Abstract
Deficits in serotonin (5-hydroxytryptamine, 5-HT) neurotransmission are implicated in abnormal emotional behaviors such as aggression, anxiety, and depression. However, the specific 5-HT receptor mechanisms involved are not well understood. The role of 5-HT2 receptors in fear potentiated startle, (FPS) was examined in rats chronically treated with p-chlorophenylalanine (PCPA) to reduce brain 5-HT. PCPA-treated rats show an enhanced magnitude of FPS. Systemic administration of the 5-HT2 receptor agonist (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride (DOI) reduced FPS in both PCPA-treated and saline (SAL)-treated control animals, normalizing the exaggerated fear response in PCPA-treated rats. In both SAL- and PCPA-treated animals, the DOI-induced reduction of learned fear was reversed by the 5-HT2 antagonist ketanserin, but not by the 5-HT2B/2C antagonist SB 206553. Together, these findings suggest 5-HT2A receptors are critical regulators of learned fear, and that 5-HT2A receptors may be an important pharmacological target to normalize exaggerated learned fear resulting from chronic 5-HT-ergic disruption.
Keywords: Fear Conditioning, Startle Reflex, 5-HT2, DOI, Ketanserin, SB 206553
1. Introduction
The neurotransmitter serotonin (5-hydroxytryptamine, 5-HT) has been widely implicated in the modulation of emotional behavior. Numerous studies have shown abnormalities in 5-HT systems in patients with mood and anxiety disorders [1]. Compounds that enhance 5-HT-ergic activity, such as selective serotonin reuptake inhibitors (SSRIs), are first-line treatments for mood and anxiety disorders. However, among the many known 5-HT receptors the specific receptor subtypes involved in the pathophysiology of psychiatric illness or the amelioration of symptoms by 5-HT compounds are not clear. Some evidence suggests that 5-HT2 receptors are involved in emotional disturbance. For instance, 5-HT2A receptor antagonists are used as adjunct treatments with SSRIs for depression and anxiety [2].
While 5-HT is known to have several biological activities, its role in emotional behavior is thought to result primarily from its activity in limbic areas such as the amygdala and hippocampus [3]. Both the amygdala and hippocampus are densely innervated by serotonergic inputs from the dorsal raphé nucleus (DRN) and median raphé nucleus (MRN), respectively, and both areas express moderate to high levels of 5-HT2 receptors [4–7]. Studies of Pavlovian fear conditioning, in which a neutral conditioned stimulus (CS) is paired with a noxious unconditioned stimulus (US), demonstrate the importance of both of the hippocampus and amygdala in fear learning [3]. Furthermore, delivery of selective serotonin reuptake inhibitors (SSRIs) directly into the amygdala attenuates conditioned fear [8,9]. However, much is still unknown about the functional role of specific 5-HT receptor subtypes in modulating emotional learning.
Serotonin mediates a net inhibitory tone in the amygdala primarily through 5-HT2 receptors [10–12]. Thus, we hypothesized that deficits in 5-HT-ergic transmission may remove critical dampers on neuronal excitability. We have previously shown that rats chronically treated with p-chlorophenylalanine (PCPA), a competitive and irreversible inhibitor of 5-HT synthesis, show enhanced fear learning; and that phenytoin, a widely used anticonvulsant, reduces fear behavior in PCPA-treated rats but has not in saline-treated controls [8]. These findings suggest that chronic low 5-HT results in a sub-seizure state of neuronal hyperexcitability in brain fear circuits [13]. However, the specific receptor mechanisms that translate low 5-HT to hyperexcitability are unknown. Here we hypothesize that increased fear learning resulting from chronic low 5-HT can be restored by 5-HT2 receptor activation. The results shown here confirm our previous findings that PCPA treatment causes enhanced fear learning, and further show that the 5-HT2 receptor agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) normalizes the exaggerated fear behavior observed in PCPA-treated animals. The behavioral effect of DOI was reversed by the 5-HT2 antagonist ketanserin, but not the 5-HT2B/2C antagonist SB 206553, suggesting a critical role for 5-HT2A receptors in exaggerated fear behavior induced by PCPA. Together, these data suggest that 5-HT2A receptors are important regulators of fear behavior, and furthermore, that exaggerated fear learning in PCPA-treated animals may result from reduced 5-HT2A receptor-mediated mechanisms in brain fear circuits.
2. Experimental Procedure
2.1. Animals
Thirty-three male Sprague-Dawley rats (Harlan, Houston, TX) weighing approximately 175 – 200 g at time of experimentation were used. Rats were individually-housed upon arrival and maintained on a 12-h light/dark cycle (lights on at 7 am) with food and water available ad libitum. Animals received either PCPA (300 mg/kg, i.p.) or isovolumetric injections of 0.9% phosphate-buffered saline (SAL) on experiment days 1, 2 and 10. Each rat was handled 5 min/day for four days prior to behavioral experimentation. Experiments were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Baylor University.
2.2. Pharmacological Agents
DL-p-chlorophenylalanine hydrochloride (PCPA, Sigma, St. Louis, MO) was dissolved in 0.9% saline (SAL) and pH adjusted to 7.2 with KOH. 3,5-dihydro-5-methyl-N-3-pyridinylbenzo[1,2-b:4,5-b’]dipyrrole-1(2H)-carboxamide hydrochloride (SB 206553, Tocris, Ellisville, MO) was dissolved in a vehicle (VEH) consisting of 40% (weight/volume) hydroxypropyl-β-cyclodextrin (Fisher Scientific, Houston, TX). Ketanserin tartrate (KET, Tocris) and (±)-2,5-Dimethoxy-4-iodoamphetamine hydrochloride (DOI, Sigma) were dissolved in 0.9% saline (SAL). Thirty minutes prior to testing, rats received i.p. injections of SAL alone followed by DOI (0.01, 0.1, 0.5, and 1.0 mg/kg) on subsequent testing days. For antagonist experiments, animals received KET (1.0 mg/kg, i.p.), SB 206553 (1.0 mg/kg, i.p.), or vehicle alone one hour before testing, followed by either DOI (1.0 mg/kg, i.p.) or SAL 30 min before testing.
2.3. HPLC
Brains were dissected from rats treated with either SAL (n = 3) or PCPA (n = 3). Tissue was collected in cold (4°C) 0.1 M phosphate-buffered saline, and 2 mm slices (approximately between 2.5 and 4.5 mm posterior to Bregma) were made on a dissecting block. Tissue samples from the amygdala, hippocampus, and cortex of both hemispheres were pooled to obtain an adequate mass of brain homogenate for HPLC quantification of amines. Samples were obtained on dry ice, weighed, flash frozen in dry ice/EtOH, and stored at −80°C until used.
Tissue samples were first protonized with 0.4 M HClO4 and 2 μM 2,3-dihydroxybenzoic acid is added as an internal standard. The samples were homogenized and centrifuged at 14,000 × g for 15 min at 4°C. The supernatant is collected then analyzed by HPLC coupled with electrochemical detection. Samples were detected on an ESA CoulArray system containing two pumps with a flow of 0.420 mL/min. The mobile phase contains 0.05 mM potassium phosphate, 8.5 mg/50 ml octylsulfate, and 14% methanol (pH 2.65). Neurotransmitters were separated using a C18 reverse-phase column. 5-HT (100 μM) and 5-HIAA (100 μM) were used as standards. Transmitter turnover was calculated as the ratio of 5-HIAA to 5-HT.
2.4. Fear-Potentiated Startle
Rats were trained and tested in an acoustic startle reflex system (Med Associates, St. Albans, VT) as described previously [8]. Briefly, steel grid rod animal holders attached to platforms were enclosed in ventilated, sound-attenuating chambers and background noise was maintained at 45 dB.
Startle responses were evoked by 50 msec white noise bursts (5 msec rise-decay time) and quantified using an accelerometer located beneath each animal platform. Changes in voltage output, proportional to the velocity of platform movement, were amplified and digitized on a scale of 0 – 2047 units and analyzed by a computer. Startle response amplitude was defined as the peak voltage change occurring during the first 200 msec after onset of the startle-eliciting stimulus.
The conditioned stimulus (CS) was a 3.7 sec light (80 Lux) delivered by an 8W fluorescent bulb (15 μsec onset) located 5 cm behind each platform. The unconditioned stimulus (US) was a 0.5 sec, 0.6 mA shock generated by a stand-alone shocker/scrambler unit and delivered through the floor bars of each animal holder. The presentation and sequencing of all stimuli were under computer control using the Startle Reflex software package provided by Med Associates. Animal holders and platforms were cleaned between subjects with 1% acetic acid.
Behavioral experimentation consisted of acclimation (days 16 – 17) to the test chambers, habituation (days 18 – 19) of the startle response, training (day 20), and testing (day 21) as described below.
Acclimation.
Rats were placed into the test chambers for 10 min and then returned to their home cages.
Habituation.
Rats were placed into the test chambers for 5 min, then presented with 30 startle-eliciting white noise bursts (10 each at 90, 95, and 105 dB, 50 msec duration). The three intensities were presented in a quasi-random order, such that each occurred once within each successive block of three stimuli, at a 30 sec inter-stimulus interval (ISI).
Training.
24 h after habituation trials, rats received 15 CS-US pairings. For each pairing the CS and US co-terminated, with the 0.5 sec shock (US) delivered 3.2 sec after onset of the 3.7 sec light (CS). The first pairing occurred 5 min after rats were placed in the test chambers, and successive presentations occurred at a variable ISI of 2 – 4 min.
Testing.
Animals were tested for cued fear behavior 24 h after training. Rats were placed into the test chambers, allowed a 5 min acclimation period, then tested for FPS. Learned fear was measured by presenting the startle-eliciting noise burst 3.2 sec after onset of the light CS (light-noise trials, CS+) in comparison to presentation of the noise burst without the light CS (noise-alone trials, CS−). Equal numbers of 90, 95, and 105 dB 50 msec noise bursts were presented in a quasi-random order. Cue-specific potentiation of the startle response (FPS) was defined as the difference between startle amplitude during the light-noise (CS+) trials and startle during the noise-alone (CS−) trials, divided by startle amplitude during noise alone, expressed as a percent (FPS = 100* [CS+ − CS−]/CS−).
The behavioral pharmacology of DOI and the effect of different antagonists were examined within-subjects. Animals were re-trained with 15 CS-US (light-footshock) pairings on intervening days (every 48 h) to prevent extinction. Pilot experiments showed consistent startle responses over many testing days when re-trained between sessions (data not shown). The antagonist pharmacology of FPS was measured using a semi-balanced design. Rats received either KET (1.0 mg/kg i.p.) (or SAL control) or SB 206553 (1.0 mg/kg i.p.) (or VEH control) 1 h prior to testing, followed by DOI (1.0 mg/kg i.p.) (or SAL control) 30 min prior to testing. Approximately half the animals received test compounds in the following order: VEH/SAL, KET/DOI, SB/DOI, VEH/ DOI (n = 4 for SAL pre-treated animals; n = 3 for PCPA pre-treated animals). To balance order effects, the other half received test compounds in the following order: VEH/SAL, SB/DOI, KET/DOI, VEH/DOI (n = 3 for SAL pretreated animals; n = 4 for PCPA pre-treated animals). The data were pooled for all SAL pre-treated and all PCPA pre-treated rats.
2.5. Statistical Analyses
Student’s unpaired t-test was used to analyze amine concentration and turnover. DOI concentration-response relationships were analyzed by two-way repeated measures analyses of variance (ANOVA) with the following factors (levels): Factor 1, Pre-treatment (SAL or PCPA, between-groups); Factor 2, DOI (0.01, 0.1. 0.5, or 1.0 mg/kg, or SAL control; within-groups). Antagonist studies were also analyzed by two-way repeated-measures ANOVA using the same SAL or PCPA pre-treatment as a between-groups factor and the agonist/antagonist administration delivered on testing day as the within-groups factor with 4 levels (VEH/SAL, VEH/DOI, KET/DOI, SB/DOI). Following ANOVA, select pairs of treatment conditions were compared using the Bonferonni multiple comparisons post-hoc test. All analyses were performed using Microsoft Excel and GraphPad Prism with alpha level of 0.05.
3. Results
3.1. Chronic PCPA Administration Decreases Brain 5-HT
Although overwhelming evidence shows that p-chlorophenylalanine (PCPA) decreases brain 5-HT, preliminary studies were conducted to confirm that the administration schedule used in this study has the intended effect of significantly lowering brain 5-HT. Individually-housed rats were treated on days 1, 2, and 10 with either p-chlorophenylalanine (PCPA, 300 mg/kg, n = 3) or phosphate-buffered saline (SAL, n = 3), as previously described [8]. On day 14, tissue samples were pooled from the amygdala, hippocampus, and cortex of both hemispheres to determine brain amine concentrations by HPLC-EC (Table 1). Systemic administration of PCPA decreased 5-HT, its primary metabolite 5-HIAA and 5-HT turnover. Analysis of amine and metabolite concentrations by HPLC showed that PCPA decreased 5-HT and 5-HIAA by 83.5% (p < 0.05, t(4) = 2.2) and 91.8% (p < 0.05, t(4) = 2.9), respectively. 5-HT turnover, expressed as the ratio of 5-HIAA/5-HT, was also significantly decreased by 66.7% in samples from PCPA-treated rats (p < 0.05, t(4) = 3.0). Therefore, this schedule of PCPA administration was used for all behavioral studies.
Table 1.
PCPA pre-treatment reduces brain 5-HT, 5-HIAA, and 5-HT turnover.
| Amine | Turnover | ||
|---|---|---|---|
|
| |||
| Pre-treatment | 5-HT | 5-HIAA | 5-HIAA/5-HT |
| SAL | 3.89 (1.76)a | 1.46 (0.60)b | 0.48 (0.15)c |
| PCPA | 0.64 (0.16)a | 0.12 (0.05)b | 0.16 (0.05)c |
Amine levels are expressed as the mean concentration (standard error) in nmol/g of tissue. Turnover is expressed as mean tissue concentration ratio (standard error) of metabolite (5-HIAA) to parent amine (5-HT). Means having the same subscript differ significantly at p < 0.05 by Student’s unpaired T-test. Abbreviations: Serotonin,5-hydroxytryptamine (5-HT); 5-hydroxy-indoleacetic acid (5-HIAA); p-chlorophenylalanine (PCPA); saline (SAL).
3.2. DOI Concentration-Response Relationship
We have previously shown that PCPA treatment interferes with fear learning [8] and that PCPA alters 5-HT-mediated inhibition of excitatory transmission in LA neurons in vitro [14]. We therefore tested the effects of the 5-HT2 agonist (±)-2,5-Dimethoxy-4-iodoam-phetamine hydrochloride (DOI) on learned fear in SAL and PCPA pre-treated rats. The concentration-response relationship of the 5-HT2A/2C receptor agonist DOI on learned fear behavior was determined using a two-factor mixed design: Factor 1 (between groups), pre-treatment (SAL or PCPA; n = 7 each); factor 2 (within groups), DOI dose. Rats were pre-treated with either SAL or PCPA as described then trained to fear a visual (light) conditioning stimulus (CS). On the first day of fear-potentiated startle testing (day 17 after first SAL or PCPA injection) animals received SAL (i.p.). On following test days (every 48 hours thereafter) animals received increasing doses of DOI. On intervening days, rats were re-trained with 15 US-CS pairings to prevent extinction of learned fear. SAL or DOI (0.01, 0.1, 0.5, and 1.0 mg/kg; i.p.) was administered 30 min before testing.
The DOI concentration-response relationship for cued conditioning in SAL pre-treated and PCPA pre-treated rats is shown in Figure 1. At the lowest dose (0.01 mg/kg) DOI slightly, but not significantly, increased learned fear in both SAL and PCPA pre-treated animals. At higher doses, DOI produced a dose-dependent decrease in potentiation of the acoustic startle reflex in both pre-treatment groups. Two-way repeated measures ANOVA revealed a significant effect of DOI dose on cued conditioning [F(4,60) = 5.32, p < 0.01]. There was no significant main effect of pre-treatment (SAL or PCPA), and no significant pre-treatment × DOI dose interaction. Post-hoc analysis revealed that PCPA pretreatment increased FPS from 27 ± 6% in SAL-treated controls to 44% ± 6% in PCPA pre-treated rat, consistent with our previous study (2). Furthermore the highest dose of DOI tested (1.0 mg/kg) produced a significant reduction in the magnitude of conditioned fear. In SAL pre-treated rats, FPS was reduced to −2% ± 1% following DOI (1.0 mg/kg). In PCPA pre-treated rats FPS was reduced by DOI (1.0 mg/kg) to 9% ± 9 %.
Figure 1.
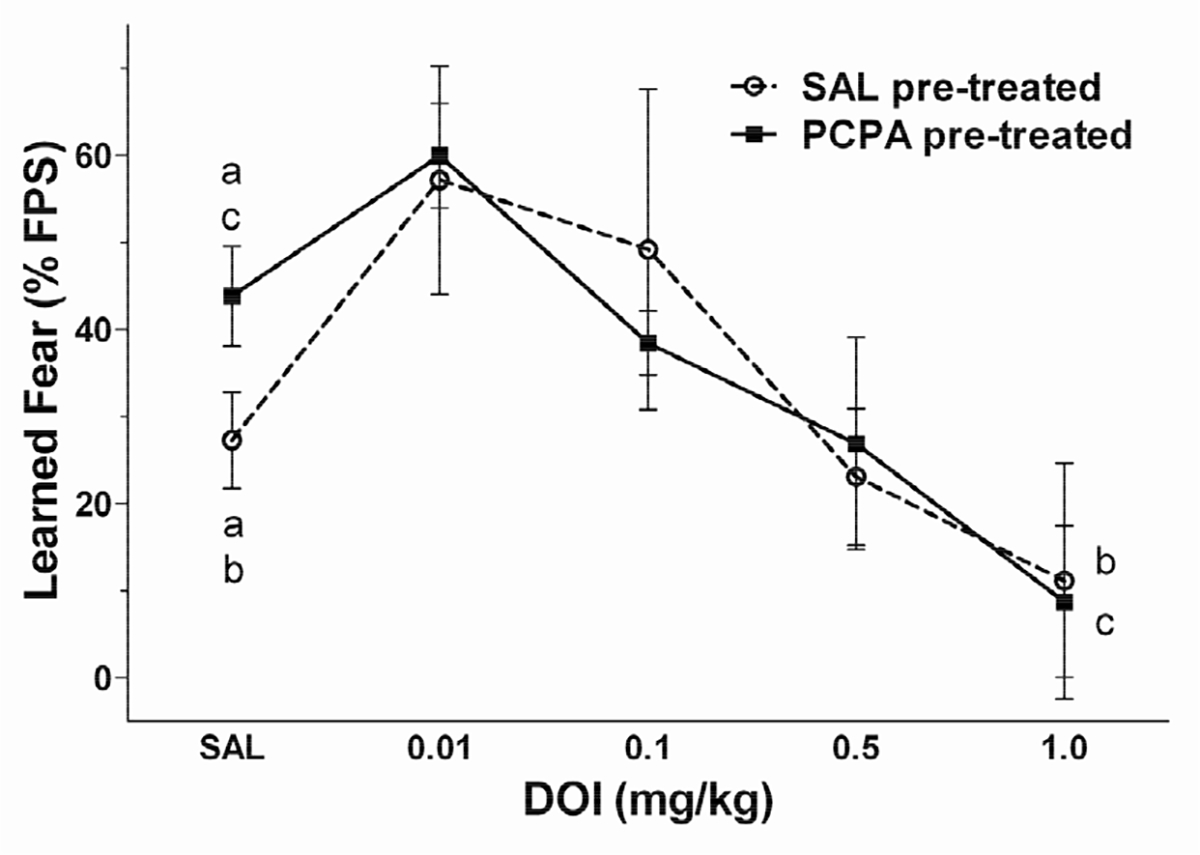
The 5-HT2 agonist (±)-2,5-dimethoxy-4-iodoamphetamine hydrochloride (DOI) decreases learned fear. Rats were pre-treated with either 0.9% saline (SAL; open circle, n = 6) or p-chlorophenylalanine (PCPA; filled square; n = 7). Learned fear was measured beginning 10 days after the last pre-treatment. On test days, rats were administered saline (SAL) or DOI (0.01, 0.1, 0.5, and 1.0 mg/kg; i.p.) 30 min prior to testing learned fear. Repeated measures analysis of variance (ANOVA) revealed only a significant main effect (p < 0.05) of DOI dose. There were no other significant main effects or significant interactions. PCPA treatment resulted in increased startle potentiation relative to SAL pre-treated control (p < 0.05). In both SAL and PCPA pre-treated animals, DOI slightly increased potentialtion of the startle reflex at the lowest dose tested (0.01 mg/kg). At higher doses of DOI, learned fear was decreased in a dose-dependent manner. DOI (1.0 mg/kg) significantly reduced learned fear in both pre-treatment groups (p < 0.05). Treatments that differed significantly (p < 0.05) are indicated by corresponding lower case letters (Bonferroni multiple comparisons post-hoc test). Data are expressed as the mean ± SEM of the % potentiation of the startle reflex.
3.3. Antagonist Studies
DOI is a nonselective 5-HT2 agonist. Both 5-HT2A and 5-HT2C receptors, but not 5-HT2B receptors, are found in critical fear-learning structures such as the amygdala. Therefore the specific 5-HT2 receptor subtype involved in DOI-mediated inhibition of fear learning was examined by comparing the effects of the 5-HT2 antagonist ketanserin (KET) and the 5-HT2B/2C-selective antagonist SB 206553. Factor 1 (between groups) was SAL or PCPA pre-treatment. Factor 2 (4 drug conditions, within groups) was the agonist/antagonist combination given on FPS testing days.
The effect of KET (1.0 mg/kg i.p.) and SB 206553 (1.0 mg/kg i.p.) on DOI (1.0 mg/kg i.p.)-induced reducetion of FPS in PCPA pre-treated and SAL pre-treated controls is shown in Figure 2. DOI-mediated reduction of FPS was blocked by the 5-HT2A/2C antagonist KET, but not by the 5-HT2B/2C-selective antagonist SB 206553. Repeated measures ANOVA revealed a significant effect of drug condition, F(3,48) = 4.16, p < 0.05. Post-hoc analyses confirmed previous findings that FPS is reduced by DOI (1.0 mg/kg) relative to both SAL and VEH control (p < 0.05). The DOI-induced inhibition of FPS was reversed by the 5-HT2A/2C antagonist KET (p < 0.05 compared to Veh/DOI), but not by the 5-HT2B/2C-selective antagonist SB 206553 (p > 0.05 compared to Veh/DOI). When administered alone, neither KET nor SB206553 affected fear learning in either SAL-or PCPA-treated rats (not shown). These results suggest that DOI-induced inhibition of fear learning involves activation of 5-HT2A receptors, and further that changes in 5-HT2A receptor function underlie PCPA-induced increase in fear-potentiated startle.
Figure 2.
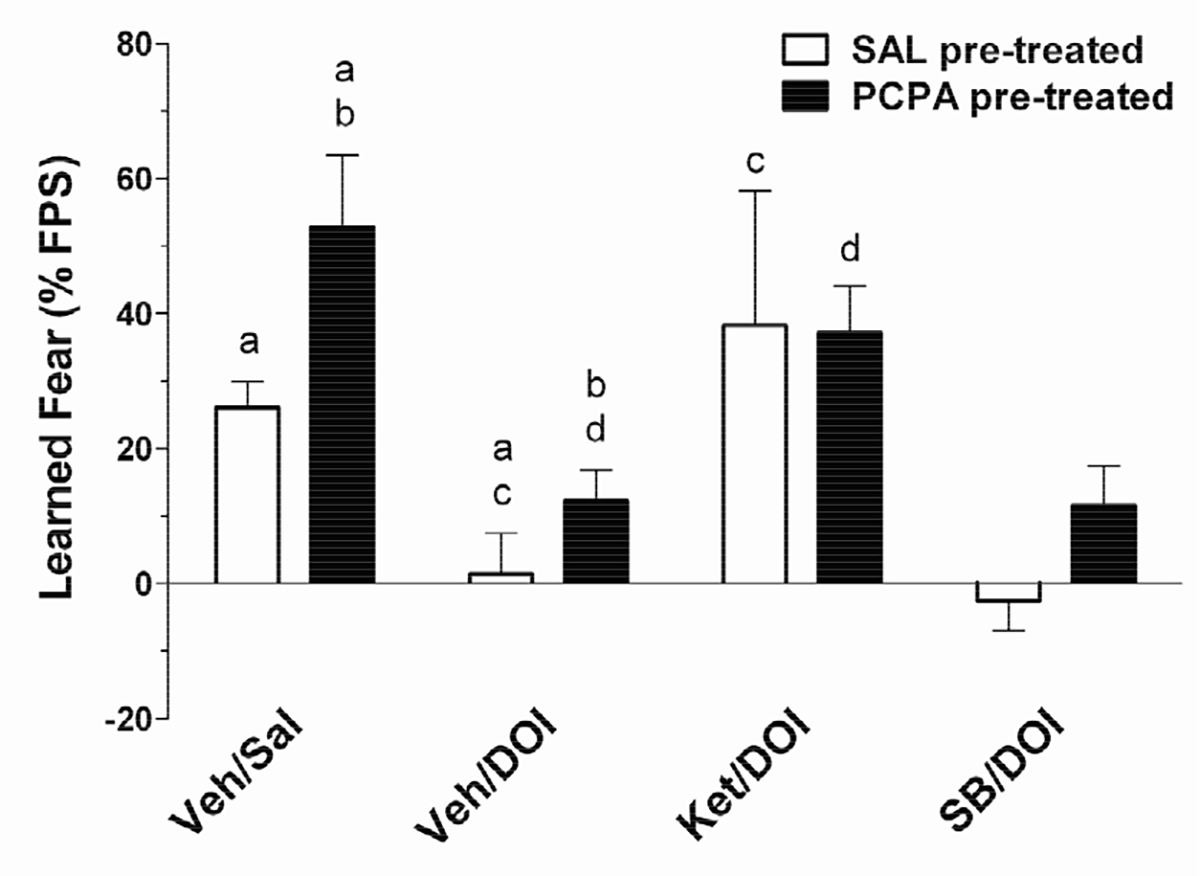
The 5-HT2 antagonist ketanserin (KET), but not the 5-HT2B/2C-selective antagonist SB 206553, reverses the DOI-induced decrease in learned fear. The behavioral pharmacology of 5-HT2 receptor antagonists on conditioned fear was tested in both SAL pre-treated (open bars; n = 7) and PCPA pre-treated (filled bars; n = 7) rats. Ketanserin (Ket), 3,5-dihydro-5-methyl-N-3-pyridinylbenzo1,2-b:4,5-b’]dipyrrole-1(2H)-carboxamide hydrochloride (SB 206553; SB) or vehicle (Veh) was administered 1 h prior to testing fear conditioning, and followed by DOI (1.0 mg/kg; or Sal control) 30 min prior to testing. Repeated measures ANOVA revealed a significant main effect (p < 0.05) of test-day treatment on learned fear. In both SAL pre-treated and PCPA-pretreated rats, DOI-induced reduction of FPS (p< 0.05) is blocked by the 5-HT2 antagonist ketanserin (Ket;1.0 mg/kg; p < 0.05) but not by the 5-HT2B/2C-selective antagonist SB 206553 (SB; 1.0 mg/kg; p > 0.05). There were no other significant main effects or significant interactions. Post-hoc comparisons that differed significantly (p < 0.05) are indicated by corresponding lower case letters (Bonferroni multiple comparisons test). Data are expressed as the mean ± SEM of the % potentiation of the startle reflex.
4. Discussion
The major findings of the current study are 1) fear learning is enhanced in PCPA-treated rats, replicating results from our previous studies [8]; 2) the 5-HT2 receptor agonist DOI attenuates conditioned fear, normalizing the PCPA-induced increase in learned fear; and 3) 5-HT2A receptors have a critical role in the normalization of exaggerated fear behavior.
While it is well-established that systemic administration of PCPA lowers brain serotonin levels [15], we confirmed here (Table 1) that the administration protocol used in the current study causes significant depletion of 5-HT. PCPA is a competitive and irreversible inhibitor of tryptophan hydroxylase (TH), the rate-limiting enzyme in 5-HT synthesis. Thus, 5-HT levels are depleted and fail to recover until more TH enzyme can be synthesized. Using a regiment of PCPA administration that chronically depleted 5-HT levels to less than 20% of normal, we replicated previous findings from our lab [8] showing that PCPA-induced low 5-HT causes an increase in fear-potentiated startle.
This study also showed that the nonselective 5-HT2 receptor agonist DOI decreased fear-potentiated startle in both PCPA- and SAL-treated rats, normalizing the increase in FPS following chronic 5-HT depletion. The pharmacological subtype of the 5-HT receptor involved in DOI-induced changes in fear behavior was characterized further by examining the antagonist pharmacology. At the lowest dose, fear conditioning was increased in both SAL and PCPA-treated animals. Higher doses then caused a dose-dependent decrease in conditioned fear. To elucidate further the subtype of 5-HT2 receptor involved, we compared the efficacy of ketanserin, a nonselective 5-HT2 antagonist, with the efficacy of SB 206553, a 5-HT2B/2C antagonist, in reversing DOI-mediated effects. In controls, DOI-mediated effects are antagonized by ketanserin, but not by SB 206553, implicating the 5-HT2A receptor in DOI-mediated reduction of learned fear behavior. Together, these results suggest a role for 5-HT2A receptors in controlling the expression of learned fear, and normalizing exaggerated fear induced by 5-HT deficits.
It is possible that our reported effect of ketanserin on DOI-mediated inhibition of learned fear is due to off-target effects of ketanserin. Although ketanserin binds 5-HT2A receptors with high affinity (about 10-fold higher than 5-HT2C receptors), ketanserin is also an effective antagonist of adrenergic α1 and histamine H1 receptors. It is therefore possible that the observed effects of ketanserin in the present study are due to interactions with these other receptor types. However, several lines of evidence argue against this possibility. First, the CNS distribution of 3H-Ket binding is tightly overlapped with the distribution of 5-HT2A mRNA measured by autoradiography [16]. Also, in behavioral studies ketanserin has selective effects on 5-HT2A receptors at or above the concentration used in the current study (1.0 mg/kg). The discriminative stimulus properties of 5-HT2A-selective compounds are inhibited by 2.5 mg/kg ketanserin [17]. These authors showed further that the blockade of discriminative drug properties by ketanserin is due to selective antagonism of 5-HT2A receptors. Similarly, the anti-hypertensive effects of low dose (0.3 mg/kg) of ketanserin, which could be mediated by α1 adrenergic recaptors (ARs), were abolished by destroying 5-HT neurons. The anti-hypertensive effects of a higher dose (3.0 mg/kg) were reduced, but still present after destruction of 5-HT neurons [18]. Results of radioactive ligand binding studies also argue against a strong role for α1 ARs. The binding affinity of ketanserin for 5-HT2A receptors is much higher that its affinity for α1 ARs. Radioligand binding studies report the pKi of ketanserin for α1 ARs is approximately 7.1 [19]. Ketanserin has higher affinity for 5-HT2 receptors, where the reported pKi is between 8.2 and 9.0 [20]. However, ketanserin has high affinity for histamine H1 receptors (pKi approx 8.7) [21] which is similar to the pKi of ketanserin for 5-HT2A receptors. It is therefore possible that the observed effects of ketanserin reported in this study could involve H1 receptors. While histamine has well-established role in motivated behaviors, its role in emotional learning has been revealed primarily in extinction studies, with no effect on expression of fear learning. Alternatively, histamine receptors other than H1 (e.g., H2 and/or H3 receptors) may be involved in histaminergic modulation of emotional behavior. For example, Bonini et al. [22] showed that consolidation of extinction of a fear memory was enhanced by histamine, while expression of fear learning was unaffected. These authors further report that histamine H2 receptors mediated the facilitation of consolidation, but H1 receptor ligands had no effect. Similarly, H1 knockout mice show no differences from wild-type controls in anxiety-like behavior in a light-dark test [23]. Nonetheless, the observed effects of 5-HT2 receptor ligands shown in this study do not definitively rule out important roles of other receptor subtypes, nor negate the possibility of complex interactions between several different (e.g., 5-HT, NE, histamine) transmitter systems. Still, our results showing that ketanserin, and not SB 206553, reversed the DOI-induced decrease in learned fear suggest that 5-HT2A receptors have a central role in the control of fear behavior in 5-HT deficient rats.
While it is well-established that serotonin regulates emotional learning [8,9,24], the role of specific 5-HT receptor subtypes is not well-understood. The 5-HT2 receptor family has been implicated in human emotional disorders. For example, patients suffering from major depression show enhanced platelet 5-HT2 receptor binding compared to healthy control subjects [25]. Chronic administration of antidepressant drugs that benefit patients with panic disorder induce a down-regulation of 5-HT2 receptors that correlates with the anxiolytic activeity of the medication [26]. Studies aimed at dissociating the effects of 5-HT2A and 5-HT2C receptor activation have been infrequent due to the limited availability of subtype-specific pharmacological agents. The 5-HT2 agonist DOI is anxiolytic in mouse models of anxiety including the four plates test (FPT), the FPT test-retest, and the elevated plus maze [27,28]. In these studies, DOI-mediated anxiolytic-like activity is reversed by pretreatment with the 5-HT2A-selective antagonist SR 46349B, but not the 5-HT2B/2C-selective antagonists SB 206553 or the 5-HT2C-selective antagonist RS 102221. Similarly, the 5-HT2 antagonist ketanserin induces an anxiogenic-like effect in the light/dark paradigm [29]. Our findings that DOI reduces fear potentiated startle, and the DOI-mediated reduction of fear behavior is antagonized by ketanserin, but not SB 206553 are consistent with these previous studies demonstrating an important role for 5-HT2A receptors in emotional behavior [27–29].
The neural mechanisms underlying learned fear are now well-known. Much research has focused on the critical role of the lateral (LA) and central (CeA) nuclei of the amygdala in the acquisition and expression conditioned fear [30]. Briefly, the LA, which receives and integrates information about both the conditioned (CS) and unconditioned (US) stimuli, is thought to be one of the primary sites of plasticity underlying fear learning [31–33]. Direct and indirect projections from the LA to the CeA coordinate appropriate fear response via projecttions from the CeA to the hypothalamus and brainstem nuclei. In the brainstem, the periaqueductal gray (PAG) and the nucleus reticularis pontis caudalis (NRPC) mediate freezing and potentiation of the acoustic startle reflex, respectively [3]. Lesion studies have shown that, while the amygdala is required for both cued and contextual fear conditioning, the hippocampus is also necessary for learned fear of a context [34,35]. While both the hippocampus and amygdala are densely innervated and functionally regulated by serotonergic inputs [36,37], the two brain regions show differential 5-HT receptor expression profiles and it is likely that 5-HT release plays a different role within each brain area. Here we show the effects of 5-HT2 receptors on amygdala-dependent (cued) fear. We found that 5-HT2A receptors have an important inhibitory role in learned fear of a light CS in control animals that may be disinhibited in PCPA-treated animals. This suggests that the role of 5-HT2A receptors in the amygdala (or other limbic or downstream brainstem targets) is dysregulated by PCPA-induced 5-HT depletion. Given the inhibitory role of serotonin in brain fear circuits [10,11], and the link between low 5-HT and abnormal emotional behavior [1,2], it has been suggested that chronic low 5-HT leads to neuronal hyperexcitability in the neural substrates mediating the fear response, and subsequent alterations in fear behavior [13]. Indeed, we have previously shown that exaggerated FPS in PCPAtreated rats is sensitive to treatment with the anticonvulsant phenytoin [8]. We have also observed physiological increases in excitability in vitro in slices taken from rats treated with PCPA in vivo, including epilepsy-like bursting activity in LA neurons in vitro following stimulation of the internal capsule (IC) at intensities that were insufficient to produce action potential firing in slices from saline-treated controls [14]. Furthermore, in contrast to slices from control animals, this previous report also showed that 5-HT does not reduce evoked excitatory postsynaptic potentials (EPSPs) in slices from PCPA-treated rats, an effect that is likely mediated by 5-HT2 receptors [10–12]. The specific role of the amygdala in the observations reported here will be an interesting follow-up in future studies.
The present data are consistent with the following mechanism by which chronic PCPA treatment may alter the brain’s normal response to fear-provoking stimuli. Chronic 5-HT deficit may reduce the functional activity of 5-HT2A receptors, which we have found to be a key regulator of fear behavior. Loss of 5-HT2A inhibition may then lead to neuronal hyperexcitability in limbic areas such as the amygdala, other limbic areas, or the brainstem, resulting in increased plasticity and enhanced fear behaviors. Alternatively, 5-HT2A receptors are desensitized by receptor antagonists [38]. Together with the current results, this raises the possibility that changes in 5-HT2A-mediated inhibition of fear may also involve receptor desensitization. While the amygdala seems a likely mediator of the behavioral changes observed in the current study, the specific brain areas involved and the specific receptor mechanisms that translate low 5-HT to disrupted fear behavior remain to be determined.
Dysfunction of 5-HT systems may contribute to mood and anxiety disorders. In the amygdala, a critical area serving learned fear, 5-HT acts as a brake on neuronal excitability. More specifically, activation of 5-HT2 receptors in the amygdala decreases excitatory transmission and increases inhibitory transmission [10–12]. PCPA treatment depletes brain 5-HT, which disinhibits the amygdala [14] by removing the 5-HT2A-mediated braking mechanism. We and others [13,39] have suggested that increased excitability in the amygdala can lead to plasticity in the neural mechanisms involved in fear. Consistent with this hypothesis, we have shown that rats with deficient 5-HT display enhanced FPS ([8]; Figure 1). Furthermore, we show that activation of 5HT2A receptors can reverse the low-5-HT induced changes in fear behavior, returning fear behavior to levels observed in control animals (Figure 2). These data add to a growing collection of evidence implicating a critical role for 5-HT2A receptors in the pathophysiology of mood and anxiety disorders.
5. Acknowledgements
This work was supported by National Institute of Mental Health grant MH-80400 and by the Office of the Vice Provost for Research at Baylor University.
Footnotes
The authors declare that they have no competing financial interests.
REFERENCES
- [1].Owens MJ and Nemeroff CB, “The Serotonin Transporter and Depression,” Depress Anxiety, Vol. 8, Suppl. 1, 1998, pp. 5–12. doi: [DOI] [PubMed] [Google Scholar]
- [2].Marek GJ, Carpenter LL, McDougle CJ, and Price LH, “Synergistic Action of 5-HT2A Antagonists and Selective Serotonin Reuptake Inhibitors in Neuropsy-Chiatric Disorders,” Neuropsychopharmacology, Vol. 28, No. 2, 2003, pp. 402–412. doi: 10.1038/sj.npp.1300057 [DOI] [PubMed] [Google Scholar]
- [3].LeDoux JE, “Emotion Circuits in the Brain,” Annual Review of Neuroscience, Vol. 23, No. 1, 2000, pp. 155–184. doi: 10.1146/annurev.neuro.23.1.155 [DOI] [PubMed] [Google Scholar]
- [4].Lopez-Gimenez JF, Mengod G, Palacios JM and Vilaro MT, “Selective Visualization of Rat Brain 5-HT2A Receptors by Autoradiography With [3H]MDL 100,907,” Naunyn Schmiedebergs Arch Pharmacol, Vol. 356, No. 4, 1997, pp. 446–454. doi: 10.1007/PL00005075 [DOI] [PubMed] [Google Scholar]
- [5].Morilak DA, Garlow SJ and Ciaranello RD, “Immunocytochemical Localization and Description of Neurons Expressing Serotonin 2 Receptors in the Rat Brain,” Neuroscience, Vol. 54, No. 3, 1993, pp. 701–717. doi: 10.1016/0306-4522(93)90241-7 [DOI] [PubMed] [Google Scholar]
- [6].Pazos A, Cortes R and Palacios JM, “Quantitative Autoradiographic Mapping of Serotonin Receptors in the Rat Brain. II. Serotonin-2 Receptors,” Brain Research, Vol. 346, No. 2, 1985, pp. 231–249. doi: 10.1016/0006-8993(85)90857-1 [DOI] [PubMed] [Google Scholar]
- [7].Pompeiano M, Palacios JM and Mengod G, “Distribution of the Serotonin 5-HT2 Receptor Family mRNAs: Comparison between 5-HT2A and 5-HT2C Receptors,” Molecular Brain Research, Vol. 23, No. 1–2, 1994, pp. 163–178. doi: 10.1016/0169-328X(94)90223-2 [DOI] [PubMed] [Google Scholar]
- [8].Hughes CR and Keele NB, “Phenytoin Normalizes Exaggerated Fear Behavior in P-Chlorophenylalanine (PCPA)-Treated Rats,” Epilepsy & Behavior, Vol. 9, No. 4, 2006, pp. 557–563. doi: 10.1016/j.yebeh.2006.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Inoue T, Li XB, Abekawa T, Kitaichi Y, Izumi T, Nakagawa S and Koyama T, “Selective Serotonin Reuptake Inhibitor Reduces Conditioned Fear through Its Effect in the Amygdala,” European Journal of Pharmacology, Vol. 497, No. 3, 2004, pp. 311–316. doi: 10.1016/j.ejphar.2004.06.061 [DOI] [PubMed] [Google Scholar]
- [10].Jiang XL, Xing GQ, Yang CH, Verma A, Zhang L and Li H, “Stress Impairs 5-HT2A Receptor-Mediated Serotonergic Facilitation of GABA Release in Juvenile Rat Basolateral Amygdala,” Neuropsychopharmacology, Vol. 34, No. 2, 2009, pp. 410–423. doi: 10.1038/npp.2008.71 [DOI] [PubMed] [Google Scholar]
- [11].Rainnie DG, “Serotonergic Modulation of Neurotransmission in the Rat Basolateral Amygdala,” Journal of Neurophysiology, Vol. 82, No. 1, 1999, pp. 69–85. [DOI] [PubMed] [Google Scholar]
- [12].Stutzmann GE and LeDoux JE, “GABAergic Antagonists Block the Inhibitory Effects of Serotonin in the Lateral Amygdala: A Mechanism for Modulation of Sensory Inputs Related to Fear Conditioning,” Journal of Neurophysiology, Vol. 19, No. 11, 1999, pp. RC8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Keele NB, “The Role of Serotonin in Impulsive and Aggressive Behaviors Associated with Epilepsy-Like Neuronal Hyperexcitability in the Amygdala,” Epilepsy & Behavior, Vol. 7, No. 3, 2005, pp. 325–335. doi: 10.1016/j.yebeh.2005.06.014 [DOI] [PubMed] [Google Scholar]
- [14].Keele NB and Randall DR, “Altered Modulation of Excitatory Neurotransmission in the Amygdala by Serotonin in an Animal Model of Impulsive Aggression,” Annals of the New York Academy of Sciences, Vol. 985, 2003, pp. 528–532. doi: 10.1111/j.1749-6632.2003.tb07119.x [DOI] [Google Scholar]
- [15].Valzelli L, Bernasconi S and Dalessandro M, “Time-Courses of P-CPA-Induced Depletion of Brain Serotonin and Muricidal Aggression in the Rat,” Pharmacological Research Communications, Vol. 15, No. 4, 1983, pp. 387–395. doi: 10.1016/S0031-6989(83)80048-4 [DOI] [PubMed] [Google Scholar]
- [16].Hannon J and Hoyer D, “Molecular Biology of 5-HT Receptors,” Behavioural Brain Research, Vol. 195, No. 1, 2008, pp. 198–213. doi: 10.1016/j.bbr.2008.03.020 [DOI] [PubMed] [Google Scholar]
- [17].Fiorella D, Rabin RA and Winter JC, “Role of 5-HT2A and 5-HT2C Receptors in the Stimulus Effects of Hallucinogenic Drugs. II: Reassessment of LSD False Positives,” Psychopharmacology (Berl), Vol. 121, No. 3, 1995, pp. 357–363. doi: 10.1007/BF02246075 [DOI] [PubMed] [Google Scholar]
- [18].Shen FM, Wang J, Ni CR, Yu JG, Wang WZ and Su DF, “Ketanserin-Induced Baroreflex Enhancement in Spontaneously Hypertensive Rats Depends on Central 5-HT (2A) Receptors,” Clinical and Experimental Pharmacology and Physiology, Vol. 34, No. 8, 2007, pp. 702–707. doi: 10.1111/j.1440-1681.2007.04626.x [DOI] [PubMed] [Google Scholar]
- [19].Korstanje C, Sprenkels R, Doods HN, Hugtenburg JG, Boddeke E, Batink HD, Thoolen MJ and Van Zwieten PA, “Characterization of Flufylline, Fluprofylline, Ritanserin, Butanserin and R 56413 With Respect to in-Vivo Alpha1-,Alpha2- and 5-HT2-Receptor Antagonism and in-Vitro Affinity for Alpha1-, Alpha2- and 5-HT2-Receptors: Comparison With Ketanserin,” Journal of Pharmacy and Pharmacology, Vol. 38, No. 5, 1986, pp. 374–379. doi: 10.1111/j.2042-7158.1986.tb04590.x [DOI] [PubMed] [Google Scholar]
- [20].Hoyer D, Engel G and Kalkman HO, “Molecular Pharmacology of 5-HT1 and 5-HT2 Recognition Sites in Rat and Pig Brain Membranes: Radioligand Binding Studies With [3H]5-HT, [3H]8-OH-DPAT, (−)[125I]Iodocyanopindolol, [3H]Mesulergine and [3H]Ketanserin,” European Journal of Pharmacology, Vol. 118, No. 1–2, 1985, pp. 13–23. doi: 10.1016/0014-2999(85)90658-2 [DOI] [PubMed] [Google Scholar]
- [21].Ghoneim OM, Legere JA, Golbraikh A, Tropsha A and Booth RG, “Novel Ligands for the Human Histamine H1 Receptor: Synthesis, Pharmacology, and Comparative Molecular Field Analysis Studies of 2-Dimethylamino-5-(6)-phenyl-1,2,3,4-tetrahydronaphthalenes,” Bioorganic & Medicinal Chemistry, Vol. 14, No. 19, 2006, pp. 6640–6658. doi: 10.1016/j.bmc.2006.05.077 [DOI] [PubMed] [Google Scholar]
- [22].Bonini JS, Da Silva WC, Da Silveira CK, Kohler CA, Izquierdo I and Cammarota M, “Histamine Facilitates Consolidation of Fear Extinction,” International Journal of Neuropsychopharmacology, Vol. 14, No. 9, 2011, pp. 1209–1217. doi: 10.1017/S1461145710001501 [DOI] [PubMed] [Google Scholar]
- [23].Zlomuzica A, Ruocco LA, Sadile AG, Huston JP and Dere E, “Histamine H1 Receptor Knockout Mice Exhibit Impaired Spatial Memory in the Eight-Arm Radial Maze,” British Journal of Pharmacology, Vol. 157, No. 1, 2009, pp. 86–91. doi: 10.1111/j.1476-5381.2009.00225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Izumi T, Inoue T, Kitaichi Y, Nakagawa S and Koyama T, “Target Brain Sites of the Anxiolytic Effect of Citalopram, a Selective Serotonin Reuptake Inhibitor,” European Journal of Pharmacology, Vol. 534, No. 1–3, 2006, pp. 129–132. doi: 10.1016/j.ejphar.2005.12.073 [DOI] [PubMed] [Google Scholar]
- [25].McKeith IG, Marshall EF, Ferrier IN, Armstrong MM, Kennedy WN, Perry RH, Perry EK and Eccleston D, “5-HT Receptor Binding in Post-Mortem Brain From Patients with Affective Disorder,” Journal of Affective Disorders, Vol. 13, No. 1, 1987, pp. 67–74. doi: 10.1016/0165-0327(87)90075–9 [DOI] [PubMed] [Google Scholar]
- [26].Neuger J, Wistedt B, Sinner B, Aberg-Wistedt A and Stain-Malmgren R, “The Effect of Citalopram Treatment on Platelet Serotonin Function in Panic Disorders,” International Clinical Psychopharmacology, Vol. 15, No. 2, 2000, pp. 83–91. doi: 10.1097/00004850-200015020-00004 [DOI] [PubMed] [Google Scholar]
- [27].Nic Dhonnchadha BA, Hascoet M, Jolliet P and Bourin M, “Evidence for a 5-HT2A Receptor Mode of Action in the Anxiolytic-Like Properties of DOI in Mice,” Behavioural Brain Research, Vol. 147, No. 1–2, 2003, pp. 175–184. doi: 10.1016/S0166-4328(03)00179-7 [DOI] [PubMed] [Google Scholar]
- [28].Ripoll N, Hascoet M and Bourin M, “Implication of 5-HT2A Subtype Receptors in DOI Activity in the Four-Plates Test-Retest Paradigm in Mice,” Behavioural Brain Research, Vol. 166, No. 1, 2006, pp. 131–139. doi: 10.1016/j.bbr.2005.07.013 [DOI] [PubMed] [Google Scholar]
- [29].Nic Dhonnchadha BA, Bourin M and Hascoet M, “Anxiolytic-Like Effects of 5-HT2 Ligands on Three Mouse Models of Anxiety,” Behavioural Brain Research, Vol. 140, No. 1–2, 2003, pp. 203–214. doi: 10.1016/S0166-4328(02)00311-X [DOI] [PubMed] [Google Scholar]
- [30].Pare D, Quirk GJ and LeDoux JE, “New Vistas on Amygdala Networks in Conditioned Fear,” Journal of Neurophysiology, Vol. 92, No. 1, 2004, pp. 1–9. doi: 10.1152/jn.00153.2004 [DOI] [PubMed] [Google Scholar]
- [31].McKernan MG and Shinnick-Gallagher P, “Fear Conditioning Induces a Lasting Potentiation of Synaptic Currents in Vitro,” Nature, Vol. 390, No. 6660, 1997, pp. 607–611. doi: 10.1038/37605 [DOI] [PubMed] [Google Scholar]
- [32].Rogan MT, Staubli UV and LeDoux JE, “Fear Conditioning Induces Associative Long-Term Potentiation in the Amygdala,” Nature, Vol. 390, No. 6660, 1997, pp. 604–607. doi: 10.1038/37601 [DOI] [PubMed] [Google Scholar]
- [33].Rumpel S, LeDoux J, Zador A and Malinow R, “Postsynaptic Receptor Trafficking Underlying a Form of Associative Learning,” Science, Vol. 308, No. 5718, 2005, pp. 83–88. doi: 10.1126/science.1103944 [DOI] [PubMed] [Google Scholar]
- [34].Kim JJ and Fanselow MS, “Modality-Specific Retrograde Amnesia of Fear,” Science, Vol. 256, No. 5057, 1992, pp. 675–677. doi: 10.1126/science.1585183 [DOI] [PubMed] [Google Scholar]
- [35].Phillips RG and LeDoux JE, “Differential Contribution of Amygdala and Hippocampus to Cued and Contextual Fear Conditioning,” Behavioral Neuroscience, Vol. 106, No. 2, 1992, pp. 274–285. doi: 10.1037/0735-7044.106.2.274 [DOI] [PubMed] [Google Scholar]
- [36].Sadikot AF and Parent A, “The Monoaminergic Innervation of the Amygdala in the Squirrel Monkey: An Immunohistochemical Study,” Neuroscience, Vol. 36, No. 2, 1990, pp. 431–447. doi: 10.1016/0306-4522(90)90439-B [DOI] [PubMed] [Google Scholar]
- [37].Vertes RP, “A PHA-L Analysis of Ascending Projections of the Dorsal Raphe Nucleus in the Rat,” The Journal of Comparative Neurology, Vol. 313, No. 4, 1991, pp. 643–668. doi: 10.1002/cne.903130409 [DOI] [PubMed] [Google Scholar]
- [38].Hanley NR and Hensler JG, “Mechanisms of Ligand-Induced Desensitization of the 5-Hydroxytrypta-mine(2A) Receptor,” Journal of Pharmacology and Experimental Therapeutics, Vol. 300, No. 2, 2002, pp. 468–477. doi: 10.1124/jpet.300.2.468 [DOI] [PubMed] [Google Scholar]
- [39].Rosen JB and Schulkin J, “From Normal Fear to Pathological Anxiety,” Psychological Review, Vol. 105, No. 2, 1998, pp. 325–350. doi: 10.1037/0033-295X.105.2.325 [DOI] [PubMed] [Google Scholar]


