Abstract
Viral infections are a common cause of encephalitis. This study investigated the relationship between the incidence of encephalitis and that of respiratory and enteric viral infections in all age groups from 2015 to 2019, using the Health Insurance Review and Assessment (HIRA) Open Access Big Data Platform. We identified monthly incidence patterns and seasonal trends using the autoregressive integrated moving average (ARIMA). The Granger causality test was used to analyze correlations between encephalitis incidence and the positive detection rate (PDR) at 1-month intervals. A total of 42,775 patients were diagnosed with encephalitis during the study period. The incidence of encephalitis was highest in the winter (26.8%). The PDRs for respiratory syncytial virus (HRSV) and coronavirus (HCoV) were associated with the trend in encephalitis diagnosis in all age groups, with a 1-month lag period. In addition, an association with norovirus was observed in patients aged over 20 years, and with influenza virus (IFV) in patients aged over 60 years. This study found that HRSV, HCoV, IFV, and norovirus tended to precede encephalitis by 1 month. Further research is required to confirm the association between these viruses and encephalitis.
Keywords: encephalitis, respiratory syncytial virus, coronavirus, influenza virus, norovirus
1. Introduction
Encephalitis is inflammation of the brain parenchyma that causes serious neurological dysfunction in both adults and children. There are numerous causes of encephalitis. Among them, the most common are viral infections such as herpes simplex virus (HSV) or autoimmune responses such as N-methyl D-aspartate receptor antibodies [1]. It presents with fever, headache, seizures, and an altered mental state. For the diagnosis of encephalitis, there must be altered consciousness persisting for longer than 24 h, evidence of central nervous system (CNS) inflammation, and at least two of the following characteristic clinical findings: (1) fever; (2) seizures or focal neurological findings attributable to the brain parenchyma; (3) CSF pleocytosis (more than four white blood cells per µL); (4) EEG findings suggestive of encephalitis; and (5) neuroimaging findings suggestive of encephalitis [2]. Encephalitis is generally more common in children, but the epidemiology of each type varies according to age, country, season, viral genetic mutations, and the patients’ immune status [3].
Studies regarding the association between encephalitis and common viruses in Korea are scarce. Thus, we aimed to analyze public health data provided by the Health Insurance Review and Assessment (HIRA) Open Access Big Data Platform and the Korea Disease Control and Prevention Agency (KDCA) to determine the relationship between the incidence of respiratory and gastrointestinal viral infections and encephalitis.
2. Materials and Methods
2.1. Study Population
We extracted data regarding encephalitis from the HIRA, which is a government-affiliated organization created to build an accurate claims review and quality assessment system for the National Health Insurance, with databases open to all academic investigators [4,5,6]. The claims data in the HIRA database include patient diagnosis, treatment, procedures, surgical history, and prescription drugs, and serve as a valuable resource for healthcare service research. We studied the HIRA data of patients with encephalitis (A85, A850, A851, A852, A858, A86, G04, G040, G041, G042, G048, G049, G05, G050, G051, G052, and G058), including a total of 42,775 incident encephalitis cases reported between 1 January 2015 and 31 December 2019.
2.2. Viral Surveillance Data
We used the data reported by the KDCA on viruses that cause acute respiratory infections and gastroenteritis, including data on more than 4000 respiratory and 2000 enteric specimens collected from 17 local environmental and health institutes and over 100 participating hospitals across Korea during each year of the study period. The causative pathogens were then identified using standardized diagnostic procedures in a central laboratory, in which the pathogen prevalence was surveyed weekly and analyzed based on genetic testing of samples from patients with influenza-like illness or acute diarrhea. The positive detection rate (PDR) data were collected from 2015 to 2019 by calculating the average monthly PDRs of seven respiratory viruses (adenovirus [HAdV], parainfluenza virus [HPIV], respiratory syncytial virus [HRSV], influenza virus [IFV], coronavirus [HCoV], rhinovirus [HRV], and bocavirus [HBoV]) and four acute diarrhea viruses (HAdV, rotavirus, norovirus, and astrovirus).
2.3. Statistical Analysis
For the incidence rate calculations, we used the 2015 Korean population data, reported by the Ministry of the Interior and Safety, as the denominator, with a total population of 51,529,338. We then constructed a model of variations in encephalitis diagnosis using the autoregressive integrated moving average (ARIMA) modeling approach, which assumes that the current observation is related to past observations over time as previously analyzed. The general multiplicative form of the ARIMA model was denoted as (p, d, q), where p, d, and q were the order values of the non-seasonal autoregressive, differencing, and moving-average parameters, respectively. Additionally, the autocorrelation function (ACF) was examined to identify the general form of the model to fit. Considering the ACF graphs, different ARIMA models were identified for model selection (Supplementary Figure S1), and the minimum Akaike information criterion model was chosen as the best-fit model (Supplementary Table S1). Moreover, the Granger approach was used to investigate the number of current values in the time series y that could be described as other values [7,8,9]. The data were analyzed using R software (R Foundation for Statistical Computing, Vienna, Austria), and significance was defined as p < 0.05.
The annual incidence was determined using the number of individuals with encephalitis as the numerator and the annual Korean population based on the HIRA database as the denominator, multiplied by the corresponding age population in 2015. The annual incidence rate of encephalitis was standardized to the 2015 population. Relative risk was calculated by dividing the overall incidence of each age group by the overall incidence of the 20–39 age group.
3. Results
3.1. Patient Characteristics
During the 5-year period, 42,775 patients were diagnosed with encephalitis (Table 1). Of these, 7710 (18.0%) were aged 0–9 years, 4224 (9.9%) were aged 10–19 years, 8890 (20.8%) were aged 20–39 years, 11,233 (26.3%) were aged 40–59 years, and 10,718 (25.1%) were aged 60 years and over. A total of 20,760 (48.5%) male and 22,015 (51.5%) female patients (M:F ratio = 1:1.06) were included in the study.
Table 1.
Characteristics of patients.
| Variables | N (%) | |
|---|---|---|
| Total number of patients | 42,775 (100.0) | |
| Age group | ||
| 0–9.99 years | 7710 (18.0) | |
| 10~19.99 years | 4224 (9.9) | |
| 20~39.99 years | 8890 (20.8) | |
| 40–59.99 years | 11,233 (26.3) | |
| ≥60 years | 10,718 (25.1) | |
| Sex | ||
| Male | 20,760 (48.5) | |
| Female | 22,015 (51.5) | |
| Location | ||
| Seoul | 11,116 (26.0) | |
| Busan | 3225 (7.5) | |
| Incheon | 1927 (4.5) | |
| Daegu | 1567 (3.7) | |
| Gwangju | 2698 (6.3) | |
| Daejeon | 1077 (2.5) | |
| Ulsan | 582 (1.4) | |
| Gyeonggi | 8656 (20.2) | |
| Gangwon | 1930 (4.5) | |
| Chungbuk | 706 (1.7) | |
| Chungnam | 1492 (3.5) | |
| Jeonbuk | 1594 (3.7) | |
| Jeonnam | 911 (2.1) | |
| Gyeongbuk | 1798 (4.2) | |
| Gyeongnam | 2660 (6.2) | |
| Jeju | 708 (1.7) | |
| Sejong | 128 (0.3) | |
| Insurance type | ||
| Medical insurance | 40,839 (95.5) | |
| Medical aid | 1918 (4.5) | |
| Free | 18 (0.0) |
The incidence rate of encephalitis was highest in the 0–9-years age group (35.1/100,000 person-years). The encephalitis incidence rate in the 0–9-years age group was 2.79-fold higher than that in the 20–39-years age group. The incidence rate per year was lowest in 2015 (7.5/100,000 person-years) (Table 2).
Table 2.
Incidence rate of encephalitis by age group.
| Age Group (Years) |
Annual Incidence * (Annual Incidence Rate) ** | Overall Incidence Rate |
Relative Risk *** |
||||
|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | |||
| 0–9 | 818 (17.8) | 2047 (44.5) | 1375 (29.9) | 1779 (38.7) | 2067 (44.9) | 35.1 | 2.79 |
| 10–19 | 331 (5.8) | 1148 (20.1) | 773 (13.5) | 1230 (21.5) | 1119 (19.6) | 16.1 | 1.28 |
| 20–39 | 673 (4.7) | 1894 (13.2) | 1463 (10.2) | 2554 (17.8) | 2468 (17.2) | 12.6 | 1.00 |
| 40–59 | 989 (5.8) | 2039 (11.9) | 2095 (12.2) | 3226 (18.8) | 2916 (17.0) | 13.1 | 1.04 |
| ≥60 | 1054 (10.9) | 1737 (18.0) | 1919 (19.9) | 2377 (24.6) | 2429 (25.2) | 19.7 | 1.56 |
| Total | 3865 (7.5) | 8865 (17.2) | 7625 (14.7) | 11,166 (21.5) | 10,999 (21.2) | 16.4 | 1.30 |
* Total number of patients by year; ** All rates are per 100,000 population, directly age-adjusted to the 2015 population; *** Relative risk compared with that of the 20–39 years age group.
3.2. Encephalitis Trend Analysis
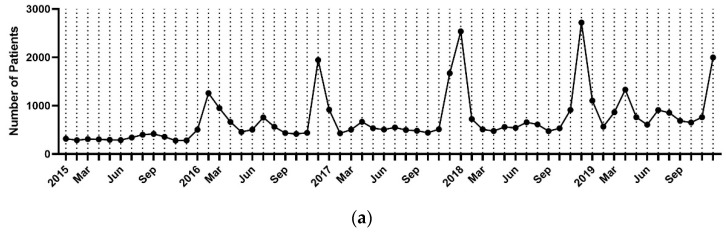
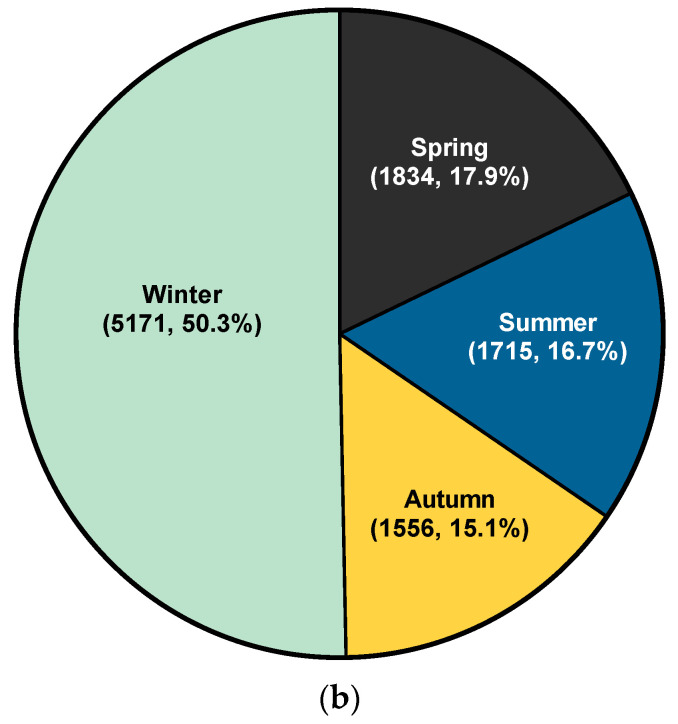
Of the 42,775 patients of all ages, 3865 were diagnosed in 2015, 8883 in 2016, 7702 in 2017, 11,239 in 2018, and 11,086 in 2019 (Table 3). In 2015, the incidence rate was highest in September, and in 2016–2019, it was highest in December (Figure 1a). Overall, the cumulative number of cases per month over the 5-year study period was highest in December and lowest in October. The average number of cases per month was 540, and the average number of cases per year between 2015 and 2019 was 71,341. Additionally, encephalitis was most often diagnosed during winter (50.3%), followed by spring (17.8%), summer (16.7%), and autumn (15.1%) (Figure 1b).
Table 3.
Monthly numbers of newly diagnosed encephalitis patients in Korea.
| Year | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Total | Average |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 316 | 285 | 308 | 304 | 294 | 288 | 341 | 399 | 415 | 357 | 278 | 280 | 3865 | 322 |
| 2016 | 503 | 1259 | 948 | 664 | 456 | 503 | 756 | 564 | 433 | 415 | 439 | 1943 | 8883 | 740 |
| 2017 | 917 | 428 | 503 | 665 | 533 | 506 | 549 | 496 | 481 | 442 | 510 | 1672 | 7702 | 642 |
| 2018 | 2535 | 723 | 508 | 476 | 559 | 540 | 656 | 610 | 473 | 530 | 911 | 2718 | 11,239 | 937 |
| 2019 | 1104 | 565 | 864 | 1330 | 760 | 606 | 909 | 854 | 685 | 651 | 761 | 1997 | 11,086 | 924 |
| Total | 5375 | 3260 | 3131 | 3439 | 2602 | 2443 | 3211 | 2923 | 2487 | 2395 | 2899 | 8610 | 42,775 | 3565 |
| Average | 946 | 513 | 488 | 512 | 447 | 466 | 550 | 491 | 391 | 362 | 403 | 917 | 71,341 | 540 |
Figure 1.
(a) Monthly trend analysis of encephalitis from 2015 to 2019. (b) Seasonal trend analysis of encephalitis incidence. Spring (March to May), summer (June to August), autumn (September to November), and winter (December to February). Cumulative incidence for 5 years, %.
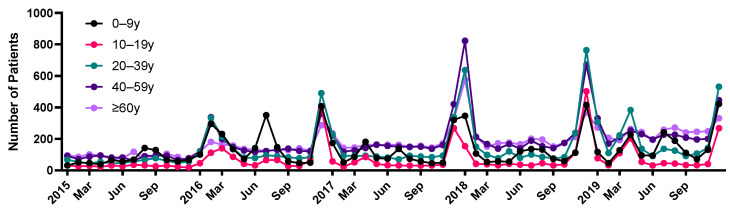
In addition, the seasonal trends in the incidence rate were similar in all age groups, increasing in the winter (Figure 2).
Figure 2.
Monthly trend analysis of encephalitis according to age.
3.3. Viral Positive Detection Rates
The PDRs of most viruses showed seasonal variation (Supplementary Table S2, Figures S2 and S3). Specifically, the PDR of HAdV was highest from August to November; the PDR of HPIV was highest in May; the PDR of HBoV was highest in May and June; and the PDR of HRSV was highest in November. Furthermore, the PDR of HCoV and norovirus were highest from November to January; the PDR of HRV and enteric HAdV were highest in September; and the PDR of human metapneumovirus (HMPV), rotavirus, and astrovirus were highest in April, March, and January, respectively.
3.4. Causal Associations between Virus Prevalence and Encephalitis
The prevalence of viruses that cause encephalitis diagnosis might increase before the peak of encephalitis diagnosis. Thus, a Granger causality test was used to assess the association between the viral PDR and the number of encephalitis diagnoses reported 1 month later. The results are shown in Table 4.
Table 4.
Causality of encephalitis incidence after 1 month with virus.
| Age Group (Years) |
HAdV | HPIV | HRSV | IFV | HCoV | HRV | HBoV | HMPV | Rotavirus | Norovirus | Adenovirus | Astrovirus |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0–9 | 0.885 | 0.894 | <0.001 | 0.521 | 0.021 | 0.447 | 0.556 | 0.377 | 0.965 | 0.112 | 0.589 | 0.668 |
| 10–19 | 0.89 | 0.345 | <0.001 | 0.569 | 0.004 | 0.882 | 0.873 | 0.83 | 0.615 | 0.134 | 0.821 | 0.493 |
| 20–39 | 0.93 | 0.485 | <0.001 | 0.572 | <0.001 | 0.263 | 0.895 | 0.658 | 0.985 | 0.046 | 0.646 | 0.932 |
| 40–59 | 0.98 | 0.683 | <0.001 | 0.139 | 0.001 | 0.072 | 0.789 | 0.646 | 0.662 | 0.011 | 0.545 | 0.898 |
| ≥60 | 0.542 | 0.66 | 0.001 | 0.041 | 0.012 | 0.097 | 0.463 | 0.497 | 0.596 | 0.002 | 0.512 | 0.913 |
| total | 0.938 | 0.63 | <0.001 | 0.74 | 0.002 | 0.302 | 0.759 | 0.587 | 0.958 | 0.026 | 0.569 | 0.901 |
HAdV, human adenovirus; HPIV, human parainfluenza virus; HRSV, human respiratory syncytial virus; IFV, influenza virus; HCoV, human coronavirus; HRV, human rhinovirus; HBoV, human bocavirus; HMPV, human metapneumovirus. Statistically significant values less than 0.05 are written in bold.
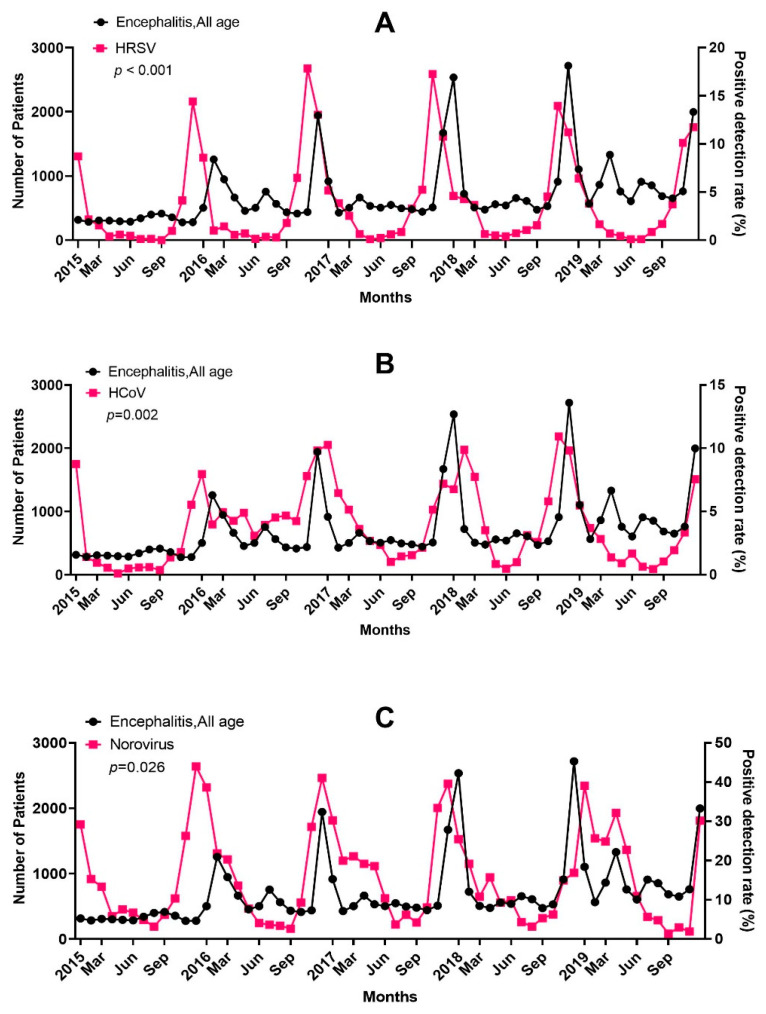
Among the seven respiratory viruses and four gastrointestinal viruses considered, the prevalence of certain viruses increased 1 month before the encephalitis incidence increased. The PDRs for HRSV (p < 0.001) and HCoV (p = 0.002) were associated with an increased incidence of encephalitis 1 month later in all age groups (Figure 3A,B). The PDR for norovirus (p = 0.026) was associated with an increased incidence of encephalitis 1 month later in patients aged 20 years and over (Figure 3C); the PDR for IFV (p = 0.041) was associated with an increased incidence of encephalitis 1 month later in patients aged 60 years and over.
Figure 3.
Relationship between the positive detection rate (PDR) of (A) respiratory syncytial virus (HRSV), (B) coronavirus (HCoV), and (C) norovirus and the incidence of encephalitis during the study period.
4. Discussion
The causes of encephalitis are diverse and include infectious, autoimmune, and unknown causes. Infectious encephalitis is a serious disease that progresses with acute inflammation and can cause severe neurological sequelae or death. The most common cause is viral infection. Therefore, this study evaluated the incidence of encephalitis and its association with viral infections. We found that certain respiratory and gastroenteritis viral PDR were significantly associated with encephalitis incidence after 1 month.
The incidence of encephalitis is high globally. It is reported to be approximately 3 to 7 per 100,000 hospitalized patients [10,11,12]. Encephalitis can affect individuals of any age; however, it is more common in children. In the United States (US), the incidence was slightly higher in men before 1997 and has been higher in women since 1998 [13,14,15]. The incidence rate of encephalitis in the US was 7.3 per 100,000 person-years between 2000 and 2010, with a peak incidence rate in infants under 1 year (13.5 per 100,000 person-years) and the lowest incidence rate in youth aged 10 to 14 years (4.1 per 100,000 person-years) [16]. In France, the incidence rate of encephalitis was 2.6 people per 100,000 in 2007 [17]. In Western countries, the annual incidence of acute encephalitis is 7.4 per 100,000 population, with an annual incidence of 10.5 per 100,000 in children and 2.2 per 100,000 in adults [18]. The annual incidence in tropical countries is 6.34 per 100, 000 population.
The combined incidence of encephalitis in 204 countries increased from 1,284,160 cases in 1990 to 1,444,720 cases in 2019, an increase of 12.50% worldwide. However, during the last 30 years, the age-standardized incidence rate declined from 23.17 to 19.33 per 100,000 person-years [19]. Although population growth and advances in medicine led to these changes, encephalitis is still associated with high mortality rates and a high incidence of neurological sequelae.
In this study, the encephalitis incidence rate in Korea was 16.4 per 100,000 population between 2015 and 2019. Overall, a total of 42,775 patients were diagnosed with encephalitis, of whom 26.3% were aged 40–59 years, and 9.9% were aged 10–19 years. The male-to-female ratio was 0.94. The female predominance is consistent with the US data. The incidence rate was 35.1 per 100,000 population in the 0–9-years age group, and 19.7 per 100,000 population in the over 60 years age group.
Acute encephalitis constitutes a neurological emergency. The main cause of encephalitis is viral infection [20]. Viral encephalitis is caused by two distinct mechanisms [21]. Primary infectious encephalitis is caused by direct invasion of the CNS, followed by viral replication in the brain, whereas immune-mediated encephalitis results from CNS damage due to an abnormal immune response. Viruses such as arboviruses can invade the CNS through the blood–brain barrier, and HSV causes cytotoxicity to neurons [16].
Therefore, the peak season for encephalitis differs depending on the type of virus. In the US, the prevalence of arbovirus- and enterovirus-associated encephalitis is high in summer and fall [15]. In contrast, IFV, HAdV, and other seasonal respiratory viruses associated with encephalitis are most prevalent in winter. In this study, the prevalence of encephalitis in Korea was high in winter. However, the COVID-19 pandemic has changed the natural epidemic course of respiratory viruses such as HRSV and IFV [22,23,24,25,26,27]. Therefore, it is likely that the seasonal epidemiology of encephalitis has also changed since 2020 owing to changes in viral epidemiology.
Several studies have investigated the association between viruses (herpesvirus, enterovirus, arbovirus, mumps, measles, rabies, and Ebola) and encephalitis [28,29]. Respiratory viruses (HRSV, IFV, HCoV, and HMPV) have been identified as a cause of CNS pathology [30]. Although there are many probable causative viruses, no definitive answer has been obtained. We found that HRSV and HCoV prevalence were associated with encephalitis incidence in all age groups. In addition, IFV was significantly associated with the incidence of encephalitis in those aged 60 years or older.
HRSV appears to be infrequently associated with encephalitis. Saravanos et al. [31] conducted a systematic review of several acute neurological complications associated with acute HRSV infection in 155 children aged less than 15 years and confirmed 6.5% of children with HRSV infection had encephalitis/encephalopathy. HRSV mainly infects the epithelial cells of the lungs, but also affects the cells of the CNS. Several studies have found that HRSV can cause CNS complications such as encephalitis [27,30,32]. To the best of our knowledge, this is the first study to reveal an association between HRSV and encephalitis across all age groups in Korea.
Several studies have investigated the association between IFV and encephalitis. Muhammad et al. [33] analyzed 1244 children aged under 12 years with influenza A H1N1, and 13.6% of the patients who presented with influenza-related neurological manifestations were diagnosed with influenza-associated encephalitis (IAE). Morishima et al. [34] reported that 87.8% of 148 patients with IAE were infected with INV A, and the incidence of IAE was highest in children under 5 years of age. Acute IAE in adults is rare [35]. Underlying neurological diseases and young age are shown to be risk factors for influenza-associated neurological complications [36,37,38]. In contrast, there was a significant association between IFV and encephalitis incidence in adults over 60 years of age in our study. There are several possible reasons for the differences in the incidence of IAE between studies. First, there are no accepted diagnostic criteria for IAE in adults [35]. Second, older adults are susceptible to infection with IFV because of their weak immune systems and underlying diseases. Third, this may be related to the limitations of our study. Additionally, influenza vaccination is important for preventing influenza-related neurological complications. However, the effectiveness of influenza vaccination in preventing encephalitis is unclear [38,39]. Therefore, further studies are warranted.
Other respiratory viruses have also been reported to cause encephalitis. HMPV is thought to cause encephalitis in both children and adults [40,41,42]. CNS involvement by HAdV and HRV has been reported but is rare [43,44]. HBoV types 1, 2, and 3 have also been reported to be associated with encephalitis [45,46,47]. In our study, there were no significant differences between HAdV, HRV, HBoV, and HMPV prevalence and encephalitis incidence, which may be related to the limitations of our study.
In addition to respiratory viruses, some studies have investigated the association between gastrointestinal viruses and encephalitis. Among them, rotavirus is known to cause CNS complications [48]. There are three mechanisms by which rotavirus can cause CNS infection: a neural route from the site of infection to the brain through neural communication, direct infection of the CNS via the lymphatic system or immune mechanisms, and an indirect route that causes CNS effects via secondary messengers such as toxin or inflammation [49].
Rotavirus has been identified in half of the pediatric seizures associated with gastroenteritis in Japan [50]. Kasai et al. [51] found that rotavirus was the third most common cause of encephalitis, accounting for 45 of 1115 cases (4.0%) in 267 hospitals between 2014 and 2017. Takanashi et al. [52] confirmed that the clinical and magnetic resonance imaging characteristics of patients with rotavirus cerebellitis were similar. In addition, several cases of encephalitis associated with rotavirus have been reported [53,54,55,56,57,58]. However, in our study, there was no significant association between rotavirus prevalence and encephalitis incidence. Infants receive 2–3 doses of the rotavirus vaccine [59,60,61]. This may have contributed to the decrease in the incidence and severity of encephalitis in children.
Norovirus is a common cause of gastroenteritis, but reports of norovirus-associated encephalitis are rare [62,63,64,65]. However, studies conducted in Japan have shown that encephalitis associated with norovirus has a poor prognosis. Shima et al. [66] conducted a multicenter study in Japan on 29 children with norovirus-related encephalitis and confirmed that norovirus is one of the main causes of encephalitis, and that, in children with norovirus infection, early onset of neurological symptoms, an elevated serum creatinine level, and an abnormal blood glucose level are associated with poor prognosis. In our study, a significant correlation was found between the prevalence of norovirus and the incidence of encephalitis in those aged 20 years or older. The incidence of norovirus infection is lower than that of rotavirus infection; however, the prognosis of norovirus infection is worse. Therefore, further studies of the association between norovirus infection and encephalitis are needed [67,68].
According to an analysis of the US Healthcare Database from 2011 to 2014, enterovirus (58.4%) was the most common cause of encephalitis in infants and children under the age of 17 years [69]. During the same period, a study of 26,429 adult patients aged 18 years or older with encephalitis confirmed that enterovirus was the most common cause, accounting for 51.6% of all cases [70].
Chen et al. [71] confirmed that CNS infection with enterovirus causes encephalitis, which is especially common in young children, considering the potential causal link between the neuroimmune system and environmental neuroinvasion. There are three mechanisms whereby enterovirus may cause encephalitis. First, neurotropic viruses, including enterovirus, reach the CNS through the blood, pass through the blood–brain barrier, and directly act on the brain. Second, neurotropic viruses invade the CNS through immune cells infected with viruses circulating in the peripheral blood and reach the CNS through peripheral nerves [72,73,74,75,76].
Astroviruses rarely cause encephalitis and are not strongly associated with encephalitis [77,78,79]. There are only a few case reports, and no large-scale studies have been conducted. In our study, there was no association between astrovirus infection and the incidence of encephalitis.
Our study has several limitations. First, it was a retrospective study, which provides a lower level of evidence than prospective studies and may have had selection bias. Second, the data collected from the HIRA did not include patient clinical records. Third, the association between the prevalence of certain viruses and the incidence of encephalitis may not be causally related and may have been a chance finding. Moreover, the profile of patients tested for viral infections was different from that of patients diagnosed with encephalitis. Therefore, it was not possible to establish a direct relationship between viral infection and encephalitis since only a temporal relationship could be assessed. Therefore, the results may differ from those of previous studies. Although time-series analysis showed a trend between viral PDRs and encephalitis incidence rates using the Granger causality test, the results do not provide definitive evidence of a causal association between viral infection and encephalitis incidence. Because viral infections are common in winter, more epidemiological data are needed to link viral epidemics to the onset of encephalitis.
5. Conclusions
This study assessed the prevalence of common viral pathogens and their correlation with encephalitis. To our knowledge, this is the largest nationwide analysis of patients with encephalitis and its association with the PDRs of viruses in Korea. In Korea, increased incidence of HRSV, HCoV, IFV, and norovirus were found to precede the occurrence of encephalitis. It is possible that these viruses were the etiology of encephalitis. Prospective studies on these viruses and encephalitis are required to further elucidate these associations.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/jcm12052003/s1, Table S1: Parameters of ARIMA models for patients with encephalitis by age; Table S2: Positive detection rates of the virus during the study period; Figure S1: Residual ACF correlogram and 95% confidence limits for encephalitis; Figure S2. Positive detection rates of viruses during the study period; Figure S3. Positive detection rates of viruses during the study period (plot).
Author Contributions
Conceptualization, S.J.L., J.M.K., S.K. and J.M.L.; methodology, S.J.L., J.M.K. and H.R.K.; formal analysis, S.W.K.; investigation, S.J.L., J.M.K. and H.R.K.; data curation, S.J.L., J.M.K. and H.R.K.; writing—original draft preparation, S.J.L. and J.M.K.; writing—review and editing, S.J.L., J.M.K., H.S.B., J.C.B., Y.K.K., S.K. and J.M.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of the Yeungnam University Medical Center (protocol code YUMC 2021-09-053).
Informed Consent Statement
Patient consent was waived due to the retrospective study design.
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was supported by 2015 Yeungnam University research grant (215A480026).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Simon D.W., Da Silva Y.S., Zuccoli G., Clark R.S. Acute encephalitis. Crit. Care Clin. 2013;29:259–277. doi: 10.1016/j.ccc.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ellul M., Solomon T. Acute encephalitis-diagnosis and management. Clin. Med. 2018;18:155–159. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar R. Understanding and managing acute encephalitis. F1000Research. 2020;9 doi: 10.12688/f1000research.20634.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim D.S. Introduction: Health of the health care system in Korea. Soc. Work Public Health. 2010;25:127–141. doi: 10.1080/19371910903070333. [DOI] [PubMed] [Google Scholar]
- 5.Kim D.S. Special issue on the national health care system of South Korea. Soc. Work Public Health. 2010;25:125–126. doi: 10.1080/19371911003648275. [DOI] [PubMed] [Google Scholar]
- 6.Kim L., Kim J.A., Kim S. A guide for the utilization of Health Insurance Review and Assessment Service National Patient Samples. Epidemiol. Health. 2014;36:e2014008. doi: 10.4178/epih/e2014008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granger C.W.J. Investigating Causal Relations by Econometric Models and Cross-spectral Methods. Econometrica. 1969;37:424. doi: 10.2307/1912791. [DOI] [Google Scholar]
- 8.Lim J.H., Kim Y.K., Min S.H., Kim S.W., Lee Y.H., Lee J.M. Seasonal Trends of Viral Prevalence and Incidence of Kawasaki Disease: A Korea Public Health Data Analysis. J. Clin. Med. 2021;10:3301. doi: 10.3390/jcm10153301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim J.H., Kim Y.K., Min S.H., Kim S.W., Lee Y.H., Lee J.M. Epidemiology and Viral Etiology of Pediatric Immune Thrombocytopenia through Korean Public Health Data Analysis. J. Clin. Med. 2021;10:1356. doi: 10.3390/jcm10071356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Granerod J., Crowcroft N.S. The epidemiology of acute encephalitis. Neuropsychol. Rehabil. 2007;17:406–428. doi: 10.1080/09602010600989620. [DOI] [PubMed] [Google Scholar]
- 11.Granerod J., Cousens S., Davies N.W., Crowcroft N.S., Thomas S.L. New estimates of incidence of encephalitis in England. Emerg. Infect. Dis. 2013;19:1455–1462. doi: 10.3201/eid1909.130064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venkatesan A. Epidemiology and outcomes of acute encephalitis. Curr. Opin. Neurol. 2015;28:277–282. doi: 10.1097/WCO.0000000000000199. [DOI] [PubMed] [Google Scholar]
- 13.Khetsuriani N., Holman R.C., Anderson L.J. Burden of encephalitis-associated hospitalizations in the United States, 1988–1997. Clin. Infect. Dis. 2002;35:175–182. doi: 10.1086/341301. [DOI] [PubMed] [Google Scholar]
- 14.Vora N.M., Holman R.C., Mehal J.M., Steiner C.A., Blanton J., Sejvar J. Burden of encephalitis-associated hospitalizations in the United States, 1998–2010. Neurology. 2014;82:443–451. doi: 10.1212/WNL.0000000000000086. [DOI] [PubMed] [Google Scholar]
- 15.George B.P., Schneider E.B., Venkatesan A. Encephalitis hospitalization rates and inpatient mortality in the United States, 2000–2010. PLoS ONE. 2014;9:e104169. doi: 10.1371/journal.pone.0104169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messacar K., Fischer M., Dominguez S.R., Tyler K.L., Abzug M.J. Encephalitis in US Children. Infect. Dis. Clin. N. Am. 2018;32:145–162. doi: 10.1016/j.idc.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernard S., Mailles A., Stahl J.P., Steering C., Investigators G. Epidemiology of infectious encephalitis, differences between a prospective study and hospital discharge data. Epidemiol. Infect. 2013;141:2256–2268. doi: 10.1017/S0950268812002518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jmor F., Emsley H.C., Fischer M., Solomon T., Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virol. J. 2008;5:134. doi: 10.1186/1743-422X-5-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H., Zhao S., Wang S., Zheng Y., Wang S., Chen H., Pang J., Ma J., Yang X., Chen Y. Global magnitude of encephalitis burden and its evolving pattern over the past 30 years. J. Infect. 2022;84:777–787. doi: 10.1016/j.jinf.2022.04.026. [DOI] [PubMed] [Google Scholar]
- 20.Stone M.J., Hawkins C.P. A medical overview of encephalitis. Neuropsychol. Rehabil. 2007;17:429–449. doi: 10.1080/09602010601069430. [DOI] [PubMed] [Google Scholar]
- 21.Lewis P., Glaser C.A. Encephalitis. Pediatr. Rev. 2005;26:353–363. doi: 10.1542/pir.26-10-353. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan S.G., Carlson S., Cheng A.C., Chilver M.B., Dwyer D.E., Irwin M., Kok J., Macartney K., MacLachlan J., Minney-Smith C., et al. Where has all the influenza gone? The impact of COVID-19 on the circulation of influenza and other respiratory viruses, Australia, March to September 2020. Euro. Surveill. 2020;25:2001847. doi: 10.2807/1560-7917.ES.2020.25.47.2001847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Brusselen D., De Troeyer K., Ter Haar E., Vander Auwera A., Poschet K., Van Nuijs S., Bael A., Stobbelaar K., Verhulst S., Van Herendael B., et al. Bronchiolitis in COVID-19 times: A nearly absent disease? Eur. J. Pediatr. 2021;180:1969–1973. doi: 10.1007/s00431-021-03968-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nagakumar P., Chadwick C.L., Bush A., Gupta A. Collateral impact of COVID-19: Why should children continue to suffer? Eur. J. Pediatr. 2021;180:1975–1979. doi: 10.1007/s00431-021-03963-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Foley D.A., Yeoh D.K., Minney-Smith C.A., Martin A.C., Mace A.O., Sikazwe C.T., Le H., Levy A., Moore H.C., Blyth C.C. The Interseasonal Resurgence of Respiratory Syncytial Virus in Australian Children Following the Reduction of Coronavirus Disease 2019-Related Public Health Measures. Clin. Infect. Dis. 2021;73:e2829–e2830. doi: 10.1093/cid/ciaa1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Agha R., Avner J.R. Delayed Seasonal RSV Surge Observed During the COVID-19 Pandemic. Pediatrics. 2021;148:e2021052089. doi: 10.1542/peds.2021-052089. [DOI] [PubMed] [Google Scholar]
- 27.Andrade C.A., Kalergis A.M., Bohmwald K. Potential Neurocognitive Symptoms Due to Respiratory Syncytial Virus Infection. Pathogens. 2021;11:47. doi: 10.3390/pathogens11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rozenberg F. Acute viral encephalitis. Handb. Clin. Neurol. 2013;112:1171–1181. doi: 10.1016/B978-0-444-52910-7.00038-6. [DOI] [PubMed] [Google Scholar]
- 29.Venkatesan A., Murphy O.C. Viral Encephalitis. Neurol. Clin. 2018;36:705–724. doi: 10.1016/j.ncl.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 30.Bohmwald K., Galvez N.M.S., Rios M., Kalergis A.M. Neurologic Alterations Due to Respiratory Virus Infections. Front. Cell Neurosci. 2018;12:386. doi: 10.3389/fncel.2018.00386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saravanos G.L., King C.L., Deng L., Dinsmore N., Ramos I., Takashima M., Crawford N., Clark J.E., Dale R.C., Jones C.A., et al. Respiratory Syncytial Virus-Associated Neurologic Complications in Children: A Systematic Review and Aggregated Case Series. J. Pediatr. 2021;239:39–49.e39. doi: 10.1016/j.jpeds.2021.06.045. [DOI] [PubMed] [Google Scholar]
- 32.Bohmwald K., Soto J.A., Andrade-Parra C., Fernandez-Fierro A., Espinoza J.A., Rios M., Eugenin E.A., Gonzalez P.A., Opazo M.C., Riedel C.A., et al. Lung pathology due to hRSV infection impairs blood-brain barrier permeability enabling astrocyte infection and a long-lasting inflammation in the CNS. Brain Behav. Immun. 2021;91:159–171. doi: 10.1016/j.bbi.2020.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muhammad Ismail H.I., Teh C.M., Lee Y.L., National Paediatric H.N.S.G. Neurologic manifestations and complications of pandemic influenza A H1N1 in Malaysian children: What have we learnt from the ordeal? Brain Dev. 2015;37:120–129. doi: 10.1016/j.braindev.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Morishima T., Togashi T., Yokota S., Okuno Y., Miyazaki C., Tashiro M., Okabe N., Collaborative Study Group on Influenza-Associated Encephalopathy in Japan Encephalitis and encephalopathy associated with an influenza epidemic in Japan. Clin. Infect. Dis. 2002;35:512–517. doi: 10.1086/341407. [DOI] [PubMed] [Google Scholar]
- 35.Meijer W.J., Linn F.H., Wensing A.M., Leavis H.L., van Riel D., GeurtsvanKessel C.H., Wattjes M.P., Murk J.L. Acute influenza virus-associated encephalitis and encephalopathy in adults: A challenging diagnosis. JMM Case Rep. 2016;3:e005076. doi: 10.1099/jmmcr.0.005076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Newland J.G., Laurich V.M., Rosenquist A.W., Heydon K., Licht D.J., Keren R., Zaoutis T.E., Watson B., Hodinka R.L., Coffin S.E. Neurologic complications in children hospitalized with influenza: Characteristics, incidence, and risk factors. J. Pediatr. 2007;150:306–310. doi: 10.1016/j.jpeds.2006.11.054. [DOI] [PubMed] [Google Scholar]
- 37.Britton P.N., Blyth C.C., Macartney K., Dale R.C., Li-Kim-Moy J., Khandaker G., Crawford N.W., Marshall H., Clark J.E., Elliott E.J., et al. The Spectrum and Burden of Influenza-Associated Neurological Disease in Children: Combined Encephalitis and Influenza Sentinel Site Surveillance from Australia, 2013–2015. Clin. Infect. Dis. 2017;65:653–660. doi: 10.1093/cid/cix412. [DOI] [PubMed] [Google Scholar]
- 38.Choi G.J., Park J.Y., Choi J.S., Choi S.R., Kim D., Lee J.H., Woo Y.J., Lee J., Kim Y.J. Influenza-associated Neurologic Complications in Hospitalized Pediatric Patients: A Multicenter Retrospective Study in Republic of Korea. Pediatr. Infect. Dis. J. 2021;40:e466–e471. doi: 10.1097/INF.0000000000003332. [DOI] [PubMed] [Google Scholar]
- 39.Espinet-Coll E., Nebreda-Duran J., Lopez-Nava Breviere G., Coordinadores del Grupo Espanol de Trabajo para el Tratamiento Endoscopico del Metabolismo y la Obesidad (GETTEMO) Gastric perforation by intragastric balloon in a patient with Nissen fundoplication. Response of the Spanish Bariatric Endoscopy Group. Gastroenterol. Hepatol. 2018;41:583–584. doi: 10.1016/j.gastrohep.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Kaida A., Iritani N., Kubo H., Shiomi M., Kohdera U., Murakami T. Seasonal distribution and phylogenetic analysis of human metapneumovirus among children in Osaka City, Japan. J. Clin. Virol. 2006;35:394–399. doi: 10.1016/j.jcv.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 41.Sanchez Fernandez I., Rebollo Polo M., Munoz-Almagro C., Monfort Carretero L., Fernandez Urena S., Rueda Munoz A., Colome Roura R., Perez Duenas B. Human Metapneumovirus in the Cerebrospinal Fluid of a Patient With Acute Encephalitis. Arch. Neurol. 2012;69:649–652. doi: 10.1001/archneurol.2011.1094. [DOI] [PubMed] [Google Scholar]
- 42.Fok A., Mateevici C., Lin B., Chandra R.V., Chong V.H. Encephalitis-Associated Human Metapneumovirus Pneumonia in Adult, Australia. Emerg. Infect. Dis. 2015;21:2074–2076. doi: 10.3201/eid2111.150608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hazama K., Shiihara T., Tsukagoshi H., Matsushige T., Dowa Y., Watanabe M. Rhinovirus-associated acute encephalitis/encephalopathy and cerebellitis. Brain Dev. 2019;41:551–554. doi: 10.1016/j.braindev.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 44.Zhao H., Liu Y., Feng Z., Feng Q., Li K., Gao H., Qian S., Xu L., Xie Z. A fatal case of viral sepsis and encephalitis in a child caused by human adenovirus type 7 infection. Virol. J. 2022;19:154. doi: 10.1186/s12985-022-01886-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitui M.T., Tabib S.M., Matsumoto T., Khanam W., Ahmed S., Mori D., Akhter N., Yamada K., Kabir L., Nishizono A., et al. Detection of human bocavirus in the cerebrospinal fluid of children with encephalitis. Clin. Infect. Dis. 2012;54:964–967. doi: 10.1093/cid/cir957. [DOI] [PubMed] [Google Scholar]
- 46.Yu J.M., Chen Q.Q., Hao Y.X., Yu T., Zeng S.Z., Wu X.B., Zhang B., Duan Z.J. Identification of human bocaviruses in the cerebrospinal fluid of children hospitalized with encephalitis in China. J. Clin. Virol. 2013;57:374–377. doi: 10.1016/j.jcv.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 47.Mori D., Ranawaka U., Yamada K., Rajindrajith S., Miya K., Perera H.K., Matsumoto T., Dassanayake M., Mitui M.T., Mori H., et al. Human bocavirus in patients with encephalitis, Sri Lanka, 2009–2010. Emerg. Infect. Dis. 2013;19:1859–1862. doi: 10.3201/eid1911.121548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee K.Y. Rotavirus infection-associated central nervous system complications: Clinicoradiological features and potential mechanisms. Clin. Exp. Pediatr. 2022;65:483–493. doi: 10.3345/cep.2021.01333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellysaz A., Hagbom M. Understanding the Central Nervous System Symptoms of Rotavirus: A Qualitative Review. Viruses. 2021;13:658. doi: 10.3390/v13040658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uemura N., Okumura A., Negoro T., Watanabe K. Clinical features of benign convulsions with mild gastroenteritis. Brain Dev. 2002;24:745–749. doi: 10.1016/S0387-7604(02)00097-9. [DOI] [PubMed] [Google Scholar]
- 51.Kasai M., Shibata A., Hoshino A., Maegaki Y., Yamanouchi H., Takanashi J.I., Yamagata T., Sakuma H., Okumura A., Nagase H., et al. Epidemiological changes of acute encephalopathy in Japan based on national surveillance for 2014–2017. Brain Dev. 2020;42:508–514. doi: 10.1016/j.braindev.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Takanashi J., Miyamoto T., Ando N., Kubota T., Oka M., Kato Z., Hamano S., Hirabayashi S., Kikuchi M., Barkovich A.J. Clinical and radiological features of rotavirus cerebellitis. Am. J. Neuroradiol. 2010;31:1591–1595. doi: 10.3174/ajnr.A2131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ushijima H., Bosu K., Abe T., Shinozaki T. Suspected rotavirus encephalitis. Arch. Dis. Child. 1986;61:692–694. doi: 10.1136/adc.61.7.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Conway S.P. Rotavirus encephalitis. Arch. Dis. Child. 1988;63:224. doi: 10.1136/adc.63.2.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shiihara T., Watanabe M., Honma A., Kato M., Morita Y., Ichiyama T., Maruyama K. Rotavirus associated acute encephalitis/encephalopathy and concurrent cerebellitis: Report of two cases. Brain Dev. 2007;29:670–673. doi: 10.1016/j.braindev.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 56.Kobayashi S., Negishi Y., Ando N., Ito T., Nakano M., Togari H., Wakuda M., Taniguchi K. Two patients with acute rotavirus encephalitis associated with cerebellar signs and symptoms. Eur. J. Pediatr. 2010;169:1287–1291. doi: 10.1007/s00431-010-1202-y. [DOI] [PubMed] [Google Scholar]
- 57.Ueda H., Tajiri H., Kimura S., Etani Y., Hosoi G., Maruyama T., Noma H., Kusumoto Y., Takano T., Baba Y., et al. Clinical characteristics of seizures associated with viral gastroenteritis in children. Epilepsy. Res. 2015;109:146–154. doi: 10.1016/j.eplepsyres.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 58.Paketci C., Edem P., Okur D., Sarioglu F.C., Guleryuz H., Bayram E., Kurul S.H., Yis U. Rotavirus encephalopathy with concomitant acute cerebellitis: Report of a case and review of the literature. Turk. J. Pediatr. 2020;62:119–124. doi: 10.24953/turkjped.2020.01.017. [DOI] [PubMed] [Google Scholar]
- 59.Soares-Weiser K., Maclehose H., Bergman H., Ben-Aharon I., Nagpal S., Goldberg E., Pitan F., Cunliffe N. Vaccines for preventing rotavirus diarrhoea: Vaccines in use. Cochrane Database Syst. Rev. 2012;11:CD008521. doi: 10.1002/14651858.CD008521.pub3. [DOI] [PubMed] [Google Scholar]
- 60.Becker-Dreps S., Bucardo F., Vilchez S., Zambrana L.E., Liu L., Weber D.J., Pena R., Barclay L., Vinje J., Hudgens M.G., et al. Etiology of childhood diarrhea after rotavirus vaccine introduction: A prospective, population-based study in Nicaragua. Pediatr. Infect. Dis. J. 2014;33:1156–1163. doi: 10.1097/INF.0000000000000427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Burnett E., Parashar U.D., Tate J.E. Real-world effectiveness of rotavirus vaccines, 2006–2019: A literature review and meta-analysis. Lancet. Glob. Health. 2020;8:e1195–e1202. doi: 10.1016/S2214-109X(20)30262-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ito S., Takeshita S., Nezu A., Aihara Y., Usuku S., Noguchi Y., Yokota S. Norovirus-associated encephalopathy. Pediatr. Infect. Dis. J. 2006;25:651–652. doi: 10.1097/01.inf.0000225789.92512.6d. [DOI] [PubMed] [Google Scholar]
- 63.Obinata K., Okumura A., Nakazawa T., Kamata A., Niizuma T., Kinoshita K., Shimizu T. Norovirus encephalopathy in a previously healthy child. Pediatr. Infect. Dis. J. 2010;29:1057–1059. doi: 10.1097/INF.0b013e3181e78889. [DOI] [PubMed] [Google Scholar]
- 64.Kimura E., Goto H., Migita A., Harada S., Yamashita S., Hirano T., Uchino M. An adult norovirus-related encephalitis/encephalopathy with mild clinical manifestation. BMJ Case Rep. 2010;2010:bcr0320102784. doi: 10.1136/bcr.03.2010.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sanchez-Fauquier A., Gonzalez-Galan V., Arroyo S., Roda D., Pons M., Garcia J.J. Norovirus-associated encephalitis in a previously healthy 2-year-old girl. Pediatr. Infect. Dis. J. 2015;34:222–223. doi: 10.1097/INF.0000000000000547. [DOI] [PubMed] [Google Scholar]
- 66.Shima T., Okumura A., Kurahashi H., Numoto S., Abe S., Ikeno M., Shimizu T., Norovirus-associated Encephalitis/Encephalopathy Collaborative Study, i A nationwide survey of norovirus-associated encephalitis/encephalopathy in Japan. Brain Dev. 2019;41:263–270. doi: 10.1016/j.braindev.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 67.Kawano G., Oshige K., Syutou S., Koteda Y., Yokoyama T., Kim B.G., Mizuochi T., Nagai K., Matsuda K., Ohbu K., et al. Benign infantile convulsions associated with mild gastroenteritis: A retrospective study of 39 cases including virological tests and efficacy of anticonvulsants. Brain Dev. 2007;29:617–622. doi: 10.1016/j.braindev.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 68.Chen S.Y., Tsai C.N., Lai M.W., Chen C.Y., Lin K.L., Lin T.Y., Chiu C.H. Norovirus infection as a cause of diarrhea-associated benign infantile seizures. Clin. Infect. Dis. 2009;48:849–855. doi: 10.1086/597256. [DOI] [PubMed] [Google Scholar]
- 69.Hasbun R., Wootton S.H., Rosenthal N., Balada-Llasat J.M., Chung J., Duff S., Bozzette S., Zimmer L., Ginocchio C.C. Epidemiology of Meningitis and Encephalitis in Infants and Children in the United States, 2011–2014. Pediatr. Infect. Dis. J. 2019;38:37–41. doi: 10.1097/INF.0000000000002081. [DOI] [PubMed] [Google Scholar]
- 70.Hasbun R., Rosenthal N., Balada-Llasat J.M., Chung J., Duff S., Bozzette S., Zimmer L., Ginocchio C.C. Epidemiology of Meningitis and Encephalitis in the United States, 2011–2014. Clin. Infect. Dis. 2017;65:359–363. doi: 10.1093/cid/cix319. [DOI] [PubMed] [Google Scholar]
- 71.Chen B.S., Lee H.C., Lee K.M., Gong Y.N., Shih S.R. Enterovirus and Encephalitis. Front. Microbiol. 2020;11:261. doi: 10.3389/fmicb.2020.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang W.X., Terasaki T., Shiroki K., Ohka S., Aoki J., Tanabe S., Nomura T., Terada E., Sugiyama Y., Nomoto A. Efficient delivery of circulating poliovirus to the central nervous system independently of poliovirus receptor. Virology. 1997;229:421–428. doi: 10.1006/viro.1997.8450. [DOI] [PubMed] [Google Scholar]
- 73.Gromeier M., Wimmer E. Mechanism of injury-provoked poliomyelitis. J. Virol. 1998;72:5056–5060. doi: 10.1128/JVI.72.6.5056-5060.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen C.S., Yao Y.C., Lin S.C., Lee Y.P., Wang Y.F., Wang J.R., Liu C.C., Lei H.Y., Yu C.K. Retrograde axonal transport: A major transmission route of enterovirus 71 in mice. J. Virol. 2007;81:8996–9003. doi: 10.1128/JVI.00236-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ong K.C., Badmanathan M., Devi S., Leong K.L., Cardosa M.J., Wong K.T. Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J. Neuropathol. Exp. Neurol. 2008;67:532–542. doi: 10.1097/NEN.0b013e31817713e7. [DOI] [PubMed] [Google Scholar]
- 76.Rhoades R.E., Tabor-Godwin J.M., Tsueng G., Feuer R. Enterovirus infections of the central nervous system. Virology. 2011;411:288–305. doi: 10.1016/j.virol.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Quan P.L., Wagner T.A., Briese T., Torgerson T.R., Hornig M., Tashmukhamedova A., Firth C., Palacios G., Baisre-De-Leon A., Paddock C.D., et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg. Infect. Dis. 2010;16:918–925. doi: 10.3201/eid1606.091536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vu D.L., Cordey S., Brito F., Kaiser L. Novel human astroviruses: Novel human diseases? J. Clin. Virol. 2016;82:56–63. doi: 10.1016/j.jcv.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 79.Koukou G., Niendorf S., Hornei B., Schlump J.U., Jenke A.C., Jacobsen S. Human astrovirus infection associated with encephalitis in an immunocompetent child: A case report. J. Med. Case Rep. 2019;13:341. doi: 10.1186/s13256-019-2302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available on request from the corresponding authors.