C. Requirement of vesicle-associated membrane protein 721 and 722 for sustained growth during immune responses in Arabidopsis. Mol. Cells. 2013;35:481–488. doi: 10.1007/s10059-013-2130-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., Park K., Kwon C., Yun H.S. Synaptotagmin 4 and 5 additively contribute to Arabidopsis immunity to Pseudomonas syringae DC3000. Plant Signal. Behav. 2022;17:2025323. doi: 10.1080/15592324.2021.2025323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Kasmi F., Krause C., Hiller U., Stierhof Y.D., Mayer U., Conner L., Kong L., Reichardt I., Sanderfoot A.A., Jürgens G. SNARE complexes of different composition jointly mediate membrane fusion in Arabidopsis cytokinesis. Mol. Biol. Cell. 2013;24:1593–1601. doi: 10.1091/mbc.e13-02-0074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S., Choi Y., Kwon C., Yun H.S. Endoplasmic reticulum stress-induced accumulation of VAMP721/722 requires CALRETICULIN 1 and CALRETICULIN 2 in Arabidopsis. J. Integr. Plant Biol. 2019;61:974–980. doi: 10.1111/jipb.12728. [DOI] [PubMed] [Google Scholar]
- 30.Yi C., Park S., Yun H.S., Kwon C. Vesicle-associated membrane proteins 721 and 722 are required for unimpeded growth of Arabidopsis under ABA application. J. Plant Physiol. 2013;170:529–533. doi: 10.1016/j.jplph.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 31.Xia L., Marquès-Bueno M.M., Bruce C.G., Karnik R. Unusual Roles of Secretory SNARE SYP132 in Plasma Membrane H+-ATPase Traffic and Vegetative Plant Growth. Plant Physiol. 2019;180:837–858. doi: 10.1104/pp.19.00266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baena G., Xia L., Waghmare S., Karnik R. SNARE SYP132 mediates divergent traffic of plasma membrane H+-ATPase AHA1 and antimicrobial PR1 during bacterial pathogenesis. Plant Physiol. 2022;189:1639–1661. doi: 10.1093/plphys/kiac149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalde M., Nühse T.S., Findlay K., Peck S.C. The syntaxin SYP132 contributes to plant resistance against bacteria and secretion of pathogenesis-related protein 1. Proc. Natl. Acad. Sci. USA. 2007;104:11850–11855. doi: 10.1073/pnas.0701083104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He H., Liu R., Ma P., Du H., Zhang H., Wu Q., Yang L., Gong S., Liu T., Huo N., et al. Characterization of Pm68, a new powdery mildew resistance gene on chro mosome 2BS of Greek durum wheat TRI 1796. Theor. Appl. Genet. 2021;134:53–62. doi: 10.1007/s00122-020-03681-2. [DOI] [PubMed] [Google Scholar]
- 35.Hewitt T., Müller M.C., Molnár I., Mascher M., Holušová K., Šimková H., Kunz L., Zhang J., Li J., Bhatt D., et al. A highly differentiated region of wheat chromosome 7AL encodes a Pm1a immune receptor that recognizes its corresponding AvrPm1a effector from Blumeria graminis. New Phytol. 2021;229:2812–2826. doi: 10.1111/nph.17075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sánchez-Martín J., Widrig V., Herren G., Wicker T., Zbinden H., Gronnier J., Spörri L., Praz C.R., Heuberger M., Kolodziej M.C., et al. Wheat Pm4 resistance to powdery mildew is controlled by alternative splice variants encoding chimeric proteins. Nat. Plants. 2021;7:327–341. doi: 10.1038/s41477-021-00869-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sánchez-Martín J., Steuernagel B., Ghosh S., Herren G., Hurni S., Adamski N., Vrána J., Kubaláková M., Krattinger S.G., Wicker T., et al. Rapid gene isolation in barley and wheat by mutant chromosome sequencing. Genome Biol. 2016;17:221. doi: 10.1186/s13059-016-1082-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu P., Guo L., Wang Z., Li B., Li J., Li Y., Qiu D., Shi W., Yang L., Wang N., et al. A rare gain of function mutation in a wheat tandem kinase confers resistance to powdery mildew. Nat. Commun. 2020;11:680. doi: 10.1038/s41467-020-14294-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li M., Dong L., Li B., Wang Z., Xie J., Qiu D., Li Y., Shi W., Yang L., Wu Q., et al. A CNL protein in wild emmer wheat confers powdery mildew resistance. New Phytol. 2020;228:1027–1037. doi: 10.1111/nph.16761. [DOI] [PubMed] [Google Scholar]
- 40.Cao A., Xing L., Wang X., Yang X., Wang W., Sun Y., Qian C., Ni J., Chen Y., Liu D., et al. Serine/threonine kinase gene Stpk-V, a key member of powdery mildew resistance gene Pm21, confers powdery mildew resistance in wheat. Proc. Natl. Acad. Sci. USA. 2011;108:7727–7732. doi: 10.1073/pnas.1016981108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hurni S., Brunner S., Buchmann G., Herren G., Jordan T., Krukowski P., Wicker T., Yahiaoui N., Mago R., Keller B. Rye Pm8 and wheat Pm3 are orthologous genes and show evolutionary conservation of resistance function against powdery mildew. Plant J. 2013;76:957–969. doi: 10.1111/tpj.12345. [DOI] [PubMed] [Google Scholar]
- 42.Chen T., Xiao J., Xu J., Wan W., Qin B., Cao A., Chen W., Xing L., Du C., Gao X., et al. Two members of TaRLK family confer powdery mildew resistance in common wheat. BMC Plant Biol. 2016;16:27. doi: 10.1186/s12870-016-0713-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zou B., Ding Y., Liu H., Hua J. Silencing of copine genes confers common wheat enhanced resistance to powdery mildew. Mol. Plant Pathol. 2018;19:1343–1352. doi: 10.1111/mpp.12617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jordan T., Seeholzer S., Schwizer S., Töller A., Somssich I.E., Keller B. The wheat Mla homologue TmMla1 exhibits an evolutionarily conserved function against powdery mildew in both wheat and barley. Plant J. 2011;65:610–621. doi: 10.1111/j.1365-313X.2010.04445.x. [DOI] [PubMed] [Google Scholar]
- 45.Jing Y., Liu J., Liu P., Ming D., Sun J. Overexpression of TaJAZ1 increases powdery mildew resistance through promoting reactive oxygen species accumulation in bread wheat. Sci. Rep. 2019;9:5691. doi: 10.1038/s41598-019-42177-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou W., Qian C., Li R., Zhou S., Zhang R., Xiao J., Wang X., Zhang S., Xing L., Cao A. TaNAC6s are involved in the basal and broad-spectrum resistance to powdery mildew in wheat. Plant Sci. 2018;277:218–228. doi: 10.1016/j.plantsci.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 47.Hu P., Liu J., Xu J., Zhou C., Cao S., Zhou W., Huang Z., Yuan S., Wang X., Xiao J., et al. A malectin-like/leucine-rich repeat receptor protein kinase gene, RLK-V, regulates powdery mildew resistance in wheat. Mol. Plant Pathol. 2018;19:2561–2574. doi: 10.1111/mpp.12729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen G., Wei B., Li G., Gong C., Fan R., Zhang X. TaEDS1 genes positively regulate resistance to powdery mildew in wheat. Plant Mol. Biol. 2018;96:607–625. doi: 10.1007/s11103-018-0718-9. [DOI] [PubMed] [Google Scholar]
- 49.Zhang X., Wang G., Qu X., Wang M., Guo H., Zhang L., Li T., Wang Y., Zhang H., Ji W. A truncated CC-NB-ARC gene TaRPP13L1-3D positively regulates powdery mildew resistance in wheat via the RanGAP-WPP complex-mediated nucleocytoplasmic shuttle. Planta. 2022;255:60. doi: 10.1007/s00425-022-03843-0. [DOI] [PubMed] [Google Scholar]
- 50.Liu J., Zhang T., Jia J., Sun J. The Wheat Mediator Subunit TaMED25 Interacts with the Transcription Factor TaEIL1 to Negatively Regulate Disease Resistance against Powdery Mildew. Plant Physiol. 2016;170:1799–1816. doi: 10.1104/pp.15.01784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yue J., Sun H., Zhang W., Pei D., He Y., Wang H. Wheat homologs of yeast ATG6 function in autophagy and are implicated in powdery mildew immunity. BMC Plant Biol. 2015;15:95. doi: 10.1186/s12870-015-0472-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng H., Dong L., Han X., Jin H., Yin C., Han Y., Li B., Qin H., Zhang J., Shen Q., et al. The TuMYB46L-TuACO3 module regulates ethylene biosynthesis in einkorn wheat defense to powdery mildew. New Phytol. 2020;225:2526–2541. doi: 10.1111/nph.16305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanderfoot A. Increases in the Number of SNARE Genes Parallels the Rise of Multicellularity among the Green Plants. Plant Physiol. 2007;144:6–17. doi: 10.1104/pp.106.092973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kim S., Kim H., Park K., Cho D.J., Kim M.K., Kwon C., Yun H.S. Synaptotagmin 5 Controls SYP132-VAMP721/722 Interaction for Arabidopsis Immunity to Pseudomonas syringae pv tomato DC3000. Mol. Cells. 2021;44:670–679. doi: 10.14348/molcells.2021.0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ichikawa M., Hirano T., Enami K., Fuselier T., Kato N., Kwon C., Voigt B., Schulze-Lefert P., Baluška F., Sato M.H. Syntaxin of Plant Proteins SYP123 and SYP132 Mediate Root Hair Tip Growth in Arabidopsis thaliana. Plant Cell Physiol. 2014;55:790–800. doi: 10.1093/pcp/pcu048. [DOI] [PubMed] [Google Scholar]
- 56.Yu X., Lund S.P., Scott R.A., Greenwald J.W., Records A.H., Nettleton D., Lindow S.E., Gross D.C., Beattie G.A. Transcriptional responses of Pseudomonas syringae to growth in epiphytic versus apoplastic leaf sites. Proc. Natl. Acad. Sci. USA. 2013;110:E425–E434. doi: 10.1073/pnas.1221892110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xing J., Li X., Wang X., Lv X., Wang L., Zhang L., Zhu Y., Shen Q., Baluška F., Šamaj J., et al. Secretion of Phospholipase Dδ Functions as a Regulatory Mechanism in Plant Innate Immunity. Plant Cell. 2019;31:3015–3032. doi: 10.1105/tpc.19.00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kim H., O’Connell R., Maekawa-Yoshikawa M., Uemura T., Neumann U., Schulze-Lefert P. The powdery mildew resistance protein RPW8.2 is carried on VAMP721/722 vesicles to the extrahaustorial membrane of haustorial complexes. Plant J. 2014;79:835–847. doi: 10.1111/tpj.12591. [DOI] [PubMed] [Google Scholar]
- 59.Cao W.-L., Yu Y., Li M.-Y., Luo J., Wang R.-S., Tang H.-J., Huang J., Wang J.-F., Zhang H.-S., Bao Y.-M. OsSYP121 Accumulates at Fungal Penetration Sites and Mediates Host Resistance to Rice Blast. Plant Physiol. 2019;179:1330–1342. doi: 10.1104/pp.18.01013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Feechan A., Jermakow A.M., Ivancevic A., Godfrey D., Pak H., Panstruga R., Dry I.B. Host Cell Entry of Powdery Mildew Is Correlated with Endosomal Transport of Antagonistically Acting VvPEN1 and VvMLO to the Papilla. Mol. Plant-Microbe Interact. 2013;26:1138–1150. doi: 10.1094/MPMI-04-13-0091-R. [DOI] [PubMed] [Google Scholar]
- 61.Bracuto V., Appiano M., Zheng Z., Wolters A.-M., Yan Z., Ricciardi L., Visser R.G.F., Pavan S., Bai Y. Functional Characterization of a Syntaxin Involved in Tomato (Solanum lycopersicum) Resistance against Powdery Mildew. Front. Plant Sci. 2017;8:1573. doi: 10.3389/fpls.2017.01573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xue F., Ji W., Wang C., Zhang H., Yang B. High-density mapping and marker development for the powdery mildew resistance gene PmAS846 derived from wild emmer wheat (Triticum turgidum var. dicoccoides) Theor. Appl. Genet. 2012;124:1549–1560. doi: 10.1007/s00122-012-1809-7. [DOI] [PubMed] [Google Scholar]
- 63.Guo H., Zhang H., Wang G., Wang C., Wang Y., Liu X., Ji W. Identification and expression analysis of heat-shock proteins in wheat infected with powdery mildew and stripe rust. Plant Genome. 2021;14:e20092. doi: 10.1002/tpg2.20092. [DOI] [PubMed] [Google Scholar]
- 64.Barry D.J., Chan C., Williams G.A. Morphological quantification of filamentous fungal development using membrane immobilization and automatic image analysis. J. Ind. Microbiol. Biotechnol. 2009;36:787–800. doi: 10.1007/s10295-009-0552-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.