Purpose of review
The quest for HIV-1 cure could take advantage of the study of rare individuals that control viral replication spontaneously (elite controllers) or after an initial course of antiretroviral therapy (posttreatment controllers, PTCs). In this review, we will compare back-to-back the immunological and virological features underlying viral suppression in elite controllers and PTCs, and explore their possible contributions to the HIV-1 cure research.
Recent findings
HIV-1 control in elite controllers shows hallmarks of an effective antiviral response, favored by genetic background and possibly associated to residual immune activation. The immune pressure in elite controllers might select against actively transcribing intact proviruses, allowing the persistence of a small and poorly inducible reservoir. Evidence on PTCs is less abundant but preliminary data suggest that antiviral immune responses may be less pronounced. Therefore, these patients may rely on distinct mechanisms, not completely elucidated to date, suppressing HIV-1 transcription and replication.
Summary
PTCs and elite controllers may control HIV replication using distinct pathways, the elucidation of which may contribute to design future interventional strategies aiming to achieve a functional cure.
Keywords: elite controllers, HIV-1 immune response, HIV-1 reservoir, posttreatment controllers
INTRODUCTION
Soon after the discovery of HIV-1 as the cause of AIDS, a rare group of people living with HIV (PLWH) who did not seem to progress towards overt immunosuppression was identified. These rare patients were initially called ‘long-term nonprogressors’ and defined by their ability to maintain high CD4+ T-cell counts over many years in the absence of antiretroviral therapy (ART) [1]. Over the past decades, a much smaller fraction of ‘nonprogressor’ was found to be able to fully suppress plasmatic HIV-1 levels, and called ‘elite controllers’ [2].
Not long after ART became widely available, a third group of individuals called posttreatment controllers (PTCs) was identified [3–5]. In contrast to most PLWH who experience viral rebound post-ART cessation [6], PTCs are able to maintain HIV-1 suppression for months or years despite discontinuing antiretroviral therapy.
To date, elite controllers and PTCs are considered to represent two facets of HIV-1 control. These two groups of individuals may suppress HIV-1 replication using distinct, nonmutually exclusive mechanisms, the characterization of which remains to be fully elucidated. In the present review, we propose to summarize some of the key observations from clinical or basic science research on the possible mechanisms involved in the viral control achieved in elite controllers and PTCs.
Box 1.
no caption available
CLINICAL AND IMMUNOVIROLOGICAL FEATURES OF ELITE CONTROLLERS
Elite controllers are a rare group of PLWH who, in the absence of ART, suppress plasmatic viral load under the limit of detection of standard assays (typically <50 copies/ml). The proportion of elite controllers is less than 1% of PLWH in multiple cohorts [7–10]; interestingly, female gender [10] and black ethnicity [10–12] were independently associated to an increased proportion of natural controllers, whereas age at HIV-1 diagnosis do not seem to be associated with HIV-1 control [13]. Longitudinal studies suggested that elite controllers may suppress HIV-1 after a variable amount of time from seroconversion (median 16.7 months, 22% of controllers from the first HIV-RNA determination) [14] but tend to show a lower zenith viral load and a higher CD4 T-cell count during primary HIV-1 infection (PHI) when compared with typical progressors [15]. Duration of viral control appears to be relatively long in elite controllers, with more than 70% of individuals still suppressed after a median of 6 years of follow-up [16]. Nevertheless, recent data suggested that on the longer period, a consistent proportion of elite controllers may show signs of disease progression [17], immune activation [18,19], and, possibly, adverse clinical outcomes [20,21]. However, whether ART is beneficial in these PLWH is still debated [22,23], and a case-by-case evaluation is suggested by current guidelines [24–26].
The exceptional clinical and virological characteristic of these PLWH led to an extensive research activity focused on determining the features associated with natural HIV-1 control. Initial observations underscored the presence of deletions in viral genes (nef[27,28] or more recently, vpr[29]) or reduced in-vitro replication capacity [30,31] of HIV-1 isolates recovered from some elite controllers, arguing towards a potential lack of viral fitness in these individuals [30]. However, additional studies showed that replication-competent HIV-1 can be isolated from elite controllers [32], and full genome sequencing of proviruses from these patients revealed genetically intact HIV-DNA [33▪▪], suggesting that viral factors alone could not entirely explain the natural control of HIV-1 infection. In addition, low-level viremia [34], viral evolution [35,36] and persistent viral replication in lymphoid tissues [37,38] could be observed in either elite controllers or macaque models of elite control, suggesting that residual viral production, mostly occurring in lymphoid tissues, may still be detected in natural controllers despite an undetectable plasmatic viral load.
In parallel, increasing number of genetic and functional studies have been performed to uncover potential immunological mechanisms associated with elite controllers. Initial genetic observations identified certain HLA variants, collectively referred to as ‘protective HLAs’, as over-represented in elite controllers [39–43]. Mechanistic analyses proposed that these ‘protective’ HLAs were more efficient in presenting conserved [42] and highly networked [44▪] HIV-1 epitopes to CD8 T cells, suggesting that HLA class-I-restricted CD8 T-cell responses represented one of the crucial immunological mechanisms by which elite controllers control HIV-1 replication.
However, other parameters may also play a role, and viral control in elite controllers might not totally rely on ‘protective HLA’ alleles. Indeed, the fact that PLWH harboring ‘protective’ HLAs do not necessarily control HIV-1 replication and that elite controllers may lose HIV-1 control following superinfection [45–47] suggests that the genetic milieu alone might not be sufficient to achieve viral suppression. Moreover, clinical evidences revealed that up to one-third of elite controllers do not express the so-called ‘protective’ HLAs [48–51] and may still display anti-HIV CD8 T-cell responses [48,49] suggesting that an efficient antiviral response might be achieved also in absence of ‘protective’ HLA alleles. Finally, the evidence that some elite controllers, harbouring or not ‘protective’ HLAs, do not show consistent CD8 T-cell-mediated anti-HIV responses while controlling viral replication suggested that class-I HLA-restricted CD8 T-cell-mediated immunity may not be the only immunological factor associated to the control of HIV-1 replication observed in elite controllers [50,52].
Therefore, a huge research effort has also been invested to further dissect immunological determinants associated to natural control of HIV-1. In particular, several studies observed superior attributes of HIV-specific CD8 T cells in elite controllers for what concern in frequency [53], breadth [48], polyfunctionality [49,54,55], stemness potential [56,57] and trafficking to lymphoid tissues [58] when compared with those of chronic progressors (reviewed in detail in the Rutishauer and Trautmann study). More recently, HIV-specific CD4 T-cell-mediated responses were also characterized in these patients, and were found to be quantitatively and qualitatively superior to those of natural progressors in terms of proliferative potential [59], polyfunctionality [60], T-follicular helper functions [61,62] and cytotoxicity [63,64]. Finally, multiple groups investigated the features of humoral and innate immunity in elite controllers, identifying peculiarities in terms of IgG-mediated seroneutralization activity [65], antibody functional profile [66], natural killer (NK) responses [67–69], and antigen-presenting cells functional profile [70,71]. Of note, the contribution of the innate components in the natural control of HIV is reviewed in details in the Calvet and Martin-Gayo study.
To further explore the mechanisms associated with the control of viral replication, several authors exploited the macaque model of natural viral control, and observed that depletion of CD8 T cells was sufficient to trigger SHIV rebound, demonstrating that CD8 T-cell-mediated antiviral immunity is most likely crucial to maintain viral suppression [72]. This view is also supported from the observation that elite controllers eventually losing the control on viral replication preferentially show an impairment in HIV-1-specific CD8 T cell compared with elite controllers persistently suppressing viral load [73▪▪,74,75]. However, supplemental longitudinal studies are needed to comprehensively characterize the balance between viral replication dynamics and specific antiviral immune features.
To summarize, elite controllers represent a heterogeneous group of PLWH who display a long-lasting natural control of HIV-1 infection, associated in most of the individuals to efficient CD8 T-cell responses arising from a favorable genetic background.
CLINICAL AND IMMUNOVIROLOGICAL FEATURES OF POSTTREATMENT CONTROLLERS
PTCs represent a small subset of HIV-infected individuals able to maintain control of HIV-1 replication after stopping ART. Initial case-reports identifying rare patients maintaining viral suppression despite ART withdrawal [3,4] were followed by several cohort studies proposing that PTCs may represent a novel model of ART-free viral suppression. After the initial description of 14 PTCs from the VISCONTI study [5], the CASCADE collaboration [76], the SPARTAC trial [77,78] and, more recently, the CHAMP cohort [79] provided a deeper characterization of these individuals.
The majority of identified PTCs were male, even though this likely reflect the demographic characteristics of the original cohorts [5,76,78,79]; notably, whether PTCs are overrepresented amongst HIV-infected female individuals and individuals of non-Caucasian ethnicity remains to be further elucidated. The observed frequency of PTCs ranged between 2.4 and 15.6% of patients undergoing treatment interruption, and exceed the expected frequency of elite controllers (<1%) despite the heterogeneity in the inclusion criteria [80]. Compared with elite controllers, PTCs rarely harbored the aforementioned ‘protective’ HLAs, displayed higher viral loads and lower CD4+ T-cell counts during PHI [5,77], and showed a more labile persistence of viral control (median 89 weeks, 22% of patients still suppressed after 5 years) [5,79].
Collectively, these studies highlighted that PTCs were probably distinct from elite controllers, and would likely not have been able to naturally control viral replication without ART. The impact of ART in promoting HIV-1 control after treatment discontinuation emerges also from the observation that early-treated PLWH have higher chances of achieving the PTC status when compared with patients treated later during the disease [79]. Indeed, ART initiation during acute infection might favor the development of viral control reducing the size of latent reservoir [81] and preserving immune functions [82]. However, multiple reports described HIV-1 rebound in patients treated days postinfection [83–86], demonstrating that even extremely early ART alone is not sufficient to prevent the establishment of a functional reservoir, and suggesting that ‘too-early’ therapy might paradoxically dampen the development of an effective and appropriate immune response. Moreover, PTCs treated during chronic infection were also identified [87], highlighting that the unknown functional modifications promoting viral control after ART initiation may arise also in patients with more advanced disease. Therefore, additional factors, not exclusively related to timing of ART initiation, are needed to prevent viral rebound. Given the importance of immune response in the natural control of HIV-1 infection, multiple studies investigated whether specific immunological features might be associated to viral suppression in PTCs.
Initial evidence from the VISCONTI cohort suggested that HIV-specific CD8 T cells from PTCs displayed a lower activation status, reduced frequency and reduced suppression of autologous CD4 T-cell infection when compared with those from elite controllers [5]. Nevertheless, additional data suggested that PTCs may be a heterogeneous population in terms of antiviral cellular immunity, as patients harboring effective HIV-specific CD8 T-cell mediated responses could be observed [88,89▪,90]. More recently, this heterogeneity was observed also for what concern humoral responses, with some PTCs showing effective antibody-mediated antiviral functions [89▪,91▪▪], whereas others not displaying any functional HIV-specific serological response [91▪▪] and, in extreme cases, showing seroreversion [92]. The drivers behind the variable antiviral immune responses detected in PTCs remain unclear to date, but preliminary evidences suggested that heterogeneous in-vivo exposure to viral antigens might be involved in shaping the immunological profile [91▪▪].
Of note, these observations arose from individuals analyzed during the phase of virological control, and may thus not reflect the processes mechanistically associated to the suppression of HIV-1 rebound. In this view, multiple studies aimed to identify biomarkers able to predict HIV-1 suppression before ATI. Given the paucity of PTCs detected to date, most of these studies focused on time-to-viral-rebound as a surrogate endpoint of acquired HIV-1 control, and explored biomarkers associated with this outcome. Several virological [93,94,95▪], immunological [96,97] and metabolic biomarkers [98] were proposed; however, to date, none of these markers was clearly validated, and ATI trials including patients recruited on the presence of one [99,100] or more [101] of these markers have led to inconclusive results. In this view, recently described animal models of PTCs may provide valuable insights in dissecting the processes associated to HIV-1 suppression and in validating new biomarkers to select patients to be included in ATI trials [102].
Taken together, PTCs represent a group of individuals that do not share the same attributes than elite controllers in terms of genetic background, viral dynamics during PHI and duration of control on viral replication. Initial studies suggested that this population might have peculiar immunological features, with a reduced preponderance of cellular and humoral antiviral responses. However, a marked heterogeneity emerged among these patients, suggesting that multiple mechanisms may be involved in achieving and maintaining viral suppression.
HIV-1 VIRAL RESERVOIR IN ELITE CONTROLLERS AND POSTTREATMENT CONTROLLERS
In addition to the characterization of clinical and immunological features of elite controllers and PTCs, many studies focused on elucidating multiple aspects of the HIV-1 reservoir in these groups of patients.
HIV-1 reservoir in elite controllers is significantly smaller in terms of total [103,104] and genetically intact proviruses [33▪▪,105] when compared with chronic progressors and to ART-treated patients, despite being subjected to the same processes of clonal expansion detected in the most of PLWH [33▪▪,38,90]. In addition, the combined study of proviral genetic intactness and integration sites highlighted that intact, but not defective, HIV-1 genomes in elite controllers were predominantly located in DNA regions significantly distant from actively transcribing chromatin, suggesting that selection processes, most likely mediated by the immune system, eliminated proviruses able to produce HIV-1 proteins and favorably positioned for transcription [33▪▪]. Of note, both intact and defective proviruses from elite controllers showed a lower frequency of mutations associated with ongoing immune pressure when compared with ART-treated patients [106▪▪], suggesting that the elimination of actively transcribing proviruses in elite controllers may be followed by a phase in which HIV-1 replication reaches a ‘dead-end’ of poorly inducible, transcriptionally silent proviruses, unable to further escape host immunity. Coherently, previous observations showed that elite controllers mostly have a poorly inducible reservoir [107]. Nevertheless, as mentioned above, viral evolution can still be detected in a subset of elite controllers despite a suppressed plasmatic viral load, suggesting that additional research efforts are needed to elucidate whether heterogeneity might be detected in reservoir composition and dynamics among natural controllers.
For what concern PTCs, the study of reservoir characteristics is less extensive; however, multiple reports [5,108] suggested that total HIV-DNA in peripheral blood is significantly lower in these patients when compared with ART-treated individuals. Of note, total intact HIV-DNA was also significantly lower in PTCs but patients with intact reservoir as high as 40% of the total were still able to control HIV-1 rebound [108]. Moreover, integration site analysis confirmed that HIV-1 reservoir from PTCs, including those harboring replication-competent proviruses, can expand in large clones despite persistent control of viral replication [90,108]. Interestingly, the observed reservoir size in PTCs did not reach the extremely low levels observed in currently reported examples of HIV-1 cure [109–111], and is similar to the one observed in very-early-treated patients not able to control viral replication [86]. Therefore, additional factors, independent of total or intact reservoir size assessed in blood, may be associated to virological control in PTCs. The VISCONTI study analyzed the cellular distribution of HIV-1-infected cells in blood, and observed an enrichment among transitional-memory CD4 T cells in PTCs, suggesting that these patients may differ in terms of cells constituting HIV-1 reservoir [5]. In addition, indirect evidence, coming from the association between low cell-associated HIV-RNA levels and longer time-to-viral-rebound [93,95▪] suggested that viral reservoir in PTCs may show limited transcriptional activity (reviewed more extensively in the Pasternak et al. study). Of note, whether this restricted HIV-1 expression is the consequence of selection processes eliminating proviruses favorably positioned for transcription, in analogy with what observed in elite controllers, or of a reservoir ‘silencing’ caused by other factors is currently unknown. Recently, the integration site landscape of HIV-1 reservoir from two PTCs was compared to that of two progressors, and no particular distribution suggestive of an active selection was identified [89▪]. On the other side, additional longitudinal observations analyzing simultaneously integration sites and intactness of proviruses suggested that selection against favorably positioned intact proviruses might occur also in PTCs [112]. However, large-sized studies comprehensively characterizing the HIV-1 reservoir in PTCs, especially focusing on cellular and tissue distribution, IS landscape and intactness of proviruses are missing, and would provide useful insights in deciphering the viral dynamics occurring in these patients.
COMPARING HIV-1 CONTROL IN ELITE CONTROLLERS AND POSTTREATMENT CONTROLLERS
Although the mechanisms responsible for viral control in elite controllers are still not fully understood, a number of evidences have demonstrated that these individuals are characterized by a strong immune response against HIV-1, with a clear involvement of an effective CD8 T-cell-mediated immunity. The enhanced immune response detected in elite controllers may lead to HIV-1 control by clearing HIV-infected cells and selecting for those that harbor HIV-DNA in less transcriptionally favorable positions, increasing the barrier to HIV-1 reactivation. Thus, the ‘locked’, poorly inducible HIV-1 reservoir observed in elite controllers might be the consequence of the peculiar immune response detected in these individuals.
On the other hand, PTCs is a more recently described group, which has not been extensively characterized from an immunological and virological standpoint. Initial evidence pointed out that, compared with elite controllers, these patients may rather display modest HIV-specific CD8 T-cell-mediated immune responses during viral control, and may thus take advantage of additional mechanisms to suppress HIV-1 replication, possibly involving currently unknown immunological mediators able to eliminate HIV-1-infected cells. The elucidation of these factors may be pivotal to design further cure strategies. However, the consistent evidence showing that PTCs would probably not have been able to control HIV-1 without an initial course of antiretroviral therapy suggests that the modifications occurring after ART introduction might be involved per se in promoting viral suppression. ART initiation is associated to an extensive immunological remodeling [113–115], whose effect might also favor HIV-1 latency instauration [116,117] by promoting effector-to-memory transition of infected cells [118]. PTCs may, thus represent the extreme end of this process: the paucity of antiviral immune responses detected ex vivo in these patients might be the consequence of a poor expression of HIV-1 in vivo, because of an excessive ‘silencing’ of HIV-1 transcription in infected cells occurring after ART initiation. Interestingly, limited levels of initiated transcripts were found prior to ATI in PTCs when compared with noncontrollers, even though these observations are not normalized on the levels of intact HIV-DNA [119]. Additional longitudinal studies, possibly involving new techniques able to simultaneously identify integration sites, intactness of proviral DNA and transcriptional activity [120▪▪] will help to elucidate whether or not immune selection mechanisms occur in these patients, and how the interplay between HIV-1 expression and antiviral response leads to acquired HIV-1 control (Fig. 1).
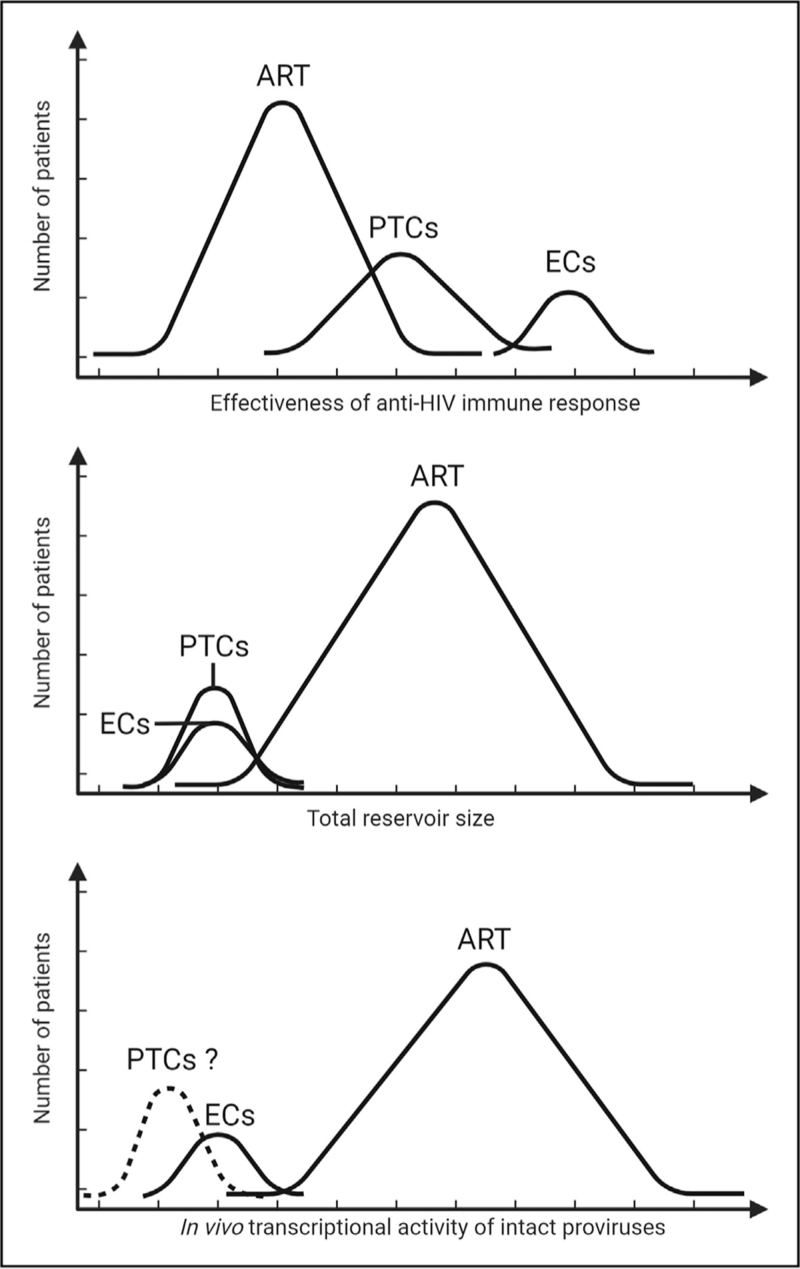
FIGURE 1.
Characteristics of elite controllers, posttreatment controllers and antiretroviral therapy-treated patients. Top panel: elite controllers show a strong, effective immune response against HIV-1, primarily based on CD8 T cells. PTCs, on the contrary, may display a less pronounced antiviral immunity, even though great heterogeneity has been detected in the immunological features of these individuals. Middle panel: both elite controllers and PTCs harbor a significantly lower frequency of HIV-1-infected cells in blood when compared with ART-treated patients. Lower panel: HIV-1 reservoir in elite controllers is dominated by poorly inducible proviruses showing low transcriptional activity. Features of HIV-1 reservoir in PTCs are largely unknown but based on preliminary data and on paucity of antiviral immune responses detected ex vivo in these patients, we can speculate that a reduced transcriptional activity of intact reservoirs might be associated to natural control of HIV-1 replication in this group. ART, antiretroviral therapy; PTCs, posttreatment controllers.
CONCLUSION
The development of effective cure strategies could exploit the immunovirological mechanisms naturally occurring in elite controllers and/or PTCs, which may represent two complementary models of ART-free HIV-1 remission. The thorough investigation and comparison of these exceptional individuals may open previously undisclosed possibilities to identify new pathways associated with HIV-1 suppression, and may elucidate the mechanisms needed to develop novel intervention strategies aiming at achieving a cure for HIV-1.
Acknowledgements
None.
Financial support and sponsorship
This work was supported by Swiss National Science Foundation Grant 320030_200912 and by Freedom Forever Association to M.P. and by an educational grant from FONDATION MACHAON to R.B.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
- 1.Buchbinder SP, Katz MH, Hessol NA, et al. Long-term HIV-1 infection without immunologic progression. AIDS 1994; 8:1123–1128. [DOI] [PubMed] [Google Scholar]
- 2.Deeks SG, Walker BD. Human immunodeficiency virus controllers: mechanisms of durable virus control in the absence of antiretroviral therapy. Immunity 2007; 27:406–416. [DOI] [PubMed] [Google Scholar]
- 3.Goujard C, Girault I, Rouzioux C, et al. HIV-1 control after transient antiretroviral treatment initiated in primary infection: role of patient characteristics and effect of therapy. Antivir Ther 2012; 17:1001–1009. [DOI] [PubMed] [Google Scholar]
- 4.Lisziewicz J, Rosenberg E, Lieberman J, et al. Control of HIV despite the discontinuation of antiretroviral therapy. N Engl J Med 1999; 340:1683–1684. [DOI] [PubMed] [Google Scholar]
- 5.Sáez-Cirión A, Bacchus C, Hocqueloux L, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the Interruption of Early Initiated Antiretroviral Therapy ANRS VISCONTI Study. PLoS Pathog 2013; 9:e1003211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davey RT, Bhat N, Yoder C, et al. HIV-1 and T cell dynamics after interruption of highly active antiretroviral therapy (HAART) in patients with a history of sustained viral suppression. Proc Natl Acad Sci U S A 1999; 96:15109–15114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hubert JB, Burgard M, Dussaix E, et al. Natural history of serum HIV-1 RNA levels in 330 patients with a known date of infection. The SEROCO Study Group. AIDS 2000; 14:123–131. [DOI] [PubMed] [Google Scholar]
- 8.Okulicz JF, Marconi VC, Landrum ML, et al. Clinical outcomes of elite controllers, viremic controllers, and long-term nonprogressors in the US Department of Defense HIV Natural History Study. J Infect Dis 2009; 200:1714–1723. [DOI] [PubMed] [Google Scholar]
- 9.Olson AD, Meyer L, Prins M, et al. An evaluation of HIV elite controller definitions within a large seroconverter cohort collaboration. PLoS One 2014; 9:e86719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang OO, Cumberland WG, Escobar R, et al. Demographics and natural history of HIV-1-infected spontaneous controllers of viremia. AIDS 2017; 31:1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sajadi MM, Constantine NT, Mann DL, et al. Epidemiologic characteristics and natural history of HIV-1 natural viral suppressors. J Acquir Immune Defic Syndr 2009; 50:403–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berg MG, Olivo A, Harris BJ, et al. A high prevalence of potential HIV elite controllers identified over 30 years in Democratic Republic of Congo. eBioMedicine 2021; 65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okulicz JF, Lambotte O. Epidemiology and clinical characteristics of elite controllers. Curr Opin HIV AIDS 2011; 6:163–168. [DOI] [PubMed] [Google Scholar]
- 14.Madec Y, Boufassa F, Porter K, et al. Natural history of HIV-control since seroconversion. AIDS 2013; 27:2451–2460. [DOI] [PubMed] [Google Scholar]
- 15.Goujard C, Chaix M-L, Lambotte O, et al. Spontaneous control of viral replication during primary HIV infection: when is ‘HIV controller’ status established? Clin Infect Dis 2009; 49:982–986. [DOI] [PubMed] [Google Scholar]
- 16.Leon A, Perez I, Ruiz-Mateos E, et al. Rate and predictors of progression in elite and viremic HIV-1 controllers. AIDS 2016; 30:1209–1220. [DOI] [PubMed] [Google Scholar]
- 17.Borrell M, Fernández I, Etcheverrry F, et al. High rates of long-term progression in HIV-1-positive elite controllers. J Int AIDS Soc 2021; 24:e25675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunt PW, Brenchley J, Sinclair E, et al. Relationship between T cell activation and CD4+ T cell count in HIV-seropositive individuals with undetectable plasma HIV RNA levels in the absence of therapy. J Infect Dis 2008; 197:126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krishnan S, Wilson EMP, Sheikh V, et al. Evidence for innate immune system activation in HIV type 1–infected elite controllers. J Infect Dis 2014; 209:931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brusca RM, Hanna DB, Wada NI, et al. Subclinical cardiovascular disease in HIV controller and long-term non-progressor populations. HIV Med 2020; 21:217–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pereyra F, Lo J, Triant VA, et al. Increased coronary atherosclerosis and immune activation in HIV-1 elite controllers. AIDS 2012; 26:2409–2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li JZ, Segal FP, Bosch RJ, et al. Antiretroviral therapy reduces T-cell activation and immune exhaustion markers in human immunodeficiency virus controllers. Clin Infect Dis 2020; 70:1636–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Plaçais L, Boufassa F, Lécuroux C, et al. Antiretroviral therapy for HIV controllers: reasons for initiation and outcomes in the French ANRS-CO21 CODEX cohort. eClinicalMedicine 2021; 37:100963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Initiation of Antiretroviral Therapy | NIH HIV Guidelines. Available at: https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/initiation-antiretroviral-therapy. Accessed 13 May 2022. [Google Scholar]
- 25. BHIVA guidelines on antiretroviral treatment for adults living with HIV-1 2022. Available at: https://www.bhiva.org/treatment-guidelines-consultation. Accessed 13 May 2022. [Google Scholar]
- 26. Prise en charge du VIH – Recommandations du groupe d’experts. Available at: https://cns.sante.fr/actualites/prise-en-charge-du-vih-recommandations-dugroupe-dexperts/. Accessed 13 May 2022. [Google Scholar]
- 27.Deacon NJ, Tsykin A, Solomon A, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 1995; 270:988–991. [DOI] [PubMed] [Google Scholar]
- 28.Zaunders J, Dyer WB, Churchill M, et al. Possible clearance of transfusion-acquired nef/LTR-deleted attenuated HIV-1 infection by an elite controller with CCR5Δ32 heterozygous and HLA-B57 genotype. J Virus Erad 2019; 5:73–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ali A, Ng HL, Blankson JN, et al. Highly attenuated infection with a Vpr-deleted molecular clone of human immunodeficiency virus-1. J Infect Dis 2018; 218:1447–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miura T, Brumme ZL, Brockman MA, et al. Impaired replication capacity of acute/early viruses in persons who become HIV controllers. J Virol 2010; 84:7581–7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miura T, Brockman MA, Schneidewind A, et al. HLA-B57/B∗5801 human immunodeficiency virus type 1 elite controllers select for rare gag variants associated with reduced viral replication capacity and strong cytotoxic T-lymphocyte recognition. J Virol 2009; 83:2743–2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blankson JN, Bailey JR, Thayil S, et al. Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 2007; 81:2508–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33▪▪.Jiang C, Lian X, Gao C, et al. Distinct viral reservoirs in individuals with spontaneous control of HIV-1. Nature 2020; 585:261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a comprehensive description of HIV-1 reservoir features in elite controllers.
- 34.Hatano H, Delwart EL, Norris PJ, et al. Evidence for persistent low-level viremia in individuals who control human immunodeficiency virus in the absence of antiretroviral therapy. J Virol 2009; 83:329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Connell KA, Brennan TP, Bailey JR, et al. Control of HIV-1 in elite suppressors despite ongoing replication and evolution in plasma virus. J Virol 2010; 84:7018–7028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mens H, Kearney M, Wiegand A, et al. HIV-1 continues to replicate and evolve in patients with natural control of HIV infection. J Virol 2010; 84:12971–12981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukazawa Y, Lum R, Okoye AA, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med 2015; 21:132–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boritz EA, Darko S, Swaszek L, et al. Multiple origins of virus persistence during natural control of HIV infection. Cell 2016; 166:1004–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kiepiela P, Leslie AJ, Honeyborne I, et al. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 2004; 432:769–775. [DOI] [PubMed] [Google Scholar]
- 40.Goulder PJR, Walker BD. HIV and HLA class I: an evolving relationship. Immunity 2012; 37:426–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Costello C, Tang J, Rivers C, et al. HLA-B∗5703 independently associated with slower HIV-1 disease progression in Rwandan women. AIDS 1999; 13:1990–1991. [DOI] [PubMed] [Google Scholar]
- 42.Migueles SA, Sabbaghian MS, Shupert WL, et al. HLA B∗5701 is highly associated with restriction of virus replication in a subgroup of HIV-infected long term nonprogressors. Proc Natl Acad Sci U S A 2000; 97:2709–2714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereyra F, Jia S X, et al. International HIV Controllers Study. The major genetic determinants of HIV-1 control affect HLA class I peptide presentation. Science 2010; 330:1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44▪.Kaseke C, Park RJ, Singh NK, et al. HLA class-I-peptide stability mediates CD8+ T cell immunodominance hierarchies and facilitates HLA-associated immune control of HIV. Cell Rep 2021; 36:109378. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides a mechanistic explanation on the protective role against HIV-1 of certain HLA alleles.
- 45.Altfeld M, Allen TM, Yu XG, et al. HIV-1 superinfection despite broad CD8+ T-cell responses containing replication of the primary virus. Nature 2002; 420:434–439. [DOI] [PubMed] [Google Scholar]
- 46.Caetano DG, Côrtes FH, Bello G, et al. A case report of HIV-1 superinfection in an HIV controller leading to loss of viremia control: a retrospective of 10 years of follow-up. BMC Infect Dis 2019; 19:588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clerc O, Colombo S, Yerly S, et al. HIV-1 elite controllers: beware of super-infections. J Clin Virol 2010; 47:376–378. [DOI] [PubMed] [Google Scholar]
- 48.Gaiha GD, Rossin EJ, Urbach J, et al. Structural topology defines protective CD8+ T cell epitopes in the HIV proteome. Science 2019; 364:480–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Betts MR, Nason MC, West SM, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood 2006; 107:4781–4789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Koofhethile CK, Ndhlovu ZM, Thobakgale-Tshabalala C, et al. CD8+ T cell breadth and ex vivo virus inhibition capacity distinguish between viremic controllers with and without protective HLA class I alleles. J Virol 2016; 90:6818–6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pereyra F, Addo MM, Kaufmann DE, et al. Genetic and immunologic heterogeneity among persons who control HIV infection in the absence of therapy. J Infect Dis 2008; 197:563–571. [DOI] [PubMed] [Google Scholar]
- 52.Sáez-Cirión A, Sinet M, Shin SY, et al. Heterogeneity in HIV suppression by CD8 T cells from HIV controllers: association with Gag-specific CD8 T cell responses. J Immunol 2009; 182:7828–7837. [DOI] [PubMed] [Google Scholar]
- 53.Gea-Banacloche JC, Migueles SA, Martino L, et al. Maintenance of large numbers of virus-specific CD8+ T cells in HIV-infected progressors and long-term nonprogressors. J Immunol 2000; 165:1082–1092. [DOI] [PubMed] [Google Scholar]
- 54.Migueles SA, Laborico AC, Shupert WL, et al. HIV-specific CD8+ T cell proliferation is coupled to perforin expression and is maintained in nonprogressors. Nat Immunol 2002; 3:1061–1068. [DOI] [PubMed] [Google Scholar]
- 55.Sáez-Cirión A, Lacabaratz C, Lambotte O, et al. HIV controllers exhibit potent CD8 T cell capacity to suppress HIV infection ex vivo and peculiar cytotoxic T lymphocyte activation phenotype. Proc Natl Acad Sci USA 2007; 104:6776–6781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perdomo-Celis F, Passaes C, Monceaux V, et al. Reprogramming dysfunctional CD8+ T cells to promote properties associated with natural HIV control. J Clin Invest 2022; 132:e157549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rutishauser RL, Deguit CDT, Hiatt J, et al. TCF-1 regulates HIV-specific CD8+ T cell expansion capacity. JCI Insight 2021; 6:e136648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nguyen S, Deleage C, Darko S, et al. Elite control of HIV is associated with distinct functional and transcriptional signatures in lymphoid tissue CD8 + T cells. Sci Transl Med 2019; 11:eaax4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007; 8:1246–1254. [DOI] [PubMed] [Google Scholar]
- 60.Ferre AL, Hunt PW, McConnell DH, et al. HIV controllers with HLA-DRB1∗13 and HLA-DQB1∗06 alleles have strong, polyfunctional mucosal CD4 + T-cell responses. J Virol 2010; 84:11020–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buranapraditkun S, Pissani F, Teigler JE, et al. Preservation of peripheral T follicular helper cell function in HIV controllers. J Virol 2017; 91:e00497–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Claireaux M, Galperin M, Benati D, et al. A high frequency of HIV-specific circulating follicular helper T cells is associated with preserved memory B cell responses in HIV controllers. mBio 2018; 9:e00317–e00318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Benati D, Galperin M, Lambotte O, et al. Public T cell receptors confer high-avidity CD4 responses to HIV controllers. J Clin Investig 2016; 126:2093–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soghoian DZ, Jessen H, Flanders M, et al. HIV-specific cytolytic CD4 T cell responses during acute HIV infection predict disease outcome. Sci Transl Med 2012; 4:123ra25–123ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Alter G, Dowell KG, Brown EP, et al. High-resolution definition of humoral immune response correlates of effective immunity against HIV. Mol Syst Biol 2018; 14:e7881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ackerman ME, Mikhailova A, Brown EP, et al. Polyfunctional HIV-specific antibody responses are associated with spontaneous HIV control. PLoS Pathog 2016; 12:e1005315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Marras F, Nicco E, Bozzano F, et al. Natural killer cells in HIV controller patients express an activated effector phenotype and do not up-regulate NKp44 on IL-2 stimulation. Proc Natl Acad Sci U S A 2013; 110:11970–11975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martin MP, Naranbhai V, Shea PR, et al. Killer cell immunoglobulin-like receptor 3DL1 variation modifies HLA-B∗57 protection against HIV-1. J Clin Invest 2018; 128:1903–1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alter G, Martin MP, Teigen N, et al. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J Exp Med 2007; 204:3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Martin-Gayo E, Buzon MJ, Ouyang Z, et al. Potent cell-intrinsic immune responses in dendritic cells facilitate HIV-1-specific T cell immunity in HIV-1 elite controllers. PLoS Pathog 2015; 11:e1004930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hartana CA, Rassadkina Y, Gao C, et al. Long noncoding RNA MIR4435-2HG enhances metabolic function of myeloid dendritic cells from HIV-1 elite controllers. J Clin Invest 2021; 131:146136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Nishimura Y, Gautam R, Chun T-W, et al. Early antibody therapy can induce long-lasting immunity to SHIV. Nature 2017; 543:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73▪▪.Collins DR, Urbach JM, Racenet ZJ, et al. Functional impairment of HIV-specific CD8+ T cells precedes aborted spontaneous control of viremia. Immunity 2021; 54:2372.e7–2384.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article provides insights into the mechanisms associated to loss of viral control in elite controllers.
- 74.Pernas M, Tarancón-Diez L, Rodríguez-Gallego E, et al. Factors leading to the loss of natural elite control of HIV-1 infection. J Virol 2018; 92:e01805–e01817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rosás-Umbert M, Llano A, Bellido R, et al. Mechanisms of abrupt loss of virus control in a cohort of previous HIV controllers. J Virol 2019; 93:e01436–e01518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lodi S, Meyer L, Kelleher AD, et al. Immunovirologic control 24 months after interruption of antiretroviral therapy initiated close to HIV seroconversion. Arch Intern Med 2012; 172:1252–1255. [DOI] [PubMed] [Google Scholar]
- 77.Martin GE, Gossez M, Williams JP, et al. Posttreatment control or treated controllers? Viral remission in treated and untreated primary HIV infection. AIDS 2017; 31:477–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.The SPARTAC Trial Investigators. Short-course antiretroviral therapy in primary HIV infection. New Engl J Med 2013; 368:207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Namazi G, Fajnzylber JM, Aga E, et al. The Control of HIV After Antiretroviral Medication Pause (CHAMP) study: posttreatment controllers identified from 14 clinical studies. J Infect Dis 2018; 218:1954–1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Etemad B, Esmaeilzadeh E, Li JZ. Learning from the exceptions: HIV remission in posttreatment controllers. Front Immunol 2019; 10:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ananworanich J, Chomont N, Eller LA, et al. HIV DNA set point is rapidly established in acute HIV infection and dramatically reduced by early ART. EBioMedicine 2016; 11:68–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schuetz A, Deleage C, Sereti I, et al. Initiation of ART during early acute HIV infection preserves mucosal Th17 function and reverses HIV-related immune activation. PLoS Pathog 2014; 10:e1004543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Henrich TJ, Hatano H, Bacon O, et al. HIV-1 persistence following extremely early initiation of antiretroviral therapy (ART) during acute HIV-1 infection: an observational study. PLoS Med 2017; 14:e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Luzuriaga K, Gay H, Ziemniak C, et al. Viremic relapse after HIV-1 remission in a perinatally infected child. N Engl J Med 2015; 372:786–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Violari A, Cotton MF, Kuhn L, et al. A child with perinatal HIV infection and long-term sustained virological control following antiretroviral treatment cessation. Nat Commun 2019; 10:412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Colby DJ, Trautmann L, Pinyakorn S, et al. Rapid HIV RNA rebound after antiretroviral treatment interruption in persons durably suppressed in Fiebig I acute HIV infection. Nat Med 2018; 24:923–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maggiolo F, Di Filippo E, Comi L, et al. Posttreatment controllers after treatment interruption in chronically HIV-infected patients. AIDS 2018; 32:623–628. [DOI] [PubMed] [Google Scholar]
- 88.Gulck EV, Bracke L, Heyndrickx L, et al. Immune and viral correlates of ‘secondary viral control’ after treatment interruption in chronically HIV-1 infected patients. PLoS One 2012; 7:e37792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89▪.Blazkova J, Gao F, Marichannegowda MH, et al. Distinct mechanisms of long-term virologic control in two HIV-infected individuals after treatment interruption of antiretroviral therapy. Nat Med 2021; 27:1893–1898. [DOI] [PubMed] [Google Scholar]; This article shows a thorough characterization of antiviral responses and features of HIV-1 reservoir in two posttreatment controllers.
- 90.Veenhuis RT, Kwaa AK, Garliss CC, et al. Long-term remission despite clonal expansion of replication-competent HIV-1 isolates. JCI Insight 2018; 3:e122795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91▪▪.Molinos-Albert LM, Lorin V, Monceaux V, et al. Transient viral exposure drives functionally-coordinated humoral immune responses in HIV-1 posttreatment controllers. Nat Commun 2022; 13:1944. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article, the authors thoroughly describe humoral immune responses in a cohort of posttreatment controllers.
- 92.Uruena A, Cassetti I, Kashyap N, et al. Prolonged posttreatment virologic control and complete seroreversion after advanced human immunodeficiency virus-1 infection. Open Forum Infect Dis 2021; 8:ofaa613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Li JZ, Etemad B, Ahmed H, et al. The size of the expressed HIV reservoir predicts timing of viral rebound after treatment interruption. AIDS 2016; 30:343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Assoumou L, Weiss L, Piketty C, et al. A low HIV-DNA level in peripheral blood mononuclear cells at antiretroviral treatment interruption predicts a higher probability of maintaining viral control. AIDS 2015; 29:2003–2007. [DOI] [PubMed] [Google Scholar]
- 95▪.Pasternak AO, Grijsen ML, Wit FW, et al. Cell-associated HIV-1 RNA predicts viral rebound and disease progression after discontinuation of temporary early ART. JCI Insight 2020; 5:e134196. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article suggests that in-vivo HIV-1 transcriptional activity is correlated to the duration of HIV-1 control without ART.
- 96.Hurst J, Hoffmann M, Pace M, et al. Immunological biomarkers predict HIV-1 viral rebound after treatment interruption. Nat Commun 2015; 6:8495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Giron LB, Papasavvas E, Azzoni L, et al. Plasma and antibody glycomic biomarkers of time to HIV rebound and viral setpoint. AIDS 2020; 34:681–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Giron LB, Palmer CS, Liu Q, et al. Noninvasive plasma glycomic and metabolic biomarkers of posttreatment control of HIV. Nat Commun 2021; 12:3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Calin R, Hamimi C, Lambert-Niclot S, et al. Treatment interruption in chronically HIV-infected patients with an ultralow HIV reservoir. AIDS 2016; 30:761–769. [DOI] [PubMed] [Google Scholar]
- 100.Castagna A, Muccini C, Galli L, et al. Analytical treatment interruption in chronic HIV-1 infection: time and magnitude of viral rebound in adults with 10 years of undetectable viral load and low HIV-DNA (APACHE study). J Antimicrob Chemother 2019; 74:2039–2046. [DOI] [PubMed] [Google Scholar]
- 101.Pannus P, Rutsaert S, De Wit S, et al. Rapid viral rebound after analytical treatment interruption in patients with very small HIV reservoir and minimal on-going viral transcription. J Int AIDS Soc 2020; 23:e25453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Strongin Z, Micci L, Fromentin R, et al. Virologic and immunologic features of simian immunodeficiency virus control post-ART interruption in rhesus macaques. J Virol 2020; 94:e00338–e420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Graf EH, Mexas AM, Yu JJ, et al. Elite suppressors harbor low levels of integrated HIV DNA and high levels of 2-LTR circular HIV DNA compared to HIV+ patients on and off HAART. PLoS Pathog 2011; 7:e1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Julg B, Pereyra F, Buzón MJ, et al. Infrequent recovery of HIV from but robust exogenous infection of activated CD4+ T cells in HIV elite controllers. Clin Infect Dis 2010; 51:233–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kwaa AK, Garliss CC, Ritter KD, et al. Elite suppressors have low frequencies of intact HIV-1 proviral DNA. AIDS 2020; 34:641–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106▪▪.Lian X, Gao C, Sun X, et al. Signatures of immune selection in intact and defective proviruses distinguish HIV-1 elite controllers. Sci Transl Med 2021; 13:eabl4097. [DOI] [PMC free article] [PubMed] [Google Scholar]; This article characterizes the effects of immune pressures on HIV-1 reservoir in elite controllers.
- 107.Noel N, Peña R, David A, et al. Long-term spontaneous control of HIV-1 is related to low frequency of infected cells and inefficient viral reactivation. J Virol 2016; 90:6148–6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharaf R, Lee GQ, Sun X, et al. HIV-1 proviral landscapes distinguish posttreatment controllers from noncontrollers. J Clin Invest 2018; 128:4074–4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hütter G, Nowak D, Mossner M, et al. Long-term control of HIV by CCR5 Delta32/Delta32 stem-cell transplantation. N Engl J Med 2009; 360:692–698. [DOI] [PubMed] [Google Scholar]
- 110.Gupta RK, Abdul-Jawad S, McCoy LE, et al. HIV-1 remission following CCR5Δ32/Δ 32 haematopoietic stem-cell transplantation. Nature 2019; 568:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.HIV-1 REMISSION WITH CCR5Δ32ΔHAPLO-CORD TRANSPLANT IN A US WOMAN: IMPAACT P1107. In: CROI Conference 2022. [Google Scholar]
- 112. LONGITUDINAL DYNAMICS OF INTACT PROVIRAL HIV-1 DNA IN POSTTREATMENT CONTROLLERS. In: CROI Conference 2021. [Google Scholar]
- 113.Lederman MM, Connick E, Landay A, et al. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J Infect Dis 1998; 178:70–79. [DOI] [PubMed] [Google Scholar]
- 114.Yero A, Shi T, Farnos O, et al. Dynamics and epigenetic signature of regulatory T-cells following antiretroviral therapy initiation in acute HIV infection. EBioMedicine 2021; 71:103570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wada NI, Jacobson LP, Margolick JB, et al. The effect of HAART-induced HIV suppression on circulating markers of inflammation and immune activation. AIDS 2015; 29:463–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Abrahams M-R, Joseph SB, Garrett N, et al. The replication-competent HIV-1 latent reservoir is primarily established near the time of therapy initiation. Sci Transl Med 2019; 11:eaaw5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Brodin J, Zanini F, Thebo L, et al. Establishment and stability of the latent HIV-1 DNA reservoir. eLife 2016; 5:e18889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Shan L, Deng K, Gao H, et al. Transcriptional reprogramming during effector-to-memory transition renders CD4+ T cells permissive for latent HIV-1 infection. Immunity 2017; 47:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Post-treatment controllers limit completed and spliced HIV transcripts after ATI. In: CROI Conference 2022. [Google Scholar]
- 120▪▪.Einkauf KB, Osborn MR, Gao C, et al. Parallel analysis of transcription, integration, and sequence of single HIV-1 proviruses. Cell 2022; 185:266.e15–282.e15. [DOI] [PMC free article] [PubMed] [Google Scholar]; In this article, the authors exploit a novel technique (PRIP-Seq), able to simultaneously characterize intactness, integration sites, and transcriptional activity of proviruses, to demonstrate selection processes occurring in ART-treated PLWH.