Abstract
Background
Children and adolescents with household exposure to multidrug- or rifampicin-resistant tuberculosis (MDR/RR-TB) are at high risk of developing TB disease. Tuberculosis preventive therapy (TPT) is recommended, but programmatic experience is limited, particularly for adolescents.
Methods
We conducted a prospective cohort study to describe MDR/RR-TB diagnosis and TPT provision for individuals aged <18 years with MDR/RR-TB exposure. Participants were assessed for TB either in homes or health facilities, with referral for chest x-ray or specimen collection at clinician discretion. The TPT regimens included levofloxacin, isoniazid, or delamanid monotherapy for 6 months, based on source patient drug-resistance profile.
Results
Between March 1, 2020 and July 31, 2021, 112 participants were enrolled; median age was 8.5 years, 57 (51%) were female, and 6 (5%) had human immunodeficiency virus. On screening, 11 (10%) were diagnosed with TB: 10 presumptive MDR/RR-TB and 1 drug-susceptible TB. Overall, 95 (94% of 101) participants started TPT: 79 with levofloxacin, 9 with isoniazid, and 7 with delamanid. Seventy-six (80%) completed TPT, 12 (13%) were lost to follow up, and 7 (7%) stopped TPT early due to adverse events. Potential adverse events were reported for 12 (13%) participants; none were serious. There were no further TB diagnoses (200 days median follow up).
Conclusions
Post-MDR/RR-TB exposure management for children and adolescents resulted in significant MDR/RR-TB detection and both high TPT initiation and completion. Tuberculosis preventive monotherapy was well tolerated and there were no further TB diagnoses after initial assessment. Key factors supporting these outcomes included use of pediatric formulations for young children, monotherapy, and community-based options for assessment and follow up.
Keywords: children, MDR/RR-TB, monotherapy, preventive therapy, tuberculosis
Children and adolescents (N = 112) with household exposure to rifampicin-resistant TB were screened in homes or healthcare facilities; 10% were diagnosed with TB and 85% received preventive monotherapy (6 months). Eighty percent completed therapy and no further TB diagnoses were made.
Each year there are approximately half a million people who develop multidrug-resistant tuberculosis (resistance to at least rifampicin and isoniazid) or rifampicin-resistant tuberculosis (MDR/RR-TB), many of whom are never diagnosed or started on treatment [1]. Although this is devastating for the individuals who are ill, it also means that persons in their households face prolonged exposure to MDR/RR-TB and risk developing both drug-resistant TB infection and disease. Such “exposed” individuals merit concerted efforts to assess their health and prevent them from becoming sick, a series of practices often referred to as “postexposure management” [2, 3]. Multidrug- or rifampicin-resistant TB postexposure management that includes both active TB diagnosis and the provision of appropriate TB preventive therapy (TPT) for vulnerable individuals has been recommended by the World Health Organization (WHO) since 2020 [4]. However, there are few examples of MDR/RR-TB postexposure management in high-burden settings [5–7].
Postexposure management is particularly important for children and adolescents, a vulnerable group that has a worrisome MDR/RR-TB diagnostic and treatment initiation gap. Although approximately 30 000 children are estimated to develop MDR/RR-TB each year [8], to date, only 15% of the targeted 115 000 children with MDR/RR-TB to be treated between 2018 and 2022 have received treatment [1]. This compares to 46% of the adult target over the same time period. Uptake of TPT for MDR/RR-TB among children at high risk of progressing to active disease is also likely to be very low, although data are scarce [9, 10]. Some of the key challenges include the difficulty in obtaining microbiological confirmation of active TB among children and therefore ruling out active TB before TPT, uncertainty in TPT regimen composition, and a lack of child friendly formulations [11].
South Africa has a high burden of MDR/RR-TB; among an estimated 21 000 individuals who developed MDR/RR-TB in 2021, 7831 were diagnosed [1]. In 2019, the National Department of Health released updated guidelines, which included a recommendation to provide TPT for selected high-risk close contacts of persons with MDR/RR-TB [12]. Building on previous experience [13], Médecins Sans Frontières ([MSF] Doctors without Borders), in collaboration with the Western Cape Provincial department of health and the City of Cape Town, implemented an MDR/RR-TB postexposure management program aimed at children and adolescents with household MDR/RR-TB exposure in Khayelitsha, Cape Town.
METHODS
Study Design, Setting and Participants
This was a prospective cohort study describing an MDR/RR-TB postexposure management program enrolling participants between March 1, 2020 and July 31, 2021. Participants were children and adolescents aged 0–18 years living in Khayelitsha, who had a recent (within 12 months) history of household exposure to someone with MDR/RR-TB.
The proportion of children and adolescents diagnosed with MDR/RR-TB at baseline and within the follow-up period as well as completion, safety, and tolerability of the preventive therapy regimen used are described. The program was implemented in the periurban township of Khayelitsha, Cape Town, South Africa. Khayelitsha has a population of approximately half a million and is characterized by low socioeconomic status and overcrowding. There are high burdens of human immunodeficiency virus (HIV), TB, and MDR/RR-TB; antenatal HIV prevalence is 31%, TB case notification is 920/100 000 per year, and approximately 150–200 individuals are diagnosed with MDR/RR-TB annually [14, 15].
Screening and Diagnosis of Disease
Household contacts of individuals newly diagnosed with MDR/RR-TB were identified from 10 primary care clinics providing MDR/RR-TB care [16]. These contacts were then assessed either in their homes or at health facilities. Initially, most contacts were screened at healthcare facilities; however, the onset of the coronavirus disease 2019 (COVID-19) pandemic led to more screening in the home. This also made accessing care more convenient for households. Initial screening of contacts included the WHO symptom screen [17], with the inclusion of fatigue/reduced playfulness, and a full physical examination, either at the home or at the facility by a medical doctor. Referral to the facility for chest radiography and the collection of specimens for bacteriology were at the clinician's discretion based on clinical presentation suggestive of disease.
All contacts were offered HIV testing. Tuberculosis disease could be diagnosed either clinically based on history, examination and/or radiography, or bacteriologically, if a submitted specimen was positive. A specimen could be either a gastric washing, induced/spontaneous sputum (sent for culture, drug sensitivity tests, and Xpert MTB/RIF Ultra), or stool sent for Xpert MTB/RIF Ultra only. Health education was provided in the local language, and in circumstances in which socioeconomic concerns were identified, families/households were referred to social auxiliary services and provided with nutritional support.
Multidrug- or Rifampicin-Resistant Tuberculosis Preventive Treatment
For household contacts in whom TB disease was ruled out, TPT was offered based on the drug susceptibility pattern of the source patient where known, suspected duration of exposure and clinical presentation [12]. Following national guidelines, tests of TB infection were not routinely conducted. In instances in which the source patient was known to be diagnosed with rifampicin monoresistant TB, defined as rifampicin resistance and isoniazid susceptibility, isoniazid preventive therapy with standard dosage was offered. For source patients with MDR/RR-TB with susceptibility to fluoroquinolones, levofloxacin monotherapy was the preferred preventive regimen (dispersible or syrup formulation for younger children). In instances in which the source patient had MDR-TB with fluoroquinolone resistance, contacts were offered either (1) monotherapy with high-dose isoniazid or delamanid or (2) regular follow up without TPT. For contacts of source patients with unknown or delayed drug susceptibility results, levofloxacin was the preferred therapy. All TPT was given daily for 6 months with the option of treatment extension by the clinician for known treatment interruptions.
Contacts in whom TB disease was ruled out were followed up at 1 month and every 2–3 months thereafter for 6 months and subsequently at either 12 months after TPT initiation or until March 31, 2022 when active follow up ceased. The majority of follow up was done by a professional nurse. All participants were screened for symptoms of TB and assessed for TB disease at follow up. Follow up was conducted via telephone when arranging in-person follow up was difficult, with additional follow up via telephone for those requiring extra support with taking their medication. Participants receiving delamanid were followed up at the facility level as they received regular electrocardiographs. For participants receiving TPT, a follow-up form was adapted from the Sentinel Project guide to include questions around adherence and adverse events [18], and possible adverse events were recorded in the medical record as per routine practice. Adverse events were graded as serious, moderate, or mild, based on South African guidance [12].
Data Collection, Definitions, and Analysis
Data on included individuals were collected prospectively from medical records and follow-up forms and entered into a RedCAP (version 9.6; www.project-redcap.org) database. Tuberculosis symptoms included cough, weight loss, fever, night sweats, and fatigue/reduced playfulness. Tuberculosis diagnosis was defined as either bacteriologically confirmed TB or unconfirmed TB based on clinician decision to initiate TB treatment. Except for cases in which a bacteriological specimen diagnosed drug-susceptible TB, MDR/RR-TB treatment was initiated based on the drug susceptibility of the presumed source patient. Initial loss to follow up (LTFU) for TPT was defined as LTFU before a TPT regimen was started. Tuberculosis preventive therapy treatment completion was defined as completing at least 80% of the prescribed doses of the 6-month regimen based on self-report and clinician assessment, with treatment duration up to 9 months [4]. Other outcomes were reported as LTFU (less than 80% of prescribed doses), treatment stopped (due to adverse events or disease development), or death (any cause). Malnutrition was defined as a weight for age z-score <−2.0 using WHO child growth standards 2006 [19].
Patient Consent Statement
Written informed consent was obtained from all guardians, and adolescents also provided assent. The study was approved by the University of Cape Town Human Research Ethics Committee (HREC 391/2017) and by the MSF Ethical Review Board (ERB 1735).
RESULTS
Participants
During the study period, 112 participants were enrolled (77% of 146 eligible contacts). These individuals were contacts of 48 source patients; each source patient had a median of 2 enrolled contacts (range, 1–6). Among source patients, 2 (4%) were diagnosed with MDR/RR-TB empirically (unconfirmed), 4 (8%) based on Xpert MTB/RIF alone (no further drug susceptibility tests available), 12 (25%) with rifampicin-monoresistant TB, 24 (50%) with fluoroquinolone-susceptible MDR-TB, and 6 (13%) with fluoroquinolone-resistant MDR-TB.
Participants were assessed and enrolled a median of 48 days (interquartile range [IQR], 23–180) after diagnosis of the source patient (defined as the date of the specimen from which MDR/RR-TB was diagnosed). Among enrolled participants, the median age was 8.5 years, 57 (51%) were female, and 6 (5%) had HIV (Table 1). Nineteen participants were found to have TB symptoms at baseline, 1 had been previously treated for TB, and 9 reported previous TPT. Further characteristics of children and adolescents identified as household contacts are summarized in Table 1.
Table 1.
Characteristics of Children and Adolescents Identified as Household Contacts
| Characteristics | Number (% of Total) |
|---|---|
| Total | 112 |
| Female | 57 (51%) |
| Median age, years (IQR) | 8.5 (4.1–11.9) |
| Age | |
| <5 | 30 (28%) |
| 5–12 | 57 (50%) |
| 13–18 | 25 (22%) |
| Known BCG vaccination | 105 (93%) |
| People with HIV | 6 (5%) |
| Previous TPT | 9 (8%) |
| Previous TB Treatment | 2 (1.8%) |
| Any TB symptom | 18 (16%) |
| Malnutritiona | 6 (5%) |
Abbreviations: BCG, Bacillus Calmette-Guérin; HIV, human immunodeficiency virus; IQR, interquartile range; TB, tuberculosis; TPT, tuberculosis preventive therapy.
Defined as weight for age < −2.0.
Tuberculosis Diagnosis
Overall, 74 (66%) participants had chest radiography as part of their assessment and 20 (18%) had at least 1 specimen taken for bacteriology (19 also had chest radiography). The remainder were assessed as growing well and asymptomatic and were therefore offered TPT directly. Among the 112 participants, 11 (9.8%) were diagnosed with confirmed or presumed TB (median age 6.5 years), all resulting from the baseline assessment. Among these 11 contacts, 10 were diagnosed with presumptive MDR/RR-TB and 1 with HIV was diagnosed with drug-susceptible TB (Figure 1). Only 2 TB diagnoses were bacteriologically confirmed (sputum); one was confirmed with rifampicin-susceptible TB and the other was indeterminate for rifampicin susceptibility (diagnosed with presumptive MDR/RR-TB). The remainder were diagnosed based on clinical and radiographic presentation. Among the 11 participants with TB diagnosed, none were classified as malnourished, but 2 were living with HIV.
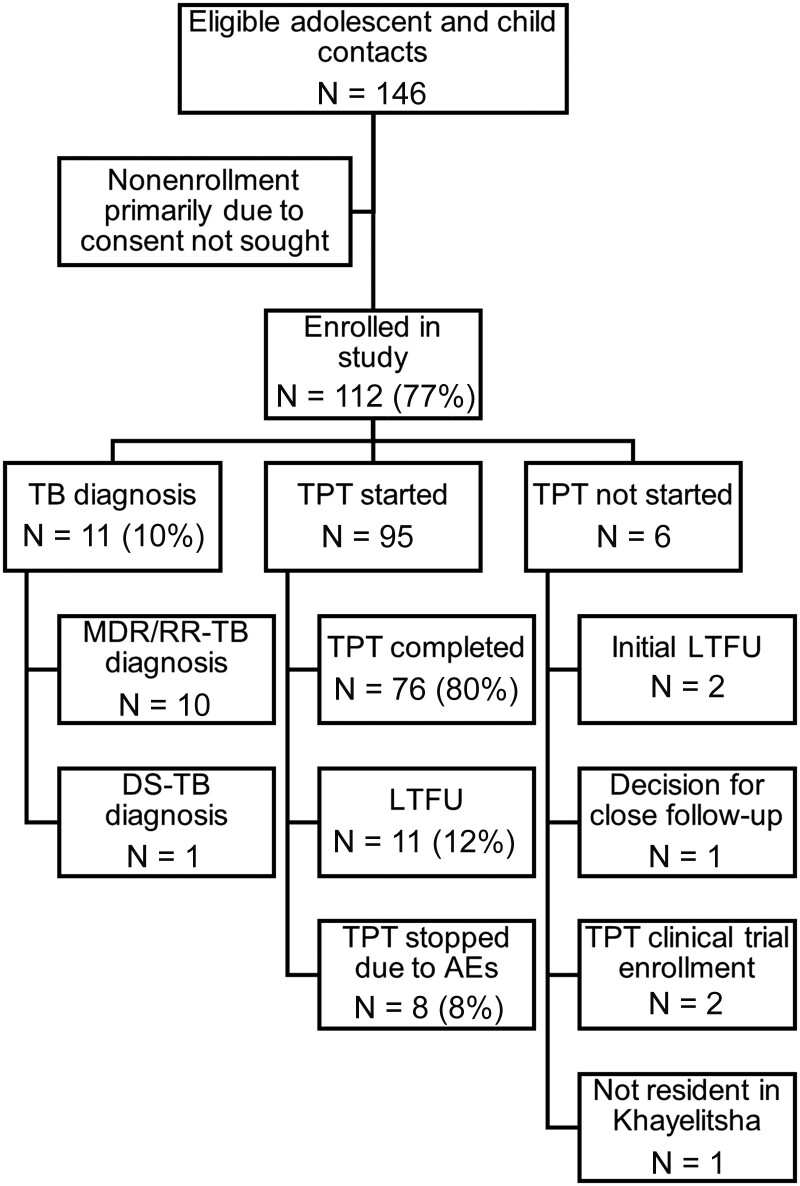
Figure 1.
Flow diagram showing tuberculosis (TB) diagnoses, tuberculosis preventive therapy (TPT) provision, and TPT outcomes. AE, adverse event; DS-TB, drug-susceptible TB; LTFU, loss to follow up.
Tuberculosis Preventive Therapy Provision and Outcomes
Among the 101 participants for whom TB was excluded, 95 (94.0%) were initiated on TPT. Reasons for not starting TPT included initial LTFU (n = 2), joint participant/clinician decision to opt for close follow up rather than TPT (n = 1), not currently a resident in Khayelitsha (n = 1), and enrolled in a clinical trial of TPT (n = 2).
Levofloxacin was the most common drug used for TPT with 79 (83.2%) participants treated with levofloxacin monotherapy (Table 2). This was predominantly in tablet form, although all children aged <5 years, or who were unable to swallow tablets, were offered levofloxacin in pediatric formulations (dispersible or syrup). Nine participants received isoniazid (4 high dose) and 7 received delamanid (these were all contacts of source patients with fluoroquinolone-resistant MDR-TB).
Table 2.
Diagnosis of TB, Provision of TPT, and TPT Outcomes
| Cohort description | Age | Age | Age | Total |
|---|---|---|---|---|
| <5 Years | 5–12 Years | 13–18 Years | ||
| N | 30 | 57 | 25 | 112 |
| TB Diagnosis | ||||
| MDR/RR-TB (based on source patient diagnosis) | 3 (10%) | 6 (11%) | 1 (4%) | 10 (9%) |
| DS-TB (bacteriologically confirmed) | 0 | 1 (2%) | 0 | 1 (1%) |
| Total | 3 (10%) | 7 (13%) | 1 (4%) | 11 (10%) |
| No TPT | 2 | 2 | 2 | 6 |
| TPT Started (Monotherapy Regimen) | ||||
| Levofloxacin (tablets) | 0 | 32 | 18 | 50 |
| Levofloxacin (dispersible/syrup) | 24 | 4 | 0 | 29 |
| Levofloxacin (any formulation) | 24 (96%) | 36 (75%) | 19 (86%) | 79 (83%) |
| Isoniazid | 1 | 5 | 4 | 9 |
| Delamanid | 0 | 7 | 0 | 7 |
| TPT Outcome | ||||
| Completed | 21 (84%) | 37 (77%) | 18 (82%) | 76 (80%) |
| LTFU | 2 (8%) | 6 (13%) | 4 (17%) | 12 (13%) |
| Stopped due to AE | 2 (8%) | 5 (10%) | 0 (0%) | 7 (7%) |
| Total | 25 | 48 | 22 | 95 |
Abbreviations: AE, adverse events; DS-TB, drug-susceptible tuberculosis; LTFU, loss to follow-up; MDR/RR-TB, multidrug- or rifampicin-resistant tuberculosis; TB, tuberculosis; TPT, TB preventive treatment.
Overall, 80% of participants completed TPT, and this did not differ by age group (Table 2) or sex. Loss to follow up was 13% overall (12/95) and 7% (7/95) stopped TPT due to adverse events (Figure 1). Loss to follow up did not differ between participants aged ≤12 years and those aged 13–18 (P = .39). The median duration of TPT among participants who were LTFU was 82 days (IQR, 52–109).
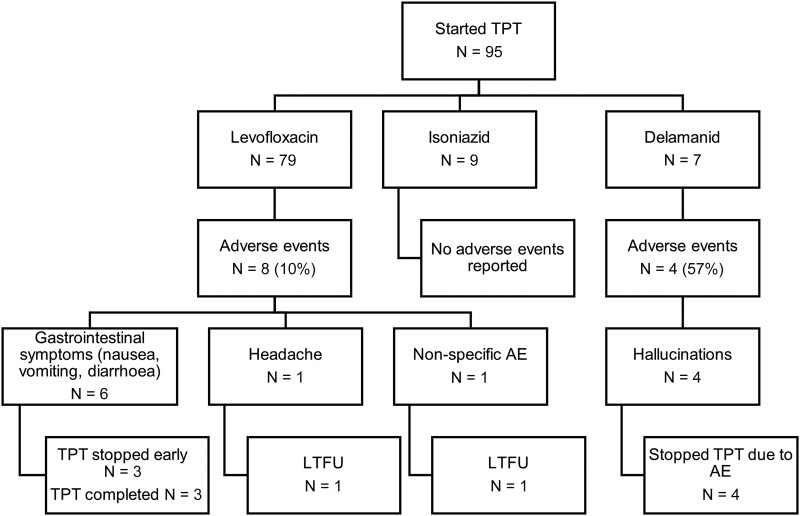
Overall, 12 (12.6%) of the 95 participants who started TPT reported adverse events (Figure 2). The median time to reported onset of adverse events was 22 days (IQR, 4–50). Among 7 participants who had TPT stopped due to adverse events, none were deemed to be serious. Four were receiving delamanid and hallucinations were the reported adverse event. These were deemed to be of moderate severity by the treating clinician, with a low threshold for stopping TPT given that these were otherwise healthy children. All 4 participants with hallucinations were from the same household. The remaining 3 participants who had treatment stopped due to adverse events were receiving levofloxacin, and all adverse events were gastrointestinal and deemed to be mild or moderate, with a similarly low threshold for stopping TPT.
Figure 2.
Adverse events (AE) among participants receiving different tuberculosis preventive therapy (TPT) regimens and TPT outcomes. AE, adverse event; LTFU, loss to follow up.
Among the remaining 5 participants with adverse events, 3 completed TPT; adverse events were recorded as nausea, vomiting, and constipation and resolved while continuing therapy. The remaining 2 participants were LTFU; one reported headache and the other reported nonspecific symptoms. There was no liver toxicity reported among those receiving isoniazid or overall (Figure 2).
Participant Follow up
The median time from assessment to last follow up for the 101 participants not diagnosed with TB at assessment was 200 days (IQR, 183–543). Among the 76 participants who completed TPT, 33 had at least 1 month of follow up after TPT completion; the median follow up after TPT completion among these 33 participants was 426 days (IQR, 135–510). There were no participants identified with TB across follow up, regardless of TPT outcome.
DISCUSSION
These data demonstrate that routine postexposure management for children and adolescents exposed to MDR/RR-TB is feasible in a high-burden setting and results in significant MDR/RR-TB case detection among this vulnerable population group. Overall, 10% of participants with household MDR/RR-TB exposure were diagnosed with drug-susceptible or MDR/RR-TB and received appropriate treatment. This is comparable to the significant yield from contact investigation in previous studies [20], and it reinforces recommendations for timely, systematic screening for children with TB exposure [21]. The majority of TB diagnoses were unconfirmed, highlighting the difficulties in bacteriological confirmation in these age groups and therefore the importance of contact investigation to diagnose and initiate appropriate MDR/RR-TB treatment. Overall, TPT with monotherapy was well tolerated, and there were high levels of completion of TPT across all age groups.
The WHO now suggests that high-risk household contacts of MDR/RR-TB patients may be offered TPT [4]. In addition, recent analyses suggest that household contact management for children exposed to MDR/RR-TB could avert a substantial proportion of the global MDR/RR-TB burden in children [9]. Children and adolescents are particularly vulnerable to active TB after household TB exposure [22, 23], and data suggest that contacts of MDR/RR-TB source patients are at elevated risk compared with contacts of drug-susceptible TB patients [24, 25]. However, there is a lack of evidence regarding appropriate preventive treatment options and models of TPT provision, across all age groups, but particularly for adolescents. Although randomized clinical trials that include children and adolescents are currently ongoing [26–28], available data from a limited number of small observational studies, predominantly among young children, suggest that TPT for MDR/RR-TB is effective [29, 30]. Most studies have used fluoroquinolone-based regimens, commonly in combination with other drugs, with varying tolerability and completion. In several studies, adverse events contributed significantly to stopping TPT early, with these attributed primarily to other drugs given in combination with the fluoroquinolone (pyrazinamide, ethionamide, or ethambutol) [29, 30], although there was no association between adverse events and loss to follow up in a more recent study [31].
Overall, 80% of participants completed TPT, whereas 13% were lost to follow up. This level of completion is similar to other reports of MDR/RR-TB TPT completion (ranging from 70% to 90%) [5, 6, 30, 32]. High treatment completion was likely facilitated by the provision of community-based options for clinical assessment and follow up. This minimized interruptions for children attending school and working parents. Additional contributors may include the use of monotherapy and the provision of pediatric formulations. Loss to follow up was somewhat higher in the older age group; however, this did not reach significance. Although there were only 22 participants in the 13- to 18-year-old age group, 18 participants successfully completed TPT. Although adherence to therapy among adolescents has been reported to be poorer than for adults or younger children [33], high treatment completion was maintained with close support and follow up. These data suggest that the risk of poor adherence among adolescents should not be a reason for not offering TPT to this neglected age group [34]. Overall, these data confirm the willingness of caregivers to provide TPT for children with household MDR/RR-TB exposure [35].
After initial investigation, no further TB diagnoses were made in the cohort, either among those receiving TPT or those with only close follow up. Although the follow-up period was relatively short overall and the sample size was small, a notable proportion had more than 1 year of follow up after TPT completion. In addition, given that most children who develop active TB do so within 90 days of initial assessment [22], it is likely that TPT was largely effective in this cohort, although this was not the primary aim of this study. However, it is possible that there was lengthy exposure to source patients before assessment, which contributed to the high disease prevalence at baseline, and low risk of incident TB during TPT and follow up. Nonetheless, these data suggest that the risk of providing inadvertent monotherapy for children with undiagnosed TB disease and the development of fluoroquinolone-resistant MDR/RR-TB was low.
Although 7% of participants stopped TPT due to adverse events, these adverse events were not deemed to be severe or serious by the treating clinician, similar to previous reports [31]. Given that the participants receiving TPT were healthy individuals without TB disease, there was a low threshold for stopping TPT, even if adverse events were mild. Delamanid was used as TPT monotherapy for 7 participants with limited TPT options (exposure to fluoroquinolone-resistant MDR with evidence of ethionamide and high-level isoniazid resistance). Four of these participants had therapy stopped due to reported hallucinations; all 4 were from the same family, and given the first occurrence of hallucination, there was a high degree of vigilance and a low threshold for the remaining participants and the caregiver to report further hallucinations. None of the participants reported any other psychiatric symptoms, and hallucinations did not reoccur after stopping delamanid. Data from PHOENIx, an ongoing randomized controlled trial, will be key to a better understanding of the safety profile of delamanid for preventive therapy [26]. In the meantime, delamanid remains a potential option for individuals with limited other TPT options, under close monitoring. Similarly, isoniazid TPT also remains an option, given recent data suggesting the benefits for child/adolescent contacts with MDR/RR-TB exposure [36]. Reported adverse events for participants receiving levofloxacin were primarily gastrointestinal and commonly short-lived. Although the treating clinician deemed that the majority of these were likely not related to TPT, therapy was stopped early for 3 of the 79 participants receiving levofloxacin. Overall, isoniazid was well tolerated, even among those receiving high-dose regimens.
Coronavirus disease 2019 provided the unplanned opportunity for conducting more household visits. These were found to be important in this program, to identify all close contacts, provide health education and counseling, and overcome the difficulties in getting apparently well children and adolescents to attend clinic visits. These visits were also important to establish a strong patient-provider relationship, both for the source patient and their close contacts. However, initial and follow-up household visits are resource intensive, suggesting that dedicated human resources are required for such programs. This has been reinforced by studies suggesting that community-based interventions for TB contact management are effective for improving completion, despite the requirement for additional resources [37].
CONCLUSIONS
The diagnosis of TB among children and adolescents has been disproportionately neglected in the global TB response, particularly for those effected by MDR/RR-TB [11]. In particular, implementation of new tools and approaches for this age group into programs has lagged behind that for adults [34]. These data demonstrate that postexposure management for both children and adolescents with MDR/RR-TB exposure is feasible under programmatic conditions. Key components from this experience include the option of providing remote/home-based care for assessment and follow up and the use of simple TPT regimens that include monotherapy and pediatric formulations. This study demonstrates that there may be substantial benefits with the use of fluoroquinolone monotherapy, both on TPT tolerability and adherence. In addition, the provision of pediatric formulations may have also reduced LTFU in those aged less than 5 years, and it shows the importance of convenient and easy-to-administer TPT regimens, findings that could also be extrapolated for older children and adolescents [38]. Overall, MDR/RR-TB postexposure management is an essential step to both closing the MDR/RR-TB diagnostic gap for children and adolescents through increased case detection as well as a key step in ending TB through preventing the development of disease.
Acknowledgments
We acknowledge the individuals with drug-resistant TB, their families, and the staff who care for them in Khayelitsha.
Financial support. This work was supported by Médecins Sans Frontières (Doctors without Borders).
Contributor Information
Ivy Apolisi, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
Helen Cox, Institute of Infectious Disease and Molecular Medicine and Wellcome Centre for Infectious Disease Research, Division of Medical Microbiology, University of Cape Town, Cape Town, South Africa.
Nolitha Tyeku, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
Johnny Daniels, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
Shaheed Mathee, Western Cape Province Department of Health, Cape Town, South Africa.
Rabia Cariem, City of Cape Town Department of Health, Cape Town, South Africa.
Bianca Douglas-Jones, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
Noluvo Ngambu, Western Cape Province Department of Health, Cape Town, South Africa.
Vanessa Mudaly, Western Cape Province Department of Health, Cape Town, South Africa.
Erika Mohr-Holland, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
Petros Isaakidis, Médecins Sans Frontières, Southern Africa Medical Unit, Cape Town, South Africa.
Colin Pfaff, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
Jennifer Furin, Global Health and Social Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Anja Reuter, Médecins Sans Frontières, Khayelitsha, Cape Town, South Africa.
References
- 1. World Health Organization . Global Tuberculosis Report 2022. Geneva: World Health Organization, 2022. [Google Scholar]
- 2. Seddon J, Amanullah F, Schaaf HS, et al. . Post-exposure management of multidrug-resistant tuberculosis contacts: evidence-based recommendations. Harvard Medical School, Centre for Global Health Delivery- Dubai, United Arab Emirates, 2015. Policy Brief No. 1. Available at: http://sentinel-project.org/2015/11/09/hms-center-for-global-health-delivery-dubai-publishes-1st-policy-brief/. Accessed 01 July 2022.
- 3. Heffernan C, Savic RM, Long RG, Raviglione MC, Ferrara G. Responding to the post-pandemic crisis: post-exposure prophylaxis for TB. Int J Tuberc Lung Dis 2022; 26:807–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. World Health Organization . WHO consolidated guidelines on tuberculosis. Module 1: prevention—tuberculosis preventive treatment. Geneva: World Health Organization, 2020. [PubMed] [Google Scholar]
- 5. Malik AA, Fuad J, Abbass W, et al. . Preventive treatment of drug-resistant TB in a rural setting. Int J Tuberc Lung Dis 2021; 25:231–3. [DOI] [PubMed] [Google Scholar]
- 6. Gureva T, Turkova A, Yablokova E, et al. . Fluoroquinolone preventive therapy for children exposed to MDR-TB. Int J Tuberc Lung Dis 2022; 26:171–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Seddon JA, Hesseling AC, Finlayson H, et al. . Preventive therapy for child contacts of multidrug-resistant tuberculosis: a prospective cohort study. Clin Infect Dis 2013;57:1676–84. [DOI] [PubMed] [Google Scholar]
- 8. Jenkins HE, Yuen CM. The burden of multidrug-resistant tuberculosis in children. Int J Tuberc Lung Dis 2018; 22:3–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dodd PJ, Mafirakureva N, Seddon JA, McQuaid CF. The global impact of household contact management for children on multidrug-resistant and rifampicin-resistant tuberculosis cases, deaths, and health-system costs in 2019: a modelling study. Lancet Glob Health 2022; 10:e1034–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moore DA. What can we offer to 3 million MDRTB household contacts in 2016? BMC Med 2016; 14:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reuter A, Seddon JA, Marais BJ, Furin J. Preventing tuberculosis in children: a global health emergency. Paediatr Respir Rev 2020; 36:44–51. [DOI] [PubMed] [Google Scholar]
- 12. National Department of Health (South Africa) . Management of rifampicin-resistant tuberculosis. A clinical reference guide, 2019. Available at: https://www.health.gov.za/wp-content/uploads/2020/11/management-of-rifampicin-resistant-tb-booklet-0220-v11.pdf. Accessed 18 March 2019.
- 13. Mohr-Holland E, Apolisi I, Reuter A, et al. . Barriers and solutions to finding rifampicin-resistant tuberculosis cases in older children and adolescents. Public Health Action 2019; 9:174–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Phelanyane F. Prevention of mother-to-child-transmission of HIV in Khayelitsha: a contemporary review of services 20 years later. PhD thesis, University of Cape Town, February 2021.. Available at: http://hdl.handle.net/11427/36002. Accessed 18 March 2022. [Google Scholar]
- 15. Western Cape Government. Provincial TB Dashboard. Available at:https://www.westerncape.gov.za/site-page/provincial-tb-dashboard. Accessed 20 September 2022.
- 16. Cox H, Hughes J, Daniels J, et al. . Community-based treatment of drug-resistant tuberculosis in Khayelitsha, South Africa. Int J Tuberc Lung Dis 2014; 18:441–8. [DOI] [PubMed] [Google Scholar]
- 17. World Health Organization . Systematic screening for active tuberculosis: an operational guide. Geneva: World Health Organization, 2015. [Google Scholar]
- 18. The Sentinel Project for Pediatric Drug-Resistant Tuberculosis. How to care for people exposed to drug-resistant tuberculosis: a practical guide. Boston, USA, 2018.. Available at: http://sentinel-project.org/2018/03/29/how-to-care-for-people-exposed-to-drug-resistant-tuberculosis-a-practical-guide/. Accessed 18 March 2022.
- 19. WHO Multicentre Growth Reference Study Group . Assessment of differences in linear growth among populations in the WHO. Acta Pædiatrica 2006; 450:56–65. [DOI] [PubMed] [Google Scholar]
- 20. Shah NS, Yuen CM, Heo M, Tolman AW, Becerra MC. Yield of contact investigations in households of patients with drug-resistant tuberculosis: systematic review and meta-analysis. Clin Infect Dis 2014; 58:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rahman MT, Malik AA, Amanullah F, Creswell J. Improving tuberculosis case detection in children: summary of innovations and findings from 18 countries. J Pediatric Infect Dis Soc 2022; 11(Suppl 3):S117–24. [DOI] [PubMed] [Google Scholar]
- 22. Martinez L, Cords O, Horsburgh CR, Andrews JR, Pediatric TBCSC . The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet 2020; 395:973–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Snow KJ, Sismanidis C, Denholm J, Sawyer SM, Graham SM. The incidence of tuberculosis among adolescents and young adults: a global estimate. Eur Respir J 2018; 51:946–53. [DOI] [PubMed] [Google Scholar]
- 24. Fox GJ, Anh NT, Nhung NV, et al. . Latent tuberculous infection in household contacts of multidrug-resistant and newly diagnosed tuberculosis. Int J Tuberc Lung Dis 2017; 21:297–302. [DOI] [PubMed] [Google Scholar]
- 25. Amanullah F, Ashfaq M, Khowaja S, et al. . High tuberculosis prevalence in children exposed at home to drug-resistant tuberculosis. Int J Tuberc Lung Dis 2014; 18:520–7. [DOI] [PubMed] [Google Scholar]
- 26. ClinicalTrials.gov . Protecting households on exposure to newly diagnosed index multidrug-resistant tuberculosis patients (PHOENIx MDR-TB). Available at:https://clinicaltrials.gov/ct2/show/NCT03568383. Accessed 21 September 2022.
- 27. Seddon JA, Garcia-Prats AJ, Purchase SE, et al. . Levofloxacin versus placebo for the prevention of tuberculosis disease in child contacts of multidrug-resistant tuberculosis: study protocol for a phase III cluster randomised controlled trial (TB-CHAMP). Trials 2018; 19:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fox GJ, Nguyen CB, Nguyen TA, et al. . Levofloxacin versus placebo for the treatment of latent tuberculosis among contacts of patients with multidrug-resistant tuberculosis (the VQUIN MDR trial): a protocol for a randomised controlled trial. BMJ Open 2020; 10:e033945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marks SM, Mase SR, Morris SB. Systematic review, meta-analysis, and cost-effectiveness of treatment of latent tuberculosis to reduce progression to multidrug-resistant tuberculosis. Clin Infect Dis 2017; 64:1670–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malik AA, Gandhi NR, Lash TL, et al. . Effectiveness of preventive therapy for persons exposed at home to drug-resistant Tuberculosis, Karachi, Pakistan. Emerg Infect Dis 2021; 27:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Malik AA, Becerra MC, Lash TL, et al. . Risk factors for adverse events in household contacts prescribed preventive treatment for drug-resistant tuberculosis exposure. Clin Infect Dis 2021; 72:1709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Denholm JT, Leslie DE, Jenkin GA, et al. . Long-term follow-up of contacts exposed to multidrug-resistant tuberculosis in Victoria, Australia, 1995–2010. Int J Tuberc Lung Dis 2012; 16:1320–5. [DOI] [PubMed] [Google Scholar]
- 33. Enane LA, Lowenthal ED, Arscott-Mills T, et al. . Loss to follow-up among adolescents with tuberculosis in Gaborone, Botswana. Int J Tuberc Lung Dis 2016; 20:1320–5. [DOI] [PubMed] [Google Scholar]
- 34. Moore BK, Dlodlo RA, Dongo JP, et al. . Evidence to action: translating innovations in management of child and adolescent TB into routine practice in high-burden countries. Pathogens 2022; 11:383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rouzier V, Murrill M, Kim S, et al. . Caregiver willingness to give TPT to children living with drug-resistant TB patients. Int J Tuberc Lung Dis 2022; 26:949–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang CC, Becerra MC, Calderon R, et al. . Isoniazid preventive therapy in contacts of multidrug-resistant tuberculosis. Am J Respir Crit Care Med 2020; 202:1159–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zawedde-Muyanja S, Reuter A, Tovar MA, et al. . Provision of decentralized TB care services: a detect-treat-prevent strategy for children and adolescents affected by TB. Pathogens 2021; 10:1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hirsch-Moverman Y, Strauss M, George G, et al. . Paediatric tuberculosis preventive treatment preferences among HIV-positive children, caregivers and healthcare providers in Eswatini: a discrete choice experiment. BMJ Open 2021; 11:e048443. [DOI] [PMC free article] [PubMed] [Google Scholar]