Abstract
Streptococcus pneumoniae is a major pathogen in humans that enters the host primarily through the respiratory tract. Targeting mucosal surfaces directly may therefore be an optimal approach for vaccination to prevent bacterial colonization and invasive disease. We have previously demonstrated the effectiveness of interleukin-12 (IL-12) delivered intransally (i.n.) as an antiviral respiratory adjuvant. In this study, we examined the effects of i.n. IL-12 treatment on induction of protective humoral immunity against S. pneumoniae. Immunization i.n. with pneumococcal surface protein A (PspA) and IL-12 resulted in enhanced lung IL-10 mRNA expression and marked augmentation of respiratory and systemic immunoglobulin G1 (IgG1), IgG2a, and IgA antibody levels compared to those in animals receiving PspA alone. In addition, i.n. vaccination with PspA and IL-12 provided increased protection against nasopharyngeal carriage. Flow cytometric analysis revealed a threefold increase in antibody-mediated, complement-independent opsonic activity in the sera of PspA- and IL-12-treated animals, which was mainly contributed by IgG2a and, to a lesser extent, IgA. Passive transfer of these immune sera conferred complete protection from death upon systemic pneumococcal challenge. These findings demonstrate the effectiveness of combining PspA and IL-12 at mucosal sites to achieve optimal antibody-mediated opsonization and killing of S. pneumoniae.
Streptococcus pneumoniae is a major pathogen in humans that causes pneumonia, bacteremia, and meningitis and is a leading cause of otitis media in young children (12). The surface of S. pneumoniae is encapsulated with polysaccharides, thus forming a formidable defense against host immune responses. Current polysaccharide vaccines are poorly immunogenic, as they are T-cell-independent antigens that do not elicit isotype switching, affinity maturation, or B-cell memory responses (24). The recently introduced pneumococcal polysaccharide-protein conjugate vaccine appears to be effective at inducing protective immunity (33). However, it only protects against capsular serotypes that are included in the vaccine preparation. Moreover, the relatively high cost of this vaccine makes it unlikely to be widely used in developing countries that have significant rates of acquired pneumococcal respiratory infections.
An alternative vaccine strategy is the use of pneumococcal proteins as immunogens to provide cross-reactive immunity (8, 29). Pneumococcal surface protein A (PspA) is a virulence factor of S. pneumoniae and is expressed on the surfaces of most clinical isolates (14). PspA has been shown to be highly immunogenic and thus is an attractive vaccine candidate against pneumococcal infections (7, 20, 34). Since S. pneumoniae enters the host primarily through the respiratory mucosa, vaccination strategies that target this site are of great interest, especially since most vaccines delivered parenterally are only partially effective at inducing mucosal immunity (39, 42). Therefore, there is a need to identify safe, noninvasive adjuvants that can be used with bacterial vaccines to induce protective mucosal immune responses.
Interleukin-12 (IL-12) is a pivotal regulatory cytokine that preferentially activates Th1 and NK cells to induce the production of gamma interferon (IFN-γ) (15, 35). We (3, 9, 10, 22, 23) and others (5, 16, 21, 25, 43) have shown that IL-12 also has a profound ability to stimulate the production of serum immunoglobulin G2a (IgG2a) and IgG3 antibody responses to a variety of protein and hapten carrier antigens. In addition, we recently reported that the parenteral use of IL-12 with a pneumococcal serotype 3 conjugate vaccine increases protection against S. pneumoniae infection (11). Specifically, IL-12 treatment at the time of vaccination enhances the expression of splenic IFN-γ and induces the production of serum IgG2a antibody. This approach is effective at inducing systemic immunity, but mucosal immune responses following local vaccination have not been examined in a bacterial infection model. Using an intranasal (i.n.) delivery method, however, it has been shown that IL-12 delivered i.n. with an influenza subunit vaccine significantly increases respiratory and systemic antibody expression and subsequent protection from lethal influenza virus infection (4). The enhanced antiviral protection mediated by IL-12 is B cell dependent and can be transferred by immune serum or bronchoalveolar lavage (BAL) fluid.
In the present study, we have evaluated the use of PspA with IL-12 delivered i.n. to induce immunity against pneumococcal infection. Our results show that IL-12 significantly augments the efficacy of PspA vaccination. The protection is antibody mediated and leads to increased opsonization and killing of S. pneumoniae.
MATERIALS AND METHODS
Mice.
BALB/c mice were obtained from Charles River Laboratories (Raleigh, N.C.) through the National Cancer Institute (Bethesda, Md.). The mice were housed and bred at the Albany Medical College and were provided food and water ad libitum. Animal care and experimental procedures were performed in compliance with the Institutional Animal Care and Use Committee guidelines.
I.n. immunization procedures.
I.n. treatments were performed as previously described (2–4). Briefly, mice were anesthetized intraperitoneally (i.p.) with a combination of ketamine HCL (Fort Dodge Laboratories, Fort Dodge, Iowa) and xylazine (Bayer Corporation, Shawnee Mission, Kans.). The mice were immunized i.n. on day 0 with 1 μg of PspA and 1 μg of recombinant murine IL-12 (Genetics Institute, Cambridge, Mass.) in phosphate-buffered saline (PBS) containing 1% normal BALB/c mouse serum (PBS-NMS) or, in the case of control mice, with PBS-NMS only. This was accompanied by i.n. inoculation of 1 μg of IL-12 on days 1, 2, and 3. The mice were boosted i.n. with 1 μg of PspA on days 14 and 28. On day 28, the mice also received either IL-12 in PBS-NMS or PBS-NMS only. No toxicity was observed with this treatment regimen. Sera were prepared by bleeding the mice from the orbital plexus.
RNA isolation and RNase protection assay.
Total RNA isolation from snap-frozen lungs was performed with the Ambion (Austin, Tex.) Total RNA Isolation kit according to the manufacturer's instructions as previously described (4). Cytokine mRNA levels were determined by utilizing the RiboQuant multiprobe RNase protection assay system (Pharmingen, San Diego, Calif.) according to the manufacturer's instructions. Briefly, 10 μg of total RNA was hybridized to a 32P-labeled RNA probe overnight at 56°C. The single-stranded nucleic acid was digested with RNase for 45 min at 30°C, subjected to phenol-chloroform extraction, and resolved on a 6% polyacrylamide gel. Transcript levels were quantified on a Storm 840 PhosphorImager (Molecular Dynamics, Sunnyvale, Calif.). Total RNA was normalized to the housekeeping gene glyceraldehyde 3-phosphate dehydrogenase, and relative cytokine mRNA levels were expressed as arbitrary values.
Collection of BAL fluid.
For collection of BAL fluid, the mice were sacrificed and their tracheas were intubated using a 0.58-mm (outside diameter) polyethylene catheter (Becton Dickinson, Sparks, Md.). The lungs were then lavaged two or three times with PBS containing 5 mM EDTA. The recovered BAL fluids were centrifuged at 12,000 × g for 5 min at 4°C, and the supernatants were stored at −70°C until use.
Detection of antibody and isotype levels by ELISA.
Serum and BAL anti-PspA antibody levels were determined by enzyme-linked immunosorbent assay (ELISA) essentially as described previously (4, 11), with minor modifications. Briefly, microtiter plates (Nalge Nunc International, Rochester, N.Y.) were coated overnight with 1 μg of PspA/ml in PBS. The plates were washed with PBS containing 0.3% Brij 35 (Sigma, St. Louis, Mo.) and blocked for 1 h at room temperature with PBS containing 5% fetal calf serum (Hyclone Laboratories, Logan, Utah) and 0.1% Brij 35. Serial dilutions of serum and BAL fluids were added, and the plates were incubated for 2 h at room temperature. The plates were washed and incubated with goat anti-mouse total Ig, IgG1, IgG2a, or IgA antibody that was conjugated to alkaline phosphatase (Southern Biotechnology Associates, Birmingham, Ala.). After incubation for 1 h, the plates were washed, and p-nitrophenyl phosphatase substrate was added to obtain color development. The plates were read at 405 nm with an ELISA microplate reader (Bio-Tek Instruments, Winooski, Vt.). In all cases, appropriate working dilutions and isotype specificities of the secondary antibody conjugates were determined using purified myeloma proteins of known isotypes (Sigma). Statistical significance was determined by Student's t test using 50% end point titers.
Bacteria.
S. pneumoniae strain TJO983, capsule type 14, PspA family 2, clade 4 was used to study bacterial carriage, and A66.1, capsule type 3, PspA family 1, clade 2 was used to study invasive disease. The immunizing PspA was family 1, clade 2. Clade 2 PspAs generally show strong immunologic cross-reactivity with each other and have sequences that are at least 75% identical in their alpha-helical regions. Family 1 and family 2 PspAs show measurable cross-reactivity but are only about 40% identical in their alpha-helical sequences (17, 26). The bacteria were grown at 37°C in Todd-Hewitt broth containing 0.5% yeast extract. Aliquots of bacteria were stored at −70°C in Todd-Hewitt broth containing 0.5% yeast extract and 10% glycerol. The identities of the pneumococci were confirmed by colony morphology on blood agar plates and by sensitivity to optochin (Sigma).
Opsonophagocytosis assay.
Bacteria were mixed with a 1-mg/ml solution of Lucifer Yellow dye (Sigma) in 0.1 M sodium bicarbonate (pH 9.5) for 2 h at room temperature with intermittent vortexing. The labeled bacteria were subsequently washed, resuspended in sterile PBS, and stored at −20°C. The opsonophagocytosis assay was then performed as described previously (28). Briefly, 5 × 105 bacteria were incubated with varying concentrations of heat-inactivated immune serum in round-bottom microtiter plates for 15 min at 37°C. The bacteria were then incubated for an additional 30 min at 37°C with the J774A.1 macrophage cell line (105 J774A.1 cells/well; American Type Culture Collection, Manassas, Va.). The cells were washed with cold 0.2% bovine serum albumin-Hanks balanced salt solution, resuspended in PBS, and analyzed by flow cytometery (FACScan; Becton Dickinson). The percentage of macrophages containing fluorescent bacteria was used as a measure of phagocytic activity. Blocking studies were performed by preincubating the J774A.1 cells with 100 μg of MOPC315 IgA or UPC10 IgG2a myeloma protein (Sigma)/ml for 1 h at 4°C prior to addition of immune serum for a further 1 h. The samples were then washed and incubated with labeled bacteria at 37°C, and phagocytosis was measured as described above.
Pneumococcal carriage and protection studies.
All mice were immunized with PspA with or without IL-12 as described above. Protection against pneumococcal nasal carriage was determined by infecting groups of mice 35 days after initial immunization and boosting with PspA on days 14 and 28. Seven days after the final boost, S. pneumoniae strain TJO983 type 14 (1.7 × 108 CFU) was administered i.n. to anesthetized mice in 15 μl of Ringer's solution. Three days after infection, the mice were sacrificed, their tracheas were cut, and 50 μl of Ringer's solution was injected and collected from the tip of the nose. Nasal colonization was determined using blood agar plates containing gentamicin alone or gentamicin plus optochin. Antibody-mediated protection against systemic infection was determined by adoptive transfer of serum from vaccinated mice to naïve mice (eight recipient mice per group). A 1:10 dilution of pooled serum was injected i.p. in a volume of 0.1 ml/animal simultaneously with i.p. challenge with S. pneumoniae strain A66.1 type 3 (4.7 × 104 CFU). The mice were monitored daily for mortality, and bacteremia levels in mice succumbing to infection were determined by plating spleen homogenates on blood agar plates. Bacterial carriage and survival data were analyzed by Wilcoxon and log-rank tests, respectively.
RESULTS
I.n. IL-12 administration augments Th1 cytokine expression in the lungs.
To determine the effects of i.n. PspA vaccination and IL-12 treatment on lung cytokine expression, BALB/c mice were treated with PspA with or without IL-12, IL-12 alone, or PBS-NMS. Levels of cytokine expression were analyzed 48 h after vaccination by RNase protection analysis. Previous studies have shown that this time interval after vaccination is optimal for detecting lung cytokine expression (4). It was found that IFN-γ expression was increased approximately twofold in the lungs of animals after i.n. treatment with PspA plus IL-12 compared to that in animals receiving vaccine alone (Table 1). However, the differences in IFN-γ mRNA expression between the mice were not statistically significant. As previously observed with other antigens (3, 4), there was very low IL-10 expression after treatment with PspA or PBS-NMS alone. However, inclusion of IL-12 during vaccination augmented IL-10 expression fivefold compared to that in animals receiving vaccine alone.
TABLE 1.
Cytokine levels in the lungs after i.n. vaccinationa
| Cytokine | PBS | IL-12 | PspA+PBS | PspA+IL-12 |
|---|---|---|---|---|
| IFN-γ | 7.75 ± 6.6 | 11.47 ± 2.6 | 9.3 ± 1.9 | 21.6 ± 16 |
| IL-10 | 0.07 ± 0.01 | 0.173 ± 0.07 | 0.045 ± 0.01 | 0.22 ± 0.03b |
Mice (three per group) were sacrificed 48 h after i.n. treatment with PspA with or without IL-12 or with PBS or IL-12 alone. Total RNA was isolated, and cytokine transcript levels were analyzed by a multiplex RNase protection assay. Relative RNA levels were quantitated on a PhosphorImager and normalized to glyceraldehyde 3-phosphate dehydrogenase. The cytokine mRNA levels are expressed as arbritary units ± standard error of the mean.
Differences in IL-10 mRNA levels between mice immunized with PspA and IL-12 and those immunized with PspA and PBS vehicle were significant (P < 0.05).
Inclusion of IL-12 during i.n. vaccination enhances systemic and mucosal antibody production.
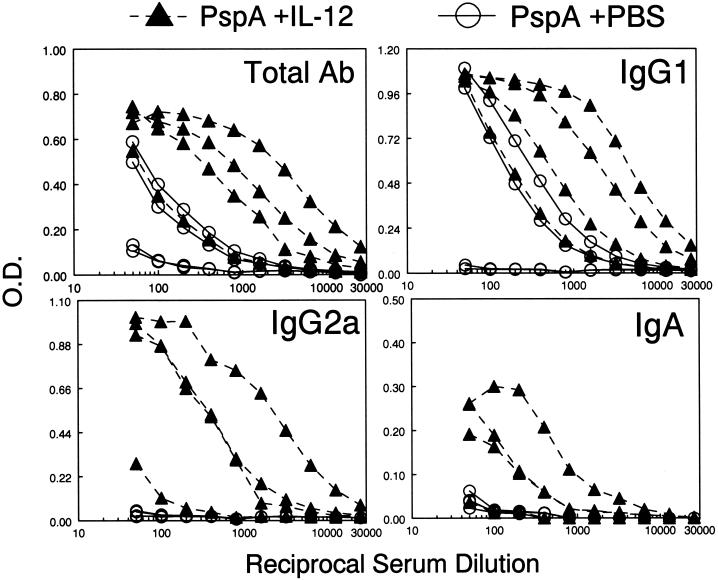
To evaluate the effect of IL-12 on antibody responses to PspA, groups of four BALB/c mice were immunized i.n. with PspA on day 0 and treated with 1 μg of soluble IL-12 or PBS vehicle on days 0, 1, 2, and 3. The mice were boosted with PspA on days 14 and 28 and received a single dose of IL-12 or vehicle on day 28. Serum and BAL antibody levels were determined by isotype-specific ELISA on day 35. Overall, animals that received PspA alone produced very low serum antibody responses (Fig. 1). However, administration of IL-12 during i.n. vaccination augmented the expression of PspA-specific total antibody by an average of 20-fold compared to that in mice immunized with PspA alone. Similarly, IgG1 and IgG2a antibody levels were considerably enhanced in the presence of IL-12. Interestingly, serum IgA levels were also increased after i.n. vaccination and IL-12 treatment. In general, mice immunized in the presence of IL-12 gave higher total, IgG1, IgG2a, and IgA antibody responses than mice vaccinated in the absence of IL-12. However, as seen in our previous studies (2, 4), there was variability in the responses, and in some cases, there was overlap among the various treatment groups. The reason for this variability is unknown and could relate to the difficulty in inoculating precisely equivalent amounts into each animal by the i.n. route.
FIG. 1.
Effects of IL-12 administered i.n. on systemic antibody responses to PspA. BALB/c mice were immunized i.n. on day 0 with PspA, treated i.n. with either IL-12 or PBS vehicle on days 0, 1, 2 and 3, and boosted with PspA on days 14 and 28. On day 28, the mice also received IL-12 or PBS vehicle. Serum anti-PspA antibody levels on day 35 were determined by isotype-specific ELISA using PspA-coated microtiter plates. Each line represents binding of antibody from an individual mouse (four mice per group). Differences in binding between mice immunized with PspA and IL-12 and those immunized with PspA and PBS vehicle were significant (P < 0.05) for total antibody (Ab) and IgG1 and (P < 0.1 for IgA and IgG2a). O.D., optical density.
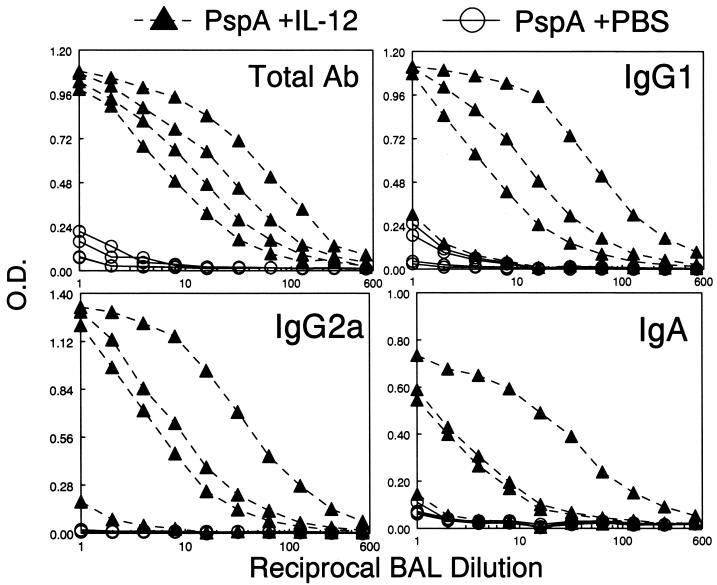
We next assessed the effects of i.n. vaccination on respiratory antibody levels. As observed in serum, there was very low antibody production in BAL fluid after vaccination with PspA alone (Fig. 2). Inclusion of IL-12 with the immunogen resulted in a 30-fold increase in total anti-PspA respiratory antibody production compared to that in mice immunized with PspA alone. The levels of IgG1, IgG2a, and IgA were all significantly increased in the presence of IL-12. Antibody titers in the BAL fluid tended to be lower than those in serum, partly because the lavage process diluted the antibodies in the respiratory fluid. There was a correlation between levels of serum and BAL fluid antibodies in individual mice, with one mouse responding poorly at both sites to IL-12 inoculation.
FIG. 2.
Effects of IL-12 administered i.n. on respiratory antibody (Ab) responses to PspA. BALB/c mice were immunized as described in the legend to Fig. 1 and sacrificed on day 35, and BAL fluid was assayed by ELISA for anti-PspA antibody levels. Each line represents binding of antibody from an individual mouse (four mice per group). Differences in binding between mice immunized with PspA and IL-12 and those immunized with PspA and PBS vehicle were significant (P < 0.05 for total Ab and P < 0.1 for other antibody isotypes). O.D., optical density.
IL-12 enhances the opsonophagocytosis of S. pneumoniae.
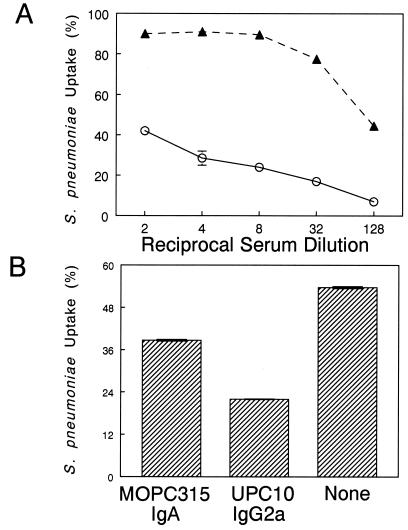
Antibody-dependent phagocytosis has been shown to be an important defense mechanism against encapsulated bacteria. To test the ability of serum antibodies to mediate opsonophagocytosis, mice were immunized i.n. with PspA with or without IL-12, and sera collected on day 35 from these animals were used in a complement-independent, flow cytometric opsonophagocytic assay using the J774A.1 murine macrophage cell line. It was found that pooled sera from PspA- and IL-12-treated mice mediated phagocytosis of S. pneumoniae in a concentration-dependent manner more efficiently than serum from animals receiving PspA alone (Fig. 3A). Of the murine antibody isotypes, IgG2a is the most effective at binding to the FcγRI on phagocytic cells (27, 36). Since IL-12 significantly augmented serum PspA-specific IgG2a and IgA antibody levels, we analyzed the contribution of these isotypes to the observed opsonophagocytosis of S. pneumoniae. The phagocytic cells were preincubated with UPC10 IgG2a or MOPC315 IgA prior to addition of immune serum from IL-12-treated mice. It was found that in the presence of IgG2a myeloma protein, bacterial uptake by phagocytic cells was inhibited by 59% (Fig. 3B). Similarly, with MOPC315 IgA, IgA-mediated phagocytosis was reduced by 27.9%. Thus, PspA vaccination in the presence of IL-12 increases the levels of opsonic antibodies, which are likely to be directly involved in killing of S. pneumoniae by arming resident macrophages.
FIG. 3.
(A) Effect of immune serum on phagocytosis of S. pneumoniae by J774A.1 cells. S. pneumoniae type 3 was opsonized with immune serum from BALB/c mice vaccinated with PspA plus PBS (open circles) or PspA plus IL-12 (solid triangles). Phagocytosis was performed with the J774A.1 macrophage cell line. The percentage of macrophages containing fluorescently labeled bacteria was used as a measure of phagocytic activity. Each condition was tested in duplicate, and the mean values are shown. Three independent experiments gave identical results. (B) UPC10 IgG2a and MOPC315 IgA inhibit the opsonic activity of immune serum from IL-12-treated mice. J774A.1 cells were incubated for 1 h at 4°C with 100 μg of MOPC315 IgA or UPC10 IgG2a and then further incubated for 1 h at 4°C with a 1:4 dilution of immune serum. The results are shown as the mean percentage of macrophages containing fluorescent bacteria ± the standard error of the mean.
IL-12 increases the protective efficacy of PspA vaccination.
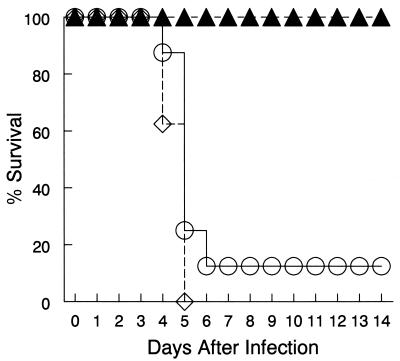
Previous work from our laboratory (4) demonstrated that i.n. immunization with an influenza subunit vaccine in the presence of IL-12 increased protection against subsequent viral infection and that this protection was principally conferred by B cells. Given that inclusion of IL-12 during i.n. PspA vaccination greatly augmented serum antibody levels, we investigated whether such antibodies would increase protection against systemic S. pneumoniae infection. Pooled day 35 sera from mice that had been vaccinated i.n. with PspA with or without IL-12 were passively transferred to naïve mice, who were then challenged i.p. with lethal doses of type 3 pneumococci. Control animals received serum from PBS-treated mice at the time of bacterial challenge. As predicted, the mice that received serum from PBS-treated mice succumbed to the infection within 5 days (Fig. 4). Transfer of immune sera from PspA-vaccinated animals resulted in only 12.5% survival. However, transfer of immune sera from PspA- and IL-12-treated mice resulted in complete protection. The mice that received serum from PspA- and IL-12-treated animals never exhibited any signs of morbidity, nor was there evidence of bacteremia in the spleens of these animals (data not shown).
FIG. 4.
Protective immunity induced by PspA vaccination. Protection against S. pneumoniae was assessed by passive transfer of pooled serum to naïve BALB/c mice (eight recipient mice per group). Sera were collected on day 35 after i.n. immunization with PspA plus IL-12 (solid triangles), PspA plus PBS (open circles), or PBS vehicle only (open diamonds). Pooled sera were given i.p. simultaneously with a bacterial challenge dose of 4.7 × 104 CFU of S. pneumoniae type 3. The mice were monitored daily for mortality. The differences in survival between mice immunized with PspA and IL-12 and those immunized with PspA alone were significant (P < 0.002).
Given that secretory IgA serves to control pneumococcal infections locally, we also examined the protective efficacy of i.n. vaccination on nasopharyngeal bacterial carriage. Groups of mice were immunized as described above and challenged i.n. with S. pneumoniae type 14 1 week after the last booster immunization. It was found that fewer pneumococci were recovered from the nasal washes of mice immunized with PspA plus IL-12 (800 CFU ± 200 [standard error of the mean]) than those receiving PspA (1,800 ± 470) or PBS vehicle alone (1,500 ± 420). Therefore, i.n. vaccination with PspA in the presence of IL-12 enhanced systemic and mucosal antibody production, and it protected the animals and reduced bacterial carriage in the respiratory tract.
DISCUSSION
We have assessed the effects of i.n. vaccination with PspA in the presence of IL-12 in inducing protective immunity against S. pneumoniae. I.n. administration of PspA plus IL-12 was found to augment the production of both secretory and systemic opsonizing antibodies and to confer increased protection against pneumococcal infection. The protection induced by IL-12 treatment was antibody mediated and involved complement-independent opsonization and phagocytosis of S. pneumoniae.
After examining over 2,000 strains of S. pneumoniae, it is apparent that over 95% of strains of pneumococci express either family 1 or family 2 PspAs (13, 17; S. Hollingshead, unpublished observation). Although these PspAs show significant differences in the sequences of their surface-exposed alpha-helical regions, the human and animal antibodies to a single PspA have been observed to cross-react with PspAs of both families. We have also observed that antibodies elicited in humans and animals to a single recombinant PspA can protect against infection with strains expressing both family 1 and family 2 PspAs (6, 7, 26). Because of this broad cross-reactivity, it should not be difficult to select one or a few PspAs that could be used in a vaccine to elicit protective antibodies reactive with the PspAs of virtually all pneumococci.
IL-12 activates Th1-mediated immunity by promoting T-cell and NK-cell activation and enhancing the production of IgG2a antibodies. However, it is now well established that IL-12 plays a major role in shaping the magnitude of overall humoral responses. We (4, 9, 10, 22, 23) and others (5, 16, 21, 25, 43) have shown that IL-12 augments the production of both Th1- and Th2-associated antibody isotypes, including IgG1 and IgA. IL-12 may mediate this function by modulating other intermediary cytokines to provide B-cell help or by directly acting on B cells. In fact, the IL-12 receptor has been shown to be present on both murine and human B cells (38, 41), and IL-12 binding to B cells results in activation of the NF-κB signaling pathway (1).
We have previously demonstrated that IL-12 delivered i.n. with an influenza subunit vaccine leads to increased mucosal and systemic antibody expression (4). This treatment regimen also results in optimal protection against subsequent influenza virus challenge. The observed protection is dependent upon the presence of B cells and can be transferred to naïve mice by either serum or BAL fluid from IL-12-treated animals. In addition, we have recently evaluated the use of IL-12 as an adjuvant for pneumococcal conjugate immunization (11). It was found that parenteral IL-12 treatment at the time of vaccination caused a fourfold increase in IgG2a production compared to that of animals receiving pneumococcal conjugate vaccine alone. Furthermore, passive transfer of immune serum obtained from mice treated with IL-12 almost completely protected naïve recipients against lethal bacterial challenge. However, mucosal immune responses following vaccination were not examined. Since S. pneumoniae primarily invades via mucosal tissues, it was important to examine the efficacy of noninvasive IL-12 delivery with a protein-based vaccine in protection from bacterial infection. Using this approach, i.n. vaccination with PspA alone resulted in weak humoral responses. However, inclusion of IL-12 at the time of vaccination markedly enhanced serum antibody levels. Specifically, the levels of total, IgG1, IgG2a, and IgA antibodies were substantially increased after IL-12 treatment. Yamamoto et al. (44) have shown that a mutant form of cholera toxin (mCT S61F) is a potential adjuvant for mucosal PspA vaccination. I.n. vaccination with PspA and mCT S61F was shown to induce protective immunity against S. pneumoniae. Although mCT S61F is an effective mucosal adjuvant, it primarily induces Th2-type immune responses. In contrast, IL-12 enhances the production of both Th1- and Th2-associated antibody isotypes. Since IgG2a antibodies are the most effective at binding to FcγR on phagocytic cells and IL-12 induces large amounts of IgG2a, soluble IL-12 may be a more promising mucosal adjuvant than mCT S61F.
Treatment with IL-12 also resulted in significant increases in respiratory antibody levels, including IgG1 and IgA anti-PspA antibody levels. Secretory IgA is the major mediator of mucosal immunity and an important component of host defenses that control invasive pneumococcal infections (18, 19). Since S. pneumoniae primarily invades via mucosal tissues in the respiratory tract, vaccines that induce local immunity are highly desirable. In fact, IL-12 delivered i.n. with PspA was found to reduce bacterial colonization in the nasal tract compared to that in animals receiving PspA alone, which may be the result of higher levels of IgA in these animals. Indeed, we (2) and others (30, 31) have recently provided evidence that IgA is an essential component of mucosal antiviral immunity. Therefore, pneumococal vaccination strategies that augment secretory IgA may be important for protective immunity against nasopharyngeal bacterial carriage as well.
Increased protection against lethal pneumoccal infection was also observed after passive transfer of sera from mice that were immunized i.n. with PspA plus IL-12. Whereas transfer of sera from mice immunized with PspA alone resulted in significant mortality, all the animals that received sera from IL-12-treated mice were completely protected, with no evidence of bacteremia. Antibody-mediated opsonization is thought to be the major protective mechanism leading to clearance of encapsulated-bacterial infection in humans. Sera from IL-12-treated mice exhibited enhanced antibody-mediated opsonization of S. pneumoniae compared to sera from mice vaccinated with PspA alone. IgG2a and, to a lesser extent, IgA were shown to be the major isotypes involved in opsonization and phagocytosis of S. pneumoniae. This assay was conducted in the absence of complement and therefore focuses on events mediated solely by antibody. Even though IgG2a is thought to be the most effective opsonizing antibody in mice (27, 36), IgA-mediated phagocytosis of S. pneumoniae has actually been demonstrated with murine alveolar macrophages (32). In humans, IgA-mediated phagocytosis by an Fc receptor mechanism was initially described in neutrophils (40). Recently, van Der and colleagues (37) have shown that purified human serum IgA specific for pneumococcal polysaccharides can initiate phagocytosis via interaction with the Fcα receptor (CD89) on phagocytes. Since IL-12 augments both of these antibody isotypes in systemic and mucosal secretions, noninvasive vaccination strategies utilizing IL-12 may be very useful in providing optimal antibody-mediated immunity in adults and possibly in protecting against pneumococcal infection in children less than 2 years of age.
In summary, the results of this study clearly demonstrate the efficacy of combining PspA and IL-12 to elicit mucosal and systemic immune responses and protection against pneumococcal infection. This approach directly targets the respiratory mucosa and may be an optimal method to induce local and systemic cross-protective immunity against S. pneumoniae infection in humans.
ACKNOWLEDGMENTS
This work was supported by National Institutes of Health grants AI41715 and HL62120 to D.W.M. B.P.A. was supported by a postdoctoral fellowship from the American Heart Association, New York State Affiliate.
We are grateful to Roberta Raeder for helpful comments and suggestions. We also thank Genetics Institute for providing rIL-12.
B.P.A. and J.M.L. contributed equally to this work.
REFERENCES
- 1.Airoldi I, Gri G, Marshall J D, Corcione A, Facchetti P, Guglielmino R, Trinchieri G, Pistoia V. Expression and function of IL-12 and IL-18 receptors on human tonsillar B cells. J Immunol. 2000;165:6880–6888. doi: 10.4049/jimmunol.165.12.6880. [DOI] [PubMed] [Google Scholar]
- 2.Arulanandam B P, Raeder R H, Nedrud J G, Bucher D J, Le J, Metzger D W. IgA immunodeficiency leads to inadequate Th cell priming and increased susceptibility to influenza virus infection. J Immunol. 2001;166:226–231. doi: 10.4049/jimmunol.166.1.226. [DOI] [PubMed] [Google Scholar]
- 3.Arulanandam B P, Metzger D W. Modulation of mucosal and systemic immunity by intranasal interleukin-12 delivery. Vaccine. 1999;17:252–260. doi: 10.1016/s0264-410x(98)00157-1. [DOI] [PubMed] [Google Scholar]
- 4.Arulanandam B P, O'Toole M, Metzger D W. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J Infect Dis. 1999;180:940–949. doi: 10.1086/314996. [DOI] [PubMed] [Google Scholar]
- 5.Bliss J, Van Cleave V, Murray K, Wiencis A, Ketchum M, Maylor R, Haire T, Resmini C, Abbas A K, Wolf S F. IL-12, as an adjuvant, promotes a T helper 1 cell, but does not suppress a T helper 2 cell recall response. J Immunol. 1996;156:887–894. [PubMed] [Google Scholar]
- 6.Briles D E, Nabors G S, Brooks-Walter A, Paton J C, Hollingshead S. The potential for using protein vaccines to protect against otitis media caused by Streptococcus pneumoniae. Vaccine. 2000;19:S87–S95. doi: 10.1016/s0264-410x(00)00285-1. [DOI] [PubMed] [Google Scholar]
- 7.Briles D E, Hollingshead S K, King J, Swift A, Braun P A, Park M K, Ferguson L M, Nahm M H, Nabors G S. Immunization of humans with recombinant pneumococcal surface protein A (rPspA) elicits antibodies that passively protect mice from fatal infection with Streptococcus pneumoniae bearing heterologous PspA. J Infect Dis. 2000;182:1694–1701. doi: 10.1086/317602. [DOI] [PubMed] [Google Scholar]
- 8.Briles D E, Paton J C, Nahm M H, Swiatlo E. Immunity to Streptococcus pneumoniae. In: Cunningham M, Fujinami R S, editors. Effect of microbes on the immune system. Philadelphia, Pa: Lippincott-Raven; 2001. pp. 263–280. [Google Scholar]
- 9.Buchanan J M, Vogel L A, Van Cleave V H, Metzger D W. Interleukin 12 alters the isotype-restricted antibody response of mice to hen eggwhite lysozyme. Int Immunol. 1995;7:1519–1528. doi: 10.1093/intimm/7.9.1519. [DOI] [PubMed] [Google Scholar]
- 10.Buchanan R M, Arulanandam B P, Metzger D W. IL-12 enhances antibody responses to T-independent polysaccharide vaccines in the absence of T and NK cells. J Immunol. 1998;161:5525–5533. [PubMed] [Google Scholar]
- 11.Buchanan R M, Briles D E, Arulanandam B P, Westerink M A, Raeder R H, Metzger D W. IL-12-mediated increases in protection elicited by pneumococcal and meningococcal conjugate vaccines. Vaccine. 2001;19:2020–2028. doi: 10.1016/s0264-410x(00)00421-7. [DOI] [PubMed] [Google Scholar]
- 12.Butler J C, Shapiro E D, Carlone G M. Pneumococcal vaccines: history, current status, and future directions. Am J Med. 1999;107:69S–76S. doi: 10.1016/s0002-9343(99)00105-9. [DOI] [PubMed] [Google Scholar]
- 13.Coral, M. C. V., N. Fonseca, E. Castaneda, J. L. Di Fabio, S. K. Hollingshead, D. E. Briles, and P. Anderson. Families of pneumococcal surface protein A (PspA) of Streptococcus pneumoniae invasive isolates recovered from Colombian children. Emerg. Infect. Dis., in press. [DOI] [PMC free article] [PubMed]
- 14.Crain M J, Waltman W D, Turner J S, Yother J, Talkington D F, McDaniel L S, Gray B M, Briles D E. Pneumococcal surface protein A (PspA) is serologically highly variable and is expressed by all clinically important capsular serotypes of Streptococcus pneumoniae. Infect Immun. 1990;58:3293–3299. doi: 10.1128/iai.58.10.3293-3299.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gately M K, Renzetti L M, Magram J, Stern A S, Adorini L, Gubler U, Presky D H. The interleukin-12/interleukin-12-receptor system: role in normal and pathologic immune responses. Annu Rev Immunol. 1998;16:495–521. doi: 10.1146/annurev.immunol.16.1.495. [DOI] [PubMed] [Google Scholar]
- 16.Germann T, Bongartz M, Dlugonska H, Hess H, Schmitt E, Kolbe L, Kolsch E, Podlaski F J, Gately M K, Rude E. Interleukin-12 profoundly up-regulates the synthesis of antigen-specific complement-fixing IgG2a, IgG2b and IgG3 antibody subclasses in vivo. Eur J Immunol. 1995;25:823–829. doi: 10.1002/eji.1830250329. [DOI] [PubMed] [Google Scholar]
- 17.Hollingshead S K, Becker R, Briles D E. Diversity of PspA: mosaic genes and evidence for past recombination in Streptococcus pneumoniae. Infect Immun. 2000;68:5889–5900. doi: 10.1128/iai.68.10.5889-5900.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Janoff E N, Fasching C, Orenstein J M, Rubins J B, Opstad N L, Dalmasso A P. Killing of Streptococcus pneumoniae by capsular polysaccharide-specific polymeric IgA, complement, and phagocytes. J Clin Investig. 1999;104:1139–1147. doi: 10.1172/JCI6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamm M E. Interaction of antigens and antibodies at mucosal surfaces. Annu Rev Microbiol. 1997;51:311–340. doi: 10.1146/annurev.micro.51.1.311. [DOI] [PubMed] [Google Scholar]
- 20.McDaniel L S, McDaniel D O, Hollingshead S K, Briles D E. Comparison of the PspA sequence from Streptococcus pneumoniae EF5668 to the previously identified PspA sequence from strain Rx1 and ability of PspA from EF5668 to elicit protection against pneumococci of different capsular types. Infect Immun. 1998;66:4748–4754. doi: 10.1128/iai.66.10.4748-4754.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKnight A J, Zimmer G J, Fogelman I, Wolf S F, Abbas A K. Effects of IL-12 on helper T cell-dependent immune responses in vivo. J Immunol. 1994;152:2172–2179. [PubMed] [Google Scholar]
- 22.Metzger D W, Buchanan J M, Collins J T, Lester T L, Murray K S, Van Cleave V H, Vogel L A, Dunnick W A. Enhancement of humoral immunity by interleukin-12. Ann N Y Acad Sci. 1996;795:100–115. doi: 10.1111/j.1749-6632.1996.tb52659.x. [DOI] [PubMed] [Google Scholar]
- 23.Metzger D W, McNutt R M, Collins J T, Buchanan J M, Van Cleave V H, Dunnick W A. Interleukin-12 acts as an adjuvant for humoral immunity through interferon-γ-dependent and -independent mechanisms. Eur J Immunol. 1997;27:1958–1965. doi: 10.1002/eji.1830270820. [DOI] [PubMed] [Google Scholar]
- 24.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 25.Morris S C, Madden K B, Adamovicz J J, Gause W C, Hubbard B R, Gately M K, Finkelman F D. Effects of IL-12 on in vivo cytokine gene expression and Ig isotype selection. J Immunol. 1994;152:1047–1056. [PubMed] [Google Scholar]
- 26.Nabors G S, Braun P A, Herrmann D J, Heise M L, Pyle D J, Gravenstein S, Schilling M, Ferguson L M, Hollingshead S K, Briles D E, Becker R S. Immunization of healthy adults with a single recombinant pneumococcal surface protein A (PspA) variant stimulates broadly cross-reactive antibodies to heterologous PspA molecules. Vaccine. 2000;18:1743–1754. doi: 10.1016/s0264-410x(99)00530-7. [DOI] [PubMed] [Google Scholar]
- 27.Neuberger M S, Rajewsky K. Activation of mouse complement by monoclonal mouse antibodies. Eur J Immunol. 1981;11:1012–1016. doi: 10.1002/eji.1830111212. [DOI] [PubMed] [Google Scholar]
- 28.Obaro S K, Henderson D C, Monteil M A. Defective antibody-mediated opsonisation of S. pneumoniae in high risk patients detected by flow cytometry. Immunol Lett. 1996;49:83–89. doi: 10.1016/0165-2478(95)02487-5. [DOI] [PubMed] [Google Scholar]
- 29.Paton J C. Novel pneumococcal surface proteins: role in virulence and vaccine potential. Trends Microbiol. 1998;6:85–87. doi: 10.1016/s0966-842x(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 30.Renegar K B, Johnson C D, Dewitt R C, King B K, Li J, Fukatsu K, Kudsk K A. Impairment of mucosal immunity by total parenteral nutrition: requirement for IgA in murine nasotracheal anti-influenza immunity. J Immunol. 2001;166:819–825. doi: 10.4049/jimmunol.166.2.819. [DOI] [PubMed] [Google Scholar]
- 31.Renegar K B, Small P A J. Immunoglobulin A mediation of murine nasal anti-influenza virus immunity. J Virol. 1991;65:2146–2148. doi: 10.1128/jvi.65.4.2146-2148.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sestini P, Nencioni L, Villa L, Boraschi D, Tagliabue A. IgA-driven antibacterial activity against Streptococcus pneumoniae by mouse lung lymphocytes. Am Rev Respir Dis. 1988;137:138–143. doi: 10.1164/ajrccm/137.1.138. [DOI] [PubMed] [Google Scholar]
- 33.Shinefield H R, Black S, Ray P, Chang I, Lewis N, Fireman B, Hackell J, Paradiso P R, Siber G, Kohberger R, Madore D V, Malinowski F J, Kimura A, Le C, Landaw I, Aguilar J, Hansen J. Safety and immunogenicity of heptavalent pneumococcal CRM197 conjugate vaccine in infants and toddlers. Pediatr Infect Dis J. 1999;18:757–763. doi: 10.1097/00006454-199909000-00004. [DOI] [PubMed] [Google Scholar]
- 34.Tart R C, McDaniel L S, Ralph B A, Briles D E. Truncated Streptococcus pneumoniae PspA molecules elicit cross-protective immunity against pneumococcal challenge in mice. J Infect Dis. 1996;173:380–386. doi: 10.1093/infdis/173.2.380. [DOI] [PubMed] [Google Scholar]
- 35.Trinchieri G. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions that bridge innate resistance and antigen-specific adaptive immunity. Annu Rev Immunol. 1995;13:251–276. doi: 10.1146/annurev.iy.13.040195.001343. [DOI] [PubMed] [Google Scholar]
- 36.Unkeless J C, Scigliano E, Freedman V H. Structure and function of human and murine receptors for IgG. Annu Rev Immunol. 1988;6:251–281. doi: 10.1146/annurev.iy.06.040188.001343. [DOI] [PubMed] [Google Scholar]
- 37.van Der P W, Vidarsson G, Vile H A, van de Winkel J G, Rodriguez M E. Pneumococcal capsular polysaccharide-specific IgA triggers efficient neutrophil effector functions via FcalphaRI (CD89) J Infect Dis. 2000;182:1139–1145. doi: 10.1086/315825. [DOI] [PubMed] [Google Scholar]
- 38.Vogel L A, Showe L C, Lester T L, McNutt R M, Van Cleave V H, Metzger D W. Direct binding of IL-12 to human and murine B lymphocytes. Int Immunol. 1996;8:1955–1962. doi: 10.1093/intimm/8.12.1955. [DOI] [PubMed] [Google Scholar]
- 39.Walker R I. New strategies for using mucosal vaccination to achieve more effective immunization. Vaccine. 1994;12:387–400. doi: 10.1016/0264-410x(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 40.Weisbart R H, Kacena A, Schuh A, Golde D W. GM-CSF induces human neutrophil IgA-mediated phagocytosis by an IgA Fc receptor activation mechanism. Nature. 1988;332:647–648. doi: 10.1038/332647a0. [DOI] [PubMed] [Google Scholar]
- 41.Wu C Y, Warrier R R, Carvajal D M, Chua A O, Minetti L J, Chizzonite R, Mongini P K, Stern A S, Gubler U, Presky D H, Gately M K. Biological function and distribution of human interleukin-12 receptor beta chain. Eur J Immunol. 1996;26:345–350. doi: 10.1002/eji.1830260212. [DOI] [PubMed] [Google Scholar]
- 42.Wu H Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]
- 43.Wynn T A, Reynolds A, James S, Cheever A W, Caspar P, Hieny S, Jankovic D, Strand M, Sher A. IL-12 enhances vaccine-induced immunity to schistosomes by augmenting both humoral and cell-mediated immune responses against the parasite. J Immunol. 1996;157:4068–4078. [PubMed] [Google Scholar]
- 44.Yamamoto M, Briles D E, Yamamoto S, Ohmura M, Kiyono H, McGhee J R. A nontoxic adjuvant for mucosal immunity to pneumococcal surface protein A. J Immunol. 1998;161:4115–4121. [PubMed] [Google Scholar]