Abstract
In this analysis of a prospective study of radiation-induced skin injury in breast cancer patients treated with radiotherapy (RT), urinary cotinine (UCot) was measured for 980 participants at baseline and end RT. High UCot was associated with a higher rate of late RISI but not acute RISI. Smoking cessation is recommended to reduce the risk of late RISI.
Introduction:
Smoking during breast radiotherapy (RT) may be associated with radiation-induced skin injury (RISI). We aimed to determine if a urinary biomarker of tobacco smoke exposure is associated with increased rates of RISI during and after breast RT.
Patients and Methods:
Women with Stage 0-IIIA breast cancer treated with breast-conserving surgery or mastectomy followed by RT to the breast or chest wall with or without regional nodal irradiation were prospectively enrolled on a multicenter study assessing acute/late RISI. 980 patients with urinary cotinine (UCot) measurements (baseline and end-RT) were categorized into three groups. Acute and late RISI was assessed using the ONS Acute Skin Reaction scale and the LENT-SOMA Criteria.
Results:
Late Grade 2+ and Grade 3+ RISI occurred in 18.2% and 1.9% of patients, respectively—primarily fibrosis, pain, edema, and hyperpigmentation. Grade 2+ late RISI was associated with UCot group (P= 006). Multivariable analysis identified UCot-based light smoker/secondhand smoke exposure (HR 1.79, P= .10) and smoking (HR 1.60, p = .06) as non-significantly associated with an increased risk of late RISI. Hypofractionated breast RT was associated with decreased risk of late RISI (HR 0.51, P=.03). UCot was not associated with acute RISI, multivariable analysis identified race, obesity, RT site/fractionation, and bra size to be associated with acute RISI.
Conclusions:
Tobacco exposure during breast RT may be associated with an increased risk of late RISI without an effect on acute toxicity. Smoking cessation should be encouraged prior to radiotherapy to minimize these and other ill effects of smoking.
Keywords: Breast cancer, Radiotherapy, Radiation-induced dermatitis, Radiation injuries, Patient reported outcomes
Introduction
Breast cancer is one of the most common malignancies in women. Many patients with breast cancer will be treated with radiotherapy (RT) either after lumpectomy, as a part of a breast-conserving therapy approach, or after mastectomy, based on clinicopathologic risk factors. Radiotherapy to the intact breast after breast-conserving lumpectomy reduces recurrence rates by over one-half and reduces breast cancer mortality.1,2 Post-mastectomy radiotherapy improves locoregional control, disease-free survival, and overall survival in patients with locally advanced breast cancer.3–5 Approximately 14% of adults (12% of women) in the United States self-report as current cigarette smokers.6 Additionally, 13% of women reported the use of any combustible tobacco product and 24% of female non-smokers surveyed in the National Health and Examination Survey had evidence of secondhand smoke (SHS) exposure7. The effects of tobacco exposure on RT-induced toxicity are particularly an issue, as 17% to 43% of breast cancer patients treated with RT are smokers.8
Radiation-induced skin injury (RISI) is a common effect of radiation therapy for breast cancer. Its acute (occurring within 12 weeks of radiation therapy) manifestations include dermatitis, desquamation, edema, pain and/or discomfort.9–11 Late effects (occurring later than 12 weeks after radiation therapy) include discoloration (hypo- or hyper-pigmentation), fibrosis, and telangiectasia.12–16 All of these sequelae manifest as changes in patient-reported cosmetic results after breast cancer radiotherapy.17,18
A prior analysis of this study has found no significant association between RISI and patient-reported smoking status.9 It is important to clarify the role of various biomarkers that have been utilized to assess the biological effect of tobacco/SHS exposure. These include urinary and blood plasma assays of cotinine, tobacco-specific nitrosamines, and polycyclic aromatic hydrocarbon-DNA adducts.19 Cotinine is a primary metabolite of nicotine with a longer half-life that has been used as a quantifiable biomarker for tobacco smoke exposure.20,21 In the NHANES study, serum cotinine was utilized as a biomarker for SHS exposure, which was defined as serum cotinine levels of 0.05–10 ng/mL in nonsmokers.22,23 Urinary cotinine excretion is also routinely used to assess for tobacco smoke exposure.24 A pragmatic, non-invasive approach to measuring recent/current tobacco exposure during cancer therapy is the measurement of urinary cotinine excretion.20 In urine, cotinine levels between 11 and 30 ng/mL may be indicative of light smoking or SHS exposure, whereas levels in active smokers typically reach upwards of 500 ng/mL.
It is unclear whether smoking status influences oncologic outcome after breast cancer treatment,25,26 but population-based evidence has identified increased risks of breast cancer recurrence and cancer-related mortality in current or former smokers.27 The link between smoking and RT normal tissue toxicity has been assessed in breast cancer,8 prostate cancer,28 and head and neck cancer.29,30 The majority of the literature on tobacco smoking and its effect on cancer and treatment outcomes is based on patient-reported smoking history. The use of urinary biomarkers to quantify tobacco exposure is widely utilized and has been shown to identify approximately 5% (range: 0.8to19.7) of misclassified current smokers who self-report as never/former smokers.31,32 This methodology has not been implemented to study the effect of smoking on skin toxicity during and after breast cancer radiotherapy. Our objective was to evaluate the impact of tobacco exposure measured by urinary cotinine on acute and late RISI.
Patients and Methods
Patient Population and Study Design
This is an analysis of a prospective clinical trial aimed to develop and validate biomarkers for acute and chronic radiation-induced skin injury (RISI) and quality of life in five racial/ethnic subgroups of breast cancer patients (Wake Forest Community Clinical Oncology Program Research Base CCCWF97609). One thousand patients with newly diagnosed Stage 0-IIIA breast cancer planned for post-operative (lumpectomy, quadrantectomy, or mastectomy) radiotherapy were enrolled between 2011 and 2013. Radiotherapy consisted of conventional fractionation or hypofractionated dosing regimens to the breast/chest wall with or without regional nodal irradiation. Patients were evaluated for skin toxicity prior to, during, and at the end of radiation therapy (end-RT). Assessments for acute effects were obtained at baseline, week 3 of RT, end-RT, and at 1- and 2-months post-RT. Late effects were assessed at 6-months and 12-months post-RT. Clinician-related outcome (CRO) assessments included the Oncology Nursing Society (ONS) Acute Skin Reaction Scale for Acute Dermatitis and the LENT-SOMA Criteria for late RT-induced skin toxicity as previously described.9 Assessment definitions are detailed in Appendix A. Patient-reported outcome (PRO) measures included the Skindex-16, FACT-B and B39 QOL Questionnaire.
Urinary Biomarker of Tobacco Exposure
Urine samples were collected prior to radiotherapy (pre-RT) and after the last fraction of radiotherapy (end-RT) and then frozen and sent to the NCORP Research Base Biospecimen Laboratory for processing and storage. Urinary cotinine concentration was measured using a cotinine direct ELISA assay (GenWay Biotech, San Diego, CA [GWB-BQK0DA]) at pre-RT and end-RT time points. Because the distribution of the values of the cotinine marker present in the urinary samples was highly skewed (mean 955.5, median 0.7, range 0 to 115,848), we categorized the variable using cutoffs of 10 and 100 into three categories – non-smoker (<10), possible former/light smokers or secondhand smoke (SHS) exposure (10 to 100), and smoker (>100). The second category was not stable across the two visits. In order to categorize patients who were actively smoking/exposed to SHS at some point during RT, a decision was made to use the maximum value of the pre-RT and end-RT visit measurements as the indicator of on-treatment smoking status. Non-smokers were those with both urinary cotinine values less than 10 ng/mL, light smokers/those with SHS exposure were defined as values between 10–100, and current smokers were those with cotinine levels greater than 100 ng/mL. Supplemental Figure 1 displays the distribution of log(cotinine) versus highest RISI by the ONS scale in relation to the selected cutoff values discriminating the three groups.
Statistical Analysis
We first conducted an exploratory analysis to operationalize the available measures of acute and late RISI (Appendix A). For the ONS score (a measure of acute RISI, range: 0 to 7), the distribution was skewed and a square root transformation was used. For the LENT-SOMA score (a measure of late RISI, range: 0 to 4), the maximum grade across the seven domains (pain, edema, soft tissue fibrosis, telangiectasia, ulceration, hyperpigmentation and hypopigmentation) was used as a single value to represent the highest-grade late toxicity. Descriptive statistics, including univariate and bivariate summaries were reported. In bivariate analysis, skin toxicity measures were also dichotomized and chi-square tests were used to evaluate the association between toxicity and the cotinine group.
The association between cotinine-based smoker status and RT-induced toxicity was also analyzed using multivariable models in which clinical and sociodemographic variables were controlled. The mixed-effects models were used to analyze ONS and LENT-SOMA outcomes, with cotinine-based smoker status as the primary independent variable. The mixed-effects models take into account the correlation between multiple observations from the same individual. We considered an extensive set of independent variables reported in Hu et al.9 Specifically, the following clinically relevant covariates were included in the models: race (White, Black, Hispanic, Asian Pacific Island, and others), age group (<60, >=60), BMI (normal, overweight, and obese), received chemotherapy (yes/no), radiation location (chest wall, breast separately; yes/no), regional lymph node (yes/no), conventionally fractionated RT (1.8–2.0 Gy/fraction) versus hypofractionation, RT dose (<50 Gy, 50–60 Gy, >60 Gy), diabetes (yes versus no), number of other comorbidities (0,1,>1), hormone therapy (yes versus no), targeted therapy (yes versus no), and patient-reported bra size (small, medium, large, and extra-large).
Results
One thousand patients were enrolled between 2011 and 2013, and 980 with evaluable urinary cotinine measures and RISI assessments were included in this analysis. Table 1 summarizes sample characteristics at baseline. The median age was 58 years, the sample population was racially diverse, the median BMI was 30.7 and the majority of patients had DCIS or early-stage (I-II) breast cancer. 35.4% of patients identified as either current or former smokers. In total, 6.3% of patients who self-identified as never smokers were categorized by urinary cotinine as either light smokers/SHS or smokers. Surgical, radiotherapeutic, and systemic treatments are detailed in Table 2. Surgical management was primarily breast-conserving surgery and the median adjuvant radiotherapy dose was 60.4 Gy (86.5% conventional fractionation, 13.5% hypofractionation).
Table 1.
Patient Baseline Characteristics Stratified by Tobacco Smoke Exposure Categorized by Urinary Cotinine.
| Totala | Non-smoker | Light Smoker or SHS | Smoker | P-value | |
|---|---|---|---|---|---|
| Age | 58.1 (10.9) | 58.1 (10.8) | 56.1 (11.3) | 55.8 (10.3) | 0.01 |
| Race | 0.23 | ||||
| White | 623 (62.3) | 502 (63.4) | 29 (53.7) | 81 (59.6) | |
| Black | 280 (28.0) | 208 (26.3) | 21 (38.9) | 45 (33.1) | |
| Asian/Pacific Islander | 64 (6.4) | 56 (7.1) | 2 (3.7) | 5 (3.7) | |
| Others | 33 (3.3) | 26 (3.3) | 2 (3.7) | 5 (3.7) | |
| Ethnicity | 0.01 | ||||
| Non Hispanic | 747 (74.7) | 572 (72.2) | 46 (85.2) | 114 (83.8) | |
| Hispanic | 241 (24.1) | 210 (26.5) | 8 (14.8) | 20 (14.7) | |
| Unknown | 12 (1.2) | 10 (1.3) | 0 (0.0) | 2 (1.5) | |
| Smoking Status | <0.0001 | ||||
| Never | 629 (64.9) | 589 (75.2) | 25 (47.2) | 15 (11.2) | |
| Former | 256 (26.4) | 194 (24.8) | 27 (50.9) | 35 (26.1) | |
| Current | 85 (8.8) | 0 (0.0) | 1 (1.9) | 84 (62.7) | |
| Smoking History | |||||
| Years smoked | 22.5 (14.1) | 18.1 (13.1) | 20.2 (13.7) | 29.9 (12.2) | <0.001 |
| Pack per day | 0.8 (1.1) | 0.8 (1.0) | 0.6 (0.5) | 0.8 (1.4) | 0.50 |
| BMI | 30.7 (7.0) | 30.7 (6.9) | 31.5 (7.7) | 30.8 (7.7) | 0.69 |
| Stage | 0.71 | ||||
| 0 | 204 (20.4) | 169 (21.3) | 6 (11.1) | 25 (18.4) | |
| 1 | 443 (44.3) | 346 (43.7) | 27 (50.0) | 63 (46.3) | |
| 2 | 280 (28.0) | 219 (27.7) | 16 (29.6) | 38 (27.9) | |
| 3A | 73 (7.3) | 58 (7.3) | 5 (9.3) | 10 (7.4) |
N from groups may not add to total because of missing value in cotinine measure.SHS: secondhand smoke exposure.
Table 2.
Treatment Characteristics.
| Totala | Non-smoker | Light Smoker or SHS | Smoker | P-value | |
|---|---|---|---|---|---|
| Surgery Type | |||||
| Lumpectomy | 883 (88.3) | 698 (88.1) | 48 (88.9) | 121 (89.0) | 0.95 |
| Quadrantectomy | 17 (1.7) | 15 (1.9) | 1 (1.9) | 1 (0.7) | 0.63 |
| Mastectomy | 99 (10.1) | 80 (10.1) | 5 (9.3) | 14 (10.3) | 0.98 |
| RT Target | |||||
| Whole breast | 910 (91.0) | 722 (91.2) | 50 (92.6) | 122 (89.7) | 0.79 |
| Chest wall | 100 (10.0) | 79 (1.0) | 4 (7.4) | 15 (11.0) | 0.75 |
| Regional Lymph Nodes | 118 (12.0) | 93 (11.7) | 7 (13.0) | 18 (13.2) | 0.86 |
| Conventional Fractionation (1.8–2 Gy per fraction) | 849 (86.5) | 682 (80.3) | 49 (5.8) | 118 (13.9) | 0.63 |
| RT Dose (median, range) | 60.4 (38.5–90.8) | 60.4 (38.5–90.0) | 60.4 (50.0–66.0) | 60.4 (38.5–66.0) | 0.36b |
| RT Number of Fractions (median, range) | 33 (5–62.8) | 33 (5–38) | 33 (16–35) | 33 (7–62.8) | 0.74b |
| Chemotherapy | 401 (40.1) | 324 (40.9) | 20 (37.0) | 49 (36.0) | 0.51 |
| Hormonal therapy | 158 (16.5) | 129 (16.9) | 9 (17.0) | 18 (14.2) | 0.74 |
| Targeted therapy | 100 (10.0) | 82 (10.6) | 3 (5.6) | 10 (7.4) | 0.32 |
N from groups may not add to total because of missing value in cotinine measure.
Nonparametric Kruskal-Wallis testRT: radiation therapy; SHS: secondhand smoke exposure
The distribution of patients with moderate to severe late RISI is presented in Figure 1. At the end of RT, ANOVA of acute RISI measured by the ONS Acute Skin Reaction Scale showed no relationship between ONS and the cotinine-based smoking category (Table 3). Further examination of the data showed that elevated cotinine level was present at Grade 5 or above for ONS (Supplemental Figure 1). An ANOVA test indicated a p-value of 0.10 on the log cotinine scale by treating different grade levels of ONS-grade as the grouping variable. While the general trend of cotinine level increases with ONS grade (e.g., log mean cotinine of 0.8, 2.0, and 3.0, at ONS Grade 0, 5, and 6, respectively), this should be interpreted with caution given sample sizes at higher grades (n = 21 and n = 6, respectively for grades 5 and 6). A summary of the collected clinician-reported outcomes at each time point is available in Supplemental Table 1.
Figure 1.
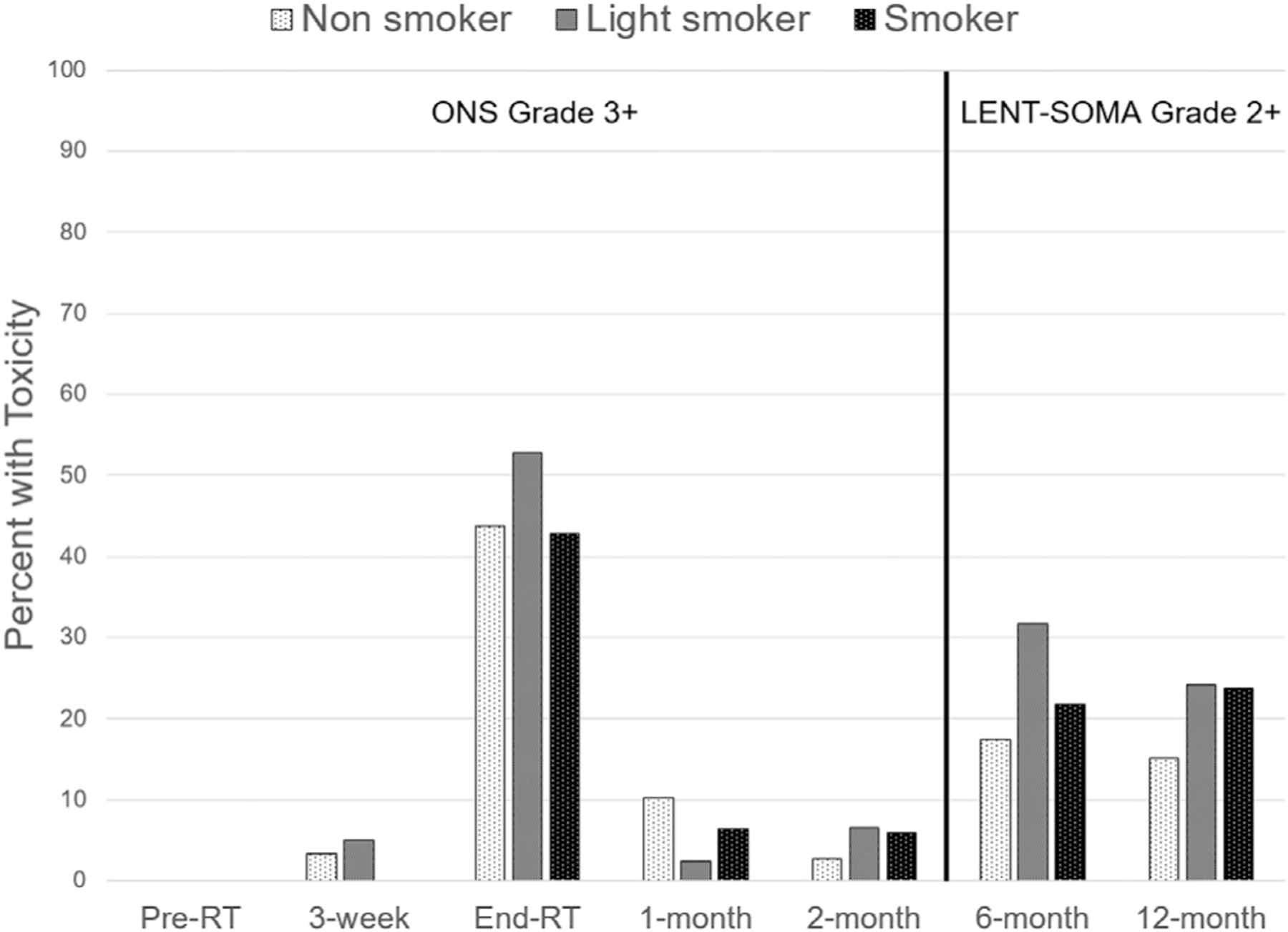
Proportion of moderate to severe radiation-induced skin injury in each urinary cotinine-based smoking group. Acute toxicity is defined as ONS Grade 3+ for baseline, 3-week, end-RT, 1-month, and 2-month visits; late toxicity is defined as LENT-SOMA Grade 2+ for 6-month and 12-month visits.
Table 3.
Moderate to Severe Radiation Skin Toxicity Measures in Smokers Identified by High Urinary Cotinine.
| Total | Non-smokers | Light smokers or SHSc | Smokers | P-value | |
|---|---|---|---|---|---|
| ONS Grade 3+ Skin Toxicitya | |||||
| Yes, n (%) | 411 (41.9) | 324 (41.2) | 28 (51.9) | 57 (42.4) | 0.30 |
| No, n (%) | 569 (58.1) | 463 (58.8) | 26 (48.1) | 78 (57.8) | |
| ONS Grade 4+ Skin Toxicitya | |||||
| Yes, n (%) | 149 (15.2) | 118 (15.0) | 11 (20.4) | 20 (14.8) | 0.56 |
| No, n (%) | 831 (84.8) | 669 (85.0) | 43 (79.6) | 115 (85.2) | |
| LENT-SOMA Grade 2+ Late Skin Toxicityb | |||||
| Yes, n (%) | 174 (18.2) | 129 (16.8) | 16 (29.6) | 28 (21.9) | 0.03 |
| No, n (%) | 781 (81.8) | 641 (83.2) | 38 (70.4) | 100 (78.1) | |
| LENT-SOMA Grade 3+ Late Skin Toxicityb | |||||
| Yes, n (%) | 18 (1.9) | 13 (1.7) | 0 (0) | 5 (3.9) | 0.13 |
| No, n (%) | 937 (98.1) | 757 (98.3) | 54 (100.0) | 123 (96.1) |
ONS Grade is based on last day of radiation therapy
LENT-SOMA toxicity is based on the maximum of 6-month and 12-month LENT-SOMA grade
SHS: secondhand smoke exposure
The late RISI measure had means (SD) of 0.91 (0.65) and 0.78 (0.67) at 6-months and 12-months post-RT, respectively, and a range of 0–4. Figure 1 shows the proportion of patients with moderate to severe late RISI at each time point and Supplementary Table 1 shows the domain level LENT-SOMA mean grade change over time. Table 3 shows the bivariate association for dichotomized LENT-SOMA grades with cotinine groups. Further ANOVA analysis showed there was a significant difference in LENT-SOMA grade across smoking groups at 12-months (P=.006) and marginal significance at 6-months (P=.06).
Table 4 summarizes the result of the multivariable analysis using all available data. For the dichotomized moderate to severe acute ONS (Grade 3+) outcome, cotinine-based smoker status is not significant. Factors that were significantly associated with acute risk include: all race groups versus White (Black, HR 1.29; Asian/Pacific Islander, HR 1.64; Others, 1.41), obesity HR 1.45) versus normal BMI, hypofractionated breast RT (HR 0.40) and conventionally fractionated chest wall RT (HR 1.38) versus conventionally fractionated breast RT, and large/extra-large bra size (HR 1.46) versus small/medium. The correlation between consecutive observations was low and ranged from −0.041 to 0.002. For the dichotomized moderate to severe LENT-SOMA (Grade 2+) outcome, cotinine-based light smoker/secondhand smoke exposure (HR 1.79, P=.10) and cotinine-based smoker status (HR 1.60, P=.06) were associated with a marginally higher risk of late RISI, though this did not reach statistical significance. Other similarly notable factors include hypofractionated breast RT (versus conventionally fractionated breast RT, HR 0.51, P=.03), and Asian/Pacific Islander race (versus white, HR 1.83, P=.06). The correlation between consecutive observations (6-month and 12-month) is 0.31.
Table 4.
Generalized Estimating Equation Regression of Moderate to Severe Acute and Late Skin Toxicity.
| Acute Skin Toxicity (ONS Grade 3+) | Late Skin Toxicity (LENT-SOMA Grade 2+) | |||||
|---|---|---|---|---|---|---|
| OR | (95% CI) | P | OR | (95% CI) | p | |
| Cotinine group (v. nonsmoker) | ||||||
| Light smoker/SHS | 1.13 | (0.82, 1.57) | 0.45 | 1.79 | (0.89, 3.60) | 0.10 |
| Smoker | 0.96 | (0.74, 1.24) | 0.75 | 1.60 | (0.98, 2.60) | 0.06 |
| Age >60 (v. <= 60) | 0.86 | (0.71, 1.03) | 0.10 | 0.91 | (0.62, 1.32) | 0.62 |
| Race (v. White) | ||||||
| Black | 1.29 | (1.06, 1.57) | 0.01 | 1.34 | (0.90, 1.99) | 0.15 |
| Asian Pacific Islander | 1.64 | (1.20, 2.25) | <0.005 | 1.83 | (0.98, 3.43) | 0.06 |
| Others | 1.41 | (0.96, 2.07) | 0.08 | 1.74 | (0.80, 3.80) | 0.16 |
| BMI group (v. normal) | ||||||
| Overweight | 1.18 | (0.88, 1.58) | 0.27 | 1.31 | (0.76, 2.24) | 0.33 |
| Obese | 1.45 | (1.08, 1.97) | 0.02 | 1.20 | (0.68, 2.14) | 0.53 |
| Chemotherapy | 0.92 | (0.75, 1.13) | 0.45 | 0.96 | (0.63, 1.46) | 0.86 |
| Radiation Treatment (v. CF Breast RT) | ||||||
| HF Breast RT | 0.40 | (0.26,0.61) | <0.0001 | 0.51 | (0.28,0.94) | 0.03 |
| CF Chest Wall | 1.38 | (1.02, 1.88) | 0.04 | 1.18 | (0.56, 2.50) | 0.66 |
| Regional Lymph Node RT | 1.09 | (0.82, 1.44) | 0.56 | 1.10 | (0.54, 2.24) | 0.80 |
| Diabetes | 1.04 | (0.82, 1.28) | 0.82 | 1.38 | (0.88, 2.17) | 0.16 |
| Number of comorbidities other than diabetes | ||||||
| 1 | 0.94 | (0.76, 1.15) | 0.54 | 0.98 | (0.65, 1.47) | 0.91 |
| >1 | 1.01 | (0.79, 1.30) | 0.92 | 1.09 | (0.63, 1.89) | 0.76 |
| Hormone therapy | 0.90 | (0.71, 1.14) | 0.39 | 0.87 | (0.54, 1.39) | 0.55 |
| Targeted therapy | 1.05 | (0.74, 1.47) | 0.79 | 1.19 | (0.65, 2.16) | 0.58 |
| Large/extra-large bra size | 1.46 | (1.18, 1.80) | <0.005 | 1.37 | (0.89, 2.10) | 0.15 |
CF, conventionally fractionated; HF, hypofractionated
Discussion
Tobacco smoke exposure remains a major public health issue in the United States that particularly affects underrepresented minorities and those in rural communities.33 While the health benefits of smoking cessation reach far beyond breast cancer outcomes and toxicity, mounting evidence suggests that active smokers experience increases in radiation toxicity such as acute dermatitis and late skin and soft tissue manifestations.12,34–36 In our patients, there were no significant differences in urinary cotinine by race, but light/SHS and smoker groups were more likely to be non-Hispanic than non-smokers. Interestingly, 50 of the 134 (37%) patients in the cotinine smoking group self-identified as never or former smokers, indicating that patients may tend to underestimate their own level of smoking in the context of clinical trial questionnaires. This highlights the importance of a urinary biomarker-based analysis of tobacco smoke exposure on breast cancer RISI.
Compared to nonsmokers, smokers are more likely to die of other comorbid diseases, but the association between smoking and breast cancer mortality is unclear.25,26 The increased all-cause and breast cancer-specific mortality risks in active or former smokers are likely even higher for those that are postmenopausal or obese at diagnosis. Additionally, continuing smokers have been found to experience significant increases in long-term risks of lung cancer and cardiac mortality after RT for breast cancer.37 A systematic review of outcomes after breast radiotherapy in smokers versus non-smokers found that 40% of the evaluated skin reaction endpoints across all studies (i.e. overall skin reaction, erythema, desquamation, edema, telangiectasia, breast pain, lymphedema, pigmentation changes, cosmetic outcomes, changes in breast shape, fibrosis, itching, and delayed healing) were significantly worse in patients with any history of smoking compared to non-smokers.8 Additionally, smokers are less likely to achieve full compliance with a course of physician-recommended radiotherapy (bivariate odds ratio = 0.20) which may, at least in part, be related to intolerance of the acute skin effects.38
As expected, ONS Acute Radiation skin reaction increased rapidly from baseline to end-RT and then decreased at 1-month and 2-month visits. Factors associated with acute RT skin reaction included race, obesity, chest wall RT target, conventionally fractionated (versus hypofractionated) RT, and large breast size. These are consistent with clinical practice and with prior reports.39 Hypofractionated RT is associated with decreased acute RISI, which is readily observed in clinical practice and has been described in the context of randomized trials.40 Interestingly, urinary cotinine smoking status was not associated with acute RISI. This finding is consistent with prior reports of hypofractionated breast RT and mimics a pattern also seen in lung cancer.41 Neither chemotherapy nor hormonal therapy were associated with acute or late RISI in this modern cohort of breast cancer patients.42 Other factors that were associated with late severe toxicity included hypofractionated breast RT (compared to conventionally fractionated breast RT), which may be related to the timing of “late” RISI assessments at 6 and 12 months designed in this protocol. Long-term late skin and subcutaneous effects have been demonstrated at 5- and 10-year follow-up to be not significantly different between conventionally fractionated and hypofractionated breast RT.43
Late RISI (defined by the LENT-SOMA criteria) in this study manifested predominantly as hyperpigmentation, fibrosis, and pain. These are just a few of the potential late skin toxicities that may develop after breast cancer RT. In this multicenter, multiethnic study, tobacco exposure categorized by urinary cotinine group was significantly associated with moderate to severe late RISI on bivariate ANOVA. However, this association was not observed upon multivariable analysis, which found the odds ratio for LENT-SOMA Grade 2+ late RISI to be 1.79 for light smokers/SHS (p = 0.10) and 1.60 for smokers (p = 0.06). We identified a signal indicating higher rates of late RISI in those with higher urinary cotinine levels. It is possible that with more extended follow-up, these trends would continue. In addition to the numerous overall health benefits of smoking cessation, attention to smoking status, use of hypofractionated RT, and its associated lower total RT doses would likely reduce the risk of late RISI in breast cancer patients treated with RT.
The mechanism of the proposed association between radiation normal tissue injury and smoking is not well defined. It may be related to impaired wound healing due to vascular effects and impairment of normal fibroblast function that is critical for tissue repair and remodeling 44. An increase in the inflammatory state as measured by C-reactive protein was associated with increased severe skin toxicity in this patient cohort, which may contribute to wound-healing difficulty further exacerbated by tobacco smoke exposure.9 We hypothesize that cigarette smoke exposure induces tissue hypoxia,45 lowers plasma total antioxidants,46 increases DNA damage, lowers DNA repair and wound healing capacity,47,48 all of which contribute to adverse acute and late normal tissue responses in patients receiving radiotherapy for breast cancer. For acute effects, it is possible that generalized tissue hypoxia associated with smoking reduces the potential for the generation of reactive oxygen species, and thus, the damaging effects are lessened. In lung cancer patients treated with RT, active smokers during treatment have a lower risk of radiation pneumonitis, thought to be secondary to either local hypoxia or possibly immunosuppressive effects of RT.41,49 Late RISI, which frequently manifests as cutaneous/subcutaneous fibrosis, pigmentation changes, atrophy, and telangiectasia, is likely a result of chronic vascular damage to endothelial cells in the irradiated region causing an overall loss of blood vessels and aberrant neovascularization.50,51 Considering the well-established negative effects of tobacco smoke on vascular health, smoking likely contributes to late RISI through potentiation of chronic RT-induced arterial damage.52 The interaction between RT-induced upregulation of inflammatory cytokines such as transforming growth factor beta (TGF-B) or interleukin 1 is less understood, though dysregulation of TGF-B is implicated in the molecular basis of late RISI as well as smoking-related premature aging.53,54
Another notable finding in this study is the association between race and increased risks of acute and late RISI. Multivariable analysis identified that non-white patients (Black, Asian Pacific Islander, and others) had a significantly increased risk of moderate to severe acute RISI. Additionally, the Asian/Pacific Islander race was associated with a trend toward increased risk of moderate to severe late RISI. Many current scoring systems for radiation skin reactions do not include hyperpigmentation, a common manifestation of RISI in patients of color. These findings are consistent with prior reports and highlight a need for further study to increase the generalizability and applicability of clinician-rated outcome measures of RISI.55 Efforts are needed to refine these scoring systems to include terminology that accurately reflects RISI in patients of all skin types in order to ensure equitable measurement and reporting of RISI across a diverse patient population.56
Our findings, in addition to the multitude of health benefits to smoking cessation, highlight an important opportunity to improve the quality of holistic care for breast cancer patients. In 2011, the National Cancer Institute outlined four primary methods to improve tobacco dependence care: forming a consensus regarding smoking status assessments, engaging with electronic medical record stakeholders to improve referral rates, expanding treatments for cancer patients with tobacco dependence, and developing methods to overcome barriers to successful cessation.57 Promising models that have been employed previously include multidisciplinary engagement, standardized screening procedures (ideally though the electronic medical record), qualified treatment professionals as well as oncology staff, and monitoring of outcomes for further quality improvement activities. A combination of behavioral therapy and pharmacotherapy is most effective at achieving cessation.58 This therapy may be most efficiently delivered through dedicated tobacco cessation programs, which have been adopted by numerous institutions and have been found to be cost-effective.59,60 Another opportunity to improve upon tobacco cessation initiatives prior to the diagnosis of cancer is in the secondary prevention setting. Many institutions pair tobacco cessation screening and therapeutic interventions with lung cancer screening for patients at high risk of lung cancer.61 The ubiquity of routine breast cancer screening provides an additional avenue to identify current smokers for cessation interventions.62 Ultimately, improvements in all aspects of smoking cessation efforts—patient identification, multidisciplinary therapy, resource allocation to minimize barriers and maximize adherence, and further quality improvement initiatives—will be required to further decrease the burden of smoking on oncology patients. Unfortunately, there are no known methodologies for the treatment or prevention of acute or late RISI related to smoking. Generally, dermatologic care during RT is comprised of routine gentle washing of the area with a mild soap and daily application of emollient moisturizers, aloe and/or topical corticosteroids.63–66 These interventions are not known to have an effect on smoking-mediated RISI, and prevention of this potential complication through smoking cessation is likely the most effective means of treatment.
This study is a secondary analysis of a prospective observational study with breast RT skin reaction as a primary outcome measure. It was not powered specifically to evaluate urinary biomarkers of tobacco exposure and their association with acute and late RT toxicity. Additionally, a limited number of severe toxicity events limits our statistical power. Limited follow-up for late toxicity (6- and 12-month assessments) in the present study also limits our ability to detect more slowly developing late RISI. These findings are limited to hypothesis generation and require validation, ideally in other large, prospective studies.
Conclusion
We identified factors associated with acute and late skin toxicity in patients treated with postoperative RT for breast cancer. Though there were no statistically significant differences in acute or late RISI by tobacco exposure as quantified by urinary cotinine, there was a signal toward worse late RISI in patients defined by urinary cotinine as light/SHS or smokers, which may persist or worsen with longer follow-up. Smoking cessation prior to breast RT is strongly recommended to reduce the risk of late RISI. Future attention to urinary biomarkers of tobacco exposure in clinical trials may facilitate further the quantifiable assessment of the effect of tobacco exposure on RT toxicity.
Clinical Practice Points
Exposure to tobacco smoke may impact tissue healing after cancer treatment. In patients with breast cancer, the impact of smoking during breast RT on acute and late radiation-induced skin injury (RISI) is unclear. The utility of a urinary biomarker of smoking (urinary cotinine, UCot) for quantitative assessment of tobacco exposure and its effects on RT toxicity also requires further exploration. We analyzed a prospective study that assessed RISI in a multiracial, multiethnic cohort of women treated with breast or chest wall RT. Univariate analysis identified higher rates of moderate to severe late RISI in the groups of patients with elevated UCot. This did persist on multivariable analysis, and hypofractionated breast RT was the only factor associated with late RISI. No association between UCot and acute RISI was observed; factors that were associated with acute RISI included race, obesity, chest wall RT, and breast size. These novel findings associate a urinary biomarker of tobacco exposure with long-term RT skin and soft tissue injury and provide additional evidence on the negative effects of smoking during breast RT. Patients should be counseled regarding these effects and smoking cessation prior to the start of breast RT should be encouraged. Further study is warranted to better understand the clinical utility of UCot testing to estimate the risk of late RISI in patients treated with breast RT.
Supplementary Material
Acknowledgments
We would like to acknowledge the following NCORP sites and PIs for their participation: Puerto Rico Minority Underserved NCORP, Southeast Clinical Oncology Research Consortium NCORP, Delaware/Christiana Care NCORP, Ozarks NCORP, Wake Forest NCORP Research Base, Upstate Carolina Consortium Community Oncology Research Program, Northwell Health NCORP, Metro Minnesota Community Oncology Research Consortium, Stroger Hospital of Cook County Minority Underserved NCORP, Wichita NCORP, Cancer Research of Wisconsin and Northern Michigan Consortium, Gulf South Minority Underserved NCORP, Wisconsin NCORP, Catholic Health Initiatives NCORP, NCORP of the Carolinas (Greenville Health System NCORP), Georgia Cares Minority Underserved NCORP, Heartland Cancer Research NCORP, Western States Cancer Research NCORP, and Iowa-Wide Oncology Research Coalition NCORP. Additionally, we would like to thank Wake Forest NCORP Research Base staff members Karen Craver, Emily V. Dressler, and Cheyenne Wagi for their efforts on behalf of this study and study participants.
Funding
This work was supported by National Cancer Institute Grants No. R01CA135288 (Jennifer Hu and James Urbanic), R03CA195643 (Jennifer Hu), U10CA081857 to the Wake Forest Research-Based CCOP (Edward Shaw, Glenn Lesser), and UG1CA189824 to the Wake Forest NCORP Research Base (Ryan Hughes, Kathryn Weaver, Glenn Lesser).
Footnotes
Ethics
The Wake Forest University IRB approved this study (IRB00011809) on 09/20/2010.
Data Availability
The data generated in this study are not publicly available due to information that could compromise patient privacy or consent but are available upon reasonable request from the corresponding author.
Disclosure
None
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.clbc.2022.09.003.
References
- 1.Early Breast Cancer Trialists’ Collaborative G, Darby S, McGale P, et al. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10 801 women in 17 randomised trials. The Lancet 2011;378(9804):1707–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002;347(16):1233–1241. [DOI] [PubMed] [Google Scholar]
- 3.Overgaard M, Hansen PS, Overgaard J, et al. Postoperative radiotherapy in high-risk premenopausal women with breast cancer who receive adjuvant chemotherapy. Danish Breast Cancer Cooperative Group 82b Trial. N Engl J Med 1997;337(14):949–955. [DOI] [PubMed] [Google Scholar]
- 4.Overgaard M, Jensen MB, Overgaard J, et al. Postoperative radiotherapy in high-risk postmenopausal breast-cancer patients given adjuvant tamoxifen: Danish Breast Cancer Cooperative Group DBCG 82c randomised trial. Lancet 1999;353(9165):1641–1648. [DOI] [PubMed] [Google Scholar]
- 5.Ragaz J, Olivotto IA, Spinelli JJ, et al. Locoregional radiation therapy in patients with high-risk breast cancer receiving adjuvant chemotherapy: 20-year results of the British Columbia randomized trial. J Natl Cancer Inst 2005;97(2):116–126. [DOI] [PubMed] [Google Scholar]
- 6.Creamer MR, Wang TW, Babb S, et al. Tobacco Product Use and Cessation Indicators Among Adults — United States, 2018. MMWR Morb Mortal Wkly Rep 2019;68:1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsai J, Homa DM, Gentzke AS, et al. Exposure to Secondhand Smoke Among Nonsmokers — United States, 1988–2014. MMWR Morb Mortal Wkly Rep 2018;67:1342–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wong G, Lam E, Karam I, et al. The impact of smoking on adjuvant breast cancer radiation treatment: A systematic review. Cancer Treatment and Research Communications 2020;24. [DOI] [PubMed]
- 9.Hu JJ, Urbanic JJ, Case LD, et al. Association Between Inflammatory Biomarker C-Reactive Protein and Radiotherapy-Induced Early Adverse Skin Reactions in a Multiracial/Ethnic Breast Cancer Population. Journal of Clinical Oncology 2018;36(24):2473–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee E, Takita C, Wright JL, et al. Genome-wide enriched pathway analysis of acute post-radiotherapy pain in breast cancer patients: a prospective cohort study. Human Genomics 2019;13(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burnett LR, Hughes RT, Rejeski AF, et al. Review of the terminology describing ionizing radiation-induced skin injury: a case for standardization. Technology in Cancer Research & Treatment 2021. [DOI] [PMC free article] [PubMed]
- 12.Lilla C, Ambrosone CB, Kropp S, et al. Predictive factors for late normal tissue complications following radiotherapy for breast cancer. Breast Cancer Res Treat 2007;106(1):143–150. [DOI] [PubMed] [Google Scholar]
- 13.Spierer MM, Hong LX, Wagman RT, Katz MS, Spierer RL, McCormick B. Postmastectomy CT-based electron beam radiotherapy: dosimetry, efficacy, and toxicity in 118 patients. Int J Radiat Oncol Biol Phys 2004;60(4):1182–1189. [DOI] [PubMed] [Google Scholar]
- 14.Hehr T, Budach W, Paulsen F, Gromoll C, Christ G, Bamberg M. Evaluation of predictive factors for local tumour control after electron-beam-rotation irradiation of the chest wall in locally advanced breast cancer. Radiotherapy and Oncology 1999;50(3):283–290. [DOI] [PubMed] [Google Scholar]
- 15.Pignol JP, Olivotto I, Rakovitch E, et al. A multicenter randomized trial of breast intensity-modulated radiation therapy to reduce acute radiation dermatitis. J Clin Oncol 2008;26(13):2085–2092. [DOI] [PubMed] [Google Scholar]
- 16.Collette S, Collette L, Budiharto T, et al. Predictors of the risk of fibrosis at 10 years after breast conserving therapy for early breast cancer: a study based on the EORTC Trial 22881–10882 ’boost versus no boost’. Eur J Cancer 2008;44(17):2587–2599. [DOI] [PubMed] [Google Scholar]
- 17.Vicini F, Cecchini R, White J, et al. Abstract GS4–04: Primary results of NSABP B-39/RTOG 0413 (NRG Oncology): A randomized phase III study of conventional whole breast irradiation (WBI) versus partial breast irradiation (PBI) for women with stage 0, I, or II breast cancer. Cancer Research 2019;79(4) SupplementGS4-04-GS04–04. [Google Scholar]
- 18.Shaitelman SF, Lei X, Thompson A, et al. Three-Year Outcomes With Hypofractionated Versus Conventionally Fractionated Whole-Breast Irradiation: Results of a Randomized, Noninferiority Clinical Trial. Journal of Clinical Oncology 2018;36(35):3495–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hecht SS. Carcinogen derived biomarkers: applications in studies of human exposure to secondhand tobacco smoke. Tob Control 2004;13(1):i48–i56 SupplSuppl 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jarvis MJ, Russell MA, Benowitz NL, Feyerabend C. Elimination of cotinine from body fluids: implications for noninvasive measurement of tobacco smoke exposure. Am J Public Health 1988;78(6):696–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benowitz NL. Cotinine as a biomarker of environmental tobacco smoke exposure. Epidemiol Rev 1996;18(2):188–204. [DOI] [PubMed] [Google Scholar]
- 22.CDC. US Department of Health and Human Services C. Fourth national report on human exposure to environmental chemicals Atlanta, GA: US Department of Health and Human Services, CDC; 2015. [Google Scholar]
- 23.NHANES 2013–2014 laboratory methods. US Department of Health and Human Services, CDC National Center for Health Statistics; 2013. https://wwwn.cdc.gov/nchs/nhanes/continuousnhanes/labmethods.aspx?BeginYear=2013. [Google Scholar]
- 24.CDC. Laboratory Procedure Manual: Cotinine and Hydroxycotinine (Total) US Department of Health and Human Services C; 2018. ed. [Google Scholar]
- 25.Fentiman IS, Allen DS, Hamed H. Smoking and prognosis in women with breast cancer. International Journal of Clinical Practice 2005;59(9):1051–1054. [DOI] [PubMed] [Google Scholar]
- 26.Holmes MD, Murin S, Chen WY, Kroenke CH, Spiegelman D, Colditz GA. Smoking and survival after breast cancer diagnosis. Int J Cancer 2007;120(12):2672–2677. [DOI] [PubMed] [Google Scholar]
- 27.Pierce JP, Patterson RE, Senger CM, et al. Lifetime cigarette smoking and breast cancer prognosis in the After Breast Cancer Pooling Project. J Natl Cancer Inst 2014;106(1) djt359–djt359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinberger E, Kollmeier M, McBride S, Novak C, Pei X, Zelefsky MJ. Cigarette smoking during external beam radiation therapy for prostate cancer is associated with an increased risk of prostate cancer-specific mortality and treatment-related toxicity. BJU International 2015;116(4):596–603. [DOI] [PubMed] [Google Scholar]
- 29.Chen AM, Chen LM, Vaughan A, et al. Tobacco Smoking During Radiation Therapy for Head-and-Neck Cancer Is Associated With Unfavorable Outcome. International Journal of Radiation Oncology, Biology, Physics 2011;79(2):414–419. [DOI] [PubMed] [Google Scholar]
- 30.Browman GP, Wong G, Hodson I, et al. Influence of cigarette smoking on the efficacy of radiation therapy in head and neck cancer. N Engl J Med 1993;328(3):159–163. [DOI] [PubMed] [Google Scholar]
- 31.Kunutsor Setor K, Spee Julia M, Kieneker Lyanne M, et al. Self-Reported Smoking, Urine Cotinine, and Risk of Cardiovascular Disease: Findings From the PREVEND (Prevention of Renal and Vascular End-Stage Disease) Prospective Cohort Study. Journal of the American Heart Association 2022;7(10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wells AJ, English PB, Posner SF, Wagenknecht LE, Perez-Stable EJ. Misclassification rates for current smokers misclassified as nonsmokers. American Journal of Public Health 1998;88(10):1503–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nighbor TD, Doogan NJ, Roberts ME, et al. Smoking prevalence and trends among a U.S. national sample of women of reproductive age in rural versus urban settings. PLOS ONE 2018;13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pignol J-P, Vu TTT, Mitera G, Bosnic S, Verkooijen HM, Truong P. Prospective Evaluation of Severe Skin Toxicity and Pain During Postmastectomy Radiation Therapy. International Journal of Radiation Oncology∗Biology∗Physics. 2015;91(1):157–164. [DOI] [PubMed] [Google Scholar]
- 35.Sharp L, Johansson H, Hatschek T, Bergenmar M. Smoking as an independent risk factor for severe skin reactions due to adjuvant radiotherapy for breast cancer. The Breast 2013;22(5):634–638. [DOI] [PubMed] [Google Scholar]
- 36.Kelemen G, Varga Z, Lázár G, Thurzó L, Kahán Z. Cosmetic Outcome 1–5 Years After Breast Conservative Surgery, Irradiation and Systemic Therapy. Pathology & Oncology Research 2012;18(2):421–427. [DOI] [PubMed] [Google Scholar]
- 37.Taylor C, Correa C, Duane FK, et al. Estimating the Risks of Breast Cancer Radiotherapy: Evidence From Modern Radiation Doses to the Lungs and Heart and From Previous Randomized Trials. J Clin Oncol 2017;35(15):1641–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Komenaka IK, Hsu C-H, Martinez ME, et al. Preoperative Chemotherapy for Operable Breast Cancer Is Associated with Better Compliance with Adjuvant Therapy in Matched Stage II and IIIA Patients. The Oncologist 2011;16(6):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johansen J, Overgaard J, Rose C, et al. Cosmetic outcome and breast morbidity in breast-conserving treatment–results from the Danish DBCG-82TM national randomized trial in breast cancer. Acta Oncol 2002;41(4):369–380. [DOI] [PubMed] [Google Scholar]
- 40.Arsenault J, Parpia S, Goldberg M, et al. Acute Toxicity and Quality of Life of Hypofractionated Radiation Therapy for Breast Cancer. International Journal of Radiation Oncology∗Biology∗Physics. 2020;107(5):943–948. [DOI] [PubMed] [Google Scholar]
- 41.McFarlane MR, Hochstedler KA, Laucis AM, et al. Predictors of Pneumonitis After Conventionally Fractionated Radiotherapy for Locally Advanced Lung Cancer. International Journal of Radiation Oncology, Biology, Physics 2021;111(5):1176–1185. [DOI] [PubMed] [Google Scholar]
- 42.Johansen J, Overgaard J, Overgaard M. Effect of adjuvant systemic treatment on cosmetic outcome and late normal-tissue reactions after breast conservation. Acta Oncol 2007;46(4):525–533. [DOI] [PubMed] [Google Scholar]
- 43.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med 2010;362(6):513–520. [DOI] [PubMed] [Google Scholar]
- 44.Wong LS, Martins-Green M. Firsthand cigarette smoke alters fibroblast migration and survival: implications for impaired healing. Wound Repair and Regeneration 2004;12(4):471–484. [DOI] [PubMed] [Google Scholar]
- 45.Jensen JA, Goodson WH, Hopf HW, Hunt TK. Cigarette smoking decreases tissue oxygen. Arch Surg 1991;126(9):1131–1134. [DOI] [PubMed] [Google Scholar]
- 46.Astori E, Garavaglia ML, Colombo G, et al. Antioxidants in Smokers. Nutr Res Rev 2021:1–77. [DOI] [PubMed]
- 47.Prantl L, Moellhoff N, Fritschen UV, et al. Impact of Smoking Status in Free Deep Inferior Epigastric Artery Perforator Flap Breast Reconstruction: A Multicenter Study. J Reconstr Microsurg 2020;36(9):694–702. [DOI] [PubMed] [Google Scholar]
- 48.Sørensen LT, Hørby J, Friis E, Pilsgaard B, Jørgensen T. Smoking as a risk factor for wound healing and infection in breast cancer surgery. Eur J Surg Oncol 2002;28(8):815–820. [DOI] [PubMed] [Google Scholar]
- 49.Blanchet MR, Israël-Assayag E, Cormier Y. Inhibitory effect of nicotine on experimental hypersensitivity pneumonitis in vivo and in vitro. Am J Respir Crit Care Med 2004;169(8):903–909. [DOI] [PubMed] [Google Scholar]
- 50.Archambeau JO, Pezner R, Wasserman T. Pathophysiology of irradiated skin and breast. International Journal of Radiation Oncology∗Biology∗Physics. 1995;31(5):1171–1185. [DOI] [PubMed] [Google Scholar]
- 51.Brush J, Lipnick SL, Phillips T, Sitko J, McDonald JT, McBride WH. Molecular Mechanisms of Late Normal Tissue Injury. Seminars in Radiation Oncology 2007;17(2):121–130. [DOI] [PubMed] [Google Scholar]
- 52.Powell JT. Vascular damage from smoking: disease mechanisms at the arterial wall. Vasc Med 1998;3(1):21–28. [DOI] [PubMed] [Google Scholar]
- 53.Rodemann HP, Bamberg M. Cellular basis of radiation-induced fibrosis. Radiotherapy and Oncology 1995;35(2):83–90. [DOI] [PubMed] [Google Scholar]
- 54.Morita A, Torii K, Maeda A, Yamaguchi Y. Molecular Basis of Tobacco Smoke-Induced Premature Skin Aging. Journal of Investigative Dermatology Symposium Proceedings 2009;14(1):53–55. [DOI] [PubMed] [Google Scholar]
- 55.Wright JL, Takita C, Reis IM, et al. Prospective evaluation of radiation-induced skin toxicity in a race/ethnically diverse breast cancer population. Cancer medicine 2016;5(3):454–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shumway DA, Kapadia N, Walker EM, et al. Development of an Illustrated Scale for Acute Radiation Dermatitis in Breast Cancer Patients. Practical Radiation Oncology 2021;11(3):168–176. [DOI] [PubMed] [Google Scholar]
- 57.Morgan G, Schnoll RA, Alfano CM, et al. National Cancer Institute Conference on Treating Tobacco Dependence at Cancer Centers. Journal of Oncology Practice 2011;7(3):178–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stead LF, Koilpillai P, Fanshawe TR, Lancaster T. Combined pharmacotherapy and behavioural interventions for smoking cessation. Cochrane Database Syst Rev 2016;3. [DOI] [PMC free article] [PubMed]
- 59.Evans WK, Truscott R, Cameron E, et al. Implementing smoking cessation within cancer treatment centres and potential economic impacts. Translational Lung Cancer Research 2019:S11–S20. [DOI] [PMC free article] [PubMed]
- 60.Slatore CG, Au DH, Hollingworth W. Cost-effectiveness of a smoking cessation program implemented at the time of surgery for lung cancer. J Thorac Oncol 2009;4(4):499–504. [DOI] [PubMed] [Google Scholar]
- 61.Bellinger C, Foley KL, Dressler EV, et al. Organizational Characteristics and Smoking Cessation Support in Community-Based Lung Cancer Screening Programs. J Am Coll Radiol 2022;19(4):529–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wang GX, Narayan AK, Park ER, Lehman CD, Gorenstein JT, Flores EJ. Screening Mammography Visits as Opportunities to Engage Smokers With Tobacco Cessation Services and Lung Cancer Screening. J Am Coll Radiol 2020;17(5):606–612. [DOI] [PubMed] [Google Scholar]
- 63.Gosselin T, Ginex PK, Backler C, et al. ONS Guidelines™ for Cancer Treatment-Related Radiodermatitis. Oncol Nurs Forum 2020;47(6):654–670. [DOI] [PubMed] [Google Scholar]
- 64.Ginex PK, Backler C, Croson E, et al. Radiodermatitis in Patients With Cancer: Systematic Review and Meta-Analysis. Oncol Nurs Forum 2020;47(6):E225–e236. [DOI] [PubMed] [Google Scholar]
- 65.Wong RK, Bensadoun RJ, Boers-Doets CB, et al. Clinical practice guidelines for the prevention and treatment of acute and late radiation reactions from the MASCC Skin Toxicity Study Group. Support Care Cancer 2013;21(10):2933–2948. [DOI] [PubMed] [Google Scholar]
- 66.Ho AY, Olm-Shipman M, Zhang Z, et al. A Randomized Trial of Mometasone Furoate 0.1% to Reduce High-Grade Acute Radiation Dermatitis in Breast Cancer Patients Receiving Postmastectomy Radiation. Int J Radiat Oncol Biol Phys 2018;101(2):325–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


