Abstract
Background
Ceftriaxone is frequently prescribed due to its convenience of dosing and robust antimicrobial activity. However, patients with hypoalbuminemia may experience suboptimal ceftriaxone exposure due to the high degree of protein binding. We aimed to evaluate the impact of hypoalbuminemia on treatment failure among hospitalized adults with Enterobacterales bacteremia who received ceftriaxone therapy.
Methods
We conducted an observational cohort study among patients with Enterobacterales bacteremia who received >72 hours of ceftriaxone initiated within 48 hours of index culture. A propensity-score model was used to match and compare patients with hypoalbuminemia. The primary outcome was treatment failure, defined as a composite of (1) escalation from ceftriaxone to ertapenem or an intravenous antibacterial agent with activity against Pseudomonas aeruginosa, or (2) inpatient death. Secondary outcomes included hospital length of stay, duration of antibiotic therapy, and time to infection resolution.
Results
Of 260 patients included, the majority developed bacteremia from a urinary source (71.5%), and Escherichia coli was the most common pathogen identified (72.3%). Patients with hypoalbuminemia experienced numerically higher rates of treatment failure, although not reaching statistical significance (12.3% vs 7.7%; P = .21). Among patients receiving care in the intensive care unit, the impact of hypoalbuminemia on treatment failure was more pronounced (24.4% vs 7.3%; P = .07).
Conclusions
Hypoalbuminemia may not have a significant impact on clinical outcomes among patients with Enterobacterales bacteremia treated with ceftriaxone. However, critically ill patients may be subject to higher incidence of treatment failure in the presence of hypoalbuminemia.
Keywords: pharmacokinetics, albumin, ceftriaxone, gram-negative bacteremia
Patients with hypoalbuminemia may experience suboptimal ceftriaxone exposure due to high protein binding. A matched cohort of patients with Enterobacterales bacteremia treated with ceftriaxone did not show a significant difference in treatment failure between the normoalbuminemia or hypoalbuminemia groups.
Ceftriaxone (CRO) is one of the most commonly utilized antibiotics among hospitalized patients in the United States due to its spectrum of activity, adverse effect profile, and lack of renal dose adjustment requirements [1]. CRO's pharmacokinetic profile displays high protein binding (83%–96%) and a prolonged half-life (6–8 hours) [2, 3]. These characteristics contribute to reduced clearance of free-drug and allow for convenient 24-hour interval dosing in the treatment of most infections [4]. Additionally, CRO's high protein binding may pose pharmacokinetic and pharmacodynamic challenges in the setting of hypoalbuminemia, as a subsequent increase in the proportion of free-drug could occur. Higher free-drug concentrations may lead to an increase in volume of distribution and drug clearance, which could result in suboptimal time of concentrations above the minimum inhibitory concentration (MIC) and, thus, increased risk for treatment failure [3, 5, 6]. However, patient weight, renal function, and MIC of the target pathogen can also impact CRO exposure and pharmacokinetic targets and may serve as important factors in mediating the impact of hypoalbuminemia [3, 7–10].
In addition to conflicting pharmacokinetic data, there is a paucity of evidence assessing the potential impact on clinical outcomes [3, 8, 9, 11]. In a subgroup analysis, Ackerman et al observed a higher incidence of treatment failure in patients with hypoalbuminemia treated with ceftriaxone. However, this study included exclusively patients in the intensive care unit (ICU) without confirmed bacterial infections, and the small sample size of the subgroup limited the statistical analysis [12]. Baalbaki et al observed similar subgroup analysis findings of bacteremic patients, with those with hypoalbuminemia being 4 times more likely to experience 90-day clinical failure (odds ratio, 4.03 [confidence interval, 1.12–14.50]; P = .033) than those with normoalbuminemia [13]. Additionally, Zusman et al reported increased mortality among patients with plasma albumin concentrations of ≤2.5 g/dL who received ertapenem, another highly protein-bound β-lactam antibiotic [14]. To further elucidate the relationship between hypoalbuminemia and CRO treatment failure, this study sought to compare clinical outcomes among patients with confirmed Enterobacterales bacteremia presenting with or without hypoalbuminemia.
METHODS
Study Design and Patient Population
This retrospective, observational cohort evaluated patients admitted to a 7-hospital health system in the greater Houston area from 1 May 2016 to 30 April 2021. Study timeframe was limited to the respective date range due to an electronic health record system change limiting historic clinical data access. Patients ≥18 years old, who had at least 1 blood culture positive for an Enterobacterales organism susceptible to CRO based on Clinical and Laboratory Standards Institute (CLSI) breakpoints updated and institutionally adopted in 2010, and who received >72 hours of CRO therapy initiated within 48 hours of index culture collection were eligible for inclusion [15]. Eligible isolates were identified by the health system's clinical laboratory via matrix-assisted laser desorption/ionization–time of flight (Bruker Daltonics, Fremont, California) and simultaneously set up for rapid antimicrobial susceptibility testing (BD Phoenix). Current CLSI breakpoints for ceftriaxone against Enterobacterales have remained unchanged since the beginning of the study period; therefore, breakpoints at the time of data analysis were applied to identify susceptible isolates. Patients without a plasma albumin level collected within 24 hours of CRO initiation, those who received >48 hours of empiric antibacterial therapy with an agent other than CRO, or those with polymicrobial blood cultures collected within 72 hours of the index culture were excluded. Additional exclusion criteria included the presence of a central nervous system infection during the hospitalization, receipt of CRO at a dosing interval other than every 24 hours, and pregnancy. Multiple encounters for an individual patient during the study period were each assessed for eligibility; however, all subsequent hospitalizations following the first encounter that met inclusion criteria were excluded. Patients were categorized into groups based on the lowest albumin concentration collected within 24 hours of CRO initiation. Dichotomization of albumin groups and definitions were prospectively defined as a plasma albumin concentration ≤2.5 g/dL and normoalbuminemia as >2.5 g/dL [16].
Data Sources and Collection
Data elements including patient demographics, vital signs, laboratory data, microbiological data, medication administration records, oxygen therapy data, and International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (ICD-10) codes were extracted retrospectively by querying a centralized electronic health record database. Additional variables such as source of infection as documented by the treating clinician, mental status (as required for the Pitt bacteremia score), and ICU admission at the time of or within 24 hours of CRO initiation were collected via chart review. Patients were considered immunosuppressed if (1) a diagnosis of AIDS was present or (2) they received ≥10 mg per day of prednisone equivalent for at least 2 weeks, antineoplastic therapy, a tumor necrosis factor alpha inhibitor, or a calcineurin inhibitor prior to or during their hospitalization [12]. All other comorbidities were identified using ICD-10 codes. Baseline Sequential Organ Failure Assessment (SOFA) scores and Pitt bacteremia scores were calculated using the most abnormal values collected for each component within 24 hours of CRO initiation. If data were missing during this timeframe, normal values were imputed, and scores for each domain were assigned accordingly. Patients with missing data for any other variables collected were excluded from all analyses. These elements and variables were all collected to form a database specifically for this planned study.
Outcomes
The primary outcome of this study was treatment failure, defined as a composite of (1) escalation from CRO to ertapenem or an intravenous (IV) antibacterial agent with activity against Pseudomonas aeruginosa, or (2) inpatient death due to any cause. Agents that could meet the primary outcome definition included amikacin, aztreonam, cefepime, ceftazidime with or without avibactam, ceftolozane-tazobactam, IV ciprofloxacin, ertapenem, gentamicin, imipenem with or without relebactam, IV levofloxacin, meropenem, piperacillin-tazobactam, or tobramycin. Secondary outcomes included hospital length of stay; total duration of antimicrobial therapy; and time to infection resolution, defined as resolution of fever (≤38°C) and leukocytosis (≤12 000 cells/µL). All outcomes were evaluated over the duration of the index hospitalization. Outcomes were further assessed within planned subgroups created based on ICU admission at the time of CRO initiation or within 24 hours of the first dose, obesity (body mass index [BMI] ≥30 kg/m2), and CRO dose exposure. Patients were divided into a corresponding category based on having received 1 g per day or 2 g per day of CRO throughout their hospitalization. One dose of the alternative regimen was permitted for each group to account for possible dose adjustments upon admission.
Statistical Analysis
To control for confounding and possible biases, a propensity score was developed for probability of hypoalbuminemia using a backward, stepwise, multivariable logistic regression approach. Baseline characteristics associated with hypoalbuminemia were identified using univariable logistic regression, and variables with a P value <.20 were eligible for inclusion in the multivariable model. Variables with a P value <.20 in the multivariable analysis were retained in the final model. Patients were matched using a 1-to-1, nearest neighbor approach. For all univariable analyses, continuous variables were compared using Welch t test or Mann-Whitney U test and categorical variables using Pearson χ2 or Fisher exact test as appropriate. All analyses were performed using R Studio version 4.1.0 (R: A Language and Environment for Statistical Computing, Vienna, Austria) and matching was executed using the MatchIt package [17]. The threshold to determine statistical significance for all analyses was P < .05.
Ethics Approval
The Houston Methodist Hospital Institutional Review Board approved this study (PRO00032592) and issued a waiver of informed consent due to the retrospective study design and the investigators’ data privacy and confidentiality plan.
RESULTS
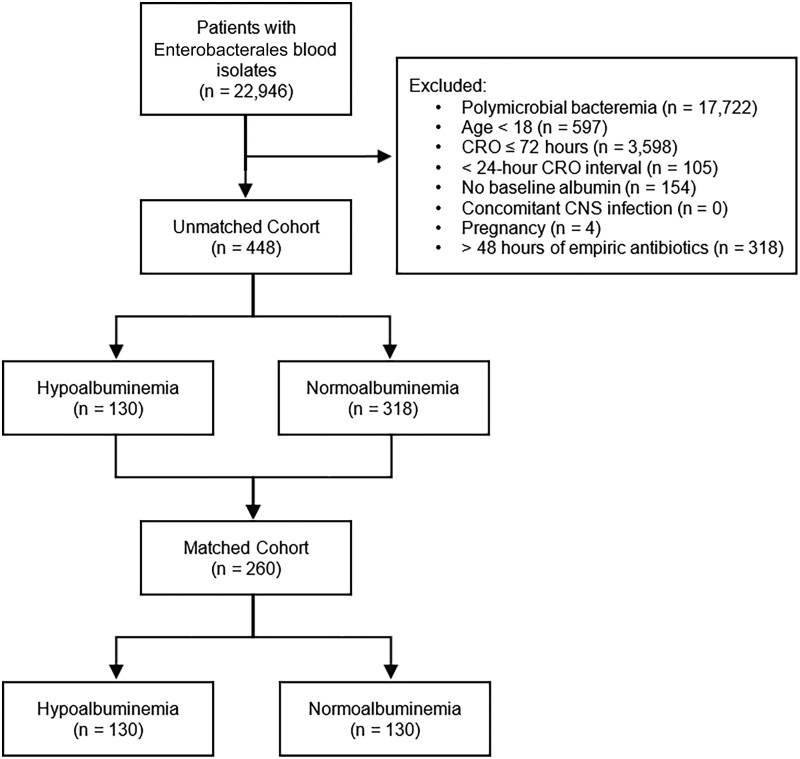
A total of 448 patients met study criteria and were included in the propensity score–matching process (130 patients with hypoalbuminemia and 318 patients without; Supplementary Appendix Table 1). Propensity score matching resulted in 130 matched pairs for primary analysis (n = 260) (Figure 1). No patients were excluded from propensity matching due to missing data. Patients were matched on age, temperature, ICU admission status, SOFA score, presence of cirrhosis, and immunosuppressed status based on the final multivariable logistic regression model (Supplementary Appendix Table 2). The median plasma albumin levels were 2.2 (interquartile range [IQR], 2.0–2.4) g/dL and 3.2 (IQR, 2.8–3.5) g/dL for hypoalbuminemia and normoalbuminemia, respectively. Remaining baseline characteristics were balanced between groups (Table 1). Urinary tract was the most common bloodstream infection source identified by clinicians (186 [71.5%]). The most common pathogen was Escherichia coli (188 [72.3%]), followed by Klebsiella pneumoniae (40 [15.4%]) and Proteus mirabilis (14 [5.4%]) (Supplementary Appendix Table 3).
Figure 1.
Study selection flow diagram. Abbreviations: CNS, central nervous system; CRO, ceftriaxone.
Table 1.
Baseline Characteristics Among Propensity-Matched Cohort by Albuminemia
| Characteristic | Hypoalbuminemia (n = 130) |
Normoalbuminemia (n = 130) |
P Value |
|---|---|---|---|
| Age, y | 65.2 (56.0–80.6) | 70.3 (56.4–82.5) | .34 |
| Female sex, No. (%) | 78 (60.0) | 77 (59.2) | .90 |
| BMI, kg/m2 | 25.9 (21.9–31.3) | 28.0 (24.0–33.7) | .09 |
| Average CRO dose, g/d | 1.6 (1.0–1.9) | 1.6 (1.0–1.8) | .60 |
| Duration of CRO, d | 4.9 (3.9–7.0) | 5.1 (3.9–7.4) | .51 |
| Time to first effective antibiotica, h | 1.6 (0.7–2.9) | 1.1 (0.3–2.6) | .06 |
| Time to CRO initiationb, h | 5.2 (1.4–32.6) | 2.1 (0.5–8.6) | <.01 |
| Infection source, No. (%) | |||
| Intravenous catheter | 5 (3.8) | 1 (0.8) | .10 |
| Intra-abdominal | 19 (14.6) | 13 (10.0) | .26 |
| LVAD driveline | 0 (0.0) | 1 (0.8) | 1.00 |
| Skin and soft tissue | 1 (0.8) | 1 (0.8) | 1.00 |
| Urinary tract | 87 (66.9) | 99 (76.1) | .10 |
| Unknown | 18 (13.8) | 15 (11.5) | .58 |
| Plasma albumin, g/dL | 2.2 (2.0–2.4) | 3.2 (2.8–3.5) | <.01 |
| Serum creatinine, g/dL | 1.6 (1.2–2.2) | 1.6 (1.1–2.4) | .58 |
| WBC count, cells × 103/µL | 15.0 (10.9–20.5) | 15.4 (10.7–19.2) | .54 |
| Temperature, °C | 38.2 (37.4–39.1) | 38.1 (37.6–38.9) | .82 |
| ICU admission, No. (%) | 41 (31.5) | 41 (31.5) | 1.00 |
| Mechanical ventilation, No. (%) | 8 (6.2) | 8 (6.2) | 1.00 |
| Vasopressor use, No. (%) | 9 (6.9) | 7 (5.4) | .61 |
| SOFA scorec | 4 (2.0–6.0) | 4 (2.0–6.0) | .57 |
| Pitt bacteremia scored | 2 (1.0–3.0) | 2 (1.0–3.0) | .99 |
| Comorbidities, No. (%) | |||
| Immunosuppression | 18 (13.8) | 18 (13.8) | 1.00 |
| Cirrhosis | 14 (10.8) | 7 (5.4) | .11 |
| Malignancy | 17 (13.1) | 13 (10.0) | .44 |
| COPD | 10 (7.7) | 14 (10.8) | .39 |
| Diabetes mellitus | 55 (42.3) | 58 (44.6) | .71 |
| Chronic kidney disease | 104 (80.0) | 102 (78.5) | .76 |
Data are presented as median (interquartile range) unless otherwise indicated.
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; CRO, ceftriaxone; ICU, intensive care unit; LVAD, left ventricular assist device; SOFA, Sequential Organ Failure Assessment; WBC, white blood cell.
Time from culture collection, excluding patients on effective therapy at the time of culture collection (n = 123 and n = 125, respectively).
Time from culture collection, excluding patients on CRO at the time of culture collection (n = 128 and n = 127, respectively).
Number of patients with missing data: partial pressure of oxygen in arterial blood, n = 232; bilirubin, n = 1.
No patients with missing data.
Incidence of treatment failure was numerically greater among the hypoalbuminemia group, although this difference did not reach statistical significance (12.3% vs 7.7%; P = .21). A similar trend was seen in the individual outcomes within the composite of treatment failure. Of the 24 patients in whom therapy was escalated, piperacillin-tazobactam was the most common agent that was initiated (10 [41.7%]). No significant differences were observed in hospital length of stay, duration of antibiotic therapy, or time to infection resolution (Table 2).
Table 2.
Clinical Outcomes Among Propensity-Matched Cohort by Albuminemia
| Outcome | Hypoalbuminemia (n = 130) | Normoalbuminemia (n = 130) | P Value |
|---|---|---|---|
| Treatment failure, No. (%) | 16 (12.3) | 10 (7.7) | .21 |
| Mortality, No. (%) | 2 (1.5) | 1 (0.8) | 1.00 |
| Escalation of therapy, No. (%) | 15 (11.5) | 9 (6.9) | .20 |
| Length of stay, d | 6.8 (5.2–9.6) | 6.4 (5.0–9.3) | .34 |
| Duration of antibiotic therapy, d | 5.4 (4.1–8.1) | 5.7 (4.0–8.0) | .93 |
| Time to infection resolutiona, d | 1.0 (0.5–2.2) | 0.9 (0.2–2.5) | .36 |
Data are presented as median (interquartile range) unless otherwise indicated.
Time from ceftriaxone initiation, excluding patients without baseline leukocytosis or fever (n = 116 and n = 113, respectively).
Among the subgroup of patients admitted to ICU at the time of CRO initiation or within 24 hours of the first dose (n = 83), treatment failure occurred more frequently in the hypoalbuminemia group (24.4% vs 7.3%; P = .07) (Supplementary Appendix Table 4). Clinical outcomes did not differ significantly among subgroups based on BMI and daily CRO dose (Supplementary Appendix Tables 5 and 6).
DISCUSSION
Treatment with CRO for CRO-susceptible, monomicrobial Enterobacterales bacteremia resulted in numerically higher incidence of treatment failure in patients with hypoalbuminemia. However, this difference did not reach the prespecified threshold for statistical significance in the overall cohort. Escalation of therapy was the primary driver of the composite outcome as 98% of patients in this study survived to hospital discharge. The strengths of this study include a relatively clean cohort due to matching patients limited specifically to monomicrobial bacteremia. Additionally, our 2 study groups had no difference in serum creatinine, eliminating confounding by this variable.
Multiple pharmacokinetic studies have reported an increase in unbound CRO associated with hypoalbuminemia [9, 11, 18]. However, this relationship can be variable and has been noted to be influenced by other factors, such as altered protein binding affinity caused by hyperbilirubinemia, changes in renal function, and the MIC of the target pathogen [3, 8, 9, 19]. These alterations in pharmacokinetics are most frequently identified in critically ill patients and may mediate the previously reported association between hypoalbuminemia and CRO treatment failure in this population [12]. Moreover, CRO protein binding kinetics have been best described as nonlinear and saturable, and thus, decreases in plasma albumin may not always result in a proportional increase in unbound CRO [11]. In contrast to previous limited clinical data, we report minimal difference in rates of treatment failure between patients with and without hypoalbuminemia overall [12]. However, with variable evidence of the impact of hypoalbuminemia on CRO-related pharmacokinetic/pharmacodynamic outcomes, our results may be a more accurate estimation of the effects on clinical outcomes among most hospitalized patients [8, 9, 20]. Our finding of a numerically greater impact of hypoalbuminemia on CRO treatment failure among patients in the ICU supports previous conclusions of a possible association in this population [12]. Though similar in pharmacokinetic profile to ertapenem, there may be important differences from CRO that contribute to differential effects on treatment outcomes in patients with hypoalbuminemia, as a previous investigation reported significantly higher rates of mortality among this subgroup [14]. Further study of the impact of hypoalbuminemia on clinical outcomes among patients treated with ertapenem may be warranted.
Our study is not without limitations. Although we attempted to adjust for selection bias by matching based on treatment propensity, sources of bias outside of the data elements collected cannot be ruled out. Although our study lacks specifically delineating complicated versus uncomplicated bacteremia designations, our cohort consisted of primarily patients with bacteremia due to E coli urinary tract infections. These patients typically experience high rates of treatment success with short durations of antibiotic therapy, which may have minimized the impact of hypoalbuminemia on clinical outcomes [21]. Mortality was an uncommon outcome in both groups, and escalation of therapy may not objectively represent treatment failure in all cases. Additionally, patients were censored at the time of hospital discharge in our study, and outcomes analyses at alternative timepoints (ie, 30 days) could yield different findings. Patients transitioned from CRO to agents with lower protein binding affinity, such as cefazolin or oral antibiotics, were not categorized as treatment failure. These transitions are often part of a routine de-escalation strategy and in some cases the decision to complete therapy with 1 of these agents may be influenced by multiple factors including treatment failure with CRO. However, the authorship team did not include this in the primary outcome as escalation to broader agents has a higher negative impact on antimicrobial resistance and stewardship practices. We observed a small numerical difference in time to first effective antibiotic (1.6 vs 1.1 hours; P = .06) and time to CRO initiation (5.2 vs 2.1 hours; P < .01). All patients were initiated on CRO within 48 hours of culture collection, received ≤48 hours of alternative empiric antibiotic therapy, and received >72 hours of CRO. Therefore, the likelihood of these differences impacting outcomes is low since all patients were initiated on effective antibiotic therapy in a timely manner and received similar durations of ceftriaxone therapy overall (4.9 vs 5.1 days; P = .51).
In summary, hypoalbuminemia did not appear to have a significant impact on clinical outcomes among patients with Enterobacterales bacteremia treated with CRO. However, critically ill patients may be subject to higher incidence of treatment failure in the presence of hypoalbuminemia. More aggressive dosing strategies or selection of alternative agents, when possible, may be preferred in this population. Future studies evaluating alterations to pharmacokinetic parameters and the impact on clinical outcomes are imperative to optimize CRO treatment strategies and continue to advance antimicrobial stewardship practices.
Supplementary Material
Contributor Information
Evan L Steere, Department of Pharmacy, Houston Methodist Hospital, Houston, Texas, USA.
Taryn A Eubank, Department of Pharmacy, Houston Methodist Hospital, Houston, Texas, USA; Department of Pharmacy Practice and Translational Research, University of Houston College of Pharmacy, Houston, Texas, USA.
Megan H Cooper, Department of Pharmacy, Houston Methodist Hospital, Houston, Texas, USA.
Sage B Greenlee, Department of Pharmacy, University of Utah Health, Salt Lake City, Utah, USA.
Ty C Drake, Department of Pharmacy, Memorial Hermann-Texas Medical Center, Houston, Texas, USA.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Author contributions. Writing–original draft: E. L. S. Writing–review and editing: T. A. E., M. H. C., S. B. G., and T. C. D. Conceptualization: T. A. E., T. C. D., S. B. G., and M. H. C. Investigation: E. L. S. and T. A. E. Methodology: T. A. E., T. C. D., S. B. G., and M. H. C. Formal analysis: E. L. S. and T. A. E. Project administration: T. A. E. and T. C. D.
Financial support. No external sources of funding were utilized to complete this study.
References
- 1. Goodman KE, Cosgrove SE, Pineles L, et al. Significant regional differences in antibiotic use across 576 US hospitals and 11 701 326 adult admissions, 2016–2017. Clin Infect Dis 2021; 73:213–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stoeckel K. Pharmacokinetics of rocephin, a highly active new cephalosporin with an exceptionally long biological half-life. Chemotherapy 1981; 27:42–6. [DOI] [PubMed] [Google Scholar]
- 3. Joynt GM, Lipman J, Gomersall CD, Young RJ, Wong EL, Gin T. The pharmacokinetics of once-daily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 2001; 47:421–9. [DOI] [PubMed] [Google Scholar]
- 4. Ulldemolins M, Roberts JA, Rello J, Paterson DL, Lipman J. The effects of hypoalbuminaemia on optimizing antibacterial dosing in critically ill patients. Clin Pharmacokinet 2011; 50:99–110. [DOI] [PubMed] [Google Scholar]
- 5. Wong G, Briscoe S, Adnan S, et al. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 2013; 57:6165–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bos JC, Prins JM, Mistício MC, et al. Pharmacokinetics and pharmacodynamic target attainment of ceftriaxone in adult severely ill sub-Saharan African patients: a population pharmacokinetic modelling study. J Antimicrob Chemother 2018; 73:1620–9. [DOI] [PubMed] [Google Scholar]
- 7. Barber KE, Loper JT, Morrison AR, Stover KR, Wagner JL. Impact of obesity on ceftriaxone efficacy. Diseases 2020; 8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Heffernan AJ, Sime FB, Kumta N, et al. Multicenter population pharmacokinetic study of unbound ceftriaxone in critically ill patients. Antimicrob Agents Chemother 2022; 66:e0218921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schleibinger M, Steinbach CL, Töpper C, et al. Protein binding characteristics and pharmacokinetics of ceftriaxone in intensive care unit patients. Br J Clin Pharmacol 2015; 80:525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Dalen R, Vree TB, Baars IM. Influence of protein binding and severity of illness on renal elimination of four cephalosporin drugs in intensive-care patients. Pharm Weekbl Sci 1987; 9:98–103. [DOI] [PubMed] [Google Scholar]
- 11. Leegwater E, Kraaijenbrink BVC, Moes D, Purmer IM, Wilms EB. Population pharmacokinetics of ceftriaxone administered as continuous or intermittent infusion in critically ill patients. J Antimicrob Chemother 2020; 75:1554–8. [DOI] [PubMed] [Google Scholar]
- 12. Ackerman A, Zook NR, Siegrist JF, Brummitt CF, Cook MM, Dilworth TJ. Comparison of clinical outcomes among intensive care unit patients receiving one or two grams of ceftriaxone daily. Antimicrob Agents Chemother 2020; 64:e00066-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Baalbaki N, Blum S, Akerman M, Johnson D. Ceftriaxone 1g versus 2g daily for the treatment of Enterobacterales bacteremia: a retrospective cohort study. J Pharm Technol 2022; 38:326–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zusman O, Farbman L, Tredler Z, et al. Association between hypoalbuminemia and mortality among subjects treated with ertapenem versus other carbapenems: prospective cohort study. Clin Microbiol Infect 2015; 21:54–8. [DOI] [PubMed] [Google Scholar]
- 15. Clinical and Laboratory Standards Institute (CLSI) . Performance standards for antimicrobial susceptibility testing. 32nd ed. Wayne, PA: CLSI; 2022:362. [Google Scholar]
- 16. Finfer S, Bellomo R, McEvoy S, et al. Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ 2006; 333:1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ho DE, Imai K, King G, Stuart EA. Matchit: nonparametric preprocessing for parametric causal inference. J Stat Softw 2011; 42:1–28. [Google Scholar]
- 18. Mimoz O, Soreda S, Padoin C, Tod M, Petitjean O, Benhamou D. Ceftriaxone pharmacokinetics during iatrogenic hydroxyethyl starch-induced hypoalbuminemia: a model to explore the effects of decreased protein binding capacity on highly bound drugs. Anesthesiology 2000; 93:735–43. [DOI] [PubMed] [Google Scholar]
- 19. Garot D, Respaud R, Lanotte P, et al. Population pharmacokinetics of ceftriaxone in critically ill septic patients: a reappraisal. Br J Clin Pharmacol 2011; 72:758–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ulldemolins M, Bastida C, Llauradó-Serra M, et al. Once-daily 1g ceftriaxone optimizes exposure in patients with septic shock and hypoalbuminemia receiving continuous veno-venous hemodiafiltration. Eur J Clin Pharmacol 2021; 77:1169–80. [DOI] [PubMed] [Google Scholar]
- 21. Yahav D, Franceschini E, Koppel F, et al. Seven versus 14 days of antibiotic therapy for uncomplicated gram-negative bacteremia: a noninferiority randomized controlled trial. Clin Infect Dis 2019;69:1091–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.