Abstract
Carnosic acid is a diterpenoid abundantly present in plants belonging to the genus Rosmarinus and Salvia of the family Lamiaceae, accounting for their application in traditional medicine. The diverse biological properties of carnosic acid that include antioxidant, anti-inflammatory, and anticarcinogenic activities have instigated studies on its mechanistic role, providing further insights into its potential as a therapeutic agent. Accumulating evidence has established the relevance of carnosic acid as a neuroprotective agent exhibiting therapeutic efficacy in combatting neuronal-injury-induced disorders. The physiological importance of carnosic acid in the mitigation of neurodegenerative disorders is just beginning to be understood. This review summarizes the current data on the mode of action through which carnosic acid exerts its neuroprotective role that may serve to strategize novel therapeutic approaches for these debilitating neurodegenerative disorders.
Keywords: neuroprotection, carnosic acid, natural sources, neurodegeneration, autophagy, oxidative stress, apoptosis, Keap1/Nrf2 signaling
1. Introduction
Neuronal injury is a major factor contributing to various neurological disorders. Despite advancements in the field of medicine and neuroscience, most neurological disorders remain incurable. Currently approved drugs for the treatment of neurological disorders focus on symptomatic relief rather than cure. Recently, there has been an interest in natural products and their therapeutic potential against these disorders. The plants from the genus Rosmarinus and Salvia, belonging to the family Lamiaceae, are the natural sources of carnosic acid (CA) and other natural compounds, which are being widely studied for their therapeutic effects against various conditions [1].
Salvia Rosmarinus, belonging to the family Lamiaceae, is native to the Mediterranean but is now found abundantly throughout the world. Commonly referred to as ‘rosemary’, it has been used as an herbal spice in food and has been a constituent of traditional therapies for various illnesses, including inflammatory diseases, headaches, and gastrointestinal issues [2,3]. Rosemary possesses significant intrinsic antioxidant activity that has been attributed to its major constituents, rosmarinic acid and carnosic acid (CA), which have demonstrated neuroprotective activity in various neurodegenerative diseases [4].
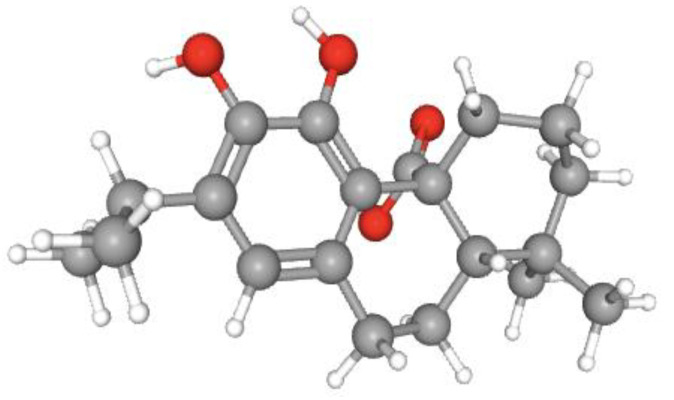
CA is the most abundant compound in rosemary leaves, accounting for 90% of its antioxidant activity [5]. CA is a diterpenoid with an abietane skeleton. Its structure comprises abieta-8,11,13-triene substituted by hydroxy groups at positions 11 and 12 and a carboxy group at position 20 [6]. This carbotricyclic polyphenolic compound is a monocarboxylic acid and a conjugate acid of a carnosate [6]. It is a lipophilic antioxidant that scavenges singlet oxygen, hydroxyl radicals, and lipid peroxyl radicals, thus preventing lipid peroxidation and disruption of biological membranes [7,8] (Figure 1).
Figure 1.
Three-dimensional structure of carnosic acid, acquired from PubChem (pubchem.ncbi.nlm.nih.gov—accessed on 18 January 2023). White represents hydrogen, grey represents carbon and red represents oxygen.
CA possesses diverse biological properties, including antioxidant, anti-inflammatory, neuroprotective, and anticarcinogenic activity [8,9,10,11]. However, the mechanisms by which CA exerts its neuroprotective effect have not been fully elucidated, and ongoing studies are providing insight into possible mechanisms of action. In this review, we aim to systematically discuss the potential neuroprotective properties and mechanisms of action of CA to provide a better understanding of its efficacy as a therapeutic agent in neural-injury-associated disorders.
2. Carnosic Acid and Mechanisms of Neuroprotection
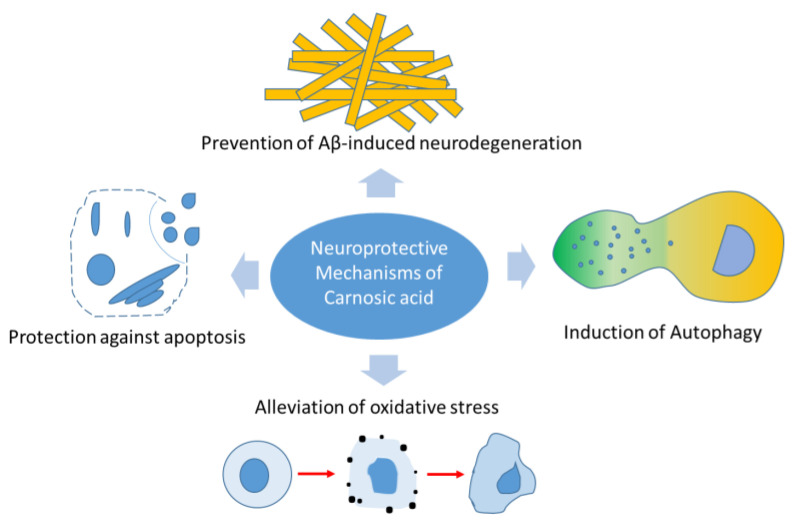
CA exerts its neuroprotective effects through a diverse range of mechanisms, some of which include the prevention of amyloid-β (Aβ)-induced neurodegeneration, induction of autophagy, alleviation of oxidative stress and via anti-apoptotic effects (Figure 2). We systematically review these mechanisms to elucidate the potential of CA in the prevention and control of neural-injury-associated disorders.
Figure 2.
Potential neuroprotective mechanisms of carnosic acid.
2.1. Induction of Autophagy
The pathogenesis of most neurodegenerative disorders bears a resemblance to the manner in which the pathogenic proteins are disposed of by neurons and glia. Autophagy, a homeostatic process by which the degradation of long-lived cellular proteins, lipids, and dysfunctional organelles occur within the lysosomal machinery, plays a crucial role in maintaining the metabolic balance between synthesis, degradation, and subsequent turnover of cytoplasmic material [12,13,14]. Since it prevents the buildup of protein aggregates and damaged mitochondria and organelles, loss of autophagy or its dysregulation may lead to atrophy and neuronal death [15]. Autophagic dysregulation is also implicated in neurodegenerative disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), amyotrophic lateral sclerosis (ALS), and lysosomal storage disorders (LSDs) [14].
A study employing human neuroblastoma SH-SY5Y cells revealed an instrumental role for CA in the reduction of Aβ-induced apoptosis and the accumulation of toxic proteins through the induction of autophagy. Aβ aggregation is a hallmark feature of AD and is a key target of AD-related therapies. The study by Liu and colleagues demonstrated that CA-induced autophagy via AMP-activated protein kinase (AMPK) is an important regulator of cellular metabolism [16]. AMPK triggers autophagy to avoid oxidative stress and mitochondrial dysfunction in cells treated with CA, highlighting a therapeutic mechanism of CA against Aβ [16]. In vitro studies that investigated the effect of pre-treating SH-SY5Y cells with CA prior to serum starvation revealed that pretreatment significantly protected these cells against nutrient depletion [17]. The cytoprotective effects of CA were afforded by the phosphorylation of protein kinase B (Akt) and extracellular signal-regulated kinase 1/2 (Erk1/2) and moderate activation of autophagy since pretreatment with LY294002 and U-0126, inhibitors of Akt and Erk1/2 phosphorylation, abolished the protective effects [17].
Another mechanism by which CA influences autophagy is through the parkin pathway. Parkin is an E3 ubiquitin ligase that catalyzes the conjugation of ubiquitin to abnormal proteins, facilitating their degradation by the ubiquitin proteasome system (UPS) [18]. Parkin gene mutations have been implicated in the pathogenesis of neurodegenerative diseases, including Parkinson’s [19,20,21]. CA was shown to prevent cell death via induction of the parkin pathway, enhancing levels of parkin protein, the UPS, and α-synuclein degradation [22]. The interaction between parkin and Beclin1 is considered to facilitate autophagosome maturation [23]. CA substantially enhances the parkin/Beclin1 interaction, inducing autophagy [24]. These effects were attenuated by wortmannin and bafilomycin A1 (an autophagosome-lysosome fusion blocker) [24]. Moreover, CA has also been shown to mitigate mitochondrial impairment, which also involves the activation of the PINK1/parkin/mitophagy pathway [25]. The neuroprotective effects of CA have also been attributed to the upregulation of OPA1 (OPA1 mitochondrial dynamin-like GTPase) via activation of the parkin/IKKγ/p65 pathway and are associated with an enhancement of mitochondrial biogenesis. This pathway is linked to the inhibition of Parkin-interacting substrate (PARIS) and induction of proliferator-activated receptor gamma coactivator-1-alpha (PGC-1α) by parkin [26,27]. This interaction has been shown to prevent the degeneration of dopaminergic neurons, demonstrating the therapeutic potential of CA against PD [27].
2.2. Alleviation of Oxidative Stress
Oxidative stress is a major contributing factor to neurodegenerative disorders [28]. Many studies have highlighted the anti-inflammatory and anti-oxidative properties of CA. Hou and colleagues [29] demonstrated the neuroprotective effect of CA on neuronal cells subjected to ischemia/hypoxia injury via the scavenging or reduction of ROS (reactive oxygen species) and NO (nitric oxide) and inhibition of COX-2 and MAPK pathways [29]. CA also displayed protective effects against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity by increasing the expression of antioxidant enzymes, including c-glutamate-cysteine ligase catalytic (GCLC) subunit, c-glutamate-cysteine ligase modifier (GCLM) subunit, superoxide dismutase (SOD), and glutathione reductase [30]. Furthermore, CA was also demonstrated to be cytoprotective against chlorpyrifos (CPF)-induced inflammation, oxidative stress, and neurotoxicity in brain and eye tissues of mice [31] as well as in SH-SY5Y cells [32]. CA protects against oxidative stress by employing various mechanisms, among which the induction of Nrf2-ARE and the activation of PI3K/Akt signaling pathways are the most significant and widely studied.
2.2.1. Induction of the Nrf2-ARE Response
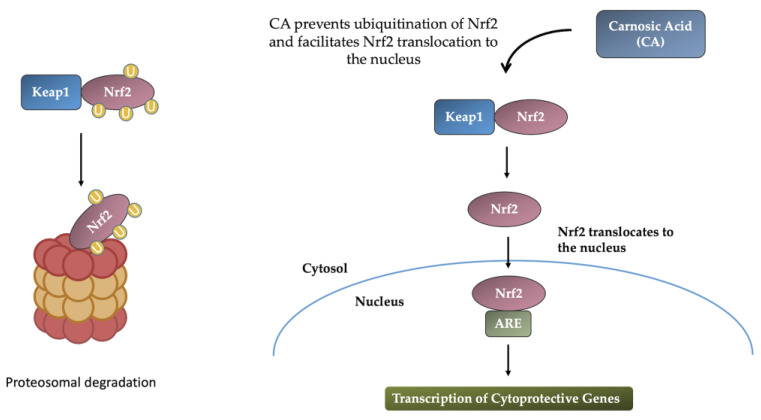
The pleiotropic transcription factor, nuclear factor erythroid 2-related factor 2 (Nrf2), is a master regulator of numerous cytoprotective genes. As an important mediator of the endogenous defense system, it serves to combat the imbalance between basal and injury-induced changes in ROS/RNS (reactive nitrogen species) and antioxidant/defense enzymes through its interaction with enhancer regions known as antioxidant response elements (AREs) of defense genes [33,34,35]. Under normal cellular conditions, Nrf2 remains sequestered in the cytoplasm by the regulator protein Keap1 (Kelch-like ECH-associated protein 1) [36], a component of the Cullin 3 (CUL3)-based E3 ubiquitin ligase complex. However, under conditions of cellular stress such as injury, toxicity, or oxidative stress, Nrf2 becomes uncoupled from Keap1 and translocates into the nucleus, where it induces the transcription of cytoprotective genes by binding with AREs [37,38].
As indicated above, CA is known to activate the Keap1/Nrf2 pathway, thereby resulting in the production of cytoprotective proteins. This highlights the significance of CA as a candidate neuroprotective agent for the treatment of neurodegenerative diseases. Activation of the neuroprotective Keap1/Nrf2 transcriptional pathway by CA involves the conversion of CA from an electrophilic precursor to an electrophilic form through a mechanism involving the release of Nrf2 from the Keap1/Nrf2 complex that results in the transcription of antioxidant enzymes (Figure 3) that protect neurons from oxidative stress and excitotoxicity [39]. It has also been shown that the hydrophilicity of CA is critical for its neuroprotective effects, which require both free carboxylic acid and catechol hydroxyl moieties [40]. The mechanism of neuroprotection of CA involves a sequence of events whereby the activation of the Keap1/Nrf2 pathway is followed by the transcription and induction of enzymes involved in glutathione (GSH) metabolism (glutathione S-transferase, alpha 4; glutathione S-transferase, alpha 2; and formylglutathione hydrolase) and phase 2 enzymes [NAD(P)H-quinone oxidoreductase1 and aldehyde dehydrogenase family 3, subfamily A1] that would lead to the activation of GSH metabolism [41]. GSH is a potent antioxidant that protects cells from the toxic effect of ROS.
Figure 3.
Induction of Nrf2-ARE response by CA. Under physiological conditions, Nrf2 binds to Keap1 and remains sequestered in the cytoplasm, where Nrf2 becomes ubiquitinated, released from Keap1, and degraded by the ubiquitin-proteosome complex. Carnosic acid blocks the ubiquitination of Nrf2 and facilitates its dissociation from Keap1, resulting in its translocation to the nucleus, where it binds to antioxidant response elements (AREs) of cytoprotective genes and facilitates transcription.
Interestingly, it has been reported that nerve growth factor (NGF), a proteinaceous neurotrophic molecule essential for the growth and functional maintenance of nervous system tissue, was markedly enhanced in T98G human glioblastoma cells following treatment with CA [42]. Investigations into the mechanisms by which CA increased the production of NGF revealed an Nrf2-dependent pathway whereby treatment increased nuclear accumulation of Nrf2 and the activation of Nrf2 target genes, including heme oxygenase 1 (HO-1) and thioredoxin reductase 1 (TXNRD1) [43]. The neuroprotective mechanism of CA was further delineated in a more recent study demonstrating a CA-mediated induction of the activating transcription factor 4 (ATF4) through the integrated stress response (ISR) pathway. This activation of Nrf2 and ATF4 by CA led to enhanced expression of NGF and other antioxidant genes, including HO-1 and TXNRD1 [44]. CA also worked synergistically with edavarone, a free radical scavenger, to enhance NGF expression in cultured human astrocytes exposed to hypoxia/re-oxygenation [45]. Application of CA to SH-SY5Y cells pretreated with the neurotoxin 6-OHDA facilitated the downregulation of the pro-apoptotic JNK and p38 signaling pathways. This down-regulation was driven by the Nrf2-mediated synthesis of GSH [46]. Similarly, by attenuating the 4-hydroxy-2-nonenal (4-HNE)-induced inhibition of mitochondrial respiration, CA can also alleviate mitochondrial dysfunction. 4-HNE is a by-product of lipid-peroxidation-induced membrane damage and plays a critical role in neurodegeneration. The attenuation of 4-HNE by CA is also associated with the induction of Nrf2-ARE [47]. CA exerts a similar neuroprotective effect through activation of Nrf2-ARE following traumatic-brain-injury-induced oxidative damage and mitochondrial dysfunction [48].
Additional evidence of the neuroprotective effects of CA was demonstrated in cultured rodent and human induced pluripotent stem cell-derived neurons treated with cyanide as well as in a non-Swiss albino mouse model of cyanide poisoning [49]. Acute exposure to cyanide in humans results in a delayed onset (up to weeks or even months) of a neurological syndrome that includes dystonia and signs and symptoms of Parkinsonism. Pretreatment of mice with 0.05% CA in food pellets for 1 week followed by twice daily intraperitoneal administration of 5–6 mg/kg potassium cyanide (KCN) for 8 days whilst maintaining oral treatment (via food) resulted in reduced neurotoxicity and improved neurobehavioral outcomes in treated mice [49]. Importantly, treatment with CA resulted in significantly reduced apoptosis in the frontal cortex, hippocampus, and striatum of KCN-poisoned mice [49]. CA was also capable of differentiating PC12 cells by activating Erk1/2 via the trkA, nerve growth factor receptor, independently of Nrf2 [50]. In addition, CA also affords neuroprotective effects by inhibiting ferroptosis via activation of the Nrf2 pathway [51]. Treatment of PC12 cells with erastin, a ferroptosis inducer, led to a dose-dependent loss in cell viability and decreased glutathione levels that were reversed following treatment with CA. In addition, CA also reversed the reduction in glutathione levels as well as the increase in reactive oxygen and nitrogen species induced by erastin [51].
A study in ovariectomized mice further demonstrated the neuroprotective role of CA in alleviating consequent depressive behaviors through the induction of serotonin and activation of Nrf2/HO-1 signaling [52]. CA also reversed the ovariectomy-induced suppression of the oxidoreductase protein, thioredoxin (Trx-1), and brain-derived neurotrophic factor (BDNF), a pivotal neurotrophic factor associated with neuronal survival. Treatment with CA for three weeks following ovariectomy also suppressed the oxidative stress markers GSH, malondialdehyde, and SOD as well as the pro-inflammatory cytokines TNF-α and IL-1β and ameliorated histopathological changes induced by ovariectomy [52]. Other evidence of the mood-altering effects of CA has been reported [53,54]. Observations of increased serotonin and BDNF suggest that CA may represent a novel therapeutic avenue for depressive behaviors that should be further explored.
2.2.2. Activation of the PI3K/Akt Signaling Pathway
The phosphoinositide-3-kinase (PI3K)/Akt signaling pathway is complex and is involved in numerous cellular functions, including cell growth, metabolism, proliferation, and survival, amongst others. These myriads of functions are driven by the ability of the pathway to regulate a broad spectrum of proteins, including NF-κB (nuclear factor kappa-light-chain-enhancer of activated B cells), another signaling molecule that is predominantly involved in cell survival, inflammation, and protection from toxicity. CA has been shown to mediate the activation of the PI3K/Akt/NF-κB pathway, leading to the upregulation of GSTP (Glutathione S-transferase pi) [55], one of the seven classes of GSTs and one that is highly expressed in glial cells of the nervous system. Upregulation of GSTP enzyme activity was shown to attenuate 6-OHDA-induced apoptosis and cell death both in SH-SY5Y neuroblastoma cells as well as in the striatum of mice [55]. Similarly, employing methylglyoxal (MG), the most potent inducer of advanced glycation end-products (AGEs), de Oliveira and colleagues [56] demonstrated that pretreatment of SH-SY5Y cells with CA prevented cells from damage caused by free radicals produced during the metabolism of MG. The cytoprotective effects of CA were exerted via activation of the PI3K/Akt/Nrf2 signaling pathway, where antioxidant enzymes were modulated by Nrf2 [56]. CA prevented MG-induced cell death by increasing levels of Bcl-2 (an apoptosis regulating protein) and decreasing levels of Bax (another apoptosis regulator), as well as by blocking cytochrome c release from mitochondria and the loss of mitochondrial membrane potential (MMP) induced by MG. CA pretreatment also inhibited caspase-3 and caspase-9 activation and decreased the fragmentation of DNA that is generally elicited by MG [56]. Similar effects of CA were observed employing the paraquat (PQ) model of PD, where cytoprotection was afforded by activation of Nrf2 through modulation of the PI3K/Akt pathway leading to an increase in levels of antioxidant enzymes [32,56,57]. It is further suggested that CA also exerts mitochondrial protection from glutamate-induced excitotoxicity. Results in SH-SY5Y cells treated with CA revealed prevention of glutamate-induced mitochondrial impairment and improved bioenergetics that was driven through the activation of Nrf2 [58].
2.3. Attenuation of Apoptosis
Although many studies highlight the role of CA in modulating autophagy, as discussed earlier, it is also found to play a critical role in the attenuation of apoptosis. Investigations have used variously in vitro and in vivo models of apoptosis to evaluate the neuroprotective role of CA and have revealed regulation at the level of apoptosis-inducible genes [59]. Studies in cultured dopaminergic cells (SN4741) employing the organochlorine pesticide dieldrin, which is known to be a risk factor for PD, revealed that neuroprotection afforded by CA was due to the repression of apoptosis-related caspase-3 and -12 and the stress signaling molecule c-Jun N-terminal kinase (JNK) [60]. Pretreatment of SN4741 cells with CA also significantly attenuated the downregulation of BDNF, a key molecule associated with dopaminergic neuron survival and maturation [60]. Treatment of these cells with dieldrin resulted in a 61% reduction in BDNF release from these cells, whereas pretreatment with 10 μM CA maintained levels of BDNF at basal expression [60]. Intriguingly, these results suggest that treatment of SN4741 cells with 10 μM CA results in a 1.5-fold increase in levels of BDNF, suggesting that prophylactic treatment with CA may support dopaminergic and other cells in the brain. In another example of the neuroprotective effects of CA, Wu and colleagues [30] reported that the cytoprotective effects of this diterpenoid were afforded by its anti-apoptotic action in 6-OHDA-treated rats and SH-SY5Y cells. This effect was mediated by Bax, a pro-apoptotic, and Bcl-2, an anti-apoptotic member of the Bcl-2 family of proteins. Treatment with CA was shown to reverse the 6-OHDA-induced reduction in the Bcl-2/Bax ratio [30]. CA also decreased 6-OHDA-induced apoptosis in SH-SY5Y cells via upregulation of GSTP through the activation of the PKA/CREB pathway and subsequent increase in the interaction between GSTP and JNK, resulting in an inhibition of JNK signaling [61].
Another study investigating the mechanism by which CA inhibits apoptosis revealed the role played by the E3 ubiquitin ligase, parkin. As indicated, parkin ubiquitinates misfolded proteins and facilitates their degradation via the ubiquitin-proteasome system [62,63]. Treatment of SH-SY5Y cells with 6-OHDA induced the expression of apoptosis-related protein in the TGF-β signaling pathway (ARTS), a pro-apoptotic protein, and reduced the expression of X-liked inhibitor of apoptosis protein (XIAP), a protein that directly blocks active sites of caspase 3 and caspase 7 and inhibits apoptosis. Pretreatment of SH-SY5Y cells with CA ameliorated the induction of ARTS and reduction of XIAP and also attenuated the activation of caspase 7 and 9, thereby reversing the apoptotic effects of 6-OHDA and shedding light on the therapeutic potential of CA in PD [64].
CA was also reported to exert a neuroprotective effect following subarachnoid hemorrhage induced by early brain injury through the inhibition of apoptosis [65]. Rats were subjected to a sub-arachnoid hemorrhage procedure, and those in the experimental group were then administered a 3 mg/kg dose of CA intraperitoneally. CA was shown to ameliorate brain edema and blood-brain barrier (BBB) disruption, as well as reduce neuronal death via apoptosis [65]. CA was also shown to increase SIRT1, a member of the highly conserved (NAD+)-dependent class of histone deacetylases responsible for combatting ROS and apoptosis, MnSOD (manganese superoxide dismutase, a metalloprotein that prevents mitochondrial dysfunction) and Bcl-2 (the founding member of a family of regulator proteins that regulate cell death) expression [65], as well as decreased p66shc, Bax, and cleaved caspase-3 expression. The anti-apoptotic effects of CA were proposed to be facilitated through the SIRT1/p66shc signaling pathway [65,66].
Importantly, CA was shown to inhibit cell growth and induce apoptosis in IMR-32 human neuroblastoma IMR-32 cells [67]. The induction of apoptosis was accompanied by ROS-mediated p38 MAPK activation resulting in a decrease in cell viability [67]. Intriguingly, these results suggest that the activity of CA is selective in its regulation of cell viability and apoptosis, whereby these processes are activated by CA to restore physiological states, implying the substantive therapeutic potential of this compound that warrants extensive investigation.
2.4. Effects of Carnosic Acid in Amyloid-β-Mediated Neurodegeneration
Brain atrophy associated with the deposition of Aβ in extracellular neuritic plaques is the most prominent neuropathological hallmark of Alzheimer’s disease (AD) [68]. Aβ-peptide, which constitutes the major component of amyloid plaques, is a 4-kDa peptide formed by the proteolytic cleavage of the amyloid precursor protein (APP) by β-secretase and the γ-secretase complex of proteins [69,70]. Cleavage of APP by β-secretase (β-site APP-cleaving enzyme-1 (BACE1)) catalyzes the critical step in the generation of Aβ. However, the constitutive pathway of APP processing is via α-secretase cleavage that results in the generation of a soluble ectodomain fragment termed soluble APPα (sAPPα), which possesses neurotrophic and neuroprotective properties [71,72,73]. The protective role of CA against neurodegeneration resulting from the presence of Aβ is well documented. An investigation of the effects of CA on Aβ production in SH-SY5Y human neuroblastoma cells revealed a critical role for this antioxidant in the suppression of Aβ42 generation, an isoform of the peptide that is known to be more hydrophobic and toxic as well as possessing faster oligomerizing properties compared to Aβ40. In the presence of CA, APP cleavage was shuttled to the α-secretase pathway, thereby precluding Aβ generation [74]. This shuttling in the presence of CA is driven by the upregulation of tumor necrosis factor-α-converting enzyme (TACE) mRNA, a member of the ADAM (a disintegrin and metalloproteinase) family of proteases, which contributes to α-secretase cleavage of APP [74]. Similarly, a substantial reduction in Aβ production by CA via the activation of TACE was evident in U373MG human astrocytoma cells [75]. Aβ also interacts with N-methyl-D-aspartate receptors (NMDARs) to induce apoptosis and synaptic dysregulation. In another study on SH-SY5Y cells, CA was shown to inhibit the phosphorylation of the NMDAR subtype 2B (NMDAR2B) receptor, thereby suppressing apoptosis and restoring expression of synaptic proteins including BDNF, postsynaptic density protein-95 (PSD-95), and synaptophysin [76]. Additionally, CA significantly attenuated apoptosis induced by Aβ42/43, further highlighting its therapeutic potential against Aβ-induced neurotoxicity [77].
In vivo, CA has been demonstrated to be protective to neurons in subfield CA1 (cornu Ammonis) of the hippocampus in an acute experimental rat model of AD (bilateral administration of Aβ into the hippocampus) where Aβ accumulation leads to neurodegeneration of the hippocampus [78]. Employing a similar in vivo paradigm, Rasoolijazi and colleagues [79] demonstrated the neuroprotective effects of CA on cognitive impairment associated with Aβ-induced neurotoxicity in the rat hippocampus. CA was shown to significantly improve short-term and spatial memory attributes in rat models of AD [79]. Furthermore, CA also delayed the deposition of Aβ and protected cells against Aβ-induced cholinergic and mitochondrial dysfunction in a Caenorhabditis elegans model of AD [80], thereby reiterating its promising potential as a neuroprotective agent against AD-associated neurodegeneration.
In recent efforts incorporating biomedical advances, nano-carrier packaged CA reduced the deposition of Aβ, subsequently restoring cognitive deficits through the inhibition of the CCAAT-enhancer-binding protein β (CEBPβ)-NFκB signaling pathway in APP/PS1 mice [81].
A recent study by Feng and colleagues [82] demonstrated a potential role of CA in the suppression of Apolipoprotein E ε4 (ApoE ε4)-associated AD. Apolipoprotein E is a major cholesterol transport protein. The ε4 allele of APOE is the strongest risk factor for late-onset AD (LOAD), the most common form of the disease that affects more than 97% of individuals diagnosed with AD. An increase in the cell surface expression of ApoE receptor 2 (ApoER2) activates the reelin signaling pathway that is important for synaptic plasticity in the adult brain. The intracellular binding of ApoE4 to ApoER2 inhibits the recycling of the receptor to the cell membrane and therefore renders neurons unresponsive to reelin [83]. CA counteracts the negative effects of ApoE ε4 by facilitating the binding of sorting nexin 17 (SNX17) to ApoER2, blocking ApoE ε4 binding and promoting the recycling of the receptor to the cell membrane [84] where reelin binds to the receptor, activating the pathway resulting in neurite growth [82].
2.5. Effects of Carnosic Acid in Models of Neuronal Injury
Intriguingly, CA also alleviated symptoms of metabolic-disease-induced brain injury through the modulation of inflammatory responses. In a high-fat-diet-induced mouse model, CA facilitated a significant decrease in the expression of various pro-inflammatory cytokines regulated by the NF-κB signaling pathway, including interleukin (IL)-1β, IL-6 and tumor necrosis factor-α (TNF-α). Additionally, it also modulated the apoptotic pathway through the increased expression of anti-apoptotic Bcl-2 and downregulation of the pro-apoptotic protein Bax and matrix metallopeptidase 9 (MMP9) [85].
Studies in levodopa-induced dyskinesia revealed that CA was capable of alleviating the detrimental effects of excessive levodopa through the attenuation of apoptotic cell death via the modulation of ERK1/2-c-Jun and induction of parkin [86]. It also attenuated inflammation, mitochondrial damage, and oxidative stress in isoflurane-treated neuronal cells through the activation of the AMPK/SIRT1 pathway [87]. CA has also been shown to exert anti-inflammatory responses in bone-marrow-derived macrophages through the modulation of the toll-like receptor 2 (TLR2) and MAPK/NF-κB signaling pathway, resulting in a decreased expression of TNF-α, IL-6, and IL-1β [88]. The anti-inflammatory response of CA was further demonstrated via an integrated proteomic and bioinformatic study that demonstrated the involvement of CA in the modulation of multiple inflammatory processes, including MAPK, NF-κB, and FoxO signaling pathways [89]. CA also inhibits the nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome, which plays a critical role in the pathogenesis of neurodegenerative disorders, including AD and PD and COVID-19, including ‘long-COVID’, thereby representing its therapeutic potential [90]. Additionally, its neuroprotective role in the prevention of prion protein (PrP) aggregation in cellular models as well as disruption of PrP aggregates in cell-free assays [91], raises interesting possibilities for considering CA as a potential adjuvant candidate against prion diseases, including Creutzfeldt–Jakob disease (CJD), Gerstmann–Straussler–Scheinker disease (GSS), and fatal familial insomnia (FFI).
Collectively, these studies demonstrate the cytoprotective characteristics afforded by CA and support its use as both a prophylactic and a neuroprotective compound that warrants continued investigation in diseases of the nervous system (summarized in Table 1).
Table 1.
Neuroprotective effects of carnosic acid and its associated mechanisms of action.
| Neuroprotective Effects | Mechanisms |
|---|---|
| Induction of autophagy | Activation of AMP-activated protein kinase (AMPK) [16] |
| Phosphorylation of protein kinase B (Akt) and extracellular signal-regulated kinase 1/2 (Erk1/2) [17,86] | |
| Induction of Parkin pathway [22,86] | |
| Enhancement of parkin/Beclin1 interaction [24] | |
| Activation of the PINK1/parkin/mitophagy pathway [25] | |
| Activation of the parkin/IKKγ/p65 pathway [26,27] | |
| Alleviation of oxidative stress | Induction of Nrf2-ARE response [39,40,41,42,43,44,45,46,47,48,49,50,51,52] |
| Activation of the PI3K/Akt signaling pathway [32,55,56,57,58] | |
| Attenuation of apoptosis | Repression of apoptosis-related caspase-3 and -12 and c-Jun N-terminal kinase (JNK) [60,61] |
| Attenuation of BDNF downregulation [60] | |
| Restoration of Bcl-2/Bax ratio [30] | |
| Activation of the PKA/CREB pathway [61] | |
| Amelioration of the induction of ARTS and reduction of XIAP [64] | |
| Activation of SIRT1/p66shc signaling pathway [65] | |
| Protection against Aβ-mediated neurodegeneration | Upregulation of tumor necrosis factor-α-converting enzyme (TACE) mRNA to suppress Aβ42 generation [74,75] |
| Inhibition of NMDAR subtype 2B (NMDAR2B) receptor phosphorylation [76] | |
| Restoration of cognitive impairment [78,79] | |
| Suppression of Aβ-induced cholinergic and mitochondrial dysfunction [80] | |
| Inhibition of the CCAAT-enhancer-binding protein β (CEBPβ)-NFκB signaling pathway [81] | |
| Suppression of Apolipoprotein E e4 (ApoE e4)-associated AD [82] | |
| Protective role in models of neuronal injury | Suppression of various pro-inflammatory cytokines [85] |
| Activation of AMPK/SIRT1 pathway [87] | |
| Modulation of the toll-like receptor 2 (TLR2), MAPK/NF-κB, and FoxO signaling pathway [88,89] | |
| Inhibition of the nucleotide-binding oligomerization domain-like receptor containing pyrin domain 3 (NLRP3) inflammasome [90] | |
| Prevention of prion protein (PrP) aggregation [91] |
3. Conclusions
The research discussed above reveals the neuroprotective effects of carnosic acid, the most abundant compound found in plants belonging to the family Lamiaceae, including rosemary and sage. When used either as a prophylactic or as a therapeutic, CA is capable of mitigating the damage caused to nervous system tissue, thereby revealing a unique role in the management of neurodegenerative disorders. A deeper understanding of the neuroprotective properties of CA will facilitate the broader applicability of this intriguing compound and may aid in its use in conjunction with mainstay treatments for neurological disorders.
Author Contributions
Conceptualization, R.M.D.H.; data curation, F.J.M.; writing—original draft preparation, F.J.M.; writing—content review and editing, S.Z. and R.M.D.H.; supervision—R.M.D.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
F.J.M. was the recipient of an International Research Support Initiative Program Scholarship No. 1-8/HEC/HRD/2018/8912 from the Higher Education Commission of Pakistan.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Birtic S., Dussort P., Pierre F.X., Bily A.C., Roller M. Carnosic acid. Phytochemistry. 2015;115:9–19. doi: 10.1016/j.phytochem.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 2.Rašković A., Milanovic I., Pavlovic N., Cebovic T., Vukmirovic S., Mikov M. Antioxidant activity of rosemary (Rosmarinus officinalis L.) essential oil and its hepatoprotective potential. BMC Complement. Altern. Med. 2014;14:225. doi: 10.1186/1472-6882-14-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Erkan N., Ayranci G., Ayranci E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008;110:76–82. doi: 10.1016/j.foodchem.2008.01.058. [DOI] [PubMed] [Google Scholar]
- 4.Taram F., Ignowski E., Duval N., Linseman D.A. Neuroprotection Comparison of Rosmarinic Acid and Carnosic Acid in Primary Cultures of Cerebellar Granule Neurons. Molecules. 2018;23:2956. doi: 10.3390/molecules23112956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Munné-Bosch S., Alegre L. Subcellular compartmentation of the diterpene carnosic acid and its derivatives in the leaves of rosemary. Plant Physiol. 2001;125:1094–1102. doi: 10.1104/pp.125.2.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Information N.C.f.B. PubChem Compound Summary for CID 65126, Carnosic Acid. [(accessed on 22 June 2021)]; Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Carnosic-acid.
- 7.Aruoma O.I., Halliwell B., Aeschbach R., Loligers J. Antioxidant and pro-oxidant properties of active rosemary constituents: Carnosol and carnosic acid. Xenobiotica. 1992;22:257–268. doi: 10.3109/00498259209046624. [DOI] [PubMed] [Google Scholar]
- 8.Haraguchi H., Saito T., Okamura N., Yagi A. Inhibition of lipid peroxidation and superoxide generation by diterpenoids from Rosmarinus officinalis. Planta Med. 1995;61:333–336. doi: 10.1055/s-2006-958094. [DOI] [PubMed] [Google Scholar]
- 9.Posadas S.J., Caz V., Largo C., De la Gandara B., Matallanas B., Reglero G., De Miguel E. Protective effect of supercritical fluid rosemary extract, Rosmarinus officinalis, on antioxidants of major organs of aged rats. Exp. Gerontol. 2009;44:383–389. doi: 10.1016/j.exger.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 10.Russo A., Lombardo L., Troncoso N., Garbarino J., Cardile V. Rosmarinus officinalis extract inhibits human melanoma cell growth. Nat. Prod. Commun. 2009;4:1707–1710. [PubMed] [Google Scholar]
- 11.Wijeratne S.S., Cuppett S.L. Potential of rosemary (Rosemarinus officinalis L.) diterpenes in preventing lipid hydroperoxide-mediated oxidative stress in Caco-2 cells. J. Agric. Food Chem. 2007;55:1193–1199. doi: 10.1021/jf063089m. [DOI] [PubMed] [Google Scholar]
- 12.Levine B., Klionsky D.J. Development by self-digestion: Molecular mechanisms and biological functions of autophagy. Dev. Cell. 2004;6:463–477. doi: 10.1016/S1534-5807(04)00099-1. [DOI] [PubMed] [Google Scholar]
- 13.Levine B., Kroemer G. Autophagy in the pathogenesis of disease. Cell. 2008;132:27–42. doi: 10.1016/j.cell.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan C.C., Yu J.T., Tan M.S., Jiang T., Zhu X.C., Tan L. Autophagy in aging and neurodegenerative diseases: Implications for pathogenesis and therapy. Neurobiol. Aging. 2014;35:941–957. doi: 10.1016/j.neurobiolaging.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 15.Murrow L., Debnath J. Autophagy as a stress-response and quality-control mechanism: Implications for cell injury and human disease. Annu. Rev. Pathol. 2013;8:105–137. doi: 10.1146/annurev-pathol-020712-163918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu J., Su H., Qu Q.M. Carnosic Acid Prevents Beta-Amyloid-Induced Injury in Human Neuroblastoma SH-SY5Y Cells via the Induction of Autophagy. Neurochem. Res. 2016;41:2311–2323. doi: 10.1007/s11064-016-1945-6. [DOI] [PubMed] [Google Scholar]
- 17.Shibata S., Ishitobi H., Miyaki S., Kawaoka T., Kayashima T., Matsubara K. Carnosic acid protects starvation-induced SH-SY5Y cell death through Erk1/2 and Akt pathways, autophagy, and FoxO3a. Int. J. Food Sci. Nutr. 2016;67:977–982. doi: 10.1080/09637486.2016.1208734. [DOI] [PubMed] [Google Scholar]
- 18.Shimura H., Hattori N., Kubo S., Mizuno Y., Asakawa S., Minoshima S., Shimizu N., Iwai K., Chiba T., Tanaka K., et al. Familial Parkinson disease gene product, parkin, is a ubiquitin-protein ligase. Nat. Genet. 2000;25:302–305. doi: 10.1038/77060. [DOI] [PubMed] [Google Scholar]
- 19.Kitada T., Asakawa S., Hattori N., Matsumine H., Yamamura Y., Minoshima S., Yokochi M., Mizuno Y., Shimizu N. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature. 1998;392:605–608. doi: 10.1038/33416. [DOI] [PubMed] [Google Scholar]
- 20.Farrer M., Chan P., Chen R., Tan L., Lincoln S., Hernandez D., Forno L., Gwinn-Hardy K., Petrucelli L., Hussey J., et al. Lewy bodies and parkinsonism in families with parkin mutations. Ann. Neurol. 2001;50:293–300. doi: 10.1002/ana.1132. [DOI] [PubMed] [Google Scholar]
- 21.Pramstaller P.P., Schlossmacher M.G., Jacques T.S., Scaravilli F., Eskelson C., Pepivani I., Hedrich K., Adel S., Gonzales-McNeal M., Hilker R., et al. Lewy body Parkinson’s disease in a large pedigree with 77 Parkin mutation carriers. Ann. Neurol. 2005;58:411–422. doi: 10.1002/ana.20587. [DOI] [PubMed] [Google Scholar]
- 22.Lin C.Y., Tsai C.W., Tsai C.W. Carnosic acid protects SH-SY5Y cells against 6-hydroxydopamine-induced cell death through upregulation of parkin pathway. Neuropharmacology. 2016;110:109–117. doi: 10.1016/j.neuropharm.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 23.Lonskaya I., Shekoyan A.R., Hebron M.L., Desforges N., Algarzae N.K., Moussa C.E. Diminished parkin solubility and co-localization with intraneuronal amyloid-beta are associated with autophagic defects in Alzheimer’s disease. J. Alzheimers Dis. 2013;33:231–247. doi: 10.3233/JAD-2012-121141. [DOI] [PubMed] [Google Scholar]
- 24.Lin C.Y., Tsai C.W. Carnosic Acid Attenuates 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Cells by Inducing Autophagy Through an Enhanced Interaction of Parkin and Beclin1. Mol. Neurobiol. 2017;54:2813–2822. doi: 10.1007/s12035-016-9873-7. [DOI] [PubMed] [Google Scholar]
- 25.Lin C.Y., Tsai C.W. PINK1/parkin-mediated mitophagy pathway is related to neuroprotection by carnosic acid in SH-SY5Y cells. Food Chem. Toxicol. 2019;125:430–437. doi: 10.1016/j.fct.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 26.Lin C.Y., Chen W.J., Fu R.H., Tsai C.W. Upregulation of OPA1 by carnosic acid is mediated through induction of IKKgamma ubiquitination by parkin and protects against neurotoxicity. Food Chem. Toxicol. 2020;136:110942. doi: 10.1016/j.fct.2019.110942. [DOI] [PubMed] [Google Scholar]
- 27.Lin C.Y., Huang Y.N., Fu R.H., Liao Y.H., Kuo T.Y., Tsai C.W. Promotion of mitochondrial biogenesis via the regulation of PARIS and PGC-1alpha by parkin as a mechanism of neuroprotection by carnosic acid. Phytomedicine. 2021;80:153369. doi: 10.1016/j.phymed.2020.153369. [DOI] [PubMed] [Google Scholar]
- 28.Olufunmilayo E.O., Gerke-Duncan M.B., Holsinger R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative disorders. Antioxidants. 2023. under review . [DOI] [PMC free article] [PubMed]
- 29.Hou C.W., Lin Y.T., Chen Y.L., Wang Y.H., Chou J.L., Ping L.Y., Jeng K.C. Neuroprotective effects of carnosic acid on neuronal cells under ischemic and hypoxic stress. Nutr. Neurosci. 2012;15:257–263. doi: 10.1179/1476830512Y.0000000021. [DOI] [PubMed] [Google Scholar]
- 30.Wu C.R., Tsai C.W., Chang S.W., Lin C.Y., Huang L.C., Tsai C.W. Carnosic acid protects against 6-hydroxydopamine-induced neurotoxicity in in vivo and in vitro model of Parkinson’s disease: Involvement of antioxidative enzymes induction. Chem. Biol. Interact. 2015;225:40–46. doi: 10.1016/j.cbi.2014.11.011. [DOI] [PubMed] [Google Scholar]
- 31.AlKahtane A.A., Ghanem E., Bungau S.G., Alarifi S., Ali D., AlBasher G., Alkahtani S., Aleya L., Abdel-Daim M.M. Carnosic acid alleviates chlorpyrifos-induced oxidative stress and inflammation in mice cerebral and ocular tissues. Environ. Sci. Pollut. Res. Int. 2020;27:11663–11670. doi: 10.1007/s11356-020-07736-1. [DOI] [PubMed] [Google Scholar]
- 32.De Oliveira M.R., Peres A., Ferreira G.C., Schuck P.F., Bosco S.M. Carnosic Acid Affords Mitochondrial Protection in Chlorpyrifos-Treated SH-SY5Y Cells. Neurotox. Res. 2016;30:367–379. doi: 10.1007/s12640-016-9620-x. [DOI] [PubMed] [Google Scholar]
- 33.Chan J.Y., Han X.L., Kan Y.W. Isolation of cDNA encoding the human NF-E2 protein. Proc. Natl. Acad. Sci. USA. 1993;90:11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moi P., Chan K., Asunis I., Cao A., Kan Y.W. Isolation of NF-E2-related factor 2 (Nrf2), a NF-E2-like basic leucine zipper transcriptional activator that binds to the tandem NF-E2/AP1 repeat of the beta-globin locus control region. Proc. Natl. Acad. Sci. USA. 1994;91:9926–9930. doi: 10.1073/pnas.91.21.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kensler T.W., Wakabayashi N., Biswal S. Cell survival responses to environmental stresses via the Keap1-Nrf2-ARE pathway. Annu. Rev. Pharmacol. Toxicol. 2007;47:89–116. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]
- 36.Itoh K., Wakabayashi N., Katoh Y., Ishii T., Igarashi K., Engel J.D., Yamamoto M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999;13:76–86. doi: 10.1101/gad.13.1.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tong K.I., Kobayashi A., Katsuoka F., Yamamoto M. Two-site substrate recognition model for the Keap1-Nrf2 system: A hinge and latch mechanism. Biol. Chem. 2006;387:1311–1320. doi: 10.1515/BC.2006.164. [DOI] [PubMed] [Google Scholar]
- 38.Jain A.K., Bloom D.A., Jaiswal A.K. Nuclear import and export signals in control of Nrf2. J. Biol. Chem. 2005;280:29158–29168. doi: 10.1074/jbc.M502083200. [DOI] [PubMed] [Google Scholar]
- 39.Lipton S.A., Rezaie T., Nutter A., Lopez K.M., Parker J., Kosaka K., Satoh T., McKercher S.R., Masliah E., Nakanishi N. Therapeutic advantage of pro-electrophilic drugs to activate the Nrf2/ARE pathway in Alzheimer’s disease models. Cell Death Dis. 2016;7:e2499. doi: 10.1038/cddis.2016.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Satoh T., Izumi M., Inukai Y., Tsutsumi Y., Nakayama N., Kosaka K., Shimojo Y., Kitajima C., Itoh K., Yokoi T., et al. Carnosic acid protects neuronal HT22 Cells through activation of the antioxidant-responsive element in free carboxylic acid- and catechol hydroxyl moieties-dependent manners. Neurosci. Lett. 2008;434:260–265. doi: 10.1016/j.neulet.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 41.Tamaki Y., Tabuchi T., Takahashi T., Kosaka K., Satoh T. Activated glutathione metabolism participates in protective effects of carnosic acid against oxidative stress in neuronal HT22 cells. Planta Med. 2010;76:683–688. doi: 10.1055/s-0029-1240622. [DOI] [PubMed] [Google Scholar]
- 42.Kosaka K., Yokoi T. Carnosic acid, a component of rosemary (Rosmarinus officinalis L.), promotes synthesis of nerve growth factor in T98G human glioblastoma cells. Biol. Pharm. Bull. 2003;26:1620–1622. doi: 10.1248/bpb.26.1620. [DOI] [PubMed] [Google Scholar]
- 43.Mimura J., Kosaka K., Maruyama A., Satoh T., Harada N., Yoshida H., Satoh K., Yamamoto M., Itoh K. Nrf2 regulates NGF mRNA induction by carnosic acid in T98G glioblastoma cells and normal human astrocytes. J. Biochem. 2011;150:209–217. doi: 10.1093/jb/mvr065. [DOI] [PubMed] [Google Scholar]
- 44.Mimura J., Inose-Maruyama A., Taniuchi S., Kosaka K., Yoshida H., Yamazaki H., Kasai S., Harada N., Kaufman R.J., Oyadomari S., et al. Concomitant Nrf2- and ATF4-activation by Carnosic Acid Cooperatively Induces Expression of Cytoprotective Genes. Int. J. Mol. Sci. 2019;20:1706. doi: 10.3390/ijms20071706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshida H., Mimura J., Imaizumi T., Matsumiya T., Ishikawa A., Metoki N., Tanji K., Ota K., Hayakari R., Kosaka K., et al. Edaravone and carnosic acid synergistically enhance the expression of nerve growth factor in human astrocytes under hypoxia/reoxygenation. Neurosci. Res. 2011;69:291–298. doi: 10.1016/j.neures.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 46.Chen J.H., Ou H.P., Lin C.Y., Lin F.J., Wu C.R., Chang S.W., Tsai C.W. Carnosic acid prevents 6-hydroxydopamine-induced cell death in SH-SY5Y cells via mediation of glutathione synthesis. Chem. Res. Toxicol. 2012;25:1893–1901. doi: 10.1021/tx300171u. [DOI] [PubMed] [Google Scholar]
- 47.Miller D.M., Singh I.N., Wang J.A., Hall E.D. Administration of the Nrf2-ARE activators sulforaphane and carnosic acid attenuates 4-hydroxy-2-nonenal-induced mitochondrial dysfunction ex vivo. Free Radic. Biol. Med. 2013;57:1–9. doi: 10.1016/j.freeradbiomed.2012.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller D.M., Singh I.N., Wang J.A., Hall E.D. Nrf2-ARE activator carnosic acid decreases mitochondrial dysfunction, oxidative damage and neuronal cytoskeletal degradation following traumatic brain injury in mice. Exp. Neurol. 2015;264:103–110. doi: 10.1016/j.expneurol.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang D., Lee B., Nutter A., Song P., Dolatabadi N., Parker J., Sanz-Blasco S., Newmeyer T., Ambasudhan R., McKercher S.R., et al. Protection from cyanide-induced brain injury by the Nrf2 transcriptional activator carnosic acid. J. Neurochem. 2015;133:898–908. doi: 10.1111/jnc.13074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kosaka K., Mimura J., Itoh K., Satoh T., Shimojo Y., Kitajima C., Maruyama A., Yamamoto M., Shirasawa T. Role of Nrf2 and p62/ZIP in the neurite outgrowth by carnosic acid in PC12h cells. J. Biochem. 2010;147:73–81. doi: 10.1093/jb/mvp149. [DOI] [PubMed] [Google Scholar]
- 51.Cheng J., Xu T., Xun C., Guo H., Cao R., Gao S., Sheng W. Carnosic acid protects against ferroptosis in PC12 cells exposed to erastin through activation of Nrf2 pathway. Life Sci. 2021;266:118905. doi: 10.1016/j.lfs.2020.118905. [DOI] [PubMed] [Google Scholar]
- 52.Samy D.M., Mostafa D.K., Saleh S.R., Hassaan P.S., Zeitoun T.M., Ammar G.A.G., Elsokkary N.H. Carnosic Acid Mitigates Depression-Like Behavior in Ovariectomized Mice via Activation of Nrf2/HO-1 Pathway. Mol. Neurobiol. 2023;60:610–628. doi: 10.1007/s12035-022-03093-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang X.Q., Tang Y.H., Zeng G.R., Wu L.F., Zhou Y.J., Cheng Z.N., Jiang D.J. Carnosic acid alleviates depression-like behaviors on chronic mild stressed mice via PPAR-gamma-dependent regulation of ADPN/FGF9 pathway. Psychopharmacology. 2021;238:501–516. doi: 10.1007/s00213-020-05699-2. [DOI] [PubMed] [Google Scholar]
- 54.Azhar M., Zeng G., Ahmed A., Dar Farooq A., Choudhary M.I., De-Jiang J., Liu X. Carnosic acid ameliorates depressive-like symptoms along with the modulation of FGF9 in the hippocampus of middle carotid artery occlusion-induced Sprague Dawley rats. Phytother. Res. 2021;35:384–391. doi: 10.1002/ptr.6810. [DOI] [PubMed] [Google Scholar]
- 55.Lin C.Y., Chen J.H., Fu R.H., Tsai C.W. Induction of Pi form of glutathione S-transferase by carnosic acid is mediated through PI3K/Akt/NF-kappaB pathway and protects against neurotoxicity. Chem. Res. Toxicol. 2014;27:1958–1966. doi: 10.1021/tx5003063. [DOI] [PubMed] [Google Scholar]
- 56.De Oliveira M.R., Ferreira G.C., Schuck P.F., Dal Bosco S.M. Role for the PI3K/Akt/Nrf2 signaling pathway in the protective effects of carnosic acid against methylglyoxal-induced neurotoxicity in SH-SY5Y neuroblastoma cells. Chem. Biol. Interact. 2015;242:396–406. doi: 10.1016/j.cbi.2015.11.003. [DOI] [PubMed] [Google Scholar]
- 57.De Oliveira M.R., Peres A., Ferreira G.C., Schuck P.F., Gama C.S., Bosco S.M.D. Carnosic Acid Protects Mitochondria of Human Neuroblastoma SH-SY5Y Cells Exposed to Paraquat Through Activation of the Nrf2/HO-1Axis. Mol. Neurobiol. 2017;54:5961–5972. doi: 10.1007/s12035-016-0100-3. [DOI] [PubMed] [Google Scholar]
- 58.De Oliveira M.R., Duarte A.R., Chenet A.L., de Almeida F.J.S., Andrade C.M.B. Carnosic Acid Pretreatment Attenuates Mitochondrial Dysfunction in SH-SY5Y Cells in an Experimental Model of Glutamate-Induced Excitotoxicity. Neurotox. Res. 2019;36:551–562. doi: 10.1007/s12640-019-00044-8. [DOI] [PubMed] [Google Scholar]
- 59.Iorio R., Celenza G., Petricca S. Multi-Target Effects of ss-Caryophyllene and Carnosic Acid at the Crossroads of Mitochondrial Dysfunction and Neurodegeneration: From Oxidative Stress to Microglia-Mediated Neuroinflammation. Antioxidants. 2022;11:1199. doi: 10.3390/antiox11061199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Park J.A., Kim S., Lee S.Y., Kim C.S., Kim D.K., Kim S.J., Chun H.S. Beneficial effects of carnosic acid on dieldrin-induced dopaminergic neuronal cell death. Neuroreport. 2008;19:1301–1304. doi: 10.1097/WNR.0b013e32830abc1f. [DOI] [PubMed] [Google Scholar]
- 61.Lin C.Y., Fu R.H., Chou R.H., Chen J.H., Wu C.R., Chang S.W., Tsai C.W. Inhibition of JNK by pi class of glutathione S-transferase through PKA/CREB pathway is associated with carnosic acid protection against 6-hydroxydopamine-induced apoptosis. Food Chem. Toxicol. 2017;103:194–202. doi: 10.1016/j.fct.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 62.Quinn P.M.J., Moreira P.I., Ambrosio A.F., Alves C.H. PINK1/PARKIN signalling in neurodegeneration and neuroinflammation. Acta Neuropathol. Commun. 2020;8:189. doi: 10.1186/s40478-020-01062-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Charan R.A., LaVoie M.J. Pathologic and therapeutic implications for the cell biology of parkin. Mol. Cell. Neurosci. 2015;66:62–71. doi: 10.1016/j.mcn.2015.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fu R.H., Huang L.C., Lin C.Y., Tsai C.W. Modulation of ARTS and XIAP by Parkin Is Associated with Carnosic Acid Protects SH-SY5Y Cells against 6-Hydroxydopamine-Induced Apoptosis. Mol. Neurobiol. 2018;55:1786–1794. doi: 10.1007/s12035-017-0443-4. [DOI] [PubMed] [Google Scholar]
- 65.Teng L., Fan L., Peng Y., He X., Chen H., Duan H., Yang F., Lin D., Lin Z., Li H., et al. Carnosic Acid Mitigates Early Brain Injury After Subarachnoid Hemorrhage: Possible Involvement of the SIRT1/p66shc Signaling Pathway. Front. Neurosci. 2019;13:26. doi: 10.3389/fnins.2019.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shan W., Gao L., Zeng W., Hu Y., Wang G., Li M., Zhou J., Ma X., Tian X., Yao J. Activation of the SIRT1/p66shc antiapoptosis pathway via carnosic acid-induced inhibition of miR-34a protects rats against nonalcoholic fatty liver disease. Cell Death Dis. 2015;6:e1833. doi: 10.1038/cddis.2015.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsai C.W., Lin C.Y., Lin H.H., Chen J.H. Carnosic acid, a rosemary phenolic compound, induces apoptosis through reactive oxygen species-mediated p38 activation in human neuroblastoma IMR-32 cells. Neurochem. Res. 2011;36:2442–2451. doi: 10.1007/s11064-011-0573-4. [DOI] [PubMed] [Google Scholar]
- 68.Parihar M.S., Hemnani T. Alzheimer’s disease pathogenesis and therapeutic interventions. J. Clin. Neurosci. 2004;11:456–467. doi: 10.1016/j.jocn.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 69.Gandy S. The role of cerebral amyloid beta accumulation in common forms of Alzheimer disease. J. Clin. Investig. 2005;115:1121–1129. doi: 10.1172/JCI25100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mattson M.P. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Furukawa K., Sopher B.L., Rydel R.E., Begley J.G., Pham D.G., Martin G.M., Fox M., Mattson M.P. Increased activity-regulating and neuroprotective efficacy of alpha-secretase-derived secreted amyloid precursor protein conferred by a C-terminal heparin-binding domain. J. Neurochem. 1996;67:1882–1896. doi: 10.1046/j.1471-4159.1996.67051882.x. [DOI] [PubMed] [Google Scholar]
- 72.Meziane H., Dodart J.C., Mathis C., Little S., Clemens J., Paul S.M., Ungerer A. Memory-enhancing effects of secreted forms of the beta-amyloid precursor protein in normal and amnestic mice. Proc. Natl. Acad. Sci. USA. 1998;95:12683–12688. doi: 10.1073/pnas.95.21.12683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stein T.D., Anders N.J., DeCarli C., Chan S.L., Mattson M.P., Johnson J.A. Neutralization of transthyretin reverses the neuroprotective effects of secreted amyloid precursor protein (APP) in APPSW mice resulting in tau phosphorylation and loss of hippocampal neurons: Support for the amyloid hypothesis. J. Neurosci. 2004;24:7707–7717. doi: 10.1523/JNEUROSCI.2211-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Meng P., Yoshida H., Matsumiya T., Imaizumi T., Tanji K., Xing F., Hayakari R., Dempoya J., Tatsuta T., Aizawa-Yashiro T., et al. Carnosic acid suppresses the production of amyloid-beta 1-42 by inducing the metalloprotease gene TACE/ADAM17 in SH-SY5Y human neuroblastoma cells. Neurosci. Res. 2013;75:94–102. doi: 10.1016/j.neures.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 75.Yoshida H., Meng P., Matsumiya T., Tanji K., Hayakari R., Xing F., Wang L., Tsuruga K., Tanaka H., Mimura J., et al. Carnosic acid suppresses the production of amyloid-beta 1-42 and 1-43 by inducing an alpha-secretase TACE/ADAM17 in U373MG human astrocytoma cells. Neurosci. Res. 2014;79:83–93. doi: 10.1016/j.neures.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 76.Liu W.Y., Li Y., Li Y., Xu L.Z., Jia J.P. Carnosic Acid Attenuates AbetaOs-Induced Apoptosis and Synaptic Impairment via Regulating NMDAR2B and Its Downstream Cascades in SH-SY5Y Cells. Mol. Neurobiol. 2023;60:133–144. doi: 10.1007/s12035-022-03032-w. [DOI] [PubMed] [Google Scholar]
- 77.Meng P., Yoshida H., Tanji K., Matsumiya T., Xing F., Hayakari R., Wang L., Tsuruga K., Tanaka H., Mimura J., et al. Carnosic acid attenuates apoptosis induced by amyloid-beta 1-42 or 1-43 in SH-SY5Y human neuroblastoma cells. Neurosci. Res. 2015;94:1–9. doi: 10.1016/j.neures.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Azad N., Rasoolijazi H., Joghataie M.T., Soleimani S. Neuroprotective effects of carnosic Acid in an experimental model of Alzheimer’s disease in rats. Cell J. 2011;13:39–44. [PMC free article] [PubMed] [Google Scholar]
- 79.Rasoolijazi H., Azad N., Joghataei M.T., Kerdari M., Nikbakht F., Soleimani M. The protective role of carnosic acid against beta-amyloid toxicity in rats. Sci. World J. 2013;2013:917082. doi: 10.1155/2013/917082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chen Y., Wang Y., Qin Q., Zhang Y., Xie L., Xiao J., Cao Y., Su Z., Chen Y. Carnosic acid ameliorated Abeta-mediated (amyloid-beta peptide) toxicity, cholinergic dysfunction and mitochondrial defect in Caenorhabditis elegans of Alzheimer’s Model. Food Funct. 2022;13:4624–4640. doi: 10.1039/D1FO02965G. [DOI] [PubMed] [Google Scholar]
- 81.Yi-Bin W., Xiang L., Bing Y., Qi Z., Fei-Tong J., Minghong W., Xiangxiang Z., Le K., Yan L., Ping S., et al. Inhibition of the CEBPbeta-NFkappaB interaction by nanocarrier-packaged Carnosic acid ameliorates glia-mediated neuroinflammation and improves cognitive function in an Alzheimer’s disease model. Cell Death Dis. 2022;13:318. doi: 10.1038/s41419-022-04765-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Feng M., Cui D., Li Y., Shi J., Xiang L., Bian H., Ma Z., Xia W., Wei G. Carnosic Acid Reverses the Inhibition of ApoE4 on Cell Surface Level of ApoER2 and Reelin Signaling Pathway. J. Alzheimers Dis. 2020;73:517–528. doi: 10.3233/JAD-190914. [DOI] [PubMed] [Google Scholar]
- 83.Chen Y., Durakoglugil M.S., Xian X., Herz J. ApoE4 reduces glutamate receptor function and synaptic plasticity by selectively impairing ApoE receptor recycling. Proc. Natl. Acad. Sci. USA. 2010;107:12011–12016. doi: 10.1073/pnas.0914984107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sotelo P., Farfan P., Benitez M.L., Bu G., Marzolo M.P. Sorting nexin 17 regulates ApoER2 recycling and reelin signaling. PLoS ONE. 2014;9:e93672. doi: 10.1371/journal.pone.0093672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu Y., Zhang Y., Hu M., Li Y.H., Cao X.H. Carnosic acid alleviates brain injury through NF-kappaB-regulated inflammation and Caspase-3-associated apoptosis in high fat-induced mouse models. Mol. Med. Rep. 2019;20:495–504. doi: 10.3892/mmr.2019.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lai C.Y., Lin C.Y., Wu C.R., Tsai C.H., Tsai C.W. Carnosic Acid Alleviates Levodopa-Induced Dyskinesia and Cell Death in 6-Hydroxydopamine-lesioned Rats and in SH-SY5Y Cells. Front. Pharmacol. 2021;12:703894. doi: 10.3389/fphar.2021.703894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liao O., Xie K., Zhang X., Jiang W., Li W., Xie A. Carnosic acid attenuates inflammation, oxidative stress and mitochondrial dysfunction in neurons via activation of AMPK/SIRT1 pathway. Trop. J. Pharm. Res. 2022;21:2359–2365. [Google Scholar]
- 88.Park M.-Y. Carnosic acid disrupts toll-like receptor 2 signaling pathway in Pam 3 CSK 4-stimulated macrophages. Toxicol. Environ. Health Sci. 2015;7:224–230. doi: 10.1007/s13530-015-0242-0. [DOI] [Google Scholar]
- 89.Wang L.C., Wei W.H., Zhang X.W., Liu D., Zeng K.W., Tu P.F. An Integrated Proteomics and Bioinformatics Approach Reveals the Anti-inflammatory Mechanism of Carnosic Acid. Front. Pharmacol. 2018;9:370. doi: 10.3389/fphar.2018.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Satoh T., Trudler D., Oh C.K., Lipton S.A. Potential Therapeutic Use of the Rosemary Diterpene Carnosic Acid for Alzheimer’s Disease, Parkinson’s Disease, and Long-COVID through NRF2 Activation to Counteract the NLRP3 Inflammasome. Antioxidants. 2022;11:124. doi: 10.3390/antiox11010124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Karagianni K., Pettas S., Kanata E., Lioulia E., Thune K., Schmitz M., Tsamesidis I., Lymperaki E., Xanthopoulos K., Sklaviadis T., et al. Carnosic Acid and Carnosol Display Antioxidant and Anti-Prion Properties in In Vitro and Cell-Free Models of Prion Diseases. Antioxidants. 2022;11:726. doi: 10.3390/antiox11040726. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the article.