Abstract
Infection of mice with the intestinal bacterial pathogen Citrobacter rodentium results in colonic mucosal hyperplasia and a local Th1 inflammatory response similar to that seen in mouse models of inflammatory bowel disease. In these latter models, and in patients with Crohn's disease, neutralization of tumor necrosis factor alpha (TNF-α) is of therapeutic benefit. Since there is no information on the role of TNF-α in either immunity to noninvasive bacterial pathogens or on the role of TNF-α in the immunopathology of infectious colitis, we investigated C. rodentium infection in TNFRp55−/− mice. In TNFRp55−/− mice, there were higher colonic bacterial burdens, but the organisms were cleared at the same rate as C57BL/6 mice, showing that TNF-α is not needed for protective antibacterial immunity. The most striking feature of infection in TNFRp55−/− mice, however, was the markedly enhanced pathology, with increased mucosal weight and thickness, increased T-cell infiltrate, and a markedly greater mucosal Th1 response. Interleukin-12 p40 transcripts were markedly elevated in C. rodentium-infected TNFRp55−/− mice, and this was associated with enhanced mucosal STAT4 phosphorylation. TNF-α is not obligatory for protective immunity to C. rodentium in mice; however, it appears to play some role in downregulating mucosal pathology and Th1 immune responses.
There is now little doubt that increased local concentrations of tumor necrosis factor alpha (TNF-α) are of prime importance in driving chronic inflammation in a number of tissues (26, 34, 51, 58, 60). In particular, in the intestine, excess TNF-α is associated with pathology (56). It is likely that excess local TNF-α can drive tissue injury via a number of pathways, including direct effects on epithelial function and permeability (16, 18, 43), upregulation of adhesion molecule expression on endothelium (4), increased production of chemokines by various cell types (33), and upregulation of matrix-degrading enzyme production by stromal cells (45). In mouse models of inflammatory bowel disease (IBD) (41, 46) and Crohn's disease in patients, neutralization of TNF-α has a clear therapeutic effect (52, 57). TNFRp55 knockout mice are resistant to experimental colitis (41), and mice overexpressing TNF-α are more susceptible to experimental colitis (41) and indeed may spontaneously develop ileitis (27). In these mouse models and probably in Crohn's disease as well, the mucosal T-cell immune response which results in the inflammatory cell infiltrate and excess TNF-α production is directed against the normal bacterial flora (12). Support for the general notion that immune responses against lumenal organisms can cause tissue injury via excess TNF-α production also comes from studies on the protozoan parasite Toxoplasma gondii (31). In certain strains of mice, infection leads to hemorrhagic necrosis of the intestine (31). This severe response, which results in the death of the host, can be ameliorated by blocking TNF-α (30).
To our knowledge, there are only two murine systems wherein it is possible to set up a natural noninvasive bacterial infection in mouse intestine which mimics human disease. These are Helicobacter pylori or Helicobacter felis in the murine stomach (28, 36) and Citrobacter rodentium infection of the colon (19). Helicobacter elicits a pronounced Th1 response in the stomach which drives the pathology (9, 39), but there have been no studies yet on the role of TNF-α in this model.
C. rodentium is a natural pathogen of mice. It has many similarities to human enteropathogenic Escherichia coli (EPEC) or enterohemorrhagic E. coli (EHEC) infection (29). EPEC, EHEC, and C. rodentium colonize the intestinal mucosa and, by subverting intestinal epithelial cell cytoskeleton function, produce a characteristic histopathological feature known as the “attaching and effacing ” (A/E) lesion (11). This requires a number of bacterial factors, including EPEC-secreted proteins (Esps), a type III secretory apparatus, and an outer membrane protein called intimin. Significant progress has been made defining the molecular basis of EPEC-host cell interactions and defining the role of EPEC's virulence determinants in the regulation of host cell cytoskeletal rearrangement (55). Not surprisingly, however, in vitro studies with epithelial cell cultures can only model the intestinal epithelium in a limited fashion and provide little insight into host immune responses. Indeed, the type and magnitude of the immune response in animals or humans infected with enteric bacterial pathogens that colonize via A/E lesion formation has been poorly described. In part, this is due to the difficulty in studying immune responses in EPEC- or EHEC-infected humans (usually children) or large animals. Nevertheless, a better understanding of these facets of the host-pathogen interaction could speed the design of vaccines and immune-based therapies which can prevent diarrhea.
In several respects, C. rodentium infection of mice represents the best small-animal model in which to study host defense against lumenal microbial pathogens relying on A/E lesion formation for colonization of the host. C. rodentium possesses both established and putative virulence determinants common to EPEC and EHEC (50), including the LEE pathogenicity island (38) and lymphostatin toxin (25). The A/E lesion induced by C. rodentium is ultrastructurally identical to those formed by EHEC and EPEC in animals and humans (10, 47). In naturally or experimentally infected susceptible mouse strains, large numbers of C. rodentium can be recovered from the colon, and infection is associated with crypt hyperplasia, goblet cell depletion, and mucosal erosion (3, 23). Extraintestinal infection of immunocompetent mice is rarely seen. Oral infection of mice with live C. rodentium or intracolonic inoculation of dead bacteria induces a CD3+ and CD4+ T-cell infiltrate into the colonic lamina propria and a highly polarized Th1 response (19, 20). Transcripts for the cytokines interleukin-12 (IL-12), TNF-α, and gamma interferon (IFN-γ) are highly expressed in colonic tissue of infected mice. The role of these cytokines in host defense and mucosal pathology is unknown.
One striking feature of C. rodentium infection is that the T-cell response and pathology in the colon are virtually identical to that seen in mouse models of IBD (19). Given the crucial role of TNF-α in these models, it is logical to investigate the role of TNF-α in C. rodentium infection. Here we report, in contrast to all other systems studied before, that the absence of TNF-α is not associated with less inflammation in C. rodentium infection. TNFRp55 knockout mice develop more severe pathology. This is associated with an increase in IL-12, Stat4 activation, an exaggerated local Th1 response, and increased bacterial loads in the gut, although these are eventually cleared.
MATERIALS AND METHODS
Animals.
Female or male 6- to 8-week-old C57BL/6J mice were purchased from Harlan Olac (Bichester, United Kingdom) or B&K Universal (Hull, United Kingdom). TNFRp55−/− mice (backcrossed to a C57BL/6 background at least 10 times) were originally purchased from Jackson Laboratories and were maintained by homozygous mating under contract at B&K Universal. The presence (in the case of C57BL/6 mice) or absence (in the case of TNFRp55−/− mice) of the TNF receptor type I p55 was analyzed by Western blotting and confirmed the genotyping (data not shown). All mice came from colonies which were specific pathogen-free. During experimental studies, groups of animals were housed in HEPA-filtered individually ventilated cages with free access to sterilized food and water. Mice were checked daily and weighed at the start and during each experiment.
Bacterial strain and challenge of mice.
DBS255(pCVD438) is a C. rodentium eae mutant complemented with the eae gene from EPEC strain E2348/69 (intimin α). This strain, which has been described previously (17, 20), expresses biologically active intimin, and is virulent in mice. Bacterial inocula were prepared by culturing bacteria overnight at 37°C in L broth containing nalidixic acid (100 μg/ml) plus chloramphenicol (50 μg/ml). Cultures were harvested by centrifugation and resuspended in a 1/10 volume of phosphate-buffered saline (PBS). Mice were orally inoculated by using a gavage needle with 2 × 109 to 3 × 109 CFU of bacteria in 200 μl of PBS. The viable count of the inoculum was determined by retrospective plating on L agar containing appropriate antibiotics. At selected time points postinfection, mice were killed by cardiac exsanguination under terminal anesthesia or by cervical dislocation. The terminal 6 cm of the colon was removed, and the colon was weighed after the removal of fecal pellets. In some experiments, the distal 1 cm of the colon was snap frozen in liquid nitrogen for subsequent analysis. The spleen, mesenteric lymph nodes (MLN), and remaining colon were removed and homogenized mechanically by using a Seward 80 stomacher (London, England). The number of viable bacteria in organ homogenates was determined by viable count on L agar containing nalidixic acid and chloramphenicol. The lower limit of sensitivity of the bacteria was 10 CFU per organ.
Immunohistochemistry.
Three-step avidin-peroxidase staining was performed on 5-μm frozen sections as described previously (59) with the monoclonal antibodies 145-2C11 (anti-CD3), YTS191 (anti-CD4), YTS169 (anti-CD8), and M5-114 (anti-major histocompatibility complex class II). Biotin-conjugated rabbit anti-rat immunoglobulin G (IgG) (Dako, High Wycombe, United Kingdom) and goat anti-hamster IgG (Vector Laboratories, Peterborough, United Kingdom) were used at 1:50 dilution in Tris-buffered saline (TBS; pH 7.6) containing 4% (vol/vol) normal mouse serum (Harlan Seralab, Oxon, United Kingdom). Avidin-peroxidase (Sigma) was used at a dilution of 1:200 in TBS. A two-step protocol was performed with rabbit anti-intimin antibody as described before (1), together with horseradish peroxidase (HRP)-conjugated swine anti-rabbit IgG secondary antibody. As controls for the specificity of the staining, serial sections were processed omitting the primary antibody or, alternatively, by using rabbit IgG (5 μg/ml; Sigma) as the first-step antibody. In both cases, no staining was observed. Peroxidase activity was detected with 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma) in a 0.5-mg/ml solution of Tris-HCl (pH 7.6) containing 0.01% H2O2. The density of positive cells in the lamina propria was determined by image analysis. Crypt length was measured by micrometry on hematoxylin-and-eosin-stained sections, with 10 measurements being taken in the distal colons of individual mice. Only well-orientated crypts were counted. Also, we quantified the bacteria in the glands by counting 100 glands and recording how many were positive for intimin staining. The data were expressed as a percentage.
RNA extraction and quantitative RT-PCR.
Total cellular RNA was isolated from frozen distal colonic tissue by homogenization of the tissue in TRIzol (Gibco/Life Technologies, Pailsey, United Kingdom) and incubation at room temperature for 5 min. RNA was extracted with chloroform (Sigma) and then centrifuged for 15 min at 12,000 × g at 4°C. The pellet was washed with 70% ethanol and resuspended in 50 μl of water. Total RNA was measured by spectrophotometric analysis. Constructs encoding standard RNAs (pCMQ1, pCMQ2, pCMQ3, and pCMQ4), kindly provided by M. F. Kagnoff, Department of Medicine, University of California, San Diego (13), were used for quantitative competitive reverse transcription-PCR (RT-PCR). pCMQ1 contains primer sites for IFN-γ; pCMQ2 contains primer sites for IL-4, and TNF-α; and pCMQ3 carries primer sites for IL-12 p40. To generate standard RNA, plasmids were linearized with SalI (pCMQ1), NotI (pCMQ2, pCMQ3, and pCMQ4) and transcribed in vitro with T7 RNA polymerase under conditions recommended by the supplier (Promega, Southampton, United Kingdom). Serial 10-fold dilutions of standard RNA (1 pg to 1 fg) were co-reverse transcribed with total cellular RNA (1 μg) at 42°C for 50 min in a 20-μl reaction volume containing 50 mM Tris (pH 8.3), 75 mM KCl, 3 mM MgCl2, 3 mM dithiothreitol, a 10 mM deoxynucleoside triphosphate mix, and 0.5 μg of oligo(dT) (Pharmacia Biotech, St. Albans, Hertfordshire, United Kingdom), using 100 U of reverse transcriptase (Superscript II; RNase H negative; Gibco). The reaction was terminated by heat inactivation at 70°C for 10 min. PCR amplification was routinely carried out in a 50-μl reaction volume (10 mM Tris, pH 9; 50 mM KCl; 1.5 mM MgCl2; 200 μM concentrations of each deoxynucleoside triphosphate; and 20 pmol of specific 5′ and 3′ primers), using 1 U of Taq polymerase (Pharmacia Biotech). The temperature profile of the amplification consisted of 35 cycles of 45 s of denaturation at 94°C, 1 min 15 s of annealing at 58°C, and 1 min 15 s of extension at 72°C. PCR products were then separated on a 1% agarose gel and visualized by ethidium bromide staining on a UV transilluminator. Band intensities were quantified by densitometry (Seescan, Cambridge, United Kingdom). The ratios of the band intensities of the PCR products from the standard RNA and target RNA were plotted against the starting number of standard RNA molecules by using a double-logarithmic scale. When the ratio of the band intensities equals 1, the number of target RNA molecules is equivalent to the number of standard RNA molecules. Data are expressed as the number of target RNA molecules/microgram of total sample RNA. The quantitative RT-PCR was sensitive to 103 cytokine mRNA transcripts per μg of total RNA.
Immunoprecipitation and Western blot analysis.
Colonic tissue samples were lysed on ice in 300 μl of lysis buffer containing 0.0625 mol of Tris (pH 6.8)/liter, 2% sodium dodecyl sulfate (SDS), 3% β-mercaptoethanol, 10% glycerol, 100 mmol of sodium fluoride/liter, 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, and 1 mmol of phenylmethylsulfonyl fluoride/liter. The tissue was then homogenized by passage through a 21-gauge needle. The lysates were then centrifuged at 4,000 × g for 30 min at 4°C. The resulting supernatants were collected and stored at −80°C prior to analysis. The protein content was determined by using the Bio-Rad assay (Bio-Rad Laboratories, Hercules, Calif.). Total proteins (750 μg/sample) were incubated with anti-Stat4 (Santa Cruz Biotechnology, Santa Cruz, Calif.) at 4°C for 2 h. Immune complexes were collected by incubation with protein A/G agarose (20 μl/sample) (Santa Cruz Biotechnology), washed three times with lysis buffer, and heated for 5 min in a boiling water bath in sample buffer for SDS-polyacrylamide gel electrophoresis. Immunoprecipitates from extracts containing the same amount of protein were analyzed by Western blotting by using antibody against phosphotyrosine (p-Tyr) (1:1,000 final dilution; Santa Cruz Biotechnology), followed by a HRP-conjugated goat anti-mouse IgG monoclonal antibody (1:10,000 dilution; Dako). Reactivity was detected by a chemiluminescence kit (Amersham International). After the analysis of phosphorylated Stat4, blots were stripped by incubation for 30 min at 50°C in stripping medium (2% SDS, 0.05 M Tris, 0.1 M β-mercaptoethanol) and then incubated with antibody against Stat4 (Santa Cruz Biotechnology), followed by an HRP-conjugated goat anti-rabbit IgG antibody (1:2,500 dilution; Dako). Antibody reactivity was again developed by using chemiluminescence (Amersham International). As a positive control, peripheral blood mononuclear cells were extracted from healthy volunteers, resuspended in RPMI 1640 containing 10% fetal calf serum, and incubated with phytohemagglutinin (PHA) (1 μg/ml) overnight. The next day, the nonadherent cells were collected, washed once with PBS, and then stimulated with 5 ng of IL-12/ml for 1 h. Both p-Stat4 and total Stat4 were analyzed as previously described (44).
Statistical analysis.
The data are presented as the mean ± the standard error of the mean (SEM) and were analyzed by the Student's t test. P values of <0.05 were considered significant.
RESULTS
Bacterial burden in the colon of C. rodentium-infected TNFRp55−/− mice.
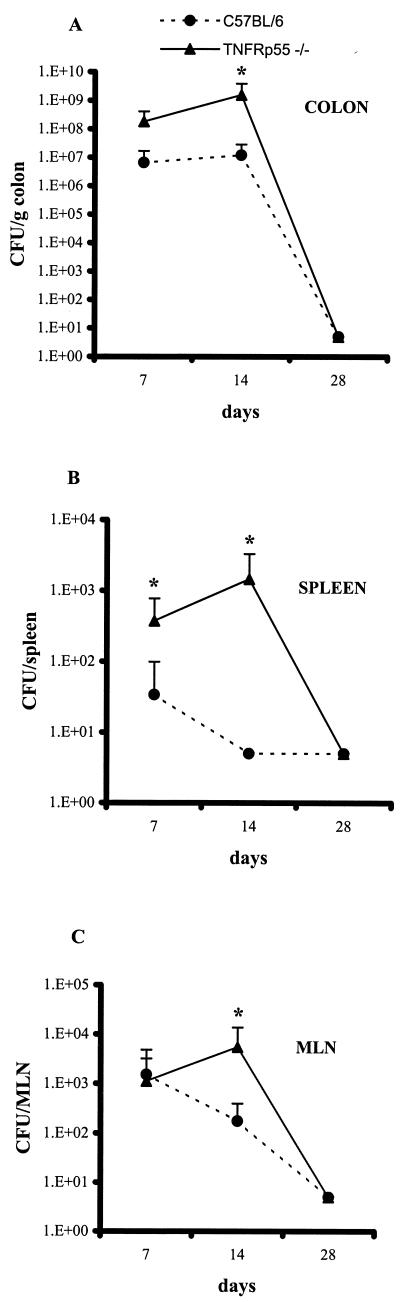
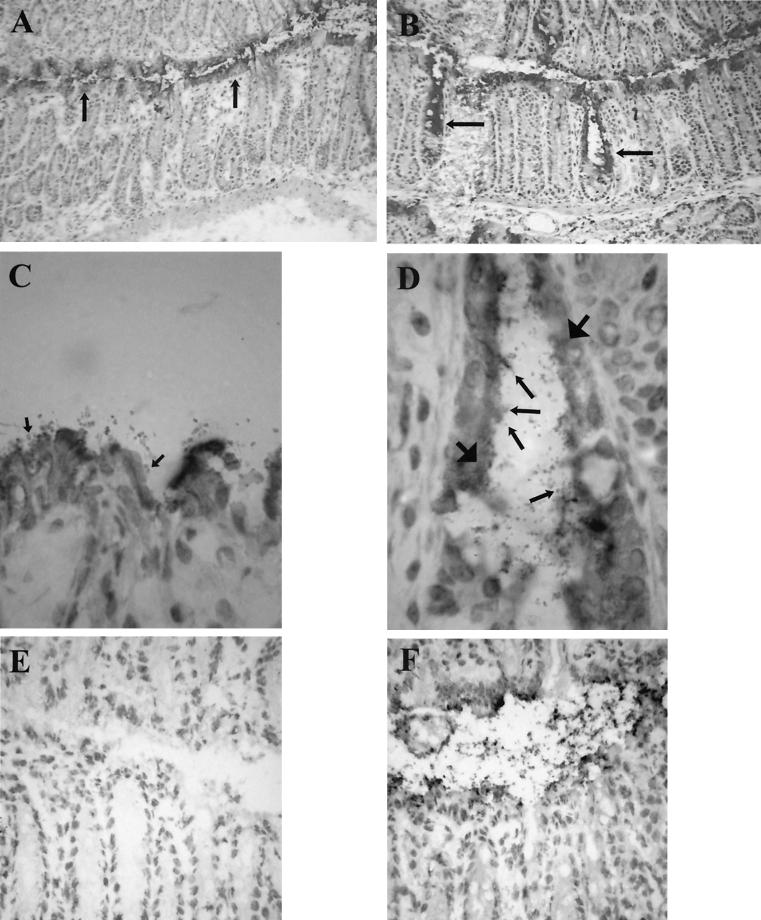
Age-matched female C57BL/6 and TNFRp55−/− mice were infected with the DBS255(pCVD438) C. rodentium strain and killed on days 7, 14, and 21 postinfection. Infected and uninfected C57BL/6 and TNFRp55−/− mice showed similar weight gains. As expected, bacteria were recovered from the colon at day 7 (Fig. 1A) in infected C57BL/6 and TNFRp55−/− mice; however, there was a greater number in the TNFRp55−/− mice which nearly achieved statistical significance (P = 0.06). At day 14, the colons of infected TNFRp55−/− mice showed a statistically significant 2-log increase in bacterial load compared to infected C57BL/6 mice (Fig. 1A, P = 0.006). Only small numbers of live bacteria were recovered from the MLN and spleens during the course of infection, but these were higher in the TNFRp55−/− mice (Fig. 1B and C). The presence of bacteria adhering to the epithelial surface was visualized by immunohistochemistry with anti-intimin antibody. In the infected C57BL/6 mice, bacteria were visualized on the surface of the epithelial cells facing the gut lumen (Fig. 2). Strikingly, in TNFRp55−/− mice, bacteria were present on the surface epithelium but were occasionally seen deep in the glands (Fig. 2). For example, in C57BL/6 mice at day 7, no mice showed bacteria deep in the glands; however, in TNFRp55−/− mice at the same time point, 4 of 6 mice showed bacteria at this site (5, 13, 14, and 15% of glands). Another striking feature was that the anti-intimin antibody, in addition to staining the bacteria, also stained epithelial cells underlying areas of bacterial colonization (Fig. 2).
FIG. 1.
Bacterial load in C. rodentium-infected C57BL/6 and TNFRp55−/− mice. Bacterial counts were determined in the colon (A), spleen (B), and MLN (C) at the indicated time points. Each point reflects the means and SEM (error bars) of five mice per group (✽, P < 0.05). The data shown are from one experiment of three, which yielded identical results. The lower limit detection of the bacteria is 10 CFU per organ.
FIG. 2.
Intimin staining of C. rodentium-infected C57BL/6 mice (magnifications: A, ×100; C, ×1,000; F, ×400) and TNFRp55−/− mice (magnifications: B, ×100; D, ×1,000). (D) Bacteria can be seen along the surface epithelium in both groups of mice (arrows) and occasionally deep in the glands in the infected TNFRp55−/− mice. (E) Rabbit isotype control IgG showed no staining (magnification, ×400). Another feature was that the anti-intimin antibody, in addition to staining the bacteria, also stained epithelial cells underlying areas of bacterial colonization (D, large arrows).
Colonic mucosal hyperplasia.
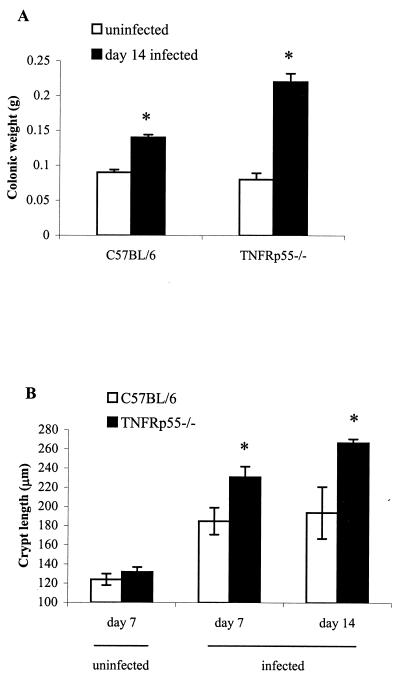
TNFRp55−/− C. rodentium-infected mice showed increased colonic weight compared to infected wild-type mice (Fig. 3A). This was associated with increased mucosal hyperplasia (Fig. 3B).
FIG. 3.
Weight of the distal colon (A) and crypt length (B) in uninfected and C. rodentium-infected C57BL/6 and TNFRp55−/− mice on days 7 and 14. Each group contained five mice (✽, P < 0.05).
Evidence for immune dysregulation in TNFRp55−/− infected with C. rodentium
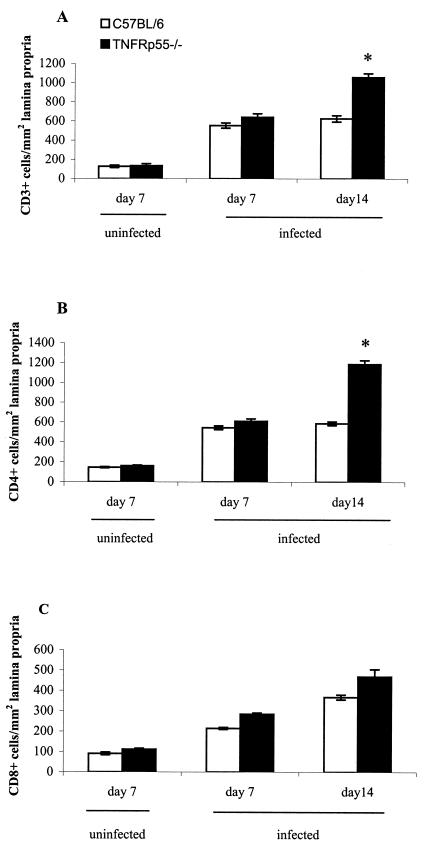
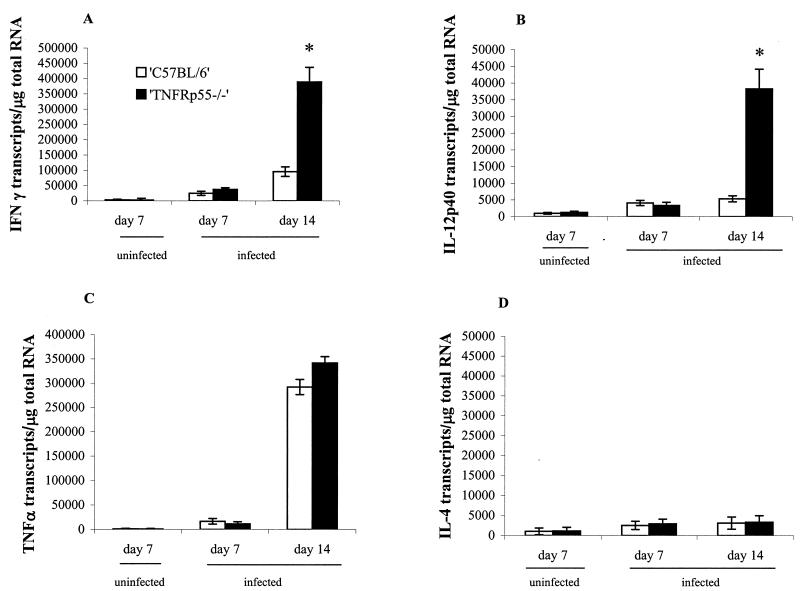
We next investigated the T-cell infiltrate and mRNA expression of regulatory cytokines. Immunohistology of the colon at day 14 postinfection revealed that there were approximately twice as many CD3+ cells present in the lamina propria of TNFRp55−/−-infected mice compared to the C57BL/6 mice (Fig. 4A). Similar changes in the number of CD4+ cells (Fig. 4B) were seen. CD8+ cells (Fig. 4C) were also increased compared to noninfected mice, but no difference was seen between the infected TNFRp55−/− mice and the infected C57BL/6 mice. Infected TNFRp55−/− mice showed a striking increase in IFN-γ mRNA transcripts (Fig. 5A) and IL-12p40 mRNA transcripts (Fig. 5B) at day 14 compared to infected C57BL/6 mice. TNF-α transcripts (Fig. 5C) were also increased, but no difference was seen between infected C57BL/6 and TNFRp55−/− mice. IL-4 mRNA did not significantly differ from uninfected controls at any time (Fig. 5D). Taken together, these data showed that the pronounced immunopathology in the infected TNFRp55−/− mice is linked to an exaggerated Th1 response in the lamina propria.
FIG. 4.
Cell counts for CD3+ (A), CD4+ (B), and CD8+ (C) cells infiltrating the lamina propria in uninfected and C. rodentium-infected C57BL/6 and TNFRp55−/− mice on days 7 and 14. Each group represents five mice (the mean ± SEM) (✽, P < 0.05).
FIG. 5.
Cytokine mRNA transcripts in gut tissue of uninfected and C. rodentium-infected C57BL/6 and TNFRp55−/− mice on days 7 and 14. Each group represents five mice (the mean ± SEM; ✽, P < 0.05). (A) IFN-γ. (B) IL-12p40. (C) TNF-α. (D) IL-4.
Stat4 activation.
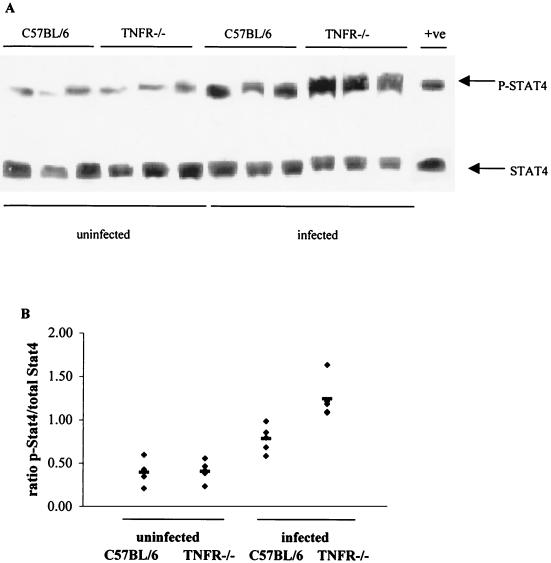
Stat4 is a key molecule in the intracellular signal transduction cascade activated in response to IL-12 stimulation. It is phosphorylated in response to IL-12 receptor ligation, resulting in the translocation of Stat4 to the nucleus and transactivation of genes that promote Th1 differentiation. An increase in the expression of phosphorylated Stat4 was seen in infected C57BL/6 mice, but this was even more enhanced in the TNFRp55−/− mice (Fig. 6).
FIG. 6.
Western blot analysis of Stat4 and tyrosine-phosphorylated Stat4 in the gut tissue of uninfected and C. rodentium-infected C57BL/6 and TNFRp55−/− mice on day 14. Three mice per group were analyzed in each of two independent experiments, and representative autoradiograph is shown in panel A. An increase in p-Stat4 expression was seen in the infected TNFRp55−/− mice at day 14. (B) The density of the bands was also quantified. In total, we analyzed 5 to 6 mice per group. As positive control (+ve), we used proteins extracted from human peripheral blood mononuclear cells preactivated with PHA and then stimulated with IL-12, the major Stat4-inducing cytokine.
DISCUSSION
In the present study, we have shown for the first time that TNF-α plays a role in controlling pathogenic bacterial populations in the gut and preventing tissue injury. We demonstrated that infection of TNFRp55−/− mice with C. rodentium resulted in more severe gut injury and pathology than in infected C57BL/6 control mice, but after 21 days the pathology resolved and the bacteria were cleared. More bacteria were also recovered in the colon and systemic tissues of infected TNFRp55−/− mice. Finally, there was a striking increase in IL-12p40 and IFN-γ mRNA transcripts in the C. rodentium-infected TNFRp55−/− mice.
It was unexpected that TNFRp55−/− mice would show increased lumenal bacterial burdens. One reason may be that TNF-α is involved in controlling the numbers of bacteria which initially colonize the gut by regulating innate immunity. In the lung and peritoneum, TNF-α produced by mast cells has been implicated in early host defenses against bacterial infections (35). The mechanism of early protection appears to be due to a mast cell-derived TNF-α-mediated emigration of neutrophils from the blood (35). However, the epithelial barrier and the low numbers of bacteria that actually colonize the epithelial surface early in infection means that early interactions with mucosal mast cells are not likely early in the case of C. rodentium infection. A more relevant cell in the context of early immunity in the gut could be the epithelial cell. Stimulation of human intestinal colonic epithelial cell lines with invasive strains of bacteria causes the increased secretion of chemoattractant and proinflammatory cytokines such as IL-8 and TNF-α (24). Proinflammatory cytokines can also be activated in uroepithelial cells after interaction with a noninvasive uropathogenic E. coli (40) and in human gut epithelial cell lines after infection with EPEC (49). Epithelial cells within the human stomach can also interact with the noninvasive bacterial pathogen, H. pylori, leading to upregulation of IL-8 gene expression in the apparent absence of bacterial entry (6). TNF-α could then amplify the early inflammatory response by attracting and activating neutrophils, macrophages, and eosinophils (8, 14). The inflammatory cells could then produce increased epithelial permeability, and antibacterial proteins in serum could act locally on the epithelial cell surface. Another potential defense mechanism could involve antimicrobial peptides, such as defensins. With the recent evidence for epithelial expression of human neutrophil defensins 1, 2, and 3 in IBD (7) and upregulation of β defensins in intestinal epithelial cells by proinflammatory cytokines (42, 53), an autocrine loop involving epithelial-cell-derived TNF acting on adjacent epithelial cells to upregulate defensin-mediated intestinal antimicrobial immunity is a possibility.
An alternative explanation for enhanced bacterial load is that it is secondary to the epithelial hyperplasia, which itself is secondary to the enhanced Th1 response we documented (although there is the possibility that it is the increased bacterial load per se that elicits the greater Th1 response). Since the bacteria colonize the surface of the mucosa, the increased surface area in the TNFRp55−/− mice could be responsible for the increase in colonic bacterial burden. It was also noteworthy that in the TNFRp55−/− mice the bacteria invaded the glands. This will contribute to the overall bacterial burden, but the reason for this is unknown. Strikingly, in areas of bacterial colonization, there was also intimin staining of epithelial cells underlying the bacteria. This strongly suggests that intimin is internalized in the epithelial cells in vivo, and this phenomenon is currently under investigation.
IL-12 is an immunoregulatory cytokine that plays a central role in the control of Th1 immune response and has been shown to induce the production of inflammatory cytokines such as IFN-γ and TNF-α (54). STATs (signal transducers and activators of transcription) are involved in the signal transduction cascades of many cytokines (22). Phosphorylated Stat4 was increased in the colon of C. rodentium infected TNFRp55−/− mice. The enhanced Th1 response could therefore lead to an increase in inflammation in the mucosa of the TNFRp55−/− mice, and indeed there were increased numbers of mucosal T cells in these mice. The link between stronger Th1 responses and epithelial hyperplasia may involve keratinocyte growth factor (KGF) production by fibroblasts, which is upregulated by proinflammatory cytokines such as IL-1β and TNF-α (5). KGF (FGF-7) is a member of the fibroblast growth factor family, is secreted by stromal cells, and plays a role in the continuous renewal of the intestinal epithelium (15). Studies in our laboratory have suggested a role for KGF in mediating crypt hyperplasia in coeliac disease (48), and in a model system we have also shown that KGF is involved in T-cell-driven crypt cell hyperplasia (2). The mechanisms involved in C. rodentium mucosal hyperplasia are currently under investigation, and the factors overexpressed in the thickened mucosa of TNFRp55−/− mice provide an ideal tool for investigating this problem.
There are other data to suggest that TNF-α directly inhibits IL-12 production, which would support the notion that it is the direct absence of TNF-α which results in higher IL-12 levels. It was recently shown that IL-12 production in thioglycolate-elicited mouse macrophages could be suppressed by TNF-α and that TNF-α-deficient mice developed a delayed but vigorous inflammatory response to heat-killed Corynebacterium parvum with very high levels of IL-12 in serum (21, 37). The reduced inflammation of Corynebacterium parvum-injected wild-type mice with lower IL-12 production suggested that TNF-α might play a role in limiting and reversing inflammation and tissue injury. Another study has also shown that TNF-α can downregulate the IL-12-dependent Th1 response during bacterial infections (32).
In conclusion, we show for the first time that TNF-α may play a role in controlling bacterial populations on the surface of the gut epithelium. Whether the enhanced immunopathology is primary or secondary to the enhanced bacterial burdens is currently under investigation.
ACKNOWLEDGMENTS
This study was supported by the European Union Training and Mobility of Researchers Programme (ERBFMRXCT9) and by the Wellcome Trust. N.S.G. was supported by the Crohn's in Childhood Research Association (CICRA).
N.S.G. and M.G.-M. contributed equally to this work.
REFERENCES
- 1.Adu-Robie J, Frankel G, Bain C, Gonçalves A G, Trabulsi L R, Douce G, Knutton S, Dougan G. Detection of intimins α, β, γ, and δ, four intimin derivatives expressed by attaching and effacing microbial pathogens. J Clin Microbiol. 1998;36:662–668. doi: 10.1128/jcm.36.3.662-668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bajaj-Elliott M, Poulsom R, Pender S L F, Wathen N C, MacDonald T T. Interaction between stromal cell-derived keratinocyte growth factor and epithelial transforming growth factor in immune-mediated crypt cell hyperplasia. J Clin Investig. 1998;102:1473–1480. doi: 10.1172/JCI2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barthold S W. The microbiology of transmissible murine colonic hyperplasia. Lab Anim Sci. 1980;30:167–173. [PubMed] [Google Scholar]
- 4.Binion D G, West G A, Ina K, Ziats N P, Emancipator S N, Fiocchi C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology. 1997;112:1895–1907. doi: 10.1053/gast.1997.v112.pm9178682. [DOI] [PubMed] [Google Scholar]
- 5.Chedid M, Rubin J S, Csaky K G, Aaronson S A. Regulation of keratinocyte growth factor gene expression by interleukin 1. J Biol Chem. 1994;269:10753–10757. [PubMed] [Google Scholar]
- 6.Crowe S E, Alvarez L, Dytoc M, Hunt R H, Muller M, Sherman P, Patel J, Jin Y, Ernst P B. Expression of interleukin 8 and CD54 by human gastric epithelium after Helicobacter pylori infection in vitro. Gastroenterology. 1995;108:65–74. doi: 10.1016/0016-5085(95)90009-8. [DOI] [PubMed] [Google Scholar]
- 7.Cunliffe R N, Rose F R A J, James P D, Mahida Y R. Expression of antimicrobial neutrophil defensin and lysozyme is induced in epithelial cells of active inflammatory bowel disease (IBD) mucosa. Gastroenterology. 1999;116:G3936. doi: 10.1136/jcp.55.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Danis V A, Franic G M, Rathjen D A, Brooks P M. Effects of granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-2, interferon-gamma (IFN-γ), tumour necrosis factor-alpha (TNF-alpha) and IL-6 on the production of immunoreactive IL-1 and TNF-alpha by human monocytes. Clin Exp Immunol. 1991;85:143–150. doi: 10.1111/j.1365-2249.1991.tb05695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Elios M M, Manghetti M, De Carli M, Costa F, Baldari C T, Burroni D, Telford J L. T helper 1 effector cells specific for Helicobacter pylori in the gastric antrum of patients with peptic ulcer disease. J Immunol. 1997;158:962–967. [PubMed] [Google Scholar]
- 10.Donnenberg M S, Tacket C O, James S P, Losonsky G, Nataro J P, Wasserman S S, Kaper J B, Levine M M. Role of the eaeA gene in experimental enteropathogenic Escherichia coli infection. J Clin Investig. 1993;92:1412–1417. doi: 10.1172/JCI116717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Donnenberg M S, Kaper J B, Finlay B B. Interactions between enteropathogenic Escherichia coli and host epithelial cells. Trends Microbiol. 1997;5:109–114. doi: 10.1016/S0966-842X(97)01000-7. [DOI] [PubMed] [Google Scholar]
- 12.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde K H, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 13.Eckmann L, Fierer J, Kagnoff M F. Genetically resistant (Ityr) and susceptible (Itys) congenic mouse strains show similar cytokine responses following infection with salmonella Dublin. J Immunol. 1996;156:2894–2900. [PubMed] [Google Scholar]
- 14.Ferrante A. Activation of neutrophils by interleukin-1 and−2 and tumor necrosis factors. Immunol Ser. 1992;57:417–436. [PubMed] [Google Scholar]
- 15.Finch P W, Rubin J S, Miki T, Ron D, Aaronson S A. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- 16.Fish S M, Proujansky R, Reenstra W W. Synergistic effects of interferon gamma and tumour necrosis factor alpha on T84 cell function. Gut. 1999;45:191–198. doi: 10.1136/gut.45.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frankel G, Phillips A D, Novakova M, Field H, Candy D C, Schauer D B, Douce G, Dougan G. Intimin from enteropathogenic Escherichia coli restores murine virulence to a Citrobacter eaeA mutant: induction of an immunoglobulin A response to intimin and EspB. Infect Immun. 1996;64:5315–5325. doi: 10.1128/iai.64.12.5315-5325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gitter A H, Bendfeldt K, Schulzke J D, Fromm M. Leaks in the epithelial barrier caused by spontaneous and TNF-alpha-induced single-cell apoptosis. FASEB J. 2000;14:1749–1753. doi: 10.1096/fj.99-0898com. [DOI] [PubMed] [Google Scholar]
- 19.Higgins L M, Frankel G, Douce G, Dougan G, MacDonald T T. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67:3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Higgins L M, Frankel G, Connerton I, Gonçalves N S, Dougan G, MacDonald T T. Role of bacterial intimin in colonic hyperplasia and inflammation. Science. 1999;285:588–591. doi: 10.1126/science.285.5427.588. [DOI] [PubMed] [Google Scholar]
- 21.Hodge-Dufour J, Marino M W, Horton M R, Jungbluth A, Burdick M D, Strieter R M, Noble P W, Hunter C A, Pure E. Inhibition of interferon-γ induced interleukin-12 production: a potential mechanism for the anti-inflammatory activities of tumor necrosis factors. Proc Natl Acad Sci USA. 1998;94:13806–13811. doi: 10.1073/pnas.95.23.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ihle J N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 23.Johnson E, Barthold S W. The ultrastructure of transmissible murine colonic hyperplasia. Am J Pathol. 1979;97:291–313. [PMC free article] [PubMed] [Google Scholar]
- 24.Jung H C, Eckmann L, Yang S K, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelials cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klapproth J M, Scaletsky I C, McNamara B P, Lai L C, Malstrom C, James S P, Donnenberg M S. A large toxin from pathogenic Escherichia coli strains that inhibits lymphocyte activation. Infect Immun. 2000;68:2148–2155. doi: 10.1128/iai.68.4.2148-2155.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kollias G, Douni E, Kassiotis G, Kontoyiannis D. The function of tumour necrosis factor in models of multi-organ inflammation, rheumatoid arthritis, multiple sclerosis and inflammatory bowel disease. Ann Rheum Dis. 1999;58(Suppl. I):I32–I39. doi: 10.1136/ard.58.2008.i32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kontoyiannis D, Pasparakis M, Pizarro T T, Cominelli F, Kollias G. Impaired on/off regulation of TNF biosynthesis in mice lacking TNF AU-rich elements: implications for joint and gut-associated immunopathologies. Immunity. 1999;10:387–398. doi: 10.1016/s1074-7613(00)80038-2. [DOI] [PubMed] [Google Scholar]
- 28.Lee A, Fox J G, Otto G, Murphy J. A small animal model of human Helicobacter pylori active chronic gastritis. Gastroenterology. 1990;99:1315–1323. doi: 10.1016/0016-5085(90)91156-z. [DOI] [PubMed] [Google Scholar]
- 29.Levine M M. Escherichia coli that cause diarrhea: enterotoxigenic, enteropathogenic, enteroinvasive, enterohemorrhagic, and enteroadherent. J Infect Dis. 1987;155:377–389. doi: 10.1093/infdis/155.3.377. [DOI] [PubMed] [Google Scholar]
- 30.Liesenfeld O, Kang H, Park D, Nguyen T A, Parkhe C V, Watanabe H, Abo T, Sher A, Remington J S, Suzuki Y. TNF-alpha, nitric oxide and IFN-gamma are all critical for development of necrosis in the small intestine and early mortality in genetically susceptible mice infected perorally with Toxoplasma gondii. Parasite Immunol. 1999;21:365–376. doi: 10.1046/j.1365-3024.1999.00237.x. [DOI] [PubMed] [Google Scholar]
- 31.Liesenfeld O, Kosek J, Remington J S, Suzuki Y. Association of CD4+ T-cell-dependent, interferon-γ mediated necrosis of the small intestine with genetic susceptibility of mice to peroral infection with Toxoplasma gondii. J Exp Med. 1996;184:597–607. doi: 10.1084/jem.184.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma X, Sun J, Papasavvas E, Riemann H, Robertson S, Marshall J, Bailer R T, Moore A, Donnelly R P, Trinchieri G, Montaner L J. Inhibition of IL-12 production in human monocyte-derived macrophages by TNF. J Immunol. 2000;164:1722–1729. doi: 10.4049/jimmunol.164.4.1722. [DOI] [PubMed] [Google Scholar]
- 33.MacDermott R P, Sanderson I R, Reinecker H C. The central role of chemokines (chemotactic cytokines) in the immunopathogenesis of ulcerative colitis and Crohn's disease. Inflamm Bowel Dis. 1998;4:54–67. doi: 10.1097/00054725-199802000-00009. [DOI] [PubMed] [Google Scholar]
- 34.Maini R N, Elliott M J, Brennan F M, Williams R O, Chu C Q, Paleolog E, Charles P J, Taylor P C, Feldmann M. Monoclonal anti-TNF-α antibody as a probe of pathogenesis and therapy of rheumatoid disease. Immunol Rev. 1995;144:195–223. doi: 10.1111/j.1600-065x.1995.tb00070.x. [DOI] [PubMed] [Google Scholar]
- 35.Malaviya R, Ikeda T, Ross E, Abraham S N. Mast cell modulation of neutrophil influx and bacterial clearance at sites of infection through TNF-α. Nature. 1996;381:77–80. doi: 10.1038/381077a0. [DOI] [PubMed] [Google Scholar]
- 36.Marchetti M, Arico B, Burroni D, Figura N, Rappuoli R, Ghiara P. Development of a mouse model of Helicobacter pylori infection that mimics human disease. Science. 1995;267:1655–1658. doi: 10.1126/science.7886456. [DOI] [PubMed] [Google Scholar]
- 37.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L J. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McDaniel T K, Jarvis K G, Donnenberg M S, Kaper J B. A genetic locus of enterocyte effacement conserved among diverse enterobacterial pathogens. Proc Natl Acad Sci USA. 1995;92:1664–1668. doi: 10.1073/pnas.92.5.1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohammadi M, Czinn S, Redline R, Nedrud J. Helicobacter-specific cell-mediated immune responses display a predominant Th1 phenotype and promote a delayed-type hypersensitivity response in the stomachs of mice. J Immunol. 1996;156:4729–4738. [PubMed] [Google Scholar]
- 40.Mulvey M A, Schilling J D, Martinez J J, Hultgren S J. From the cover: bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci USA. 2000;97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Neurath M F, Fuss I, Pasparakis M, Alexopoulou L, Haralambous S, Meyer zum Büschenfelde K H, Strober W, Kollias G. Predominant pathogenic role of tumour necrosis factor in experimental colitis in mice. Eur J Immunol. 1997;27:1743–1750. doi: 10.1002/eji.1830270722. [DOI] [PubMed] [Google Scholar]
- 42.O'Neil D A, Porter E M, Elewaut D, Anderson G M, Eckmann L, Ganz T, Kagnoff M F. Expression and regulation of the human beta-defensins hBD-1 and hBD-2 in intestinal epithelium. J Immunol. 1999;163:6718–6724. [PubMed] [Google Scholar]
- 43.Panja A, Goldberg S, Eckmann L, Krishen P, Mayer L. The regulation and functional consequence of proinflammatory cytokine binding on human intestinal epithelial cells. J Immunol. 1998;161:3675–3684. [PubMed] [Google Scholar]
- 44.Parrello T, Monteleone G, Cucchiara S, Monteleone I, Sebkova L, Doldo P, Luzza F, Pallone F. Up-regulation of the IL-12 receptor beta 2 chain in Crohn's disease. J Immunol. 2000;165:7234–7239. doi: 10.4049/jimmunol.165.12.7234. [DOI] [PubMed] [Google Scholar]
- 45.Pender S L F, Fell J M, Chamow S M, Ashkenazi A, MacDonald T T. A p55 TNF receptor immunoadhesin prevents T cell-mediated intestinal injury by inhibiting matrix metalloproteinase protection. J Immunol. 1998;160:4098–4103. [PubMed] [Google Scholar]
- 46.Powrie F, Leach M W, Mauze S, Menon S, Caddle L B, Coffman R L. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 47.Rothbaum R J, Partin J C, Saalfield K, McAdams A J. An ultrastructural study of enteropathogenic Escherichia coli infection in human infants. Ultrastruct Pathol. 1983;4:291–304. doi: 10.3109/01913128309140582. [DOI] [PubMed] [Google Scholar]
- 48.Salvati V M, Bajaj-Elliott M, Poulsom R, Mazzarella G, Nilsen E M, Lundin K E A, Troncone R, MacDonald T T. Keratinocyte growth factor in celiac disease. Gut. 2001;49:176–181. doi: 10.1136/gut.49.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Savkovic S D, Koutsouris A, Hecht G. Attachment of a noninvasive enteric pathogen, enteropathogenic Escherichia coli, to cultured human intestinal epithelial monolayers induces transmigration of neutrophils. Infect Immun. 1996;64:4480–4487. doi: 10.1128/iai.64.11.4480-4487.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schauer D B, Falkow S. The eae gene of Citrobacter freundii biotype 4280 is necessary for colonization in transmissible murine colonic hyperplasia. Infect Immun. 1993;61:4654–4661. doi: 10.1128/iai.61.11.4654-4661.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharief M K, Phil M, Hentges R. Association between tumor necrosis factor-a and disease progression in patients with multiple sclerosis. N Engl J Med. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- 52.Targan S R, Hanauer S B, van Deventer S J H, Mayer L, Present D H, Braakman T, DeWoody K L, Schaible T F, Rutgeerts P J. A short-term study of chimeric monoclonal antibody cA2 to tumor necrosis factor α for Crohn's disease. N Engl J Med. 1997;337:1029–1035. doi: 10.1056/NEJM199710093371502. [DOI] [PubMed] [Google Scholar]
- 53.Tarver A P, Clark D P, Diamond G, Russell J P, Erdjument-Bromage H, Tempst P, Cohen K S, Jones D E, Sweeney R W, Wines M, Hwang S, Bevins C L. Enteric beta-defensin: molecular cloning and characterization of a gene with inducible epithelial cell expression associated with Cryptosporidium parvum infection. Infect Immun. 1998;66:1045–1056. doi: 10.1128/iai.66.3.1045-1056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trinchieri G. Interleukin 12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T helper cells type I and cytotoxic lymphocytes. Blood. 1994;84:4008–4027. [PubMed] [Google Scholar]
- 55.Vallance B A, Finlay B B. Exploitation of host cells by enteropathogenic Escherichia coli. Proc Natl Acad Sci USA. 2000;97:8799–8806. doi: 10.1073/pnas.97.16.8799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Deventer S J H. Tumor necrosis factor and Crohn's disease. Gut. 1997;40:443–448. doi: 10.1136/gut.40.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Dullemen H M, van Deventer S J H, Hommes D W, Bijl H A, Jansen J, Tytgat G N J, Woody J. Treatment of Crohn's disease with anti-tumor necrosis factor chimeric monoclonal antibody (cA2) Gastroenterology. 1995;109:129–135. doi: 10.1016/0016-5085(95)90277-5. [DOI] [PubMed] [Google Scholar]
- 58.Vassalli P. The pathophysiology of tumor necrosis factors. Annu Rev Immunol. 1992;10:411–452. doi: 10.1146/annurev.iy.10.040192.002211. [DOI] [PubMed] [Google Scholar]
- 59.Viney J, MacDonald T T, Kilshaw P J. T cell receptor expression in intestinal intraepithelial lymphocyte subpopulations of normal and athymic mice. Immunology. 1989;66:583–587. [PMC free article] [PubMed] [Google Scholar]
- 60.Weil D. What's new about tumour necrosis factors? Eur Cytokine Network. 1992;3:347–351. [PubMed] [Google Scholar]