Abstract
With a view to exploring the role of transforming growth factor β (TGF-β) during mycobacterial infection, recombinant clones of bacillus Calmette-Guérin (BCG) were engineered to express the natural antagonist of TGF-β, latency-activated peptide (LAP). Induction of TGF-β activity was reduced when macrophages were infected with BCG expressing the LAP construct (LAP-BCG). There was a significant reduction in the growth of LAP-BCG in comparison to that of control BCG following intravenous infection in a mouse model. The enhanced control of mycobacterial replication was associated with an increase in the production of gamma interferon by splenocytes challenged during the acute stage of infection but with a diminished recall response assessed after 13 weeks. Organ weight and hydroxyproline content, representing tissue pathology, were also lower in mice infected with LAP-BCG. The results are consistent with the hypothesis that TGF-β has a detrimental effect on mycobacterial immunity. While a reduction in TGF-β activity augments the initial response to BCG vaccination, early bacterial clearance may adversely affect the induction of a long-term memory response by LAP-BCG.
Although bacillus Calmette-Guérin (BCG) vaccination confers clear benefit against disseminated forms of tuberculosis in children (15), its efficacy against the predominant adult pulmonary disease has varied widely in clinical trials (4). Recent progress in mycobacterial genetics (5) and an improved understanding of the immunological mechanisms required for protection (1) provide an opportunity to develop recombinant vaccines designed to promote an enhanced immune response.
Cytokine-secreting recombinant BCG (rBCG) vaccines have been shown to enhance the host immune responses to mycobacterial antigens (11, 12). To date, work has focused on the expression of proinflammatory cytokines, such as interleukin 2 and gamma interferon (IFN-γ). A complementary approach is to evaluate rBCG expressing antagonists to endogenous immunosuppressive cytokines, such as transforming growth factor β (TGF-β). This approach has the added attraction of reducing the profibrotic contribution of TGF-β and thus potentially reducing BCG-associated scarring.
TGF-β1 is a product of activated macrophages and other inflammatory cells (26). It is one of five isoforms of a multifunctional cytokine which is produced in response to tissue injury and which exhibits a wide array of immunomodulatory and biological activities (25). TGF-β is a potent endogenous immunosuppressive agent, deactivating macrophages and modulating T-cell function (24). In addition, TGF-β promotes tissue fibrosis in human and rodent diseases by enhancing the synthesis of extracellular matrix components (14, 16). TGF-β is produced early in the delayed-type hypersensitivity reaction to the mycobacterial protein extract purified protein derivative (PPD) (21, 27) and in tuberculous granulomas (22). It has also been implicated in the regulation of the immune response against Mycobacterium avium (2).
TGF-β is produced in an inactive form noncovalently bound to its natural antagonist, latency-associated peptide (LAP). The cDNA sequences of a number of mammalian LAP genes have been established and shown to exhibit extensive homology (6, 18). When LAP is expressed independently in tissue culture cells, it acts as a functional binding protein for mature TGF-β1 (as well as related family members), masking its biological activity (8). Recombinant LAP is a potent inhibitor of the effects of TGF-β on the replication of Mycobacterium tuberculosis in monocytes in vitro (9), and continuous infusion of recombinant LAP has been shown to reduce the growth of BCG in a murine infection model (29).
In this study, we tested the hypothesis that the expression of LAP by rBCG would result in a reduction in TGF-β activity, augmenting the protective immune response of the host and reducing local inflammatory side effects of BCG vaccination.
MATERIALS AND METHODS
Construction of BCG strains expressing LAP.
Murine cDNA for LAP was cloned from pSVTGFβ1 (provided by Hal Moses, Vanderbilt University School of Medicine, Nashville, Tenn., and described elsewhere [23]) into plasmids pRBD3a and pRBD4a (11, 12). These expression vectors contain a kanamycin resistance marker and origins of replication suitable for maintenance in mycobacteria and in Escherichia coli. Transcription is under the control of the mycobacterial hsp60 promoter, and the insert gene is fused to the signal sequence derived from the gene encoding the BCG alpha antigen, targeting the expressed protein for secretion through the mycobacterial cell membrane and wall. LAP cDNA was modified by PCR to replace the endogenous signal sequence-encoding DNA using the following primers: LAP-R-R1, CGGAATTCCTATCTCCGGTGCCGTGAGCTG; and LAP-F BAM, CGCGGATCCGGGAGGCCAGCCGCGGGAC.
Fragments were digested with EcoRI and BamHI and cloned into pRBD3a or pRBD4a digested with the same enzymes. Recombinant plasmids amplified in E. coli were transformed into Mycobacterium bovis BCG (Pasteur) by electroporation. Kanamycin-resistant colonies were selected and grown in Middlebrook 7H9 medium supplemented with albumin-dextrose-catalase (Difco Laboratories) prior to screening for LAP expression. The cell pellet from cultures that had reached an optical density at 600 nm of 0.5 to 1.0 was lysed by sonication for five 10-s intervals using a Soniprep 150 sonicator (Measurement Scientific Equipment; Sanyo Gallen-Kamp) at a power setting of 14. Sonicates were boiled with sample buffer and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Culture supernatants were also analyzed by Western blotting after passage through a Millipore Millex prefilter and a 0.22-μm-pore-size filter. For selected samples, filtrates from cultures prepared in Sauton medium were screened by Western blotting after concentration approximately 50-fold by freeze-drying. Western blots were developed using polyclonal goat anti-human affinity-purified LAP antibody (R&D Systems) at a dilution of 1:250, followed by peroxidase-conjugated anti-goat immunoglobulin G (Sigma). For the two positive clones, plasmids were electroduced into E. coli and bulk samples were prepared for oligonucleotide sequencing using an ABI Prism dye terminating cycle sequencer (Perkin-Elmer). The anticipated sequence of the LAP insert was confirmed in both cases.
BCG transformed by electroporation with plasmid pRBD3a or pRBD4a minus cytokine cDNA was used as a control.
THP-1 infection model.
Cells (5 × 106) from the THP-1 human macrophage cell line were suspended in 50 ml of RPMI 1640 medium (supplemented with glutamine but without antibiotics) in a 225-ml tissue culture flask (Nunc). After 4 and 7 days, cells were removed from the flask, centrifuged, and resuspended at a density of 5 × 105/ml in a 24-well plate (Nunc). Cells were activated with phorbol myristate acetate (5 ng/ml) and IFN-γ (10 ng/ml) for 72 h. Before infection with BCG or BCG expressing the LAP construct (LAP-BCG), the medium was removed from the now adherent THP-1 cells, which were then washed once with phosphate-buffered saline (PBS) and infected for different periods of time as described in Results. Cell-free culture supernatants were harvested and stored at −70°C for measurement of TGF-β bioactivity.
Murine infection model.
Female C57BL6/J mice were purchased from Harlan OLAC (Bicester, United Kingdom) and raised under specific-pathogen-free conditions. The mice were maintained for at least 1 week and used for experiments at 6 to 8 weeks of age. On the day of injection, stored BCG or LAP-BCG samples were thawed and diluted in sterile PBS to a density of 107 CFU/ml. Cultures were thoroughly dispersed by repeated gentle passage through a 27-gauge needle. BCG strains in a total volume of 200 μl were injected into a lateral tail vein (five mice per group). Control mice were injected with the same volume of sterile PBS alone. Mice were weighed and killed by cervical dislocation on two different days postinfection. The spleen, liver, and lungs were removed and weighed, and histopathological changes were monitored by use of formalin-fixed and paraffin-embedded sections stained with Ziehl-Neelsen (Z-N) and hematoxylin-eosin stains. Hydroxyproline content was determined by the methods described by Stegemann and Stadler (20) and Wangoo et al. (28). Hydroxyproline content was expressed as content per total lung or liver.
To monitor mycobacterial growth, weighed pieces of each organ were homogenized and plated in serial dilutions on Middlebrook 7H11 medium supplemented with oleic acid-albumin-dextrose-catalase (Difco), amphotericin (10 μg/ml) (Squibb), and kanamycin (50 μg/ml). Colonies were counted after 3 weeks.
To monitor the in vitro response to mycobacterial antigens, spleens were removed aseptically from naive and BCG-infected mice, teased through a cell strainer (Falcon; Becton-Dickinson), and washed three times with Hanks' balanced salt solution. Splenocytes from each group were pooled and resuspended at a concentration of 106 cells/ml in supplemented RPMI 1640 medium as previously described (19). Splenocytes were stimulated with mycobacterial PPD (Evans) at a concentration of 10 μg/ml. Supernatants were harvested at 72 h and stored at −70°C.
Cytokine assays.
TGF-β bioactivity was measured using the Mv1Lu mink epithelial cell line (ATCC CCL-64). Cells grown to confluence were detached using trypsin-EDTA, washed with Iscove's modified Dulbecco medium (Sigma) containing 2% fetal calf serum, resuspended in the same medium, and dispersed in 96-well plates at a density of 3 × 104 cells/well. After 12 to 18 h, serial twofold dilutions of TGF-β standard (R&D Systems) or test sample were added to triplicate wells, and incubation was continued for a further 24 h. On day 3, cells were pulsed with 3H-thymidine (1 μCi/well; Amersham Life Science), and the plates were incubated for a further 4 to 6 h. Cells were then washed, incubated with trypsin-EDTA, and harvested using a Filtermate cell harvester (Packard). Radioactivity was measured using a Top Count microplate scintillation counter (Packard). TGF-β activity was interpolated from a standard curve generated over a concentration range of 0.005 to 2 ng/ml.
The IFN-γ concentration in supernatants was measured by a sandwich enzyme-linked immunosorbent assay (ELISA) technique using paired antibodies from Pharmingen, San Diego, Calif.
Statistical analysis.
Data were analyzed using the Student t test. Statistical significance was taken as a P value of <0.05.
RESULTS
Construction of rBCG expressing LAP.
cDNA encoding murine LAP was cloned in shuttle vectors pRBD3a and pRBD4a as a translational fusion to the signal sequence from BCG alpha antigen, downstream of the mycobacterial hsp60 promoter. The two constructs differed by the presence of a hemagglutinin (HA) epitope tag in pRBD4a. These vectors have previously been used to generate rBCG strains expressing a range of biologically active mammalian cytokines (11, 12). The LAP constructs and appropriate control vectors were introduced into M. bovis BCG (Pasteur) by electroporation, and kanamycin-resistant colonies were characterized by Western blot analysis (Fig. 1). A single band reactive with antiserum to human LAP was observed in sonicated preparations of clones transformed with the pRBD3a and pRBD4a LAP constructs (3a/LAP and 4a/LAP, respectively); there was no antibody reactivity to corresponding extracts prepared from control BCG transformed with vector alone. In spite of the fact that both constructs included a signal peptide shown previously to promote the secretion of proteins expressed in recombinant mycobacteria (11, 12), we were unable to detect immunologically active LAP in filtrates prepared from cultures of either of the positive clones. Recombinant LAP may have structural features that preclude its secretion from BCG. Alternatively, secreted LAP may be subject to degradation and its steady-state concentration may be reduced below the level detectable in the immunoassay.
FIG. 1.
LAP expression by rBCG. Western blot analysis demonstrated the presence of LAP in cell lysates from rBCG clones 3a/LAP and 4a/LAP (lanes 5 and 6, respectively). The band corresponding to 4a/LAP runs slightly slower than that corresponding to 3a/LAP because of the addition of the HA tag. LAP was not detected in corresponding culture filtrate preparations. No reactivity was observed in cell extract or culture filtrate from control BCG transformed with the pBRD3a vector (lane 3 or 4, respectively). Lanes 1 and 2 show molecular weight markers and the antibody response to 50 ng of recombinant human LAP, respectively. LAP expression in rBCG extracts was estimated to be approximately 2.5 ng per 107 cells.
Characterization of LAP-BCG constructs in tissue culture models.
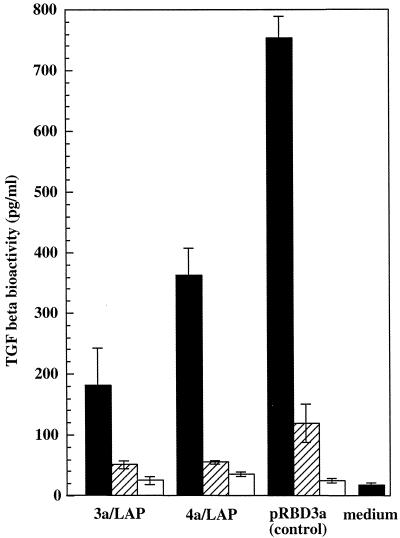
To test for the presence of biologically active LAP, culture filtrates from the recombinant BCG transformed with two LAP constructs 3a/LAP and 4a/LAP (3a/LAP-BCG and 4a/LAP-BCG, respetively) were tested for their ability to inhibit the activity of recombinant TGF-β in a bioassay using the Mv1Lu mink lung epithelial cell line. Again, we were unable to detect LAP activity in the filtrate preparations. Evidence of the biological activity of recombinant LAP was obtained, however, when LAP-BCG constructs were used to infect the THP-1 human macrophage cell line. Cells were infected with live rBCG at a multiplicity of infection of 10, 1, or 0.1 CFU per cell, and TGF-β activity in culture supernatants was measured using the Mv1Lu bioassay. The highest TGF-β activity was observed at the highest multiplicity of infection, but at each dose, cytokine activity in 16-h supernatants was significantly lower in cultures infected with LAP-BCG constructs than in cultures infected with the pRBD3a vector control strain (Fig. 2). The pattern of lower TGF-β activity was also observed in supernatants prepared 40 h after infection (data not shown). These results suggest that, although undetectable in filtrate preparations from bacterial cultures, LAP expressed by rBCG strains has a suppressive effect on the expression of biologically active TGF-β by infected THP-1 cells.
FIG. 2.
TGF-β activity following rBCG infection of THP-1 cells. TGF-β activity was measured using the Mv1Lu assay and supernatants prepared from THP-1 cells infected for 16 h with 3a/LAP, 4a/LAP, or vector control BCG at a multiplicity of infection of 10 (solid bars), 1 (hatched bars), or 0.1 (open bars) bacterium/cell. A significant reduction in TGF-β activity was observed with each of the LAP-BCG constructs (P < 0.05). The standard error is shown for triplicate samples.
Growth of LAP-BCG constructs in a murine infection model.
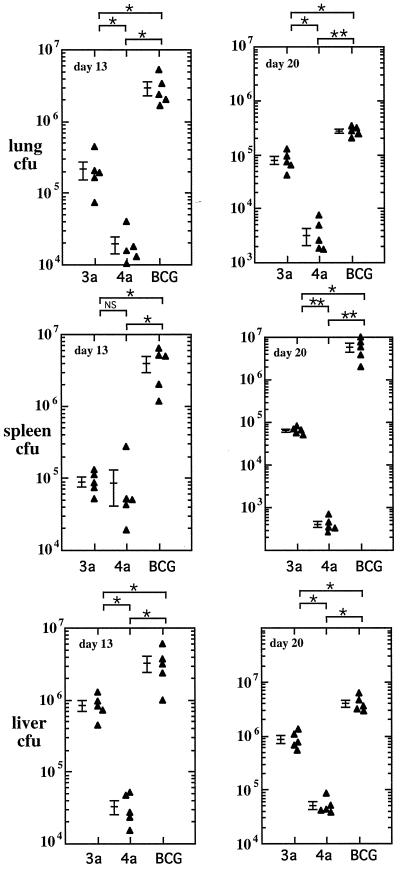
Infection of mice with BCG results in a self-limiting infection characterized by an initial acute phase of bacterial replication and subsequent control by acquired cell-mediated immunity. To test the effect of recombinant LAP expression on the growth of BCG in this model, C57BL/6 mice were infected by intravenous injection of 2 × 106 CFU of 3a/LAP-BCG, 4a/LAP-BCG, or control BCG transformed with the pRBD3a vector (pRBD3a-BGG). Mice were sacrificed on days 13 and 20 after infection, and the bacterial loads in the lungs, liver, and spleen were assessed (Fig. 3). The highest counts were found in the liver, with 3.2 × 107 CFU of pRBD3a-BCG at day 13, falling to 4.1 × 106 CFU by day 20. Similarly, in the lungs, a high bacterial load at day 13 (3.0 × 106) had declined 10-fold by day 20 (2.7 × 105). Counts in the spleen rose slightly from 3.9 × 106 on day 13 to 5.9 × 106 on day 20. In each organ, at both time points, the bacterial load was significantly lower in mice infected with the LAP-BCG constructs. This finding was particularly marked with 4a/LAP-BCG, with mean organ counts consistently being 1 to 2 log units lower than those observed with the pBRD3a-BCG control. At day 104, CFU were only just detectable in organs of the 3a/LAP- and 4a/LAP-infected mice (total spleen counts, 6 ± 3.8 CFU for 3a/LAP-BCG and 0.3 ± 0.6 CFU for 4a/LAP-BCG) compared to BCG-infected control mice (total spleen counts, 114 ± 35 CFU). Furthermore, plating of samples of homogenized organs in the presence or absence of kanamycin confirmed that a higher percentage was drug sensitive, suggesting either instability of the plasmid within mycobacteria or even positive selection from nonexpressing clones. The same pattern was observed in two independent experiments.
FIG. 3.
Growth of rBCG in organs of infected mice. Mice were infected with 2 × 106 CFU of rBCG carrying the 3a/LAP or 4a/LAP construct or vector control BCG. Mice were sacrificed after 13 and 20 days, and the bacterial loads in lung, liver, and spleen homogenates were measured. There was a significant reduction in the growth of rBCG clones expressing LAP. Data points show CFU results for individual mice. P values were <0.05 (single asterisks) and <0.001 (double asterisks); NS, not significant. Bars show means and standard errors.
Immunogenicity of LAP-BCG constructs.
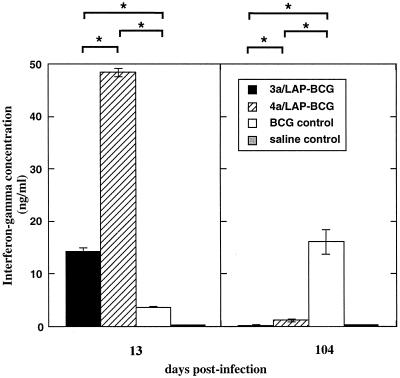
The restricted growth of the LAP-BCG constructs in the mouse model could have been caused by a direct detrimental effect of LAP expression on bacterial replication in murine tissues. Alternatively, LAP-mediated inhibition of TGF-β function could have resulted in an enhanced immune response and consequent restriction of infection. To evaluate the latter possibility, 13-day splenocytes from the different groups were compared with respect to PPD-induced expression of IFN-γ (Fig. 4). The IFN-γ response was significantly higher in splenocytes from mice infected with the LAP-BCG constructs than in those receiving the pRBD3a-BCG control; the highest level of IFN-γ production was found in mice infected with 4a/LAP-BCG, the construct associated with the lowest bacterial load. A similar pattern of responses was observed in two separate experiments. The reciprocal relationship between bacterial load and IFN-γ response is consistent with the proposal that the expression of recombinant LAP is associated with enhanced immunogenicity. PPD-induced expression of IFN-γ was also measured in a group of mice 104 days after infection. In contrast to the results obtained at the early time point, the highest response was found in mice receiving control BCG (Fig. 4). A possible explanation for this result is that the rapid clearance of the LAP-BCG constructs has a detrimental effect on the establishment of a long-term memory response.
FIG. 4.
IFN-γ production by splenocytes from infected mice. Splenocytes from mice infected for 13 days or 104 days with the different rBCG clones were challenged in vitro with PPD, and IFN-γ in cell supernatants was measured. Results show the mean and standard error for triplicate assays with splenocytes pooled from five mice per group. On day 13, IFN-γ production was significantly higher for splenocytes from mice infected with LAP-BCG. On day 104, the IFN-γ response was higher in mice that received control BCG. P values were <0.05 (single asterisks) and <0.001 (double asterisks).
Pathogenicity of LAP-BCG constructs.
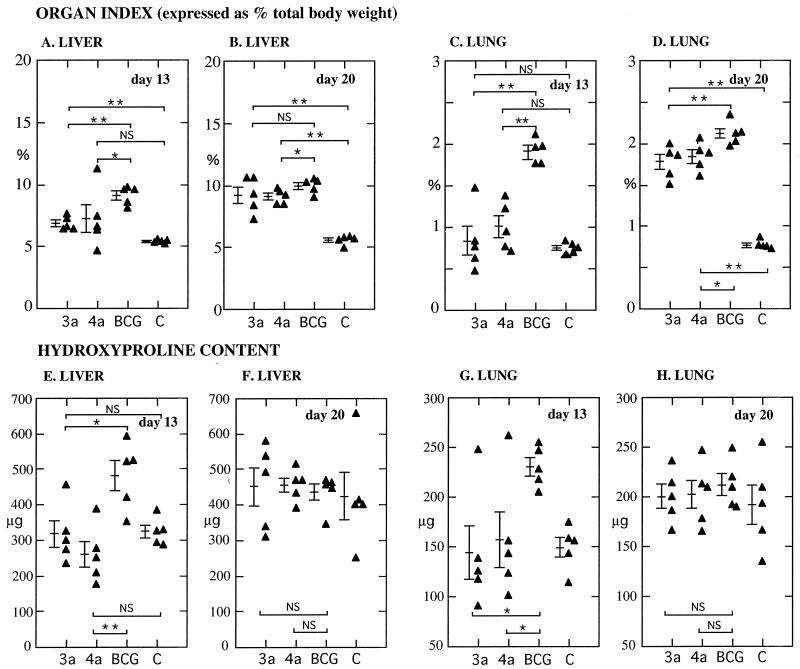
In light of the important role of TGF-β in fibrotic reactions, it was of interest to compare pathological manifestations in mice infected with LAP-BCG and control BCG. At day 13 after infection, mean organ indices (organ weight expressed as a percentage of total body weight) were significantly lower for the liver and lungs of mice infected with 3a/LAP-BCG or 4a/LAP-BCG than in those receiving pRBD3a-BCG (Fig. 5). Increasing organ indices in the LAP-BCG-infected mice over the next week of infection resulted in a marked reduction in this difference by day 20. Similarly, hydroxyproline content—a marker of new collagen formation—was lower in the lungs and liver of mice infected with LAP-BCG at day 13; there was no significant difference by day 20.
FIG. 5.
Pathology in mice infected with rBCG. (A to D) Organ indices (organ weight expressed as a percentage of total body weight) for the lungs and liver of mice infected for 13 or 20 days with different rBCG constructs (C, saline control). On day 13, organ weights were significantly lower for mice infected with LAP-expressing constructs. (E to H) Similarly, hydroxyproline content—expressed as micrograms per organ—was lower for LAP-BCG constructs on day 13. P values were <0.05 (single asterisks) and <0.005 (double asterisks); NS, not significant. Bars show means and standard errors.
Hematoxylin-eosin- and Z-N-stained sections from all mice were examined at the two time points. At day 13 after infection, liver sections from all BCG-infected groups showed intense inflammation throughout the organ—predominantly mononuclear cells, with occasional neutrophils. Some of the inflammatory foci had the appearance of early granuloma formation. Multiple acid-fast bacilli were visible within these inflammatory foci on Z-N staining. In the lungs, the inflammation was again marked, with interstitial changes and intravascular marginating cells, predominantly migrating macrophages. There were no obvious differences among the three BCG-infected groups. At day 20 after infection, the inflammatory changes in the liver were more intense; at this stage, the inflammation was granulomatous, comprising central epithelioid cells with a cuff of lymphocytes. The lung inflammation was also marked and predominantly interstitial. There were no convincing differences among the three groups for both organs examined, although there might have been a tendency for the granulomas to be better formed in LAP-BCG-infected mice. It was not possible to quantify this finding. Again, Z-N staining revealed multiple acid-fast bacilli at the centers of the granulomas in all three groups. Naive mice exhibited normal histologic findings.
DISCUSSION
The pRBD shuttle vector system has been successfully exploited to express a range of mammalian cytokines in BCG (10–12), and the present study extends this repertoire to include LAP, a natural cytokine antagonist. rBCG strains provide important tools for analysis of the contributions of individual cytokines to protective and pathological immune responses during mycobacterial infection and may also have a role as improved prophylactic or therapeutic vaccines. LAP-BCG constructs were characterized by reduced expression of functionally active TGF-β following infection of macrophage cultures in vitro and by an enhanced immune response during murine infection. The mechanism by which recombinant LAP exerts an immunomodulatory effect remains to be clarified. Neutralization of TGF-β released from stimulated cells by LAP secreted by BCG prior to phagocytosis represents the simplest hypothesis but, in light of our inability to detect LAP in culture filtrates, the possible contribution of LAP released by lysis of intracellular mycobacteria cannot be ruled out. The native LAP molecule is a disulfide-linked homodimer modified by glycosylation. Previous studies with bacterially expressed LAP have demonstrated that glycosylation is not required for functional activity (30). Dimerization is essential, however, and the requirement for disulfide bond formation may impose an important limitation in the secretion of active LAP by rBCG (17). Murray et al. have previously reported an inverse relationship between the number of disulfide bonds and the amount of detectable cytokine in mycobacterial supernatants (11).
Wilkinson et al. recently reported a reduction of approximately 50% in the growth of BCG following aerosol infection in the lungs of mice treated with exogenous LAP delivered by an implanted osmotic pump (29). We observed an analogous reduction in bacterial load during infection with LAP-expressing rBCG. The more marked difference in bacterial growth in the latter model (1 to 2 log units) may reflect a greater potency of local delivery of LAP; alternatively, it may reflect a more pronounced effect of TGF-β modulation on the systemic growth of mycobacteria following intravenous infection. There was a difference between the two LAP-BCG constructs in terms of their ability to multiply in mycobacteria, with the 4a/LAP-BCG construct being more easily controlled in mice than the 3a/LAP-BCG construct. We postulate that this result may have been due to the presence of the HA tag in the 4a/LAP construct affecting the stability, expression, or possibly the biological activity of recombinant LAP in these constructs. Further work is required to elucidate the exact mechanism. The reduced growth of LAP-BCG was associated with an enhanced immune response in the early phase of infection, as evidenced by increased mycobacterium-specific IFN-γ production by splenocytes. Again, this effect parallels results obtained by systemic delivery of recombinant LAP (29). The enhanced IFN-γ response is consistent with the anticipated inhibition of the immunosuppressive effect of TGF-β.
In addition to its role in the suppression of macrophage activation, TGF-β has an important profibrotic function (3). Fibrosis may have some beneficial effect in sealing off tuberculous lesions but may also seed the potential for cavitation and subsequent reactivation (13). During BCG vaccination, fibrosis has an obvious detrimental effect in the promotion of scarring (7). Comparison of LAP-BCG with control BCG demonstrated a reduction in the pathological manifestations of infection, as assessed by organ weight and by hydroxyproline content at early time points. Two possible explanations can be proposed to account for these observations. First, increased local concentrations of LAP may directly inhibit the fibrotic function of TGF-β. Alternatively, the slower evolution of pathological manifestations may be an indirect consequence of the reduced bacterial load resulting from enhanced immunogenicity. We were unable to distinguish between these two possibilities in the present experimental model. Analysis of the longer-term effects of infection with equivalent LAP-expressing strains of M. tuberculosis would be of interest in this regard.
Overall, the results of these experiments indicate that a reduction of TGF-β activity has a beneficial effect on immunity to mycobacterial infection. While this notion suggests a potential role for LAP-expressing rBCG as an improved vaccine, preliminary experiments to test the longer-term effects of LAP-BCG vaccination highlight an important caveat. Although the immune response to LAP-BCG was significantly enhanced during the acute phase of infection, the recall response was substantially lower than that obtained with control BCG when tested after several months. A likely explanation for this observation is that initial mycobacterial replication has an important influence on BCG vaccination and that reduced replication of LAP-BCG results in suboptimal immunization. The association of an enhanced early response to vaccination with a reduced memory response may have general relevance in relation to the development of improved live vaccines for tuberculosis.
ACKNOWLEDGMENTS
This work was supported by the British Medical Association (support given to B.G.M.) and by project grants (to A.W., R.J.S., and D.B.Y.) and a program grant (to D.B.Y.) from the Wellcome Trust.
We thank Peter Murray for the generous gift of the plasmid DNA from which the LAP-BCG clones were derived and Rod Shipley for technical support.
REFERENCES
- 1.Barnes P F, Wizel B. Type 1 cytokines and the pathogenesis of tuberculosis. Am J Respir Crit Care Med. 2000;161:1773–1774. doi: 10.1164/ajrccm.161.6.16167. [DOI] [PubMed] [Google Scholar]
- 2.Bermudez L E. Production of transforming growth factor-β by Mycobacterium avium-infected human macrophages is associated with unresponsiveness to IFN-γ. J Immunol. 1993;150:1838–1845. [PubMed] [Google Scholar]
- 3.Brocklemann T J, Limper A H, Colby T V, McDonald J A. Transforming growth factor beta 1 is present at sites of extracellular matrix gene expression in human pulmonary fibrosis. Proc Natl Acad Sci USA. 1991;88:6642–6646. doi: 10.1073/pnas.88.15.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. JAMA. 1994;271:698–702. [PubMed] [Google Scholar]
- 5.Cole S T, Brosch R, Parkhill J, Garnier T, Churcher C, Harris D, Gordon S V, Eiglmeier K, Gas S, Barry C E, I. I I, Tekaia F. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature. 1998;393:537–544. doi: 10.1038/31159. [DOI] [PubMed] [Google Scholar]
- 6.Derynck R, Jarrett J A, Chen E Y, Goeddel D V. The murine transforming growth factor-β precursor. J Biol Chem. 1986;261:4377–4379. [PubMed] [Google Scholar]
- 7.Fang J W S, Ko B M L, Wilson J A. BCG vaccination scars: incidence and acceptance amongst British high-school children. Child Care Health Dev. 1993;19:37–43. doi: 10.1111/j.1365-2214.1993.tb00711.x. [DOI] [PubMed] [Google Scholar]
- 8.Gentry L E, Nash B W. The pro domain of pre-pro-transforming growth factor β1 when independently expressed is a functional binding protein for the mature growth factor. Biochemistry. 1990;29:6851–6857. doi: 10.1021/bi00481a014. [DOI] [PubMed] [Google Scholar]
- 9.Hirsch C, Ellner S, Blinkhorn, R. J J, Toossi Z. In vitro restoration of T cell responses in tuberculosis and augmentation of monocyte effector function against Mycobacterium tuberculosis by natural inhibitors of transforming growth factor beta. Proc Natl Acad Sci USA. 1997;94:3926–3931. doi: 10.1073/pnas.94.8.3926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong D, Kunimoto D Y. Secretion of human interleukin-2 by recombinant Mycobacterium bovis BCG. Infect Immun. 1995;63:799–803. doi: 10.1128/iai.63.3.799-803.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray P J, Aldovini A, Young R A. Manipulation and potentiation of antimycobacterial immunity using recombinant bacille Calmette-Guerin strains that secrete cytokines. Proc Natl Acad Sci USA. 1996;93:934–939. doi: 10.1073/pnas.93.2.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.O'Donnell M A, Aldovini A, Duda R B, Yang H, Szilvasi A, Young R A, DeWolf W C. Recombinant Mycobacterium bovis BCG secreting functional interleukin-2 enhances gamma interferon production by splenocytes. Infect Immun. 1994;62:2508–2514. doi: 10.1128/iai.62.6.2508-2514.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orme I. The immunopathogenesis of tuberculosis: a new working hypothesis. Trends Microbiol. 1998;6:94–97. doi: 10.1016/s0966-842x(98)01209-8. [DOI] [PubMed] [Google Scholar]
- 14.Roberts A B, Sporn M B, Assoian R K, Smith J M, Roche N S, Wakefield L M. Transforming growth factor type β: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–4171. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rodrigues L C, Diwan V K, Wheeler J G. Protective efficacy of BCG against tuberculous meningitis and miliary tuberculosis: meta-analysis. Int J Epidemiol. 1993;22:1154–1158. doi: 10.1093/ije/22.6.1154. [DOI] [PubMed] [Google Scholar]
- 16.Sanderson N, Factor V, Nagy P, Kopp J, Kandaiah P, Wakefield L, Roberts A B, Sporn M B, Thorgeirsson S S. Hepatic expression of mature transforming growth factor β1 in transgenic mice results in multiple tissue lesions. Proc Natl Acad Sci USA. 1995;92:2572–2576. doi: 10.1073/pnas.92.7.2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sha X, Yang L, Gentry L E. Identification and analysis of discrete functional domains in the pro region of pre-pro-transforming growth factor beta 1. J Cell Biol. 1991;114:827–839. doi: 10.1083/jcb.114.4.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sharples K, Plowman G D, Rose T M, Twardzik D R, Purchio A F. Cloning and sequence analysis of simian transforming growth factor-β cDNA. DNA. 1987;6:239–244. doi: 10.1089/dna.1987.6.239. [DOI] [PubMed] [Google Scholar]
- 19.Snewin V A, Gares M P, Hasan Z, Brown I N, Young D B. Assessment of immunity to mycobacterial infection with luciferase reporter constructs. Infect Immun. 1999;67:4586–4593. doi: 10.1128/iai.67.9.4586-4593.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stegemann H, Stadler K. Determination of hydroxyproline. Clin Chim Acta. 1967;18:267–273. doi: 10.1016/0009-8981(67)90167-2. [DOI] [PubMed] [Google Scholar]
- 21.Toossi Z, Young T G, Averill L E, Hamilton B D, Shiratsuchi H, Ellner J J. Induction of transforming growth factor β1 by purified protein derivative of Mycobacterium tuberculosis. Infect Immun. 1995;63:224–228. doi: 10.1128/iai.63.1.224-228.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toossi Z, Gogate P, Shiratsuchi H, Young T, Ellner J J. Enhanced production of TGF-β by blood monocytes from patients with active tuberculosis and presence of TGF-β in tuberculous granulomatous lung lesions. J Immunol. 1995;154:465–473. [PubMed] [Google Scholar]
- 23.Torre-Amione G, Beauchamp R D, Koeppen H, Park B H, Schreiber H, Moses H L, Rowley D A. A highly immunogenic tumor transfected wth a murine transforming growth factor type β1 cDNA escapes immune surveillance. Proc Natl Acad Sci USA. 1990;87:1486–1490. doi: 10.1073/pnas.87.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsunawaki S, Sporn M, Ding A, Nathan C. Deactivation of macrophages by transforming growth factor β. Nature. 1988;334:260–262. doi: 10.1038/334260a0. [DOI] [PubMed] [Google Scholar]
- 25.Wahl S, Hunt D A, Wakefield L M, McCartney-Francis N, Wahl L M, Roberts A B, Sporn M B. Transforming growth factor type β induces monocyte chemotaxis and growth factor production. Proc Natl Acad Sci USA. 1987;8:354–360. doi: 10.1073/pnas.84.16.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wahl S M, McCartney-Francis N, Mergenhagen S E. Inflammatory and immunomodulatory roles for TGF-β. Immunol Today. 1989;10:258–261. doi: 10.1016/0167-5699(89)90136-9. [DOI] [PubMed] [Google Scholar]
- 27.Wangoo A, Cook H T, Taylor G M, Shaw R J. Enhanced expression of type 1 procollagen and Transforming growth factor-β in tuberculin induced delayed type hypersensitivity. J Clin Pathol. 1995;48:339–345. doi: 10.1136/jcp.48.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wangoo A, Brown I, Marshall B G, Young D B, Shaw R J. BCG-associated inflammation and fibrosis: modulation by recombinant BCG expressing interferon gamma. Clin Exp Immunol. 2000;119:92–98. doi: 10.1046/j.1365-2249.2000.01100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkinson K A, Martin T D, Reba S M, Aung H, Redline R W, Boom W H, Toossi Z, Fulton S A. Latency-associated peptide of transforming growth factor β enhances mycobacterial immunity in the lung during Mycobacterium bovis BCG infection in C57BL/6 mice. Infect Immun. 2000;68:6505–6508. doi: 10.1128/iai.68.11.6505-6508.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Dignam J D, Gentry L E. Role of carbohydrate in the binding of beta1-latency-associated peptide to ligands. Biochemistry. 1997;36:11923–11932. doi: 10.1021/bi9710479. [DOI] [PubMed] [Google Scholar]