SUMMARY
OBJECTIVE:
This study aimed to evaluate the diagnostic efficiency of contrast-to-noise and signal-to-noise ratios created by the contrast medium in detecting lymph nodes.
METHODS:
In this study, 57 short-axis subcentimeter lymph nodes in 40 cardiac computed tomography patients with noncontrast- and contrast-enhanced phases were evaluated. The contrast-to-noise ratios and signal-to-noise ratios of noncontrast- and contrast-enhanced lymph node-mediastinal fat and aortic-mediastinal fat tissues were determined. In addition, lymph nodes in noncontrast- and contrast-enhanced series were evaluated subjectively.
RESULTS:
There was a significant difference in lymph node-mediastinal fat signal-to-noise values between the contrast and noncontrast phases (p=0.0002). In the contrast phase, aortic density values were found to be 322.04±18.51 HU, lymph node density values were 76.41±23.41 HU, and mediastinal adipose tissue density values were −65.73±22.96 HU. Aortic-mediastinal fat contrast-to-noise ratio value was 20.23±6.92 and the lymph node-mediastinal fat contrast-to-noise ratio value was 6.43±2.07. A significant and moderate correlation was observed between aortic-mediastinal fat and lymph node-mediastinal fat contrast-to-noise ratio values in the contrast phase (r=0.605; p<0.001). In the contrast-enhanced series, there was a significant increase in the subjective detection of lymph nodes (p=0.0001).
CONCLUSION:
In the detection of paratracheal lymph nodes, the contrast agent increases the detection of short-axis subcentimeter lymph nodes quantitatively and qualitatively. Contrast enhances and facilitates the detection of paratracheal lymph nodes.
KEYWORDS: Lymph nodes, Contrast media, Computed tomography, Mediastinum
INTRODUCTION
Lung cancer is the leading cause of death from cancer 1 . Mediastinal lymph node evaluation is important in the diagnosis, treatment, and follow-up of lung cancer because lung cancer often causes mediastinal and hilar lymph node involvement 1,2 . In particular, lymphoscintigraphic evaluations revealed that the dominant route in lymphatic drainage was the bilateral paratracheal area 3 . Therefore, the detection of paratracheal lymph nodes is an important factor in tumor staging 3 . Lymph node diagnosis, staging, and treatment protocol may vary 4,5 . A short axis >1 cm is an important criterion in the definition of the pathological lymph node 6 . However, some studies have shown that lymph nodes with a short axis <1 cm can also be pathological 7−9 . Therefore, determining the early metastatic involvement of the lymph nodes is very important for the patient's prognosis 5 . In addition, suspicious lymph nodes should not be overlooked in lymph node surgeries. Detection of large lymph nodes is relatively easy during imaging and surgical procedures, while detection of small lymph nodes is quite difficult. The most accurate detection of small lymph nodes can change the staging of the patient 7,10 . Thus, a patient's surgery, treatment protocol, and prognosis may change.
Many imaging methods are used in the detection, staging, and follow-up of mediastinal lymph nodes. Methods such as computed tomography (CT), positron emission tomography, magnetic resonance imaging (MRI), and endobronchial ultrasonography can be used 1,11−13 . However, these methods have difficulties in detecting small lymph nodes, and their effectiveness decreases 10 . Therefore, optimizing shooting techniques and increasing the efficiency of these methods by determining the factors affecting image quality are important problems in engineering and radiology sciences.
MRI has the advantage over CT that it is radiation free. However, since MRI does not provide evaluation of lung parenchyma, CT is still used more effectively in the lung and mediastinal area 14 . CT is an effective and important test in the diagnosis of early-stage lung cancer 15 . However, since it has the disadvantage of containing radiation dose, it should be done with optimal and correct techniques. Thus, the patient is exposed to less radiation dose 15 . For this reason, protocols are being made for lung and mediastinal evaluation, and these protocols are being developed day by day 16 .
In our study, we aimed to evaluate the effect of vascular contrast material on contrast-to-noise ratio (CNR) and signal-to-noise ratio (SNR) in CT and its effectiveness in subcentimetric lymph node diagnosis.
METHODS
Patient selection
This study was approved by the Atatürk University Scientific Ethics Committee (dated February 24, 2022; decision no. 2/26). In this study, the images of the patients who had no known malignancy and who had 62 cardiac extractions were reviewed retrospectively. Notably, 5 patients with body mass index >30, 3 patients with mediastinal calcified lymphadenopathies, 3 patients whose lymph node evaluation was not performed due to artifacts, and 11 patients whose mediastinal paratracheal lymph nodes were not detected were excluded from the study. Only individuals with mediastinal subcentimetric lymph nodes were used in the study. Patients with lung pathology that may cause mediastinal lymph node formation and an increase in number were excluded from the study. Thus, the cardiac patient group in which only cardiac imaging was performed due to cardiac complaints and the mediastinal area was evaluated was selected. In total, 40 individuals who met the criteria were evaluated. In addition, 57 lymph nodes with a short axis <1 cm were evaluated in 40 patients.
Computed tomography protocol
CT examinations were performed with a 256-section CT scanner (Somatom Definition Flash®, Siemens Healthcare, Forscheim, Germany). Prospective ECG-gated high-pitch “flash spiral” technique was used to acquire the images with the following parameters: 120 kVp; 3 mm slice thickness; 256×0.6 mm slice collimation; z-flying focal spot; 280 ms gantry rotation time; 3.4 pitch; 75 msn temporal resolution; tube current of 80–140 mA; and topogram-based automatic tube current selection (CareDose 4D®, Siemens Healthcare). The contrast amount was 90–100 mL, and the injection rate was 4–5 mL/s.
Image analysis
Noncontrast and 15-s images of 40 patients in the aorta were evaluated in the mediastinal window (window width, 350 HU; level, 50 HU). Evaluations were made for lymph nodes located 1 cm below the short axis at the paratracheal level. Hounsfield unit mean density and standard deviation (SD) values were determined by applying 0.10–0.15 cm2 to a region of interest (ROI) from the aortic lumen, paratracheal adipose tissue, and paratracheal lymph nodes in the cortical area. Aortic-mediastinal fat and paratracheal lymph node-mediastinal fat CNRs and SNRs were determined with the following formulas for contrast and noncontrast series. The SNR (Equation 1) is found by dividing the signal intensity (SI) by the SD. The CNR (Equation 2) is found by dividing the difference between the lesion and background ROI values by the square root of half the sum of the squares of the SD.
| (1) |
| (2) |
In addition, the lymph nodes were evaluated subjectively by two observers according to the clarity of the lymph node mediastinal fat border separation. Image quality was evaluated by two reviewers for lymph node and mediastinal adipose tissue separation. They were instructed to report lesion conspicuity on a 4-point scale (1, barely perceptible with presence debatable; 2, subtle finding but likely a lesion; 3, definite lesion detected; and 4, strikingly evident and easily detected) 17,18 . The conspicuity of undetected lesions was recorded as 0. The final data were obtained by averaging the data of two reviewers.
Statistical analysis
The normality of the data was checked using the D’Agostino-Pearson test. The correlation between aortic-mediastinal adipose tissue CNR data and lymph node-mediastinal adipose tissue CNR values was evaluated using the Spearman's correlation test. The relationship between CNR, SNR, and subjective evaluation between the contrast and noncontrast groups was evaluated using the Wilcoxon test.
RESULTS
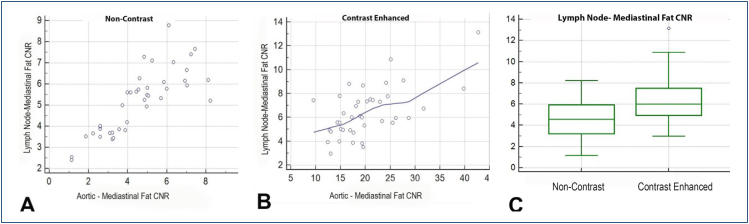
The mean age of 40 patients was 49.05 years; 20 patients were male and 20 patients were female, with a female-to-male ratio of 1. In 40 patients, 57 lymph nodes with a short axis <1 cm were evaluated. In the noncontrast phase, aortic density value was 39.53±13.35 HU, lymph node density value was 29.3±15.85 HU, and mediastinal adipose tissue density value was −69.74±26.52 HU. Aortic-mediastinal fat CNR value was 5.18±1.49; lymph node-mediastinal fat CNR value was 4.58±1.85. In the noncontrast phase, a significantly high correlation was observed between aortic-mediastinal fat and lymph node-mediastinal fat CNR values (r=0.833; p<0.0001) (Figure 1A). The noncontrast lymph node SNR was 2.23±1.68, and the aortic SNR was 3.85±4.63. No correlation was observed between lymph node SNR values and aortic SNR values in noncontrast series (r=0.164, p=0.31).
Figure 1. There was a correlation between aortic contrast-to-noise ratio values and lymph node contrast-to-noise ratio values in noncontrast-enhanced (A) and contrast-enhanced (B) series. In the contrast-enhanced series, there was a significant increase in lymph contrast-to-noise ratio (C).
In the contrast phase, aortic density value was found to be 322.04±18.51 HU, lymph node density value was 76.41±23.41 HU, and mediastinal adipose tissue density value was −65.73±22.96 HU. Aortic-mediastinal fat CNR value was 20.23±6.92; lymph node-mediastinal fat CNR value was 6.43±2.07. A significant and moderate correlation was observed between aortic-mediastinal fat and lymph node-mediastinal fat CNR values in the contrast phase (r=0.605; p<0.001) (Figure 1B). The contrast-enhanced lymph node SNR was 6.43±2.07, and the aortic SNR was 20.2±6.92. A significant moderate correlation was observed between contrast-enhanced lymph node SNR values and aortic SNR values (r=0.5, p=0.001) (Table 1).
Table 1. Statistical analysis of contrast-to-noise ratio and signal-to-noise ratio values.
| CNR | Correlation value | SNR | Correlation value | |||
|---|---|---|---|---|---|---|
| Aortic | Lymph node | aortic | lymph node | |||
| Contrast phase | 20.23±6.92 | 6.43±2.07 | r=0.605 p<0.001 |
20.2±6.92 | 6.43±2.07 | r=0.5 p=0.001 |
| Noncontrast phase | 5.18±1.49 | 4.58±1.85 | r=0.833 p<0.0001 |
3.85±4.63 | 6.43±2.07 | r=0.164 p=0.31 |
| p-value | – | 0.0002 | – | – | <0.0001 | – |
There was a significant difference in lymph node-mediastinal fat CNR values between the contrast and noncontrast phases (p=0.0002) (Figure 1C). As the signal rate created by the contrast increases, there is an increase in the mediastinal lymph node signal (Figure 2). Contrast-enhanced lymph node SNR values were also significantly higher than nonenhanced lymph node SNR values (p<0.0001) (Table 1).
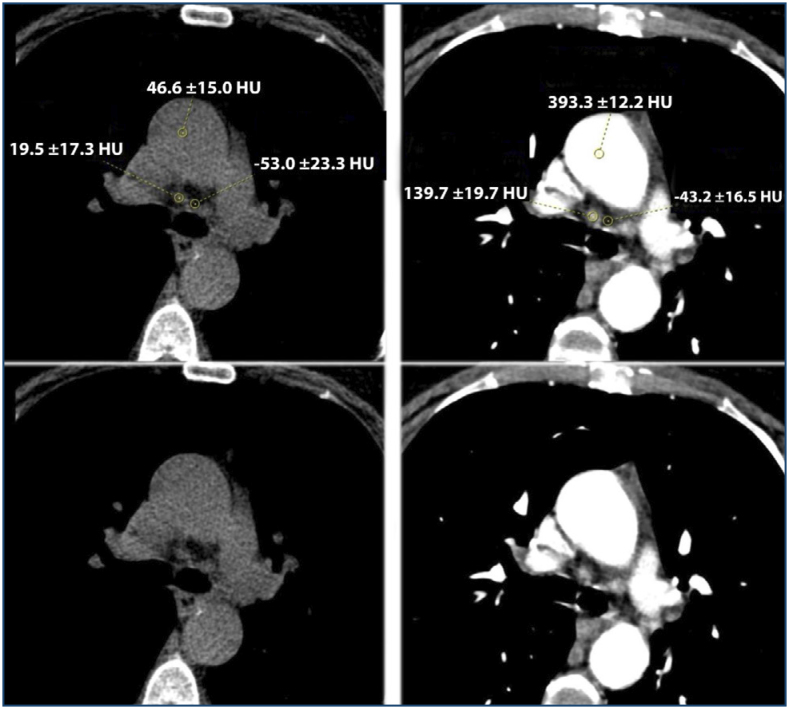
Figure 2. Noncontrast-enhanced aortic-mediastinal fat contrast-to-noise ratio value 5.18, lymph node-mediastinal fat contrast-to-noise ratio ratio 4.58; the contrast-enhanced aortic-mediastinal fat contrast-to-noise ratio value was 20.23, and the contrast-enhanced lymph node-mediastinal fat ratio was 6.43. In subjective evaluations, the mean of the lymph node was 3.19 in the noncontrast-enhanced image, and 3.41 in the contrast-enhanced image.
In the subjective evaluation of lymph nodes among reviewers, an agreement was high in terms of noncontrast- and contrast-enhanced groups (kappa 0.81 and 0.86, respectively). In the pre-contrast subjective evaluation of lymph nodes, the mean rating was 3.19±0.9, and in the post-contrast evaluation, the mean rating was 3.41±0.7. There was a significant increase in the subjective detection of the lymph node in the contrast-enhanced series (p=0.0001).
DISCUSSION
Our study showed that as the vascular contrast agent and its signal strength increase, the signal strength of lymph nodes also increases. Thus, the detectability of lymph nodes increases significantly. Detection and follow-up of lymph nodes are important in many lung diseases, especially lung cancer 1,13 . Therefore, imaging methods, techniques, and evaluation criteria are also very important 10 . CT is an important one among these methods. In fact, with the detection power of CT, changes in staging guidelines have to be made 19 . However, lymph node detection in CT has not reached optimal levels. At this point, the specificity of CT is still 81%, while the sensitivity is still 55%. 20 Although PET-CT increases this sensitivity and specificity, its effectiveness in small lymph nodes is still low 21 . In addition, PET-CT contains a high radiation dose, and false-positive rates are high 22 . Therefore, lymph node biopsy is still the gold standard for diagnosis 23 . At this point in the biopsy, it is an invasive procedure and can cause complications 23 . Some studies have shown that even respiratory activity during a CT scan can affect the detection of lymph nodes 24 . Therefore, it is necessary to increase the efficiency of the techniques and to develop them continuously, and new protocols are created. This is the first study in the literature to determine the effect of vascular CNR on lymph node CNR.
Studies are carried out to use MRI in the staging of lung cancer. Many studies show that MRI plays an important role in detecting mediastinal lymph nodes 25 . However, studies that will establish many standardizations regarding MRI are still required 1,25 . In addition, CT is a feature that can be used as an advantage as it provides faster evaluation than MRI.
Huang et al. observed that the densities of metastatic and nonmetastatic lymph nodes increased significantly in contrast-enhanced CT images 6 . In our study, these data supported the increase in lymph node density in contrast-enhanced series. In addition, we showed that the CNR of the lymph nodes with the ground mediastinal adipose tissue increased. Huang et al. found no significant difference between the arterial and venous phases of the contrast medium in lymph nodes 6 . In our study, we examined the effect of the signal strength created by the contrast agent in the aorta on the contrast signal strength of the lymph nodes, independent of the contrast phase. We found a moderately significant correlation between the vascular CNR and the lymph node CNR.
Choi et al. found a significant increase in the CNR of lymph nodes in contrast-enhanced images 18 . This study supports the increase in lymph node CNR, as we obtained in the contrast-enhanced series in our study. In addition, our study is important in terms of showing the effect of signal strength in the aortic lumen on the lymph node signal CNR in contrast-enhanced series. Aortic contrast signal strength can be affected by individual differences between individuals, as well as by contrast delivery protocols (weight, blood pressure, cardiac rate, etc.). Choi et al. found an increase in the degree of lesion salience in the subjective evaluation of the lymph node in the contrast-enhanced series of reviewers 18 . Our study shows a significant increase in subjective evaluation and supports these data.
Our study also showed a high correlation between aortic-mediastinal fat CNR values and lymph node-mediastinal fat CNR values in noncontrast series. In addition, the lymph node SNR value in the contrast-enhanced phase was also significantly higher than in the noncontrast phase. Many studies in the literature show that nonmetastatic lymph nodes are vascular dense 26,27 . The presence of this dense vascularity may explain the high correlation of lymph nodes with major vascular structures such as the aorta in terms of CNR. However, in contrast-enhanced series, the moderate correlation between aortic CNR and lymph node CNR indicates that the increase in the signal created directly by the contrast in the vascular structure is not as much as the increase in the signal created in the lymph node. As seen in our data, the density increase created by the contrast in the aorta is about 8 times, while the increase in the density created in the lymph node is about 2.5 times. However, this density increase rate provided data that would affect the qualitative evaluation.
As a limitation of our study, we evaluated the detectability of nonmetastatic lymph nodes. Therefore, since the metastatic lymph nodes are on a variable pathological spectrum (increased or decreased vascularity, necrosis, etc.), the effect of aortic signal on the CNR varies 28,29 .
CONCLUSION
The contrast agent increases the detection of lymph nodes quantitatively and qualitatively. In addition, an increased aortic CNR facilitates lymph node detection. Contrast enhances and facilitates the detection of paratracheal lymph nodes.
Footnotes
Funding: none.
REFERENCES
- 1.Zhang L, Wu F, Zhu R, Wu D, Ding Y, Zhang Z, et al. Application of computed tomography, positron emission tomography-computed tomography, magnetic resonance imaging, endobronchial ultrasound, and mediastinoscopy in the diagnosis of mediastinal lymph node staging of non-small-cell lung cancer: a protocol for a systematic review. Medicine (Baltimore) 2020;99(9):e19314. doi: 10.1097/MD.0000000000019314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yu J, Ouyang W, Li C, Shen J, Xu Y, Zhang J, et al. Mapping patterns of metastatic lymph nodes for postoperative radiotherapy in thoracic esophageal squamous cell carcinoma: a recommendation for clinical target volume definition. BMC Cancer. 2019;19(1):927–927. doi: 10.1186/s12885-019-6065-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakao Y, Suzuki K, Takeo S, Hayashi A, Tsuchida M, Hirono T, et al. Oncological issues in staging mediastinal lymph node metastasis for left lung cancer. Asian J Surg. 2022;45(1):143–147. doi: 10.1016/j.asjsur.2021.04.003. [DOI] [PubMed] [Google Scholar]
- 4.Iskender I, Kadioglu SZ, Cosgun T, Kapicibasi HO, Sagiroglu G, Kosar A, et al. False-positivity of mediastinal lymph nodes has negative effect on survival in potentially resectable non-small cell lung cancer. Eur J Cardiothorac Surg. 2012;41(4):874–879. doi: 10.1093/ejcts/ezr054. [DOI] [PubMed] [Google Scholar]
- 5.Camidge DR, Mandair D, Morgan R, Amini A, Rusthoven CG. Quantifying the medical impact of a missed diagnosis of non-small cell lung cancer on chest imaging. Clin Lung Cancer. 2022;23(5):377–385. doi: 10.1016/j.cllc.2022.03.006. [DOI] [PubMed] [Google Scholar]
- 6.Huang S, Meng H, Cen R, Ni Z, Li X, Suwal S, et al. Use quantitative parameters in spectral computed tomography for the differential diagnosis of metastatic mediastinal lymph nodes in lung cancer patients. J Thorac Dis. 2021;13(8):4703–4713. doi: 10.21037/jtd-21-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Almeida FA, Uzbeck M, Ost D. Initial evaluation of the nonsmall cell lung cancer patient: diagnosis and staging. Curr Opin Pulm Med. 2010;16(4):307–314. doi: 10.1097/MCP.0b013e32833ab0b6. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen P, Bhatt M, Bashirzadeh F, Hundloe J, Ware R, Fielding D, et al. Comparison of objective criteria and expert visual interpretation to classify benign and malignant hilar and mediastinal nodes on 18-F FDG PET/CT. Respirology. 2015;20(1):129–137. doi: 10.1111/resp.12409. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current diagnosis and management of small-cell lung cancer. Mayo Clin Proc. 2019;94(8):1599–1622. doi: 10.1016/j.mayocp.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 10.Wang H, Zhou Z, Li Y, Chen Z, Lu P, Wang W, et al. Comparison of machine learning methods for classifying mediastinal lymph node metastasis of non-small cell lung cancer from (18)F-FDG PET/CT images. EJNMMI Res. 2017;7(1):11–11. doi: 10.1186/s13550-017-0260-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayub II, Mohan A, Madan K, Hadda V, Jain D, Khilnani GC, et al. Identification of specific EBUS sonographic characteristics for predicting benign mediastinal lymph nodes. Clin Respir J. 2018;12(2):681–690. doi: 10.1111/crj.12579. [DOI] [PubMed] [Google Scholar]
- 12.Liu J, Hoffman J, Zhao J, Yao J, Lu L, Kim L, et al. Mediastinal lymph node detection and station mapping on chest CT using spatial priors and random forest. Med Phys. 2016;43(7):4362–4362. doi: 10.1118/1.4954009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Udoji TN, Phillips GS, Berkowitz EA, Berkowitz D, Ross C, Bechara RI. Mediastinal and hilar lymph node measurements. Comparison of multidetector-row computed tomography and endobronchial ultrasound. Ann Am Thorac Soc. 2015;12(6):914–920. doi: 10.1513/AnnalsATS.201312-430OC. [DOI] [PubMed] [Google Scholar]
- 14.Ohno Y, Nishio M, Koyama H, Miura S, Yoshikawa T, Matsumoto S, et al. Dynamic contrast-enhanced CT and MRI for pulmonary nodule assessment. AJR Am J Roentgenol. 2014;202(3):515–529. doi: 10.2214/AJR.13.11888. [DOI] [PubMed] [Google Scholar]
- 15.Svahn TM, Sjöberg T, Ast JC. Dose estimation of ultra-low-dose chest CT to different sized adult patients. Eur Radiol. 2019;29(8):4315–4323. doi: 10.1007/s00330-018-5849-5. [DOI] [PubMed] [Google Scholar]
- 16.Byrne SC, Hammer MM. Use of diagnostic ct and patient retention in a lung cancer screening program. J Am Coll Radiol. 2022;19(1 Pt A):47–52. doi: 10.1016/j.jacr.2021.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Shuman WP, Green DE, Busey JM, Mitsumori LM, Choi E, Koprowicz KM, et al. Dual-energy liver CT: effect of monochromatic imaging on lesion detection, conspicuity, and contrast-to-noise ratio of hypervascular lesions on late arterial phase. AJR Am J Roentgenol. 2014;203(3):601–606. doi: 10.2214/AJR.13.11337. [DOI] [PubMed] [Google Scholar]
- 18.Choi JW, Cho YJ, Ha JY, Lee SB, Lee S, Choi YH, et al. Generating synthetic contrast enhancement from non-contrast chest computed tomography using a generative adversarial network. Sci Rep. 2021;11(1):20403–20403. doi: 10.1038/s41598-021-00058-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leyn P, Dooms C, Kuzdzal J, Lardinois D, Passlick B, Rami-Porta R, et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg. 2014;45(5):787–798. doi: 10.1093/ejcts/ezu028. [DOI] [PubMed] [Google Scholar]
- 20.Silvestri GA, Gonzalez AV, Jantz MA, Margolis ML, Gould MK, Tanoue LT, et al. Methods for staging non-small cell lung cancer: diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143(5 Suppl):e211S–e250S. doi: 10.1378/chest.12-2355. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Welch K, Wang L, Kong FM. Negative predictive value of positron emission tomography and computed tomography for stage T1-2N0 non-small-cell lung cancer: a meta-analysis. Clin Lung Cancer. 2012;13(2):81–89. doi: 10.1016/j.cllc.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seol HY, Kim YS, Kim SJ. Predictive value of 18F-fluorodeoxyglucose positron emission tomography or positron emission tomography/computed tomography for assessment of occult lymph node metastasis in non-small cell lung cancer. Oncology. 2021;99(2):96–104. doi: 10.1159/000509988. [DOI] [PubMed] [Google Scholar]
- 23.Yasufuku K, Pierre A, Darling G, Perrot M, Waddell T, Johnston M, et al. A prospective controlled trial of endobronchial ultrasound-guided transbronchial needle aspiration compared with mediastinoscopy for mediastinal lymph node staging of lung cancer. J Thorac Cardiovasc Surg. 2011;142(6):1393.e1–1400.e1. doi: 10.1016/j.jtcvs.2011.08.037. [DOI] [PubMed] [Google Scholar]
- 24.Lin WY, Hsu WH, Lin KH, Wang SJ. Role of preoperative PET-CT in assessing mediastinal and hilar lymph node status in early stage lung cancer. J Chin Med Assoc. 2012;75(5):203–208. doi: 10.1016/j.jcma.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 25.Pereiro-Brea T, Alegría AM, Valdés L, Golpe-Gómez A, Carreira-Villamor JM, Ruano-Raviña A. Magnetic resonance imaging for the study of mediastinal adenopathies in lung cancer: comparison with standard tests. J Cancer Res Ther. 2021;17(4):917–924. doi: 10.4103/jcrt.JCRT_1626_20. [DOI] [PubMed] [Google Scholar]
- 26.Kato T, Uehara K, Ishigaki S, Nihashi T, Arimoto A, Nakamura H, et al. Clinical significance of dual-energy CT-derived iodine quantification in the diagnosis of metastatic LN in colorectal cancer. Eur J Surg Oncol. 2015;41(11):1464–1470. doi: 10.1016/j.ejso.2015.08.154. [DOI] [PubMed] [Google Scholar]
- 27.Rizzo S, Radice D, Femia M, Marco P, Origgi D, Preda L, et al. Metastatic and non-metastatic lymph nodes: quantification and different distribution of iodine uptake assessed by dual-energy CT. Eur Radiol. 2018;28(2):760–769. doi: 10.1007/s00330-017-5015-5. [DOI] [PubMed] [Google Scholar]
- 28.Li X, Meng X, Ye Z. Iodine quantification to characterize primary lesions, metastatic and non-metastatic lymph nodes in lung cancers by dual energy computed tomography: an initial experience. Eur J Radiol. 2016;85(6):1219–1223. doi: 10.1016/j.ejrad.2016.03.030. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Yan F, Pan Z, Lin X, Luo X, Shi C, et al. Evaluation of dual energy spectral CT in differentiating metastatic from non-metastatic lymph nodes in rectal cancer: Initial experience. Eur J Radiol. 2015;84(2):228–234. doi: 10.1016/j.ejrad.2014.11.016. [DOI] [PubMed] [Google Scholar]