Abstract
The cysteine protease of group A streptococci has been suggested to contribute to the pathogenesis of invasive infection through degradation of host tissue, activation of the host inflammatory response, release of protective molecules from the bacterial cell surface, or other mechanisms. However, studies of the effects on virulence of inactivating the cysteine protease gene speB have yielded conflicting results. In some reports, a speB mutant was relatively avirulent in mouse models of invasive infection whereas little or no attenuation of virulence was observed in other studies of similar mutant strains. Possible reasons for these discordant results include differences in the streptococcal strains from which the speB mutants were derived, differences in the infection models employed, or unintended effects on another virulence determinant(s) that arose during the derivation of a speB mutant. We attempted to clarify these issues by characterizing the phenotypic properties and relative virulence in mice of two speB mutant strains, both derived from wild-type strain AM3: speB mutant AM3speB, which has been shown to be markedly attenuated in virulence in mice after intraperitoneal or subcutaneous challenge, and AM3speBΩ, a new mutant strain derived for this investigation. Both mutant strains were negative for protease activity, as expected, and both produced wild-type amounts of type 3 M protein and streptolysin O. However, AM3speB produced significantly less cell-associated hyaluronic acid capsule than did parent strain AM3 or strain AM3speBΩ. Compared to wild-type strain AM3, AM3speB was more sensitive to opsonophagocytic killing in vitro and was significantly less virulent in mice after intraperitoneal challenge. By contrast, AM3speBΩ was fully resistant to phagocytosis and did not differ significantly from the wild-type strain in mouse virulence after an intraperitoneal or subcutaneous challenge. We concluded that previous reports attributing loss of virulence in strain AM3speB to inactivation of speB are in error. Within the limitations of the models used, we found no effect of cysteine protease on invasive streptococcal infection.
The manifestations of group A streptococcus (GAS) infections in humans are diverse in both clinical presentation and morbidity. Pharyngitis and impetigo are common childhood illnesses with few complications. Infrequently, GAS causes invasive disease, of which the most serious presentation is necrotizing soft-tissue infection with associated shock and multisystem organ failure (24).
The molecular details of the interaction between the bacterium and the host that determines the natural history of GAS infection remain poorly understood. GAS has the potential to produce a number of cell-associated and extracellular products that may contribute to pathogenesis. Virtually all GAS strains contain the speB gene that encodes a cysteine protease. Indirect support for a role of SpeB in invasive GAS infection includes the observations that the protease mediates degradation of the host tissue components vitronectin, fibronectin, and collagen; that the protease activates interleukin-1β, a proinflammatory cytokine; and that the protease may function as an adhesin by binding GAS to a variety of cell surface or ground substance molecules, including integrins, fibronectin, and laminectin (5, 7–9, 25).
More direct evidence supporting an effect of SpeB in GAS infection is the demonstration that SpeB is directly toxic after intravenous injection and may enhance GAS virulence in animal models of human disease (10, 11, 22, 26). However, the interpretation of these experiments is confounded by the use of partially purified protein preparations and by uncertainty about the biologically relevant concentration of SpeB. A more rigorous method for evaluating SpeB's role in GAS pathogenesis is to compare the virulence of wild-type and isogenic speB mutants in animal models of human infection. Lukomski et al. challenged mice intraperitoneally with isogenic M3 and M49 wild-type or protease-deficient strains and found that the protease-deficient mutants were attenuated (16). It is of note that the attenuation was particularly pronounced in the M3 background but was of a much smaller magnitude in the M49 background. A follow-up study using the M3 strain AM3 extended the findings of the intraperitoneal challenge studies, demonstrating that the protease-deficient mutant also was less virulent than the wild-type strain in a murine model of invasive soft-tissue infection (15). Although the mechanism of the protease effect was not evaluated in these experiments, subsequent work suggested that the loss of the protease increased bacterial susceptibility to phagocytic killing, possibly due to diminished expression of the antiphagocytic hyaluronic acid capsule (14, 30).
In contrast to these results, we previously found no contribution of the protease to invasive soft-tissue infection by using a different M type 3 GAS wild-type strain and an isogenic protease-deficient mutant, although in the same study, we observed a marked loss of virulence in mutants lacking either M3 protein or the hyaluronic acid capsule (4). Because SpeB is expressed predominantly during stationary-phase growth in vitro (6), it has been argued that the conflict between the results of our experiments and those of the previously cited studies is due to difference between the growth phases of the inocula used (14). Diminished virulence of protease-deficient GAS in vivo may only be seen when the bacterial challenge comprises organisms isolated during stationary-phase growth.
To further investigate the role of the protease in GAS infection and to clarify both the potential effect of the loss of protease activity on capsule expression and the influence of the growth phase of the inoculum on the role of SpeB in invasive infection, we independently derived a protease-deficient mutant in the background of GAS M3 strain AM3 and determined its capsule expression, susceptibility to phagocytic killing, and virulence in mice. Our results indicate that the amount of cell-associated hyaluronic acid capsule is not influenced by the GAS cysteine protease. In addition, our findings demonstrate that, regardless of the growth phase of the bacterial inoculum, inactivation of speB does not significantly attenuate murine invasive infection after either an intraperitoneal or a subcutaneous challenge.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
GAS strain AM3 is an M3 isolate recovered from a patient with puerperal sepsis (23). Strain AM3speB is a cysteine protease-deficient plasmid integration mutant derived from AM3 in which the speB gene was insertionally inactivated (16). Both AM3 and AM3speB were kindly provided by Andreas Podbielski (Department of Medical Microbiology and Hygiene, University Hospital Rostock, Rostock, Germany). GAS strain AM3speBΩ is a cysteine protease-deficient interposon mutant derived from AM3 that contains a kanamycin resistance cassette inserted within the speB gene. Strain AM3RV is a cysteine protease-producing revertant derived from a protease-deficient plasmid integration mutant. The derivation of AM3speBΩ and AM3RV is described below. GAS strains were grown in Todd-Hewitt broth (Difco Laboratories, Detroit, Mich.) supplemented with yeast extract (Difco) to a final concentration of 2.5% (wt/vol) (THY) or grown on either Trypticase soy blood agar medium (Becton Dickinson Microbiological Systems, Cockeysville, Md.) (TSA-blood) or THY agar medium supplemented with 5% (vol/vol) defibrinated sheep blood (PML Microbiologicals, Richmond, British Columbia, Canada) (THY-blood). GAS was cultured at 37°C in 5% CO2. When specified, erythromycin was added to either broth or agar medium to a final concentration of 1 μg/ml.
DNA manipulation.
Plasmid purification was performed with Qiagen mini- or maxipreps in accordance with the manufacturer's (Qiagen Inc., Valencia, Calif.) protocol. Preparation of electrocompetent GAS and transformation of GAS by electroporation were performed as previously described (2). GAS chromosomal DNA was purified in accordance with the method of O'Connor and Cleary (18). DNA probes for Southern hybridization analysis were conjugated to horseradish peroxidase, and hybridization was detected with a chemiluminescent substrate (ECL kit) in accordance with the manufacturer's (Amersham Life Science, Arlington Heights, Ill.) instructions.
Derivation of cysteine protease-deficient mutant AM3speBΩ and cysteine protease-producing revertant AM3RV.
AM3speBΩ and AM3RV were derived from AM3 by allelic-exchange mutagenesis (4). Plasmid pJspeBΩ contains the ΩKm-2 interposon cloned within the speB gene in temperature-sensitive shuttle vector pJRS233 (4, 19). Plasmid pJspeBΩ was electroporated into AM3 cells. Transformants that contained the free plasmid were selected after growth at 30°C in THY broth supplemented with erythromycin. A single transformant was grown in 10 ml of THY broth with erythromycin at 37°C until the culture reached stationary phase (A600, >0.5). The stationary-phase culture was serially diluted and subcultured on THY-blood with erythromycin at 37°C. Individual colonies were stored as possible strains that contained the plasmid integrated within speB on the AM3 chromosome. One candidate protease-deficient integrant strain was serially passed eight times in THY broth at 30°C, and the final broth was diluted and plated on TSA-blood plates. Individual colonies were replica plated on TSA-blood and THY-blood with erythromycin. Excision of pJspeBΩ from the AM3 chromosome is expected to either complete the exchange of the mutant speB allele or reconstitute the wild-type genotype. Erythromycin-sensitive colonies were identified as possible allelic-exchange mutants or wild-type revertants. Individual erythromycin-sensitive colonies were assayed for cysteine protease activity. One erythromycin-sensitive, protease-deficient strain was designated AM3speBΩ. One erythromycin-sensitive, protease-positive strain was designated AM3RV.
Determination of hyaluronic acid expression.
GAS capsule expression from exponential-phase bacterial cultures was quantified by measurement of cell-associated hyaluronic acid by using the carbocyanine dye 1-ethyl-2-[3-(1-ethyl-naphtho[1,2d]thiazolin-2-ylidene)-2-methyl-propenyl]naphtho[1,2d]-thiazolium bromide (Stains-All; Sigma Chemical Co., St Louis, Mo.) as previously described (21). The measurements reported are the means of three experiments performed in duplicate.
Determination of M protein expression.
Acid extracts of GAS surface M protein were prepared from 300-ml exponential-phase broth cultures as described by Lancefield (13). The amount of M protein in the extracts was evaluated by an Ouchterlony immunodiffusion assay using M type 3-specific antiserum as previously described (29). A more-than-twofold difference in the antigen concentration required to produce a precipitation line was considered a significant difference in M protein expression between GAS strains.
Determination of cysteine protease production
GAS cysteine protease activity was determined by a plate assay as previously described (4). To quantitatively compare the amounts of protease produced by GAS strains, bacteria were grown for 16 h and diluted to an A600 of 0.5 to equalize bacterial numbers and a 5-μl aliquot of each cell suspension was stabbed into the assay medium. After a 24-h incubation at 37°C, photographs of the inoculation site were obtained and the surface area of proteolysis was determined from five images per strain by using the National Institutes of Health software program Image.
Determination of streptolysin O production.
The streptolysin O (SLO) activity in GAS culture supernatants was determined by a modification of the method of Alouf (1). A standardized suspension of sheep red blood cells (RBC) was prepared by diluting 6 ml of fresh defibrinated sheep blood (PML Microbiologicals) in 100 ml of phosphate-buffered saline (PBS), pH 6.5, supplemented with 0.1% (wt/vol) bovine serum albumin (Sigma). Additional PBS-bovine serum albumin buffer was added as required such that the lysis of a 500-μl aliquot of the RBC suspension by its dilution in 14.5 ml of 0.1% (wt/vol) Na2CO3 (Sigma) resulted in an A541 of 0.2. GAS was inoculated into 10 ml of THY broth to an A600 of 0.05, and the culture was incubated at 37°C for 9 h. The culture supernatants were recovered after centrifugation and filter sterilized. Dithiothreitol (Sigma) was added to a 1-ml aliquot of culture supernatant to a final concentration of 40 mM, and the supernatant was incubated at 37°C for 10 min. A 25-μl aliquot of the culture supernatant was incubated with 500 μl of the standardized RBC suspension at 37°C for 45 min. Intact RBC were removed by centrifugation, and the A541 of the supernatant was determined. Absorbance values were compared with a standard curve of RBC lysed in water. The highest dilution of the culture supernatant that lysed 50% of the RBC suspension was reported. The specificity of the SLO effect was confirmed by complete inhibition of RBC lysis when the assay included cholesterol (Sigma) at a concentration of 17 μg/ml. It is of note that the complete suppression of the hemolytic activity present in early stationary-phase GAS culture supernatants by cholesterol is consistent with our observation that negligible streptolysin S is produced under these culture conditions. By contrast, streptolysin S is the predominant hemolysin recovered from late stationary-phase GAS cultures. A more-than-twofold difference in the culture supernatant concentration that lysed 50% of the RBC suspension was considered a significant difference in SLO expression between GAS strains.
Phagocytosis assay.
The direct bactericidal test of Lancefield (12) was used to determine the ability of GAS strains to resist opsonophagocytic killing in human whole blood as previously described (29). In this assay, approximately 1,000 organisms are inoculated in heparinized whole blood and mixed with rotation for 3 h at 37°C. Aliquots are removed at the initiation and completion of the experiment and cultured on blood agar medium for enumeration of the bacterial population. Results of the phagocytic assays are reported as the log of the fold change in the CFU count (total CFU after incubation/total starting CFU).
Murine invasive infection models.
In the acute sepsis model, female 6- to 8-week-old ICR (CD-1) mice (Harlan, Indianapolis, Ind.) were injected intraperitoneally with either 104 exponential-phase or 107 stationary-phase bacteria suspended in 1 ml of THY broth. To prepare the exponential-phase inoculum, GAS bacteria were seeded in 10 ml of THY to an initial A600 of 0.05. The broth culture was harvested when the A600 reached 0.15. To prepare the stationary-phase inoculum, GAS was suspended in 10 ml of THY to an initial A600 of 0.05. The culture was harvested after a 16-h incubation, and the cells were washed once in sterile PBS before resuspension in THY broth. An aliquot of each inoculum was removed and subcultured on TSA-blood plates for enumeration of the challenge dose. Animals were monitored twice daily for morbidity over 72 h (exponential-phase bacterial challenge) or 120 h (stationary-phase bacterial challenge). Moribund animals and animals surviving to the experimental endpoint were euthanatized by carbon dioxide inhalation. Spleens were removed from dead animals and homogenized in 1 ml of THY broth. A 100-μl aliquot of splenic homogenate was cultured on TSA-blood plates for enumeration of hematogenously disseminated GAS on the basis of its characteristic β-hemolysis. In the invasive soft-tissue infection model, anesthetized female 6- to 8-week-old ICR (CD-1) mice were injected subcutaneously with 50 μl of either 107 exponential-phase or 108 stationary-phase GAS bacteria suspended in sterile PBS. Animals were monitored twice daily for 2 weeks. Moribund animals and animals surviving to the completion of the experiment were euthanatized, and spleen cultures were obtained as described above.
Statistical analysis.
Statistical analysis was performed with GraphPad Prism version 2.0 (GraphPad, San Diego, Calif.). Differences between strains in the amount of cell-associated hyaluronic acid capsule and susceptibility to phagocytic killing were compared for significance by one-way analysis of variance with Bonferroni's multiple-comparison posttest analysis (20). Analysis for significant differences in mouse survival following a GAS challenge employed the log-rank test (20). A P value of <0.05 was considered significant.
RESULTS
Derivation of a cysteine protease-deficient mutant in the background of GAS strain AM3.
GAS strain AM3 is an M3 isolate originally recovered from a patient with puerperal sepsis (23). AM3speB is a protease-deficient plasmid integration mutant derived from AM3 (16). To independently derive protease-deficient mutant AM3speBΩ in the AM3 background, we inserted the ΩKm-2 element into the AM3 speB gene by allelic-exchange mutagenesis. Southern hybridization analysis confirmed the interruption of speB in AM3speBΩ (data not shown). Determination of protease expression using a solid-phase plate assay indicated that AM3speBΩ was completely deficient in protease activity (Table 1).
TABLE 1.
Virulence determinant expression in wild-type strain AM3 and protease-deficient mutants AM3speB and AM3speBΩ
| Strain | Amt of HA capsule (fg/CFU ± SEM)a | M protein dilutionb | SLO dilutionc | Cysteine protease activityd (mm2 ± SEM) |
|---|---|---|---|---|
| AM3 | 34 ± 3.0 | 1:4 | 1:10 | 129 ± 5.4 |
| AM3speB | 20 ± 1.7 | 1:4 | 1:20 | ND |
| AM3speBΩ | 33 ± 3.8 | 1:8 | 1:10 | ND |
The amount of cell-associated hyaluronic acid (HA) capsule was determined by Stains-All assay. The data shown are mean values derived from three experiments performed in duplicate. Capsule production by AM3speB was significantly less than that by either AM3 or AM3speBΩ (P < 0.05)
Highest dilution of M protein extract that produced a precipitin line in an Ouchterlony immunodiffusion assay with anti-M3 serum. The data shown are from a single representative experiment.
Highest dilution of culture supernatant that lysed 50% of a sheep RBC suspension. The data shown are from a single representative experiment.
Area of protein precipitate surrounding a GAS stab inoculated into agar medium containing casein. The data shown are mean values derived from the measurement of five inoculation sites. ND, not detectable.
Quantitative analysis of virulence factor expression in AM3, AM3speB, and AM3speBΩ.
Because the current techniques for targeted mutagenesis of GAS involve multiple in vitro manipulations that may unpredictably influence the bacterial expression of products unrelated to the target gene, we quantified the expression of several recognized or putative GAS virulence determinants in the parent and mutant strains (Table 1). The results demonstrate that the expression of the hyaluronic acid capsule was significantly less in AM3speB than in either AM3 or AM3speBΩ but that the production of the other measured virulence determinants was similar between strains, except for the absence of protease activity in the two speB mutants. These results confirm a previous report that AM3speB produced less capsule than did parent AM3 strain (30). Because AM3 and AM3speBΩ made equivalent amounts of capsule, these results also indicate that the decreased capsule expression in AM3speB was not linked to inactivation of the protease.
Resistance of AM3 and the isogenic cysteine protease-deficient mutants to phagocytic killing in vitro.
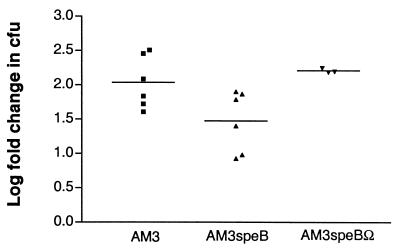
To determine whether the decrease in capsule expression seen in AM3speB relative to AM3 and AM3speBΩ was associated with increased bacterial susceptibility to phagocytic killing, we determined bacterial survival after incubation in human whole blood (Fig. 1). The fold increase in the number of AM3speB CFU was 0.5 log less than that of the number of either AM3 or AM3speBΩ CFU. Although small, this difference was significant and was similar in magnitude to the differences in susceptibility to phagocytic killing that are characteristic of isogenic encapsulated-unencapsulated GAS strain pairs in this assay (3, 17, 28). A 0.5-log decrease in the recovery of acapsular bacteria in the assay predicts attenuation in in vivo murine models of invasive infection (17, 28).
FIG. 1.
Growth of GAS in whole blood. Values represent the mean log fold increase in CFU after 3 h of rotation in fresh human blood. Each point represents a single experiment. The differences in net growth between AM3 and AM3speB and between AM3speBΩ and AM3speB were significant (P < 0.05).
GAS cysteine protease is not required for virulence in murine models of invasive infection.
To determine whether the cysteine protease contributes to virulence in a murine model of human GAS sepsis, we challenged mice intraperitoneally with either the parent AM3 strain or either of the cysteine protease-deficient mutant strains and determined mouse mortality. Because the expression of the cysteine protease is known to be sensitive to growth conditions in vitro, with nearly exclusive expression during stationary-phase growth in broth culture, we performed experiments with either exponential- or stationary-phase organisms as the challenge inoculum.
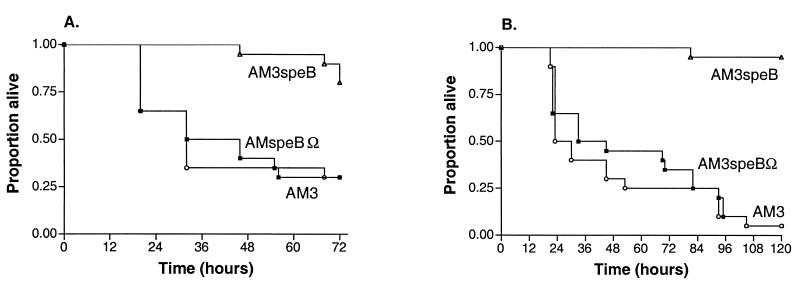
Regardless of the growth phase of the inoculum, the survival of animals challenged with cysteine protease-deficient mutant strain AM3speBΩ was not significantly different from the survival of animals challenged with wild-type strain AM3 (Fig. 2). Because bacteria cultured from the spleen of each animal that died after a challenge with strain AM3speBΩ tested negative for protease activity, the virulence of this mutant was not due to reversion to the wild-type phenotype. These results demonstrate that the protease has no significant effect on mouse mortality after an intraperitoneal challenge. By contrast, animals challenged with protease-deficient mutant AM3speB in either the exponential or the stationary phase were significantly more likely to survive than were animals challenged with parent strain AM3 or cysteine protease-deficient mutant AM3speBΩ (Fig. 2). These findings confirm a previous report demonstrating that the virulence of strain AM3speB was attenuated in mice challenged intraperitoneally but indicate that this attenuation was not due to loss of protease activity, since protease-deficient mutant AM3speBΩ was fully virulent (16).
FIG. 2.
Mouse survival after an intraperitoneal challenge with GAS. The curves represent Kaplan-Meier survival function estimates after an intraperitoneal challenge with either 104 exponential-phase (A) or 107 stationary-phase (B) GAS bacteria. Each curve represents combined data from two experiments with 10 mice per strain. Survival was significantly longer in animals challenged with AM3speB than in animals challenged with either AM3 or AM3speBΩ, and this effect was independent of the growth phase of the inoculum (exponential phase, P = 0.003; stationary phase, P < 0.001).
Because the effect of the protease may be restricted to a specific host environment, we performed similar experiments to determine whether the protease is required for the pathogenesis of murine invasive soft-tissue infection. Animals were challenged subcutaneously with either exponential- or stationary-phase GAS and mortality was determined over 14 days (Fig. 3 and 4). This model results in local tissue necrosis with delayed bacteremia and simulates human necrotizing fasciitis (4, 15). GAS was recovered from the spleen of each animal that died during these studies. Measurement of the protease activity of each spleen isolate confirmed that the speB genotype and the protease phenotype of the various challenge strains were stable.
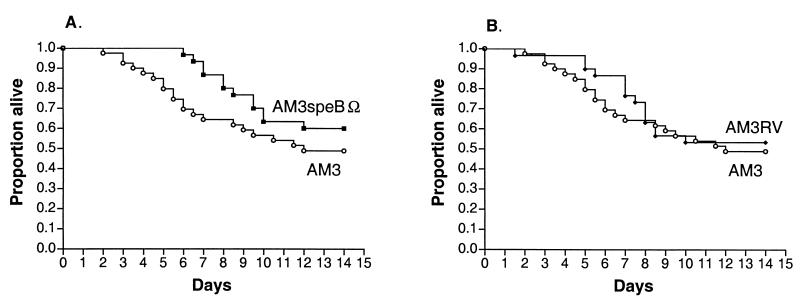
FIG. 3.
Mouse survival after a subcutaneous challenge with exponential-phase GAS. The curves represent Kaplan-Meier survival function estimates after a mouse challenge with 107 exponential-phase GAS bacteria. Each curve represents combined data from two experiments with 10 mice per strain. The survival of animals challenged with AM3 was not significantly different from that of animals challenged with AM3speBΩ.
FIG. 4.
Mouse survival after a subcutaneous challenge with stationary-phase GAS. The curves represent Kaplan-Meier survival function estimates after a mouse challenge with 108 stationary-phase GAS bacteria. Each curve represents combined data from three experiments with 10 mice per strain. For clarity, the survival curve for animals challenged with AM3 is compared with the survival curve for animals challenged with AM3speBΩ (A) or with the survival curve for animals challenged with AM3RV (B). The survival of animals challenged with AM3 was not significantly different from that of animals challenged with AM3speBΩ or AM3RV.
After subcutaneous inoculation with exponential-phase organisms, there was no significant difference in the mortality of animals challenged with wild-type strain AM3 and those challenged with cysteine protease-deficient mutant AM3speB (Fig. 3). After a subcutaneous challenge with stationary-phase GAS, there appeared to be a trend toward delayed mortality in animals challenged with AM3speBΩ compared with animals challenged with AM3, although this difference was not statistically significant (Fig. 4A).
To determine whether the trend toward delayed mortality in animals challenged with stationary-phase AM3speBΩ was specific to the mutation in speB or was due to a nonspecific attenuation of virulence that occurred during the process of mutagenesis, we repeated the experiment with GAS strain AM3RV. AM3RV is a wild-type revertant strain that underwent in vitro passage identical to that of AM3speBΩ. By contrast to AM3speBΩ, AM3RV reverted to the protease-positive phenotype after plasmid excision from the GAS chromosome during mutagenesis and therefore is a suitable control for the effect of in vitro passage on in vivo virulence. The protease activity of AM3RV was equivalent to that of wild-type parent strain AM3 (AM3, 129± 5.4 mm2; AM3RV 127 ± 5.3 mm2). As did animals challenged with stationary-phase AM3speBΩ, animals challenged with stationary-phase AM3RV showed a nonsignificant trend toward delayed mortality compared with those challenged with wild-type strain AM3 (Fig. 4B).
Taken together, the results of the mouse subcutaneous challenge experiments do not support a significant contribution of the cysteine protease in the pathogenesis of invasive soft-tissue infection in the murine model and suggest that a slight attenuation of virulence independent of the genetic manipulation of speB may have occurred in both AM3speBΩ and AM3RV during the in vitro passage required for derivation of the mutant strain.
DISCUSSION
Our findings confirm the previous observation that protease-deficient strain AM3speB produces less hyaluronic acid capsule than does parent strain AM3 (30). However, since protease-deficient mutant AM3speBΩ produced an amount of capsule equivalent to that produced by AM3, the reduced capsule expression in AM3speB is not due to inactivation of speB, as had been postulated (30). Because electroporation of recombinant DNA into GAS is both a necessary step in targeted mutagenesis and an event that occurs more efficiently in the absence of capsule, we speculate that the decreased capsule expression in AM3speB may have been selected for during bacterial transformation. A similar phenomenon has been reported in Streptococcus pneumoniae (27).
Decreased production of the antiphagocytic capsule in AM3speB was associated with increased bacterial susceptibility to opsonophagocytic killing in vitro. Because the whole-blood phagocytic assay is a specific, but not a particularly sensitive, predictor of in vivo virulence, even small increases in GAS susceptibility to phagocytic killing in this assay can be associated with marked attenuation in animal models of invasive infection (3, 17, 28). Although there may be additional mechanisms, it seems likely that decreased production of capsular polysaccharide contributes to the diminished virulence of AM3speB noted in this and previous studies (15, 16). In any event, the attenuated phenotype of AM3speB in mice after an intraperitoneal or subcutaneous challenge noted previously was not due to the speB mutation, since inactivation of speB in AM3speBΩ did not significantly diminish virulence in the same animal models.
The nearly equivalent mortality of mice challenged either intraperitoneally or subcutaneously with wild-type strain AM3 or protease-deficient mutant AM3speBΩ is consistent with our prior study, in which an speB mutant in a different M3 background was fully virulent in murine invasive soft-tissue infection. Arguably, our earlier result may have been due to the use of an exponential-phase challenge inoculum in which the protease was not being expressed. However, because protease-deficient mutant AM3speBΩ was fully virulent when animals were challenged with either exponential- or stationary-phase organisms, the present study indicates that regardless of the growth phase of the bacterial challenge inoculum, the cysteine protease does not influence mouse mortality in these models of invasive infection.
Our results contrast with reports of the diminished virulence of an M49 speB mutant in mice after an intraperitoneal challenge and of an M1 and M49 speB mutant in mice after a subcutaneous challenge (11, 16). It is possible that the protease is required for the virulence of these M1 and M49 strains but not for the virulence of the M3 strains we tested. Although our findings do not exclude the possibility of such a strain-dependent phenotype, they do suggest alternative explanations for the attenuated behavior of the M1 and M49 speB mutants. First, as noted above, derivation of the mutants may have inadvertently selected for less-encapsulated strains. Second, the repeated in vitro passage required for derivation of the mutant may have nonspecifically diminished bacterial virulence. Although not a statistically significant effect, in our study, there appeared to be a slight decrease in mouse mortality after subcutaneous challenge with either AM3speBΩ or wild-type revertant strain AM3RV, both strains that had been serially cultured in vitro, compared with the survival of mice challenged with wild-type strain AM3. This observation is consistent with an attenuating effect of in vitro passage on GAS virulence, and although it is small, the decrease in bacterial virulence after in vitro passage is likely to be greater when the wild-type strain is intrinsically more mouse virulent than AM3, a wild-type strain that requires a significantly larger challenge inoculum to cause disease in these models than do more aggressive GAS strains.
The absence of an effect of the protease on virulence in two different M3 backgrounds and two different animal models strongly suggests that the protease makes no significant contribution to invasive infection. Nevertheless, because the animal models used are only approximations of human disease, it remains possible that the protease participates in the pathogenesis of invasive infection, although these studies suggest that such an effect, if present, is probably small. Our experiments do not address the potential influence of the protease on the evolution of infections localized to the pharynx or the skin. In this regard, a recent study by Svensson et al. indicates a possible role for the protease in impetigo (26).
ACKNOWLEDGMENTS
We thank Sarah Henderson, Thuyanh Le, and Joy Rosenblatt for expert technical assistance. We thank Gabriele Sierig for performing the SLO assays.
This work was supported by Public Health Service grants AI29952 (M.R.W.) and AI01343 (C.D.A.) and contract AI75326 from the National Institute of Allergy and Infectious Diseases.
REFERENCES
- 1.Alouf J E. Streptococcal toxins (streptolysin O, streptolysin S, erythrogenic toxin) Pharmacol Ther. 1980;11:661–717. doi: 10.1016/0163-7258(80)90045-5. [DOI] [PubMed] [Google Scholar]
- 2.Ashbaugh C D, Albertí S A, Wessels M R. Molecular analysis of the capsule gene region of group A Streptococcus: the hasAB genes are sufficient for capsule expression. J Bacteriol. 1998;180:4955–4959. doi: 10.1128/jb.180.18.4955-4959.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashbaugh C D, Moser T J, Shearer M H, White G L, Kennedy R C, Wessels M R. Bacterial determinants of persistent throat colonization and the associated immune response in a primate model of human group A streptococcal infection. Cell Microbiol. 2000;2:283–292. doi: 10.1046/j.1462-5822.2000.00050.x. [DOI] [PubMed] [Google Scholar]
- 4.Ashbaugh C D, Warren H B, Carey V J, Wessels M R. Molecular analysis of the role of the group A streptococcal cysteine protease, hyaluronic acid capsule, and M protein in a murine model of human invasive soft-tissue infection. J Clin Investig. 1998;102:550–560. doi: 10.1172/JCI3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burns E H J, Marciel A M, Musser J M. Activation of a 66-kilodalton human endothelial cell matrix metalloprotease by Streptococcus pyogenes extracellular cysteine protease. Infect Immun. 1996;64:4744–4750. doi: 10.1128/iai.64.11.4744-4750.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaussee M S, Phillips E R, Ferretti J J. Temporal production of streptococcal erythrogenic toxin B (streptococcal cysteine proteinase) in response to nutrient depletion. Infect Immun. 1997;65:1956–1959. doi: 10.1128/iai.65.5.1956-1959.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hytonen J, Haataja S, Gerlach D, Podbielski A, Finne J. The SpeB virulence factor of Streptococcus pyogenes, a multifunctional secreted and cell surface molecule with strepadhesin, laminin-binding and cysteine protease activity. Mol Microbiol. 2001;39:512–519. doi: 10.1046/j.1365-2958.2001.02269.x. [DOI] [PubMed] [Google Scholar]
- 8.Kapur V, Majesky M W, Li L L, Black R A, Musser J M. Cleavage of interleukin 1 beta (IL-1 beta) precursor to produce active IL-1 beta by a conserved extracellular cysteine protease from Streptococcus pyogenes. Proc Natl Acad Sci USA. 1993;90:7676–7680. doi: 10.1073/pnas.90.16.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kapur V, Topouzis S, Majesky M W, Li L L, Hamrick M R, Hamill R J, Patti J M, Musser J M. A conserved Streptococcus pyogenes extracellular cysteine protease cleaves human fibronectin and degrades vitronectin. Microb Pathog. 1993;15:327–346. doi: 10.1006/mpat.1993.1083. [DOI] [PubMed] [Google Scholar]
- 10.Kellner A, Robertson T. Myocardial necrosis produced in animals by means of crystalline streptococcal protease. J Exp Med. 1954;99:495–504. doi: 10.1084/jem.99.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuo C F, Wu J J, Lin K Y, Tsai P J, Lee S C, Jin Y T, Lei H Y, Lin Y S. Role of streptococcal pyrogenic exotoxin B in the mouse model of group A streptococcal infection. Infect Immun. 1998;66:3931–3935. doi: 10.1128/iai.66.8.3931-3935.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lancefield R. Differentiation of group A streptococci with a common R antigen into three serological types with special reference to the bactericidal test. J Exp Med. 1957;107:525–544. doi: 10.1084/jem.106.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lancefield R C. The antigenic complex of Streptococcus haemolyticus. I. Demonstration of a type specific substance in extracts of Streptococcus haemolyticus. J Exp Med. 1928;47:91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lukomski S, Burns E H J, Wyde P R, Podbielski A, Rurangirwa J, Moore-Poveda D K, Musser J M. Genetic inactivation of an extracellular cysteine protease (SpeB) expressed by Streptococcus pyogenes decreases resistance to phagocytosis and dissemination to organs. Infect Immun. 1998;66:771–776. doi: 10.1128/iai.66.2.771-776.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lukomski S, Montgomery C A, Rurangirwa J, Geske R S, Barrish J P, Adams G J, Musser J M. Extracellular cysteine protease produced by Streptococcus pyogenes participates in the pathogenesis of invasive skin infection and dissemination in mice. Infect Immun. 1999;67:1779–1788. doi: 10.1128/iai.67.4.1779-1788.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lukomski S, Sreevatsan S, Amberg C, Reichardt W, Woischnik M, Podbielski A, Musser J M. Inactivation of Streptococcus pyogenes extracellular cysteine protease significantly decreases mouse lethality of serotype M3 and M49 strains. J Clin Investig. 1997;99:2574–2580. doi: 10.1172/JCI119445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moses A E, Wessels M R, Zalcman K, Alberti S A, Natanson-Yaron S, Menes T, Hanski E. Relative contributions of hyaluronic acid capsule and M protein to virulence in a mucoid strain of the group A Streptococcus. Infect Immun. 1997;65:64–71. doi: 10.1128/iai.65.1.64-71.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O'Connor S P, Cleary P P. Localization of the streptococcal C5a peptidase to the surface of group A streptococci. Infect Immun. 1986;53:432–444. doi: 10.1128/iai.53.2.432-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perez-Casal J, Price J A, Maguin E, Scott J R. An M protein with a single C repeat prevents phagocytosis of Streptococcus pyogenes: use of a temperature-sensitive shuttle vector to deliver homologous sequences to the chromosome of S. pyogenes. Mol Microbiol. 1993;8:809–819. doi: 10.1111/j.1365-2958.1993.tb01628.x. [DOI] [PubMed] [Google Scholar]
- 20.Rosner B. Fundamentals of Biostatistics. 3rd ed. Boston, Mass: PWS-KENT; 1990. [Google Scholar]
- 21.Schrager H, Wessels M R. Hyaluronic acid capsule and the role of streptococcal entry into keratinocytes in invasive skin infection. J Clin Investig. 1996;98:1954–1958. doi: 10.1172/JCI118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shanley T P, Schrier D, Kapur V, Kehoe M, Musser J M, Ward P A. Streptococcal cysteine protease augments lung injury induced by products of group A streptococci. Infect Immun. 1996;64:870–877. doi: 10.1128/iai.64.3.870-877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stamp T C, Hendry E B. The immunizing activity of certain chemical fractions isolated from haemolytic streptococci. Lancet. 1937;i:257–259. [Google Scholar]
- 24.Stevens D L. Streptococcal toxic shock syndrome associated with necrotizing fasciitis. Annu Rev Med. 2000;51:271–288. doi: 10.1146/annurev.med.51.1.271. [DOI] [PubMed] [Google Scholar]
- 25.Stockbauer K E, Magoun L, Liu M, Burns E H, Gubba S, Renish S, Pan X, Bodary S C, Baker E, Coburn J, Leong J M, Musser J M. A natural variant of the cysteine protease virulence factor of group A streptococcus with an arginine-glycine-aspartic acid (RGD) motif preferentially binds human integrins αvβ3 and αIIbβ3. Proc Natl Acad Sci USA. 1999;96:242–247. doi: 10.1073/pnas.96.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Svensson M D, Scaramuzzino D A, Sjobring U, Olsen A, Frank C, Bessen D E. Role for a secreted cysteine proteinase in the establishment of host tissue tropism by group A streptococci. Mol Microbiol. 2000;38:242–253. doi: 10.1046/j.1365-2958.2000.02144.x. [DOI] [PubMed] [Google Scholar]
- 27.Weiser J N, Kapoor M. Effect of intrastrain variation in the amount of capsular polysaccharide on genetic transformation of Streptococcus pneumoniae: implications for virulence studies of encapsulated strains. Infect Immun. 1999;67:3690–3692. doi: 10.1128/iai.67.7.3690-3692.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wessels M R, Bronze M S. Critical role of the group A streptococcal capsule in pharyngeal colonization and infection in mice. Proc Natl Acad Sci USA. 1994;91:12238–12242. doi: 10.1073/pnas.91.25.12238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wessels M R, Moses A E, Goldberg J B, DiCesare T J. Hyaluronic acid capsule is a virulence factor for mucoid group A streptococci. Proc Natl Acad Sci USA. 1991;88:8317–8321. doi: 10.1073/pnas.88.19.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woischnik M, Buttaro B A, Podbielski A. Inactivation of the cysteine protease SpeB affects hyaluronic acid capsule expression in group A streptococci. Microb Pathog. 2000;28:221–226. doi: 10.1006/mpat.1999.0341. [DOI] [PubMed] [Google Scholar]