Background:
Current guidelines recommend interpreting concentrations of NPs (natriuretic peptides) irrespective of the time of presentation to the emergency department. We hypothesized that diurnal variations in NP concentration may affect their diagnostic accuracy for acute heart failure.
Methods:
In a secondary analysis of a multicenter diagnostic study enrolling patients presenting with acute dyspnea to the emergency department and using central adjudication of the final diagnosis by 2 independent cardiologists, the diagnostic accuracy for acute heart failure of BNP (B-type NP), NT-proBNP (N-terminal pro-B-type NP), and MR-proANP (midregional pro-atrial NP) was compared among 1577 daytime presenters versus 908 evening/nighttime presenters. In a validation study, the presence of a diurnal rhythm in BNP and NT-proBNP concentrations was examined by hourly measurements in 44 stable individuals.
Results:
Among patients adjudicated to have acute heart failure, BNP, NT-proBNP, and MR-proANP concentrations were comparable among daytime versus evening/nighttime presenters (all P=nonsignificant). Contrastingly, among patients adjudicated to have other causes of dyspnea, evening/nighttime presenters had lower BNP (median, 44 [18–110] versus 74 [27–168] ng/L; P<0.01) and NT-proBNP (median, 212 [72–581] versus 297 [102–902] ng/L; P<0.01) concentrations versus daytime presenters. This resulted in higher diagnostic accuracy as quantified by the area under the curve of BNP and NT-proBNP among evening/nighttime presenters (0.97 [95% CI, 0.95–0.98] and 0.95 [95% CI, 0.93–0.96] versus 0.94 [95% CI, 0.92–0.95] and 0.91 [95% CI, 0.90–0.93]) among daytime presenters (both P<0.01). These differences were not observed for MR-proANP. Diurnal variation of BNP and NT-proBNP with lower evening/nighttime concentration was confirmed in 44 stable individuals (P<0.01).
Conclusions:
BNP and NT-proBNP, but not MR-proANP, exhibit a diurnal rhythm that results in even higher diagnostic accuracy among evening/nighttime presenters versus daytime presenters.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifiers: NCT01831115, NCT02091427, and NCT02210897.
Keywords: circadian rhythm; diagnostic techniques, cardiovascular; dyspnea; heart failure; natriuretic peptides
What is New?
This study describes for the first time that natriuretic peptide concentrations among patients presenting to the emergency department with acute, noncardiac dyspnea follow a diurnal rhythm with peak levels at midday and through levels at nighttime. Diurnal variations of BNP (B-type natriuretic peptide) and NT-proBNP (N-terminal pro-B-type natriuretic peptide) were confirmed in 44 stable individuals.
WHAT ARE THE CLINICAL IMPLICATIONS?
Despite statistically impacting the diagnostic accuracy for acute heart failure depending on presentation time, the diagnostic accuracy of the clinically established cutoff values for BNP and NT-proBNP for acute heart failure remains very high at all times. MR-proANP (midregional pro-atrial natriuretic peptide) concentrations do not exhibit diurnal variations.
Acute heart failure (AHF) is the most common cause of unplanned hospitalization and associated with high morbidity and mortality.1–3 The clinical introduction of NPs (natriuretic peptides) as quantitative markers of hemodynamic stress and heart failure has substantially improved the rapid detection and rule out of AHF among patients presenting with acute dyspnea.4–7 Current European and American clinical practice guidelines endorse the diagnostic use of NPs with a class I recommendation.2,3 They also suggest interpreting NP concentrations and applying NP cutoff levels irrespective of the time of presentation. This strategy has recently been challenged by pilot studies suggesting NP concentrations to display naturally occurring diurnal variations in healthy individuals and in stable patients, with lower NP concentrations in the evening/night versus during the day.8–11 It is currently unknown whether diurnal variations in NPs affect diagnostic accuracy in patients presenting with acute dyspnea.
We, therefore, aimed to quantify diurnal variations and the possible effect on their diagnostic accuracy for AHF in BNP (B-type NP), NT-proBNP (N-terminal pro-B-type NP), and MR-proANP (midregional pro-atrial NP) concentrations among daytime versus evening/nighttime presenters in a large, multicenter diagnostic study using central adjudication. To validate our findings, we examined diurnal variation with hourly blood sampling over 1 full day (25 hours) in 44 stable volunteers with and without chronic kidney disease (CKD).
Methods
BASEL V Emergency Department Patient Population
BASEL V (Basics in Acute Shortness of Breath Evaluation Study; NCT01831115) was a prospective, diagnostic, multicenter study enrolling adult patients presenting to the emergency department (ED) of 2 university hospitals (Basel and Zurich) in Switzerland with nontraumatic acute dyspnea.12,13 While enrollment was independent of renal function, patients with terminal renal failure on chronic renal replacement therapy were excluded. For this secondary analysis, patients were eligible if the time of presentation was recorded and at least 1 NP measurement was performed at presentation (Figure S1). The study was performed according to the principles of the Declaration of Helsinki and approved by the local ethics committees. The authors designed the study, gathered, analyzed, and report the data according to the Standards for Reporting of Diagnostic Accuracy Studies guidelines for studies of diagnostic accuracy. The authors declare that all supporting data are available within the article.
Central Adjudication of AHF (Reference Standard)
Two independent cardiologists centrally adjudicated the final diagnosis mainly responsible for acute dyspnea using all medical information pertaining to the patient including detailed clinical assessment, NPs, cardiac imaging, response to therapy, autopsy data for deceased patients, and 90-day follow-up according to the European Society of Cardiology clinical practice guidelines. In situations of diagnostic disagreement, cases were reviewed and adjudicated in conjunction with a third cardiologist.
Biomarker Sampling
At presentation to the ED, blood samples for determination of NP concentrations were collected into tubes containing potassium EDTA. After centrifugation, samples were either immediately analyzed (the NP in clinical use at the respective site) or frozen at −80 °C for later analysis in batch (the other NPs).
Maastricht Serial Sampling Patient Population
Hourly blood sampling over 1 full day (25 hours) was performed in 44 individuals divided into 2 study groups as described previously.14–17 Briefly, the first study group consisted of 24 individuals without clinically diagnosed CKD (21% women), and the second group (30% women) consisted of 20 patients with clinically diagnosed CKD stage ≥3 (estimated glomerular filtration rate, <60 mL/min per 1.73 m2). The estimated glomerular filtration rate was calculated according to the CKD Epidemiology Collaboration formula.18 Exclusion criteria included current dialysis treatment, myocardial infarction in the 12 months before the study, active cardiac disease (cardiomyopathy, angina pectoris, or myocarditis), and anemia (hemoglobin, <10.5 g/dL).14 Subjects arrived at the laboratory by public transport or car after an overnight fast. During 25 hours, from 8.30 AM till 9.30 AM the next day, subjects were restricted to the laboratory environment, and samples were collected every hour from an antecubital venous catheter. Extension lines for blood sampling were used to prevent disturbance of participants’ sleep during the night. Meals were consumed at 8:30 AM, 12:30 PM, and 6:00 PM (breakfast, lunch, and dinner, respectively). Subjects went to bed at 11:30 PM, and lights were off between 11:35 PM and 7:00 AM. Participants were asked to refrain from exhaustive physical activities and exercise training, 2 days before the test day. Hemoglobin and hematocrit values were used to quantify possible plasma volume changes due to changes in hydration status or posture during the diurnal variation study.19 The serial sampling study was approved by the Institutional Review Board and Ethics Committee of the Maastricht University Medical Center and registered at https://www.clinicaltrials.gov (NCT02091427 and NCT02210897). All participants provided written informed consent. This analysis includes 21 and 19 patients with and without CKD, respectively, after exclusion of 4 individuals due to the absence of both BNP and NT-proBNP measurements.
Measurement of NPs
For both cohorts, BNP was measured by a microparticle enzyme immunoassay (AxSym or Architect; Abbott Laboratories, Abbott Park, IL), which had a limit of detection (LoD) of 10 ng/L and an assay range of 10 to 5000 ng/L (package insert). Forty-four (2% of the study population) patients tested at or below the LoD. NT-proBNP levels were determined by a quantitative electrochemiluminescence immunoassay (Elecsys proBNP; Roche Diagnostics AG, Zug, Switzerland). According to the package insert, this method has an LoD of 5 ng/L and an assay range of 5 to 35000 ng/L. Eighteen patients (0.7% of the study population) tested at or below the LoD. In the BASEL V ED patient population, MR-proANP was measured with an automated sandwich chemiluminescence immunoassay on the KRYPTOR System (BRAHMS AG, Hennigsdorf/Berlin, Germany) as described previously.20 The functional assay sensitivity (interassay coefficient of variance, <20%) is 20 pmol/L, and the LoD is 6.0 pmol/L. No patient tested at the LoD.
Statistical Analysis
Comparisons between groups were made using the independent Student t test and ANOVA, or Mann-Whitney test and Kruskal-Wallis test, Wilcoxon signed-rank test, and Pearson χ2 test, as appropriate. The Jonckheere-Terpstra trend test was used to assess time-dependent trends in NP levels. Receiver operating characteristic curves were constructed to assess sensitivity, specificity, and 95% CIs of NP levels to diagnose AHF. The comparison of the areas under independent receiver operating characteristic curves (AUCs) was performed as recommended by Hanley et al.21 For diurnal variation analyses in the BASEL V ED cohort, patients were separated into quintiles according to their ED presentation time. Patients presenting during the first 3 presentation time quintiles (9:30–15:40) were defined as daytime presenters. Patients presenting during the last 2 presentation time quintiles (15:41–9:29) were defined as evening/nighttime presenters. Additionally, a chronological definition of day and night was used with daytime lasting from 8:00 to 19:59 and nighttime lasting from 20:00 to 7:59.22
Linear regression models were used to assess the relative percentage difference of circulating NP concentrations between daytime and evening/nighttime presenters with a 95% CI (adjusted for age, sex, body mass index [BMI], and systolic blood pressure). This was calculated using the following formula: (eβ)×100 (β coefficient from linear regression).
Diurnal rhythms of NPs in the Maastricht serial sampling cohort were analyzed by fitting the data to a cosine curve by using the method of cosinor rhythmometry, which was extensively described previously.14–16,23,24 Briefly, the cosinor model is described with Z(t)=M+A·cos (ωt+φ)+e(t); where Z(t) represents the measured NP concentration at a given time (t), M represents the mesor (value around which oscillation occurs), A represents the amplitude (half the difference between the peak and the nadir value), ω represents the angular frequency (degrees per unit time with 360° representing a complete cycle), φ represents the acrophase (timing of maximal value in degrees), and e(t) represents the error between the cosinor model and the measurement. Evidence of a diurnal rhythm was indicated by a significant cosinor model fit on normalized analyte values (normalized to the 24-hour mean value). Cosine curves on group level were expressed as deviation (%) from the 24-hour mesor concentration. For both cohorts, all hypothesis testing was 2 tailed, and P<0.05 was considered statistically significant unless otherwise stated. Secondary analyses were performed in both cohorts assessing the impact of body weight on NP rhythm. In line with a previous study, patients with a BMI between 18 and 25 kg/m2 were considered lean, and patients with a BMI above 30 kg/m2 were considered obese.11 Statistical analyses were performed with SPSS for Windows 23.0 (IBM, Armonk, NY), MedCalc 11.2.1.0 (MedCalc Software, Ostend, Belgium), R (version 3.3.1), and the R-packages Cosinor (version 1.1) and Cosinor2 (version 0.1).24
Results
BASEL V ED Patient Population
Baseline Characteristics
Among 2485 eligible patients, the median age was 76 years, and 43% of patients were women (Table 1; Table S1). Median New York Heart Association functional class at presentation was (3 [interquartile range (IQR), 3–4]) independent of presentation time. Overall, baseline characteristics were comparable in patients presenting during daytime versus in the evening/nighttime. However, systolic blood pressure displayed a diurnal variation with peak levels at night (0:00–9:29) and trough levels at midday (11:16–13:15). This was observed in all patients, patients with AHF, and patients with other causes of dyspnea (P<0.01 for all). In No-AHF patients, the difference in mean systolic blood pressure between peak and through levels was 8 mm Hg. Consequently, systolic blood pressure was lower in daytime presenters compared with evening/nighttime presenters (all patients: 136±27 versus 141±26, P<0.01; no-AHF patients: 137±24 versus 142±23, P=0.02). AHF was the adjudicated final diagnosis in a similar proportion of patients presenting during daytime versus in the evening/nighttime (59% versus 58%). Circulating BNP, NT-proBNP, and MR-proANP concentrations were 490 (96–1204), 2015 (IQR, 305–6395), and 273 (125–472) ng/L in all patients; 948 (521–1704) ng/L, 5052 (IQR, 2313–9954) pmol/L, and 410 (278–605) ng/L in patients with an adjudicated diagnosis of AHF; and 64 (IQR, 22–150) ng/L, 236 (75–714) ng/L, and 103 (60–191) pmol/L in patients with other causes of dyspnea. In lean and obese patients BNP, NT-proBNP, and MR-proANP concentrations were 547 (102–2636) versus 338 (86–1323) ng/L (P<0.01), 2218 (313–7245) versus 1379 (283–4101) ng/L (P<0.01), and 286 (127–505) versus 242 (118–395) pmol/L (P<0.01) in all patients; 1056 (583–1928) versus 646 (316–1214) ng/L (P<0.01), 5851 (2826–11633) versus 3164 (1306–5923) ng/L (P<0.01), and 441 (298–640) versus 332 (233–474) pmol/L (P<0.01) in patients with an adjudicated diagnosis of AHF; and 64 (23–147) versus 59 (19–154) ng/L (P=0.59), 250 (80–759) versus 197 (60–625) ng/L (P=0.09), and 103 (63–192) versus 98 (48–176) pmol/L (P=0.17) in patients with other causes of dyspnea.
Table 1.
Baseline Characteristics
Diurnal Variations of NP Levels
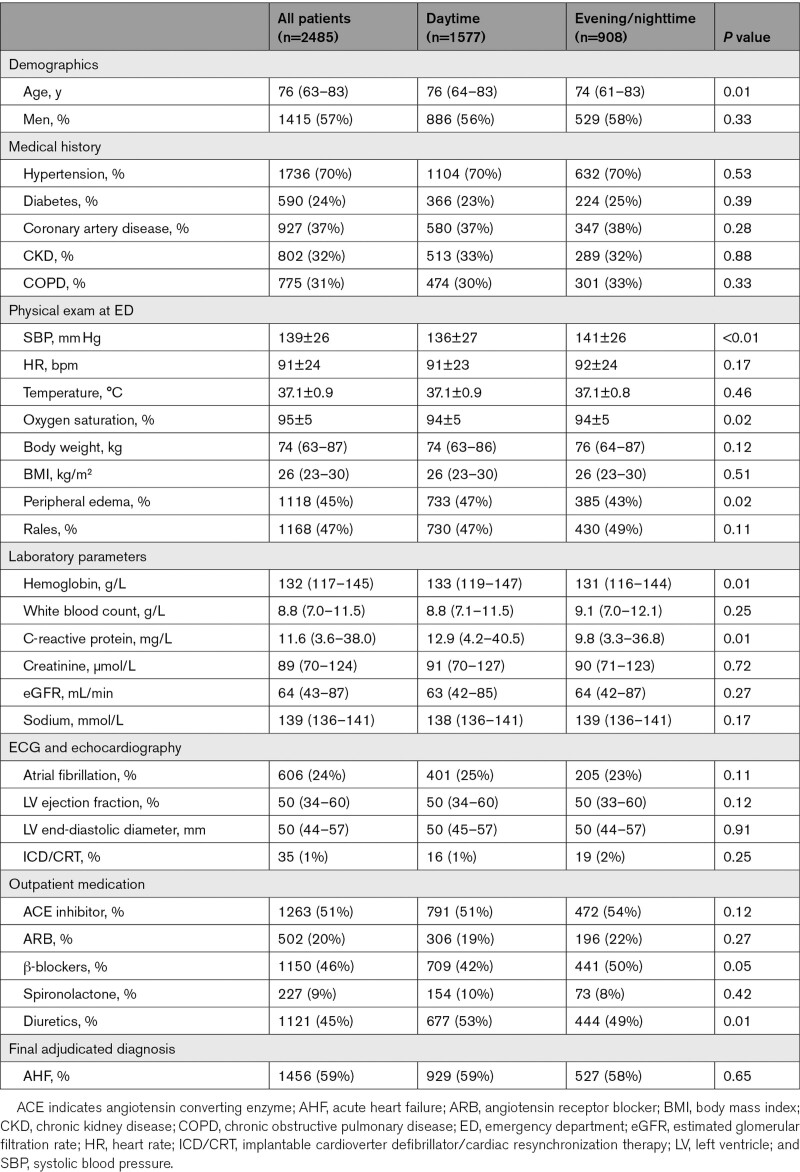
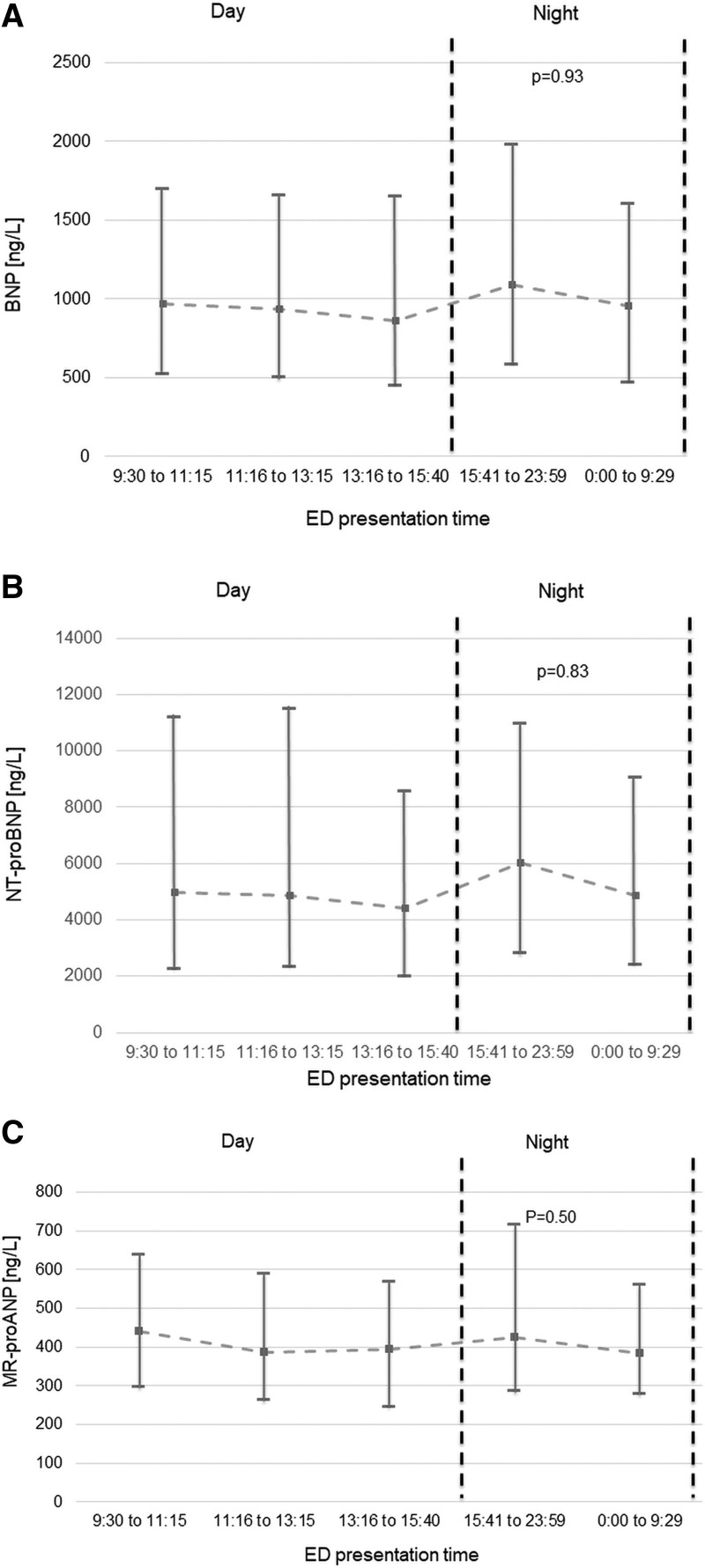
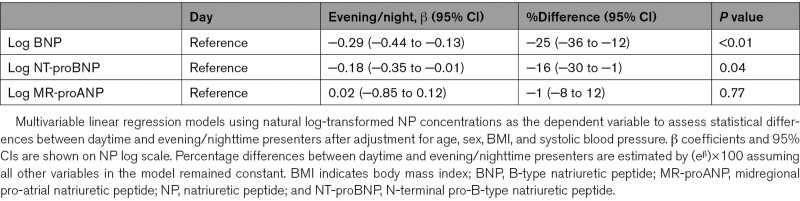
Among patients adjudicated to have AHF, BNP, NT-proBNP, and MR-proANP concentrations were comparable among daytime versus evening/nighttime presenters (all P=nonsignificant; Figure 1). When using the chronological day and night, 316 patients (107 with no-AHF causes of dyspnea) presented during nighttime. BNP, NT-proBNP, and MR-proANP concentrations were similar in AHF patients presenting during chronological day and night. In no-AHF patients, chronic obstructive pulmonary disease/asthma (34%), pneumonia (21%), pulmonary cancers/metastases (10%), and pulmonary embolism (9%) were the most common final adjudicated diagnoses. Among patients adjudicated to have other causes of dyspnea, evening/nighttime presenters had lower BNP (median, 44 [18–110] versus 75 [27–168] ng/L; P<0.01) and NT-proBNP (median, 167 [IQR, 58–533] versus 269 [92–827] ng/L; P<0.01) concentrations versus daytime presenters (Figure 2). Hence, median BNP and NT-proBNP concentrations in evening/nighttime presenters were 59% and 62% of the concentrations observed in daytime presenters. When dividing presentation time according to chronological night and day, results were similar (BNP: 33 [15–85] versus 69 [25–160] ng/L, P<0.01; NT-proBNP: 117 [37–417] versus 252 [83–761] ng/L, P<0.01). Median BNP and NT-proBNP concentrations in chronological nighttime presenters were 48% and 46% of the concentrations observed in daytime presenters. This observation was independent of body mass (BNP lean: median evening/nighttime 50 [18–108] ng/L versus daytime 70 [31–181] ng/L, P<0.01; BNP obese: median evening/nighttime 40 [17–102] ng/L versus daytime 79 [20–226] ng/L, P=0.01; NT-proBNP lean: median evening/nighttime 156 [57–522] ng/L versus daytime 333 [114–933] ng/L, P<0.01; NT-proBNP obese: evening/nighttime 162 [60–553] ng/L versus daytime 244 [60–646] ng/L, P=0.08). Hence, median BNP and NT-proBNP concentrations in evening/nighttime presenters were 71% and 46% of the concentrations in lean and 51% and 66% in obese daytime presenters, respectively. Similarly, BNP (P<0.01 for both) and NT-proBNP (P<0.01 for both) concentrations were lower in evening/nighttime presenters independent of the need for outpatient β-blocker therapy. This difference was not observed for MR-proANP concentrations (P=0.52; Pchronological=0.23); consequently, MR-proANP concentrations of patients presenting during evening/nighttime and during chronological night were 93% and 110% of daytime presenters. Table 2 shows percentage differences of circulating NP levels after adjustment for age, sex, BMI, and systolic blood pressure.
Figure 1.
Whisker plots displaying spot measurements of natriuretic peptide concentrations with acute heart failure according to quintiles of presentation time. A, BNP (B-type natriuretic peptide), (B) NT-proBNP (N-terminal pro-B-type natriuretic peptide), and (C) MR-proANP (midregional pro-atrial natriuretic peptide). P values are derived from Jonckheere-Terpstra trend test. Results are displayed as median concentrations; whisker bars display interquartile ranges. Dashed line highlights median concentrations according to different presentation times. No serial measurements were performed. ED indicates emergency department.
Figure 2.
Whisker plots displaying spot measurements of natriuretic peptide concentrations with noncardiac dyspnea according to quintiles of presentation time. A, BNP (B-type natriuretic peptide), (B) NT-proBNP (N-terminal pro-B-type natriuretic peptide), and (C) MR-proANP (midregional pro-atrial natriuretic peptide). P values are derived from the Jonckheere-Terpstra trend test. Results are displayed as median concentrations; whisker bars display interquartile ranges. Dashed line highlights median concentrations according to different presentation times. No serial measurements were performed. ED indicates emergency department.
Table 2.
Differences in NP Levels Between Daytime and Evening/Nighttime Presenters
Diagnostic Performance of NPs in Daytime and Evening/Nighttime Presenters
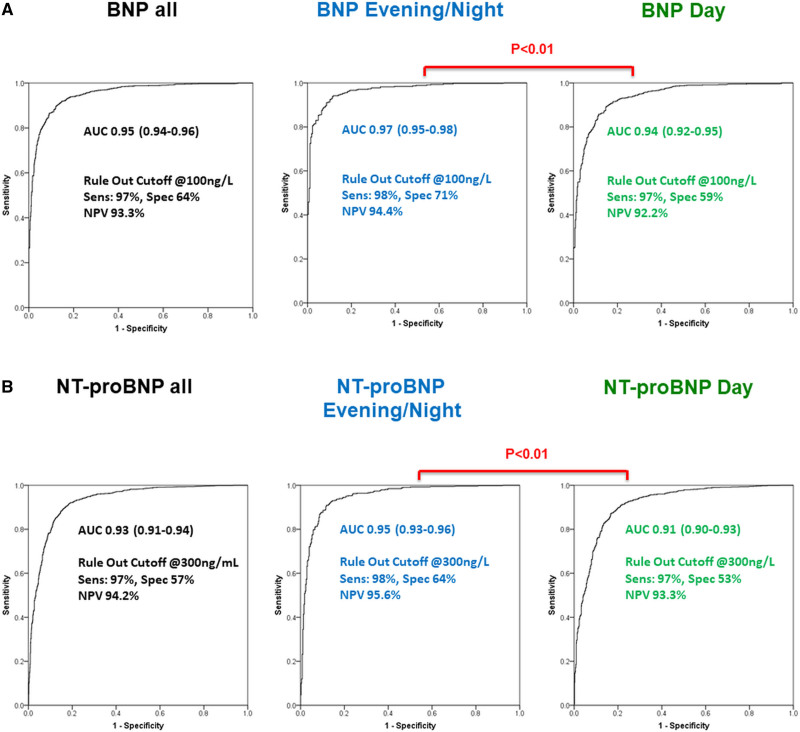
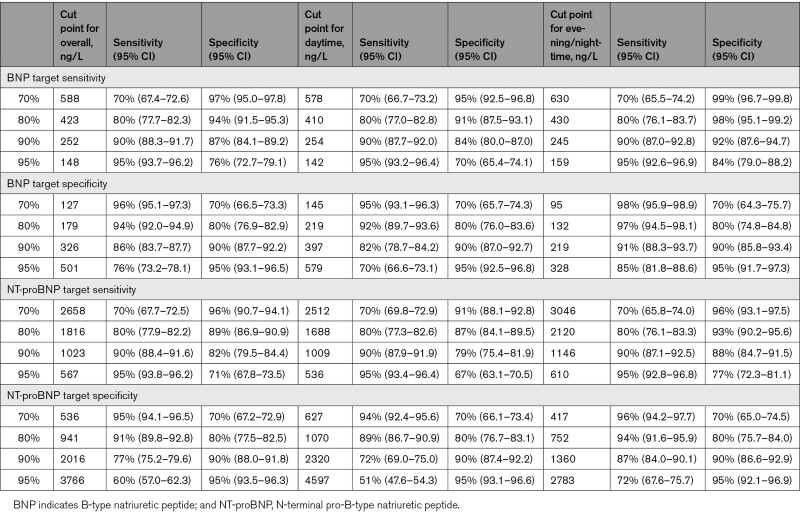
Diagnostic accuracy for AHF as quantified by the AUC was significantly higher for BNP (Figure 3A) and NT-proBNP (Figure 3B) in evening/nighttime presenters compared with daytime presenters (0.97 [95% CI, 0.95–0.98] and 0.95 [95% CI, 0.93–0.96] versus 0.94 [95% CI, 0.92–0.95] and 0.91 [95% CI, 0.90–0.93]). Again, similar results were obtained when separating patients according to chronological presentation time (20:00–7:59: BNP, 0.98 [95% CI, 0.97–0.99]; NT-proBNP, 0.97 [95% CI, 0.96–0.99] versus 8:00–19:59: BNP, 0.94 [95% CI, 0.93–0.95]; NT-proBNP, 0.92 [95% CI, 0.91–0.93]) Secondary analyses including patients with a complete pair of BNP and NT-proBNP samples revealed similar results. Likewise, the diagnostic accuracy for AHF was higher for BNP (lean AUC: evening/nighttime, 0.98 versus daytime, 0.95; obese AUC: evening/nighttime, 0.93 versus daytime, 0.89) and NT-proBNP (lean AUC: evening/nighttime, 0.96 versus daytime, 0.92; obese AUC: evening/nighttime, 0.91 versus daytime, 0.88) in evening/nighttime presenters compared with daytime presenters independent of BMI. The AUC for MR-proANP to detect AHF as the main cause of dyspnea was similar in evening/nighttime and daytime presenters (AUC, 0.92 [95% CI, 0.90–0.94] versus 0.90 [95% CI, 0.89–0.92]). Importantly, the diagnostic accuracy of BNP, NT-proBNP, and MR-proANP for AHF remained very high (>0.9) at all times. Table 3 displays diagnostic test characteristics of BNP and NT-proBNP at prespecified sensitivity and specificity target levels in the overall cohort, as well as evening/nighttime versus daytime presenters. No differences between evening/nighttime versus daytime presenters existed for the sensitivity of the 3 NPs at the guideline-recommended rule out cutoff levels at 100 (98% versus 97%) ng/L, 300 (98% versus 98%) ng/L, and 120 (97% versus 97%) pmol/L, respectively. Similar results were obtained when using chronological night and day (BNP, 98% versus 97%; NT-proBNP, 98% versus 98%; MR-proANP, 97% versus 97%). When assessing guideline-recommended rule in cutoff values, specificities for AHF in nighttime and daytime were as follows: BNP, 97% versus 90%; NT-proBNP<50 years, 93% versus 89%, NT-proBNP50–75 years, 89% versus 81%, NT-proBNP>75 years, 82% versus 80%. Single calculated cutoff values maximizing the sum of sensitivity and specificity were 184 ng/L for BNP (sensitivity, 94%; specificity, 89%) in evening/nighttime presenters and 274 ng/L (sensitivity, 89%; specificity, 85%) in daytime presenters, as well as 1101 ng/L (sensitivity, 91%; specificity, 88%) and 1224 ng/L (sensitivity, 87%; specificity, 83%) for NT-proBNP.
Figure 3.
Receiver operating characteristic (ROC) curves displaying the diagnostic accuracy of natriuretic peptides with acute heart failure in daytime and nighttime presenters. A, BNP (B-type natriuretic peptide) and (B) NT-proBNP (N-terminal pro-B-type natriuretic peptide). P values are derived from comparison of areas under independent ROC curves as recommended by Hanley et al.21 AUC indicates area under the curve; NPV, negative predictive value; Sens, sensitivity; and Spec, specificity.
Table 3.
Diagnostic Test Characteristics of BNP and NT-proBNP at Prespecified Sensitivity and Specificity Targets
External Validation in Maastricht Serial Sampling Cohort
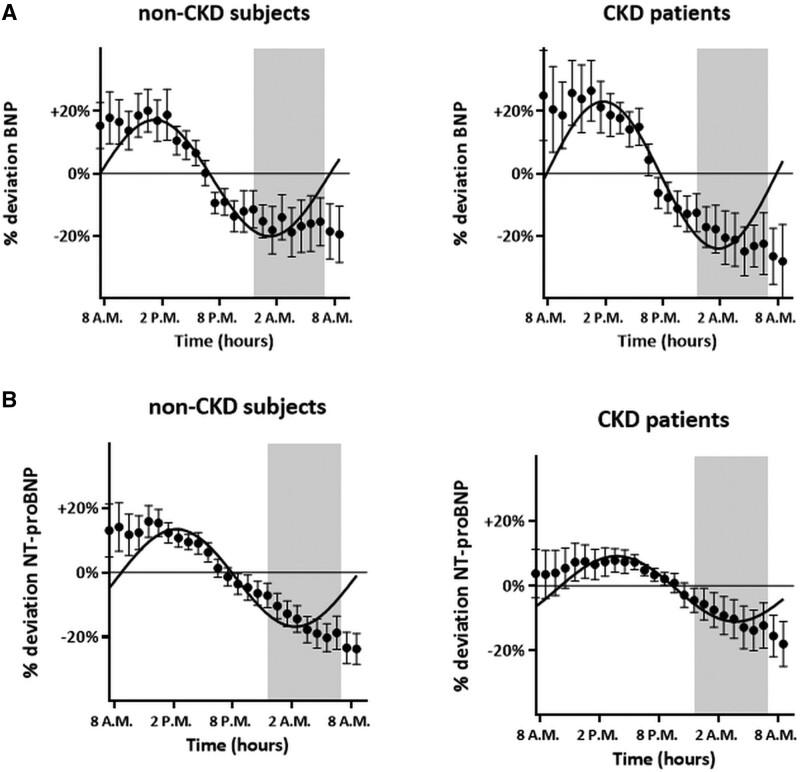
The baseline characteristics of the Maastricht cohort are described in Table S2. In most individuals (35 of 40), we found rhythmic diurnal BNP variation, characterized by the highest concentrations through daytime (mean, 21.3 ng/L at 15.30 PM and 31.4 ng/L at 14.30 PM for non-CKD and CKD subjects, respectively) and the lowest concentrations during nighttime (mean, 16 ng/L at 4:30 AM and 21.4 ng/L at 5:30 AM the next day, respectively; Figure 4A; individual curves in Figures S2 and S3). During the evening/night, individuals had lower BNP (median, 9 [IQR, 6–27] versus 12 [IQR, 6–32] ng/L for non-CKD and median, 13 [IQR, 5–27] versus 18 [IQR, 8–36] ng/L for CKD subjects) and NT-proBNP (median, 10 [IQR, 4–38] versus 12 [IQR, 4–40] ng/L for non-CKD and median, 48 [IQR, 14–92] versus 61 [IQR, 17–101] ng/L for CKD subjects) concentrations versus daytime presenters (Figures S2 through S5). Median BNP and NT-proBNP concentrations during the evening/nighttime were 75%/83% and 72%/79% of the concentrations observed during daytime for non-CKD and CKD subjects, respectively. When dividing presentation time according to chronological day and night, results were similar: during the evening/night, individuals had lower BNP (median, 10 [IQR, 6–27] versus 11 [IQR, 6–30] ng/L for non-CKD and median, 12 [IQR, 5–27] versus 17 [IQR, 8–36] ng/L for CKD subjects) and NT-proBNP (median, 10 [IQR, 4–37] versus 12 [IQR, 5–38] ng/L for non-CKD and median, 47 [IQR, 15–92] versus 61 [IQR, 16–100] ng/L for CKD subjects) concentrations versus daytime presenters. Median BNP and NT-proBNP concentrations during chronological night were 91%/71% and 83%/77% of the concentrations observed during day for non-CKD and CKD subjects, respectively.
Figure 4.
Diurnal variation of natriuretic peptides in nondyspneic participants according to renal function. A, BNP (B-type natriuretic peptide) and (B) NT-proBNP (N-terminal pro-B-type natriuretic peptide). CKD indicates chronic kidney disease.
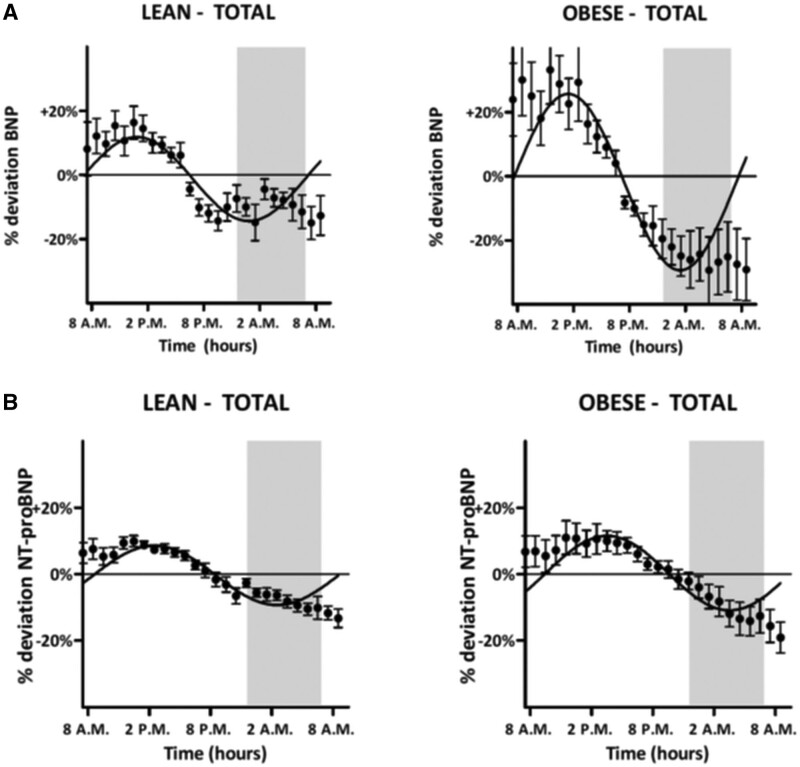
These observed patterns correlated significantly to the fitted cosine model (range R2, 0.18–0.83; all P<0.05; Tables S3 and S4). Additionally, we found similar rhythmic diurnal NT-proBNP variation in most individuals (38 of 40), again characterized by the highest concentrations throughout daytime (mean, 292.8 ng/L at 13:30 PM and 649.4 ng/L at 13:30 PM for non-CKD and CKD subjects, respectively) and lower concentrations during morning time (mean, 227.2 ng/L at 9:30 AM and 504.0 ng/L at 9:30 AM, respectively; Figure 4B; individual curves in Figures S4 and S5). These observed patterns correlated significantly to the fitted cosine model (range R2, 0.27–0.99; all P≤0.05; Tables S5 and S6). No significant differences for BNP versus NT-proBNP were found between subjects with or without CKD and between lean and obese patients (Figure 5). There was substantial overlap in phase shifts between lean and obese patients (BNP, 1.75 [95% CI, 1.55–1.95] versus 1.59 [95% CI, 1.41–1.77]; NT-proBNP, 1.30 [95% CI, 1.16–1.44] versus 1.04 [95% CI, 0.84–1.24]) corresponding to a mean phase difference of 6 minutes for BNP and 16 minutes for NT-proBNP between lean and obese patients. For all of the diurnal rhythms, correction for possible posture-induced changes in plasma volume did not abrogate the diurnal rhythm (data not shown).
Figure 5.
Diurnal variation of natriuretic peptides in nondyspneic participants according to body mass classes. A, BNP (B-type natriuretic peptide) and (B) NT-proBNP (N-terminal pro-B-type natriuretic peptide).
Discussion
In a large, multicenter diagnostic study using central adjudication, we quantified diurnal variations in NP concentrations and their possible effect on the diagnostic accuracy for AHF among evening/nighttime versus daytime presenters. We report 8 major findings.
First, BNP and NT-proBNP, the 2 most widely used NPs, exhibit a diurnal rhythm in patients adjudicated to have noncardiac causes of acute dyspnea, which is characterized by peak levels around midday and trough levels at night. This confirms previous pilot studies in healthy individuals.9,11 Second, the extent of the diurnal variation was moderate with peak/trough differences of 34 and 114 ng/L for BNP and NT-proBNP, respectively. Similarly, the percentage difference of BNP and NT-proBNP concentrations attributable to presentation time was about 20% after adjustment for comorbidities. Third, no clinically relevant impact of body mass on the diurnal rhythm of NP concentrations was observed. Fourth, in patients with AHF, no diurnal rhythm was observed, possibly because AHF-induced hemodynamic stress overshadowed the moderate naturally occurring daytime/nighttime variations. Fifth, the diurnal rhythm of circulating BNP and NT-proBNP concentrations in patients with noncardiac dyspnea significantly affected the diagnostic accuracy of these NPs for AHF, with even higher AUCs for BNP and NT-proBNP in evening/nightime presenters (due to lower concentrations in non-AHF patients) versus daytime presenters. Importantly, the diagnostic accuracy of BNP and NT-proBNP for AHF remained high (AUC, >0.9) at all times, and the accurate rule out of AHF at the clinically used cutoff levels of 100 and 300 ng/L was not affected by presentation time (sensitivity, ≥97%). Similarly, the specificities at recommended rule in cutoff values remained high at all time points and were comparable to previously published values.7,25–27 Hence, the impact of diurnal NP variations on their diagnostic accuracy for AHF was low. We, therefore, believe that the small differences in sensitivity and specificity in daytime versus evening/nighttime presenters do not justify the added complexity of adjusting established cutoff values. Sixth, external validation using hourly measurements of BNP and NT-proBNP in volunteers confirmed a diurnal rhythm for BNP and NT-proBNP with substantially lower concentrations during the night. Reduction in stable body posture–induced plasma volume changes did not underlie these diurnal variation of circulating BNP and NT-proBNP concentrations, suggesting diurnal variations in cardiac filling pressure independent NP release mechanisms.28 Moreover, no significant differences were found between subjects with or without CKD. Seventh, diurnal blood pressure variations appeared to be in antiphase of BNP and NT-proBNP variations with higher blood pressure values observed in patients presenting during evening/nighttime. While this observation is in agreement with some older studies,10,29 it seems contradict a recent study establishing synchronicity between circulating NP concentrations and blood pressure levels in healthy volunteers undergoing serial blood pressure and NP measurements following a normal sleep/wake rhythm.11 The absence of a normal sleep/wake rhythm in ED patients most likely explains this apparent difference. Importantly, NP variations persisted after adjustment for systolic blood pressure. It needs to be noted that the etiology underlying the diurnal variations of NP concentrations and its central or peripheral regulation remains unknown.
Eighth, MR-proANP concentrations did not display statistically significant diurnal variations in patients with noncardiac dyspnea, and the diagnostic accuracy of MR-proANP for AHF was similar in daytime and evening/nighttime presenters. This extends a recent study establishing a circadian variation of MR-proANP in healthy subjects under controlled light laboratory conditions and a standardized diet.11 These physiological changes appear to be muted when assessing ED presentation time in acutely ill patients deprived of a normal sleep/wake rhythm rather than intraindividual variations in healthy volunteers undergoing serial measurements. Interestingly, our findings are supported by experimental studies documenting that ANP mRNA did not follow a diurnal oscillation in the atrial and ventricular mouse heart biopsies, whereas BNP mRNA followed a pronounced diurnal rhythm with the lowest levels during the 12-hour dark period,30 similar to the variations observed in this study. In parallel, differences in the half-life of BNP (20 minutes) versus NT-proBNP (120 minutes)31 might explain the relatively smaller amplitude of the diurnal NT-proBNP variations versus BNP variation observed in this study.
Study Strengths and Limitations
This study has important methodological strengths including its large sample size, its highly representative population of patients presenting to the ED with acute dyspnea,1,3 central adjudicated final diagnosis, and external validation in a well-described cohort of stable participants.
This study also has a number of limitations. First, MR-proANP concentrations were only available in a subset of patients in the BASEL V ED cohort and not in the Maastricht stable participant cohort. However, sensitivity analyses including only patients with a full set of BNP/NT-proBNP and MR-proANP samples confirmed the results of the overall cohort. However, we cannot comment on any potential diurnal variations in MR-proANP concentrations in healthy participants following a normal wake-sleep pattern. Second, although using a very strong methodology for the central adjudication of the final diagnosis, a very small number of patients may still have been misclassified as either AHF or non-AHF in this study. It is extremely unlikely that this inherent limitation would have influenced the main findings. Third, while enrollment was independent from renal function and a substantial number of patients with renal dysfunction were included in both cohorts, this study did not include patients with terminal kidney failure on chronic hemodialysis. Accordingly, we cannot comment on diurnal variations in NP concentrations in this vulnerable patient population. Fourth, we enrolled a real-life population of patients presenting with acute dyspnea to Swiss EDs. Our study population, therefore, mirrors the primarily White population of Switzerland. Further studies are needed to validate our findings in other ethnic groups. Fifth, even though the same sample storage methods were used in this study as in the large, multicenter study establishing the diagnostic accuracy and the currently used cutoff levels for MR-proANP,6 we cannot fully exclude that the lack peptidase inhibitors during storage might have caused some sample degradation. Sixth, a history of obstructive sleep apnea, ambulatory sleep quality, and shift work were not assessed in this study. However, obesity-associated dyspnea was the final adjudicated diagnosis in only 2% of the study population, and over 70% of all patients were of retirement age; it is, therefore, unlikely that these limitations would have relevantly influenced our results.
Finally, parameters of sympathetic nervous system and renin-angiotensin-aldosteron system activation were not measured in this study.
Conclusions
In healthy volunteers and in ED patients with noncardiac causes of acute dyspnea, BNP and NT-proBNP concentrations exhibit significant diurnal variations, characterized by peak levels around midday and trough levels at night. Despite statistically impacting the diagnostic accuracy for AHF depending on presentation time, the diagnostic accuracy of BNP and NT-proBNP for AHF remains very high (>0.9) at all times. Importantly, the accurate rule out of AHF at the clinically used cutoff levels of 100 and 300 ng/L is independent of presentation time (sensitivity, ≥97% at all times). MR-proANP concentrations do not exhibit diurnal variations.
Article Information
Acknowledgments
The authors thank the patients who participated in the study, the staff of the participating emergency departments, the research coordinators, and the laboratory technicians.
Sources of Funding
This study was supported by research grants from the European Union, the Swiss National Science Foundation, the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, the University of Basel, the University Hospital Basel, Critical Diagnostics, Abbott, Alere, BRAHMS, Roche, and Singulex. The sponsors had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Disclosures
Dr Breidthardt received research grants from the Swiss National Science Foundation (PASMP3-134362), University Hospital Basel, Abbott, and Roche, as well as speaker or advisory fees from AstraZeneca, Daiichi Sankyo, Roche, and Vifor. These payments were made directly to University Hospital Basel, and no personal payments were received. Dr Kozhuharov received research grants from the Swiss National Science Foundation (P400PM-194477), Gottfried und Julia Bangerter-Rhyner-Stiftung, and the European Society of Cardiology. Dr Mueller received research grants from the Swiss National Science Foundation and the Swiss Heart Foundation, the European Union, the Cardiovascular Research Foundation Basel, the University of Basel, 8sense, Abbott, Alere, Astra Zeneca, Beckman Coulter, Biomerieux, BRAHMS, Critical Diagnostics, Nanosphere, Roche, Siemens, Singulex, and University Hospital Basel, as well as speaker or consulting honoraria from Abbott, Alere, AstraZeneca, BG Medicine, Biomerieux, Bristol Myers Squibb, Boehringer Ingelheim, BRAHMS, Cardiorentis, Daiichi Sankyo, Novartis, Roche, Sanofi, Singulex, and Siemens. Dr Meex received research funding and lecture fees from Abbott Laboratories and Roche Diagnostics. The other authors report no conflicts.
Supplemental Material
Figures S1–S5
Tables S1–S6
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AHF
- acute heart failure
- AUC
- area under the curve
- BASEL V
- Basics in Acute Shortness of Breath Evaluation Study
- BMI
- body mass index
- BNP
- B-type natriuretic peptide
- CKD
- chronic kidney disease
- ED
- emergency department
- IQR
- interquartile range
- LOD
- limit of detection
- MR-proANP
- midregional pro-atrial natriuretic peptide
- NP
- natriuretic peptide
- NT-proBNP
- N-terminal pro-B-type natriuretic peptide
T. Breidthardt and W.P.T.M. van Doorn contributed equally as first authors.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCHEARTFAILURE.121.009165.
For Sources of Funding and Disclosures, see page 586.
Contributor Information
William P.T.M. van Doorn, Email: william.van.doorn@mumc.nl.
Noreen van der Linden, Email: noreen.vander.linden87@gmail.com.
Matthias Diebold, Email: matthias.diebold@usb.ch.
Desiree Wussler, Email: desireenadine.wussler@usb.ch.
Isabelle Danier, Email: isabelledanier@googlemail.com.
Tobias Zimmermann, Email: tobias.zimmermann@usb.ch.
Samyut Shrestha, Email: samyut.shrestha@usb.ch.
Nikola Kozhuharov, Email: Nikola.Kozhuharov@usb.ch.
Maria Belkin, Email: maria.belkin@usb.ch.
Caroline Porta, Email: CarolineAlessandra.Porta@usb.ch.
Ivo Strebel, Email: ivo.strebel@usb.ch.
Eleni Michou, Email: Eleni.michou@usb.ch.
Danielle M. Gualandro, Email: Danielle.Gualandro@usb.ch.
Albina Nowak, Email: albina.nowak@usz.ch.
S.J.R. Meex, Email: steven.meex@mumc.nl.
Christian Mueller, Email: christian.mueller@usb.ch.
References
- 1.Mebazaa A, Yilmaz MB, Levy P, Ponikowski P, Peacock WF, Laribi S, Ristic AD, Lambrinou E, Masip J, Riley JP, et al. Recommendations on pre-hospital and early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine–short version. Eur Heart J. 2015;36:1958–1966. doi: 10.1093/eurheartj/ehv066 [DOI] [PubMed] [Google Scholar]
- 2.Jessup M, Drazner MH, Book W, Cleveland JC, Jr, Dauber I, Farkas S, Ginwalla M, Katz JN, Kirkwood P, Kittleson MM, et al. 2017 ACC/AHA/HFSA/ISHLT/ACP advanced training statement on advanced heart failure and transplant cardiology (revision of the ACCF/AHA/ACP/HFSA/ISHLT 2010 clinical competence statement on management of patients with advanced heart failure and cardiac transplant): a report of the ACC Competency Management Committee. J Am Coll Cardiol. 2017;69:2977–3001. doi: 10.1016/j.jacc.2017.03.001 [DOI] [PubMed] [Google Scholar]
- 3.McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, et al. ; ESC Scientific Document Group. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42:3599–3726. doi: 10.1093/eurheartj/ehab368 [DOI] [PubMed] [Google Scholar]
- 4.Januzzi JL, Jr, Camargo CA, Anwaruddin S, Baggish AL, Chen AA, Krauser DG, Tung R, Cameron R, Nagurney JT, Chae CU, et al. The N-terminal Pro-BNP investigation of dyspnea in the emergency department (PRIDE) study. Am J Cardiol. 2005;95:948–954. doi: 10.1016/j.amjcard.2004.12.032 [DOI] [PubMed] [Google Scholar]
- 5.Mueller C, Scholer A, Laule-Kilian K, Martina B, Schindler C, Buser P, Pfisterer M, Perruchoud AP. Use of B-type natriuretic peptide in the evaluation and management of acute dyspnea. N Engl J Med. 2004;350:647–654. doi: 10.1056/NEJMoa031681 [DOI] [PubMed] [Google Scholar]
- 6.Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, et al. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol. 2010;55:2062–2076. doi: 10.1016/j.jacc.2010.02.025 [DOI] [PubMed] [Google Scholar]
- 7.Mueller C, McDonald K, de Boer RA, Maisel A, Cleland JGF, Kozhuharov N, Coats AJS, Metra M, Mebazaa A, Ruschitzka F, et al. ; Heart Failure Association of the European Society of Cardiology. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur J Heart Fail. 2019;21:715–731. doi: 10.1002/ejhf.1494 [DOI] [PubMed] [Google Scholar]
- 8.Bruins S, Fokkema MR, Römer JW, Dejongste MJ, van der Dijs FP, van den Ouweland JM, Muskiet FA. High intraindividual variation of B-type natriuretic peptide (BNP) and amino-terminal proBNP in patients with stable chronic heart failure. Clin Chem. 2004;50:2052–2058. doi: 10.1373/clinchem.2004.038752 [DOI] [PubMed] [Google Scholar]
- 9.Goetze JP, Jørgensen HL, Sennels HP, Fahrenkrug J. Diurnal plasma concentrations of natriuretic propeptides in healthy young males. Clin Chem. 2012;58:789–792. doi: 10.1373/clinchem.2011.178921 [DOI] [PubMed] [Google Scholar]
- 10.Sothern RB, Vesely DL, Kanabrocki EL, Bremner FW, Third JL, Boles MA, Nemchausky BM, Olwin JH, Scheving LE. Blood pressure and atrial natriuretic peptides correlate throughout the day. Am Heart J. 1995;129:907–916. doi: 10.1016/0002-8703(95)90111-6 [DOI] [PubMed] [Google Scholar]
- 11.Parcha V, Patel N, Gutierrez OM, Li P, Gamble KL, Musunuru K, Margulies KB, Cappola TP, Wang TJ, Arora G, et al. Chronobiology of natriuretic peptides and blood pressure in lean and obese individuals. J Am Coll Cardiol. 2021;77:2291–2303. doi: 10.1016/j.jacc.2021.03.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wussler D, Kozhuharov N, Sabti Z, Walter J, Strebel I, Scholl L, Miró O, Rossello X, Martín-Sánchez FJ, Pocock SJ, et al. External validation of the MEESSI acute heart failure risk score: a cohort study. Ann Intern Med. 2019;170:248–256. doi: 10.7326/M18-1967 [DOI] [PubMed] [Google Scholar]
- 13.Breidthardt T, Moreno-Weidmann Z, Uthoff H, Sabti Z, Aeppli S, Puelacher C, Stallone F, Twerenbold R, Wildi K, Kozhuharov N, et al. How accurate is clinical assessment of neck veins in the estimation of central venous pressure in acute heart failure? Insights from a prospective study. Eur J Heart Fail. 2018;20:1160–1162. doi: 10.1002/ejhf.1111 [DOI] [PubMed] [Google Scholar]
- 14.Klinkenberg LJ, van Dijk JW, Tan FE, van Loon LJ, van Dieijen-Visser MP, Meex SJ. Circulating cardiac troponin T exhibits a diurnal rhythm. J Am Coll Cardiol. 2014;63:1788–1795. doi: 10.1016/j.jacc.2014.01.040 [DOI] [PubMed] [Google Scholar]
- 15.Klinkenberg LJ, Wildi K, van der Linden N, Kouw IW, Niens M, Twerenbold R, Rubini Gimenez M, Puelacher C, Daniel Neuhaus J, Hillinger P, et al. Diurnal rhythm of cardiac troponin: consequences for the diagnosis of acute myocardial infarction. Clin Chem. 2016;62:1602–1611. doi: 10.1373/clinchem.2016.257485 [DOI] [PubMed] [Google Scholar]
- 16.van der Linden N, Cornelis T, Kimenai DM, Klinkenberg LJJ, Hilderink JM, Lück S, Litjens EJR, Peeters FECM, Streng AS, Breidthardt T, et al. Origin of cardiac troponin T elevations in chronic kidney disease. Circulation. 2017;136:1073–1075. doi: 10.1161/CIRCULATIONAHA.117.029986 [DOI] [PubMed] [Google Scholar]
- 17.Hilderink JM, van der Linden N, Kimenai DM, Litjens EJR, Klinkenberg LJJ, Aref BM, Aziz F, Kooman JP, Rennenberg RJMW, Bekers O, et al. Biological variation of creatinine, cystatin C, and eGFR over 24 hours. Clin Chem. 2018;64:851–860. doi: 10.1373/clinchem.2017.282517 [DOI] [PubMed] [Google Scholar]
- 18.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, Kusek JW, Manzi J, Van Lente F, Zhang YL, et al. ; CKD-EPI Investigators. Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med. 2012;367:20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dill DB, Costill DL. Calculation of percentage changes in volumes of blood, plasma, and red cells in dehydration. J Appl Physiol. 1974;37:247–248. doi: 10.1152/jappl.1974.37.2.247 [DOI] [PubMed] [Google Scholar]
- 20.Morgenthaler NG, Struck J, Thomas B, Bergmann A. Immunoluminometric assay for the midregion of pro-atrial natriuretic peptide in human plasma. Clin Chem. 2004;50:234–236. doi: 10.1373/clinchem.2003.021204 [DOI] [PubMed] [Google Scholar]
- 21.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–843. doi: 10.1148/radiology.148.3.6878708 [DOI] [PubMed] [Google Scholar]
- 22.Pang PS, Teerlink JR, Boer-Martins L, Gimpelewicz C, Davison BA, Wang Y, Voors AA, Severin T, Ponikowski P, Hua TA, et al. Day vs night: does time of presentation matter in acute heart failure? A secondary analysis from the RELAX-AHF trial. Am Heart J. 2017;187:62–69. doi: 10.1016/j.ahj.2017.02.024 [DOI] [PubMed] [Google Scholar]
- 23.Nelson W, Tong YL, Lee JK, Halberg F. Methods for cosinor-rhythmometry. Chronobiologia. 1979;6:305–323. [PubMed] [Google Scholar]
- 24.Tong YL. Parameter estimation in studying circadian rhythms. Biometrics. 1976;32:85–94. [PubMed] [Google Scholar]
- 25.Januzzi JL, Jr, Chen-Tournoux AA, Christenson RH, Doros G, Hollander JE, Levy PD, Nagurney JT, Nowak RM, Pang PS, Patel D, et al. ; ICON-RELOADED Investigators. N-terminal pro-B-type natriuretic peptide in the emergency department: the ICON-RELOADED study. J Am Coll Cardiol. 2018;71:1191–1200. doi: 10.1016/j.jacc.2018.01.021 [DOI] [PubMed] [Google Scholar]
- 26.Ibrahim I, Kuan WS, Frampton C, Troughton R, Liew OW, Chong JP, Chan SP, Tan LL, Lin WQ, Pemberton CJ, et al. Superior performance of N-terminal pro brain natriuretic peptide for diagnosis of acute decompensated heart failure in an Asian compared with a Western setting. Eur J Heart Fail. 2017;19:209–217. doi: 10.1002/ejhf.612 [DOI] [PubMed] [Google Scholar]
- 27.Kozhuharov N, Sabti Z, Wussler D, Nowak A, Badertscher P, Twerenbold R, Wildi K, Stallone F, Vogt F, Hilti J, et al. ; BASEL V Investigators. Prospective validation of N-terminal pro B-type natriuretic peptide cut-off concentrations for the diagnosis of acute heart failure. Eur J Heart Fail. 2019;21:813–815. doi: 10.1002/ejhf.1471 [DOI] [PubMed] [Google Scholar]
- 28.Puelacher C, Rudez J, Twerenbold R, Moreno Weidmann Z, Osswald S, Eckstein F, Lurati-Buse G, Pargger H, Mueller C. B-type natriuretic peptide secretion without change in intra-cardiac pressure. Clin Biochem. 2015;48:318–321. doi: 10.1016/j.clinbiochem.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 29.Portaluppi F, Montanari L, Bagni B, degli Uberti E, Trasforini G, Margutti A. Circadian rhythms of atrial natriuretic peptide, blood pressure and heart rate in normal subjects. Cardiology. 1989;76:428–432. doi: 10.1159/000174529 [DOI] [PubMed] [Google Scholar]
- 30.Goetze JP, Georg B, Jørgensen HL, Fahrenkrug J. Chamber-dependent circadian expression of cardiac natriuretic peptides. Regul Pept. 2010;160:140–145. doi: 10.1016/j.regpep.2009.12.010 [DOI] [PubMed] [Google Scholar]
- 31.de Lemos JA, McGuire DK, Drazner MH. B-type natriuretic peptide in cardiovascular disease. Lancet. 2003;362:316–322. [DOI] [PubMed] [Google Scholar]