Abstract
There has been considerable recent research into protein based Streptococcus pneumoniae vaccines as alternatives to the existing capsular antigen vaccines. PiuA and PiaA (formerly Pit1A and Pit2A) are recently identified lipoprotein components of S. pneumoniae iron uptake ABC transporters which are required for full virulence and are likely to be expressed on the surface of the bacterial cell membrane. We investigated the efficacy of recombinant PiuA and PiaA proteins at eliciting protective immunity in mice against systemic infection with S. pneumoniae. Both recombinant PiuA and PiaA generated antibody responses that cross-reacted with each other but not with pneumolysin and reacted with identical proteins from nine different S. pneumoniae serotypes. Mice immunized with recombinant PiuA and PiaA were protected against systemic challenge to a degree similar to those immunized with an existing protein vaccine candidate, PdB (a genetically modified pneumolysin toxoid). Immunization with a combination of both PiuA and PiaA resulted in additive protection and was highly protective against systemic infection with S. pneumoniae. PiuA and PiaA are therefore promising additional candidates for a novel S. pneumoniae vaccine using protein antigens.
Infection with the gram-positive pathogen Streptococcus pneumoniae is responsible for most cases of community-acquired pneumonia (2, 12) and many cases of meningitis, otitis media, and septicemia. It has been estimated that up to 25% of deaths among children under 5 years old in the developing world are due to S. pneumoniae infections (24). Prevention of this excess mortality and morbidity will require a relatively cheap vaccine which is effective at preventing infection by the majority of clinically relevant S. pneumoniae serotypes in the groups with the highest risk of infection. The existing polyvalent vaccine contains capsular polysaccharide antigen from 23 different serotypes of S. pneumoniae and confers serotype-specific protection in adults. However, since the carbohydrate antigens present in this vaccine are T cell independent they do not elicit adequate protective immune response among infants and the elderly, the age groups at greatest risk of fatal pneumococcal infection (2, 7, 10). To circumvent this problem, a vaccine containing capsular antigens from seven S. pneumoniae serotypes conjugated to proteins has been developed. The conjugated capsular antigen vaccine protects infants from S. pneumoniae infections due to the seven serotypes represented in the vaccine (4, 8), but populations given the conjugated vaccine have had a large increase in the number of infections resulting from replacement carriage by invasive nonvaccine serotypes (8, 15). Furthermore, although the S. pneumoniae serotypes represented in the conjugated vaccine account for most infections in infants in the United States, these serotypes are much less representative of the prevalent clinical serotypes affecting adults in other parts of the world, particularly in developing countries (9).
As a consequence of the difficulties with vaccines based on capsular carbohydrate antigens there has been much interest in developing a S. pneumoniae vaccine based on protein antigens. Protein antigens are more likely to elicit strong T-cell-dependent protective responses in infants and the elderly, and some may be well conserved between all serotypes of S. pneumoniae, thus overcoming the two major drawbacks of the capsular antigen vaccines. To date, the pneumococcal proteins that have shown the greatest potential as vaccine antigens are genetically modified derivatives of the thiol-activated toxin pneumolysin (Ply) (1, 16, 17) and surface proteins of S. pneumoniae, including the choline binding protein PspA (5, 13, 16, 22, 25) and the ABC Mn2+ transporter component PsaA (21). These have generally provided systemic and sometimes mucosal immunity to animals challenged with S. pneumoniae but have yet to become available for clinical use.
We have recently described two ABC transporter systems of S. pneumoniae, Piu and Pia, required for iron uptake and for full virulence in both systemic and pulmonary models of infection (6). By using PCR, we showed that pia and piu were present in all S. pneumoniae serotypes investigated. Both pia and piu contain one gene each encoding likely lipoproteins, PiuA and PiaA, respectively, which have high degrees of similarity to iron receptors known to be expressed on bacterial cell surfaces. In the present study, we assessed the potential of PiuA and PiaA as vaccine candidates by immunizing mice with purified recombinant PiuA and PiaA protein, followed by systemic challenge with S. pneumoniae. Immunization with both PiuA and PiaA elicited antibody responses and resulted in a high degree of protection against systemic challenge with S. pneumoniae. PiuA and PiaA warrant further investigation as candidate antigens for protein-based S. pneumoniae vaccines.
MATERIALS AND METHODS
Bacterial strains, media, DNA isolation, and manipulation.
All of the experiments were performed by using the virulent S. pneumoniae type 2 strain D39 (3). During laboratory manipulations D39 was cultured on Columbia agar supplemented with 5% horse blood or in Todd-Hewitt broth supplemented with 0.5% yeast extract (THY) in an atmosphere of 5% CO2 and 95% air at 37°C. Inocula of D39 for challenge experiments were prepared from cultures grown in serum broth (10% horse serum in meat extract broth) as previously described (16). S. pneumoniae lysates for sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels were prepared by culturing S. pneumoniae cells in 3 ml of THY to an optical density at 580 nm (OD580) of 0.4, pelleting by centrifugation at 20,000 × g for 5 min, followed by lysis by using 100 μl of 0.1% deoxycholate in phosphate-buffered saline (PBS, pH 7.4). Plasmid DNA was isolated from Escherichia coli by using Qiagen plasmid kits (Qiagen), and S. pneumoniae chromosomal DNA was isolated by using Wizard genomic DNA isolation kits (Promega). Standard protocols were used for cloning, transformation, restriction digests, and ligations of plasmid DNA (20). Plasmids were maintained in E. coli DH5α and grown at 37°C on Luria-Bertani (LB) medium with appropriate selection (20).
Cloning, expression, and purification of His6-PiuA and His6-PiaA fusion proteins.
The piuBCDA (pneumococcal iron uptake) and piaABCD (pneumococcal iron acquisition) iron uptake genes were formerly known as pit1BCDA and pit2ABCD, respectively (6). Recombinant PiuA and PiaA proteins (excluding the N-terminal lipoprotein peptidase signal sequence) were expressed and purified by using the QIAexpress (Qiagen) expression system. Fragments of piuA and piaA were amplified by using high-fidelity PCR and S. pneumoniae chromosomal DNA as the template and the following oligonucleotide primer pairs: Sit1.1 (5′-CTACTTGGTGCATGGATCCCAAACTCA-3′) and Sit1.2 (5′-TGAGTCTGCAGTGGCTTATTTCAAAGC-3′) for piuA and Sit2.1 (5′-GTTTTTAGCGCTGGATCCTCTAATTCTG-3′) and Sit2.2 (5′-ATTGTAATAAGAAGAGCTCGGCAGATTATA-3′) for piaA. The following restriction sites (underlined sequences) were incorporated into the primers: BamHI (Sit1.1 and Sit2.1), PstI (Sit1.2), and SacI (Sit2.2). The PCR products were ligated into the polylinker of the QIAexpress vector pQE31 by using these restriction sites to make the plasmids pPC38 (Sit1.1/Sit1.2 product encoding PiuA) and pPC39 (Sit2.1/Sit2.2 product encoding PiaA). The resulting constructs encode fusion proteins corresponding to amino acids 24 to 323 of PiuA (pPC38) and amino acids 22 to 354 of PiaA (pPC39) combined with the His6 tag at the N terminus. In-frame fusions of the piuA and piaA fragments with the N-terminal His6 tag of pQE31 were confirmed by automated dye-terminator sequencing of recombinant DNA from selected pPC38 and pPC39 clones using the QIAexpress Sequencing Primer QE1 (5′-GGCGTATCACGAGGCCCTTTCG-3′). The E. coli K-12 expression strain M15 carrying a kanamycin resistance repressor plasmid pREP4 (required to repress expression of the cloned genes prior to induction) was transformed with plasmids pPC38 or pPC39 and cultured overnight in LB broth containing 100 μg of ampicillin and 25 μg of kanamycin/ml. The resulting cultures were used to inoculate 900 ml of LB broth containing appropriate antibiotics and grown at 37°C for 2 h with vigorous shaking. High-level expression of His6-PiaA and His6-PiuA was achieved by the addition of isopropyl-β-d-thiogalactoside (IPTG) to a final concentration of 2 mM, and the cultures were incubated for a further 4 h. The cells were harvested by centrifugation at 6,000 × g for 10 min and resuspended in lysis buffer (50 mM sodium phosphate, pH 8.0; 2 M NaCl; 40 mM imidazole). The cells were lysed in a French pressure cell (SLM Aminco, Inc.) at 12,000 lb/in2, and the lysates were centrifuged at 100,000 × g for 1 h. Then, 20 mM β-mercaptoethanol was added to the resultant supernatants, which were loaded onto 2-ml nickel-nitrilotriacetic acid resin columns (ProBond; Invitrogen) previously equilibrated with five column volumes of lysis buffer. The columns were washed with 10 column volumes of 10 mM sodium phosphate, 20 mM imidazole, and 1 M NaCl (pH 6.0), and the proteins were eluted with a 30-ml gradient of 0 to 500 mM imidazole in 10 mM sodium phosphate (pH 6.0). Fractions of 3 ml were collected and analyzed by SDS-PAGE to identify fractions containing abundant purified protein. The selected fractions were dialyzed extensively against 10 mM sodium phosphate (pH 7.0) to remove the imidazole. The purified His6-PiaA and His6-PiuA proteins were then resuspended in 50 mM sodium phosphate (pH 7.0), glycerol was added to a final concentration of 50%, and the proteins stored at −15°C.
Immunization experiments.
Purified His6-PiuA and His6-PiaA proteins and PdB were used as antigens for the immunization experiments. PdB is a modified version of pneumolysin that has only 0.1% of the cytotoxic activity of the native toxin due to a Trp-433→Phe substitution, but it retains full immunogenicity. Preparation of PdB has been described previously (1, 17). The concentrations of these proteins were calculated using Bradford reagent (Bio-Rad), and their purity was ascertained by SDS-PAGE after staining with Coomassie brilliant blue R250. Proteins were prepared for immunization as 100-μg ml−1 concentrations in 10% alum adjuvant (Imject Alum No. 77161; Pierce, Chicago, Ill.). Groups of 12 to 15 male BALB/c mice (5 to 6 weeks old) were used for all immunization experiments. For the active protection experiments, each mouse was immunized by intraperitoneal (i.p.) injection of 100 μl of each protein preparation in alum adjuvant on days 0, 10, and 20. Sera were collected from mice by retro-orbital bleeding on day 27 and pooled for each group. On day 34 mice were challenged i.p. with ca. 106 CFU of S. pneumoniae strain D39 organisms. Mice were observed four times hourly for the first 96 h, twice daily for the next 96 h, and then daily until 21 days after challenge. For passive immunization experiments, mice were given 100 μl of pooled antisera diluted in PBS i.p., followed 30 min later by i.p. challenge with ca. 103 CFU of D39. One group of 15 mice was used for each vaccination group used for the active immunization experiments. For each passive immunization group, mice were monitored by using the schedule described above. Data on survival of mice for the active immunization experiment were analyzed by using Kaplan-Meier graphs and log rank tests. For the passive immunization experiment, the median survivals of groups of mice were compared by using Mann-Whitney U test (one tailed).
ELISA and Western blotting.
Specific antibody titers were measured by enzyme-linked immunosorbent assays (ELISA) by using 96-well polystyrene microtiter dish wells (Nunc) coated with purified antigens as described previously (16). Bound antibodies were detected by using alkaline phosphatase-conjugated goat anti-mouse immunoglobulin G (IgG) (Bio-Rad) and disodium p-nitrophenol phosphate as the substrate. Absorbance at OD405 was measured, and endpoint antibody titers were defined as the reciprocal of the dilution of serum giving 50% of the highest absorbance reading above the background level (16). For Western blot analysis, purified proteins or S. pneumoniae culture lysates were separated by SDS–12% PAGE, electroblotted onto nitrocellulose, and reacted with specific antisera by standard protocols (20).
RESULTS AND DISCUSSION
SDS-PAGE gel analysis of recombinant His6-PiuA and His6-PiaA.
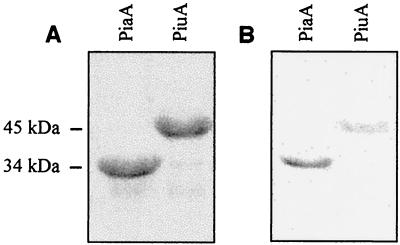
Genes encoding PiuA and PiaA were amplified by PCR and ligated into the expression vector pQE31 for expression and purification. The recombinant His6-PiuA and His6-PiaA proteins were both judged to be >95% pure when assessed by SDS-PAGE and staining with Coomassie brilliant blue (Fig. 1A). Both purified proteins reacted with anti-His6-tag monoclonal antibody (Roche) in a Western blot, confirming that the purified proteins were the products of their respective expression vectors and therefore were recombinant PiuA and PiaA (Fig. 1B). The mass of His6-PiuA determined by SDS-PAGE was 34 kDa, a value close to the expected mass for PiuA excluding the residues removed by cleavage of the N terminus at the lipoprotein signal peptidase motif and after the addition of the six histidine residues of the His6 tag (Fig. 1A). The predicted mass of PiaA after cleavage of the N terminus at the lipoprotein signal peptidase motif and the addition of the His6 tag is ca. 36 kDa. However, the mass of the His6-PiaA fusion protein when measured by SDS-PAGE is ca. 45 kDa.
FIG. 1.
(A) Coomassie blue-stained SDS-PAGE gel of purified His6-PiuA and His6-PiaA proteins showing their relative masses and purity. (B) Western blot of purified His6-PiuA and His6-PiaA proteins probed with monoclonal anti-His6 tag antibody (1 in 3,000 dilution).
Analysis of mouse antisera.
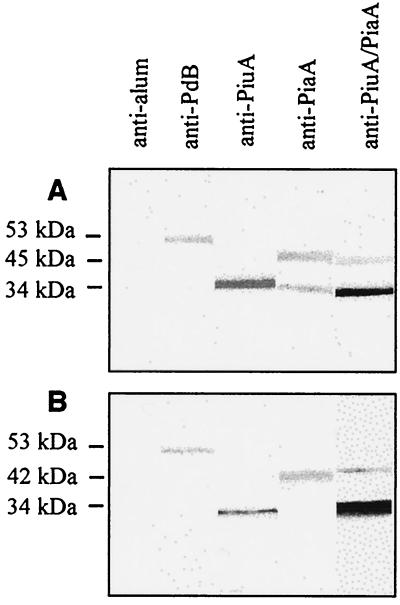
The following antigens were tested in immunization and S. pneumoniae challenge experiments: a noncytotoxic derivative of pneumolysin, PdB (positive control); His6-PiuA; His6-PiaA; His6-PiuA combined with His6-PiaA; or PBS-alum alone (negative control). Sera were collected from four to five mice from each group 7 days after the last immunization, and sera from each group were pooled. The sera were analyzed for specific antibodies to each of the antigens used in the immunization experiments by Western blotting against purified proteins (Fig. 2A) and by ELISA (Table 1). Specific antibody responses to recombinant His6-PiuA, His6-PiaA, and PdB were demonstrated for sera from the corresponding immunization groups (Fig. 2A; Table 1). No positive bands were seen with antisera from mice immunized with the alum adjuvant only. The anti-PiaA titer was low compared to the anti-PdB and anti-PiuA titers, suggesting that at least in BALB/c mice His6-PiaA is a relatively weak immunogen (Table 1). To confirm that the antisera raised against the recombinant His6-PiuA and His6-PiaA fusion proteins were directed against S. pneumoniae proteins, a whole-cell lysate preparation of D39 was reacted with each of the antisera in a Western blot. Both anti-PiuA and anti-PiaA sera reacted with bands of ca. 34 and 42 kDa from the S. pneumoniae lysates, corresponding to PiuA and PiaA, respectively (Fig. 2B). Anti-PiuA and anti-PiaA did not cross-react with any other pneumococcal proteins in the crude D39 isolates. However, despite the low level of identity between PiuA and PiaA at the amino acid level (22%) (6), anti-PiuA did cross-react with recombinant His6-PiaA when measured by ELISA (Table 1) and anti-PiaA serum cross-reacted with recombinant His6-PiuA by Western blotting (Fig. 2A).
FIG. 2.
Western blots of purified His6-PiuA, His6-PiaA, and PdB proteins (A) and S. pneumoniae whole-cell lysates (B) probed with anti-alum, anti-PiuA, anti-PiaA, antiPiuA-PiaA, or anti-PdB sera (1 in 3,000 dilution). The relative molecular mass is indicated on the left.
TABLE 1.
Antibody titers as measured by ELISA obtained from mice immunized with purified His6-PiuA, His6-PiaA, or PdBa
| Immunization group | Antibody titer against:
|
||
|---|---|---|---|
| His6-PiuA | His6-PiaA | PdB | |
| Alum-PBS | ND | ND | ND |
| His6-PiuA | 5,000 | 126 | ND |
| His6-PiaA | ND | 250 | ND |
| His6-PiuA + His6-PiaA | 4,000 | 600 | ND |
| PdB | ND | ND | 6,000 |
ND, not detected (<20).
To assess whether antibodies raised against PiuA or PiaA from one S. pneumoniae strain will also recognize PiuA and PiaA from diverse S. pneumoniae strains, whole-cell lysates from eight other S. pneumoniae strains (representing capsular serotypes 1, 5, 6B, 7F, 8, 12, 17, and 20) were probed with anti-PiuA and PiaA. Bands of identical sizes for both anti-PiuA and anti-PiaA were seen in all strains (data not shown), indicating that there was little functionally significant antigenic variation in PiuA and PiaA between different S. pneumoniae strains. This result reinforces the genetic data showing that piuA and piaA are present in all strains of S. pneumoniae that have been investigated (6). Hence, a vaccine based on PiuA and PiaA is likely to provide protection against most of the clinically relevant strains and therefore avoid the difficulties with the existing polysaccharide-based vaccines, which provide only serotype-specific protection.
Systemic challenge of actively and passively immunized mice with S. pneumoniae
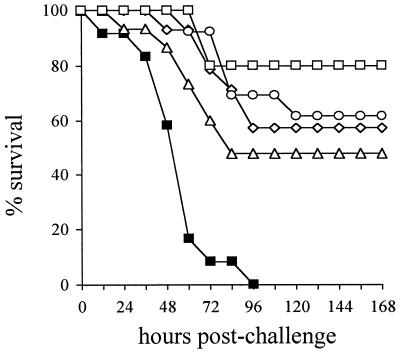
In order to assess the efficacy of active immunization with His6-PiuA and His6-PiaA in protecting against systemic infection of mice with S. pneumoniae strain D39, two separate i.p. challenge experiments were carried out. Similar results were obtained in both experiments, and the data from one of the experiments are presented here. Mice were immunized by i.p. inoculation of three doses of 10 μg of antigen at 10-day intervals. Immunized mice were challenged i.p. with ca. 106 CFU of D39; this dose is ca. 104-fold higher than the 50% lethal dose for D39 in naive BALB/c mice. As expected, there was 100% mortality within 96 h for mice immunized with the alum adjuvant only (Fig. 3). After 14 days the mortalities for the groups of mice immunized with PdB, His6-PiuA, and His6-PiaA were 43, 52, and 39%, respectively, showing that immunization with these proteins protects against systemic infection with S. pneumoniae (P < 0.01 for all three proteins compared to the adjuvant only group by using the log rank test) (Fig. 3). The 13% difference in mortality between groups immunized with His6-PiuA and His6-PiaA was not statistically significant (P = 0.26). Mice immunized with a combination of His6-PiuA and His6-PiaA were highly protected against systemic infection with S. pneumoniae with only a 20% mortality, demonstrating statistically significant additive protection compared to immunization with His6-PiuA alone (His6-PiuA combined with His6-PiaA versus the PBS-alum group [P < 0.0001], versus PiuA [P = 0.03], and versus PiaA [P = 0.27]) (Fig. 3).
FIG. 3.
Survival curves for groups of 12 to 15 mice vaccinated with His6-PiuA (triangles), His6-PiaA (circles), PdB (diamonds), His6-PiuA combined with His6-PiaA (open squares) or alum-PBS alone (filled squares) after IP challenge with 106 CFU of S. pneumoniae. Symbols: ▪, alum; ▵, PiuA; ⋄, PdB; ○, PiaA; □, PiuA + PiaA.
To confirm that the protective effect of immunization with PiuA or PiaA was antibody mediated, groups of 15 mice were passively immunized by i.p. injection of 100 μl of antisera diluted in PBS (1 in 7 dilution for anti-PiuA, anti-PiaA, and anti-PdB and a 1 in 5 dilution for anti-PiuA-PiaA and for anti-PBS-alum only) followed 30 min later by i.p. challenge with 103 CFU of S. pneumoniae D39. Passive immunization with anti-PiuA-PiaA significantly delayed mortality compared to alum antisera (median time to death, 72 h versus 40 h [P < 0.01]), showing that the protective effect of immunization with PiuA combined with PiaA was, at least in part, antibody mediated. Anti-PiuA and anti-PdB sera slightly delayed mortality compared to alum antisera (44 h versus 40 h), although this was only statistically significant for anti-PdB (P < 0.03). The median time of death for mice given anti-PiaA serum and alum antisera was 40 h. The minimal protection provided by anti-PdB serum compared to previously reported results and the lack of protection provided by anti-PiuA serum or anti-PiaA serum alone can be explained by the small volume of diluted antisera used. However, the limited volume of antisera available prevented repetition of these experiments with higher antibody concentrations.
PiuA and PiaA are iron uptake ABC transporter lipoproteins (6) and are probably attached to the extracellular surface of the bacterial membrane, where they are shielded to some extent by the bacterial cell wall and the capsule from interaction with antibodies. Despite this, our results demonstrate that immunization with PiuA and PiaA individually provide a degree of protection against systemic challenge with S. pneumoniae similar to that provided by immunization with the well-recognized vaccine candidate PdB (1, 16). Furthermore, immunization with a combination of PiuA and PiaA resulted in higher degree of protection than that obtained with PiuA alone. These data, along with evidence that immunization with the metal-binding ABC transporter lipoprotein PsaA also protects mice from S. pneumoniae infection (21), demonstrates that the lipoprotein components of ABC transporters can be effective antigens for vaccines. Gram-positive bacteria contain numerous ABC transporters, many of which are required for virulence (11, 14), and these could represent potential vaccine candidates for other gram-positive bacterial pathogens. Gram-negative bacteria also contain many ABC transporters, but the lipoprotein component is usually within the periplasmic space and hence is probably less exposed to antibodies.
Antibodies PdB and another S. pneumoniae vaccine candidate, PspA, are thought to prevent systemic S. pneumoniae infection by inhibiting the in vivo function of pneumolysin or PspA (16). PspA antibodies also opsonize the bacterium, aiding clearance by phagocytes. In contrast to pneumolysin and PspA, Piu and Pia affect virulence by allowing growth of the bacteria in the relatively iron restricted conditions found in vivo (6) rather than by inhibiting the host immune response or by cytotoxicity (18, 19, 23). Due to the likely position of PiuA and PiaA beneath the S. pneumoniae capsule and cell wall, antibodies directed against PiuA and PiaA are probably ineffective opsonins. Presumably, the protective effect of PiuA and PiaA antibodies is due to blockade of Piu and Pia function, but further investigation is required to confirm this possibility. Considering the relatively low level of the PiaA antibody titer, we find it surprising that immunization with this protein protected as well as (if not better than) immunization with His6-PiuA. Disruption of piaA leads to a greater defect in virulence than disruption of piuA (6), and piaA mRNA transcripts are present in greater quantities than piuA mRNA transcripts during culture in THY broth (J. S. Brown and D. W. Holden, unpublished data). Hence, PiaA antibodies may provide better protection than PiuA antibodies because antibody-mediated inhibition of PiaA function will have a stronger effect on in vivo growth than inhibition of PiuA function. A strain containing mutations in both piu and pia is highly attenuated compared to strains with isolated piu and pia disruptions (6), which may explain the additive protective effect of immunization with a combination of PiuA and PiaA.
Recent research on alternatives to the existing S. pneumoniae capsular antigen vaccines has identified a small number of possible protein vaccine candidates, such as PdB and PspA. Although further research is needed to evaluate whether antibodies directed against Piu and Pia proteins also protect against mucosal colonization and pulmonary infection, the data we present here suggest that PiuA and PiaA are additional vaccine candidates that should be considered for inclusion in a protein-based S. pneumoniae vaccine.
ACKNOWLEDGMENTS
This work was supported by a Wellcome Trust Advanced Research Fellow grant awarded to J.S.B.
J.S.B. and A.D.O. contributed equally to this study.
REFERENCES
- 1.Alexander J E, Lock R A, Peeters C C A M, Poolman J T, Andrew P W, Mitchell T J, Hansman D, Paton J C. Immunization of mice with pneumolysin toxoid confers a significant degree of protection against at least nine serotypes of Streptococcus pneumoniae. Infect Immun. 1994;62:5683–5688. doi: 10.1128/iai.62.12.5683-5688.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alonso De Velasco E, Verheul A F M, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Avery O T, MacLeod C M, McCarty M. Studies on the chemical nature of the substance inducing transformation of pneumococcal types. Induction of transformation by a desoxyribonucleic acid fraction isolated from pneumococcus type III. J Exp Med. 1944;79:137–158. doi: 10.1084/jem.79.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Black S, Shinefield H, Fireman B, Lewis E, Ray P, Hansen J R, Elvin L, Ensor K M, Hackell J, Siber G, Malinoski F, Madore D, Chang I, Kohberger R, Watson W, Austrian R, Edwards K. Efficacy, safety, and immunogenicity of heptavalent pneumococcal vaccine in children. Pediatr Infect Dis J. 2000;19:187–195. doi: 10.1097/00006454-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 5.Briles D E, Tart R C, Wu H Y, Ralph B A, Russell M W, McDaniel L S. Systemic and mucosal protective immunity to pneumococcal surface protein A. Ann N Y Acad Sci. 1998;797:118–126. doi: 10.1111/j.1749-6632.1996.tb52954.x. [DOI] [PubMed] [Google Scholar]
- 6.Brown J S, Gilliland S M, Holden D W. A Streptococcus pneumoniae pathogenicity island encoding an ABC transporter involved in iron uptake and virulence. Mol Microbiol. 2001;40:572–585. doi: 10.1046/j.1365-2958.2001.02414.x. [DOI] [PubMed] [Google Scholar]
- 7.Douglas R M, Paton J C, Duncan S J, Hansman D. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;l48:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 8.Eskola J, Kilpi T, Palmu A, Jokinen J, Haapakoski J, Herva E, Takala A, Käyhty H, Karma P, Kohberger R, Siber G, Mäkelä H. Efficacy of a pneumococcal conjugate vaccine against acute otitis media. N Engl J Med. 2001;344:403–409. doi: 10.1056/NEJM200102083440602. [DOI] [PubMed] [Google Scholar]
- 9.Hausdorff W P, Bryant J, Paradiso P R, Siber G R. Which pneumococcal serotypes cause the most invasive disease: implications for conjugate vaccine formulation and use, part 1. Clin Infect Dis. 2000;30:100–121. doi: 10.1086/313608. [DOI] [PubMed] [Google Scholar]
- 10.Koskela M, Leinonen M, Häivä V M, Timonen M, Mäkelä P H. First and second dose antibody responses to pneumococcal polysaccharide vaccine in infants. Pediatr Infect Dis. 1986;5:45–50. doi: 10.1097/00006454-198601000-00009. [DOI] [PubMed] [Google Scholar]
- 11.Lau G, Haataja S, Lonetto M, Kensit S E, Marra A, Bryant A P, McDervitt D, Morrison D A, Holden D W. A functional genomic analysis of type 3 Streptococcus pneumoniae virulence. Mol Microbiol. 2001;40:555–571. doi: 10.1046/j.1365-2958.2001.02335.x. [DOI] [PubMed] [Google Scholar]
- 12.Lim W S, MacFarlane J T, Boswell T C J, Harrison T G, Rose D, Leinonen M, Saikku P. Study of community acquired pneumonia aetiology (SCAPA) in adults admitted to hospital: implications for management guidelines. Thorax. 2001;56:296–301. doi: 10.1136/thorax.56.4.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McDaniel L S, Sheffield J S, Delucchi P, Briles D E. PspA, a surface protein of Streptococcus pneumoniae, is capable of eliciting protection against pneumococci of more than one capsular serotype. Infect Immun. 1991;59:222–228. doi: 10.1128/iai.59.1.222-228.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mei J-M, Nourbakhsh F, Ford C W, Holden D W. Identification of Staphylococcus aureus virulence genes in a murine model of bacteraemia using signature-tagged mutagenesis. Mol Microbiol. 1997;26:399–407. doi: 10.1046/j.1365-2958.1997.5911966.x. [DOI] [PubMed] [Google Scholar]
- 15.Obaro S K. Prospects for pneumococcal vaccination in African children. Acta Trop. 2000;75:141–153. doi: 10.1016/s0001-706x(99)00094-7. [DOI] [PubMed] [Google Scholar]
- 16.Ogunniyi A D, Folland R L, Briles D E, Hollingshead S K, Paton J C. Immunization of mice with combinations of pneumococcal virulence proteins elicits enhanced protection against challenge with Streptococcus pneumoniae. Infect Immun. 2000;68:3028–3033. doi: 10.1128/iai.68.5.3028-3033.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paton J C, Lock R A, Hansman D. Effect of immunization with pneumolysin on survival time of mice challenged with Streptococcus pneumoniae. Infect Immun. 1983;40:548–552. doi: 10.1128/iai.40.2.548-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paton J C. The contribution of pneumolysin to the pathogenicity of Streptococcus pneumoniae. Trends Microbiol. 1996;4:103–106. doi: 10.1016/0966-842X(96)81526-5. [DOI] [PubMed] [Google Scholar]
- 19.Rubins J B, Charboneau D, Paton J C, Mitchell T J, Andrew P W, Janoff E N. Dual function of pneumolysin in the early pathogenesis of murine pneumococcal pneumonia. J Clin Investig. 1994;95:142–150. doi: 10.1172/JCI117631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 21.Talkington D F, Brown B G, Tharpe J A, Koenig A, Russell H. Protection of mice against fatal pneumococcal challenge by immunization with pneumococcal surface adhesin A (PsaA) Microb Pathog. 1996;21:17–22. doi: 10.1006/mpat.1996.0038. [DOI] [PubMed] [Google Scholar]
- 22.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 23.Tu A-H T, Fulgham R L, McCrory M A, Briles D E, Szalai A J. Pneumococcal surface protein A inhibits complement activation by Streptococcus pneumoniae. Infect Immun. 1999;67:4720–4724. doi: 10.1128/iai.67.9.4720-4724.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.World Health Organization. Global programme for vaccines and immunization (vaccine research and development). Report of the Technical Review Group meeting, July 1997–June 1998. Achievements and plan of activities: meningococcal and pneumococcal disease vaccines. Geneva, Switzerland: World Health Organization; 1997. pp. 26–30. [Google Scholar]
- 25.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]