Abstract
Chlamydia trachomatis is an obligate intracellular pathogen. Infection of susceptible individuals with this bacterium can trigger the development of reactive arthritis, an acute inflammation that is associated with the expression of the class I major histocompatibility antigen, HLA-B27. Other facultative intracellular pathogens, such as Yersinia and Salmonella spp., are also known triggers of reactive arthritis. Previous studies report conflicting results concerning whether the presence of HLA-B27 modulates the infection of cells with these enteric pathogens. In the present study, we have examined whether the expression of HLA-B27 can influence the infection of cell lines with C. trachomatis and also whether the replication of these bacteria is altered in HLA-B27-expressing cell lines. To do this, we have used a sensitive flow cytometric approach. We fixed and permeabilized cells and used fluorescein isothiocyanate-conjugated monoclonal antibody specific for chlamydia lipopolysaccharide to detect intracellular bacteria. The staining pattern obtained closely resembled the intracellular life cycle of chlamydia, with the appearance of brightly staining cells correlating to the microscopic detection of mature inclusion bodies. Moreover, since the percentage of cells that stained with the antibody was proportional to the infectious inoculum used, we were able to use the technique to quantitate the number of infectious organisms recoverable from infected cell lines. An important component of our study was the use of heparin to prevent reinfection of cells and thus enable the infection to be followed from a discrete time point. Our results suggest that HLA-B27 influences neither the infection nor replication of C. trachomatis serovar L2 within cell lines. Consequently, the role of HLA-B27 in the pathogenesis of reactive arthritis may lie downstream of the invasion and replication stages of the triggering pathogenic infection.
The major histocompatibility complex (MHC) class I molecule HLA-B27 is strongly associated with the spondyloarthropathies (2, 23). However, the role which this molecule may play in disease has yet to be elucidated. A link between HLA-B27 and bacterial infection is indicated from animal models (8, 16), where disease fails to occur if disease-susceptible HLA-B27 transgenic animals are maintained in germfree (25) or pathogen-free (16) environments. Moreover, in humans, the expression of HLA-B27 is prevalent in individuals who develop reactive arthritis, a joint inflammation triggered by infection with various pathogens, including Salmonella, Yersinia, and Chlamydia spp. Indeed, studies examining the development of arthritis in patients after an outbreak of salmonella food poisoning suggest that HLA-B27 may also be associated with the severity of the disease (20).
There are various theories concerning how HLA-B27 and bacteria may interact in disease (1). For example, a number of studies have examined whether the presence of HLA-B27 influences infection of cells with facultative intracellular gram-negative enteric pathogens. Clearly, if HLA-B27 influences the infection and/or the survival of pathogenic bacteria within cells, then the way in which bacteria are cleared and/or the manner in which the immune response is triggered may be affected and predispose to pathogenic disease, such as reactive arthritis. However, studies addressing this issue have reported conflicting results. For example, an inhibitory effect of HLA-B27 on the in vitro infection of cell lines with Yersinia and Salmonella spp. has been found (11, 12; R. Inman, B. Chiu, and U. Payne, Arthritis Rheum. 39:S297 [abstract], 1996). It was further suggested that HLA-B27 may prevent the invasion of these bacteria by competing, either indirectly or directly, for the bacterial-invasion-mediated protein(s) (12). This result is in disagreement with other studies that find no effect on invasion (22) but an adverse effect of HLA-B27 on replication or clearance of bacteria (19, 26). It was suggested that, if the results were paralleled in vivo, the increased survival of salmonellae within HLA-B27 cells may contribute to an increased antigenic load leading to the development of reactive arthritis in these susceptible individuals. However, further studies with salmonella have failed to note any impact of the expression of HLA-B27 on either invasion or replication of salmonella within nontransformed primary fibroblast cultures (10).
In the present study, we examined whether HLA-B27 influences the infection of cell lines with the obligate intracellular pathogen Chlamydia trachomatis serovar L2. We also examined whether replication of the bacteria is altered within HLA-B27-expressing cells. To do this, we have used a flow cytometric approach to visualize and quantitate intracellular chlamydiae infecting a panel of cell lines. The results of this study indicate that the presence of an HLA-B27 transgene does not specifically influence infection of cell lines or the replication of chlamydiae within the infected cell lines studied. Moreover, using a panel of B lymphoblastoid cell lines (BLCL) which express or lack native HLA-B27, no effects of HLA-B27 on Chlamydia infection could be identified. Our results suggest that HLA-B27 does not have a major influence on the infection or replication of C. trachomatis serovar L2 within transformed cell lines.
MATERIALS AND METHODS
Cell lines.
The C1R parent cell line and transfectants expressing HLA-B2705 or HLA-A201 (6, 30) were a generous gift from K. Granfors, National Public Health Institute, Turku, Finland. BLCL were derived by transforming peripheral blood mononuclear cells (PBMC) with Epstein-Barr virus (EBV) obtained from the supernatant of the cell line B95.8, as previously described (29). PBMC were obtained from four healthy donors (one of whom was HLA-B27+) or from HLA-B27+ spondyloarthropathy patients. HLA-B27 expression was determined serologically or by PCR (results not shown). HeLa cells were used for propagating chlamydiae, and the elementary bodies (EB) were purified as described previously (3). The cell lines were maintained in RPMI 1640 (Gibco-BRL, Paisley, Scotland) supplemented with 2 mM glutamine (Sigma-Aldrich Company, Ltd., Poole, England), 20 mM HEPES (Sigma), and 5% heat-inactivated fetal bovine serum (First Link UK, Ltd., Brierley Hill, England). Transfected cell lines were maintained in 1 mg of G418 (Gibco-BRL)/ml. All cell lines tested negative for mycoplasma by using PCR and/or enzyme-linked immunosorbent assay techniques (Roche Diagostics, Ltd., Lewes, England).
Infection protocol.
The infectious EB titer of the purified C. trachomatis L2 was determined using immunofluorescent microscopy by counting inclusion bodies formed on infected monolayer cultures of the HeLa cell line, stained 24 to 48 h after infection with an anti-chlamydia lipopolysaccharide (LPS)-fluorescein isothiocyanate (FITC) monoclonal antibody (Dako, Ltd., Ely, England). These EB were used to infect the C1R cell lines and BLCL. Briefly, the cell lines were added to 24-well plates (Gibco-BRL) in 2-ml volumes at 0.5 × 106 to 1 × 106/ml and C. trachomatis L2 was added at the indicated multiplicity of infection. Control wells lacking chlamydiae (mock) were also included. The plates were spun at 1,400 × g for 30 to 60 min. Most of the medium was removed, and fresh medium supplemented as described above was added. Unless otherwise indicated, 200 U of heparin (Monoparin; CP Pharmaceuticals Ltd., Wrexham, England)/ml was also added to the cells after the centrifugation step. The cells were then incubated for 18 to 26 h or for the indicated periods of time.
Quantification of intracellular chlamydiae.
Infected cells were washed to remove exogenous heparin. The cells were then lysed by using sonication, and the lysate was centrifuged at 380 × g for 5 min to pellet the cell debris. The bacterium-rich supernatant was transferred to Eppendorf tubes and spun at 16,000 × g for 1 h at 4°C to pellet the chlamydiae. For immediate use, the chlamydia-rich pellet was resuspended in phosphate-buffered saline, and serial dilutions were used to infect aliquots of C1R cells, as described above. Alternatively, as in the experiment depicted in Fig. 5, the pellet was resuspended in sucrose-phosphate-glutamate buffer and frozen at −70°C until required. To check that copurifying cell debris was not toxic, a control preparation from mock-infected cells was also prepared. Intracellular staining followed by flow cytometric analysis (see below) was performed on the cells 18 to 24 h after the infection.
FIG. 5.
Quantification of infectious bacteria recovered from C1R, C1R-B2705, or C1R-A201 cell lines at 24 h. Serial dilutions of C. trachomatis prepared from C1R (squares), C1R-A201 (triangles), or C1R-B2705 (circles) cells 24 h after their infection with C. trachomatis were used to infect C1R cells. After 24 h, the percentage of positively staining cells was determined after flow cytometry analysis of the infected C1R cells and is plotted against the dilution of C. trachomatis used for the infection. The percentage of staining cells treated with a control preparation prepared from mock-infected cells (diamond) and the percentage of mock-infected C1R cells (inverted triangle) staining with anti-EB–FITC are also indicated. The results shown are representative of three such experiments.
Staining protocol.
For intracellular staining, cells were harvested and fixed and permeabilized by using the commercial Fix and Permeabilisation Reagent (BD Pharmingen, Oxford, England) according to the manufacturer's instructions. The cells were stained with a predetermined optimal amount of FITC-conjugated monoclonal antibody specific for chlamydia LPS (Dako, Ltd.) or an appropriate isotype control. No staining with the isotype control was detected (results not shown). To confirm that the staining was due to intracellular staining, infected cells that were fixed only with 4% formaldehyde were also incubated with the antibodies; no or very little staining was apparent with the anti-chlamydia monoclonal antibody (results not shown). A Becton Dickinson Facsort machine was used to collect the cells, with 10,000 events usually being acquired (except for Fig. 2, wherein 5,000 events were collected), and the data were subsequently analyzed by using WinMDI software (Joseph Trotter, http://facs.scripps.edu/). For quantitating the infectious inoculum of bacteria, the percentage of cells giving fluorescence above that of the negative control was calculated and plotted against the dilution of bacterial preparation used to infect the cells.
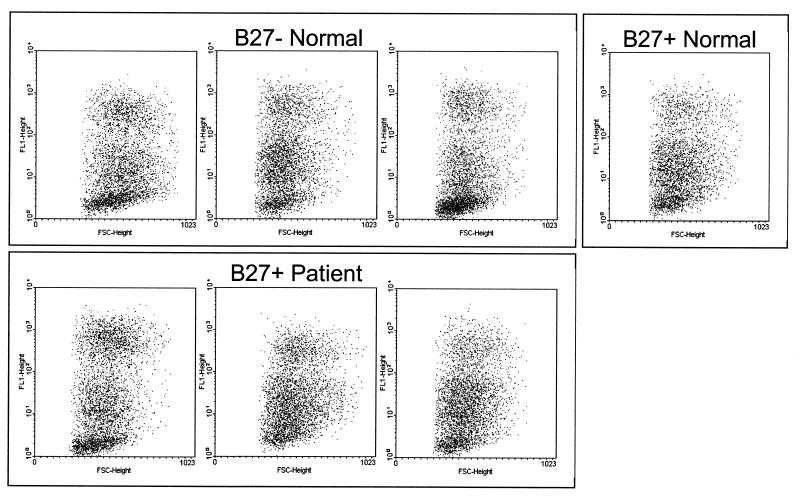
FIG. 2.
HLA-B27 expression does not affect infection of BLCL with C. trachomatis. Dot plots of forward scatter against fluorescence are shown for various BLCL infected with C. trachomatis at a multiplicity of 10:1 and stained after 24 h with anti-EB–FITC. The presence or absence of HLA-B27 expression and the donor origin of the cell lines are indicated. No staining was apparent with mock-infected cells (not shown). The results shown are representative of five similar experiments.
RESULTS
Heparin inhibits reinfection of cell lines with Chlamydia.
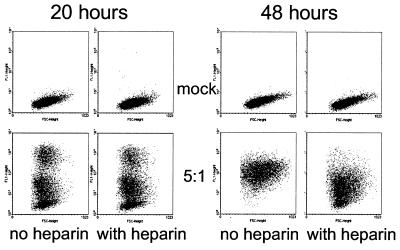
We used intracellular staining, followed by fluorescence-activated cell sorting (FACS) analysis to visualize infection of cell lines with C. trachomatis L2. As shown in Fig. 1, no staining was evident with mock-infected cells while a proportion of the cells (at 20 h, 66% in the absence of heparin and 68% in the presence of heparin) that had been exposed to C. trachomatis stained with the anti-LPS antibody. Infected cells did not stain when they were fixed only, indicating that the C. trachomatis was intracellular (results not shown). Both bright and intermediately staining populations of infected cells could be seen, with the former likely to represent mature inclusion bodies. Because we wished to monitor the course of an infection in cell lines that differed for the expression of HLA-B27, we tested whether the addition of heparin would inhibit ongoing infection within the cell cultures. As is apparent, after 48 h, in the absence of heparin, all of the cells became infected with C. trachomatis. However, a substantial number of uninfected cells remained (22%) in cultures to which heparin had been added, while there was a loss of brightly staining cells. This suggests that, in the absence of heparin, cells not infected at early time points could later be infected by C. trachomatis elementary bodies released into the culture from the early infected cells. Heparin inhibits this secondary infection. Accordingly, to facilitate a synchronous infection, heparin was added in all of the following experiments.
FIG. 1.
Heparin inhibits the reinfection of cell lines in culture. Dot plots of forward scatter against fluorescence are shown for mock-infected C1R cells or C1R cells infected with C. trachomatis at a multiplicity of five EB to one cell and stained after 20 or 48 h with anti-EB–FITC. This result is representative of four similar experiments.
HLA-B27 does not affect infection of BLCL with Chlamydia.
Various BLCL were exposed to Chlamydia, and the percentage of infected cells was determined by flow cytometry after 24 h (Fig. 2). Although the cell lines showed minor variations in their intracellular staining patterns, there was no difference associated with either the presence or absence of HLA-B27 expression. Thus, HLA-B27 does not appear to influence the ability to infect a BLCL with C. trachomatis.
HLA-B27 does not affect the course of an infection with Chlamydia or the rate of replication of bacteria within infected cell lines.
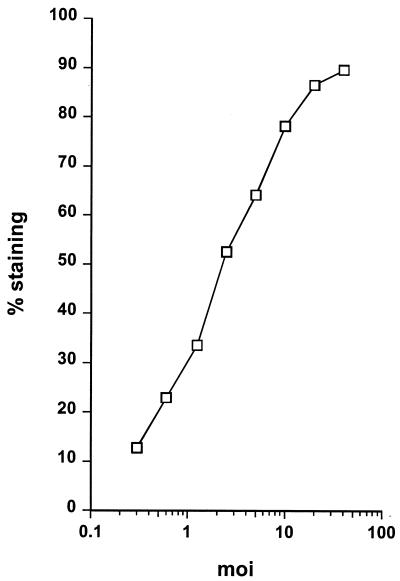
Aliquots of cells were infected with various numbers of C. trachomatis, incubated, and stained after 24 h. As shown in Fig. 3, the percentage of cells giving positive staining was directly proportional to the number of bacteria used to infect the cells. Thus, we reasoned that intracellular staining and FACS analysis can provide a useful technique for quantitating the numbers of bacteria used to initiate an infection. It was therefore possible to compare the numbers of bacteria recovered from infected cell lines differing for expression of HLA-B27. Thus, in addition to assessing whether HLA-B27 modulates cell infection by C. trachomatis, we could also assay whether HLA-B27 influenced replication of C. trachomatis.
FIG. 3.
The percentage of infected cells is proportional to the infectious inoculum used. The percentage of C1R cells giving positive staining with anti-EB–FITC 24 h after their infection with the indicated amounts of C. trachomatis was determined from analysis of flow cytometry data. Fewer than 1% of mock-infected cells stained with the anti-EB–FITC (result not shown). The results shown are representative of three similar experiments.
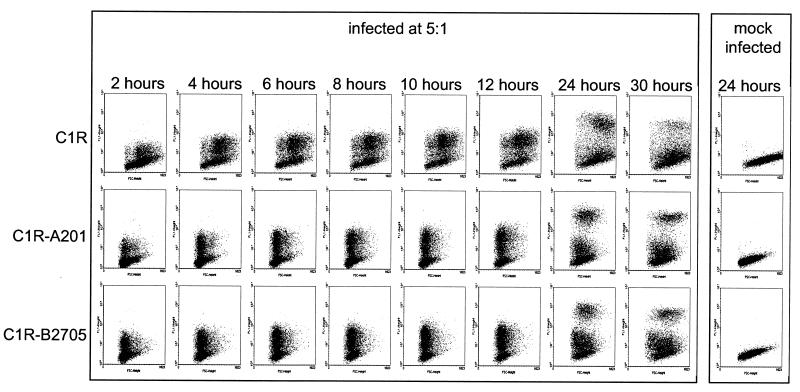
We analyzed aliquots of cell lines infected with C. trachomatis over a 30-h time period (Fig. 4). The parental cell line C1R was compared with cell lines transfected with HLA-A201 or HLA-B2705. In all cases, infection could be detected as early as 2 h after the addition of the C. trachomatis. At this time point, inclusion bodies could not be detected microscopically, suggesting that the flow cytometry technique is more sensitive than conventional microscopy. A very bright staining subpopulation, which correlated with the microscopic detection of mature inclusion bodies, was not apparent until the 24-h time point. A higher proportion of the parental cell line was infected compared to the transfectants. However, cells transfected with either HLA-B2705 or HLA-A201 exhibited similar infection, indicating that HLA-B2705 did not have a specific inhibitory effect. The numbers of infectious bacteria that could be recovered from the infected cell lines 24 h after infection were compared (Fig. 5). In agreement with the higher percentage of parental cell line infection, more infectious bacteria were recovered from the infected C1R cells. Similar numbers of infectious bacteria were recovered from transfected cell lines expressing either HLA-B2705 or HLA-A201. Thus, we conclude that replication of C. trachomatis is similar in these transfected cell lines and not influenced by the expression of HLA-B2705.
FIG. 4.
Time course of C. trachomatis infection in C1R, C1R-A201, and C1R-B2705 cell lines. Dot plots of forward scatter against fluorescence are shown for the indicated cell lines, stained with anti-EB–FITC at the indicated times after addition of C. trachomatis at a multiplicity of 5:1. Examples of the negative staining obtained with mock-infected cells are also shown. Similar results to those shown were observed in a repeat experiment (not shown).
DISCUSSION
In the present study we used a flow cytometric approach to analyze infections of cell lines with the obligate intracellular pathogen, C. trachomatis. C. trachomatis is more commonly examined using direct microscopy and counting the inclusion bodies which are formed by the replicating bacteria (7, 18). This latter technique has severe limitations in the number of cells that can be examined and can lead to large errors when one is quantitating infectious bacteria. Moreover, if replication of C. trachomatis within a cell is not optimal, then it may not be possible to visualize inclusion bodies. The use of flow cytometry provides a way to analyze accurately a large number of cells for evidence of chlamydia infection. Moreover, since staining is apparent as early as 2 h postincubation with C. trachomatis, abnormal and suboptimal infections can still be detected using this technique. Although the use of intracellular staining and flow cytometry has been previously reported for analyzing the infection of cell lines with Chlamydia, the technique has not been extensively used since its initial description 14 years ago (27). The advent of commercially available reagents for fixation and permeabilization has greatly simplified this method, and we would advocate its use as a standard technique in studying chlamydial infections. The staining pattern obtained by using intracellular staining and flow cytometry (see Fig. 4) closely resembles the infectious cycle of C. trachomatis (21). Thus, dim staining was seen in the first few hours after addition of the C. trachomatis with the appearance of bright staining populations correlating to the microscopic detection of mature inclusion bodies. Similarly, the reduction in the numbers of bright staining cells at a late time in the infectious cycle is consistent with the lysis of these cells and release of the mature elementary bodies. As expected by the correlation of staining with the infectious cycle, the total percentage of cells staining positive for evidence of Chlamydia infection was found to be dependent upon the infectious inoculum used. Thus, flow cytometry also provided a method for quantifying bacteria present within a preparation. We believe that this technique provides an ideal tool to address cellular issues relating to C. trachomatis infection.
In the present study, we examined whether HLA-B27 expression exerts an influence on the infection of cell lines with C. trachomatis and/or the replication of C. trachomatis within the cell. We infected various cell lines, which differed for the expression of HLA-B27, with C. trachomatis and monitored the infection by intracellular staining and flow cytometry. An important technical consideration in this study was the inclusion of heparin after the initial infection. Heparin has been shown to inhibit uptake of C. trachomatis by various cell lines (31) and its inclusion in the present study inhibited secondary infection of cells with C. trachomatis serovar L2. It should be noted that heparin may not be as successful in inhibiting infection of cell lines with other serovars of C. trachomatis. For example, although trachoma and LGV serovars have both been shown to use a glucoaminoglycan-dependent infection pathway (5), trachoma serovars are less dependent on the host cell heparin sulfate than is L2 for their infectivity (4, 24). However, our use of serovar L2 in the present study enabled us to examine discrete time points after the inception of a C. trachomatis infection. Using BLCL, variations in infectivity pattern were noted, but there was no correlation between any particular C. trachomatis staining profile and the expression of HLA-B27 or with the disease status of the donor of the blood from which the cell lines were established. When we used the C1R cell line (6, 30), an EBV-positive mutant of the human plasma cell leukemia-derived line Hmy2, which expresses very low levels of MHC class I, we found that this cell line was more susceptible to infection with C. trachomatis than other conventional BLCL. Precisely why this cell line is more readily infected than BLCL is unknown, but it may be due to the loss of regulatory genes from the mutated C1R cell line and/or related to the differing status of EBV infectivity. The expression of either the HLA-B2705 or HLA-A201 transgene by C1R cells reduced their infectivity with C. trachomatis. However, no significant differences were found in the kinetics of the infection or the percentage of infected cells between C1R-B2705 and C1R-A201 cells. Similarly, in agreement with the larger percentage of parental cells exhibiting evidence of intracellular C. trachomatis, a greater number of C. trachomatis organisms were harvested from these cells after 24 h in comparison to the two transfectants which yielded similar numbers of C. trachomatis. Thus, the expression of HLA class I, and not specifically HLA-B27, may exert an inhibitory effect on C. trachomatis infection. However, it is also noteworthy, as is apparent from the greater numbers of the parental C1R cells with a large forward-scatter signal (see Fig. 4), that more of the parental C1R cells were in the exponential phase of growth compared to the MHC class I transfectants. This, rather than the lack of MHC class I, may have led to the enhanced infection with C. trachomatis. Nonetheless, the results with both the BLCL and the transfectants show that no effect of HLA-B27 on the infection or replication of C. trachomatis could be discerned. This result is in disagreement with a study that described a decreased replication of C. trachomatis within C1R cells that correlated with the expression of HLA-B2705 intracellular domain (17). However, the methods used for quantitating the C. trachomatis in that study were subject to large errors, and no heparin was added to the cultures to prevent continuing infection. Indeed, in a previous report the same authors using the same methods found no effect of HLA-B27 expression on C. trachomatis infection (J. G. Kuipers et al., Abstr. 62nd Annu. Sci. Meet. Am. Coll. Rheumatol., Arthritis Rheum. 41[9S], abstr. 699, 1998).
The apparent lack of a modulating role of HLA-B27 expression on C. trachomatis infection seen in the present study does not necessarily exclude that such a mechanism functions in reactive arthritis disease. It may be that our use of transformed cell lines and/or the use of the invasive L2 serovar of C. trachomatis prevented the detection of this proposed inhibitory mechanism of HLA-B27. We used the L2 serovar because of its relative ease of culture and susceptibility to inhibition of infection by heparin. This highly invasive serovar is not generally associated with reactive arthritis, although there have been reports of joint symptoms and erythema nodosum, a rash associated with reactive arthritis, after LGV infection (13). While we cannot detect an effect of HLA-B2705 on C. trachomatis replication, we have a robust technique to permit the reciprocal study of the effects of bacterial infection on HLA-B27 expression. Indeed, alterations in the expression of HLA-B27 (28) after the infection of cells with Yersinia and Salmonella spp. have been reported. Moreover, infection of cells with C. trachomatis also leads to a downregulation of MHC class I (32) which allows the recognition of infected cells by NK cells (C. E. Hook and J. S. H. Gaston, Abstr. 4th Meet. Eur. Soc. Chlamydia Res., p. 184, 2000). It is also possible that C. trachomatis infection may increase the expression of aberrant forms of HLA-B27, such as homodimers or free heavy chains, which have been suggested as pathologic targets of immune responses (14, 15). Indeed, an alteration in the splicing of B27 mRNA leading to the production of soluble HLA-B27 has been detected after infection of cell lines with Yersinia and Salmonella spp. (9).
ACKNOWLEDGMENT
This work was supported by the Medical Research Council (United Kingdom).
REFERENCES
- 1.Allen R L, Bowness P, McMichael A. The role of HLA-B27 in spondyloarthritis. Immunogenetics. 1999;50:220–227. doi: 10.1007/s002510050596. [DOI] [PubMed] [Google Scholar]
- 2.Brewerton D, Caffrey M, Hart F, James D, Nicholls A, Sturrock R. Ankylosing spondylitis and HL-A27. Lancet. 1973;i:996–999. doi: 10.1016/s0140-6736(73)91360-3. [DOI] [PubMed] [Google Scholar]
- 3.Brunst M, Shahmanesh M, Sukthankar A, Pearce J H, Gaston J S. Isolation and characterisation of T lymphocytes from the urethra of patients with acute urethritis. Sex Transm Infect. 1998;74:279–283. doi: 10.1136/sti.74.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J C, Stephens R S. Chlamydia trachomatis glycosaminoglycan-dependent and independent attachment to eukaryotic cells. Microb Pathog. 1997;22:23–30. doi: 10.1006/mpat.1996.0087. [DOI] [PubMed] [Google Scholar]
- 5.Chen J C R, Stephens R S. Trachoma and biovars of Chlamydia trachomatis share the same glycosaminoglycan-dependent mechanism for infection of eukaryotic cells. Mol Microbiol. 1994;11:501–507. doi: 10.1111/j.1365-2958.1994.tb00331.x. [DOI] [PubMed] [Google Scholar]
- 6.Edwards P A, Smith C M, Neville A M, O'Hare M J. A human-hybridoma system based on a fast-growing mutant of the ARH-77 plasma cell leukemia-derived line. Eur J Immunol. 1982;12:641–648. doi: 10.1002/eji.1830120804. [DOI] [PubMed] [Google Scholar]
- 7.Furness G, Fraser E F. One-step growth curves for inclusion blennorrhea virus in HeLa cell monolayers. J Gen Microbiol. 1962;223:613–619. doi: 10.1099/00221287-27-2-299. [DOI] [PubMed] [Google Scholar]
- 8.Hammer R E, Maika S D, Richardson J A, Tang J-P, Taurog J D. Spontaneous inflammatory disease in transgenic rats expressing HLA-B27 and human β2m: an animal model of HLA-B27-associated human disorders. Cell. 1990;63:1099–1112. doi: 10.1016/0092-8674(90)90512-d. [DOI] [PubMed] [Google Scholar]
- 9.Huang F, Yamaguchi A, Tsuchiya N, Ikawa T, Tamura N, Virtala M M K, Granfors K, Yasaei P, Yu D T Y. Induction of alternative splicing of HLA-B27 by bacterial invasion. Arthritis Rheum. 1997;40:694–703. doi: 10.1002/art.1780400414. [DOI] [PubMed] [Google Scholar]
- 10.Huppertz H I, Heesemann J. Invasion and persistence of Salmonella in human fibroblasts positive or negative for endogenous HLA B27. Ann Rheum Dis. 1997;56:671–676. doi: 10.1136/ard.56.11.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kapasi K, Inman R. HLA B27 expression modulates gram-negative bacterial invasion into transfected L cells. J Immunol. 1992;148:3554–3559. [PubMed] [Google Scholar]
- 12.Kapasi K, Inman R D. ME1 epitope of HLA-B27 confers class I-mediated modulation of gram-negative bacterial invasion. J Immunol. 1994;153:833–840. [PubMed] [Google Scholar]
- 13.Keat A. Sexually transmitted arthritis syndromes. Med Clin N Am. 1990;74:1617–1631. doi: 10.1016/s0025-7125(16)30498-9. [DOI] [PubMed] [Google Scholar]
- 14.Khare S D, Bull M J, Hanson J, Luthra H S, David C S. Spontaneous inflammatory disease in HLA-B27 transgenic mice is independent of MHC class II molecules: a direct role for B27 heavy chains and not B27-derived peptides. J Immunol. 1998;160:101–106. [PubMed] [Google Scholar]
- 15.Khare S D, Hansen J, Luthra H S, David C S. HLA-B27 heavy chains contribute to spontaneous inflammatory disease in B27/human β2-microglobulin (β2m) double transgenic mice with disrupted mouse β2m. J Clin Investig. 1996;98:2746–2755. doi: 10.1172/JCI119100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khare S D, Luthra H S, David C S. Spontaneous inflammatory arthritis in HLA-B27 transgenic mice lacking β2-microglobulin: a model of human spondyloarthropathies. J Exp Med. 1995;182:1153–1158. doi: 10.1084/jem.182.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuipers J G, Bialowons A, Dollmann P, Jendro M C, Koehler L, Ikeda M, Yu D T, Zeidler H. The modulation of chlamydial replication by HLA-B27 depends on the cytoplasmic domain of HLA-B27. Clin Exp Rheumatol. 2001;19:47–52. [PubMed] [Google Scholar]
- 18.Kuo C C, Grayston T. Interaction of Chlamydia trachomatis organisms and HeLa 229 cells. Infect Immun. 1976;13:1103–1109. doi: 10.1128/iai.13.4.1103-1109.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laitio P, Virtala M, Salmi M, Pelliniemi L J, Yu D T Y, Granfors K. HLA-B27 modulates intracellular survival of Salmonella enteritidis in human monocytic cells. Eur J Immunol. 1997;27:1331–1338. doi: 10.1002/eji.1830270606. [DOI] [PubMed] [Google Scholar]
- 20.Leirisalo-Repo M. Prognosis, course of disease, and treatment of the spondyloarthropathies. Rheum Dis Clin N Am. 1998;24:737–751. doi: 10.1016/s0889-857x(05)70039-9. [DOI] [PubMed] [Google Scholar]
- 21.Moulder J W. Interaction of chlamydiae and host cells in vitro. Microbiol Rev. 1991;55:143–190. doi: 10.1128/mr.55.1.143-190.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ortiz-Alvarez O, Yu D T, Petty R E, Finlay B B. HLA-B27 does not affect invasion of arthritogenic bacteria into human cells. J Rheumatol. 1998;25:1765–1771. [PubMed] [Google Scholar]
- 23.Schlosstein L, Terasaki P I, Bluestone R, Pearson C M. High association of an HL-A antigen, W27, with ankylosing spondylitis. N Engl J Med. 1973;288:704–706. doi: 10.1056/NEJM197304052881403. [DOI] [PubMed] [Google Scholar]
- 24.Taraktchoglou M, Pacey A A, Turnbull J E, Eley A. Infectivity of Chlamydia trachomatis serovar LGV but not E is dependent on host cell heparan sulfate. Infect Immun. 2001;69:968–976. doi: 10.1128/IAI.69.2.968-976.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taurog J D, Richardson J A, Croft J T, Simmons W A, Zhou M, Fernandezsueiro J L, Balish E, Hammer R E. The germfree state prevents development of gut and joint inflammatory disease in HLA-B27 transgenic rats. J Exp Med. 1994;180:2359–2364. doi: 10.1084/jem.180.6.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Virtala M, Kirveskari J, Granfors K. HLA-B27 modulates the survival of Salmonella enteritidis in transfected L cells, possibly by impaired nitric oxide production. Infect Immun. 1997;65:4236–4242. doi: 10.1128/iai.65.10.4236-4242.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Waldman F M, Hadley W K, Fulwyler M J, Schachter J. Flow cytometric analysis of Chlamydia trachomatis interaction with L cells. Cytometry. 1987;8:55–59. doi: 10.1002/cyto.990080109. [DOI] [PubMed] [Google Scholar]
- 28.Wuorela M, Jalkanen S, Kirveskari J, Laitio P, Granfors K. Yersinia enterocolitica serotype O:3 alters the expression of serologic HLA-B27 epitopes on human monocytes. Infect Immun. 1997;65:2060–2066. doi: 10.1128/iai.65.6.2060-2066.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young J L, Goodall J C, Beacock-Sharp H, Gaston J. Human γδ T-cell recognition of Yersinia enterocolitica. Immunology. 1997;91:503–510. doi: 10.1046/j.1365-2567.1997.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zemmour J, Little A M, Schendel D J, Parham P. The HLA-A/B “negative” mutant cell line C1R expresses a novel HLA-B35 allele, which also has a point mutation in the translation initiation codon. J Immunol. 1992;148:1941–1948. [PubMed] [Google Scholar]
- 31.Zhang J P, Stephens R S. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell. 1992;69:861–869. doi: 10.1016/0092-8674(92)90296-o. [DOI] [PubMed] [Google Scholar]
- 32.Zhong G M, Liu L, Fan T, Fan P Y, Ji H Z. Degradation of transcription factor RFX5 during the inhibition of both constitutive and interferon gamma-inducible major histocompatibility complex class I expression in Chlamydia-infected cells. J Exp Med. 2000;191:1525–1534. doi: 10.1084/jem.191.9.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]