Abstract
Phase 1 and 2 clinical trials of group B streptococcal (GBS) capsular polysaccharide (CPS)-protein conjugate vaccines in healthy adults have demonstrated their safety and improved immunogenicity compared with uncoupled CPSs. Two recent trials sought to determine (i) whether adsorption of conjugate vaccine to aluminum hydroxide would improve immunogenicity and (ii) whether the CPS-specific immunoglobulin G (IgG) response could be boosted by administration of a second dose. Adsorption of GBS type III CPS-tetanus toxoid (III-TT) conjugate vaccine to alum did not improve the immune response to a 12.5-μg dose in healthy adult recipients. Four weeks after vaccination, the geometric mean antibody concentrations (GMCs) for the 15 recipients of III-TT with or without alum were 3.3 and 3.6 μg/ml, respectively. In the second trial, 36 healthy adults vaccinated previously with GBS III-TT conjugate were given a second 12.5-μg dose 21 months later. At 4 weeks after the second dose, the GMCs of type III CPS-specific IgG were similar to those measured 4 weeks after the primary vaccination, suggesting a lack of a booster response. However, 8 (22%) of the 36 participants who had undetectable III CPS-specific IgG (<0.05 μg/ml) before the first dose of III-TT conjugate exhibited a booster response to the second dose, with a fourfold-greater GMC of type III CPS-specific IgG than after the initial immunization. These results suggest that prior natural exposure to type III GBS or a related antigen may be responsible for the brisk IgG response to CPS noted in most adults after vaccination. However, a second dose of GBS III-TT conjugate vaccine may be required for adults whose initial CPS-specific IgG concentrations are very low and would also restore the initial peak-specific III CPS-IgG in responders to previous vaccination.
Improved vaccines against group B streptococci (GBS) have been developed by covalent coupling of variably immunogenic capsular polysaccharide (CPS) antigens to immunogenic protein carriers (13). Phase 1 and 2 clinical trials in healthy, nonpregnant adults have shown that capsular types Ia, Ib, II, and III (1, 2, 9) and type V (L. C. Paoletti, C. J. Baker, and D. L. Kasper, Abstr. First Annu. Conf. Vaccine Res., abstr. P16, 1998) GBS conjugate vaccines are well tolerated and have superior immunogenicity to uncoupled, homologous polysaccharides. Serotype-specific antibodies elicited by these conjugate vaccines are primarily of the immunoglobulin G (IgG) class, are active in promoting opsonization for killing of homologous GBS by human peripheral blood neutrophils in vitro, and prevent lethal GBS infection in newborn mice treated before challenge (15).
With these principles established, research has focused on ways of further improving immune responses to GBS conjugate vaccines. Each of the monovalent conjugate vaccines tested to date elicit a rise in CPS-specific IgG concentrations 2 weeks after administration of a single dose without adjuvant; peak concentrations are achieved 4 to 8 weeks postimmunization (1, 2, 9). These results support the hypothesis that maternal vaccination with GBS conjugates administered early in the third trimester would elicit specific antibodies available for placental transport and at concentrations capable of preventing invasive GBS infection in neonates and young infants (2). Alternatively, vaccination of women of childbearing age might benefit future offspring if antibody concentrations remained at protective levels for several years. Both vaccination scenarios involve placental transfer of GBS-specific IgG to provide protection to the young infant and require that maternal GBS-specific IgG concentrations be higher than those potentially suitable for protection in a single nonpregnant adult. Two doses of vaccine may therefore be necessary, particularly if nonpregnant women are the chosen target population and there is a long time lapse between vaccination and pregnancy or if there is a failure or a low response to primary vaccination.
On the basis of the observed improvement in CPS-specific IgG response in mice (8) and nonhuman primates (12) vaccinated with GBS conjugate vaccines adsorbed with alum, we hypothesized a heightened response in healthy adults given a GBS conjugate vaccine adsorbed to alum. If successful, alum would serve to reduce the effective dose of both vaccine components, particularly that of the carrier protein, which could be at prohibitively high concentrations if a multivalent GBS vaccine is formulated based on the most immunogenic dose (∼60 μg) of polysaccharide (1, 2, 9).
Another method of improving immune response could be administration of a second dose. Support for the hypothesis that a second dose would provide a booster response was reported for baboons in which a heightened antibody response was observed after each of three doses of GBS type III polysaccharide-tetanus toxoid conjugate (III-TT) administered with alum, suggesting induction of a memory response upon repeat vaccination (12). However, in a previous study, healthy nonpregnant women of childbearing age who received a primary dose of GBS III-TT vaccine without alum and a dose of uncoupled type III CPS 2 months later failed to show an improved type III CPS-specific IgG response (C. M. Mink, H.-K. Guttormsen, K. R. Lottenbach, J. C. Cannon, L. C. Paoletti, P. McInnes, and D. L. Kasper, Abstr. 35th Intersci. Conf. Antimicrob. Agents Chemother., abstr. G6, 1995).
We present the results of two clinical trials with healthy, nonpregnant adults that sought to address these questions. The first study examined the effect of alum adjuvant on the immune response to GBS III-TT conjugate vaccine. The second study determined the effect of two doses of GBS III-TT conjugate vaccine separated by a nearly 2-year interval, since we hypothesized that a second dose of GBS III-TT conjugate vaccine would serve to restore or improve III CPS-specific IgG concentrations achieved after the primary dose.
MATERIALS AND METHODS
Vaccines.
GBS III-TT vaccine lots 1-1-95 and 3-1-96 were prepared according to good manufacturing practices at The Salk Institute, Swiftwater, Pa., by using CPS purification, conjugation, and conjugate vaccine purification methods described in detail elsewhere (9). The degree of sialic acid oxidation of type III CPS used to prepare the vaccines was 32 and 30%, respectively. Purified III-TT vaccines were lyophilized in multidose vials with sucrose excipient. GBS III-TT lot 1-1-95 was composed of 61% (wt/wt) CPS and 39% protein; therefore, a 50-μg CPS dose contained 32 μg of TT. Lot 3-1-96 was composed of 44% (wt/wt) CPS and 56% (wt/wt) protein; therefore, a 12.5-μg CPS dose contained 15.9 μg of TT. Purified TT used as the carrier protein for both lots of vaccine was purchased from the Massachusetts Public Health Laboratory, Jamaica Plains, Mass. Both lots of GBS III-TT passed tests for general safety, microbial sterility, and pyrogenicity as required by the Food and Drug Administration (FDA).
Adsorption studies.
Aluminum hydroxide gel in 0.9% saline (alum) was prepared for the National Institutes of Health at the Massachusetts Biologic Laboratories, University of Massachusetts Medical School, Jamaica Plains, under good manufacturing practices using Alhydrogel 1.3% (aluminum hydroxide gel adjuvant manufactured by Superfos Biosector a/s, Vedbæk, Denmark) as the starting material. The elemental aluminum concentration in the vialed alum was 500 μg/ml, and vials were maintained at 2 to 8°C. Alum passed tests for general safety, microbial sterility, and pyrogenicity as required by the FDA.
Complete adsorption of the III-TT vaccine by the alum preparation was ensured by mixing a series of five tubes containing 0.6 ml of III-TT vaccine lot 1-1-95 reconstituted with 0.45% saline plus 0.01% thimerosal with 0.6 ml of alum to a final CPS concentration of 12.5 μg/0.5 ml. A second set of tubes was prepared to test the adsorption of alum to III-TT at a final CPS concentration of 3.125 μg/0.5 ml. Tubes were incubated at room temperature (25°C) for 30 min and then processed immediately or stored at 2 to 8°C for 1, 2, 4, or 7 days. Control tubes that contained 0.9% saline instead of alum were treated similarly. The solutions were clarified by centrifugation (13,600 × g) for 4 min at room temperature, and supernatant fluids were collected and further clarified by a second centrifugation. The amount of type III CPS and TT remaining in solution (i.e., not absorbed) was measured by inhibition enzyme-linked immunosorbent assays (ELISAs) specific for each component. The type III CPS inhibition ELISA was performed as follows. Supernatant fluids from the adsorbed and nonadsorbed vaccines were serially diluted threefold on a microtiter plate. A standard curve was prepared with GBS type III CPS serially diluted twofold starting at 625 ng/ml. Standard rabbit reference serum raised to III-TT vaccine was diluted 1:100,000, and 60 μl was added to all wells except blank control wells. Supernatant-antibody mixture (100 μl) was transferred to microtiter plates coated with 0.1 μg of GBS type III CPS–poly-l-lysine per well and allowed to react for 1 h at 37°C. The plates were washed three times, and 100 μl of goat anti-rabbit IgG (gamma and light chains)-alkaline phosphatase conjugate, diluted 1:3,000, was added to each well for 1 h at 37°C; the plates were then washed again three times before the addition of 200 μl of substrate solution (Sigma 104 substrate tablets; 1 mg/ml). The plates were incubated at 37°C for 75 min in the dark, and the absorbance values at 405 nm were measured. The concentration of type III CPS that resulted in the inhibition of 50% (IC50) of antibody binding was determined from the linear portion of the standard curve. The dilution of the adsorbed and nonadsorbed samples that resulted in an IC50 was determined. The amount of type III CPS in the supernatant fluid was calculated by multiplying the type III CPS IC50 by the reciprocal of the dilution of test sample that resulted in the IC50. The percent adsorption was calculated as follows: [1 − (amount of adsorbed type III CPS divided by the amount of nonadsorbed type III CPS)] × 100. The same methods were used to measure the amount of TT in solution except that the standard curve was prepared with TT serially diluted twofold starting at a concentration of 25 μg/ml, and TT-specific rabbit serum was used at a 1:10,000 dilution.
Study designs.
Data from two separate phase 1 clinical trials are presented. These studies were approved by the Institutional Review Board for Human Subject Research at Baylor College of Medicine, Houston, Tex., and at Children's Hospital, Boston, Mass. The first trial, performed in Boston and Houston, was designed to determine the safety and effect of alum adsorption on the immunogenicity of III-TT lot 1-1-95. For this trial, 60 healthy nonpregnant adults, ages 18 to 50 years, were randomized into four groups (n = 15 per group) to receive 0.5 ml of GBS III CPS intramuscularly at one of the following CPS doses: 50 μg without alum, 12.5 μg with alum, 12.5 μg without alum, or 3.125 μg with alum. Blood was obtained before and 4 weeks after vaccination. These 60 volunteers included 33 women and 27 men; 39 were white, 9 were Hispanic, 8 were black, 3 were Asian, and 1 was other or unknown.
The second trial performed in Houston was designed to assess the safety of and immune response to a second dose of GBS III-TT vaccine. Thirty-six healthy adults (24 to 49 years of age), vaccinated 21 months previously with 12.5 μg of III-TT lot 3-1-96 either alone or in combination with a GBS type II CPS-TT conjugate vaccine, received a second dose of 12.5 μg of III-TT lot 3-1-96. Adjuvant was not used in this trial. There were 21 women and 15 men; 21 were white, 8 were Asian, 4 were black, and 3 were Hispanic. Blood was obtained before and at 4, 8, and 26 weeks after subjects received the primary dose of GBS III-TT and before (21 months after the first dose) and at 4, 8, and 20 weeks after they received the second dose.
For assessment of reactogenicity and safety in the alum trial, subjects and study personnel, except for the nurse administering the vaccine injections, were blinded to vaccine group assignment. The second dose study of GBS III-TT conjugate vaccine was of open-label design. Study personnel in the clinic observed volunteers for 15 to 30 min after vaccination. Subjects in the first trial were instructed regarding the completion a vaccine diary form, how to record oral temperature, and regarding the occurrence of systemic symptoms or injection site symptoms or signs daily for 8 days. For the second trial, the vaccine diary was completed for 3 days unless symptoms existed, which were monitored until resolution. Any volunteer with a temperature of >100.0°F or with grade 3 symptoms within 48 h of vaccination came to the clinic for examination by a study physician.
Quantification of antibody in serum.
GBS type III CPS-specific IgG in sera from all subjects was measured by an ELISA as described previously (7). TT-specific IgG was quantified by ELISA. Microtiter plates were coated with 2 μg of TT (lot M1A-A305; Massachusetts Biologic Laboratories, Jamaica Plains, Mass.)/ml. The ELISA was standardized by using a reference plasma pool with an assigned TT-specific IgG concentration of 31.2 μg/ml. Fourfold serial dilutions were performed on each serum sample. After overnight incubation at 4°C, plates were washed with phosphate-buffered saline (PBS)–0.05% Tween 20, and mouse anti-human IgG-alkaline phosphatase conjugate (Southern Biotechnology Associates, Birmingham, Ala.) was added followed by p-nitrophenyl phosphate substrate (Sigma Diagnostics, St. Louis, Mo.) to detect TT-specific antibody. The reaction was stopped with 1 M NaOH, and optical densities were measured at 405 nm on a Vmax Microplate Reader (Molecular Devices, Sunnyville, Calif.).
Statistical methods.
Comparison of antibody data was performed using two-tailed paired and unpaired t tests on log-transformed data and Mann-Whitney U tests by using Statview, version 5.0.1 (SAS Institute, Inc., Cary, N.C.).
RESULTS
Adsorption of alum to GBS III-TT vaccine.
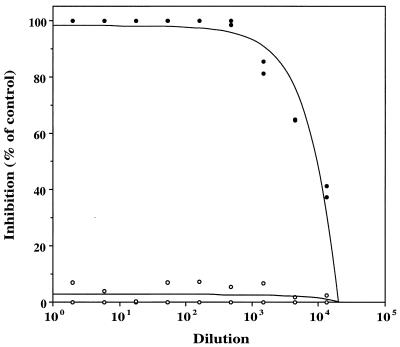
Alum preparation at a concentration of 500 μg of completely (100%) adsorbed III-TT vaccine lot 1-1-95/ml (Fig. 1) at CPS concentrations of 25 and 6.25 μg/ml. Both vaccine components were adsorbed by the alum preparation within 30 min and remained completely adsorbed over 7 days at 2 to 8°C.
FIG. 1.
Adsorption of alum to GBS III-TT vaccine. Inhibition of GBS type III-specific antibody binding to type III CPS-coated microtiter plates by 12.5 μg of III-TT lot 1-1-95 incubated with (●) or without (○) alum. These data were obtained after 1 day of adsorption and are representative of adsorption measured at each time point tested.
Safety and immunogenicity of GBS III-TT adsorbed to alum.
The GBS III-TT conjugate vaccine was well tolerated by healthy adults (Table 1). There were no vaccine-associated systemic symptoms. Most (>86%) recipients, regardless of the dose received or the presence of alum, experienced mild or no pain at the injection site and no redness or swelling at the injection site.
TABLE 1.
Reactogenicity of GBS III-TT conjugate vaccine in healthy adults
| Vaccine and CPS dose (μg) | No. of recipients | % Patients witha:
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pain
|
Redness or swelling
|
Systemic reactions | ||||||||
| 0 | 1 | 2 | 3 | 0 | 1 | 2 | 3 | |||
| GBS III-TT lot 1-1-95 | ||||||||||
| 5.0 | 15 | 26.7 | 73.3 | 0 | 0 | 86.7 | 0 | 6.7 | 6.7 | 0 |
| 12.5 (with alum) | 15 | 26.7 | 60.0 | 6.7 | 6.7 | 86.7 | 13.3 | 0 | 0 | 0 |
| 12.5 | 15 | 53.3 | 33.3 | 6.7 | 6.7 | 100 | 0 | 0 | 0 | 0 |
| 3.125 (with alum) | 15 | 26.7 | 66.7 | 6.7 | 0 | 100 | 0 | 0 | 0 | 0 |
| GBS III-TT lot 3-1-96 | ||||||||||
| 12.5 (first dose) | 36 | 27.8 | 30.5 | 41.7 | 0 | 94.4 | 2.8 | 0 | 2.8 | 2.8 |
| 12.5 (second dose) | 36 | 16.7 | 63.9 | 16.7 | 2.8 | 91.7 | 8.3 | 0 | 0 | 0 |
Pain was scored as follows: 0, no pain; 1, mild, more than baseline but not interfering with usual activities; 2, moderate, more than baseline, causing difficulty with some activities; and 3, severe, more than baseline, interfering with usual activities. Redness or swelling was scored as follows: 0, none to <1 cm; 1, 1 to 3 cm; 2, >3 to 5 cm; and 3, >5 cm.
The immune responses of volunteers to GBS III-TT conjugate vaccine in the presence or absence of alum are summarized in Table 2. The geometric mean concentration (GMC) of GBS type III CPS-specific IgG in preimmunization sera from each of the four groups of volunteers was similarly low and rose significantly 4 weeks following a single dose of GBS III-TT conjugate vaccine (P < 0.001). The GMCs in sera from the 15 recipients of a 12.5-μg dose of III-TT with alum did not differ (P = 0.95) from the GMCs in the sera from recipients of the same vaccine dose without adjuvant. A dose response to the vaccine was noted, with recipients of the 50-μg dose administered without alum achieving the highest postimmunization GBS III CPS-specific IgG concentrations in serum and recipients of the 3.12-μg dose with alum having the lowest.
TABLE 2.
Immune response of healthy adults to GBS III-TT conjugate vaccine with or without aluminum hydroxide
| Vaccine CPS, dose (μg) | Adsorbed alum (μg)a | No. of recipients | GMC (μg/ml) of GBS type III CPS-specific IgG (95% CI; range)b at wk after vaccination:
|
|
|---|---|---|---|---|
| 0 | 4c | |||
| 50 | 0 | 15 | 0.48 (0.15–1.48; 0.025–22.84) | 41.83d(16.53–106; 247–550) |
| 12.5 | 125 | 15 | 0.21 (0.06–0.67; 0.025–7.85) | 3.28e (0.68–15.95; 0.025–448) |
| 12.5 | 0 | 15 | 0.13 (0.05–0.32; 0.025–1.29) | 3.59 (1.05–12.33; 0.13–56.72) |
| 3.125 | 125 | 15 | 0.13 (0.04–0.44; 0.025–11.89) | 1.03 (0.24–4.46; 0.025–84.77) |
Aluminum hydroxide gel.
95% CI, 95% confidence interval.
P < 0.001 when concentrations before and after vaccination are compared.
P < 0.05 when compared with other groups.
P = 0.95 when compared with the same vaccine dose administered without alum.
The immune responses of subjects to the tetanus carrier protein in the GBS III-TT conjugate vaccine are shown in Table 3. The TT-specific IgG in sera from each of the 60 III-TT conjugate vaccine recipients increased substantially 4 weeks after vaccination. Alum did not augment the immune response to this carrier protein; the GMCs of TT-specific IgG were similar in the sera of recipients of the 12.5-μg dose with or without alum (P = 0.089). The highest TT-specific IgG GMCs were elicited in recipients of the highest TT dose and, conversely, the lowest GMCs were found in sera from recipients of the lowest TT dose.
TABLE 3.
Immune response of healthy adults to the protein carrier in GBS III-TT conjugate vaccine in the absence or presence of aluminum hydroxide
| Vaccine CPS dose (μg) | Adsorbed alum (μg)a | No. of recipients | GMC (μg/ml) of TT-specific IgG (95% CI; range)b at wk after vaccination:
|
|
|---|---|---|---|---|
| 0 | 4 | |||
| 50 | 0 | 15 | 84.3c (59.3–119.7; 15.0–277) | 950d (526–1,714; 285–12,462) |
| 12.5 | 125 | 15 | 66.9e(40.4–110.9; 13.3–287) | 181f (113–292; 32.3–1,450) |
| 12.5 | 0 | 15 | 51.2 (33.2–78.9; 5.9–149) | 295 (207.0–419.7; 118–1,277) |
| 3.125 | 125 | 15 | 97.4 (66.1–143.5; 26.2–310) | 151 (112.7–203.7; 72.5–392) |
Aluminum hydroxide.
95% CI, 95% confidence interval.
P > 0.05 compared with the 12.5-μg dose with alum at week 0.
P = 0.001 compared to all other groups at week 4.
P = 0.39 compared with the 12.5-μg dose without alum.
P = 0.089 compared with the 12.5-μg dose without alum; P = 0.49 compared with the 3.125-μg dose with alum.
Safety and immunogenicity of two doses of GBS III-TT vaccine.
GBS III-TT conjugate vaccine was well tolerated by the 36 adults given two doses separated by an interval of 21 months (Table 1). One subject developed an oral temperature of 100.4°F associated with chills, malaise, and headache 18 h after receiving the first dose of GBS III-TT conjugate combined with GBS II-TT. These symptoms completely resolved within 10 h of onset. This patient reported no systemic symptoms upon receiving a second dose of vaccine. None of the other 35 volunteers reported vaccine-associated systemic symptoms with the first or second doses. The majority (58.3%) of recipients of the first dose of GBS III-TT conjugate vaccine experienced no or mild pain, and 94.4% had no redness or swelling at the injection site (Table 1). After the second dose, 80.6% had no or mild pain, and 91.7% had no redness or swelling at the injection site.
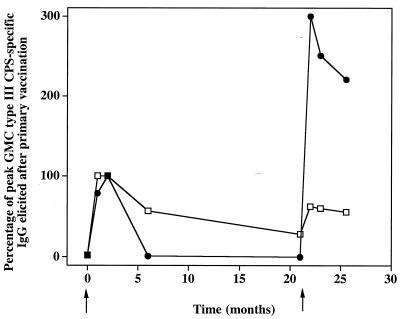
Table 4 summarizes the immune response after the first and second doses of GBS III-TT conjugate vaccine in the 36 adult volunteers. The GMC of type III CPS-specific IgG increased significantly from 0.21 μg/ml before vaccination to a peak of 9.3 μg/ml 2 months after the first dose. Twenty-one months later and before subjects had received the second dose of vaccine, the GMC fell to 2.8 μg/ml. One month after the second dose of III-TT, the type III CPS-specific IgG GMC increased threefold, from 2.8 to 8.4 μg/ml (P < 0.0001). The GMCs of 9.3 μg/ml achieved 2 months after the first vaccine dose and of 7.8 μg/ml achieved after the second vaccine dose, respectively, were similar (P = 0.32). However, an improved immune response after a second dose of vaccine was observed in eight (22%) of the 36 recipients of III-TT conjugate vaccine. For these eight individuals, the type III CPS-specific IgG in their preimmunization sera was below the lower limit of detection (<0.05 μg/ml) (Table 5). In these individuals, the preimmunization GMC rose from below the level of detection (i.e., 0.05 μg/ml) to 1.4 μg/ml 2 months after the first vaccine dose. Twenty-one months later, the GMC was 0.5 μg/ml, and the GMC increased to 4.2 μg/ml 1 month after a second dose of III-TT conjugate vaccine (Table 5). This latter value was 300% higher than the peak GMC achieved after the primary vaccine dose (P = 0.008) (Fig. 2). In contrast, sera from the remaining 28 subjects whose sera had >0.05 μg/ml of type III CPS-specific IgG before the subjects had received an initial vaccine dose had a GMC of 0.37 μg/ml before and 16 μg/ml after their first vaccine dose (Table 5). One month after their second dose of III-TT vaccine, the GMC for this group was 10.3 μg/ml, a concentration lower (P = 0.05) than the GMC at 1 month after administration of the primary vaccine dose (Table 5 and Fig. 2).
TABLE 4.
Immune response of 36 healthy adults given two doses of GBS III-TT conjugate vaccine
| Time after primary infection (mo) |
GMC (μg/ml) of III CPS-specific IgG (95% CI; range)a |
|---|---|
| 0 | 0.21b (0.1–0.4; 0.03–11.0) |
| 1 | 8.8cd (4.3–18.1; 0.04–412) |
| 2 | 9.3d (4.8–18.2; 0.1–545) |
| 6 | 5.4 (3.0–9.8; 0.07–210) |
| 21 | 2.8 (1.5–5.2; 0.1–144) |
| 22 | 8.4e (5.4–13.0; 0.7–181) |
| 23 | 7.8f (4.9–12.2; 0.7–171) |
| 25.5 | 7.1 (4.6–10.8; 0.7–142) |
The first vaccine dose was administered at 0 month and the second was given 21 months later. 95% CI, 95% confidence interval.
P < 0.0001 compared with all other time points.
P < 0.0001 compared with values at 21 months.
P > 0.32 compared with values at 22, 23, and 25.5 months.
P = 0.84 compared with values at 1 month and P < 0.0001 compared with values at 21 months.
P = 0.32 compared with values at 2 months.
TABLE 5.
Immune response of healthy adults to two doses of GBS III-TT conjugate vaccine by preimmunization status
| Initial antibody concn (μg/ml) | GMC (μg/ml) of type III CPS-specific IgG (95% CI; range) at the indicated mo after primary vaccinationa
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 6 | 21 | 22 | 23 | 25.5 | |
| <0.05 (n = 8) | NDb (0.03) | 1.1c (0.3–3.7; 0.04–4.4) | 1.4d (0.5–3.8; 0.1–4.5) | 0.9 (0.3–2.1; 0.07–1.8) | 0.5 (0.2–1.1; 0.1–1.2) | 4.2 (1.6–10.9; 0.7–24.2) | 3.5 (1.3–9.5; 0.7–27.1) | 3.1 (1.3–7.1; 0.7–16.5) |
| >0.05 (n = 28) | 0.37b (0.19–0.74; 0.05–10.9) | 16.0e (7.6–33.5; 0.12–413) | 16.0f (7.9–32.5; 0.25–545) | 9.2 (5.0–16.8; 0.56–209) | 4.6 (2.4–8.8; 0.21–144) | 10.3 (6.2–16.9; 1.1–181) | 9.7 (5.8–16.2; 0.7–172) | 9.0 (5.5–14.5; 0.65–143) |
The first vaccine dose was administered at 0 month, and the second was given 21 months later. 95% CI, 95% confidence interval.
Not detectable (below the level of 0.05-μg/ml detection; 0.025 μg/ml was used for computational purposes). The P value was ≤0.0002 compared with all other time points in the group.
P = 0.12 compared with values at 21 months, and P ≤ 0.02 compared with values at 22, 23, and 25.5 months in this group.
P < 0.03 compared to values at 22, 23, and 25.5 months in this group.
P < 0.0001 compared with values at 21 months, and P ≤ 0.05 compared with values at 22, 23, and 25.5 months in this group.
P < 0.0001 compared with values at 21 months, and P < 0.03 compared with values at 22, 23, and 25.5 months in this group.
FIG. 2.
Immune response in healthy adults to two doses of GBS III-TT conjugate vaccine. The data presented are the percentages of peak type III CPS-specific GMCs elicited after the first dose in 28 of 36 subjects with prevaccination concentrations of type III CPS-specific IgG of >0.05 μg/ml (□) and in 8 subjects with prevaccination concentrations of <0.05 μg/ml (●).
DISCUSSION
The most cost-effective and potentially lasting method of preventing invasive group B streptococcal infections in all age groups is active immunization (11). The decline in the incidence of early-onset GBS disease in neonates that has been associated with the widespread use of maternal intrapartum antibiotic prophylaxis could also be associated with the emergence of antibiotic-resistant organisms (4, 16, 18). Phase 1 and phase 2 trials in healthy adults have tested conjugate vaccines for the five serotypes of GBS that account for an estimated 98% of invasive disease cases in the United States. Further, successful preclinical studies of GBS types VI and VIII conjugate vaccines (serotypes prevalent thus far only in Japan [10]) suggest the ability, if necessary, to extend vaccine coverage (14).
Vaccines against invasive GBS disease must be safe and sufficiently immunogenic to evoke protective, and durable, concentrations of GBS-specific antibodies. As a prelude to examining potential target populations for these vaccines, we examined the effect of alum adjuvant or a second dose of GBS III conjugate vaccine in healthy adults. Whether GBS conjugate vaccines are administered to women before or late in pregnancy to prevent maternal and infant infections, to healthy adults older than 65 years of age, or to those with defined underlying medical conditions (18), information regarding conditions that would optimize immune response to vaccination is needed.
Adsorption of aluminum hydroxide gel to GBS III-TT conjugate vaccine at levels exceeding 80% as recommended by the World Health Organization (6) did not enhance the immune response of healthy adults to either the CPS or the tetanus component of the vaccine. This result was unexpected based on previous experiments demonstrating anamnestic responses in mice and baboons given GBS III-TT conjugate with alum (8, 12). With baboons, the presence of alum was required for immunogenicity, with the III CPS-specific IgG response after each of three doses exceeding that after the previous dose (12). Unlike mice and baboons, adults vaccinated with GBS III-TT conjugate were not immunologically naive to tetanus. Each had received a primary series of TT during infancy, with periodic booster doses, but none in the 12 months prior to study enrollment. The concentrations of TT-specific IgG in serum before vaccination with GBS III-TT conjugate ranged from 5 to 310 μg/ml, confirming immune priming to the carrier protein. Priming mice with carrier protein before administration of a conjugate vaccine can either augment or suppress immune response (17); perhaps the latter effect explains the lack of adjuvant effect of alum in our study population. A recent study with children of an 11-valence pneumococcal conjugate vaccine with both diphtheria and TT as carrier proteins also found that alum had no significant effect on immunogenicity (21). Despite the effectiveness of aluminum-based adjuvants in improving immune response to toxoids (6) and the mechanism described to explain its potent effect in vivo (20), little information exists regarding the effect of alum adjuvant on the immunogenicity of conjugate vaccines.
Although multiple doses of conjugate vaccines have been administered to children, there are few studies of this design have been conducted with adults. In one study of healthy adults who received multiple doses of Pseudomonas aeruginosa type 5 O-polysaccharide–toxin A conjugate vaccine, responses to O-polysaccharide antibody were not boosted, although improved responses to toxin A were stimulated (3). In contrast, significantly higher geometric mean Haemophilus influenzae type b CPS-specific IgG concentrations were noted after a second dose of H. influenzae type b conjugate vaccine in a study with adult patients undergoing bone marrow transplantation (5). These differences in response may be related to the immunocompromised state of the host.
In our study, a second dose of GBS III-TT vaccine restored specific antibody levels to those obtained after the primary vaccination. The ability of a second dose to augment the immune response was apparent only in the minority of healthy adults who had very low concentrations (<0.05 μg/ml) of CPS-specific IgG. In this group, the second dose resulted in specific IgG GMC that was threefold higher than that obtained after a single dose. Therefore, for adults with very low levels of type III CPS-specific IgG, two doses of III-TT vaccine may be required to achieve high levels of specific antibody. We speculate that prior exposure to GBS type III CPS or an immunochemically similar antigen may account for the brisk response after the first dose in the majority of volunteers with >0.05 μg of type III CPS-specific IgG/ml before immunization. For this population, a single dose of III-TT vaccine may be sufficient to induce high levels of type III CPS-specific IgG.
The amount of type III CPS-specific IgG elicited by 15 adults before and 4 weeks after receiving GBS III-TT lot 1-1-95 (12.5-μg CPS dose, no adjuvant) or lot 91-1 (14.5-μg CPS dose, no alum) (9) vaccines were similar (P > 0.6). This indicates the reproducibility in the manufacture of these GBS conjugate vaccines that were prepared 4 years apart in two different laboratories.
Of the 26 candidate vaccines analyzed recently by the Committee to Study Priorities for Vaccine Development for the Institute of Medicine, GBS conjugate vaccines were listed as one of seven in the most favorable (level I) rating or those likely to save money and quality-adjusted life years (19). Of course, successful implementation of a vaccination program relies on proper vaccine formulation and administration. Results from the studies presented here may assist in determining the optimal formulation of a multivalent GBS vaccine and in establishing a vaccination schedule that would ensure a high degree of protective immunity.
ACKNOWLEDGMENTS
Kenneth Johnson, Melissa Hickman, and Claire Skeeter provided invaluable technical assistance on many aspects of this study.
This work was supported by NIH-NIAID contracts AI-25152 and AI-75326.
REFERENCES
- 1.Baker C J, Paoletti L C, Rench M A, Guttormsen H K, Carey V J, Hickman M E, Kasper D L. Use of capsular polysaccharide-tetanus toxoid conjugate vaccine for type II group B Streptococcus in healthy women. J Infect Dis. 2000;182:1129–1138. doi: 10.1086/315839. [DOI] [PubMed] [Google Scholar]
- 2.Baker C J, Paoletti L C, Wessels M R, Guttormsen H-K, Rench M A, Hickman M E, Kasper D L. Safety and immunogenicity of capsular polysaccharide-tetanus toxoid conjugate vaccines for group B streptococcal types Ia and Ib. J Infect Dis. 1999;179:142–150. doi: 10.1086/314574. [DOI] [PubMed] [Google Scholar]
- 3.Cryz S J, Sadoff J C, Furer E. Immunization with a Pseudomonas aeruginosa immunotype 5 O polysaccharide-toxin A conjugate vaccine: effect of a booster dose on antibody levels in humans. Infect Immun. 1988;56:1829–1830. doi: 10.1128/iai.56.7.1829-1830.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fernandez M, Hickman M E, Baker C J. Antimicrobial susceptibilities of group B streptococci isolated between 1992 and 1996 from patients with bacteremia or meningitis. Antimicrob Agents Chemother. 1998;42:1517–1519. doi: 10.1128/aac.42.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guinan E C, Molrine D C, Antin J H, Lee M C, Weinstein H J, Sallan S E, Parsons S K, Wheeler C, Gross W, McGarigle C, et al. Polysaccharide conjugate vaccine responses in bone marrow transplant patients. Transplantation. 1994;57:677–684. doi: 10.1097/00007890-199403150-00009. [DOI] [PubMed] [Google Scholar]
- 6.Gupta R K, Siber G R. Adjuvants for human vaccines—current status, problems and future prospects. Vaccine. 1995;13:1263–1276. doi: 10.1016/0264-410x(95)00011-o. [DOI] [PubMed] [Google Scholar]
- 7.Guttormsen H K, Baker C J, Edwards M S, Paoletti L C, Kasper D L. Quantitative determination of antibodies to type III group B streptococcal polysaccharide. J Infect Dis. 1996;173:142–150. doi: 10.1093/infdis/173.1.142. [DOI] [PubMed] [Google Scholar]
- 8.Guttormsen H K, Wetzler L M, Finberg R W, Kasper D L. Immunologic memory induced by a glycoconjugate vaccine in a murine adoptive lymphocyte transfer model. Infect Immun. 1998;66:2026–2032. doi: 10.1128/iai.66.5.2026-2032.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kasper D L, Paoletti L C, Wessels M R, Guttormsen H K, Carey V J, Jennings H J, Baker C J. Immune response to type III group B streptococcal polysaccharide-tetanus toxoid conjugate vaccine. J Clin Investig. 1996;98:2308–2314. doi: 10.1172/JCI119042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lachenauer C S, Kasper D L, Shimada J, Ichiman Y, Ohtsuka H, Kaku M, Paoletti L C, Ferrieri P, Madoff L C. Serotypes VI and VIII predominate among group B streptococci isolated from pregnant Japanese women. J Infect Dis. 1999;179:1030–1033. doi: 10.1086/314666. [DOI] [PubMed] [Google Scholar]
- 11.Mohle-Boetani J C, Schuchat A, Plikaytis B D, Smith J D, Broome C V. Comparison of prevention strategies for neonatal group B streptococcal infection. A population-based economic analysis. JAMA. 1993;270:1442–1448. [PubMed] [Google Scholar]
- 12.Paoletti L C, Kennedy R C, Chanh T C, Kasper D L. Immunogenicity of group B Streptococcus type III polysaccharide-tetanus toxoid vaccine in baboons. Infect Immun. 1996;64:677–679. doi: 10.1128/iai.64.2.677-679.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paoletti L C, Madoff L C, Kasper D L. Surface structures of group B Streptococcus important in human immunity. In: Fischetti V A, Novick R P, Ferretti J J, Portnoy D A, Rood J I, editors. Gram-postive pathogens. Washington, D.C.: ASM Press; 2000. pp. 137–153. [Google Scholar]
- 14.Paoletti L C, Pinel J, Johnson K D, Reinap B, Ross R A, Kasper D L. Synthesis and preclinical evaluation of glycoconjugate vaccines against group B Streptococcus types VI and VIII. J Infect Dis. 1999;180:892–895. doi: 10.1086/314955. [DOI] [PubMed] [Google Scholar]
- 15.Paoletti L C, Pinel J, Rodewald A K, Kasper D L. Therapeutic potential of human antisera to group B streptococcal glycoconjugate vaccines in neonatal mice. J Infect Dis. 1997;175:1237–1239. doi: 10.1086/593678. [DOI] [PubMed] [Google Scholar]
- 16.Pearlman M D, Pierson C L, Faix R G. Frequent resistance of clinical group B streptococci isolates to clindamycin and erythromycin. Obstet Gynecol. 1998;92:258–261. doi: 10.1016/s0029-7844(98)00155-0. [DOI] [PubMed] [Google Scholar]
- 17.Peeters C C, Tenbergen-Meekes A M, Poolman J T, Beurret M, Zegers B J, Rijkers G T. Effect of carrier priming on immunogenicity of saccharide-protein conjugate vaccines. Infect Immun. 1991;59:3504–3510. doi: 10.1128/iai.59.10.3504-3510.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schrag S J, Zywicki S, Farley M M, Reingold A L, Harrison L H, Lefkowitz L B, Hadler J L, Danila R, Cieslak P R, Schuchat A. Group B streptococcal disease in the era of intrapartum antibiotic prophylaxis. N Engl J Med. 2000;342:15–20. doi: 10.1056/NEJM200001063420103. [DOI] [PubMed] [Google Scholar]
- 19.Stratton K R, Durch J S, Lawrence R S, editors. Vaccines for the 21st century: a tool for decisionmaking. Washington, D.C.: National Academy Press; 2000. [PubMed] [Google Scholar]
- 20.Ulanova M, Tarkowski A, Hahn-Zoric M, Hanson L L. The common vaccine adjuvant aluminum hydroxide upregulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect Immun. 2001;69:1151–1159. doi: 10.1128/IAI.69.2.1151-1159.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wuorimaa T, Dagan R, Eskola J, Janco J, Ahman H, Leroy O, Kayhty H. Tolerability and immunogenicity of an eleven-valent pneumococcal conjugate vaccine in healthy toddlers. Pediatr Infect Dis J. 2001;20:272–277. doi: 10.1097/00006454-200103000-00011. [DOI] [PubMed] [Google Scholar]