Abstract
Salmonella isolates that lack or overproduce DNA adenine methylase (Dam) elicited a cross-protective immune response to different Salmonella serovars. The protection afforded by the Salmonella enterica serovar Typhimurium Dam vaccine was greater than that elicited in mice that survived a virulent infection. S. enterica serovar Typhimurium Dam mutant strains exhibited enhanced sensitivity to mediators of innate immunity such as antimicrobial peptides, bile salts, and hydrogen peroxide. Also, S. enterica serovar Typhimurium Dam− vaccines were not immunosuppressive; unlike wild-type vaccines, they failed to induce increased nitric oxide levels and permitted a subsequent robust humoral response to diptheria toxoid antigen in infected mice. Dam mutant strains exhibited a low-grade persistence which, coupled with the nonimmunosuppression and the ectopic protein expression caused by altered levels of Dam, may provide an expanded source of potential antigens in vaccinated hosts.
Many pathogenic bacterial species are composed of multiple strains (serotypes) that can cause disease in animal hosts vaccinated against only a single pathogenic strain. Thus, it is desirable to develop bacterial vaccines that can stimulate cross-protective host immune responses to several pathogenic strains. Much of the work regarding the construction of live bacterial vaccines has been performed with Salmonella spp. since they establish an infection by direct interaction with the gut-associated lymphoid tissue, resulting in strong mucosal responses. Salmonella spp. also invade and proliferate within host cells and thus are capable of eliciting strong cell-mediated immune responses (9, 20, 23, 39).
Conceptually, cross-protective immunity could be elicited by live vaccines that express multiple antigens. The rationale is that although different serotypes possess different antigen repertoires, some of the protective antigens may be shared among heterologous serotypes and that expression of these shared antigens may lead to cross-protective immunity. We have recently shown that Salmonella DNA adenine methylase (Dam) mutants ectopically express multiple genes that are normally induced during infection (18). These Dam mutants are markedly attenuated but highly effective as live vaccines against Salmonella infection of mice (12, 18) and chickens (E. L. Dueger, J. K. House, D. M. Heithoff, and M. J. Mahan, submitted for publication). Similarly, Dam mutants are attenuated for virulence in Vibrio cholerae and Yersinia pseudotuberculosis and elicit protective immune responses against Yersinia bacteremia (26; S. M. Julio, D. M. Heithoff, D. Provenzano, K. E. Klose, R. L. Sinsheimer, D. A. Low, and M. J. Mahan, submitted for publication). Here we show that Dam− and Dam-overproducing (DamOP) strains were sensitive to mediators of innate immunity and conferred cross-protective immunity to heterologous Salmonella serotypes, which are designated principally by variations in lipopolysaccharide O antigen structure.
MATERIALS AND METHODS
Bacterial strains and phage.
Salmonella enterica serovar Typhimurium strains used in this study (Table 1) were derived from strain ATCC 14028 (CDC 6516-60). Strains used in infection studies were grown overnight in Luria broth (LB) at 37°C with shaking. The dam-102::Mud-Cm allele was obtained from John Roth and transduced into virulent S. enterica serovar Typhimurium strain 14028 and S. enterica serovar Enteritidis O1,9,12; CDC SSU7998, obtained from the Salmonella Genetic Stock Center, SARB, #16 (3, 36), resulting in the Dam− strain, MT2223. S. enterica serovar Dublin Lane was obtained from Don Guiney (6). The construction of S. enterica serovar Typhimurium damΔ232 (MT2188) was described previously (18). Salmonella Dam overproducer strains were constructed by introduction of the E. coli Dam overproducer plasmid, pTP166 (29). The high-frequency generalized transducing bacteriophage P22 mutant HT105/1, int-201, was used for all transductional crosses (37), and phage-free, phage-sensitive transductants were isolated as previously described (5).
TABLE 1.
Bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| ATCC 14028 | S. enterica serovar Typhimurium | CDC 6516-60 |
| SARB16 | S. enterica serovar Enteritidis | CDC SSU7998 |
| Lane | S. enterica serovar Dublin | 6 |
| MT2128 | pTP166 (dam overproducer plasmid) in ATCC 14028 | 18, 29 |
| MT2188 | damΔ232 in ATCC 14028 | 18 |
| MT2223 | dam102::Mud-Cm in SARB16 | John Roth; this work |
| MT2257 | pTP166 in damΔ232 in ATCC 14028 | 29; this work |
| MT2265 | aroA551::Tn10 in ATCC 14028 | John Roth; this work |
Media and chemicals.
Luria broth (7) was the laboratory medium used in these studies. Final concentrations of antibiotics (Sigma) were as follows: ampicillin, 50 μg/ml; tetracycline, 20 μg/ml; and chloramphenicol, 20 μg/ml.
Virulence and protection assays.
A 50% lethal dose (LD50) assay was used to determine the lethal dose required to kill 50% of the animals; this virulence assay was performed as described previously (18). Briefly, mutant and wild-type cells were grown overnight in Luria broth with shaking. BALB/c mice, 6 to 8 weeks old, were perorally infected or perorally immunized by gastrointubation 0.2 M sodium phosphate buffer (pH 8.0). The protective capacity of Dam derivatives was determined by challenging immunized mice with the virulent strain. Mice were examined daily following challenge for morbidity and mortality. To determine the number of bacteria in host tissues, moribund mice were sacrificed and bacteria were recovered from host tissues and plated for determination of colony counts. Host tissues assayed include Peyer's patches (the four Peyer's patches proximal to the ileal-cecal junction), mesenteric lymph nodes, liver, and spleen.
Two-dimensional protein gel electrophoresis.
Two-dimensional protein gel electrophoresis was performed by the method of O'Farrell (31) on whole-cell protein extracts of log-phase cells grown in Luria broth. Isoelectric focusing using pH 5 to 7 ampholines (Bio-Rad Laboratories, Hercules, Calif.) was carried out at 800 V for 17 h. The second dimension consisted of 12.5% polyacrylamide slab gels which were run for 5.5 h at 175 V. Proteins were visualized by silver staining (30).
RESULTS
Salmonella Dam mutant strains ectopically produce proteins in vitro.
Recent work has shown that Salmonella Dam mutants ectopically express multiple genes (18) that are normally induced only during infection (17, 27). Therefore, we determined whether Salmonella Dam mutant strains ectopically expressed proteins in vitro since such inappropriate protein expression could lead to reduced virulence and the elicitation of protective immune responses. To this end, we analyzed protein expression in Dam−, Dam+, and DamOP S. enterica serovar Typhimurium strains by two-dimensional gel electrophoresis of crude whole-cell protein extracts. Dam− and DamOP strains expressed specific proteins, different from each other and different from those produced by wild-type Salmonella grown in vitro (Fig. 1A and C). In addition, at least one protein was preferentially expressed in wild-type Salmonella compared to the two Dam mutant strains (Fig. 1B). This latter expression pattern is similar to that of the Dam-regulated uropathogenic Escherichia coli pyelonephritis-associated pilus (pap) operon, in which under- and overexpression of Dam blocks Pap pilus production (2, 4, 41). Taken together, these data indicate that Salmonella strains that lack or overproduce Dam ectopically produce proteins in vitro. Such ectopic protein expression may contribute to the virulence attenuation of Dam mutant strains and the elicitation of protective immunity via an expanded source of potential antigens in vaccinated hosts.
FIG. 1.
Ectopic protein expression by Dam and DamOP Salmonella strains. Two-dimensional protein gel electrophoresis was performed on whole-cell protein extracts of Dam− (MT2188) (A), Dam+ (ATCC 14028) (B), and DamOP (MT2128) (C) S. enterica serovar Typhimurium strains (30, 31). Arrows indicate representative examples of proteins that are preferentially expressed in the strains indicated.
Salmonella Dam-based vaccines confer cross-protective immune responses to heterologous serotypes.
Since Dam is a global regulator of gene expression (18), we questioned whether immunization of mice with Salmonella Dam− strains might elicit cross-protective immunity to heterologous Salmonella serotypes. Vaccination with a single oral dose of Dam− S. enterica serovar Enteritidis significantly protected mice (P < 0.05) against challenge with either serovar Dublin or Typhimurium at 10,000 times the LD50 (Table 2). Reciprocally, vaccination with Dam− serovar Typhimurium significantly protected mice (P < 0.05) against challenge with either serovar Dublin at 10,000 times the LD50 or serovar Enteritidis at 1,000 times the LD50 (Table 2). Since DamOP strains of serovar Typhimurium are also attenuated (18), they were also evaluated for the elicitation of protective immune responses to homologous and heterologous Salmonella serotypes. We found that 75% of mice immunized with DamOP serovar Typhimurium survived a challenge at 1,000 times the LD50 with either serovar Dublin or serovar Typhimurium (P < 0.05) (Table 2). Live attenuated Salmonella strains have previously been shown to elicit cross-protective immunity that is often short-lived and dependent on nonspecific immune responses due to persistent infection with the vaccine strain (21, 22). However, in this study, mice were challenged with virulent Salmonella 6 weeks after they had cleared the Dam− or DamOP vaccine strains. That is, mice infected with an oral dose of 109 Dam− Salmonella had cleared the vaccine strain from the Peyer's patches, mesenteric lymph nodes, liver, and spleen 5 weeks postimmunization (data not shown). Accordingly, these data suggest that the cross-protection reported here was not mediated through nonspecific immune responses conferred by vaccine persistence in host tissues.
TABLE 2.
Oral immunization with Salmonella Dam-based vaccines elicits cross-protective immune responses against heterologous serotypesa
| Vaccine strain | Challenge dose (no. of bacteria) | No. of survivors/total no. after oral challenge with serovar:
|
||
|---|---|---|---|---|
| Dublin | Typhimurium | Enteritidis | ||
| No bacteria | 109 | 0/20 | 0/19 | 0/19 |
| dam102::Mud-Cm Enteritidis | 109 | 9/26 | 7/25 | 5/26 |
| No bacteria | 109 | 0/25 | 0/10 | 0/10 |
| damΔ232 Typhimurium | 109 | 4/19 | 11/11 | 0/10 |
| No bacteria | 108 | 0/28 | 0/38 | 0/25 |
| damΔ232 Typhimurium | 108 | 11/23 | 20/20 | 4/18 |
| No bacteria | 108 | 0/10 | 0/10 | ND |
| Dam overproducer Typhimurium | 108 | 6/8 | 6/8 | ND |
BALB/c mice were perorally immunized via gastrointubation with a dose of 109 Dam− or Dam-overproducing Salmonella (18). The mice were challenged with the virulent Salmonella serotypes indicated 11 weeks postimmunization, which was 6 weeks after the vaccine strains were cleared from murine tissues, including Peyer's patches, mesenteric lymph nodes, liver, and spleen. The Dam-overproducing serovar Typhimurium strain (MT2257) contains E. coli dam on recombinant plasmid pTP166 in a damΔ232 background (18, 29). The oral LD50s of challenge strains are as follows: S. enterica serovars Typhimurium (18) and Enteritidis, 105 organisms (data not shown); serovar Dublin, 5 × 104 organisms (6). The cross-protection conferred was significant according to the two-tailed Fisher exact test (P < 0.05).
ND, not done.
Mice immunized with Dam− Salmonella exhibit enhanced protection compared to mice that recovered from a sublethal infection with the Dam+ virulent strain.
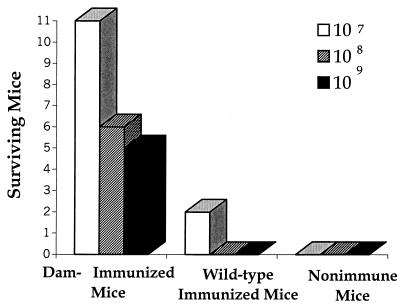
It is generally thought that individuals who have recovered from a natural infection exhibit the strongest state of immunity to reinfection. Thus, the protection conferred by immunization with Dam− Salmonella was compared to that elicited following recovery from a sublethal infection (at the LD50) with the virulent wild-type strain. At an immunizing dose of 105 bacteria, animals vaccinated with the Dam− S. enterica serovar Typhimurium were significantly more protected (P < 0.05) against a lethal challenge with virulent organisms than were animals that recovered from a sublethal infection with Dam+ Typhimurium (Fig. 2). Additionally, mice immunized at this dose (105) with Dam− Typhimurium were protected similarly over a range of virulent challenge doses (107 to 109 bacteria). Previous work showed that mice immunized with 109 Dam− organisms are fully protected against a challenge with 109 virulent organisms (18). Therefore, an immunizing dose of 105 Dam− bacteria appears to be a suboptimal number of organisms to elicit protection against high challenge doses.
FIG. 2.
Mice immunized with Dam− Salmonella exhibit high levels of protection compared to mice recovering from a sublethal infection with the virulent parental strain. The protective immunity elicited in mice immunized with 105 Dam− S. enterica serovar Typhimurium organisms (MT2188) was compared to either that elicited in mice that survived an infection of 105 bacteria (LD50) of the virulent parental Dam+ strain (14028) or to nonimmunized mice. Twenty immunized or nonimmune mice were challenged 5 weeks postinfection with doses of 107, 108, or 109 virulent serovar Typhimurium organisms (ATCC 14028). The enhanced protection conferred by Dam− Salmonella was significant at all challenge doses according to the two-tailed Fisher exact test (P < 0.05).
Mice immunized with Dam− Salmonella show hindered proliferation of virulent Dam+ Salmonella in mucosal and systemic tissues.
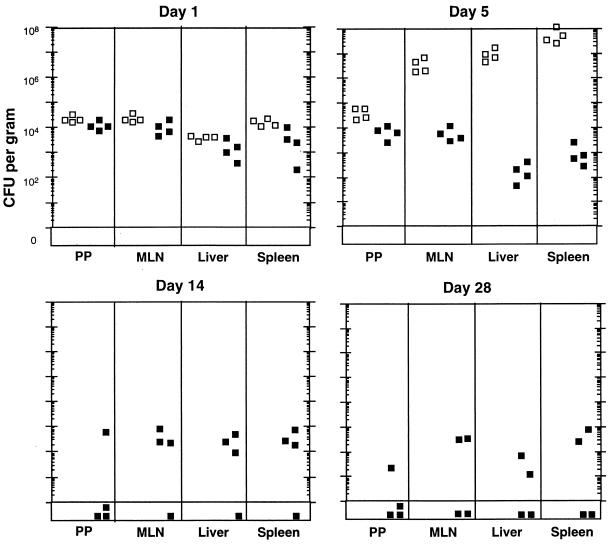
Since mice immunized with Dam-based vaccines showed no overt signs of disease after challenge with virulent bacteria, we determined the fates of virulent S. enterica serovar Typhimurium in vaccinated versus nonvaccinated mice. Following a challenge dose with 10,000-fold the LD50, nonvaccinated mice showed a rapid increase in bacterial number in the Peyer's patches, mesenteric lymph nodes, liver, and spleen and succumbed to the infection on day 6 (Fig. 3). In contrast, although mice vaccinated with Dam− serovar Typhimurium carried high loads (103 to 104 CFU/g) of virulent bacteria in both mucosal and systemic tissues (days 1 to 5), these immunized mice hindered the further proliferation of, and in some cases eliminated, wild-type bacteria from both mucosal and systemic tissues (on day 28, no bacteria were detected in two of four immunized mice). These results indicate that for the high challenge dose tested, the Dam− vaccine protects mice from the lethal effects of Salmonella by blocking the proliferation of Salmonella at mucosal and systemic sites.
FIG. 3.
Mice immunized with Dam− Salmonella block the proliferation of virulent Salmonella in mucosal and systemic tissues. Virulent S. enterica serovar Typhimurium (109 14028 organisms) was perorally administered to nonvaccinated mice (open boxes) or to mice perorally vaccinated with 109 Dam− serovar Typhimurium organisms (MT2188) (solid boxes). Vaccinated mice were challenged 5 weeks postimmunization. The fate of the virulent organisms was determined in host tissues at the times indicated after challenge. All nonvaccinated mice were dead by day 6. PP, Peyer's patches; MLN, mesenteric lymph nodes.
Salmonella Dam− strains are not immunosuppressive in infected mice.
To begin to understand the immunological basis of protection, we examined the contribution of innate immune responses to infection by Salmonella Dam mutant strains. One of the host responses that follows wild-type Salmonella infection is the activation of macrophages and the concomitant release of nitric oxide (NO), which has been shown (via peroxynitrite) to have antibacterial activity (8). In addition to being involved in innate immune functions, Salmonella-induced NO has immunosuppressive effects on the adaptive immune response via lymphocyte inactivation (10), resulting in a condition wherein animals experience a transient state of nonspecific immunosuppression (9). Therefore, we compared both the levels of induced NO and the extent of immunosuppression induced by Dam− S. enterica serovar Typhimurium to those induced by wild-type infection or by administration of a serovar Typhimurium live attenuated vaccine deficient in aromatic amino acid biosynthesis (AroA) (24). The results show that NO levels measured in splenocytes derived from mice infected with Dam− Salmonella are well below those observed after infection with virulent Dam+ Salmonella or after an AroA− immunization (Table 3). The inability to induce high NO levels suggests that Dam− Salmonella strains are not immunosuppressive in mice.
TABLE 3.
Dam− Salmonella strains do not exhibit elevated NO levelsa
| Time of splenocyte collectionb | Nitrate production (μM) inc:
|
|||
|---|---|---|---|---|
| Control | Dam+ (103 CFU) | Dam− (104 CFU) | AroA− (104 CFU) | |
| Day 3 | NDd | 29.17 ± 1.56 | ND | 2.24 ± 0.24 |
| Day 5 | 0.99 ± 0.09 | NSe | 3.38 ± 0.17 | 28.5 ± 0.86 |
BALB/c mice were inoculated intraperitoneally with saline (control) or Dam+ (14028), Dam− (MT2188), or AroA− (MT2265) S. enterica serovar Typhimurium at the doses indicated.
Splenocytes from inoculated mice were collected 3 and 5 days postinoculation and cultured at 107 cells/ml for 48 h.
NO production was determined by measuring nitrite, a stable degradation product of NO, in the supernatants of the cultured cells by the procedure described by Schwacha et al. (38). Data are presented as mean ± standard error of four samples.
ND, not detected.
NS, no survivors.
To measure immunosuppression directly, orally vaccinated mice (Dam− or AroA− S. enterica serovar Typhimurium) were subcutaneously immunized 7 days later with diphtheria toxoid (DT) and the antibodies to DT were measured over a 4-week period. Vaccination with AroA− Salmonella caused more than a fivefold suppression in the antibody response to DT, whereas mice immunized with the Dam− vaccine exhibited anti-DT antibody titers similar to those of non-Salmonella-exposed mice (Table 4). Thus, based on analyses of NO levels and immune response to DT, Dam− Salmonella strains are not immunosuppressive. These results are consistent with the observation that mice vaccinated with Dam− Salmonella strains exhibited a higher level of protection than was observed in mice that recovered from a virulent infection (Fig. 2).
TABLE 4.
Dam− Salmonella strains are not immunosuppressive in infected micea
| Time of blood collectionb | Anti-DT IgG antibody concn (ng/ml)c after immunization with:
|
||
|---|---|---|---|
| Hib-DT | AroA− + Hib-DT | Dam− + Hib-DT | |
| Day 14 | 325.1 ± 73.3 | 389.2 ± 107.6 | 402.4 ± 55.0 |
| Day 21 | 688.3 ± 118.5 | 258.8 ± 48.4 | 484.3 ± 44.1 |
| Day 28 | 887.3 ± 112.1 | 175.3 ± 55.9 | 929.5 ± 120.1 |
BALB/c mice were orally immunized with 107 CFU of AroA− (MT2265) or Dam− (MT2188) S. enterica serovar Typhimurium. One week later, the mice were subcutaneously immunized with 50 μl of a vaccine formulation consisting of Haemophilus influenzae oligosaccharide-diptheria toxoid [(Hib-DT); 1 μg of DT protein per mouse] and aluminum hydroxide (Alum, 13.7 μg).
Blood samples were taken on days 14, 21, and 28 postimmunization. Serum samples were prepared and analyzed for the presence of DT-specific antibodies by enzyme-linked immunosorbent assay on DT-coated plates as described previously (11).
Data are presented as mean ± standard error for five mice.
Salmonella Dam mutant strains are sensitive to components of innate immunity.
To assess the contribution of innate immune responses to infection by Dam mutant Salmonella strains, we tested whether these strains were sensitive to components of innate immunity, including antimicrobial peptides (defensins NP-1 and bactinecin [15]), detergents (bile salts [13]), and mediators of oxidative damage (H2O2 [1]). Our results show that Dam− S. enterica serovar Typhimurium strains are more sensitive to these agents than are wild-type Dam+ bacteria (Table 5). Analysis of S. enterica Typhimurium DamOP strains showed that they are as sensitive to bile salts and hydrogen peroxide as are Dam− strains but, unlike Dam− Salmonella strains, are relatively resistant to the antimicrobial peptides evaluated, which may reflect differences in virulence gene expression between these strains. These data suggest that an enhanced sensitivity to innate immune functions contributes to the attenuated virulence of Salmonella Dam mutant strains.
TABLE 5.
Dam− and DamOP Salmonella strains are sensitive to antimicrobial peptides, bile salts, and hydrogen peroxidea
| Salmonella strain | Inhibitory concn (μg/ml) of antimicrobial peptideb:
|
Inhibitory concn (%) of bile saltsc | Inhibitory concn (mM) of H2O2d | ||
|---|---|---|---|---|---|
| NP-1 | Bactinecin | Protamine sulfate | |||
| Dam+ | >100 | 37.5 | 470 | 1.25 | 50 |
| Dam− | 18.5 | 2.5 | 26 | 0.15 | 12.5 |
| DamOP | 41.0 | 18.5 | 250 | 0.15 | 12.5 |
Values given depict the mean of four samples. Differences in the values of Dam+ versus Dam− and Dam+ versus DamOP were significant using an unpaired t test (P < 0.01). Differences in the values for sensitivity to antimicrobial peptides in Dam− versus DamOP were deemed significant (P < 0.01).
S. enterica serovar Typhimurium was exposed for 1 h to the antimicrobial peptide indicated in phosphate-buffered saline solution. The values refer to the peptide concentration leading to 50% inhibition in CFU.
S. enterica serovar Typhimurium was grown in LB overnight at 37°C without shaking. The values refer to bile concentration representing the MIC.
S. enterica serovar Typhimurium was grown to log phase in LB, resuspended in phosphate-buffered saline, and exposed to hydrogen peroxide for 15 min. The values refer to the H2O2 concentration representing the MIC.
DISCUSSION
One of the problematic aspects of vaccine design is that there are often many different pathogenic isolates of a given species that contribute to disease. Thus, vaccination against one strain may not elicit protection against another strain or even against a variant of the parental strain. This is a principal reason why protective immunity against some microbes may require annual vaccinations with different strains, why vaccine efficacy may depend on the specific pathogenic isolates endemic to a given geographical region, and why mutant variants can cause disease in populations that are immune to infection with the parental strain. Some of these problems may be circumvented by the use of vaccine strains that ectopically express multiple antigens that are shared among different pathogenic strains. Here we show that Salmonella Dam− and DamOP live attenuated vaccines elicited cross-protective immunity to heterologous Salmonella serotypes.
Previous reports have shown that the cross-protective response to S. enterica serovar Typhimurium live attenuated vaccines is highly dependent on nonspecific immune responses attributed to the persistence of the vaccine strain within host tissues (reviewed in references 16, 21, and 22). In this study, the cross-protective immunity elicited was not attributed to the persistence of the vaccine strain since mice were protected against heterologous challenge 6 weeks after the Dam− vaccine strain was eliminated from immunized animals. Notably, Salmonella Dam− vaccines were not immunosuppressive; unlike wild-type infection or aroA vaccination, they failed to induce increased NO levels and permitted a subsequent robust humoral response to DT antigen in infected mice.
Insights into the possible mechanisms by which Dam regulates gene expression come from regulatory analysis of the E. coli pap operon (2, 4, 41), which codes for pili that are essential for virulence in two animal models of pyelonephritis (32, 35). Dam target sites in the pap promoter are protected from methylation by the binding of regulatory proteins at or near these sites, forming specific DNA methylation patterns similar to those observed in eukaryotes (4, 14, 19, 34, 40). These DNA methylation patterns regulate gene expression by modulating the binding of regulatory proteins to Dam target sites. Notably, DNA methylation conveys additional information to DNA without altering the sequence, and such an epigenetic methylation signal is transmitted to future generations. This provides a cellular memory mechanism in which the behavior of daughter cells can be influenced by the environment that their parent cells experienced. This methylation-directed cellular memory system may be important for the infection cycle, which can be viewed analogously to a developmental program (25). Supporting this notion, the cell cycle-regulated methyltransferase, CcrM, is thought to play an important role in coordinating gene expression with the cell cycle in Caulobacter, which undergoes a morphogenetic alteration between a motile swarmer cell and a sessile stalked cell (33).
The role of Dam in virulence and in the elicitation of protective immune responses may rely on its capacity as a global regulator of gene expression (18, 24a, 26, 28). One possible consequence of Dam dysregulation is an expanded repertoire of antigens that contribute to the heightened immunity observed in vaccinated hosts. Additionally, the nonimmunosuppressive nature and low-grade persistence of Dam mutant vaccines in host tissues (12) may provide a stable source of antigens over the time needed to transition to the development of strong adaptive immune responses. Since DNA adenine methylases are highly conserved in a wide variety of virulent bacteria (24a, 26), dysregulation of Dam activity is potentially a general strategy for the generation of vaccines against bacterial pathogens. In addition, Salmonella Dam mutants may serve as a platform to express passenger bacterial and viral antigens that elicit protective immune responses to the cognate pathogen.
ACKNOWLEDGMENTS
We thank John House for critically reviewing the manuscript and Erica Dueger for helping with statistical analyses.
This work was supported by private donations from Jim and Deanna Dehlsen, University of California Biotech Program, the Santa Barbara Cottage Hospital Research Program, and USDA grant 2000-02539 (to M.J.M), National Institutes of Health (NIH) grant AI23348 (to D.A.L.), NIH grants CA25917 and DK55491 (to R.A.D.), and a postdoctoral grant from the Cancer Center of Santa Barbara (to D.M.H.).
REFERENCES
- 1.Babior B M. Phagocytes and oxidative stress. Am J Med. 2000;109:33–44. doi: 10.1016/s0002-9343(00)00481-2. [DOI] [PubMed] [Google Scholar]
- 2.Blyn L B, Braaten B A, Low D A. Regulation of pap pilin phase variation by a mechanism involving differential dam methylation states. EMBO J. 1990;9:4045–4054. doi: 10.1002/j.1460-2075.1990.tb07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyd E F, Wang F S, Beltran P, Plock S A, Nelson K, Selander R K. Salmonella reference collection B (SARB): strains of 37 serovars of subspecies. J Gen Microbiol. 1993;139:1125–1132. doi: 10.1099/00221287-139-6-1125. [DOI] [PubMed] [Google Scholar]
- 4.Braaten B A, Nou X, Kaltenbach L S, Low D A. Methylation patterns in pap regulatory DNA control pyelonephritis-associated pili phase variation in E. coli. Cell. 1994;76:577–588. doi: 10.1016/0092-8674(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 5.Chan R K, Botstein D, Watanabe T, Ogata Y. Specialized transduction of tetracycline resistance by phage P22 in Salmonella typhimurium. II. Properties of a high-frequency-transducing lysate. Virology. 1972;50:883–898. doi: 10.1016/0042-6822(72)90442-4. [DOI] [PubMed] [Google Scholar]
- 6.Chikami G K, Fierer J, Guiney D. Plasmid-mediated virulence in Salmonella dublin demonstrated by use of Tn5-oriT construct. Infect Immun. 1985;50:420–424. doi: 10.1128/iai.50.2.420-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Plainview, N.Y: Cold Spring Harbor Laboratory Press; 1980. [Google Scholar]
- 8.De Groote M A, Granger D, Xu Y, Campbell G, Prince R, Fang F C. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc Natl Acad Sci USA. 1995;92:6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisenstein T K. Mucosal immune defense: the Salmonella typhimurium model. In: Paterson Y, editor. Intracellular bacterial vaccine vectors. New York, N.Y: Wiley-Liss, Inc.; 1999. pp. 51–109. [Google Scholar]
- 10.Eisenstein T K, Huang D, Meissler J J, Jr, al-Ramadi B. Macrophage nitric oxide mediates immunosuppression in infectious inflammation. Immunobiology. 1994;191:493–502. doi: 10.1016/S0171-2985(11)80455-9. [DOI] [PubMed] [Google Scholar]
- 11.Enioutina E Y, Visic D, McGee Z A, Daynes R A. The induction of systemic and mucosal immune responses following the subcutaneous immunization of mature adult mice: characterization of the antibodies in mucosal secretions of animals immunized with antigen formulations containing a vitamin D3 adjuvant. Vaccine. 1999;17:3050–3064. doi: 10.1016/s0264-410x(99)00147-4. [DOI] [PubMed] [Google Scholar]
- 12.Garcia del Portillo F, Pucciarelli M G, Casadesus J. DNA adenine methylase mutants of Salmonella typhimurium show defects in protein secretion, cell invasion, and M cell cytotoxicity. Proc Natl Acad Sci USA. 1999;96:11578–11583. doi: 10.1073/pnas.96.20.11578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunn J S. Mechanisms of bacterial resistance and response to bile. Microbes Infect. 2000;2:907–913. doi: 10.1016/s1286-4579(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 14.Hale W B, van der Woude M W, Low D A. Analysis of nonmethylated GATC sites in the Escherichia coli chromosome and identification of sites that are differentially methylated in response to environmental stimuli. J Bacteriol. 1994;176:3438–3441. doi: 10.1128/jb.176.11.3438-3441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hancock R E, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8:402–410. doi: 10.1016/s0966-842x(00)01823-0. [DOI] [PubMed] [Google Scholar]
- 16.Harrison J A, Villarreal-Ramos B, Mastroeni P, Demarco de Hormaeche R, Hormaeche C E. Correlates of protection induced by live Aro−Salmonella typhimurium vaccines in the murine typhoid model. Immunol. 1997;90:618–625. doi: 10.1046/j.1365-2567.1997.00158.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heithoff D M, Conner C P, Hanna P C, Julio S M, Hentschel U, Mahan M J. Bacterial infection as assessed by in vivo gene expression. Proc Natl Acad Sci USA. 1997;94:934–939. doi: 10.1073/pnas.94.3.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heithoff D M, Sinsheimer R L, Low D A, Mahan M J. An essential role for DNA adenine methylation in bacterial virulence. Science. 1999;284:967–970. doi: 10.1126/science.284.5416.967. [DOI] [PubMed] [Google Scholar]
- 19.Hendrich B, Bird A. Mammalian methyltransferases and methyl-CpG-binding domains: proteins involved in DNA methylation. Curr Top Microbiol Immunol. 2000;249:55–74. doi: 10.1007/978-3-642-59696-4_4. [DOI] [PubMed] [Google Scholar]
- 20.Hone D M, Shata M T, Pascual D W, Lewis G K. Mucosal vaccination with Salmonella vaccine vectors. In: Paterson Y, editor. Intracellular bacterial vaccine vectors. New York, N.Y: Wiley-Liss, Inc.; 1999. pp. 171–221. [Google Scholar]
- 21.Hormaeche C E, Joysey H S, Desilva L, Izhar M, Stocker B A D. Immunity conferred by AroA−Salmonella live vaccines. Microb Pathog. 1991;10:149–158. doi: 10.1016/0882-4010(91)90075-l. [DOI] [PubMed] [Google Scholar]
- 22.Hormaeche C E, Mastroeni P, Harrison J A, Demarco de Hormaeche R, Svenson S, Stocker B A D. Protection against oral challenge three months after i.v. immunization of BALB/c mice with live Aro Salmonella typhimurium and Salmonella enteritidis vaccines is serotype (species)-dependent and only partially determined by the main LPS O antigen. Vaccine. 1996;14:251–259. doi: 10.1016/0264-410x(95)00249-z. [DOI] [PubMed] [Google Scholar]
- 23.Jones B D, Falkow S. Salmonellosis: host immune responses and bacterial virulence determinants. Annu Rev Genet. 1996;14:533–561. doi: 10.1146/annurev.immunol.14.1.533. [DOI] [PubMed] [Google Scholar]
- 24.Lee J C, Gibson C W, Eisenstein T K. Macrophage-mediated mitogenic suppression induced in mice of the C3H lineage by a vaccine strain of Salmonella typhimurium. Cell Immunol. 1985;91:75–91. doi: 10.1016/0008-8749(85)90033-4. [DOI] [PubMed] [Google Scholar]
- 24a.Low, D. A., N. J. Weyland, and M. J. Mahan. Roles of DNA methylation in regulating bacterial gene expression and virulence. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 25.Mahan M J, Heithoff D M, Sinsheimer R L, Low D A. Assessment of bacterial pathogenesis by analysis of gene expression in the host. Annu Rev Genet. 2000;34:139–164. doi: 10.1146/annurev.genet.34.1.139. [DOI] [PubMed] [Google Scholar]
- 26.Mahan M J, Low D A. DNA methylation regulates bacterial gene expression and virulence. ASM News. 2001;67:356–361. [Google Scholar]
- 27.Mahan M J, Slauch J M, Mekalanos J J. Selection of bacterial virulence genes that are specifically induced in host tissues. Science. 1993;259:686–688. doi: 10.1126/science.8430319. [DOI] [PubMed] [Google Scholar]
- 28.Marinus M G. Methylation of DNA, edition VIII. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 782–791. [Google Scholar]
- 29.Marinus M G, Poteete A, Arraj J A. Correlation of DNA adenine methylase activity with spontaneous mutability in Escherichia coli K-12. Gene. 1984;28:123–125. doi: 10.1016/0378-1119(84)90095-7. [DOI] [PubMed] [Google Scholar]
- 30.Merril C R, Goldman D, Van Keuren M L. Gel protein stains: silver stain. Methods Enzymol. 1984;104:441–447. doi: 10.1016/s0076-6879(84)04111-2. [DOI] [PubMed] [Google Scholar]
- 31.O'Farrell P H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975;250:4007–4021. [PMC free article] [PubMed] [Google Scholar]
- 32.O'Hanley P M, Low D, Romero I, Lark D, Vosti K, Falkow S, Schoolnik G. Gal-Gal binding and hemolysin phenotypes and genotypes associated with uropathogenic Escherichia coli. N Engl J Med. 1985;313:414–447. doi: 10.1056/NEJM198508153130704. [DOI] [PubMed] [Google Scholar]
- 33.Reisenauer A, Kahng L S, McCollum S, Shapiro L. Bacterial DNA methylation: a cell cycle regulator? J Bacteriol. 1999;181:5135–5139. doi: 10.1128/jb.181.17.5135-5139.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinquist S, Smith C L. The Escherichia coli chromosome contains specific, unmethylated dam and dcm sites. Proc Natl Acad Sci USA. 1992;89:4539–4543. doi: 10.1073/pnas.89.10.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roberts J A, Suarez G M, Kaak B, Kallenius G, Svenson S B. Experimental pyelonephritis in the monkey. VII. Ascending pyelonephritis in the absence of vesicoureteral reflux. J Urol. 1985;133:1068–1075. doi: 10.1016/s0022-5347(17)49382-7. [DOI] [PubMed] [Google Scholar]
- 36.Sanderson K E, Hessel A, Stocker B A D. Strains of Salmonella typhimurium and other Salmonella species used in genetic analysis. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 2496–2503. [Google Scholar]
- 37.Schmeiger H. Phage P22-mutants with increased or decreased transduction abilities. Mol Gen Genet. 1972;119:75–88. doi: 10.1007/BF00270447. [DOI] [PubMed] [Google Scholar]
- 38.Schwacha M G, Meissler J J, Jr, Eisenstein T K. Salmonella typhimurium infection in mice induces nitric oxide-mediated immunosuppression through a natural killer cell-dependent pathway. Infect Immun. 1998;66:5862–5866. doi: 10.1128/iai.66.12.5862-5866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sirard J C, Niedergang F, Kraehenbuhl J P. Live attenuated Salmonella: a paradigm of mucosal vaccines. Immunol Rev. 1999;171:5–26. doi: 10.1111/j.1600-065x.1999.tb01340.x. [DOI] [PubMed] [Google Scholar]
- 40.Tavazoie S, Church G M. Quantitative whole-genome analysis of DNA-protein interactions by in vivo methylase protection in E. coli. Nat Biotechnol. 1998;16:566–571. doi: 10.1038/nbt0698-566. [DOI] [PubMed] [Google Scholar]
- 41.van der Woude M, Braaten B, Low D A. Epigenetic phase variation of the pap operon in Escherichia coli. Trends Microbiol. 1996;4:5–9. doi: 10.1016/0966-842x(96)81498-3. [DOI] [PubMed] [Google Scholar]