Abstract
Candida auris is an emerging fungus that represents a serious health threat globally. In Italy, the first case was detected in July 2019. Then, one case was reported to the Ministry of Health (MoH) on January 2020. Nine months later, a huge number of cases were reported in northern Italy. Overall, 361 cases were detected in 17 healthcare facilities between July 2019 and December 2022 in the Liguria, Piedmont, Emilia-Romagna, and Veneto regions, including 146 (40.4%) deaths. The majority of cases (91.8%) were considered as colonised. Only one had a history of travel abroad. Microbiological data on seven isolates showed that all but one strain (85.7%) were resistant to fluconazole. All the environmental samples tested negative. Weekly screening of contacts was performed by the healthcare facilities. Infection prevention and control (IPC) measures were applied locally. The MoH nominated a National Reference Laboratory to characterise C. auris isolates and store the strains. In 2021, Italy posted two messages through the Epidemic Intelligence Information System (EPIS) to inform on the cases. On February 2022, a rapid risk assessment indicated a high risk for further spread within Italy, but a low risk of spread to other countries.
Keywords: Candida auris, outbreak, epidemic, epidemiology, Italy, healthcare-associated infections
1. Introduction
The dissemination of Candida auris [1,2,3,4] and other multidrug-resistant organisms [5,6] is a growing public health threat worldwide, aggravated by the COVID-19 pandemic [7], that requires an improvement in microbiology laboratory capacity, integrated surveillance systems, and policy action.
C. auris has caused severe infections around the world. The earliest isolates of nosocomial infection were identified in 1996 in Korea [8] and in 2009 in Japan [9], while the identification of the first European case dated back to 2007 in France, and it was due to an isolate belonging to the South Indian clade I [10].
Since then, the reporting of cases was increasing rapidly worldwide. In the European Economic Area (EU/EEA), 620 cases were reported in the period January 2013–December 2017 [11], and 349 cases were reported between January 2018 and May 2019 [12]. In the period October 2021–September 2022, the United States reported 1994 clinical cases and 5071 screening cases [2], and its transmission is steadily increasing [13].
Worldwide, the following risk factors for C. auris infection have been described: male gender, prematurity, underlying diseases such as diabetes, kidney or ear disease, trauma, history of central venous catheters, and use of broad-spectrum antibiotics [14]. In the United States, C. auris has been predominantly identified among patients with extensive exposure to ventilator units by skilled nursing facilities and long-term acute care hospitals [15]. The clinical manifestation of C. auris infection depends upon the site of infection. It can cause wound infections and otitis. C. auris was found in urine and respiratory specimens, although its contribution to clinical disease in these sites is unclear, while bloodstream infection typically causes sepsis and severe illness. Invasive infections due to C. auris were associated with a 30–60% in-hospital crude mortality rate. In addition, C. auris can colonise patients’ skin and other body sites asymptomatically, and it is the only Candida species that is transmissible from patient to patient. Most of the C. auris strains were resistant to at least one out of the three major classes of antifungal drugs, one-third were resistant to two antifungal drug classes, and some strains were resistant to all of the three classes [16]. Particularly, antifungal-resistant C. auris strains increased by 60% in 2020 vs. 2019 in the US due to the COVID-19 epidemic. C. auris has caused numerous healthcare-associated outbreaks [17], also spreading among hospitalised patients with COVID-19 [18,19]. C. auris can persist in the healthcare environment for weeks, with reduced susceptibility against clinically relevant concentrations of chlorhexidine and hydrogen peroxide, and eradication is achieved using povidone iodine only [20,21].
The control of C. auris requires timely detection and adherence to recommended infection prevention and control (IPC) practices. Yeast identification methods used at many clinical laboratories often misidentify C. auris as other yeasts (e.g., Candida haemulonii) [22,23], thereby making its control challenging. A consensus case definition, which was approved in 2018 [24] and last updated in 2019 [25], allows for the standardised public health tracking and surveillance of C. auris cases, providing opportunity for rapid response to contain its spread.
In Italy, cases of Candida spp., including C. auris, are subjected to mandatory notification according to the Ministerial Decree of 15 December 1990 “Information system for infectious and diffusive diseases” referring to infectious diseases included in the Class V. The first case of invasive C. auris infection was identified in 2019 [26]; then, other scientific articles described hospital outbreaks that occurred locally in recent years [27,28].
This report describes the demographic, geographic, and temporal variables associated with C. auris cases reported in Italy, together with the available microbiological results, with the aim to inform and raise awareness on this health threat and facilitate its prevention and control at the country and global level.
2. Materials and Methods
Cases were reported by routine surveillance from the local healthcare facilities to the regional health authorities and/or the MoH through the regional electronic systems or email referring to the case definitions described by the European Centre for Disease Prevention and Control (ECDC) [17]. On October 2021, following the notification of cases from the region Liguria, the Italian MoH queried this region and the neighbours (Emilia-Romagna, Lombardy, Piedmont, and Tuscany) specifically for retrospective identification and notification of cases. Passive notification of cases from all the interested Regions through the country continued. Here, we carried out a national descriptive study of cases infected or colonised by C. auris identified in Italy between July 2019 and December 2022.
Confirmed cases were individuals with C. auris identified by Matrix-Assisted Laser Desorption Ionisation Time of Flight (MALDI-TOF Bruker), VITEK 2 YST (bioMérieux), or Polymerase Chain Reaction (PCR) performed in clinical samples or surveillance cultures according to the referred diagnostic methods [3,22]. Contact tracing was performed for patients admitted in hospital wards where a confirmed case was hospitalised. All the cases, with the exception of the only one imported, had epidemiological (being hospitalised in the same ward/hospital regardless of temporal link).
Each involved region sent to the MoH the demographic, microbiological, and clinical data by one excel file. Data were anonymised at the local level. We performed a descriptive analysis by the R statistical software (ver. 4.1.1). The ECDC Map Maker tool (EMMa) was used to create the map of the areas with resident cases. The number (N) of the subjects with data available was detailed in Table 1 for each demographic and clinical characteristic.
Table 1.
Main demographic and clinical characteristics of confirmed C. auris cases, Liguria, Piedmont and Emilia-Romagna regions, Italy, July 2019–December 2022 (N = 360 out of 361 total cases).
| Patients’ Characteristics (%) | Liguria N = 297 |
Piedmont N = 48 |
Emilia-Romagna N = 15 |
Total N = 360 |
|---|---|---|---|---|
| Median age (years, range) | N = 295 64 (0–91) |
N = 48 64 (22–82) |
N = 15 61.5 (47–87) |
N = 358 64 (0–91) |
| Gender | N = 297 | N = 48 | N = 12 | N = 357 |
| Males | 195 (65.7) | 32 (66.7) | 10 (83.3) | 237 (66.3) |
| Females | 102 (34.3) | 16 (33.3) | 2 (16.7) | 120 (33.6) |
| Sample type | N = 294 | N = 48 | N = 15 | N = 357 |
| Blood | 25 (8.5) | 2 (4.2) | 3 (20) | 30 (8.4) |
| BAL/bronchial aspirate | 40 (13.6) | 3 (6.3) | 6 (40) | 49 (13.7) |
| CVC | 0 | 1 (2.1) | 1 (6.7) | 2 (0.6) |
| Sputum | 1 (0.3) | 0 | 0 | 1 (0.3) |
| Surgical wound | 1 (0.3) | 0 | 0 | 1 (0.3) |
| Swab (inguinal/axillary and/or rectal) | 217 (73.8) | 40 (83.3) | 3 (20) | 260 (72.8) |
| Urine | 10 (3.4) | 2 (4.2) | 2 (13.3) | 14 (3.9) |
| Hospital ward | N = 296 | N = 48 | N = 15 | N = 359 |
| Emergency room | 6 (2) | 0 | 0 | 6 (1.7) |
| Geriatrics | 0 | 0 | 2 (13.3) | 2 (0.6) |
| ICU | 228 (77.0) | 46 (95.8) | 10 (66.7) | 284 (79.1) |
| Internal medicine | 31 (10.5) | 1 (2.1) | 0 | 32 (8.9) |
| Rehabilitation unit | 2 (0.7) | 0 | 3 (20) | 5 (1.4) |
| Surgery | 29 (9.8) | 1 (2.1) | 0 | 30 (8.4) |
| Main comorbidity/medical issue | N = 289 | N = 48 | N = 5 | N = 342 |
| No symptoms due to C. auris | 54 (18.7) | 48 (100) | 14 (93.3) | 116 (33.9) |
| Acute respiratory disease | 19 (6.6) | 5 (10.4) | 2 (40.0) | 26 (7.6) |
| Bacteraemia | 9 (3.0) | 2 (4.2) | 2 (40.0) | 13 (3.8) |
| Chronic respiratory disease | 4 (1.4) | 1 (2.1) | 0 | 5 (1.5) |
| Cardiac disease | 21 (7.3) | 5 (10.4) | 0 | 26 (7.6) |
| SARS-CoV-2 positive/COVID-19 | 89 (30.8) | 14 (29.2) | 0 | 103 (30.1) |
| Diabetes | 3 (1.0) | 2 (4.2) | 0 | 5 (1.5) |
| Immunosuppressive disorder/Cancer | 4 (1.4) | 6 (12.5) | 0 | 10 (2.9) |
| Intestinal/renal/mediastinal disorder | 0 | 10 (20.8) | 0 | 10 (2.9) |
| Neurologic disorder | 62 (21.5) | 2 (4.2) | 1 (20.0) | 65 (19.0) |
| Sepsis/septic shock | 32 (11.1) | 0 | 0 | 32 (9.4) |
| Surgery | 38 (13.1) | 0 | 0 | 38 (11.1) |
| Trauma | 14 (4.8) | 1 (2.1) | 0 | 15 (4.4) |
| Median time between diagnosis and death (days) | 6.0 * | 10.0 § | n.a. | n.a. |
| In-hospital lethality | 119 (40.1) | 20 (41.7) | 6 (50.0) | N = 145 (40.3) |
* the denominator is 29 deaths; § the denominator is 20 deaths; BAL broncho-aspirated lavage; ICU intensive care unit; CVC central venous catheter; n.a. data not available.
Antifungal susceptibility testing was performed by using MICRONAUT-AM broth microdilution panels (MERLIN Diagnostika GmbH, Bornheim, Germany). According to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) clinical breakpoints [29], we defined antifungal resistance as referred to Fuconazole, while non-species related breakpoints have been determined on the basis of PK/PD.
All the high-touch surfaces in the patient area were sampled and tested regularly after the notification of each colonised/infected case.
Incidence rates were calculated by dividing the number of cases by the number of the resident population per region. The year 2021 was selected as the mid-year in the referred period mid 2019–end of 2022. Regional demographic data were obtained by the Italian National Institute of Statistics (ISTAT, https://demo.istat.it/index_e.php, accessed on 1 December 2022). The resident population included both Italian and foreign individuals resident in Italy even if temporarily absent.
3. Results
3.1. Notification of Cases
The first confirmed case due to C. auris occurred in a hospital of the Liguria region in 2019 [26] and was reported to the MoH on January 2020. A literature search in PubMed revealed some cases occurred in the same hospital in 2020 [30,31], while the Region Liguria where the hospital was located reported cases on February, September, and October 2021. Then, following the Ministerial request of retrospective investigation, Liguria reported a huge outbreak with 277 cases infected or colonised by C. auris occurring between November 2020 and October 2021 [32]. Moreover, 83 more cases were reported in the period November 2021–December 2022 (19 by Liguria, 48 by Piedmont, 15 by Emilia-Romagna, and 1 by Veneto) for a total of 361 cases.
The last C. auris cases were reported on December 2021 in Emilia-Romagna, on June 2022 in Veneto, on September 2022 in Liguria and on December 2022 in Piedmont.
3.2. Characteristics of Cases Colonised or Infected by C. auris
The index case identified in Liguria was reported by Crea et al. in 2019 [26]. The patient had no history of recent travel abroad or hospital admission.
Table 1 describes the main demographic and clinical characteristics of confirmed C. auris cases reported in Liguria, Piedmont, and Emilia-Romagna regions, while only one case was reported by the Veneto region in June 2022. The number of the subjects (N) for whom data were available was detailed for each variable and considered as a denominator for the proportion of the specific variable listed. Overall, the mean age of the cases was 61.8 years (median age 64 years, age range: 0–91, N = 358), and most of them (66.3%, N = 237) were males. All the cases were hospitalised; most of them (79.1%, N = 284) were admitted to an Intensive Care Unit (ICU). One-third of the cases had no symptoms related to C. auris (33.9%, N = 116), had COVID-19 or tested SARS-CoV-2 positive (30.1%, N = 103), and some of them suffered from neurologic disorders (19.0%, N = 65), underwent surgery (8.4%, N = 30), and had sepsis/septic shock (9.4%, N = 32), cardiac (7.6%, N = 26) or acute respiratory disease (7.6%, N = 26).
Particularly, the variations in time elapsed between the date of sampling and the date of diagnosis follow: Liguria, median time 2.0 days (mean 2.8 days, range 0–7 days, calculated for N = 26 cases); Piedmont, median time 0 days (mean 0.1 days, range 0–7 days, N = 48); Emilia-Romagna, median time 2.5 days (mean 2.8 days, range 2–5 days, calculated for N = 12 cases).
Patients who died with/by C. auris were mid-age adults (Liguria region: mean age 64.9 years old, median age 70.6, age range 0–84, data available for N = 28 cases; Piedmont region: mean age 64.6 years old, median age 63, age range 43–82, N = 20; Emilia-Romagna region: mean age 61.7 years old, median age 59.5, age range 47–87, N = 6). These patients were hospitalised in the following wards: in Liguria, almost two-thirds of the patients (65.5%) in ICU, 4 (13.8%) Surgery, 4 (13.8%) Internal Medicine, 2 (6.9%) Emergency Department; in Piedmont, they were all admitted to ICU; in Emilia-Romagna, 5 were hospitalised in ICU and 1 was in Geriatrics.
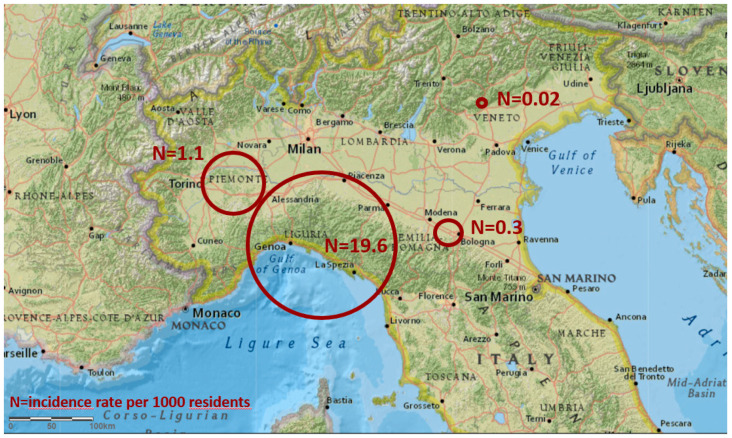
Overall, 361 cases were reported by 17 healthcare facilities located in 4 regions (297 cases distributed in 13 facilities in Liguria, 48 cases in 1 hospital in Piedmont, 15 cases in 2 facilities in Emilia-Romagna, 1 case in 1 hospital in Veneto). Figure 1 describes the areas where the confirmed cases were reported, colonised with or infected by C. auris.
Figure 1.
Map of the areas from where confirmed cases colonised with or infected by C. auris were reported, located in 4 neighbouring regions, North Italy, July 2019–December 2022 (N = 361). The size of the red circles represents the regional incidence rate per 1000 residents (N).
Overall, 82.3% of the cases were located in Liguria region, 13.3% in Piedmont, 4.2% in Emilia-Romagna and 0.3% in Veneto.
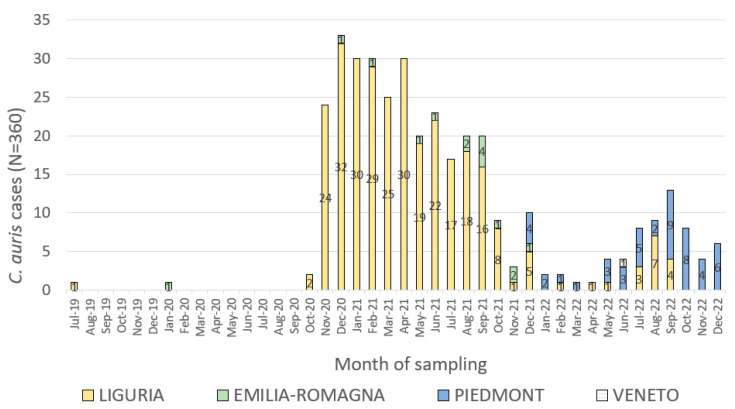
Figure 2 shows the epicurve of the cases occurred in the neighbouring four regions involved in the C. auris outbreaks in north Italy. Cases for whom the date of sampling was available are shown in the epicurve.
Figure 2.
Epicurve of confirmed C. auris cases occurred in Liguria, Piedmont, Emilia-Romagna and Veneto regions by date of sampling and region of residence, Italy, July 2019–December 2022 (N = 360 out of 361 cases).
The shape of the epicurve suggested an ongoing source epidemic that raised to a peak in December 2020, with a second lower peak occurred in September 2022.
Table 2 reports the number of C. auris cases and the incidence rate per 1000 residents by region together with data on the number of residents and the number of beds in hospital facilities and nursing homes.
Table 2.
Incidence rate of C. auris cases per 1000 residents and number of healthcare beds by region, Liguria, Piedmont, Emilia-Romagna and Veneto regions, Italy, July 2019–December 2022.
| Region | N. of Cases | Incidence per 1000 Residents | N. of Residents (1 Jan 2021) |
N. of Hospital/Healthcare Beds (1 Jan 2019) |
|---|---|---|---|---|
| Liguria | 297 | 19.6 | 1,518,495 | 5723 |
| Piedmont | 48 | 1.1 | 4,274,945 | 16,513 |
| Emilia-Romagna | 15 | 0.3 | 4,438,937 | 17,308 |
| Veneto | 1 | 0.02 | 4,869,830 | 17,472 |
| Total | 361 | 0.024 | 15,102,207 | 57,016 |
Table 2 describes the total number of cases and the size of the population resident in the four interested regions.
3.3. Laboratory Testing
Among the microbiological methods reported for the diagnosis of C. auris infection/colonisation [3,22], MALDI-TOF was used mostly (126 out of 142 cases, 88.7%), while Biomerieux Vitek or PCR were used in very few cases (three out 79 reported, 3.8%, and two out of 79 reported, 2.5%, respectively).
Antifungal susceptibility testing was performed on strains from infected patients rather than colonised individuals. All but one (85.7%, N = 7) isolate were resistant to at least one antifungal treatment (according to EUCAST breakpoints, fluconazole). Table 3 shows the microbiological results of the C. auris isolates tested against the main antifungal drugs. Data were available for seven strains from the Emilia-Romagna region, July 2019–December 2022.
Table 3.
Minimum inhibitory concentration resulted from susceptibility testing of C. auris strains isolated in Emilia-Romagna region, Italy, July 2019–December 2022 (N = 7).
| Antifungal Drugs | MIC Range (mcg/mL) |
|---|---|
| Triazoles | |
| Fluconazole | ≤0.0019–8 |
| Itraconazole | ≤0.0312–1 |
| Posaconazole | ≤0.0078–0.25 |
| Voriconazole (and other 2° generation azoles) | ≤0.0078–2 |
| Polyenes | |
| Amphotericin B | ≤0.0312–2 |
| Anidulafungin | ≤0.0019–0.125 |
| Caspofungin | 0.0156–0.25 |
| Micafungin | ≤0.0019–0.12 |
| Flucytosine | ≤0.0625–0.5 |
MIC minimum inhibitory concentration.
3.4. Environmental Investigation
Environmental investigations were conducted in Emilia-Romagna and Liguria regions. All the environmental samples tested negative for C. auris.
3.5. Control Measures
In each healthcare facility involved, the local infection prevention and control (IPC) teams put in place several health measures to control the spread of C. auris: standard and contact precautions, single room isolation or patient cohorting, dedicated staff and equipment where available, weekly screening of close contacts (sharing room, ward, non-disposable devices, and/or staff) until two weeks after the last case was discharged, internal audits, chlorine-based environmental cleaning and reprocessing of medical devices. The cleaning staff was instructed on the specific disinfection measures. Regular meetings of the health professionals were carried out as well as protocols for cases’ management were produced in the facilities involved. C. auris was included among the alert organisms. The patients and their families were informed on C. auris and provided with behaviour information at discharge.
In May 2018, following the Rapid Risk Assessment (RRA) by ECDC on C. auris infection, the Italian MoH published a circular letter to raise awareness on this health threat. The MoH informed the regions and autonomous provinces on the online platform of the ECMM (European Confederation of Medical Mycology) project FungiScope™ CandiReg [33], which enables international surveillance and facilitates the conduct of epidemiological studies, and nominated the National Reference Laboratory (Fondazione Policlinico Universitario A. Gemelli IRCCS Università Cattolica del Sacro Cuore) to support the characterisation of isolates and storage of strains. Then, the MoH organised meetings with the Italian regions involved and published five circular letters to give details for the notification of all the confirmed cases colonised/infected by C. auris isolated both in public and accredited healthcare facilities, microbiological information on this health threat, and epidemiological update while highlighting specific recommendations.
To apprise the European countries, the MoH shared the first message through the web-based communication platform Epidemic Intelligence Information System (EPIS), which was later replaced by the European surveillance portal for infectious diseases (EpiPulse), on June 2021. A second update was posted on December 2021. In addition, dedicated meetings with the competent international health authorities (i.e., World Health Organisation—WHO, Transatlantic Taskforce on Antimicrobial Resistance—TATFAR, ECDC) were organised.
Italy contributed to the third C. auris survey [34] conducted by ECDC on April 2022.
4. Discussion
Multiple transmission chains starting from different sources may have occurred simultaneously in the C. auris outbreaks described in Italy. Possibly, patients had common procedures in the same healthcare facility. The transfer of cases colonised with or infected by C. auris between facilities might have been unreported. Almost 1400 public or private healthcare facilities are located in Italy, of which over 10% are distributed in the two main regions with C. auris cases. Moreover, the capacity for the microbiological identification of C. auris and for the active surveillance of cases as well as the ability for patient isolation are highly heterogeneous through the country. However, Katja Saris et al. did not find any information of cases isolated in Italy in the period Jan 2016–July 2018 published in the scientific literature, including gray literature [35]. Some samples were processed by molecular typing, highlighting a recent introduction of C. auris in Italy and a rapid spread to some extent likely to be facilitated by the COVID-19 epidemic [30,31]. Particularly, around one-third of the cases overall had a positive SARS-CoV-2 test. Nevertheless, based on the regional trends of the epidemic, we observed that C. auris patients were diagnosed in periods when SARS-CoV-2 cases were less numerous than in others. We assumed that the decrease in C. auris cases between late-2021 and mid-2022 and then at the end of 2022 was attributed to several and repeated health interventions put in place in each involved facility.
Given the high number of C. auris cases identified in Italy, the spread to several healthcare facilities and the difficulties to stop recurrence, on February 2022, the ECDC defined the risk of further spread within the country as high, while the risk of spread into other European areas was defined as low, unless there was a cross-border transfer of hospitalised patients. However, the epidemiological situation is rapidly deteriorating at the global level [34,36,37,38], and continued vigilance is needed. C. auris is an urgent health threat due to its global and rapid emergence, challenging microbiological identification, high mortality, and persistent transmissions. In the ECDC point prevalence survey on healthcare-associated infections in European acute care hospitals performed in 2011–2012, Candida spp. was the fifth most common pathogen associated with septicaemias, which was isolated in 7.4% of all documented cases. Results from the ECDC surveys described over 1800 C. auris cases reported by 15 EU/EEA countries from 2013 to 2021.
The COVID-19 pandemic likely intensified the spread of C. auris and hindered the detection of additional cases [39]. The increased spread in hospitals could be a result of staff and supply shortages as well as changes in IPC practices (e.g., re-use or extended use of gowns and gloves). Although there is evidence of limited recombination in nature among the five clades after their divergence [40], clinical isolates were shown to be highly resistant to antifungal treatments [41] and surface disinfection. In particular, the marine environment was recently identified as a natural niche for this pathogen, while stored apples treated with antifungal agents were pointed as a possible reservoir for the transmission of C. auris-resistant strains to humans [42]. Thus, effective measures to contain C. auris spread require a multi-disciplinary approach [43].
5. Conclusions
The epidemiological situation regarding C. auris in northern Italy is alarming. Large and prolonged outbreaks cannot be ruled out for the years 2021 and 2022. Outbreak investigations should be initiated to detect possible sources and transmission routes, to better describe the outbreak strain and to identify risk factors in hospitalised patients.
The spread of C. auris underlined the importance of several practices: prompt outbreak investigation, availability of dedicated staff and isolation rooms, correct microbiological identification [44,45], effective treatment [46], trained health personnel, rigorous application of IPC measures including hand hygiene and research for innovative disinfection procedures [47], accurate patient screening and retrospective surveillance, precise information of cases and their family as well as between healthcare facilities in case of patient transfer. Prompt reporting of cases to the competent health authorities may facilitate local and national alert and shared diagnostic protocols, with the aim of limiting further spread of multidrug-resistant pathogens nationally and beyond. C. auris cases and outbreaks occurred in several EU/EEA countries within a few years after the first cases were reported in the EU/EEA with evidence of inter-facility spread in two EU/EEA countries and an assessment of endemicity in at least one region in one country [11].
The rapid emergence of C. auris and the high likelihood for undetected cases warrant strengthened microbiological and epidemiological surveillance as well as coordinated preparedness.
Acknowledgments
The authors thank all the health personnel who performed the microbiological analysis in the involved regions and those who reported cases at local, regional and national level, particularly: Piedmont region, Microbiological laboratory A.O.U. Città della Salute e della Scienza, Torino: Alessandro Bondi, Antonio Curtoni, Teresa Zaccaria; Local Health Authority ASL Città di Torino: Piera Bassi, Rosa Maria Teresa Cristaudo, Erica Di Tolla, Rita Madonna, Mariaelisabetta Scarvaglieri, Carmela Scinica, Angela Strippoli.
Author Contributions
Conceptualisation, F.M., G.R. and M.S.; Data curation, C.S. (Camilla Sticchi), R.R., L.F. (Lorenza Ferrara), E.V., L.F. (Loredana Ferrero), D.F., G.F., E.F., C.S. (Carlo Silvestre), S.Z., S.A., G.D., C.G., M.L.M., E.R., F.T., F.R., M.T. and M.S.; Formal analysis, M.S.; Investigation, C.S. (Camilla Sticchi), R.R., L.F. (Lorenza Ferrara), E.V., L.F. (Loredana Ferrero), D.F., G.F., E.F., C.S. (Carlo Silvestre), S.Z., G.D., C.G., M.L.M., E.R., F.R. and M.T.; Methodology, F.M., G.R. and M.S.; Project administration, M.S.; Software, C.S. (Camilla Sticchi), R.R., L.F. (Lorenza Ferrara), E.V., L.F. (Loredana Ferrero), D.F., G.F., E.F., C.S. (Carlo Silvestre), S.Z., S.A., F.T. and M.S.; Supervision, F.M. and G.R.; Validation, C.S. (Camilla Sticchi), R.R., L.F. (Lorenza Ferrara), E.V., L.F. (Loredana Ferrero), D.F., G.F., E.F., C.S. (Carlo Silvestre), S.Z., S.A., G.D., C.G., M.L.M., E.R., F.T., F.R. and M.T.; Visualisation, M.S.; Writing—original draft, M.S.; Writing—review and editing, C.S. (Camilla Sticchi), R.R., L.F. (Lorenza Ferrara), E.V., L.F. (Loredana Ferrero), S.A., F.T., F.R., M.T., F.M. and G.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study did not require ethical approval. The study was conducted in accordance with the Declaration of Helsinki. Ethical review and approval were waived for this study due to the use of anonymised data at the local level.
Informed Consent Statement
Patient consent is not applicable to our study that provided description and analysis of data collected and anonymised by local healthcare facilities for public health reasons.
Data Availability Statement
Data are unavailable due to privacy protection and ethical restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding. The APC was funded by personal waiver to MS.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sanyaolu A., Okorie C., Marinkovic A., Abbasi A.F., Prakash S., Mangat J., Hosein Z., Haider N., Chan J. Candida auris: An Overview of the Emerging Drug-Resistant Fungal Infection. Infect. Chemother. 2022;54:236–246. doi: 10.3947/ic.2022.0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention Tracking Candida Auris. [(accessed on 1 December 2022)]; Available online: https://www.cdc.gov/fungal/candida-auris/tracking-c-auris.html.
- 3.Ahmad S., Alfouzan W. Candida auris: Epidemiology, Diagnosis, Pathogenesis, Antifungal Susceptibility, and Infection Control Measures to Combat the Spread of Infections in Healthcare Facilities. Microorganisms. 2021;9:807. doi: 10.3390/microorganisms9040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pruden A., Vikesland P.J., Davis B.C., de Roda Husman Ana M. Seizing the moment: Now is the time for integrated global surveillance of antimicrobial resistance in wastewater environments. Curr. Opin. Microbiol. 2021;64:91–99. doi: 10.1016/j.mib.2021.09.013. [DOI] [PubMed] [Google Scholar]
- 5.OECD. ECDC. EFSA. EMA Antimicrobial Resistance in the EU/EEA: A One Health Response. 2022. [(accessed on 1 December 2022)]. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/antimicrobial-resistance-policy-brief-2022.pdf.
- 6.Eze N., Cecchini M., Hashiguchi T.O. Antimicrobial Resistance in Long-Term Care Facilities. OECD Publishing; Paris, France: 2022. OECD Health Working Papers; No. 136. [DOI] [Google Scholar]
- 7.Centers for Disease Control and Prevention COVID-19: U.S. Impact on Antimicrobial Resistance, Special Report 2022. [(accessed on 1 December 2022)];2022 Available online: https://stacks.cdc.gov/view/cdc/117915.
- 8.Lee W.G., Shin J.H., Uh Y., Kang M.G., Kim S.H., Park K.H., Jang H.-C. First three reported cases of nosocomial fungemia caused by Candida auris. J. Clin. Microbiol. 2011;49:3139–3142. doi: 10.1128/JCM.00319-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Satoh K., Makimura K., Hasumi Y., Nishiyama Y., Uchida K., Yamaguchi H. Candida auris sp. nov., a novel ascomycetous yeast isolated from the external ear canal of an inpatient in a Japanese hospital. Microbiol. Immunol. 2009;53:41–44. doi: 10.1111/j.1348-0421.2008.00083.x. [DOI] [PubMed] [Google Scholar]
- 10.Desnos-Ollivier M., Fekkar A., Bretagne S. Earliest case of Candida auris infection imported in 2007 in Europe from India prior to the 2009 description in Japan. J. Mycol. Med. 2021;31:101139. doi: 10.1016/j.mycmed.2021.101139. [DOI] [PubMed] [Google Scholar]
- 11.Kohlenberg A., Struelens M.J., Monnet D.L., Plachouras D., Candida auris survey collaborative group Candida auris: Epidemiological situation, laboratory capacity and preparedness in European Union and European Economic Area countries, 2013 to 2017. Euro. Surveill. 2018;23:18–00136. doi: 10.2807/1560-7917.ES.2018.23.13.18-00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plachouras D., Lotsch F., Kohlenberg A., Monnet D.L., Candida auris survey collaborative group Candida auris: Epidemiological situation, laboratory capacity and preparedness in the European Union and European Economic Area*, January 2018 to May 2019. Euro. Surveill. 2020;25:2000240. doi: 10.2807/1560-7917.ES.2020.25.12.2000240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rossi A., Chavez J., Iverson T., Hergert J., Oakeson K., LaCross N., Njoku C., Gorzalski A., Gerrity D. Candida auris Discovery through Community Wastewater Surveillance during Healthcare Outbreak, Nevada, USA, 2022. Emerg. Infect. Dis. 2023;29:422–425. doi: 10.3201/eid2902.221523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu S., Zhu F., Jiang W., Wang Y., Quan Y., Zhang G., Gu F., Yang Y. Retrospective Analysis of the Clinical Characteristics of Candida auris Infection Worldwide From 2009 to 2020. Front. Microbiol. 2021;12:658329. doi: 10.3389/fmicb.2021.658329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossow J., Ostrowsky B., Adams E., Greenko J., McDonald R., Vallabhaneni S., Forsberg K., Perez S., Lucas T., Alroy K.A., et al. Factors Associated With Candida auris Colonization and Transmission in Skilled Nursing Facilities With Ventilator Units, New York, 2016–2018. Clin. Infect. Dis. 2021;72:e753–e760. doi: 10.1093/cid/ciaa1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giacobbe D.R., Magnasco L., Sepulcri C., Mikulska M., Koehler P., Cornely O.A., Bassetti M. Recent advances and future perspectives in the pharmacological treatment of Candida auris infections. Expert Rev. Clin. Pharmacol. 2021;14:1205–1220. doi: 10.1080/17512433.2021.1949285. [DOI] [PubMed] [Google Scholar]
- 17.European Centre for Disease Prevention and Control (ECDC) Candida Auris in Healthcare-Settings—Europe, First Update. ECDC; Stockholm, Sweden: 2018. [Google Scholar]
- 18.Vinayagamoorthy K., Pentapati K.C., Prakash H. Prevalence, risk factors, treatment and outcome of multidrug resistance Candida auris infections in Coronavirus disease (COVID-19) patients: A systematic review. Mycoses. 2022;65:613–624. doi: 10.1111/myc.13447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thoma R., Seneghini M., Seiffert S.N., Vuichard Gysin D., Scanferla G., Haller S., Flury D., Boggian K., Kleger G.-R., Filipovic M., et al. The challenge of preventing and containing outbreaks of multidrug-resistant organisms and Candida auris during the coronavirus disease 2019 pandemic: Report of a carbapenem-resistant Acinetobacter baumannii outbreak and a systematic review of the literature. Antimicrob. Resist Infect. Control. 2022;11:12. doi: 10.1186/s13756-022-01052-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Welsh R.M., Bentz M.L., Shams A., Houston H., Lyons A., Rose L.J., Litvintseva A.P. Survival, Persistence, and Isolation of the Emerging Multidrug-Resistant Pathogenic Yeast Candida auris on a Plastic Health Care Surface. J. Clin. Microbiol. 2017;55:2996–3005. doi: 10.1128/JCM.00921-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kean R., McKloud E., Townsend E.M., Sherry L., Delaney C., Jones B.L., Williams C., Ramage G. The comparative efficacy of antiseptics against Candida auris biofilms. Int. J. Antimicrob. Agents. 2018;52:673–677. doi: 10.1016/j.ijantimicag.2018.05.007. [DOI] [PubMed] [Google Scholar]
- 22.Keighley C., Garnham K., Harch S.A.J., Robertson M., Chaw K., Teng J.C., Chen S.C.-A. Candida auris: Diagnostic Challenges and Emerging Opportunities for the Clinical Microbiology Laboratory. Curr. Fungal. Infect. Rep. 2021;15:116–126. doi: 10.1007/s12281-021-00420-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dennis E.K., Chaturvedi S., Chaturvedi V. So Many Diagnostic Tests, So Little Time: Review and Preview of Candida auris Testing in Clinical and Public Health Laboratories. Front. Microbiol. 2021;12:757835. doi: 10.3389/fmicb.2021.757835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Council of State and Territorial Epidemiologists . Standardized Case Definition for Candida Auris Causing Clinical Infection and Colonization in People. Council of State and Territorial Epidemiologists; Atlanta, GA, USA: 2018. (17-ID-03) [Google Scholar]
- 25.Centers for Disease Control and Prevention Candida Auris. [(accessed on 1 December 2022)]; Available online: https://www.cdc.gov/fungal/candida-auris/index.html.
- 26.Crea F., Codda G., Orsi A., Battaglini A., Giacobbe D.R., Delfino E., Ungaro R., Marchese A. Isolation of Candida auris from invasive and non-invasive samples of a patient suffering from vascular disease, Italy, July 2019. Euro. Surveill. 2019;24:1900549. doi: 10.2807/1560-7917.ES.2019.24.37.1900549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mikulska M., Magnasco L., Signori A., Sepulcri C., Dettori S., Tutino S., Vena A., Miletich F., Ullah N., Morici P., et al. Sensitivity of Serum Beta-D-Glucan in Candidemia According to Candida Species Epidemiology in Critically Ill Patients Admitted to the Intensive Care Unit. J. Fungi. 2022;8:921. doi: 10.3390/jof8090921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briano F., Magnasco L., Sepulcri C., Dettori S., Dentone C., Mikulska M., Ball L., Vena A., Robba C., Patroniti N., et al. Candida auris Candidemia in Critically Ill, Colonized Patients: Cumulative Incidence and Risk Factors. Infect. Dis. Ther. 2022;11:1149–1160. doi: 10.1007/s40121-022-00625-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.The European Committee on Antimicrobial Susceptibility Testing Breakpoint Tables for Interpretation of MICs for Antifungal agents, Version 10.0. 2020. [(accessed on 1 December 2022)]. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/
- 30.Magnasco L., Mikulska M., Giacobbe D.R., Taramasso L., Vena A., Dentone C., Dettori S., Tutino S., Labate L., Di Pilato V., et al. Spread of Carbapenem-Resistant Gram-Negatives and Candida auris during the COVID-19 Pandemic in Critically Ill Patients: One Step Back in Antimicrobial Stewardship? Microorganisms. 2021;9:95. doi: 10.3390/microorganisms9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Pilato L., Codda G., Ball L., Giacobbe D.R., Willison E., Mikulska M., Magnasco L., Crea F., Vena A., Pelosi P., et al. Molecular Epidemiological Investigation of a Nosocomial Cluster of C. auris: Evidence of Recent Emergence in Italy and Ease of Transmission during the COVID-19 Pandemic. J. Fungi. 2021;7:140. doi: 10.3390/jof7020140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piatti G., Sartini M., Cusato C., Schito A.M. Colonization by Candida auris in critically ill patients: Role of cutaneous and rectal localization during an outbreak. J. Hosp. Infect. 2022;120:85–89. doi: 10.1016/j.jhin.2021.11.004. [DOI] [PubMed] [Google Scholar]
- 33.Koehler P., Arendrup M.C., Arikan-Akdagli S., Bassetti M., Bretagne S., Klingspor L., Lagrou K., Meis J.F., Rautemaa-Richardson R., Schelenz S., et al. ECMM CandiReg-A ready to use platform for outbreaks and epidemiological studies. Mycoses. 2019;62:920–927. doi: 10.1111/myc.12963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kohlenberg A., Monnet D.L., Plachouras D., Candida auris survey collaborative group. Candida auris survey collaborative group Increasing number of cases and outbreaks caused by Candida auris in the EU/EEA, 2020 to 2021. Euro. Surveill. 2022;27:2200846. doi: 10.2807/1560-7917.ES.2022.27.46.2200846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saris K., Meis J.F., Bano J.R., Tacconelli E., van de Belt Tom H., Voss A. Does Online Search Behavior Coincide with Candida auris Cases? An Exploratory Study. J. Fungi. 2019;5:44. doi: 10.3390/jof5020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Du H., Bing J., Nobile C.J., Huang G. Candida auris infections in China. Virulence. 2022;13:589–591. doi: 10.1080/21505594.2022.2054120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Okoye C.A., Nweze E., Ibe C. Invasive candidiasis in Africa, what is the current picture? Pathog. Dis. 2022;80:ftac012. doi: 10.1093/femspd/ftac012. [DOI] [PubMed] [Google Scholar]
- 38.Zerrouki H., Ibrahim A., Rebiahi S., Elhabiri Y., Benhaddouche D., de Groot T., Meis J.F., Rolain J., Bittar F. Emergence of Candida auris in intensive care units in Algeria. Mycoses. 2022;65:753–759. doi: 10.1111/myc.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vaseghi N., Sharifisooraki J., Khodadadi H., Nami S., Safari F., Ahangarkani F., Meis J.F., Badali H., Morovati H. Global prevalence and subgroup analyses of coronavirus disease (COVID-19) associated Candida auris infections (CACa): A systematic review and meta-analysis. Mycoses. 2022;65:683–703. doi: 10.1111/myc.13471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang Y., Xu J. Population genomic analyses reveal evidence for limited recombination in the superbug Candida auris in nature. Comput. Struct. Biotechnol. J. 2022;20:3030–3040. doi: 10.1016/j.csbj.2022.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacobs S.E., Jacobs J.L., Dennis E.K., Taimur S., Rana M., Patel D., Gitman M., Patel G., Schaefer S., Iyer K., et al. Candida auris Pan-Drug-Resistant to Four Classes of Antifungal Agents. Antimicrob. Agents Chemother. 2022;66:e0005322. doi: 10.1128/aac.00053-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yadav A., Jain K., Wang Y., Pawar K., Kaur H., Sharma K.K., Tripathy V., Singh A., Xu J., Chowdhary A. Candida auris on Apples: Diversity and Clinical Significance. mBio. 2022;13:e0051822-22. doi: 10.1128/mbio.00518-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aldejohann A.M., Wiese-Posselt M., Gastmeier P., Kurzai O. Expert recommendations for prevention and management of Candida auris transmission. Mycoses. 2022;65:590–598. doi: 10.1111/myc.13445. [DOI] [PubMed] [Google Scholar]
- 44.de Jong A.W., van den Ende B.G., Hagen F. Molecular Tools for Candida auris Identification and Typing. Methods Mol. Biol. 2022;2517:33–41. doi: 10.1007/978-1-0716-2417-3_3. [DOI] [PubMed] [Google Scholar]
- 45.Johnson E.M., Borman A.M. Susceptibility Testing of Candida auris Isolates. Methods Mol. Biol. 2022;2517:55–71. doi: 10.1007/978-1-0716-2417-3_5. [DOI] [PubMed] [Google Scholar]
- 46.Izadi A., Aghaei Gharehbolagh S., Sadeghi F., Talebi M., Darmiani K., Zarrinnia A., Zarei F., Peymaeei F., Khojasteh S., Borman A.M., et al. Drug repurposing against Candida auris: A systematic review. Mycoses. 2022;65:784–793. doi: 10.1111/myc.13477. [DOI] [PubMed] [Google Scholar]
- 47.Bandara H.M.H.N., Samaranayake L.P. Emerging strategies for environmental decontamination of the nosocomial fungal pathogen Candida auris. J. Med. Microbiol. 2022;71:001548. doi: 10.1099/jmm.0.001548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are unavailable due to privacy protection and ethical restrictions.